- The First Affiliated Hospital, College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
Limb-girdle muscular dystrophy type 2A (LGMD R1 Calpain 3-Related, LGMD2A/R1), an autosomal recessive disorder, is characterized by progressive muscle weakness with a prominent presentation in the proximal limb girdle muscles. LGMD2A/R1, which is caused by variants in calcium-activated neutral proteinase 3 (CAPN3), is the most common. The present study aimed at identifying the clinically significant variants in a Chinese family with LGMD2A/R1 and exploring the genotype–phenotype correlations. Clinical symptoms, laboratory findings, and physical examinations were obtained. Genomic DNA was extracted from the peripheral blood samples of this family. Whole-exome sequencing (WES) and Sanger sequencing were used to explore and validate the pathogenic genes. In this study, the proband and his sister, who had two identical mutations in the CAPN3 gene sequence, exhibited diverse clinical features, including disease onset and progression. The mutation c.2120 A>G (p. D707G) is pathogenic and has been reported in the Human Gene Mutation Database (HGMD) and the ClinVar database. c.1783-72 C>G may be a novel pathogenic mutation of LGMD2A/R1 based on the American College of Medical Genetics (ACMG) guidelines, which widens the gene variant pool in CAPN3 and improves diagnosis and genetic counseling.
1 Introduction
Limb-girdle muscular dystrophy (LGMD) is a genetically inherited disease, classified into autosomal dominant (AD-LGMD) and autosomal recessive (AR-LGMD) (Bushby, 1995). LGMD mainly affects the skeletal muscle, which progressively leads to the weakness of proximal muscle by loss of muscle fibers (Fanin and Angelini, 2015). Limb-girdle muscular dystrophy type 2A (LGMD R1 Calpain 3-Related, LGMD2A/R1), a prevalent form in many countries, is mainly caused by the calcium-activated neutral proteinase 3 (CAPN3) gene mutations (Fanin et al., 2009). CAPN3 is a calcium-dependent cysteine protease responsible for assembling and remodeling the sarcomere, regulating calcium outflow from the sarcoplasmic reticulum, and repairing sarcolemma (Straub et al., 2018).
Diagnosing LGMD2A/R1 is difficult due to laboratory examination results and clinical manifestations that overlap other LGMDs (Fanin et al., 2009). Furthermore, the clinical manifestations of LGMD2A/R1 are highly variable in onset of age, degree of muscular weakness, and disease progression. Genotype–phenotype correlation is quite challenging due to the genetic heterogeneity of CAPN3 and variations in clinical manifestations. Thus, the LGMD2A/R1 remains to be explored.
In the present study, we reported two siblings diagnosed as LGMD2A/R1. The siblings exhibited diverse clinical features with two identical mutations in the CAPN3 gene sequence, one of which has been reported. The novel mutation c.1783-72 C>G may be pathogenic.
2 Case report and methods
2.1 Subjects
In this study, the clinical and genetic data of a Chinese family were collected. The case study was approved by the Ethics Committee of the First Affiliated Hospital and the College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China (ethical approval number: 2022-03-B127). Informed consent was acquired from the patient (or relative/guardian) AND that patient (or relative/guardian) consented to the publishing of all images, clinical data, and other data included in the manuscript.
The proband (a 33-year-old male) was born to non-consanguineous Chinese parents. He presented with progressive weakness, decreased stride frequencies, increased unsteadiness in both lower limbs, and, at the age of 15, needed knee support while standing. Over time, he began to fall to the ground frequently and required help to rise. Three years ago, he could not walk, comb his hair, or get dressed unaided. The weakness and atrophy of the proximal limbs, a wide-based gait, hyperlordosis, scapular winging, and anterior pelvic tilt were found through physical examination. The strength was measured with the Medical Research Council muscle testing/MRC scale (MacAvoy and Green, 2007): deltoid 2/5, biceps/triceps 3/5, quadriceps and biceps femoris 3/5, and gluteus maximus 2/5. The facial and bulbar muscles were not involved. The cardiovascular and respiratory systems were normal. At 16 years of age, his blood analysis results showed elevated creatine kinase levels of 3,000 U/L (normal value < 150 U/L), which decreased to 1,700 U/L at 21. Electromyography (EMG) showed severe generalized myopathy with low-amplitude short-duration motor units. Nerve conduction was spared. The electrocardiogram (ECG) and ultrasound cardiogram (UCG) results were normal. He is now wheelchair-dependent and unable to take care of himself. Due to regular follow-up, the proband has a stable mood and sleeps well. The patient was treated with oral inosine and functional exercise. He consequently developed concomitant hyperthyroidism, and he was put on oral propylthiouracil tablets.
The sister of the proband was a 36-year-old woman. She complained of paroxysmal myalgia after exercise and hyperCKemia for 7 years. Progressively, she presented with proximal lower limb weakness. Her physical examination showed bilateral atrophy of the proximal lower limbs, scapular winging, bilateral calf hypertrophy, and slight weakness (the MRC scale: deltoid 4+/5, musculus biceps brachii 5−/5, forearm flexors 5/5, forearm extensor 4/5, iliopsoas and gluteus maximus 5−/5, pronator and supinator 5/5) and a positive Gower’s maneuver result. Her creatine kinase level was 1,800 U/L at the first evaluation and 3,000 U/L at the 1-month follow-up. EMG results showed features of myopathy. Within 3 years of follow-up, she had difficulty climbing the stairs and needed to hang on to the railing with both hands. On physical examination, the patient’s deltoid muscle, proximal thigh, and right calf were atrophic (Figure 1A). The proximal limb weakness progressively aggravated (the MRC scale: deltoid 4/5, musculus biceps brachii 4/5, forearm flexors 4/5, forearm extensor 3/5, iliopsoas and gluteus maximus 4-/5, pronator and supinator 5/5). Muscular MRI of the lower limb showed that the glutei/iliopsoas muscles, vastus lateralis/gracilis, adductors/biceps, femoris/semitendinosus and semimembranosus muscles, and gastrocnemius/soleus were affected (Figure 1B). Muscle fibers became thinner, accompanied by fat infiltration and edema. She had no sleeping or emotional disorder. She took vitamin E tablets and trimetazidine hydrochloride tablets orally. Meanwhile, she was prescribed functional training.
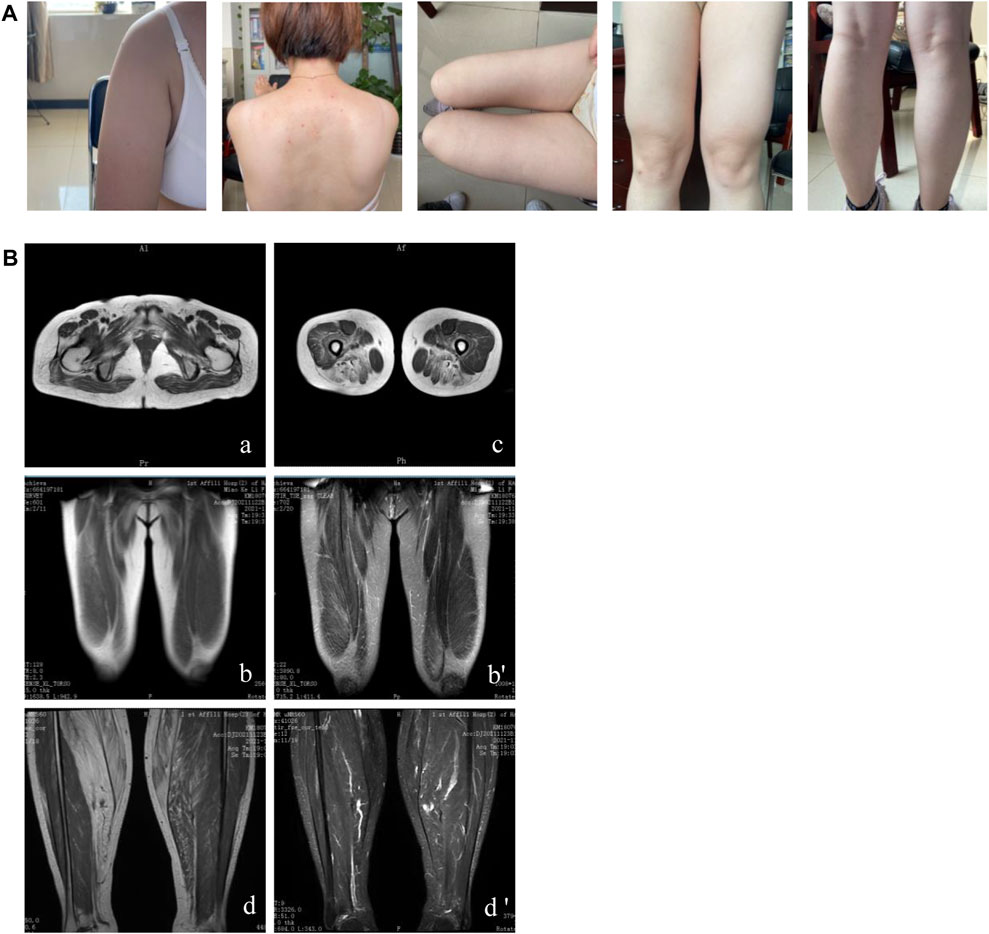
Figure 1. Clinical features and muscular MRI of patient II-3. (A) Winged scapulae, atrophy of the deltoid, inner muscles of the thigh and right calf, and left calf pseudo-hypertrophy are shown. (B) The affected glutei/iliopsoas muscles (A), vastus lateralis/gracilis (B), adductors/biceps femoris/semitendinosus and semimembranosus muscles (C), and gastrocnemius/soleus (D) were presented through muscular MRI.
2.2 Muscle biopsies
Samples of the quadriceps femoris muscle of patient Ⅱ-3 were collected for muscle biopsies according to the conventional methods.
2.3 Whole-exome sequencing
Peripheral blood samples were collected from all the members of the family, and genomic DNA was extracted. Agilent SureSelect Human All Exon V6 reagent was used for capture and enrichment, and Illumina NovaSeq 6000 was applied for whole-exon gene sequencing in the proband. The results were aligned to the human reference genome hg19. Variations were annotated according to the American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al., 2015). Some databases, including the Human Gene Mutation Database (HGMD), ClinVar database, 1,000 Genomes, Exome Aggregation Consortium (ExAC), and genome Aggregation Database (gnomAD), were applied to determine the known or potential novel mutations. Predictions for the pathogenicity of mutations were performed with SIFT, PolyPhen-2, and MutationTaster.
2.4 Sanger sequencing
Sanger sequencing to predict and analyze the mutation splicing sites. The primers were designed to target CAPN3 (Forward: 5′-CTCCAAGTGCCTTCTGAATGACCACAGGCG-3′; Reverse: 5′-CATCTCGTAGCTGTTGATGGTGCCGGACTG-3′). Genetic validation was performed at the Institute of Neurology, the First Affiliated Hospital of Fujian Medical University.
3 Results
3.1 Muscle biopsy histology results
Muscle biopsy histology was performed in patient Ⅱ-3 (Figure 2). Hematoxylin and eosin (H&E) show dystrophic features with fiber size variation, scattered small circular atrophic muscle fibers, and partial compensatory hypertrophic muscle fibers. Nuclear translocation was observed in some muscle fibers. The denatured necrotic muscle fibers were observed with a small amount of mononuclear phagocyte infiltration. The inflammatory cell infiltration was not observed in the connective tissue and adipose tissue. Adenosine triphosphatase (ATPase) staining indicated that the fibers of type Ⅰ and type Ⅱ showed mosaic distribution, and no grouping phenomenon was observed. Gomori’s trichrome (GT) staining showed no typical or atypical ragged-red fibers (RRF) and rimmed vacuole (RV) in muscle fibers, and periodic acid-Schiff (PAS) staining showed no obvious glycogen increase in muscle fibers. No special changes were observed in succinic dehydrogenase (SDH) staining and cytochrome c oxidase (COX) staining. We concluded that pathological changes were consistent with muscular dystrophy. Limb-girdle muscular dystrophy was suggested, and further gene sequencing was recommended.
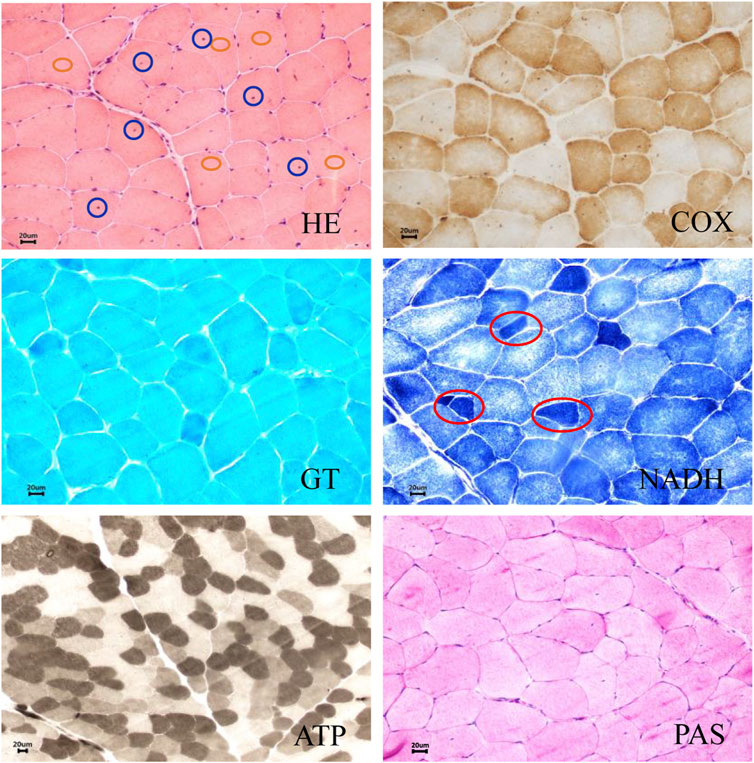
Figure 2. Muscle biopsy histology of patient II-3. Hematoxylin and eosin (H&E) show dystrophic features with fiber size variation, scattered small circular atrophic muscle fibers, and partial compensatory hypertrophic muscle fibers. Intranuclear migration was observed in some muscle fibers. The denatured necrotic muscle fibers were observed with a small amount of mononuclear phagocyte infiltration. Inflammatory cell infiltration was not observed in the connective tissue and adipose tissue. Adenosine triphosphatase (ATPase) staining showed that the fibers of type Ⅰ and type Ⅱ showed mosaic distribution, and no grouping phenomenon was observed. Gomori’s trichrome (GT) staining showed no typical or atypical ragged-red fibers (RRF) and rimmed vacuole (RV) in muscle fibers, and periodic acid-Schiff (PAS) staining showed no obvious glycogen increase in muscle fibers. No special changes were observed in succinic dehydrogenase (SDH) staining and cytochrome c oxidase (COX) staining. The blue circle represents intranuclear migration. The red circle represents muscle fiber atrophy. The orange circle represents muscle fiber hypertrophy. Scale bar: 20 µm.
3.2 Genetic screening results
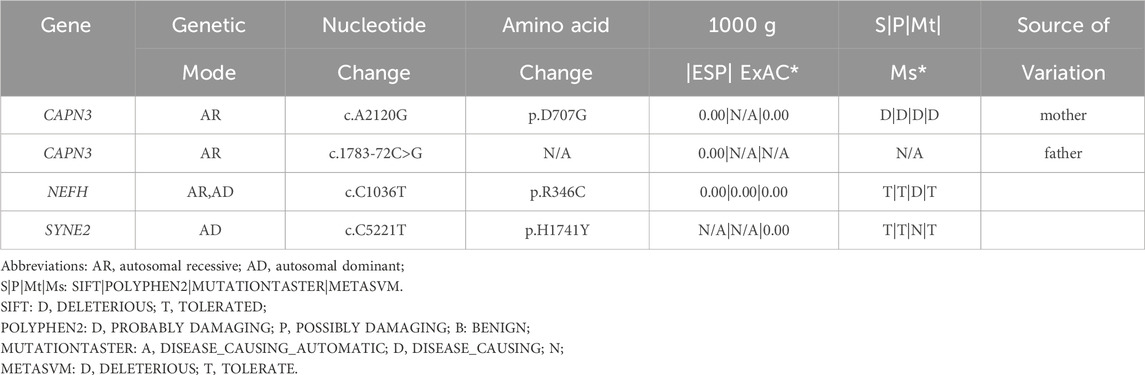
The family pedigree was obtained (Figure 3A). The phenotypes of the proband and his sister were different (Table 1). However, the gene analysis results showed that two siblings possessed two identical mutations in the CAPN3 gene that were derived from their parents (Table 2). The mutation c.1783-72 C>G from the father (Figure 3B) was not previously reported. According to the ACMG guidelines (Richards et al., 2015), its pathogenicity grade is PM3+PP1+PP3+PP4, which may be a “likely pathogenic” mutation. Another mutation, c.2120 A>G, from the mother, leading to amino acid changes (p.D707G) (Figure 3C), is the LGMD2A/R1 pathogenic mutation reported in the HGMD Professional and ClinVar databases.

Figure 3. Compound heterozygous CAPN3 variants causing limb-girdle muscular dystrophy type 2A in a Chinese family (A) The family pedigree of the limb-girdle muscular dystrophy type 2A. The proband and his sister carried the c.1783-72 C>G and c.2120 A>G (p. D707G) variants of CAPN3. The c.1783-72 C>G and c.2120 A>G (p. D707G) were inherited from the siblings’ father and mother, respectively. The mutation of c.1783-72 C>G (B) and c.2120 A>G (p. D707G) (C) in CAPN3 were obtained. The red arrow indicates the variant site. The normal sequence is shown below. (D) A skip of exon 2 near chr15-42698052-C-G (16 bp in total) in CAPN3 was detected by Sanger sequencing. The normal sequence is shown below.
3.3 Genetic validation results
The cDNA of the proband’s sister was amplified based on the result of the WES. Sanger sequencing showed that exon 2 had a skip (16 bp total) near the variant site chr15-42698052-C-G that validated the mutation c.1783-72 C >G detected by WES (Figure 3D).
4 Discussion
In the present study, the proband and his sister presented the typical clinical features of LGMDR1, including progressive proximal muscle weakness, calf hypertrophy, and winged scapula (Mahmood and Jiang, 1954). The sister of the proband may have benign clinical features, including paroxysmal myalgia after exercise and elevated serum creatine kinase level at onset. Although she developed muscular weakness, she was able to live independently. Meanwhile, the proband’s condition progressed rapidly, and he lived dependently. The age of onset, severity of the disease, and rate of progression significantly vary within this Chinese family, which suggests intra-familial phenotypic variability. This variability is consistent with reports of the same genetic variant in LGMDR1 that had a different clinical presentation (Schessl et al., 2008).
Great variation exists between the genotype and phenotype in LGMDR1. The significant differences in clinical manifestations among patients with limb-girdle muscular dystrophy with the same genetic mutations within one family can be attributed to several factors. First, genetic modifiers or variations in other genes may influence the expression and severity of the disease. These modifier genes can interact with the mutated gene, altering the disease phenotype. Second, environmental factors play a role. Different exposures to toxins, infections, diet, and physical activity levels can impact the progression and symptoms of the disorder. Epigenetic modifications, such as DNA methylation and histone acetylation, can also modify gene expression and contribute to the variability in clinical manifestations. Furthermore, individual variations in the immune system response and the body’s ability to cope with muscle damage and regeneration can lead to differences in the disease course and symptoms. In addition, stochastic events during development and aging processes may also contribute to the heterogeneity of the clinical picture among affected family members.
In our study, the mutation c. 2120 A>G is the LGMDR1 pathogenic mutation and has been reported in the HGMD Professional and ClinVar databases (Narihiro et al., 1999). The c.1783-72 C>G mutation has not been reported. According to ACMG (Richards et al., 2015), the pathogenicity grade of c.1783-72 C>G was PM3+PP1+PP3+PP4, suggesting that it may be a new pathogenic mutation of LGMDR1. The mutation c.1783-72 C>G could lead to exon 2 skipping identified by Sanger sequencing, which might affect the normal transcription of CAPN3 in one chromosome. c.2120A > G (p. D707G) and c.1783-72 C> G performed a compound heterogenous mutation, which abolished the function of CAPN3 to cause limb-girdle muscular dystrophy.
The underlying mechanisms of LGMDR1 have not been clear until now. In the skeletal muscle, the CAPN3 encodes a Ca2+/Na+-dependent cysteine protease (calpain-3) that plays a key role in sarcomere formation, muscle remodeling, and the regulation of cytoskeleton (Tidball and Spencer, 2000; Yasuko et al., 2010). Except for its proteolytic activity, calpain-3 has a nonproteolytic structural function acting on Ca2+ handling in the skeletal muscle (Lasa-Elgarresta et al., 2019). A previous study reported that the knockout of CAPN3 could damage the mitochondrial function in an animal model (Kramerova et al., 2009). Furthermore, mitochondrial deficiencies were found in LGMDR2A with calpain-3 mutations (El-Khoury et al., 2019). Thus, abnormality of the CAPN3 gene may lead to defects of muscular fiber formation and weakness in LGMDR1 by affecting mitochondrial function and/or regulation of Ca2+ in muscle.
Some limitations exist. First, gene sequencing was not done on muscle tissue. Second, the proband could not participate in further imaging examination and muscle biopsy due to disability.
5 Conclusion
In conclusion, we reported two cases in which the same genotype of LGMDR1 had different phenotypes, including age of onset, disease severities, and rate of progression in a Chinese family. Two compound heterozygosity variants were found. c.1783-72 C > G mutation of CANP3 may be a novel pathogenic mutation of LGMDR1. This broadens the understanding of LGMDR1.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital and the College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GD: conceptualization, writing–original draft, and writing–review and editing. WF: data curation, formal analysis, investigation, and writing–review and editing. YC: conceptualization, funding acquisition, investigation, and writing–original draft. RR: investigation, methodology, software, and writing–review and editing. XY: data curation, formal analysis, methodology, and writing–review and editing. CC: software, supervision, validation, and writing–review and editing. HJ: resources, supervision, validation, and writing–review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Joint Co-construction Project of the Henan Medical Science and Technology Research Plan (Grant LHGJ20210590).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bushby, K. M. (1995). Diagnostic criteria for the limb-girdle muscular dystrophies: report of the ENMC Consortium on Limb-Girdle Dystrophies. Neuromuscul. Disord. 5 (1), 71–74. doi:10.1016/0960-8966(93)e0006-g
El-Khoury, R., Traboulsi, S., Hamad, T., Lamaa, M., Sawaya, R., and Ahdab-Barmada, M. (2019). Divergent features of mitochondrial deficiencies in LGMD2A associated with novel calpain-3 mutations. J. Neuropathology Exp. Neurology 78 (1), 88–98. doi:10.1093/jnen/nly113
Fanin, M., and Angelini, C. (2015). Protein and genetic diagnosis of limb girdle muscular dystrophy type 2A: the yield and the pitfalls. Muscle Nerve 52 (2), 163–173. doi:10.1002/mus.24682
Fanin, M., Nascimbeni, A. C., Tasca, E., and Angelini, C. (2009). How to tackle the diagnosis of limb-girdle muscular dystrophy 2A. Eur. J. Hum. Genet. 17 (5), 598–603. doi:10.1038/ejhg.2008.193
Kramerova, I., Kudryashova, E., Wu, B., Germain, S., Vandenborne, K., Romain, N., et al. (2009). Mitochondrial abnormalities, energy deficit and oxidative stress are features of calpain 3 deficiency in skeletal muscle. Hum. Mol. Genet. 18 (17), 3194–3205. doi:10.1093/hmg/ddp257
Lasa-Elgarresta, J., Mosqueira-Martín, L., Naldaiz-Gastesi, N., Sáenz, A., López de Munain, A., and Vallejo-Illarramendi, A. (2019). Calcium mechanisms in limb-girdle muscular dystrophy with CAPN3 mutations. Int. J. Mol. Sci. 20 (18), 4548–8. doi:10.3390/ijms20184548
MacAvoy, M. C., and Green, D. P. (2007). Critical reappraisal of Medical Research Council muscle testing for elbow flexion. J. hand Surg. 32 (2), 149–153. doi:10.1016/j.jhsa.2006.10.020
Mahmood, O., and Jiang, X. (1954). Limb-girdle muscular dystrophies: where next after six decades from the first proposal (Review). Mol. Med. Rep. 9 (5), 1515--–1532. doi:10.3892/mmr.2014.2048
Narihiro, M., Ichizo, N., Kobayashi, O., Ikezoe, K., Goto, Y., and Nonaka, I. (1999). Mutations of calpain 3 gene in patients with sporadic limb-girdle muscular dystrophy in Japan. J. Neurological Sci. 171 (1), 31–37. doi:10.1016/s0022-510x(99)00245-2
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of medical genetics and genomics and the association for molecular pathology. Genet. Med. 17 (5), 405–424. doi:10.1038/gim.2015.30
Schessl, J., Walter, M. C., Schreiber, G., Schara, U., Kirschner, J., Lochmüller, H., et al. (2008). Phenotypic variability in siblings with Calpainopathy (LGMD2A). Acta Myol. 27 (2), 54–58.
Straub, V., Murphy, A., Udd, B., Corrado, A., Aymé, S., Bönneman, C., et al. (2018). 229th ENMC international workshop: limb girdle muscular dystrophies – nomenclature and reformed classification Naarden, The Netherlands, 17–19 March 2017. Neuromuscul. Disord. 28 (8), 702–710. doi:10.1016/j.nmd.2018.05.007
Tidball, J. G., and Spencer, M. J. (2000). Calpains and muscular dystrophies. Int. J. Biochem. Cell Biol. 32 (1), 1–5. doi:10.1016/s1357-2725(99)00095-3
Keywords: limb-girdle muscular dystrophy type 2A, CAPN3, progressive muscle weakness and wasting, gene splicing, autosomal recessive disorder
Citation: Feng W, Cao Y, Ren R, Yang X, Cao C, Jiang H and Du G (2024) Case report: A novel mutation of the CAPN3 gene in a Chinese family with limb-girdle muscular dystrophy type 2A. Front. Genet. 15:1410727. doi: 10.3389/fgene.2024.1410727
Received: 07 May 2024; Accepted: 26 July 2024;
Published: 12 August 2024.
Edited by:
Elaine Cristina Leite Pereira, University of Brasilia, BrazilReviewed by:
Mehmet Ali Ergün, Gazi University, TürkiyeCorrado Italo Angelini, University of Padua, Italy
Copyright © 2024 Feng, Cao, Ren, Yang, Cao, Jiang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ganqin Du, ZGdxOTlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Wanjun Feng†
Wanjun Feng† Yanyan Cao
Yanyan Cao Chunyan Cao
Chunyan Cao Ganqin Du
Ganqin Du