
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 18 June 2024
Sec. Applied Genetic Epidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fgene.2024.1391542
Introduction: Observational studies have found a correlation between the consumption of tobacco and alcohol and the likelihood of developing renal cell carcinoma. However, whether these associations indicate causal relationships is unclear.
Methods: To establish if these connections indicate causal relationships, we performed a Mendelian Randomization (MR) analysis using a two-sample approach. For the number of daily cigarettes, lifetime smoking index, smoking initiation, and weekly drinking, we employed 44, 108, 174, and 76 single nucleotide polymorphisms (SNPs) as instrumental variables. Outcome data were obtained from the FinnGen Alliance, which included a combined total of 429,290 individuals. The MR analysis was conducted using the inverse-variance weighted (IVW) method to estimate causal effects. To address potential violations of MR assumptions due to directional pleiotropy, we performed MR-Egger regression and MR-PRESSO (Mendelian Randomization Pleiotropy RESidual Sum and Outlier) analysis.
Results: Genetically influenced smoking initiation was directly associated with the risk of developing renal cell carcinoma (OR = 1.55, 95% CI: 1.04–2.33; p = 0.03). No causal relationship was found between daily cigarette consumption and lifetime smoking index with the risk of renal cell cancer. Genetic predisposition for weekly alcohol consumption showed a reduced risk of renal cell cancer (OR = 0.45, 95% CI: 0.26–0.81; p = 0.007).
Discussion: Our study suggests a potential causal relationship between alcohol consumption and reduced risk of renal cell cancer, while no such association was observed with smoking. Further research is needed to confirm these findings.
Over the last couple of years, there has been a notable rise in the prevalence of renal cell carcinoma (RCC), with an annual increase of around 1.1%. (Palumbo et al., 2021). Compared to 1990, there was a 154.78% increase in RCC cases by 2019. (Zi et al., 2021). During the three-decade study duration, there was a yearly rise in the age-adjusted mortality rate and age-adjusted rate of RCC, along with the disability-adjusted life rate (estimated annual percentage changes = 0.35 and 0.12). (Zi et al., 2021). Previous studies have identified several risk factors for RCC, but the accurate pathogenesis remains unclear. (Capitanio et al., 2019; Yuan et al., 2021). Since RCC is a deadly condition, it is imperative to identify intervention strategies that can decrease the likelihood of developing this illness.
Smoking and drinking are health risk factors. Many studies have shown that smoking is a risk factor for kidney cancer, while alcohol consumption has a protective effect against the occurrence of kidney cancer. (Capitanio et al., 2019; Bukavina et al., 2022; Kim et al., 2023). However, the nature of the relationship between smoking, drinking, and the risk of RCC remains uncertain. Moreover, the outcomes of current observational epidemiological studies could be impacted by combined and reciprocal causality, which holds significant importance for implementing clinical intervention strategies and developing recommendations for public policy. While a randomized controlled trial (RCT) is the optimal approach to deduce causation, (Bhide et al., 2018), it is impractical and morally wrong to carry out an RCT to evaluate the influence of smoking and alcohol intake on RCC. In observational epidemiological research, Mendelian randomization (MR) employs single nucleotide polymorphisms (SNPs) as instrumental variables to evaluate the effects of the outcomes of interest and mitigate bias. Theoretically, MR and RCT share similarities and random distribution occurs in the assignment. The impact of reverse causality on MR is minimized due to the fact that genetic variation remains constant from conception. Furthermore, MR is more resistant to environmental confounding in contrast to conventional observational research. The reason for this is that in MR, it is assumed that genetic instrumental variables (IVs) only impact the outcomes through exposure and are not related to any confounding factors. (Hemani et al., 2018a; Saunders et al., 2022).
To investigate the impact of genetics on the susceptibility to kidney cell carcinoma and its potential causal association with alcohol consumption, we employed a two-sample MR analysis, focusing on a single variable.
To be considered valid instrumental variables, genetic variants must meet three essential criteria: 1) they must exhibit a strong correlation with the exposure; 2) they should not be linked to any potential confounding factors associated with the exposure outcomes; and 3) besides the exposure, they should not have any impact on the outcomes of other variables (Figure 1). (Hemani et al., 2018a)
To fulfill the initial requirement of Mendelian randomization (MR), uncorrelated individual nucleotide variations (SNPs) linked to the exposure at the genome-wide significance threshold (p < 5 × 10−8) and free from genetic linkage (r2 < 0.01 and cluster window >10,000 kb) were utilized as instrumental factors (IVs). (Didelez and Sheehan, 2007). To validate the initial hypothesis, we computed the ratio of phenotypic variability elucidated by the complete collection of SNPs and the F statistic. (Burgess et al., 2016a). The strength of the instrument depends on the precision and extent to which the IV is associated with the risk factor. If the associated F statistic exceeds 10, it is deemed satisfactory.
We acquired the summary-level data for the entire genetic makeup (Supplementary Table S1). (Saunders et al., 2022). To ensure the uniformity of the sample, we specifically excluded individuals of non-European descent for the two sample MR analysis. (Hemani et al., 2018a). The SNPs of daily cigarettes was obtained from the GWAS and Sequencing Consortium of Alcohol and Nicotine Use (GSCAN), encompassing 784,353 individuals. It is defined as the average daily smoking among both current and former smokers. (Saunders et al., 2022). Data from the GWAS abstract were used to obtain the lifetime smoking index. (Wootton et al., 2020). This encompasses information about smoking intensity, duration, and smoking cessation, which are amalgamated into a comprehensive lifelong smoking index. The GWAS researcher has created a framework that includes time, duration of smoking, daily number of cigarettes, and fixed values for decay rate and delay time to comprehend the non-linear hazards linked to smoking. (Wootton et al., 2020). After excluding individuals who lack phenotype data and have not been eliminated by genotype, GWAS still has 462,690 participants. In addition, the SNP used for smoking initiation was derived from GSCAN, involving a total of 3,383,199 European blood participants, representing the likelihood of regular smoking. (Saunders et al., 2022). We obtained the GWAS data for weekly drinking from the GSCAN, which included data from 2,965,643 individuals. (Saunders et al., 2022). The weekly intake of alcoholic drinks by the participants in the study is measured, including a variety of alcoholic beverages.
We obtained the summary data of the genetic correlation with kidney cell carcinoma, its subtypes, and urothelial carcinoma from the FinnGen League (Supplementary Table S1). The Finnish consortium has recently released the R10 dataset, comprising a grand total of 429,290 individuals of Finnish origin, both male and female. Individuals with excessive hybridization, lacking high-quality genotypes, ambiguous gender, and non-Finnish blood were excluded (https://finngen.gitbook.io/documentation/). The size of all genetic correlation effects is calculated by logical regression and adjusts ages, gender, and genetic main components.
In order to examine the third MR hypothesis, we assessed the heterogeneity of independent SNP effects by employing the Cochran Q statistic and the MR Egger intercept test to detect directional pleiotropy. (Hemani et al., 2018b; Bowden et al., 2018). After the MR-Egger regression adjusts for pleiotropy, it can provide estimated values, but the statistical power is reduced. The MR-Egger intercept’s p-value determines the presence of directional pleiotropy. The primary statistical analysis method employed was the inverse-variance weighted (IVW) approach. In cases where horizontal pleiotropy is either equalized or absent, the IVW technique can provide an impartial estimation. (Hemani et al., 2018b). The genome-wide association studies (GWAS) utilized individuals of exclusively European descent, and the genetic principal component was employed to account for population structure in these GWAS. To address pleiotropy, we utilized various methods including MR-PRESSO, penalized weighted median, IVW radial regression, and weighted median as robust approaches. (Hemani et al., 2018b). The objective of the MR-PRESSO approach is to identify possible anomalies and produce estimates once they are eliminated. Distortion tests are used to detect discrepancies between estimates before and following the elimination of outliers. Assuming that the estimated weight of the MR effect is not more than 50% of the pleiotropic SNP effect, the weighted median method penalizes it to provide a consistent effect estimation. The weight depends on the strength of its correlation with the exposure. IVW radial regression employs an enhanced second-order weight to test and remove peripheral SNPs. At least 50% of the specified analysis in the weighted median comes from effective instrumental variables, and the weighted mode, which necessitates the largest subset to determine the same causal effect, constitutes effective tools. (Hartwig et al., 2017). Statistical analyses were conducted utilizing the R software, version 4.3.2, with the TwoSampleMR (0.5.8) and MR-PRESSO (1.0) packages.
For daily cigarettes, lifetime smoking index, smoking initiation, and drinks per week, instrumental variables (IVs) consist of 44, 108, 174, and 76 SNPs in total. The minimum F-statistics are 25.50, 31.36, 20.29, and 22.61.
Supplementary Table S1 presents the units for RCC. Supplementary Table S2 provides a description of the phenotype in the genome-wide association (GWAS) study of exposure and its outcomes. To summarize, the examination shows no variation when considering smoking and drinking as factors, and the MR-Egger intercept analysis does not detect any biased effects in any of the assessments (Supplementary Table S3). MR-PRESSO analysis did not detect any abnormal values (Supplementary Table S4).
The FinnGen consortium study found that individuals with a genetic inclination towards starting smoking had a higher likelihood of developing renal cell cancer, as suggested by multiple estimates accompanied by wider confidence intervals (CIs) (Figure 2).
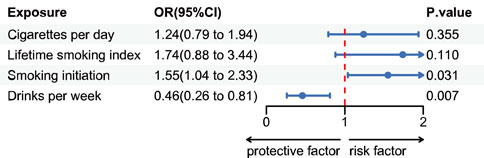
Figure 2. Estimates for the association of genetic liability for cigarettes per day, lifetime smoking index, smoking initiation and drinks per week with risk of renal cell cancer. Odds ratios per SD increment in the exposure from single-variable inverse variance weighted analysis.
With a one-standard deviation increase in the number of smoking initiations, the odds ratio (OR) for kidney cell cancer exhibited a value of 1.55 (IVW 95% CI 1.04–2.33; p = 0.03) and 2.03 (WM 95% CI 1.11–3.72; p = 0.02). While not statistically significant in the MR-Egger regression analysis, this causal relationship remained relatively consistent in the supplementary analysis (Supplementary Figure S1). There is no potential causal link between the anticipated number of cigarettes smoked daily based on genetics and the lifetime smoking index with RCC. The odds ratio (OR) is 1.24 (IVW 95% CI: 0.79–1.93; p = 0.35) and 1.74 (IVW 95% CI: 0.88–3.43; p = 0.11), respectively. These non-significant associations remain consistent in the supplementary analysis (Supplementary Figure S1). Genetic predisposition to drinks per week demonstrated a protective effect against kidney cell cancer. The odds ratio (OR) for kidney cell cancer decreased to 0.45 (IVW 95% CI 0.26–0.81; p = 0.007) with a weekly increase of one standard deviation in the number of alcoholic beverages consumed. This association remained almost consistent in supplementary analyses (Supplementary Figure S1).
Using two-sample Mendelian randomization (MR), we assessed the potential relationship between the risk of smoking and drinking and RCC. A positive relationship was observed between the initiation of smoking and the risk of kidney cell carcinoma, although no significant association statistics were found for daily cigarette consumption and the lifelong smoking index. This suggests a potential risk discrepancy among different exposure levels. Therefore, we cannot conclusively determine the association between smoking and kidney cell carcinoma. Additionally, we identified drinking as a protective factor against RCC. Across various exposure and analysis models, the MR estimation results were consistent in both size and direction.
Previous observational studies have shown that smoking was a major contributing factor to the development of RCC. (Bukavina et al., 2022; Li and Hecht, 2022; Campi et al., 2023). The majority of research indicates that individuals who smoke are twice as likely to develop renal cell cancer. The incidence of RCC appears to increase exponentially in people who smoke for more than 22 pack-years. (Campi et al., 2023). After conducting a comprehensive analysis of 56 research papers, it has been determined that individuals who currently engage in smoking are subjected to a 39% elevated likelihood of developing kidney cancer (KC). The authors also reported a 20% higher risk for former smokers and a 26% higher risk for ever-smokers compared to never-smokers. The correlation between smoking and the risk of KC is directly proportional to the amount smoked, with a significant rise in risk for individuals who smoke up to 30 cigarettes daily. For individuals who smoked 5, 10, 20, and 30 cigarettes per day, the relative risk (RR) was 1.18 (95% CI 1.22–1.52), 1.61 (95% CI 1.40–1.86), and 1.72 (95% CI 1.52–1.92), respectively. (Liu et al., 2019). The likelihood of developing kidney cancer (KC) decreases steadily over time after quitting smoking cigarettes. At 10, 20, and 30 years after quitting, the risk ratio (RR) for individuals who used to smoke compared to those who currently smoke is 0.94 (95% CI 0.87–1.01), 0.88 (95% CI 0.76–1.02), and 0.82 (95% CI 0.66–1.02) correspondingly. (Liu et al., 2019). The exact mechanism of increasing RCC (RCC) risk has not yet been fully understood. Tobacco contains a large number of chemicals, with over 50 of them classified as human carcinogens. (Li and Hecht, 2022). The mutagenic activity of tobacco carcinogens can be attributed to smoke, which serves as a risk factor for RCC. These carcinogens are converted into reactive metabolic products, which can combine with DNA and cause structural changes. (Buendia Jimenez et al., 2015). ANKS1B is a gene that encodes the primary form of a protein involved in transmitting signals through tyrosine kinase pathways, characterized by the presence of an Ankyrin repeat and sterile alpha motif domain. Compared with the patient-matched normal kidney tissue, ANKS1B is expressed at a decreased level in the kidney tumor tissue of RCC patients. (Eckel-Passow et al., 2014). A single nucleotide polymorphism in ANKS1B was found in the tissue from patients with lung cancer, and a correlation with carcinogenic metabolism was established, (Lin et al., 2012), which indicates that ANKS1B may have a similar effect in RCC and plays potential tumor inhibitory Gene. Polymorphism in other genes, such as N-acetyl metastases 2 (NAT2) and glutathione S-metastases (GSTM1) is also related to the increased cancer risk of RCC in the smokers. Compared to individuals with a fast NAT2 acetyl gene or GSTM1-positive genotype, those with a slow NAT2 acetyl genome or GSTM1-NULL genome have a higher risk of RCC. (Cumberbatch et al., 2016). NAT2 and GSTM1 play a role in the breakdown and elimination of cancer-causing substances present in tobacco, thus forming the basis of this connection. Individuals with a slow NAT2 acetyl genome and GSTM1-NULL genotype exhibit impaired metabolic ability, leading to an increased risk of these cancers. (Cumberbatch et al., 2016).
Our discovery regarding the association between drinking and RCC is in line with previous observational research. Several potential studies have suggested that the consumption of alcohol in small to moderate amounts has a dose-dependent protective impact on the progression of RCC. (Lew et al., 2011; Minami et al., 2021; van de Pol et al., 2021). Typically, the intake of a minimum of 15 g of alcohol daily is linked to a lower chance of RCC, with an estimated reduction of 28%. (Cumberbatch et al., 2016). Bagnardi et al. conducted a comprehensive meta-analysis. (Bagnardi et al., 2015). They observed a statistical inverse correlation between RCC and alcohol consumption. According to the researchers, in a total of 24 studies, there is a decreased risk indicated by a relative risk (RR) of 0.92 (95% CI 0.86–0.99), specifically linked to moderate alcohol consumption. Several mechanisms have been proposed by which alcohol consumption might reduce the risk of RCC. First, light to moderate alcohol consumption has been reported to enhance insulin sensitivity, (Davies et al., 2002; Bonnet et al., 2012; Schrieks et al., 2015), which may play a role in diabetes management. Given that a meta-analysis has shown a significant positive association between diabetes and RCC incidence, improved insulin sensitivity through alcohol consumption could serve as an indirect protective factor against RCC. (Larsson and Wolk, 2011). Second, alcoholic beverages contain antioxidant phenolic compounds that can reduce oxidative stress. These compounds help to remove oxidized carcinogenic agents, reduce lipid peroxidation and cell proliferation, and promote apoptosis. (Gago-Dominguez et al., 2002; Lee et al., 2007). Third, alcohol’s diuretic effect might contribute to controlling hypertension, a known risk factor for RCC. (Lee et al., 2007). However, it is important to note that an increase in total fluid intake has not been conclusively established to influence RCC risk. (Lee et al., 2007; Hu et al., 2009).
There are several advantages in our research. The primary advantage is the implementation of Mendelian Randomization (MR) design, which helps in minimizing residual confounding factors and reverse causal relationships. Additionally, our study is conducted using a substantial number of RCC cases from two independent research groups. The entire analysis is restricted to individuals of European ancestry, aiming to minimize the potential for stratification bias within the population. To minimize the chance of instrumental weakness, we implemented rigorous selection criteria (p < 5 × 10−8) when choosing instruments. Furthermore, all related F-statistics surpassed 10, suggesting that the genetic instruments employed exhibit robust strength and are not affected by biases caused by weaker instruments. (Burgess et al., 2016b). In our research, sensitivity analysis conducted through the MR-Egger model reveals no evidence of horizontal pleiotropy in single-variable analysis, suggesting the validity of our results. To evaluate and reduce heterogeneity and multifactorial effects, we conducted a sensitivity analysis.
Our research has some limitations. First, the standard Mendelian Randomization (MR) assumes a linear relationship between risk factors and outcomes. In cases where the real correlation is not linear, the measured estimation value can be deceptive, despite still indicating the presence and direction of the average causal impact within the group. (Burgess and Thompson, 2015). Secondly, in our research, the instrumental variables IV) for smoking and drinking may be associated with other risk factors for kidney cell carcinoma. Therefore, the relationship between genetic mutations and kidney cell carcinoma may be easily influenced by these factors. Third, exposure assessment relies on self-reported information, making it prone to underestimation. The inherent measurement error in exposure assessment does not impact the instrumental variable analysis. Fourth, the impact of different types of cigarettes or alcohol on smoking and drinking can vary. Furthermore, it is important to exercise caution prior to summarizing the results. Moreover, it is crucial to prioritize the discovery and advancement of novel biomarkers, alongside additional risk factors, that have the ability to identify and forecast clinical results in individuals diagnosed with RCC.
Our MR study demonstrated that alcohol consumption can lower the risk of RCC, but there is no compelling evidence to suggest a causal relationship between smoking and the risk of RCC. Further longitudinal and experimental studies are still required to validate our findings.
The data presented in the study are deposited in the Data Repository for the University of Minnesota (DRUM), DOI: https://doi.org/10.13020/przg-dp88; University of Bristol Research Data Repository, DOI: 10.5523/bris.10i96zb8gm0j81yz0q6ztei23d and Finngen R10 Study, https://r10.risteys.finngen.fi/endpoints/C3_KIDNEY_NOTRENALPELVIS_EXALLC.
The studies involving humans were approved by The Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
HC: Conceptualization, Investigation, Writing–original draft, Data curation, Formal Analysis, Resources, Writing–review and editing. JD: Methodology, Software, Writing–original draft. HX: Funding acquisition, Project administration, Writing–original draft. YZ: Visualization, Writing–original draft. CL: Supervision, Validation, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1391542/full#supplementary-material
Bagnardi, V., Rota, M., Botteri, E., Tramacere, I., Islami, F., Fedirko, V., et al. (2015). Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br. J. Cancer 112 (3), 580–593. doi:10.1038/bjc.2014.579
Bhide, A., Shah, P. S., and Acharya, G. (2018). A simplified guide to randomized controlled trials. Acta Obstet. Gynecol. Scand. 97 (4), 380–387. doi:10.1111/aogs.13309
Bonnet, F., Disse, E., Laville, M., Mari, A., Hojlund, K., Anderwald, C. H., et al. (2012). Moderate alcohol consumption is associated with improved insulin sensitivity, reduced basal insulin secretion rate and lower fasting glucagon concentration in healthy women. Diabetologia 55 (12), 3228–3237. doi:10.1007/s00125-012-2701-3
Bowden, J., Spiller, W., Del Greco M, F., Sheehan, N., Thompson, J., Minelli, C., et al. (2018). Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int. J. Epidemiol. 47 (4), 2100–1278. doi:10.1093/ije/dyy265
Buendia Jimenez, I., Richardot, P., Picard, P., Lepicard, E. M., De Meo, M., and Talaska, G. (2015). Effect of increased water intake on urinary DNA adduct levels and mutagenicity in smokers: a randomized study. Dis. Markers 2015, 478150. doi:10.1155/2015/478150
Bukavina, L., Bensalah, K., Bray, F., Carlo, M., Challacombe, B., Karam, J. A., et al. (2022). Epidemiology of renal cell carcinoma: 2022 update. Eur. Urol. 82 (5), 529–542. doi:10.1016/j.eururo.2022.08.019
Burgess, S., Davies, N. M., and Thompson, S. G. (2016b). Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40 (7), 597–608. doi:10.1002/gepi.21998
Burgess, S., Dudbridge, F., and Thompson, S. G. (2016a). Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat. Med. 35 (11), 1880–1906. doi:10.1002/sim.6835
Burgess, S., and Thompson, S. G. (2015). Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181 (4), 251–260. doi:10.1093/aje/kwu283
Campi, R., Rebez, G., Klatte, T., Roussel, E., Ouizad, I., Ingels, A., et al. (2023). Effect of smoking, hypertension and lifestyle factors on kidney cancer - perspectives for prevention and screening programmes. Nat. Rev. Urol. 20 (11), 669–681. doi:10.1038/s41585-023-00781-8
Capitanio, U., Bensalah, K., Bex, A., Boorjian, S. A., Bray, F., Coleman, J., et al. (2019). Epidemiology of renal cell carcinoma. Eur. Urol. 75 (1), 74–84. doi:10.1016/j.eururo.2018.08.036
Cumberbatch, M. G., Liu, W., Zhao, X., Cumberbatch, M. G., Rota, M., Catto, J. W. F., et al. (2016). Reply to wentao liu, xiaokun Zhao, zhaohui zhong’s letter to the editor re: marcus G. Cumberbatch, matteo rota, james W.F. Catto, carlo La vecchia. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur urol 2016;70:458-66. Eur. Urol. 70 (4), e106–e107. doi:10.1016/j.eururo.2015.08.033
Davies, M. J., Baer, D. J., Judd, J. T., Brown, E. D., Campbell, W. S., and Taylor, P. R. (2002). Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. Jama 287 (19), 2559–2562. doi:10.1001/jama.287.19.2559
Didelez, V., and Sheehan, N. (2007). Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 16 (4), 309–330. doi:10.1177/0962280206077743
Eckel-Passow, J. E., Serie, D. J., Bot, B. M., Joseph, R. W., Cheville, J. C., and Parker, A. S. (2014). ANKS1B is a smoking-related molecular alteration in clear cell renal cell carcinoma. BMC Urol. 14, 14. doi:10.1186/1471-2490-14-14
Gago-Dominguez, M., Castelao, J. E., Yuan, J. M., Ross, R. K., and Yu, M. C. (2002). Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States). Cancer Causes Control 13 (3), 287–293. doi:10.1023/a:1015044518505
Hartwig, F. P., Davey Smith, G., and Bowden, J. (2017). Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46 (6), 1985–1998. doi:10.1093/ije/dyx102
Hemani, G., Bowden, J., and Davey Smith, G. (2018b). Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 27 (R2), R195–R208. doi:10.1093/hmg/ddy163
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018a). The MR-Base platform supports systematic causal inference across the human phenome. Elife 7, e34408. doi:10.7554/eLife.34408
Hu, J., Mao, Y., DesMeules, M., Csizmadi, I., Friedenreich, C., Mery, L., et al. (2009). Total fluid and specific beverage intake and risk of renal cell carcinoma in Canada. Cancer Epidemiol. 33 (5), 355–362. doi:10.1016/j.canep.2009.10.004
Kim, L. H., Bang, A., Sarich, P., Nair-Shalliker, V., Patel, M. I., and Smith, D. P. (2023). Alcohol consumption and socioeconomic status associated with the risk of kidney cancer in a large Australian cohort study. Ann. Epidemiol. 84, 16–24. doi:10.1016/j.annepidem.2023.04.014
Larsson, S. C., and Wolk, A. (2011). Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia 54 (5), 1013–1018. doi:10.1007/s00125-011-2051-6
Lee, J. E., Hunter, D. J., Spiegelman, D., Adami, H. O., Albanes, D., Bernstein, L., et al. (2007). Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J. Natl. Cancer Inst. 99 (10), 801–810. doi:10.1093/jnci/djk181
Lew, J. Q., Chow, W. H., Hollenbeck, A. R., Schatzkin, A., and Park, Y. (2011). Alcohol consumption and risk of renal cell cancer: the NIH-AARP diet and health study. Br. J. Cancer 104 (3), 537–541. doi:10.1038/sj.bjc.6606089
Li, Y., and Hecht, S. S. (2022). Carcinogenic components of tobacco and tobacco smoke: a 2022 update. Food Chem. Toxicol. 165, 113179. doi:10.1016/j.fct.2022.113179
Lin, J., Lu, C., Stewart, D. J., Gu, J., Huang, M., Chang, D. W., et al. (2012). Systematic evaluation of apoptotic pathway gene polymorphisms and lung cancer risk. Carcinogenesis 33 (9), 1699–1706. doi:10.1093/carcin/bgs192
Liu, X., Peveri, G., Bosetti, C., Bagnardi, V., Specchia, C., Gallus, S., et al. (2019). Dose-response relationships between cigarette smoking and kidney cancer: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 142, 86–93. doi:10.1016/j.critrevonc.2019.07.019
Minami, T., Inoue, M., Sawada, N., Yamaji, T., Iwasaki, M., and Tsugane, S. (2021). Alcohol consumption, tobacco smoking, and subsequent risk of renal cell carcinoma: the JPHC study. Cancer Sci. 112 (12), 5068–5077. doi:10.1111/cas.15129
Palumbo, C., Pecoraro, A., Knipper, S., Rosiello, G., Luzzago, S., Deuker, M., et al. (2021). Contemporary age-adjusted incidence and mortality rates of renal cell carcinoma: analysis according to gender, race, stage, grade, and histology. Eur. Urol. Focus 7 (3), 644–652. doi:10.1016/j.euf.2020.05.003
Saunders, G. R. B., Wang, X., Chen, F., Jang, S. K., Liu, M., Wang, C., et al. (2022). Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature 612 (7941), 720–724. doi:10.1038/s41586-022-05477-4
Schrieks, I. C., Heil, A. L. J., Hendriks, H. F. J., Mukamal, K. J., and Beulens, J. W. J. (2015). The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care 38 (4), 723–732. doi:10.2337/dc14-1556
van de Pol, J. A. A., George, L., van den Brandt, P. A., Baldewijns, M. M. L. L., and Schouten, L. J. (2021). Etiologic heterogeneity of clear-cell and papillary renal cell carcinoma in The Netherlands Cohort Study. Int. J. Cancer 148 (1), 67–76. doi:10.1002/ijc.33193
Wootton, R. E., Richmond, R. C., Stuijfzand, B. G., Lawn, R. B., Sallis, H. M., Taylor, G. M. J., et al. (2020). Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol. Med. 50 (14), 2435–2443. doi:10.1017/S0033291719002678
Yuan, S., Fang, C., Leng, W. D., Wu, L., Li, B. H., Wang, X. H., et al. (2021). Oral microbiota in the oral-genitourinary axis: identifying periodontitis as a potential risk of genitourinary cancers. Mil. Med. Res. 8 (1), 54. doi:10.1186/s40779-021-00344-1
Keywords: smoke, alchohol, Mendelian randomization, renal cell caecinoma, causal relationship
Citation: Cui H, Du J, Xue H, Zhao Y and Li C (2024) The causal relationship between smoking, alcohol consumption, and renal clear cell carcinoma: a Mendelian randomization study. Front. Genet. 15:1391542. doi: 10.3389/fgene.2024.1391542
Received: 28 February 2024; Accepted: 03 June 2024;
Published: 18 June 2024.
Edited by:
Zili Zhang, Nanjing University of Chinese Medicine, ChinaReviewed by:
Vesna Coric, University of Belgrade, SerbiaCopyright © 2024 Cui, Du, Xue, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengwen Li, bGljaGVuZ3dlbjE5NzRAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.