- 1Department of First Clinical Medical School, Shanxi Medical University, Taiyuan, China
- 2Department of Endocrinology, The First Hospital of Shanxi Medical University, Taiyuan, China
Acute intermittent porphyria (AIP) is caused by mutations in the gene encoding hydroxymethylbilane synthase (HMBS), a key enzyme in the heme biosynthesis pathway. AIP is an autosomal dominant disorder characterized by low penetrance and a highly heterogenous clinical presentation. The estimated prevalence of AIP is 5–10 cases per 100,000 persons, with acute attacks manifesting in less than 1% of the at-risk population. This low frequency of attacks suggests significant roles for oligogenic inheritance and environmental factors in the pathogenesis of the disease. In recent years, identification of several modifier genes has advanced our understanding of the factors influencing AIP penetrance and disease severity. This review summarizes these factors including the impact of specific HMBS mutations, oligogenic inheritance, mitochondrial DNA copy number, age, sex, the influence of sex hormones, and the role of environmental factors. Further studies into the etiology of AIP disease penetrance should inform pathogenesis, potentially allowing for the development of more precise diagnostic and therapeutic approaches.
1 Introduction
The porphyrias are a group of disorders of porphyrin metabolism caused by defective enzyme activity in one of the eight enzymes of the heme biosynthesis pathway. These are primarily hereditary disorders, and divided into eight subtypes (Kauppinen, 2005). According to the tissue in which the particular enzyme defect is located, porphyrias are classified as hepatic porphyrias, caused by disorders of porphyrin metabolism in the liver, and erythropoietic porphyrias, disorders of porphyrin metabolism in the bone marrow. Hepatic porphyrias have also been subdivided into acute hepatic porphyrias (AHPs) and chronic hepatic porphyrias according to the characteristics of the disease attacks.
Acute intermittent porphyria (AIP), the most common form of acute hepatic porphyria, is an autosomal dominant disorder caused by a partial deficiency in the activity of the third enzyme in the heme biosynthesis pathway, hydroxymethylbilane synthase (HMBS), also known as porphobilinogen deaminase (PBGD) (Puy et al., 2010). AIP rarely attacks before puberty, with typical symptoms occurring between the ages of 20 and 40, more in females than males. In patients with AIP, certain endogenous or exogenous factors such as fluctuations in female sex hormones, low caloric intake, alcohol consumption, stress, infections, and porphyrinogenic drugs lead to the upregulation of hepatic δ-aminolevulinic acid synthase 1 (ALAS1). This results in the accumulation of the porphyrin precursors δ-aminolevulinic acid (ALA) and porphobilinogen (PBG) in AIP patients, ultimately causing the development of clinical symptoms (Anderson et al., 2005; Schulenburg-Brand et al., 2022).
Patients with pathogenic HMBS can be categorized into those with manifest AIP (MAIP) and those with latent AIP (LAIP) (Barreda-Sánchez et al., 2019). A patient with LAIP is someone who has never experienced acute porphyria-related manifestations (Sardh and Barbaro, 1993). Patients with MAIP usually present with acute neurovisceral attacks, including severe abdominal pain, nausea and vomiting, constipation, tachycardia, hypertension, psychiatric disorders (e.g., insomnia, anxiety, and depression), and severe neurologic symptoms (neuropathy and seizures) (Puy et al., 2010; Souza et al., 2021).
AIP management includes avoidance of triggers, oral or intravenous glucose and intravenous hemin infusion, symptom treatment, acute attack prevention, and management of long-term complications (Schulenburg-Brand et al., 2022; Wang et al., 2023). Intravenous glucose (300 g/d in adults) is used in early acute attacks or when hemin is unavailable, but hemin infusion is more effective (Schulenburg-Brand et al., 2022; Wang et al., 2023). Hemin infusion rapidly downregulates ALAS1 expression, decreases ALA and PBG accumulation, and resolves symptoms in 48–72 h, although its effectiveness in preventing recurrent attacks is unclear (Yarra et al., 2019; Blaylock et al., 2020). Lists of safe and unsafe drugs for patients with AIP are available online (http://www.drugs-porphyria.org/; https://porphyrianet.org/).
Givosiran (Givlaari), an ALAS1-directed siRNA, is approved for treating adults with AHP in the United States and those 12 years and older in the EU (Balwani et al., 2020; Scott, 2020). Monthly givosiran injections significantly reduced the rate of acute attacks in a phase 3 study in patients with recurrent AIP (Balwani et al., 2020). A Phase III clinical trial of Givosiran in AHP patients showed sustained improvements in clinical manifestations and quality of life with a favorable safety profile (Kuter et al., 2023). Thus, patients with recurrent acute attacks (4 or more per year) should be considered for prophylactic treatment with heme therapy or subcutaneous givosiran (Wang et al., 2023). Liver transplantation may be considered for patients with persistent severe disease who have failed other treatment options. In addition, gene therapy, hPBGD mRNA, and pharmacological chaperone therapy are promising treatments for AIP (Bustad et al., 2020; Kizilaslan et al., 2023).
In addition to variable penetrance, there is considerable heterogeneity in disease severity, even within the same family. Most symptomatic AIP patients have only a few attacks in their lifetime; however, about 3%–8% of symptomatic AIP patients have recurrent attacks (Gouya et al., 2020). The variation in penetrance and clinical phenotype would indicate that other genetic and/or environmental factors are either preventing or precipitating AIP attacks in HMBS heterozygotes (outlined in Table 1).
2 Genetic factors
2.1 Complexity of mutations in the HMBS gene
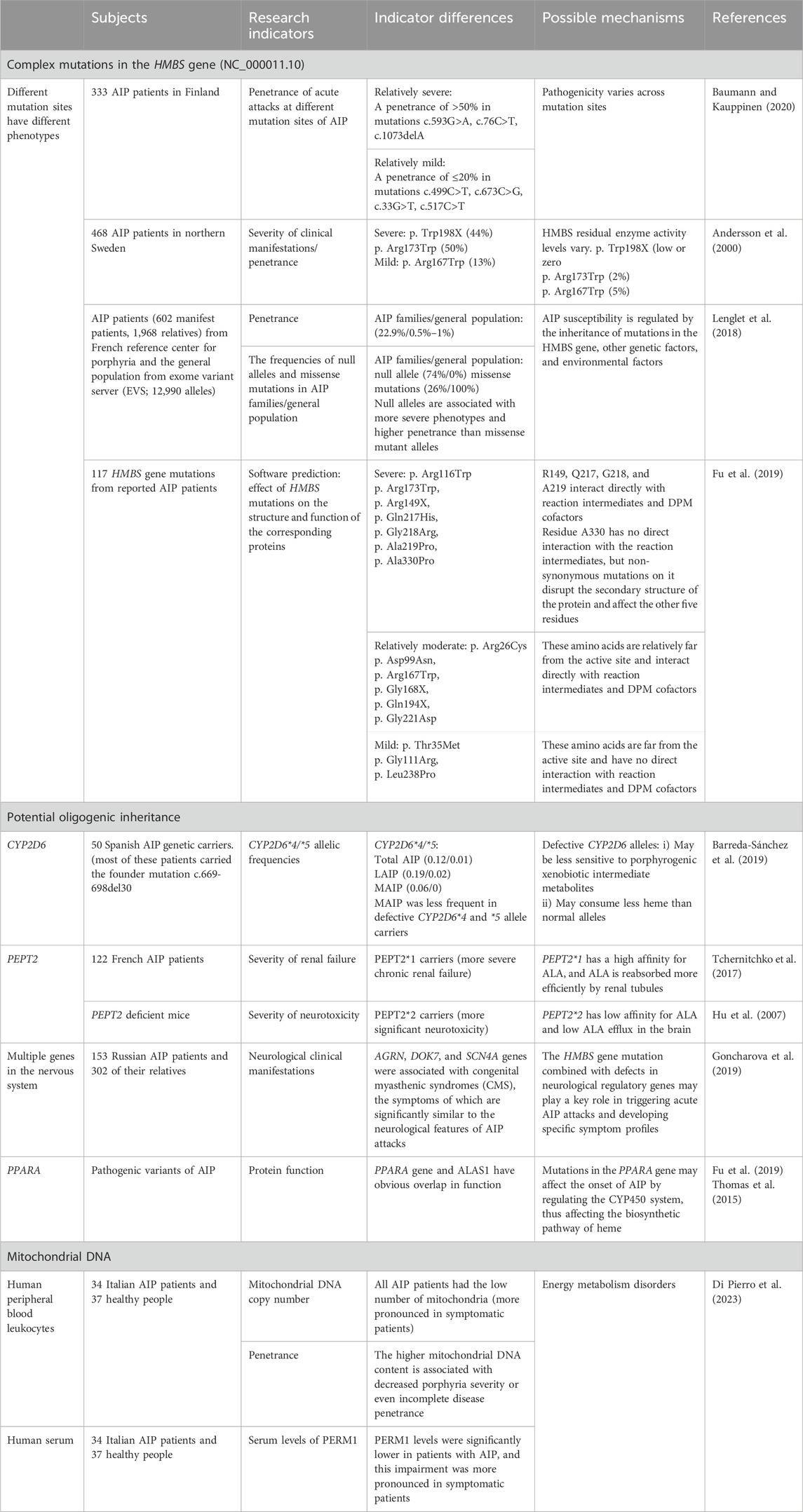
HMBS is the gene responsible for AIP, with mutations resulting in loss of enzyme function. The HMBS gene is located on chromosome 11 (11q24.1–24.2), and the coding region consists of 15 exons. Transcription from different promoters generates two types of transcripts, the housekeeping transcripts and the erythroid-specific transcripts, and this article focuses on the housekeeping transcript (NM_000190.4). To date, more than 500 different HMBS pathogenic mutations have been reported in the Human Gene Mutation Database (Stenson et al., 2017). HMBS mutations include missense mutations, nonsense mutations, splice mutations, frameshift mutations, large/small deletions, large/small insertions, and large/small duplications. There are 31 CpG dinucleotides in the HMBS 1,086 base pair coding sequence (Chen et al., 2016). The primary sources of DNA sequence variation are replication errors and deamidation of CpG dinucleotides, which are hotspots for mutations (Cooper and Youssoufian, 1988). Most AIP mutations are found only in single or a few unrelated families, demonstrating the molecular genetic heterogeneity of AIP (Whatley et al., 1999; Martinez di Montemuros et al., 2000; Ulbrichova et al., 2009; Chen et al., 2019a). However, there exist some relatively common mutations: one reason is due to CpG dinucleotide mutation hotspots in the gene (Tjensvoll et al., 2003), such as the mutations encoding p.Arg173Trp, p.Arg167 Gln, and p.Arg116Trp; and the second reason is the founder effect, such as HMBS c.593G>A (p.Trp198X) in Sweden and Norway (Tjensvoll et al., 2003), c.669_698del30 in the Murcia region of Spain (Guillén-Navarro et al., 2004).
Although correlation between AIP genotypes and phenotypes has not been established (Fraunberg et al., 2005; Bustad et al., 2013; Bonkovsky et al., 2014), studies suggest that different mutations can lead to different phenotypes and differences in clinical penetrance (Chen et al., 2016; Zhang et al., 2021). For instance, the c.1073delA, c.593G>A (p. Trp198X), and c.76C>T (p. Arg26Cys) mutations have been associated with higher penetrance (greater than 50%) or a more severe clinical presentation (Andersson et al., 2000; Fraunberg et al., 2005; Baumann and Kauppinen, 2020). Conversely, the c.33G>T, c.499C>T (p. Arg167Trp), and c.673C>G (p. Arg225Gly) mutations have been associated with lower penetrance (less than or equal to 20%) or milder phenotypes (Andersson et al., 2000; Fraunberg et al., 2005; Baumann and Kauppinen, 2020). However, in northern Sweden, the p. Arg173Trp mutation had a higher clinical penetrance compared to Finland (Andersson et al., 2000; Baumann and Kauppinen, 2020). Recently, a study of protein function prediction (Fu et al., 2019) showed that some mutations, including p. Arg149X, p. Gln217His, p. Gly218Arg, p. Ala219Pro, and p. Ala330Pro, lead to severe clinical manifestations. Moreover, concordant with a previous study, it was also shown that patients with p. Arg116Trp and p. Arg173Trp mutations had severe clinical symptoms (Bustad et al., 2013). In contrast, the p. Arg26Cys, p. Asp99Asn, p. Arg167Trp, p. Gly168X, p. Gln194X, and p. Gly221Asp mutations result in relatively moderate disease while mutations p. Thr35Met, p. Gly111Arg, and p. Leu238Pro exhibited a mild clinical phenotype. The penetrance of AIP in different Finland families varied even though the genotypes were the same (Baumann and Kauppinen, 2020), again suggesting that modifier genes and environmental factors also affect AIP phenotype.
2.2 Potential oligogenic inheritance model
A study of AIP in France using patients from the French Reference Center for Porphyria and sequencing data derived from the general population evaluated the prevalence and penetrance of AIP, and the influence of genetic factors on the phenotypic expression of HMBS mutations (Lenglet et al., 2018). Among the patients and their families of the French Reference Center for Porphyria, 496 patients with MAIP and 1,672 LAIP relatives were identified, and the penetrance of AIP in these AIP families was estimated to be 22.9%. The Exome Variant Server (EVS) database was then used to estimate the prevalence of deleterious HMBS mutations and AIP penetrance in the general population. A total of 42 missense mutations were identified in the AIP family (n = 20), and the EVS database (n = 22), and their pathogenicity was assessed by computerized and functional studies, which revealed a minimum estimated prevalence of AIP of 1/1,299 in the general population. Thus, given the estimated prevalence of pathogenic HMBS mutations in France, between 50,000 and 100,000 AIP patients (rather than 496) would be expected, suggesting the penetrance of HMBS mutations in the general population is only 0.5%–1%. This is in keeping with a study using whole exome sequencing of more than 40,000 Caucasians indicating that the AIP penetrance in HMBS heterozygotes is ∼1% (Chen et al., 2016).
Further analysis of intra-familial correlations for AIP revealed that correlations were generally strong and moderated by the individual’s kinship and region of residence. Disease penetrance in affected families was estimated to range from 58% (parent-offspring) to 73% (siblings).
Finally, Lenglet et al. analyzed the effects of the specific mutations, and then assessed the frequency of missense mutations and null alleles in the EVS database (general population) (n = 6,495) and in families of AIP patients, including simplex families (n = 106), multiplex families (n = 147), and patients with recurrent AIP attacks (4 or more per year; n = 37). Notably, these different mutations were associated with variable clinical phenotypes. Null alleles (little or no expression of HMBS) were more frequent in multiplex families than in simplex families, and patients with recurrent AIP also had a higher frequency of null genes than less severe forms, but the difference was not statistically significant. In the general population, the null allele frequency was 0%, and the frequency of missense mutations 100%. In familial AIP patients, the null allele frequency was 74%, and missense mutations 26%, suggesting that null alleles are associated with more severe phenotypes and higher penetrance than other mutant alleles.
These researchers proposed an oligogenic genetic model with environmental modifiers to better explain the penetrance and heritability of AIP (Lenglet et al., 2018).
2.2.1 Protective variants
2.2.1.1 CYP2D6 gene
Recent research has indicated a higher frequency of specific cytochrome P450 (CYP) alleles among individuals with certain types of porphyria compared to the general population, suggesting a potential role of these alleles in disease susceptibility (Christiansen et al., 2000; Gardlo et al., 2003). An investigative study conducted in Argentina focused on analyzing CYP2D6 polymorphisms in patients with porphyria, encompassing 121 participants, including 51 healthy volunteers, 50 porphyria patients, and 19 individuals with elevated iron levels. The results showed that the CYP2D6*3 and CYP2D6*4 alleles, particularly, were linked to the onset of porphyria (Lavandera et al., 2006). Furthermore, a recent study in Spain investigated the correlation between hepatic CYP genes and acute attacks of AIP (Barreda-Sánchez et al., 2019). The study examined CYP2C9*2, *3, CYP2C19*2, CYP2D*4, *5, CYP3A4*1B, and CYP3A5*3 alleles in fifty Spanish carriers of the AIP gene. Most fifty patients with AIP (78%) carried the founder mutation c.669_698del30. The Spanish study suggested that CYP genes may be penetrance modifiers in AIP because CYP2D6*4 and *5 are more frequent in LAIP than MAIP, despite the similar allele frequencies of CYP2D6*4 and *5 in the total AIP carriers and the general population (Menoyo et al., 2006), suggests CYP2D6*4 and *5 defective alleles play a protective role in the clinical onset of AIP, modulating the penetrance of AIP.
2.2.2 Damaging variants
2.2.2.1 PEPT2 gene
Human peptide transporter protein 2 (PEPT2) is expressed in proximal renal tubular cells, and its genetic variation modulates the severity of porphyrin-related nephropathy and neurological impairment (Yasuda et al., 2019). ALA, a highly hydrophilic compound, is freely filtered in the urine and is reabsorbed by human peptide transporter protein 2 (PEPT2) (Tchernitchko et al., 2017). It has been demonstrated that the toxicity of porphyrin metabolites is associated with the pathogenesis of typical renal tubulointerstitial lesions (Pallet et al., 2015). During AIP attacks, ALA accumulates and promotes renal tubular cell death and tubulointerstitial damage. ALA and PBG promote phenotypic changes and apoptosis in renal epithelial cells, and an epithelial-to-mesenchymal transition that leads to primary tubulointerstitial nephropathy.
Recently, a study showed that in French Caucasians with HMBS mutations, PEPT2 variants affect the severity and prognosis of porphyria-associated nephropathy (Tchernitchko et al., 2017). Haplotype analysis demonstrated the existence of two significant variants of PEPT2, PEPT2*1 and PEPT2*2. PEPT2*1 has a high affinity for ALA, and PEPT2*2 has a low affinity for ALA. Individuals carrying the high-affinity transporter (PEPT2*1) may suffer from more severe chronic renal failure because ALA is reabsorbed more efficiently by the renal tubules and is, therefore, more nephrotoxic.
The development of neuropsychiatric symptoms in AIP may be associated with an increase in brain ALA (Pischik and Kauppinen, 2009; Fu et al., 2019). It has been found that the protein encoded by the PEPT2 gene is responsible for the transport of ALA from the cerebrospinal fluid (Hu et al., 2007). Since PEPT2*2 has a lower affinity for ALA than PEPT2*1, more significant neurotoxicity may occur in PEPT2*2 carriers because they have lower ALA efflux from the brain (Ennis et al., 2003; Jaramillo-Calle et al., 2019).
2.2.2.2 Multiple genes in the nervous system
During acute attacks of AIP, the toxic porphyrin precursors ALA and PBG accumulate in tissues, causing neurologic injury. Recently, a study from Russia performed long-term follow-up and genetic analysis of 153 patients with AIP and 302 of their relatives, focusing on the HMBS gene mutation spectrum and the degree of penetrance (Goncharova et al., 2019). In whole-exome sequencing of six AIP patients the researchers found several pathogenic gene variants associated with neurodegenerative diseases, including UNC13A, ALG8, FBXO38, AGRN, DOK7, and SCN4A. AGRN, DOK7, and SCN4A genes were associated with congenital myasthenic syndromes (CMS), the symptoms of which are significantly similar to the neurological features of AIP attacks. Thus, HMBS gene mutation combined with defects in neurological regulatory genes may play a key role in triggering acute AIP attacks and developing specific symptom profiles (Goncharova et al., 2019). However, this hypothesis requires confirmatory studies.
2.2.2.3 PPARA gene
Peroxisome proliferator-activated receptor (PPARα) is a ligand-activated transcription factor and member of the nuclear hormone receptor superfamily. A recent study on pathogenic variants of AIP showed a significant functional overlap of the PPARA gene with ALAS1 by protein functional analysis (Fu et al., 2019).
Most drugs are metabolized by the cytochrome p450 system. Studies have shown that some drugs induce ALAS1 either directly or indirectly by inducing synthesis of these heme-containing CYP450 proteins (Granick, 1963; Granick, 1966), leading to the overproduction of heme precursors (Podvinec et al., 2004; Bissell et al., 2017). A study showed that PPARA genes directly regulate transcription of CYP2C8 and CYP3A4 (Thomas et al., 2013; Thomas et al., 2015), consistent with findings that the PPARA gene can specifically regulate CYP1-3 class of genes (Richert et al., 2003; Barbier et al., 2004; Sérée et al., 2004). Consequently, mutations in PPARA may influence AIP attacks by modulating the CYP450 system, thus impacting the heme biosynthesis pathway (Thomas et al., 2015). Despite these findings, the importance of PPARα in expression of the AIP phenotype remains unexplored.
2.3 Mitochondrial DNA
Mitochondria are essential organelles in all nucleated cells, involved in cellular respiration, metabolism, macromolecular biosynthesis, and regulation of apoptosis, cell proliferation, and motility. Mutations, deletions, and insertions in mitochondrial DNA play a crucial role in the pathogenesis of inherited mitochondrial diseases.
Liver transcriptome analysis in an animal model of AIP showed dysregulation of genes associated with mitochondrial biogenesis (Chen et al., 2019b), suggesting that a lack of functionally normal mitochondria may lead to (or be a toxic effect of) acute attacks of AIP. In addition, abnormalities in mitochondrial morphology and function have been reported in studies of animal models of AIP (Homedan et al., 2014; Homedan et al., 2015) and patients with porphyria (Ostrowski et al., 1983; Dixon et al., 2019), emphasizing the potential role of impaired energy supply in the clinical manifestations of the disease.
Recently, an Italian study reported a link between decreased mitochondrial DNA content and porphyria (Di Pierro et al., 2023). The study performed a relative quantification of mitochondrial DNA (mtDNA) copy number in human peripheral leukocytes to indirectly estimate the average mitochondrial content per cell in patients with symptomatic and asymptomatic AIP and compared these results with those of healthy controls. The results showed that AIP patients had decreased numbers of mitochondria and significantly lower serum PERM1 (a marker of mitochondrial biogenesis) levels; a reduction more pronounced in symptomatic patients, suggesting that higher mitochondrial DNA content may modulate porphyria severity or penetrance, perhaps through affecting an individual’s ability to respond to endogenous or environmental stressors.
If confirmed in further studies, mitochondrial DNA copy number per cell and/or mitochondrial biogenesis may become biomarkers of porphyria. The metabolic and genetic factors responsible for these results remain to be determined.
3 Gender, age, sex hormones
The mutation that causes AIP is autosomal and inherited equally between men and women (Schuurmans et al., 2001; Kauppinen and von und zu Fraunberg, 2002). However, acute attacks affect females more often than males, and recurrent attacks are more common in females (Chen et al., 2016).
Most AIP onset attacks occur between the ages of 20 and 40, with onset in childhood rare (Varshney et al., 2018). In addition to a three-year-old Nigerian boy diagnosed with AIP (Sykes, 2001), a Chinese study has reported childhood AIP onset with two female cases and one male case, ages 1 year and 7 months, 1 year and 5 months and 5 years old (mutations: c.579_583del, c.499C > T, c.422 + 1G > A, respectively) (Ren et al., 2023).
A recent Finland study (Baumann and Kauppinen, 2020) found that in families with AIP, the penetrance of hospitalized attacks in female patients was as high as 41%, and the penetrance of all acute symptoms associated with AIP was 50%. Similar findings have been reported in families with the founder mutation p. Trp198X in northern Sweden (Andersson et al., 2000). In addition, a study in Cape Town, South Africa, showed a male-to-female ratio of 1:6 for acute attacks in patients with AIP and a high correlation between AIP attacks and menstruation (Hift and Meissner, 2005). Another Finland study evaluating the prognosis of 206 adult patients with porphyria found that nearly one-third of the women had symptoms of porphyria associated with their menstrual cycle (Kauppinen and Mustajoki, 1992), while a study in northern Sweden showed that symptoms in AIP patients change during the menstrual cycle (Ahangari et al., 2015).
The above studies suggest that female hormones trigger AIP attacks, which may explain the higher penetrance in female patients with AIP than in male patients.
Hift and Meissner (2005) reported that the effect of sex hormones is more pronounced in AIP than in other types of porphyria, with 50% of female AIP attacks associated with the menstrual cycle (De Block et al., 1999; Innala et al., 2010; Linenberger and Fertrin, 2020), and 10%–30% of these attacks occurring during the luteal phase (Ahangari et al., 2015).
Progesterone and its metabolites induce the hepatic ALAS1 enzyme (Podvinec et al., 2004; Phillips, 2019) and are associated with increased heme metabolism (Bonkovsky et al., 2019; Spiritos et al., 2019), and may be a more potent inducer than estrogen (Andersson et al., 2002). While estrogen has a weak effect on AIP, when combined with progesterone, it can induce porphyria attacks (Bissell et al., 2017). Monitoring serum progesterone levels can clarify the luteal phase and progesterone-induced attacks in patients with AIP who experience acute attacks.
Gonadotropin-releasing hormone (GnRH) analogs are synthetic hypothalamic hormone analogs that include gonadotropin-releasing hormone agonists (GnRH-a) and gonadotropin-releasing hormone antagonists (GnRH-A). Studies have shown that GnRH-a therapy inhibits endogenous hormone production and successfully prevents attacks in patients with AIP associated with the menstrual cycle (Castelo-Branco et al., 2001; Innala et al., 2010; Xu et al., 2022). However, the administration of GnRH-a has an initial rebound effect of transiently stimulating the pituitary gland to secrete elevated Follicle-stimulating Hormone (FSH) and Luteinising Hormone (LH), known as the “flare-up” effect, which leads to a transient increase in ovarian hormone production that lasts for about 7 days. This flare-up effect promotes the maturation of primordial follicles and improves therapeutic efficacy but may increase the risk of ovarian hyperstimulation syndrome (OHSS) (Lambalk et al., 2017). GnRH-A inhibits ovarian function by directly inhibiting GnRH, which decreases the levels of FSH and LH secreted by the pituitary gland without any flare-up effect and has a more rapid onset of action.
Elagolix is an oral GnRH-A that is FDA-approved for the treatment of moderate to severe pain associated with endometriosis (Lamb, 2018), however unlike leuprolide, it induces CYP3A and a fluorescence-based drug porphyrogenicity screening assay in Leghorn Male Hepatoma (LMH) cells demonstrated that it is porphyrogenic and associated with increased acute porphyria attacks in women (Ma and Bonkovsky, 2022).
In addition, animal model studies have found an increased local vasoconstrictor response in AIP mice and a lower sensitivity to vasodilation in female mice (pro-constrictor response), suggesting the sex difference may be due to differing sensitivities of mesenteric arteries to porphyrin precursors (Pulgar et al., 2019).
4 Environmental factors
AIP attacks can be triggered by low energy intake, dieting, and alcohol consumption (Storjord et al., 2019). Alcohol, as a porphyrinogenic agent, enhances the induction of ALAS1, causing acute AIP attacks (Goldberg et al., 1981; Doss et al., 2000). Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) is an enzyme that regulates ALAS1 in the liver and can be activated by fasting (Herzig et al., 2001). Fasting can cause a significant increase in PGC-1α and ALAS1 (Scassa et al., 1998), which may lead to AIP attacks. Smoking may contribute to recurrent AIP attacks by influencing drug metabolism and steroid hormone metabolism (Michnovicz et al., 1986; Klaiber and Broverman, 1988; Lip et al., 1991).
The activity of ALAS1 is positively regulated by substances that induce hepatic CYPs (Manceau et al., 2017). As discussed in Section 2.2.2.3, most porphyrogenic drugs cause AIP attacks through impacting CYP450 metabolism, particularly through induction of, or irreversible inactivation of, CYP3A4 and CYP2C9 (Brodie, 1980; Moore and Hift, 1997; Thunell, 2000; Thunell et al., 2007). Studies have shown that phenobarbital is extremely porphyrinogenic and induces drug-oxidizing enzymes in the liver, exacerbating AIP (Kappas et al., 1977; Holroyd and Seward, 1999). Phenobarbital-induced AIP mice have upregulated CYP2C40, CYP2C68, and CYP2C69, equivalent to human CYP2C8, CYP2C9, and CYP2C19 respectively (Nelson et al., 2004; Zanger et al., 2008), but downregulates CYP21A1 (Chen et al., 2019b), equivalent to human CYP21A2 or a homolog of CYP17A1 (−37), a crucial enzyme in corticosteroid and sex hormone synthesis (Idkowiak et al., 1993). Diclofenac and fluoxetine, CYP3A4 inhibitors, are associated with acute porphyrias (Pelkonen et al., 2008). CYP2C8 and CYP3A4/5 metabolize hydroxychloroquine in vivo (Rendic and Guengerich, 2020). AIP attacks have been reported during hydroxychloroquine treatment for systemic lupus erythematosus (Esteve-Valverde et al., 2020), and with use of the HIV antiretroviral drugs, atazanavir/ritonavir (Le Tiec et al., 2005; Bharti et al., 2016).
Heme is a potent inhibitor of ALAS1 expression and activity. Studies have shown that heme oxygenase (HO-1) is induced by stress, hypoglycemia, toxins, heavy metals, and organic chemicals (Thunell, 2000; Li et al., 2007; Abraham and Kappas, 2008), leading to increased heme catabolism and a decrease in the intracellular heme pool, which activates ALAS1 transcription. Chronic administration of enflurane or isoflurane and treatment with griseofulvin have been shown to induce an increase in HO-1 activity. Chronic exposure to enflurane and isoflurane anesthesia, as well as veronal treatment, increased HO mRNA expression (Rodriguez et al., 2005; Ferrándiz and Devesa, 2008). Furthermore, a mouse model of AIP has confirmed the porphyrinogenicity of isoflurane and sevoflurane (Ruspini et al., 2018).
5 Concluding remarks and future perspectives
In conclusion, it is important to consider the low penetrance of AIP and highly variable clinical phenotype, as revealed in population studies. This variability may result in underdiagnosis of the disease, or undertreatment, or even be potentially life-threatening for some patients. A comprehensive understanding of the prevalence and penetrance of this rare disease aids early diagnosis, the establishment of standardized management protocols, and identification of potential therapeutic targets. We have described analyses of genetic susceptibility factors and modifier genes implicated in the regulation of AIP penetrance and phenotype, however, most of these findings require further studies to validate their clinical relevance. Nonetheless the characterization of confounding variables holds promise for enhancing the clinical care of AIP patients and may pave the way for the development of novel, safe, and productive treatment strategies.
Author contributions
J-JL: Conceptualization, Writing–original draft, Writing–review and editing. SL: Writing–original draft, Writing–review and editing. B-XD: Writing–original draft, Writing–review and editing. JY: Writing–review and editing. YR: Conceptualization, Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Key research and development project of Shanxi Province, China (201903D321127), Special Project on the Transformation and Guidance of Scientific and Technological Achievements in Shanxi Province, China (201804D131044).
Acknowledgments
The authors thank the Key research and development project of Shanxi Province, China (201903D321127), Special Project on the Transformation and Guidance of Scientific and Technological Achievements in Shanxi Province, China (201804D131044) for the support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abraham, N. G., and Kappas, A. (2008). Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 60, 79–127. doi:10.1124/pr.107.07104
Ahangari, A., Bäckström, T., Innala, E., Andersson, C., and Turkmen, S. (2015). Acute intermittent porphyria symptoms during the menstrual cycle. Intern Med. J. 45, 725–731. doi:10.1111/imj.12784
Anderson, K. E., Bloomer, J. R., Bonkovsky, H. L., Kushner, J. P., Pierach, C. A., Pimstone, N. R., et al. (2005). Recommendations for the diagnosis and treatment of the acute porphyrias. Ann. Intern Med. 142, 439–450. doi:10.7326/0003-4819-142-6-200503150-00010
Andersson, C., Floderus, Y., Wikberg, A., and Lithner, F. (2000). The W198X and R173W mutations in the porphobilinogen deaminase gene in acute intermittent porphyria have higher clinical penetrance than R167W. A population-based study. Scand. J. Clin. Lab. Invest 60, 643–648. doi:10.1080/003655100300054891
Andersson, C., Nilsson, A., and Bäckström, T. (2002). Atypical attack of acute intermittent porphyria--paresis but no abdominal pain. J. Intern Med. 252, 265–270. doi:10.1046/j.1365-2796.2002.01020.x
Balwani, M., Sardh, E., Ventura, P., Peiro, P. A., Rees, D. C., Stolzel, U., et al. (2020). Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N. Engl. J. Med. 382, 2289–2301. doi:10.1056/NEJMoa1913147
Barbier, O., Fontaine, C., Fruchart, J. C., and Staels, B. (2004). Genomic and non-genomic interactions of PPARalpha with xenobiotic-metabolizing enzymes. Trends Endocrinol. Metab. 15, 324–330. doi:10.1016/j.tem.2004.07.007
Barreda-Sánchez, M., Buendía-Martínez, J., Glover-López, G., Carazo-Díaz, C., Ballesta-Martínez, M. J., López-González, V., et al. (2019). High penetrance of acute intermittent porphyria in a Spanish founder mutation population and CYP2D6 genotype as a susceptibility factor. Orphanet J. Rare Dis. 14, 59. doi:10.1186/s13023-019-1031-7
Baumann, K., and Kauppinen, R. (2020). Penetrance and predictive value of genetic screening in acute porphyria. Mol. Genet. Metab. 130, 87–99. doi:10.1016/j.ymgme.2020.02.003
Bharti, S., Malhotra, P., and Hirsch, B. (2016). Acute intermittent porphyria precipitated by atazanavir/ritonavir. Int. J. STD AIDS 27, 1234–1235. doi:10.1177/0956462416633981
Bissell, D. M., Anderson, K. E., and Bonkovsky, H. L. (2017). Porphyria. N. Engl. J. Med. 377, 862–872. doi:10.1056/NEJMra1608634
Blaylock, B., Epstein, J., and Stickler, P. (2020). Real-world annualized healthcare utilization and expenditures among insured US patients with acute intermittent porphyria (AIP) treated with hemin. J. Med. Econ. 23, 537–545. doi:10.1080/13696998.2020.1724118
Bonkovsky, H. L., Dixon, N., and Rudnick, S. (2019). Pathogenesis and clinical features of the acute hepatic porphyrias (AHPs). Mol. Genet. Metab. 128, 213–218. doi:10.1016/j.ymgme.2019.03.002
Bonkovsky, H. L., Maddukuri, V. C., Yazici, C., Anderson, K. E., Bissell, D. M., Bloomer, J. R., et al. (2014). Acute porphyrias in the USA: features of 108 subjects from porphyrias consortium. Am. J. Med. 127, 1233–1241. doi:10.1016/j.amjmed.2014.06.036
Bustad, H. J., Toska, K., Schmitt, C., Vorland, M., Skjaerven, L., Kallio, J. P., et al. (2020). A pharmacological chaperone therapy for acute intermittent porphyria. Mol. Ther. 28, 677–689. doi:10.1016/j.ymthe.2019.11.010
Bustad, H. J., Vorland, M., Rønneseth, E., Sandberg, S., Martinez, A., and Toska, K. (2013). Conformational stability and activity analysis of two hydroxymethylbilane synthase mutants, K132N and V215E, with different phenotypic association with acute intermittent porphyria. Biosci. Rep. 33, e00056. doi:10.1042/bsr20130045
Castelo-Branco, C., Vicente, J. J., and Vanrell, J. A. (2001). Use of gonadotropin-releasing hormone analog with tibolone to prevent cyclic attacks of acute intermittent porphyria. Metabolism 50, 995–996. doi:10.1053/meta.2001.25587
Chen, B., Solis-Villa, C., Erwin, A. L., Balwani, M., Nazarenko, I., Phillips, J. D., et al. (2019a). Identification and characterization of 40 novel hydroxymethylbilane synthase mutations that cause acute intermittent porphyria. J. Inherit. Metab. Dis. 42, 186–194. doi:10.1002/jimd.12040
Chen, B., Solis-Villa, C., Hakenberg, J., Qiao, W., Srinivasan, R. R., Yasuda, M., et al. (2016). Acute intermittent porphyria: predicted pathogenicity of HMBS variants indicates extremely low penetrance of the autosomal dominant disease. Hum. Mutat. 37, 1215–1222. doi:10.1002/humu.23067
Chen, B., Wang, M., Gan, L., Zhang, B., Desnick, R. J., and Yasuda, M. (2019b). Characterization of the hepatic transcriptome following phenobarbital induction in mice with AIP. Mol. Genet. Metab. 128, 382–390. doi:10.1016/j.ymgme.2018.12.010
Christiansen, L., Bygum, A., Jensen, A., Thomsen, K., Brandrup, F., Hørder, M., et al. (2000). Association between CYP1A2 polymorphism and susceptibility to porphyria cutanea tarda. Hum. Genet. 107, 612–614. doi:10.1007/s004390000415
Cooper, D. N., and Youssoufian, H. (1988). The CpG dinucleotide and human genetic disease. Hum. Genet. 78, 151–155. doi:10.1007/bf00278187
De Block, C. E., Leeuw, I. H., and Gaal, L. F. (1999). Premenstrual attacks of acute intermittent porphyria: hormonal and metabolic aspects - a case report. Eur. J. Endocrinol. 141, 50–54. doi:10.1530/eje.0.1410050
Di Pierro, E., Perrone, M., Franco, M., Granata, F., Duca, L., Lattuada, D., et al. (2023). Mitochondrial DNA copy number Drives the penetrance of acute intermittent porphyria. Life (Basel) 13, 1923. doi:10.3390/life13091923
Dixon, N., Li, T., Marion, B., Faust, D., Dozier, S., Molina, A., et al. (2019). Pilot study of mitochondrial bioenergetics in subjects with acute porphyrias. Mol. Genet. Metab. 128, 228–235. doi:10.1016/j.ymgme.2019.05.010
Doss, M. O., Kühnel, A., and Gross, U. (2000). Alcohol and porphyrin metabolism. Alcohol Alcohol 35, 109–125. doi:10.1093/alcalc/35.2.109
Ennis, S. R., Novotny, A., Xiang, J., Shakui, P., Masada, T., Stummer, W., et al. (2003). Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 959, 226–234. doi:10.1016/s0006-8993(02)03749-6
Esteve-Valverde, E., Tapiz-Reula, A., Ruiz, D., and Alijotas-Reig, J. (2020). Systemic lupus erythematosus and hydroxychloroquine-related acute intermittent porphyria. Rheumatol. Int. 40, 777–783. doi:10.1007/s00296-019-04500-8
Ferrándiz, M. L., and Devesa, I. (2008). Inducers of heme oxygenase-1. Curr. Pharm. Des. 14, 473–486. doi:10.2174/138161208783597399
Fraunberg, M., Pischik, E., Udd, L., and Kauppinen, R. (2005). Clinical and biochemical characteristics and genotype-phenotype correlation in 143 Finnish and Russian patients with acute intermittent porphyria. Med. Baltim. 84, 35–47. doi:10.1097/01.md.0000152455.38510.af
Fu, Y., Jia, J., Yue, L., Yang, R., Guo, Y., Ni, X., et al. (2019). Systematically analyzing the pathogenic variations for acute intermittent porphyria. Front. Pharmacol. 10, 1018. doi:10.3389/fphar.2019.01018
Gardlo, K., Selimovic, D., Bolsen, K., Ruzicka, T., Abel, J., and Fritsch, C. (2003). Cytochrome p450A1 polymorphisms in a Caucasian population with porphyria cutanea tarda. Exp. Dermatol 12, 843–848. doi:10.1111/j.0906-6705.2003.00095.x
Goldberg, A., McColl, K. E., and Moore, M. R. (1981). Alcohol and porphyria. Lancet 2, 925. doi:10.1016/s0140-6736(81)91406-9
Goncharova, M., Pshenichnikova, O., Luchinina, Y., Pustovoit, Y., Karpova, I., and Surin, V. (2019). Molecular genetic study of acute intermittent porphyria in Russia: HMBS gene mutation spectrum and problem of penetrance. Clin. Genet. 96, 91–97. doi:10.1111/cge.13558
Gouya, L., Ventura, P., Balwani, M., Bissell, D. M., Rees, D. C., Stolzel, U., et al. (2020). EXPLORE: a prospective, multinational, natural history study of patients with acute hepatic porphyria with recurrent attacks. Hepatology 71, 1546–1558. doi:10.1002/hep.30936
Granick, S. (1963). Induction of the synthesis of δ-Amino-levulinic acid synthetase in liver parenchyma cells in culture by chemicals that induce acute porphyria. J. Biol. Chem. 238, 2247–2249. doi:10.1016/s0021-9258(18)67967-0
Granick, S. (1966). The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J. Biol. Chem. 241, 1359–1375. doi:10.1016/s0021-9258(18)96783-9
Guillén-Navarro, E., Carbonell, P., Glover, G., Sánchez-Solís, M., and Fernández-Barreiro, A. (2004). Novel HMBS founder mutation and significant intronic polymorphism in Spanish patients with acute intermittent porphyria. Ann. Hum. Genet. 68, 509–514. doi:10.1046/j.1529-8817.2003.00114.x
Herzig, S., Long, F., Jhala, U. S., Hedrick, S., Quinn, R., Bauer, A., et al. (2001). CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183. doi:10.1038/35093131
Hift, R. J., and Meissner, P. N. (2005). An analysis of 112 acute porphyric attacks in Cape Town, South Africa: evidence that acute intermittent porphyria and variegate porphyria differ in susceptibility and severity. Med. Baltim. 84, 48–60. doi:10.1097/01.md.0000152454.56435.f3
Holroyd, S., and Seward, R. L. (1999). Psychotropic drugs in acute intermittent porphyria. Clin. Pharmacol. Ther. 66, 323–325. doi:10.1016/s0009-9236(99)70041-x
Homedan, C., Laafi, J., Schmitt, C., Gueguen, N., Lefebvre, T., Karim, Z., et al. (2014). Acute intermittent porphyria causes hepatic mitochondrial energetic failure in a mouse model. Int. J. Biochem. Cell Biol. 51, 93–101. doi:10.1016/j.biocel.2014.03.032
Homedan, C., Schmitt, C., Laafi, J., Gueguen, N., Desquiret-Dumas, V., Lenglet, H., et al. (2015). Mitochondrial energetic defects in muscle and brain of a Hmbs/mouse model of acute intermittent porphyria. Hum. Mol. Genet. 24, 5015–5023. doi:10.1093/hmg/ddv222
Hu, Y., Shen, H., Keep, R. F., and Smith, D. E. (2007). Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J. Neurochem. 103, 2058–2065. doi:10.1111/j.1471-4159.2007.04905.x
Idkowiak, J., Cragun, D., Hopkin, R. J., and Arlt, W. (1993). “Cytochrome P450 oxidoreductase deficiency,” in GeneReviews(®). Editors M. P. ADAM, J. FELDMAN, G. M. MIRZAA, R. A. PAGON, S. E. WALLACE, L. J. H. BEANet al. (Seattle, WA: University of Washington, Seattle).
Innala, E., Bäckström, T., Bixo, M., and Andersson, C. (2010). Evaluation of gonadotropin-releasing hormone agonist treatment for prevention of menstrual-related attacks in acute porphyria. Acta Obstet. Gynecol. Scand. 89, 95–100. doi:10.3109/00016340903390729
Jaramillo-Calle, D. A., Solano, J. M., Rabinstein, A. A., and Bonkovsky, H. L. (2019). Porphyria-induced posterior reversible encephalopathy syndrome and central nervous system dysfunction. Mol. Genet. Metab. 128, 242–253. doi:10.1016/j.ymgme.2019.10.011
Kappas, A., Bradlow, H. L., Bickers, D. R., and Alvares, A. P. (1977). Induction of a deficiency of steroid delta 4-5 alpha-reductase activity in liver by a porphyrinogenic drug. J. Clin. Invest 59, 159–164. doi:10.1172/jci108614
Kauppinen, R., and Mustajoki, P. (1992). Prognosis of acute porphyria: occurrence of acute attacks, precipitating factors, and associated diseases. Med. Baltim. 71, 1–13. doi:10.1097/00005792-199201000-00001
Kauppinen, R., and von und zu Fraunberg, M. (2002). Molecular and biochemical studies of acute intermittent porphyria in 196 patients and their families. Clin. Chem. 48, 1891–1900. doi:10.1093/clinchem/48.11.1891
Kizilaslan, E. Z., Ghadge, N. M., Martinez, A., Bass, M., Winayak, R., Mathew, M., et al. (2023). Acute intermittent porphyria's symptoms and management: a narrative review. Cureus 15, e36058. doi:10.7759/cureus.36058
Klaiber, E. L., and Broverman, D. M. (1988). Dynamics of estradiol and testosterone and seminal fluid indexes in smokers and nonsmokers. Fertil. Steril. 50, 630–634. doi:10.1016/s0015-0282(16)60196-6
Kuter, D. J., Bonkovsky, H. L., Monroy, S., Ross, G., Guillen-Navarro, E., Cappellini, M. D., et al. (2023). Efficacy and safety of givosiran for acute hepatic porphyria: final results of the randomized phase III ENVISION trial. J. Hepatology S0168-8278, 1150–1158. doi:10.1016/j.jhep.2023.06.013
Lamb, Y. N. (2018). Elagolix: first global approval. Drugs 78, 1501–1508. doi:10.1007/s40265-018-0977-4
Lambalk, C. B., Banga, F. R., Huirne, J. A., Toftager, M., Pinborg, A., Homburg, R., et al. (2017). GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum. Reprod. Update 23, 560–579. doi:10.1093/humupd/dmx017
Lavandera, J. V., Parera, V. E., Batlle, A., and Buzaleh, A. M. (2006). CYP2D6 polymorphisms in patients with porphyrias. Mol. Med. 12, 259–263. doi:10.2119/2005–00047.Lavandera
Lenglet, H., Schmitt, C., Grange, T., Manceau, H., Karboul, N., Bouchet-Crivat, F., et al. (2018). From a dominant to an oligogenic model of inheritance with environmental modifiers in acute intermittent porphyria. Hum. Mol. Genet. 27, 1164–1173. doi:10.1093/hmg/ddy030
Le Tiec, C., Barrail, A., Goujard, C., and Taburet, A. M. (2005). Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir. Clin. Pharmacokinet. 44, 1035–1050. doi:10.2165/00003088-200544100-00003
Li, C., Hossieny, P., Wu, B. J., Qawasmeh, A., Beck, K., and Stocker, R. (2007). Pharmacologic induction of heme oxygenase-1. Antioxid. Redox Signal 9, 2227–2239. doi:10.1089/ars.2007.1783
Linenberger, M., and Fertrin, K. Y. (2020). Updates on the diagnosis and management of the most common hereditary porphyrias: AIP and EPP. Hematol. Am. Soc. Hematol. Educ. Program 2020, 400–410. doi:10.1182/hematology.2020000124
Lip, G. Y., McColl, K. E., Goldberg, A., and Moore, M. R. (1991). Smoking and recurrent attacks of acute intermittent porphyria. Bmj 302, 507. doi:10.1136/bmj.302.6775.507
Ma, C. D., and Bonkovsky, H. L. (2022). Elagolix is porphyrogenic and may induce porphyric attacks in patients with the acute hepatic porphyrias. Mol. Genet. Metab. Rep. 33, 100915. doi:10.1016/j.ymgmr.2022.100915
Manceau, H., Gouya, L., and Puy, H. (2017). Acute hepatic and erythropoietic porphyrias: from ALA synthases 1 and 2 to new molecular bases and treatments. Curr. Opin. Hematol. 24, 198–207. doi:10.1097/moh.0000000000000330
Martinez di Montemuros, F., Di Pierro, E., Fargion, S., Biolcati, G., Griso, D., Macrì, A., et al. (2000). Molecular analysis of the hydroxymethylbilane synthase (HMBS) gene in Italian patients with acute intermittent porphyria: report of four novel mutations. Hum. Mutat. 15, 480. doi:10.1002/(sici)1098-1004(200005)15:5<480::Aid-humu12>3.0.Co;2-j
Menoyo, A., del Rio, E., and Baiget, M. (2006). Characterization of variant alleles of cytochrome CYP2D6 in a Spanish population. Cell Biochem. Funct. 24, 381–385. doi:10.1002/cbf.1258
Michnovicz, J. J., Hershcopf, R. J., Naganuma, H., Bradlow, H. L., and Fishman, J. (1986). Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N. Engl. J. Med. 315, 1305–1309. doi:10.1056/nejm198611203152101
Moore, M. R., and Hift, R. J. (1997). Drugs in the acute porphyrias--toxicogenetic diseases. Cell Mol. Biol. (Noisy-le-grand) 43, 89–94.
Nelson, D. R., Zeldin, D. C., Hoffman, S. M., Maltais, L. J., Wain, H. M., and Nebert, D. W. (2004). Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14, 1–18. doi:10.1097/00008571-200401000-00001
Ostrowski, J., Kostrzewska, E., Michalak, T., Zawirska, B., Medrzejewski, W., and Gregor, A. (1983). Abnormalities in liver function and morphology and impaired aminopyrine metabolism in hereditary hepatic porphyrias. Gastroenterology 85, 1131–1137. doi:10.1016/s0016-5085(83)80081-x
Pallet, N., Mami, I., Schmitt, C., Karim, Z., François, A., Rabant, M., et al. (2015). High prevalence of and potential mechanisms for chronic kidney disease in patients with acute intermittent porphyria. Kidney Int. 88, 386–395. doi:10.1038/ki.2015.97
Pelkonen, O., Turpeinen, M., Hakkola, J., Honkakoski, P., Hukkanen, J., and Raunio, H. (2008). Inhibition and induction of human cytochrome P450 enzymes: current status. Arch. Toxicol. 82, 667–715. doi:10.1007/s00204-008-0332-8
Phillips, J. D. (2019). Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 128, 164–177. doi:10.1016/j.ymgme.2019.04.008
Pischik, E., and Kauppinen, R. (2009). Neurological manifestations of acute intermittent porphyria. Cell Mol. Biol. (Noisy-le-grand) 55, 72–83. doi:10.1170/T841
Podvinec, M., Handschin, C., Looser, R., and Meyer, U. A. (2004). Identification of the xenosensors regulating human 5-aminolevulinate synthase. Proc. Natl. Acad. Sci. U. S. A. 101, 9127–9132. doi:10.1073/pnas.0401845101
Pulgar, V. M., Yasuda, M., Gan, L., Desnick, R. J., and Bonkovsky, H. L. (2019). Sex differences in vascular reactivity in mesenteric arteries from a mouse model of acute intermittent porphyria. Mol. Genet. Metab. 128, 376–381. doi:10.1016/j.ymgme.2019.01.005
Puy, H., Gouya, L., and Deybach, J. C. (2010). Porphyrias. Lancet 375, 924–937. doi:10.1016/s0140-6736(09)61925-5
Ren, Y., Li, S., Lei, J. J., Li, R., Dong, B. X., and Yang, J. (2023). Clinical feature and genetic analysis of HMBS gene in Chinese patients with acute intermittent porphyria: a systematic review. Front. Genet. 14, 1291719. doi:10.3389/fgene.2023.1291719
Rendic, S., and Guengerich, F. P. (2020). Metabolism and interactions of chloroquine and hydroxychloroquine with human cytochrome P450 enzymes and drug transporters. Curr. Drug Metab. 21, 1127–1135. doi:10.2174/1389200221999201208211537
Richert, L., Lamboley, C., Viollon-Abadie, C., Grass, P., Hartmann, N., Laurent, S., et al. (2003). Effects of clofibric acid on mRNA expression profiles in primary cultures of rat, mouse and human hepatocytes. Toxicol. Appl. Pharmacol. 191, 130–146. doi:10.1016/s0041-008x(03)00231-x
Rodriguez, J. A., Martinez Mdel, C., Gerez, E., Batlle, A., and Buzaleh, A. M. (2005). Heme oxygenase, aminolevulinate acid synthetase and the antioxidant system in the brain of mice treated with porphyrinogenic drugs. Cell Mol. Biol. (Noisy-le-grand) 51, 487–494. doi:10.1170/T654
Ruspini, S. F., Zuccoli, J. R., Lavandera, J. V., Martínez, M. D. C., Oliveri, L. M., Gerez, E. N., et al. (2018). Effects of volatile anaesthetics on heme metabolism in a murine genetic model of Acute Intermittent Porphyria. A comparative study with other porphyrinogenic drugs. Biochim. Biophys. Acta Gen. Subj. 1862, 1296–1305. doi:10.1016/j.bbagen.2018.02.013
Sardh, E., and Barbaro, M. (1993). “Acute intermittent porphyria,” in GeneReviews(®). Editors M. P. ADAM, J. FELDMAN, G. M. MIRZAA, R. A. PAGON, S. E. WALLACE, L. J. H. BEANet al. (Seattle, WA: University of Washington, Seattle).
Scassa, M. E., Varone, C. L., Montero, L., and Cánepa, E. T. (1998). Insulin inhibits delta-aminolevulinate synthase gene expression in rat hepatocytes and human hepatoma cells. Exp. Cell Res. 244, 460–469. doi:10.1006/excr.1998.4206
Schulenburg-Brand, D., Stewart, F., Stein, P., Rees, D., and Badminton, M. (2022). Update on the diagnosis and management of the autosomal dominant acute hepatic porphyrias. J. Clin. Pathol. 75, 537–543. doi:10.1136/jclinpath-2021-207647
Schuurmans, M. M., Schneider-Yin, X., Rüfenacht, U. B., Schnyder, C., Minder, C. E., Puy, H., et al. (2001). Influence of age and gender on the clinical expression of acute intermittent porphyria based on molecular study of porphobilinogen deaminase gene among Swiss patients. Mol. Med. 7, 535–542. doi:10.1007/bf03401859
Sérée, E., Villard, P. H., Pascussi, J. M., Pineau, T., Maurel, P., Nguyen, Q. B., et al. (2004). Evidence for a new human CYP1A1 regulation pathway involving PPAR-alpha and 2 PPRE sites. Gastroenterology 127, 1436–1445. doi:10.1053/j.gastro.2004.08.023
Souza, P. V. S., Badia, B. M. L., Farias, I. B., Pinto, W., and Oliveira, A. S. B. (2021). Acute hepatic porphyria: pathophysiological basis of neuromuscular manifestations. Front. Neurosci. 15, 715523. doi:10.3389/fnins.2021.715523
Spiritos, Z., Salvador, S., Mosquera, D., and Wilder, J. (2019). Acute intermittent porphyria: current perspectives and case presentation. Ther. Clin. Risk Manag. 15, 1443–1451. doi:10.2147/tcrm.S180161
Stenson, P. D., Mort, M., Ball, E. V., Evans, K., Hayden, M., Heywood, S., et al. (2017). The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet. 136, 665–677. doi:10.1007/s00439-017-1779-6
Storjord, E., Dahl, J. A., Landsem, A., Ludviksen, J. K., Karlsen, M. B., Karlsen, B. O., et al. (2019). Lifestyle factors including diet and biochemical biomarkers in acute intermittent porphyria: results from a case-control study in northern Norway. Mol. Genet. Metab. 128, 254–270. doi:10.1016/j.ymgme.2018.12.006
Sykes, R. M. (2001). Acute intermittent porphyria, seizures, and antiepileptic drugs: a report on a 3-year-old Nigerian boy. Seizure 10, 64–66. doi:10.1053/seiz.2000.0473
Tchernitchko, D., Tavernier, Q., Lamoril, J., Schmitt, C., Talbi, N., Lyoumi, S., et al. (2017). A variant of peptide transporter 2 Predicts the severity of porphyria-associated kidney disease. J. Am. Soc. Nephrol. 28, 1924–1932. doi:10.1681/asn.2016080918
Thomas, M., Burk, O., Klumpp, B., Kandel, B. A., Damm, G., Weiss, T. S., et al. (2013). Direct transcriptional regulation of human hepatic cytochrome P450 3A4 (CYP3A4) by peroxisome proliferator-activated receptor alpha (PPARα). Mol. Pharmacol. 83, 709–718. doi:10.1124/mol.112.082503
Thomas, M., Winter, S., Klumpp, B., Turpeinen, M., Klein, K., Schwab, M., et al. (2015). Peroxisome proliferator-activated receptor alpha, PPARα, directly regulates transcription of cytochrome P450 CYP2C8. Front. Pharmacol. 6, 261. doi:10.3389/fphar.2015.00261
Thunell, S. (2000). Porphyrins, porphyrin metabolism and porphyrias. I. Update. Scand. J. Clin. Lab. Invest 60, 509–540. doi:10.1080/003655100448310
Thunell, S., Pomp, E., and Brun, A. (2007). Guide to drug porphyrogenicity prediction and drug prescription in the acute porphyrias. Br. J. Clin. Pharmacol. 64, 668–679. doi:10.1111/j.0306-5251.2007.02955.x
Tjensvoll, K., Bruland, O., Floderus, Y., Skadberg, Ø., Sandberg, S., and Apold, J. (2003). Haplotype analysis of Norwegian and Swedish patients with acute intermittent porphyria (AIP): extreme haplotype heterogeneity for the mutation R116W. Dis. Markers 19, 41–46. doi:10.1155/2003/384971
Ulbrichova, D., Schneider-Yin, X., Mamet, R., Saudek, V., Martasek, P., Minder, E. I., et al. (2009). Correlation between biochemical findings, structural and enzymatic abnormalities in mutated HMBS identified in six Israeli families with acute intermittent porphyria. Blood Cells Mol. Dis. 42, 167–173. doi:10.1016/j.bcmd.2008.11.001
Varshney, G. A., Saini, P. A., and Ghure, U. (2018). A rare case of acute intermittent porphyria with ichthyosis vulgaris in a young boy. J. Fam. Med. Prim. Care 7, 261–263. doi:10.4103/jfmpc.jfmpc_141_17
Wang, B., Bonkovsky, H. L., Lim, J. K., and Balwani, M. (2023). AGA clinical practice update on diagnosis and management of acute hepatic porphyrias: expert review. Gastroenterology 164, 484–491. doi:10.1053/j.gastro.2022.11.034
Whatley, S. D., Woolf, J. R., and Elder, G. H. (1999). Comparison of complementary and genomic DNA sequencing for the detection of mutations in the HMBS gene in British patients with acute intermittent porphyria: identification of 25 novel mutations. Hum. Genet. 104, 505–510. doi:10.1007/s004390050995
Xu, J., Yi, C., He, J., and Huang, J. (2022). Long-term remission of acute intermittent porphyria treated with gonadotropin-releasing hormone analogues and estrogen: a case report. Clin. Lab. 68. doi:10.7754/Clin.Lab.2022.211218
Yarra, P., Faust, D., Bennett, M., Rudnick, S., and Bonkovsky, H. L. (2019). Benefits of prophylactic heme therapy in severe acute intermittent porphyria. Mol. Genet. Metab. Rep. 19, 100450. doi:10.1016/j.ymgmr.2019.01.002
Yasuda, M., Chen, B., and Desnick, R. J. (2019). Recent advances on porphyria genetics: inheritance, penetrance and molecular heterogeneity, including new modifying/causative genes. Mol. Genet. Metab. 128, 320–331. doi:10.1016/j.ymgme.2018.11.012
Zanger, U. M., Turpeinen, M., Klein, K., and Schwab, M. (2008). Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 392, 1093–1108. doi:10.1007/s00216-008-2291-6
Keywords: acute intermittent porphyria, hydroxymethylbilane synthase, gene mutation, penetrance, heterogeneity, oligogenic inheritance
Citation: Lei J-J, Li S, Dong B-X, Yang J and Ren Y (2024) Acute intermittent porphyria: a disease with low penetrance and high heterogeneity. Front. Genet. 15:1374965. doi: 10.3389/fgene.2024.1374965
Received: 23 January 2024; Accepted: 29 July 2024;
Published: 12 August 2024.
Edited by:
John G. Quigley, University of Illinois Chicago, United StatesReviewed by:
Kitiwan Rojnueangnit, Thammasat University Hospital, ThailandPaulo Victor Sgobbi Souza, Federal University of São Paulo, Brazil
Copyright © 2024 Lei, Li, Dong, Yang and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Ren, cmVueWlfMF8wQDE2My5jb20=
 Jia-Jia Lei
Jia-Jia Lei Shuang Li
Shuang Li Bai-Xue Dong
Bai-Xue Dong Jing Yang
Jing Yang Yi Ren
Yi Ren