- Tissue Typing Laboratory, Department of Medical Biology, Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkiye
The Histocompatibility and Immunogenetics laboratories provide disease association and pharmacogenetic analyses as well as the tests required for transplantation immunology and transfusion medicine. They perform Human Leukocyte Antigen (HLA) genotyping in patients/recipients and potential donor candidates for solid organ and stem cell transplants using various molecular methods, and determine mismatches. In addition, they also perform HLA antibody tests to detect anti-HLA antibodies in patients and flow cross-matches to evaluate donor-recipient compatibility. Evidence-based clinical guidelines have emphasized the importance of laboratory tests in clinical practices for a long time. Understanding the principles of Quality Control and External Quality Assurance is a fundamental requirement for the effective management of Tissue Typing laboratories. When these processes are effectively implemented, errors in routine assays for transplantation are reduced and quality is improved. In this review, the importance of Quality Assurance, Quality control and proficiency testing in Histocompatibility and Immunogenetic testing, the necessity of external proficiency testing (EPT) for accreditation, and existing and potential EPT programmes will be reviewed and evaluated in the light of the literature.
Introduction
Histocompatibility and Immunogenetics Laboratories (Tissue Typing Labs) play an active role in both solid organ and hematopoetic stem cell transplants. Histocompatibility testing is essential for donor identification and risk assessment in solid organ and hematopoietic stem cell transplant. Additionally, it is useful for identifying donor specific alleles for monitoring donor specific antibodies in post-transplant patients.
Post-transplant chimerism test to evaluate engraftment especially in hematopoietic stem cell transplantation (HSCT) transplant patients and donor specific antibody monitoring in renal transplants are routine tasks. In addition, Human Leukocyte Antigen (HLA) Laboratories provide disease association and pharmacogenetic analyses as well as the tests for transfusion medicine.
In recent surveys performed with expert clinicians in Germany and United States, it was reported that 60%–70% of clinical decisions were influenced by the results of laboratory tests performed both in hospitals and in external centers (Rohr et al., 2016). Evidence based clinical guidelines indicate that at least 80% of guidelines, targeting to make a diagnosis or manage a disease, require laboratory tests (Goodman et al., 2005). Laboratories have been aware of this for a long time and try to reduce the risk of misinterpretation of the test results obtained from different laboratories (Shahangian and Cohn, 2000) However, these concepts should be based on well-designed and well-implemented Quality Control (QC) and External Quality Assurance (EQA) systems (Badrick, 2003; Badrick, 2021).
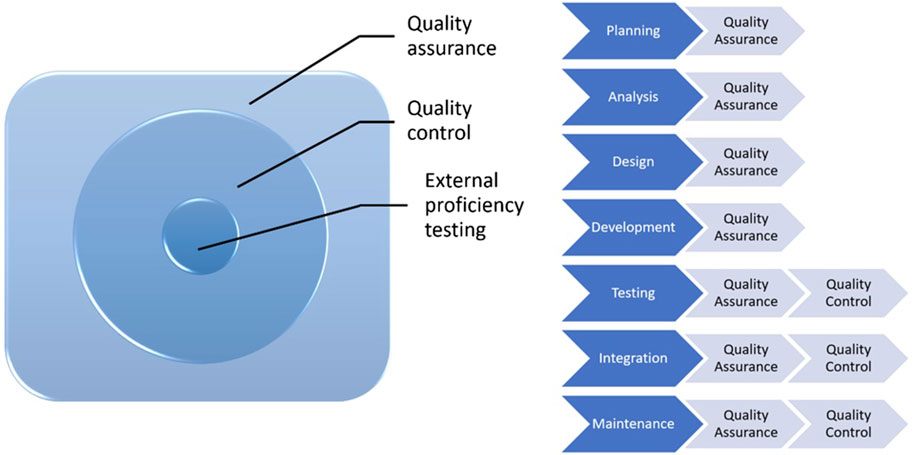
Quality assurance (QA) is a subgroup of quality management (Figure 1). It is proactive, concerns the whole process, includes a series of activities and procedures, that occur during operations and help in providing a high quality analysis, and prevents errors. All health institutions should establish QA policies for laboratories to meet these standards for each analysis. It should be kept in mind that quality control is a part of quality assurance.
QC involves establishment of a quality standard or specifications for each aspect of a test procedure, specification of how the test procedure complies with quality standards and taking the necessary corrective measures to bring up the procedures to standard. It is an active and team-wide process that identifies the errors related to all outputs during the procedure and after the procedure.
Internal Quality Control tests have been designed to control if a test or procedure will produce the same result in case of in-laboratory variations or when performed by varying technicians.
External Quality Control (EQC) is defined as an evaluation study performed by an exteral provider using samples with known or unknown content or concentration with the objective of providing or improving the reliability of laboratory test results. EQC programmes are conducted by independent institutions and comparatively evaluate the performances of test results and reports of laboratories. With EQC programmes, laboratories’ performances are compared with the performances of other laboratories and evaluated on an international scale.
Demonstrating compliance of Tissue Typing laboratories with good practices in providing clinical transplantation services has gained importance, and there are many legal and regulatory requirements targeting to provide appropriate review and documentation of services. Therefore, laboratories should provide assurance related to the quality of the services they give in line with regulatory objectives (Harmer et al., 2018).
The increase in the number of laboratories and methods in years proved the necessity of meeting a high standard for the results reported by different laboratories. In this context, standardization studies and survey programmes were established many years ago.
In a study which summarized the studies, in which HLA standardization by way of international cell exchange was performed, tissue typing performed using a blind design in 468 people in a 12-year period was examined. The number of participant laboratories was reported to be increased from 85 in the beginning to 285. It has been emphasized that these standardization studies help to standardize typing reactives of tissue typing laboratories globally, to determine new specificities and to evaluate the status of improvement in tissue typing in renal transplant practices (Loon et al., 1987) The Histocompatibility Survey Programmes was organized in 1982 by American Society for Histocompatibility and Immunogenetics (ASHI) and College of American Pathologists (CAP) as a joint project to evaluate laboratory performance in HLA typing, lymphotoxicity crossmatch and antibody analysis (Marrari and Duquesnoy, 1994).
The risk of post-transplant complications is reduced by way of detailed analysis of the patient’s anti HLA profile and appropriate donor-recipient matching. Precise characterization of alloantibodies in sensitized patients and complete HLA typing at the allelic level are mandatory at the time of transplantation (Morath et al., 2012). Furthermore, knowledge of HLA sensitivities and identification of anti-HLA antibodies among potential kidney recipients is essential to control graft loss (Duquesnoy et al., 2016).
Appropriate techniques should be used to increase the reliability of the tests performed for histocompatibility which is accepted to be effective in graft loss, and a meticulous quality control system should be implemented. With this objective, various EPT programmes were established globally (Table 1). Successful performance in EPT was accepted as a prerequisite for accreditation of a laboratory (Doxiadis et al., 2000; Bogunia-Kubik et al., 2006; Bogunia-Kubik and Lange, 2008; Bogunia-Kubik and Lange, 2010; Balza et al., 2023; European Federation for Immunogenetics, 2023).
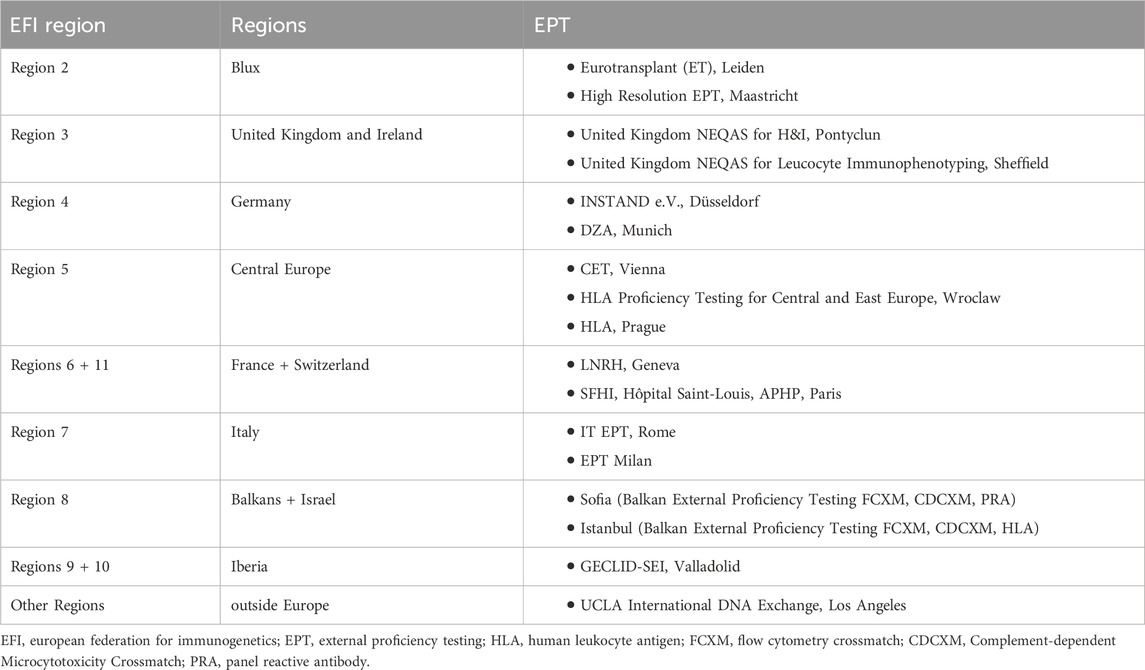
TABLE 1. EPT provider and EFI regions (based on data from European Federation of Immunogenetics, 2023).
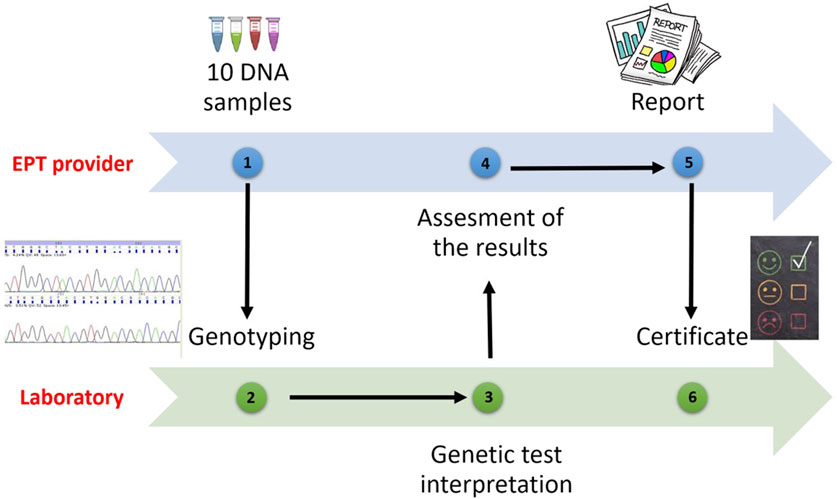
All laboratories applying to receive accreditation from the European Federation for Immunogenetics (EFI) or wishing to resume their accreditations, are obliged to participate in EPT programs related to laboratory practices involving the categories for which they will be accredited (HLA typing, antibody screening and detection, cross match, etc.) (European Federation for Immunogenetics, 2023) (Figure 2).
If there are no programmes specified for a certain category, the laboratory should participate in an EPT workshop or trial provided by an EPT provider or be involded in an inter-laboratory sample exchange programmes. The laboratory shall have a predetermined policy for testing EPT specimens, documenting relevant EPT programmes or workshops prospectively on an annual basis. Thus, participation in external proficiency testing workshops will give the opportunity to validate HLA typing results. It will contribute to the training of laboratories by making comparisons with other participants. It is also expected that the error rates of participating laboratories will decrease over the years.
According to the EFI standards, EPT samples should be tested and interpreted one by one or in association using the techniques used routinely for clinical samples. If the same sample is being tested for multiple accreditation categories, the results should be analyzed independent from each other. The annual minimum sample number for EPT is shown in the table (Table 2). If the same sample is being tested with multiple techniques in the same accreditation category, the laboratory should give the provider only one report, but keep the results obtained with different techniques ready for inspection (European Federation for Immunogenetics, 2023).
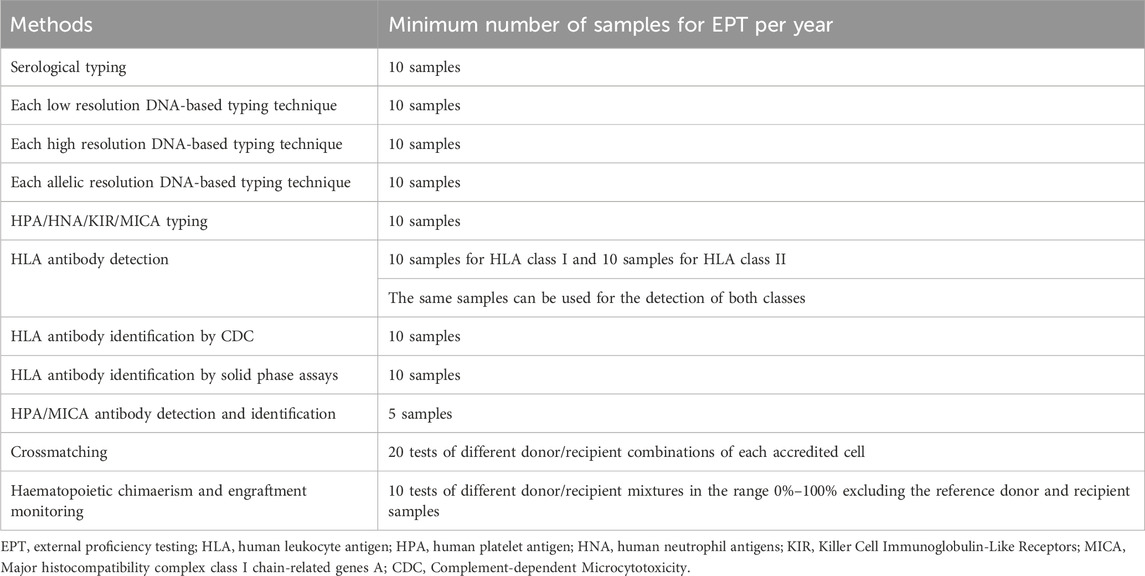
TABLE 2. EPT methods and samples (based on data from European Federation of Immunogenetics, 2023).
In recent years, experimental transplant models have shown that mechanisms other than T-lymphocyte anti-donor responses could be effective (Oberbarnscheidt et al., 2014; Dai et al., 2017). In addition, a few translational genetic association studies have showed that incompatibilities originating from interaction of two different genomes could lead to complex immune responses in solid organ transplants and non-HLA antibodies could also be effective in rejection (Sankaran et al., 1999; Grinyó et al., 2008; Menon et al., 2015; Zhang et al., 2021; Jethwani et al., 2022).
Numerous studies in solid organ transplantation provide evidence that high levels of donor-derived cell-free DNA (DD-cfDNA) correlate with clinically relevant endpoints. Increased DD-cfDNA has been associated with episodes of graft injury and rejection. Efforts are ongoing to further improve sensitivity and specificity. DD-cfDNA could be used as a biomarker in the near future as it is quantitative and has the potential to be cost-effective. Although there are EPT programs on cell-free DNA in different fields, EPT programs in transplantation are not yet available (Samoila et al., 2020; Edwards et al., 2022)
Multi-center studies have shown that specification of non-HLA loci with long-term allograft results and identification of non-HLA antibodies in patients might enable sensitive matching of organs in patients who have multiple potential donors. Performance of routine tests in HLA laboratories addressing these parameters will undoubtedly contribute to successful organ transplantation to a great extent. When EPT programmes are examined, it is observed that there is currently no study dedicated to these analyses.
Conclusion
The role of the Histocompatibility and Immunogenetic laboratories in stem cell and organ transplants has expanded to provide HLA antibody detection and tracking for selection of compatible donors and monitoring desensitization therapies.
In the future, they will request new programmes in accordance with clinical needs in order to perform new routine tests successfully in parallel with accreditation categories and to evaluate and improve laboratory performance.
Author contributions
FO: Conceptualization, Data curation, Formal Analysis, Investigation, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Badrick, T. (2021). Integrating quality control and external quality assurance. Clin. Biochem. 95, 15–27. doi:10.1016/j.clinbiochem.2021.05.003
Balza, G., Gardalini, M., Neri, R., Mele, L., Peretti, G., Di Matteo, R., et al. (2023). Chimerism analysis after allogeneic hematopoietic stem cell transplantation: standardization and quality assurance. Work. Pap. Public Health 11, 9640. doi:10.4081/wpph.2023.9640
Bogunia-Kubik, K., and Lange, A. (2006). “Proficiency testing of HLA class I for central-east europe,” in Focus on immunology research: standardization of donor-recipient matching in transplantation. Editor A. Lange (Hauppauge, NY: Nova Publishers Inc.), 113–126.
Bogunia-Kubik, K., and Lange, A. (2008). Development of the HLA proficiency testing for central and east europe. Int. J. Immunogenet 35, 409–416. doi:10.1111/j.1744-313X.2008.00789.x
Bogunia-Kubik, K., and Lange, A. (2010). Human leukocyte antigen proficiency testing for Central and Eastern Europe: a summary of over 10 years of activity. Transplant. Proc. 42, 3263–3265. doi:10.1016/j.transproceed.2010.07.020
Dai, H., Friday, A. J., Abou-Daya, K. I., Williams, A. L., Mortin-Toth, S., Nicotra, M. L., et al. (2017). Donor sirpa polymorphism modulates the innate immune response to allogeneic grafts. Sci. Immunol. 12, eaam6202. doi:10.1126/sciimmunol.aam6202
Doxiadis, , Witvliet, M., Verduyn, W., de Lange, P., Tanke, J., Schreuder, G. M., et al. (2000). The relevance of proficiency testing for laboratories involved in cadaveric organ transplantation and its consequences for graft survival. Clin. Transplant. 14, 99–103.
Duquesnoy, R. J., Hönger, G., Hösli, I., Marrari, M., and Schaub, S. (2016). Detection of newly antibody-defined epitopes on HLA class I alleles reacting with antibodies induced during pregnancy. Int. J. Immunogenetics 43, 200–208. doi:10.1111/iji.12280
Edwards, R. L., Menteer, J., Lestz, R. M., and Baxter-Lowe, L. A. (2022). Cell-free DNA as a solid-organ transplant biomarker: technologies and approaches. Biomark. Med. 16, 401–415. doi:10.2217/bmm-2021-0968
European Federation for Immunogenetics (2023). Standards for histocompatibility and immunogenetics testing-version 8.2. Available at: https://efi-web.org/fileadmin/Efi_web/Standardv8_280819.pdf (Accessed September 04, 2023).
Goodman, C., Faulkner, E., Gould, C., Karnes, E., Smith, A., Aguiar, C., et al. (2005). The value of diagnostics innovation, adoption and diffusion into health care. Available at: http://earlydetect.com/Graph/TheValueofDiagnostics0.pdf.
Grinyó, J., Vanrenterghem, Y., Nashan, B., Vincenti, F., Ekberg, H., Lindpaintner, K., et al. (2008). Association of four DNA polymorphisms with acute rejection after kidney transplantation. Transpl. Int. 21, 879–891. doi:10.1111/j.1432-2277.2008.00679.x
Harmer, A., Mascaretti, L., and Petershofen, E. (2018). Accreditation of histocompatibility and immunogenetics laboratories: achievements and future prospects from the European federation for immunogenetics accreditation programme. HLA 92, 67–73. doi:10.1111/tan.13289
Jethwani, P., Rao, A., Bow, L., and Menon, M. C. (2022). Donor-recipient non-HLA variants, mismatches and renal allograft outcomes: evolving paradigms. Front. Immunol. 13, 822353. doi:10.3389/fimmu.2022.822353
Loon, J., Terasaki, P. I., Takemura, S., and Park, M. S. (1987). Standardization of HLA through an international cell exchange. Clin. Transpl., 453–458.
Marrari, M., and Duquesnoy, R. J. (1994). Progress report on the ASHI/CAP proficiency survey program in histocompatibility testing. I. HLA-A,B,C typing, antibody screening, and lymphocytotoxicity crossmatching. American society for histocompatibility and immunogenetics. College of American Pathologists. Hum. Immunol. 39, 87–95. doi:10.1016/0198-8859(94)90106-6
Menon, M. C., Chuang, P. Y., Li, Z., Wei, C., Zhang, W., Luan, Y., et al. (2015). Intronic locus determines SHROOM3 expression and potentiates renal allograft fibrosis. J. Clin. Invest. 125, 208–221. doi:10.1172/JCI76902
Morath, C., Opelz, G., Zeier, M., and Süsal, C. (2012). Prevention of antibody-mediated kidney transplant rejection. Transpl. Int. 25, 633–645. doi:10.1111/j.1432-2277.2012.01490.x
Oberbarnscheidt, M. H., Zeng, Q., Li, Q., Dai, H., Williams, A. L., Shlomchik, W. D., et al. (2014). Non-self recognition by monocytes initiates allograft rejection. J. Clin. Invest. 124, 3579–3589. doi:10.1172/JCI74370
Rohr, U. P., Binder, C., Dieterle, T., Giusti, F., Messina, C. G. M., Toerien, E., et al. (2016). The value of in vitro diagnostic testing in medical practice: a status report. PLoS One 11, e0149856. doi:10.1371/journal.pone.0149856
Samoila, A., Sosa, J., Padilla, J., Wutkowski, M., Vanness, K., Viale, A., et al. (2020). Developing quality programs for cell-free DNA (cfDNA) extraction from peripheral Blood. J. Appl. Lab. Med. 5, 788–797. doi:10.1093/jalm/jfaa050
Sankaran, D., Asderakis, A., Ashraf, S., Roberts, I. S., Short, C. D., Dyer, P. A., et al. (1999). Cytokine gene polymorphisms predict acute graft rejection following renal transplantation. Kidney Int. 56, 281–288. doi:10.1046/j.1523-1755.1999.00536.x
Shahangian, S., and Cohn, R. D. (2000). Variability of laboratory test results. Am. J. Clin. Pathol. 113, 521–527. doi:10.1309/LF4J-0B04-HUYK-NKE2
Keywords: external proficiency testing, EPT, HLA, quality control, QA
Citation: Oguz FS (2024) External proficiency testing for histocompatibility and immunogenetics in today and future. Front. Genet. 15:1294330. doi: 10.3389/fgene.2024.1294330
Received: 14 September 2023; Accepted: 15 January 2024;
Published: 26 February 2024.
Edited by:
Martin Petrek, Palacký University, CzechiaReviewed by:
Ilias Doxiadis, University Hospital Leipzig, GermanyMathijs Groeneweg, Maastricht University Medical Centre, Netherlands
Anikó Szilvási, Hungarian National Blood Transfusion Service, Hungary
Copyright © 2024 Oguz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatma Savran Oguz, b2d1enNmQGlzdGFuYnVsLmVkdS50cg==
 Fatma Savran Oguz
Fatma Savran Oguz