- 1Noncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran
- 2College of Science, University of Tikrit University, Tikrit, Iraq
- 3State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangdong Provincial Clinical Research Center for Cancer, Guangzhou, China
Colorectal cancer (CRC) is one of the main fatal cancers. Cell signaling such as Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling substantially influences the process of gene expression and cell growth. Long non-coding RNAs (lncRNAs) play regulatory roles in cell signaling, cell proliferation, and cancer fate. Hence, lncRNAs can be considered biomarkers in cancers. The inhibitory or activating effects of different lncRNAs on the JAK/STAT pathway regulate cancer cell proliferation or tumor suppression. Additionally, lncRNAs regulate immune responses which play a role in immunotherapy. Mechanisms of lncRNAs in CRC via JAK/STAT regulation mainly include cell proliferation, invasion, metastasis, apoptosis, adhesion, and control of inflammation. More profound findings are warranted to specifically target the lncRNAs in terms of activation or suppression in hindering CRC cell proliferation. Here, to understand the lncRNA cross-talk in CRC through the JAK/STAT signaling pathway, we collected the related in vitro and in vivo data. Future insights may pave the way for the development of novel diagnostic tools, therapeutic interventions, and personalized treatment strategies for CRC patients.
Background
Colorectal cancer (CRC) is one of the leading causes of cancer-related death, with a survival rate of nearly 50% (Misale et al., 2012; Hugen et al., 2016). Cell signaling pathways such as Hippo, Janus kinase signal transducer and activator of transcription (JAK/STAT), NOTCH, SHH/GLI, TGF/SMAD, and Wnt/β-catenin play substantial roles in cancer initiation and progression (Takebe et al., 2015; Park et al., 2020; Zou et al., 2020; Zhou et al., 2022). These pathways contribute to the epithelium maintenance and stem cell multiplication and differentiation. STAT signaling participates in numerous cellular processes such as immune regulation and cancer progression (Aggarwal et al., 2009; Yu et al., 2014). STATs including STAT1–6 may exacerbate cell growth and cancer development (STAT3 and STAT5) and regulate the anti-tumor immune responses for tumor control (STAT1 and STAT2) (Owen et al., 2019; O'Shea et al., 2015). For instance, cytokines such as IL-2, IL-15, IL-12, INF-α/β, and INF-γ have been associated with the STATs (Heinrich et al., 2003; Morris et al., 2018). In protumor conditions, Th2, Tregs, Th17, and myeloid-derived suppressor cells (MDSCs) secrete IL-1, IL-10, IL-17, and TGF-β through STAT3 and STAT5 signaling, which precludes immune function in the tumor microenvironment (TME) (Seif et al., 2017; Yoshimura et al., 2018; Kang et al., 2019). STATs 1, 2, 3, and 5 enter the nucleus and control the interferon gene transcription. STAT1 and STAT4 mainly contribute to cytokine production and cell-specific activity, which results in the anti-tumor effects of immune cells (MHC-I and checkpoint inhibitor upregulation) (Erdogan et al., 2022). The JAK/STAT pathway can be regulated in several cancers by microRNAs, circular RNAs, and long non-coding RNAs (lncRNAs) (Yan et al., 2020).
Transcription of 85% of mammalian genomes infers the critical role of RNAs in biological systems. lncRNAs, comprising >200 bp, have wide biological activities such as transcriptional or post-transcriptional functions and interference with RNA processing, thereby promoting or inhibiting tumorigenesis (Yang et al., 2014; Chi et al., 2019; Wu W. et al., 2022). These RNAs lack open reading frames (ORFs) and interact with proteins, miRNAs, and other lncRNAs. Mechanisms of lncRNA functions include natural antisense transcripts (NATs), chromatin interactions and remodeling, and ceRNAs (Beermann et al., 2016). Numerous lncRNAs are upregulated or downregulated in cancers like CRC, influencing cell signaling pathways (He and Wu, 2023). Genetic or epigenetic factors underlie the lncRNA expression varieties in cancer (Shaker et al., 2017). lncRNAs KCNQ1OT1, HOX transcript antisense RNA (HOTAIR), and metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) have been dysregulated in colorectal cancer (Kogo et al., 2011; Xu et al., 2011; Niinuma et al., 2012). The lncRNA HOTAIR/miR-326/FUT6 axis facilitates the development of CRC via PI3K/AKT/mTOR signaling (Pan et al., 2019). lncRNA-H19 regulates miR-29b-3p and leads to CRC progression (Ding et al., 2018).
JAK/STAT activation or regulation by lncRNAs has been unveiled in CRC (ITIH4-AS1), chronic myeloid leukemia (MEG3) (Li et al., 2018), gastric cancer (LINC00691), osteosarcoma (lncRNA 135528), and non-small-cell lung cancer cells (PART1). In the CRC cells/tissues, the JAK/STAT pathway has been regulated by RP11-468E2.5, LINC01116, AB073614, SUMO1P3, HAND2-AS1, MIR100HG, TRG-AS1, PCED1B-AS1, FAM30A, AL365361.1, AC090559.1, LINC01094, LINC00346, TPT1-AS1, and HOXA11-AS. It was observed that the downregulation of the RE1-silencing transcription (REST) factor led to the upregulation of ITIH4-AS1 and contributed to CRC via FUS-mediated JAK/STAT3 signaling activation (Liang et al., 2019; Ming et al., 2021). In the current review, the available data on the lncRNA-mediated JAK/STAT pathway in CRC are analyzed to indicate how pivotal lncRNAs could be in CRC progression and management.
JAK/STAT and colorectal cancer
The sensing, cytokine receptor binding, and phosphorylation of the JAK/STAT pathway lead to related gene regulation. The activation of STATs results in the transcriptional regulation of immune responses, apoptosis, inflammation, proliferation, and angiogenesis. Genetic mutations in the JAK-STAT pathway lead to aberrant activation, even without the cytokine induction, which results in persistent activation and tumorigenesis. For instance, persistent overexpression of STAT5 occurs in the neoplasia stage. It was revealed that circular RNA circSPARC promotes the invasion of CRC cells via JAK/STAT pathway regulation (Wang J. et al., 2021). A previous study showed that activating transcription factor 1 (ATF1) was associated with CRC progression via regulating the JAK/STAT, TNF, and Wnt pathways. ATF1 was also associated with the two lncRNAs PVT1 and CCAT1, which played a role in the exacerbation of CRC (Wang H. et al., 2021). CCAT5 also upregulates STAT3 in CRC (Wang et al., 2020). The ADAM10-JAK-STAT signaling pathway can also be regulated by miR-365a-3p, thus inhibiting CRC cell proliferation (Hong et al., 2020). STAT3 also mediates the activation and infiltration of tumor-specific T cells. A bibliometric analysis deciphered a myriad of lncRNAs associated with CRC progression, invasion, and metastasis (He and Wu, 2023). The downregulation of STAT3 by miR-34a inhibits CRC cell metastasis. In addition, interactions between CASC2, miR-18a, PIAS3 (an mRNA molecule), and the STAT3 signaling pathway caused CRC cell multiplication and tumor development (Huang et al., 2016a).
Long non-coding RNA levels change in cancer and cancer therapy
lncRNAs are involved in cancer cell multiplication and metastasis through a myriad of cellular processes and common signaling pathways, where lncRNAs can bind to some specific DNA or protein complexes (Chen et al., 2021). These include phosphatidylinositol-3-kinase (PI3K), P53, KRAS, epidermal growth factor receptor (EGFR), Wnt/β catenin, epithelial–mesenchymal transition (EMT), and TGF-β signaling pathways (Lin et al., 2021). The lncRNA regulator of reprogramming (lnc-ROR) modulates the exacerbation of various cancerous conditions (Lulli et al., 2022; Tabarestani et al., 2022). The TME metabolic conditions also regulate the expression of lncRNAs, which leads to the expression of various enzymes and signaling pathways. A previous study unveiled that the aberrant expression of LINC00152 develops CRC conditions (Ou J. et al., 2021; Li S. et al., 2022). lncRNA HOXB-AS3 encodes a peptide which inhibits CRC cell growth via metabolic regulation (Huang et al., 2017). lncRNA SNHGS mediates CRC cell resistance to oxaliplatin via deregulation of STAU1 (Chen F. et al., 2022). Moreover, expression levels of lncRNA RP11-462C24.1 regulate PI3K/AKT and HSP70 signaling in the CRC tissues (Zhang H. et al., 2021). lncRNA ADAMTS9-AS2 affects the development of various cancers, such as the tumor metastasis of glioma cells and gastric and lung cancers, while lncRNA DANCR develops tumorigenesis by osteosarcoma via repression of miR-33a-5p (Yao et al., 2014; Liu et al., 2018; Ren et al., 2020). lncRNA LINC01116 can target miR-520a-3p and VEGFA and arrest cell proliferation and preclude brain and osteosarcoma tumorigenesis [lnc3 introduction (Ye et al., 2020)]. It also regulates miR-9-5p-STMN1 and EZH2-regulated TPM1, hence promoting CRC development (Bi et al., 2020a; Liang et al., 2021a). lncRNA OTUD6B-AS1 was associated with thyroid and renal carcinoma inhibition via regulation of Wnt/β-catenin signaling (Wang et al., 2019). Additionally, its overexpression has reduced CRC cell proliferation mainly via regulation of miR-21-5p and proline-rich nuclear receptor coactivator 2 (PNRC2) (Cai et al., 2021; Wang W. et al., 2021). MEG3 is an lncRNA, which inhibits carcinogenesis through the regulation of JAK/STAT-activating miR-9 (He et al., 2017; Moradi et al., 2019). Other cell-proliferative and metastatic lncRNAs include PCGEM1, SNHG15, POU3F3, PVT1, MALAT1, TUG1, urothelial carcinoma-associated 1 (UCA1), and PCAT-1 (Jin et al., 2020; Zhu and Zhu, 2022). Tumor-suppressive lncRNAs include growth arrest-specific transcript 5 (GAS5), MEG3, DGCR5, PTENP1, and NKILA (Yu and Hann, 2019). Immune escape is mediated by MALAT1, UCA1, and SNHG1 (Liu et al., 2020). Angiogenesis is mediated by MEG3, TUG1, and CASC3. Some lncRNAs cause metastasis (CCAT2, H19, and NKILA), cell stemness (ASAP-1-IT-1, BCAR4, and ARSR), drug resistance (PVT1, HOTAIR, and AX747207), and DNA damage (DDSR1, ANRIL, and NEAT1). Moreover, lncRNAs regulate miRNAs which regulate cell signaling and cancer progression. The regulation of intestinal epithelium maintenance by lncRNAs has been documented (Xiao et al., 2019) (Figure 1).
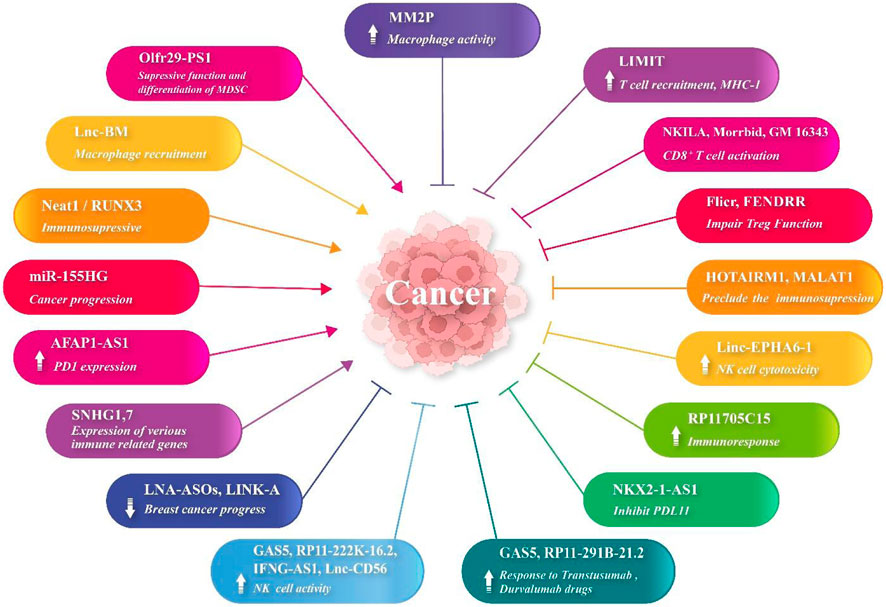
Low immune response and immune evasion lead to cancer cell multiplication, invasion, and metastasis. Although various lncRNAs (such as Neat1 and RUNX3) (Pandey et al., 2022) have participated in immunosuppression in cancers, some of them have exhibited immunostimulatory properties (Eptaminitaki et al., 2021). lncRNAs are of significance in the multiplication and activation of CD4+ and CD8+ T cells and NK cells (Li et al., 2021). MHC-I and immunogenicity of tumor (LIMIT) is an lncRNA, which provokes the expression of the MHC-I gene via detachment of heat shock factor-1 (HSF1) and HSP90 proteins. RNA-guided CRISPR activation results in MHC-I overexpression, T-cell recruitment, and checkpoint blockade response (Li et al., 2021). lncRNA AFAP1-AS1 increases PD-1 molecule expression in nasopharyngeal carcinoma (Tang et al., 2017). lncRNA Olfr29-ps1 targets miR-214-3p, which decreases MDSC activity and differentiation mediated by MyD88 (Shang et al., 2019). lncRNA RP11705C15 provokes efficient immune responses against NSCLC cells following anti PD-1 immunotherapy (Xu et al., 2018). lncRNA MIR-155HG is also associated with the overall survival (OS) of patients suffering from various cancers (Li et al., 2016). lncRNA Flicr impairs the Treg functions and, hence, enhances the immune responses. CD8+ T-cell activation is mediated by NKILA, Morrbid, and GM16343. In addition, MM2P enhances macrophage activation. HOTAIRM1 and MALAT1 preclude MDSC-mediated immunosuppression. GAS5, RP11-222K-16.2, IFNG-AS1, and lnc-CD56 enhance NK cell activity. In hepatocellular carcinoma, FENDRR hinders the activity and multiplication of Tregs. lnc-BM regulates macrophage recruitment into the brain and HCC TME. In NSCLC, linc EPHA6-1 enhances NK cell cytotoxicity. In lung adenocarcinoma, NKX2-1-AS1 inhibits PDL-1 expression. Furthermore, lncRNAs GAS5 and RP11-291B-21.2 enhance the responses to trastuzumab and durvalumab, respectively. An in silico study demonstrated that high expression levels of two lncRNAs SNHG1 and SNHG7 (using GEO analysis and GSEA) in HCC tissue were associated with the expression of various immune-related genes. These genes were mainly related to infiltration and checkpoint inhibitors (Chen E. et al., 2022). The combination of locked nucleic acid (LNA)-ASOs and LINK-A lncRNA has suppressed breast cancer progression (Hu Q. et al., 2019). Moreover, the incorporation of lncRNA into CAR T cells has played the role of an adjuvant in the regulation of T-cell apoptosis and suppressed tumor immune evasion and enhanced the immunotherapy (Huang et al., 2018).
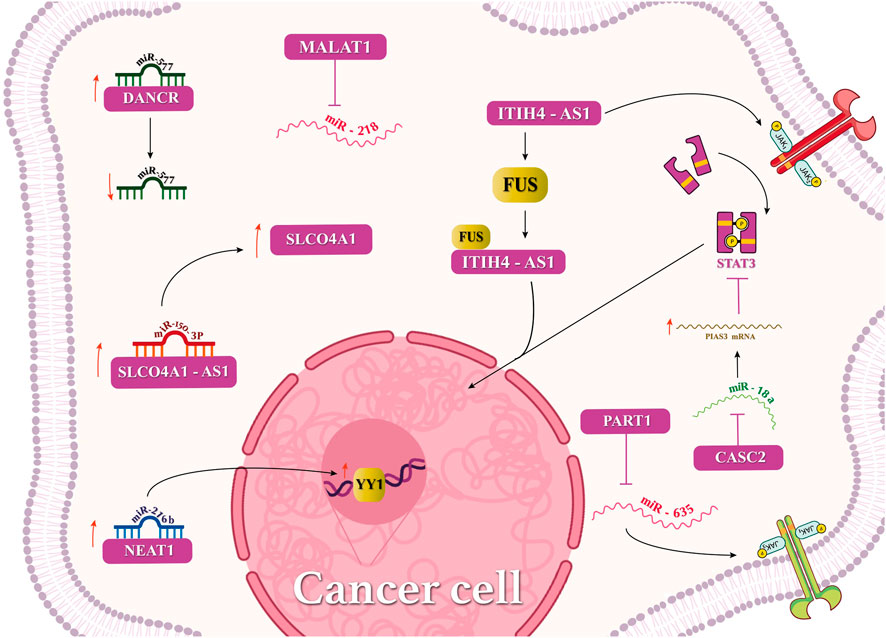
Interactions of lncRNAs with miRNAs, mRNAs, or circular RNAs have been investigated in CRC (Figure 2), highlighting meaningful effects in the disease fate. lncRNA MALAT1 interacts with miRNA-218, which accelerates metastasis and EMT. Additionally, MALAT1 suppresses miR-106-5p and promotes the SLAIN2 expression. lncRNA CYTOR and LINC00707 binding to miR-3679-5p and miR-206, respectively, has promoted the development of CRC. Furthermore, lncRNAs DANCR and UICLM have sponged miR-577 and miR-215, respectively, causing the metastasis of CRC. CCAT2 has modulated the expression of miR-145, leading to the mutation inhibition of CRC cells (Niu et al., 2021; Tan et al., 2021). LAMTOR5 or LAMTOR5-AS1, a proposed biomarker in CRC, which is highly expressed in elderly patients, has sponged hsa-miR-20a-5p and hsa-miR-let-7b-3p (Zaniani et al., 2021). LAMTOR5 was not a suitable biomarker for CRC. An in silico study revealed that lncRNAs SNHG5, GATA2-AS1, and H19 with various miRNAs facilitated CRC (Shaath et al., 2021). The high expression of LINC00963 but not miR-1281 has been reported in CRC, and their interaction has caused the downregulation of miRNA-1281. lncRNA NEAT1 has sponged miRNA-216b and activated YIN-YANG-1 (YY1), thus accelerating CRC tumorigenesis (Zhu et al., 2022). Interactions of SLCO4A1, miR-150-3p, and SLCO4A1-AS1 have been investigated in colon cancer tissues. SLCO4A1-AS1 binds to miR-150-3p and enhances the expression of SLCO4A1, which increases CRC tumorigenicity (Wu et al., 2021).
The lncRNA–miRNA interaction regulates several cancers. For instance, the binding of lncRNA MALAT1 and miR-200c leads to the progression to EMT. The binding of lncRNAs Sox2ot and XIST to miR-200a and miR-429, respectively, exerts similar effects. lncRNA PCED1B-AS1 and each of miR-411-3p and miR-29b also mediate such effects. THAP9-AS1 has sponged miRNA-484 and has led to Hippo pathway regulation in cancer. DANCR has sponged the tumor-suppressive miRNAs 33b, 135a-5p, 613, 34c, 34a-5p, 149,496, 1972, and 335-5p to regulate cancers via several signaling pathways (Farooqi et al., 2021). Regarding CRC (Yang et al., 2021), lncRNA XIST regulates miRNA-200b-3p, which hinders the ZEB1 function and inhibits metastasis and EMT. lncRNA MALAT1 sponges miR-126-5p and prohibits VEGFA in the angiogenesis process (Sun et al., 2019). lncRNA HOTAIR binds to miR-218, which may control NF-KB signaling and resistance to 5-fluorouracil or facilitate cancer development (Li et al., 2017; Hu X. et al., 2019; Wei et al., 2020). lncRNA HAGLROS binds to miR-100 and controls ATG5 in autophagy, which activates the PI3K/AKT and mTOR pathways. In the stemness process, lncRNA BCAR4 regulates miR-665 and controls STAT3, which, in turn, regulates the expression of NANOG, OCT4, SOX2, CD44, CD133, and Lgr5 proteins. Several other miRNAs, such as miR-137 (by SNHG1), miR-181a-5p (by ZEB1-AS1), miR-200a, miR-29b-3p, and miR-138 (by the H19 oncogene), miR-1271 (by HCG18), miR-204 (by PlncRNA-1), miR-203a-3p and miR-214 (by HOTAIR), miR-34a and miR-200b-3p (by XIST), miR-129-5p (by HIF1A-AS2), miR-497 (by TTN-AS1), miR-203 (by B3GALT5-AS1), miR-215 (UICLM), miR-101-3p (SNHG6), miR-489/TWIST1 (by CHRF), miR-600/KIAA1199 (by TUG1), and miR-150-5p (by ZFAS1), have been reported (Huang et al., 2016a; Bian et al., 2016; Wu et al., 2019; Chodary Khameneh et al., 2022).
JAK/STAT pathway targeting in immunotherapies
The enhanced expression of cytokines and their receptors mainly result in the aberrant regulation of JAK1/2, STAT1, STAT3, STAT5, and STAT6, causing inflammation and cancer development, cancer recurrence, and decreased overall survival. JAK1 and STAT3 are necessary for T-cell activation. Hence, the impairment in the system affects efficient cancer cell combating and even leads to T-cell lymphoma (Waldmann and Chen, 2017). INF-JAK/STAT pathway targeting can provide efficient outcomes in immunotherapy and radiotherapy, considering sufficient strength and duration of treatment. JAK1 signaling has participated in PDL-1-mediated melanoma immunotherapy (Luo et al., 2018; Shi and Bonner, 2021). Moreover, JAK/STAT targeting, particularly of combination therapy, is promising for glioblastoma treatment (Ou A. et al., 2021; Shi and Bonner, 2021). STAT3 activation suppresses immune cells and targets interleukin-6 (IL-6), which activates MDSCs and shifts Th1/Th2 balance to Th2 type. Therefore, JAK/STAT inhibitors are promising for cancer therapy (Sabaawy and Zeeshan, 2021). The suppression of STAT5, an activator of CD4+/CD25+ Tregs, can activate immune cells such as NK cells (Gotthardt et al., 2016). Targeting JAK/STAT signals leads to the inhibition of chronic inflammation, anti-tumor cell suppression, and more effective cancer therapy (Sabaawy et al., 2021). The human microbiota may affect the expression of lncRNAs, such as HOTAIR, LINC00491, KCNQ1OT1, and LINC00355, by CRC cells (Yang et al., 2020; Khodaii et al., 2022). RPS6, PMAIP1, BCL2, and FAM129A genes were associated with immune cell infiltration (Tan et al., 2021).
Long non-coding RNAs and JAK/STAT regulation
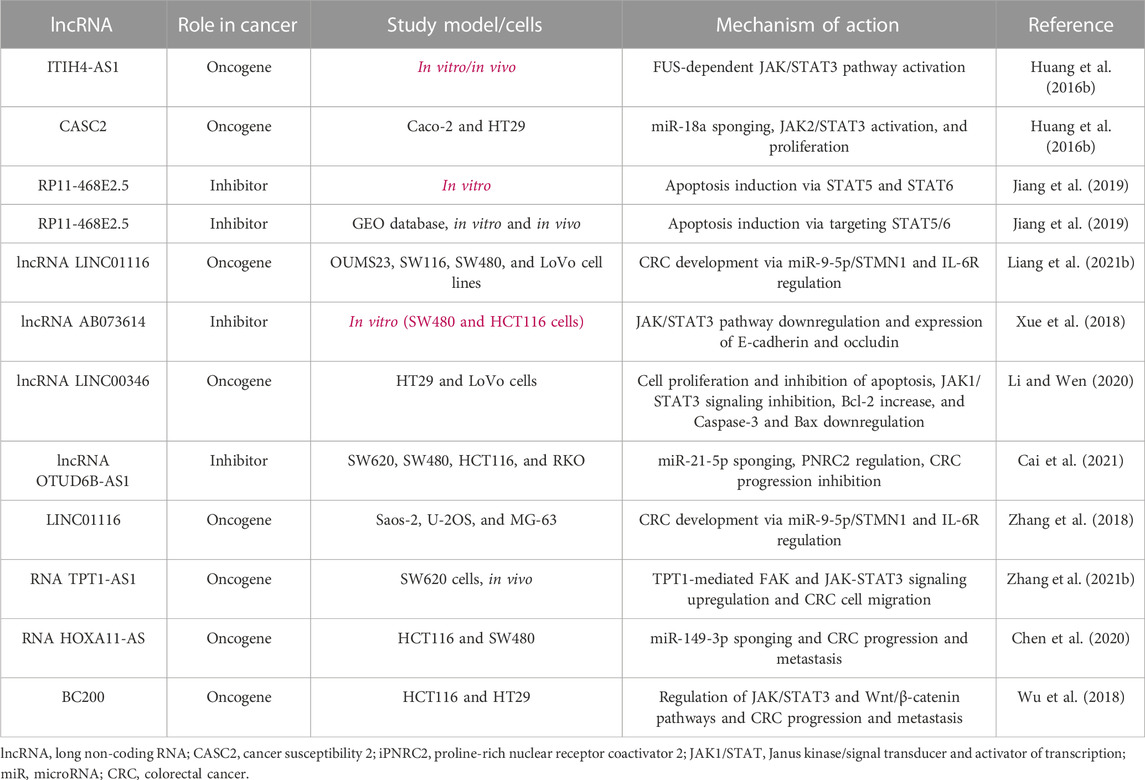
lncRNA 135528 can regulate the JAK/STAT pathway and then CXCL10, which precludes tumorigenesis (Wang K. et al., 2021). lncRNA HOTAIR leads to liver cancer progression via SETD2 regulation. In addition, lncRNA HOTTIP leads to pancreatic cancer progression by the regulation of HOXA9 expression. lncRNA XIST/miR-200c also regulates bladder cancer. lncRNA PART1 provokes the JAK-STAT signaling pathway, which facilitates the non-small-cell lung cancer cell proliferation in vitro and in vivo. The main regulation mechanisms and results of the JAK/STAT signaling pathway include FUS-dependent JAK/STAT3 pathway activation (ITIH4-AS1), miR-18a/STAT3 sponging (CASC2), apoptosis induction via STAT5 and STAT6 (RP11-468E2.5), apoptosis induction via targeting STAT5/6 (RP11-468E2.5), CRC development via miR-9-5p/STMN1 and IL-6R regulation (lncRNA LINC01116), JAK/STAT3 pathway downregulation, expression of E-cadherin and occludin (lncRNA AB073614), cell proliferation and inhibition of apoptosis, JAK1/STAT3 signaling inhibition, Bcl-2 increase, caspase-3 and Bax downregulation (lncRNA LINC00346), miR-21-5p sponging, PNRC2 regulation, CRC progression inhibition (lncRNA OTUD6B-AS1), CRC development via miR-9-5p/STMN1 and IL-6R regulation (LINC01116), TPT1-mediated FAK and JAK-STAT3 signaling upregulation, CRC cell migration, miR-149-3p sponging, and CRC progression and metastasis (RNA TPT1-AS1 and RNA HOXA11-AS) (Huang et al., 2016b).
LINC00691 has regulated the JAK/STAT pathway and led to gastric cancer cell proliferation and invasion, which was confirmed using MKN-45 and HGC-27 cell lines and bioinformatics study, gene expression, luciferase gene reporter, Western blot, and in vivo (BALB/c nude mice) analyses (Liang et al., 2020). The inhibitor ruxolitinib could reverse the LINC00691 effects.
Long non-coding RNAs and JAK/STAT regulation in colorectal cancer
It was observed that lncRNA RP11-468E2.5 exerted anticancer effects against CRC via apoptosis induction and precluding angiogenesis in silico, in vitro (tissue samples from 169 patients), and in vivo. lncRNA RP11-468E2.5 targeted the JAK/STAT signaling pathway via inhibition of STAT5 and STAT6. RP11-468E2.5 targeting using siRNA reversed the effects and inhibited apoptosis. Moreover, lncRNA LINC01116 has accelerated CRC progression via regulation of miR-9-5p/STMN1 and interleukin-6 receptor (IL-6R) (Bi et al., 2020b). LINC01116 exhibited a high expression level in CRC tissues, and its knockdown could hinder cancer progression. LINC01116 has also promoted cancer cell proliferation and migration (Xu et al., 2021). On the other hand, lncRNA AB073614 has exerted mesenchymal CRC cell tumorigenesis via JAK/STAT3 pathway regulation. It was highly expressed in CRC tissue, and its suppression in SW480 and HCT116 cells hindered the cell proliferation and invasion via expression of E-cadherin and occludin proteins. The phosphorylated STAT3 expression was also mitigated. lncRNA AB073614 has also decreased tumor growth, invasion, and metastasis; cell cycle arrest; and promotion of apoptosis. Other roles included regulation of EMT and Wnt-β catenin pathway (Liao et al., 2019; Liao et al., 2021; Yang et al., 2021).
It has been unraveled that lncRNA SUMO1P3 contributes to CRC cell proliferation via CPEB3 silencing and inhibition of apoptosis. Relevantly, CPEB3 affects the JAK/STAT pathway and decreases tumorigenesis of CRC cells (Fang et al., 2020). An in silico study using the R limma package and multivariate Cox regression unraveled that four lncRNAs, which were mostly associated with the JAK/STAT pathway, were also associated with stages II–III CRC. These included HAND2-AS1, MIR100HG, TRG-AS1, PCED1B-AS1, FAM30A, AL365361.1, AC090559.1, and LINC01094 (Brenner et al., 2014).
lncRNA LINC00346 has been associated with CRC cell proliferation and invasion, Bcl-2 increase, and caspase-3 and Bax downregulation in HT29 and LoVo CRC cells. Its silencing was associated with apoptosis induction and inhibition of cancer progression via inhibiting the JAK/STAT signaling pathway. Interestingly, tofacitinib (JAK1 inhibitor) could reverse its cancer-promoting effects (Li and Wen, 2020). Moreover, triptolide, an inhibitor of JAK1 and phosphorylated STAT3, hindered the proliferation of CRC cells (Wang et al., 2009). Zhang L. et al. (2021) assessed 72 CRC and 36 adjacent normal tissues and revealed that lncRNA TPT1-AS1 facilitates CRC progression and metastasis through upregulation of JAK/STAT3 and FAK pathways by the expression of tumor protein translationally controlled 1 (TPT1). Additionally, in vivo findings revealed CRC cell proliferation by clone formation and tumor size/weight assay in nude mice. Chen et al. (2020)showed that the HOXA11-AS/miR-149-3p axis caused CRC cell proliferation and HCT116 cell migration. They also demonstrated that, with HOXA11-AS being its molecular sponge, miR-149-3p could lead to increased E-cadherin expression (Table 1; Figure 3). HOTAIR is another lncRNA overexpressed in CRC and promotes tumor growth and metastasis. HOTAIR interacts with the JAK-STAT pathway by binding to STAT3, leading to its activation and subsequent promotion of CRC cell proliferation and invasion (Liu et al., 2019). UCA1 is upregulated in CRC and activates the JAK-STAT pathway. UCA1 promotes CRC cell proliferation, migration, and invasion by enhancing STAT3 phosphorylation and nuclear translocation (Liu et al., 2021). GAS5 is downregulated in CRC and acts as a tumor suppressor. GAS5 inhibits the JAK-STAT pathway by interacting with JAK2 and preventing its phosphorylation. This leads to decreased STAT3 activation and suppression of CRC cell growth and invasion (Lin et al., 2022; Shakhpazyan et al., 2023).
MALAT1 is upregulated in CRC and has been implicated in promoting tumor growth and metastasis. MALAT1 activates the JAK-STAT pathway by interacting with STAT3 and enhancing its phosphorylation, thereby contributing to CRC progression.
Future prospects
In recent years, there has been a growing body of research that has shed light on the significant contribution of non-coding RNAs to the pathogenesis of different types of cancers (Wu J. et al., 2022; Gao et al., 2022; Liu et al., 2023; Xie et al., 2023). Specifically, lncRNAs and the JAK-STAT pathway have emerged as pivotal factors in the initiation and advancement of CRC (Li B. et al., 2022). The understanding of the biological functions and mechanisms of lncRNAs in cancer is essential for future studies to succeed in early diagnosis (as biomarkers) and achievement of appropriate therapeutic options (Dai et al., 2017; Xie et al., 2022). RNA sequencing revealed several lncRNAs as CRC biomarkers including CRCAL-1-4 (Yamada et al., 2018). The traits and pathophysiology of various cancers have been related to lncRNAs which can be considered “genetic debris” prognostic or diagnostic biomarkers. Their specific therapy is possible considering tissue-specific expression. Discovering lncRNA cross-talk and its effects on CRC progression is also helpful in understanding regulatory mechanisms. Consequently, these verification aspects contribute to the guide of inhibitory or activating drugs/compounds. As the detection of lncRNA expression is convenient in patients’ samples, they can be considered biomarkers of diagnosis or prognosis of cancers/disease type. The minimal side effects due to the lncRNA targeting in cancers (for oncogenic circuit blocking), lncRNA-based cancer therapy such as restricted expression is promising. Cancer-suppressing lncRNA expression can be enhanced using various approaches such as nanovectors. The knockout of lncRNAs using CRISPR-CAS9 is another approach for cancer therapy, which has reduced metastasis and increased the survival rate of mice (Zhuo et al., 2019). Targeting of the JAK/STAT pathway can lead to the activation of anti-tumor immune cells, and combination therapies are promising in this regard (Sabaawy et al., 2021). In addition, functional characterization of lncRNAs, identification of lncRNA biomarkers, therapeutic targeting of lncRNAs, elucidating the cross-talk between lncRNAs and the JAK-STAT pathway, and integration of lncRNAs and the JAK-STAT pathway into personalized medicine open new avenues in future.
Conclusion
lncRNAs play substantial roles in the regulation of cancer-driving pathways, and their roles have been recently demonstrated in the field of oncology. lncRNAs can be considered for cancer theranostic aims. By unveiling the biological roles of lncRNAs in the regulation of epithelial cell proliferation and mechanisms of development to neoplasia and more, cancer diagnosis will face a promising future when lncRNA radiotracing technology comes to clinical use. The understanding of lncRNA interactions or their cross-talk is also important for efficient precluding of cancer development. Several lncRNAs play a role as oncogenic or tumor-suppressor agents via regulation of JAK/STAT signaling. Hence, the targeting of this pathway is crucial for efficacious CRC anti-tumor therapy.
Author contributions
AG: conceptualization, data curation, and writing–original draft. HO: software and writing–original draft. YM: investigation and writing–original draft. PM: investigation and writing–original draft. XD: writing–review and editing. FD: data curation, visualization, and writing–original draft. EZ: data curation, visualization, and writing–original draft. MK: supervision and writing–original draft. BP: supervision and writing–review and editing. ZW: writing–review and editing. HT: conceptualization, project administration, and writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Fasa University of Medical Sciences, Fasa, Iran.
.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, B. B., Kunnumakkara, A. B., Harikumar, K. B., Gupta, S. R., Tharakan, S. T., Koca, C., et al. (2009). Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann. N. Y. Acad. Sci. 1171, 59–76. doi:10.1111/j.1749-6632.2009.04911.x
Beermann, J., Piccoli, M. T., Viereck, J., and Thum, T. (2016). Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol. Rev. 96 (4), 1297–1325. doi:10.1152/physrev.00041.2015
Bi, C., Cui, H., Fan, H., and Li, L. (2020a). LncRNA LINC01116 promotes the development of colorectal cancer by targeting miR-9-5p/STMN1. OncoTargets Ther. 13, 10547–10558. doi:10.2147/OTT.S253532
Bi, C., Cui, H., Fan, H., and Li, L. (2020b). LncRNA LINC01116 promotes the development of colorectal cancer by targeting miR-9-5p/STMN1. Onco Targets Ther. 13, 10547–10558. doi:10.2147/OTT.S253532
Bian, Z., Jin, L., Zhang, J., Yin, Y., Quan, C., Hu, Y., et al. (2016). LncRNA—UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci. Rep. 6 (1), 23892. doi:10.1038/srep23892
Brenner, H., Kloor, M., and Pox, C. P. (2014). Colorectal cancer. Lancet 383 (9927), 1490–1502. doi:10.1016/S0140-6736(13)61649-9
Cai, Y., Li, Y., Shi, C., Zhang, Z., Xu, J., and Sun, B. (2021). LncRNA OTUD6B-AS1 inhibits many cellular processes in colorectal cancer by sponging miR-21-5p and regulating PNRC2. Hum. Exp. Toxicol. 40 (9), 1463–1473. doi:10.1177/0960327121997976
Chen, D., Zhang, M., Ruan, J., Li, X., Wang, S., Cheng, X., et al. (2020). The long non-coding RNA HOXA11-AS promotes epithelial mesenchymal transition by sponging miR-149-3p in Colorectal Cancer. J. Cancer 11 (20), 6050–6058. doi:10.7150/jca.49809
Chen, E., Yi, J., Jiang, J., Zou, Z., Mo, Y., Ren, Q., et al. (2022b). Identification and validation of a fatty acid metabolism-related lncRNA signature as a predictor for prognosis and immunotherapy in patients with liver cancer. BMC cancer 22 (1), 1037. doi:10.1186/s12885-022-10122-4
Chen, F., Zhang, F., Leng, Y. F., Shi, Y. J., Zhang, J. M., and Liu, Y. Q. (2022a). The crucial roles of long noncoding RNA SNHGs in lung cancer. Clin. Transl. Oncol. 24, 2272–2284. doi:10.1007/s12094-022-02909-5
Chen, W., Liu, S., and Wang, F. (2021). Potential impact and mechanism of Long Non-coding RNAs on cancer and associated T cells. J. Cancer 12 (16), 4873–4882. doi:10.7150/jca.58859
Chi, Y., Wang, D., Wang, J., Yu, W., and Yang, J. (2019). Long non-coding RNA in the pathogenesis of cancers. Cells 8 (9), 1015. doi:10.3390/cells8091015
Chodary Khameneh, S., Razi, S., Shamdani, S., Uzan, G., and Naserian, S. (2022). Weighted correlation network analysis revealed novel long non-coding RNAs for colorectal cancer. Sci. Rep. 12 (1), 2990. doi:10.1038/s41598-022-06934-w
Dai, M., Chen, X., Mo, S., Li, J., Huang, Z., Huang, S., et al. (2017). Meta-signature LncRNAs serve as novel biomarkers for colorectal cancer: integrated bioinformatics analysis, experimental validation and diagnostic evaluation. Sci. Rep. 7 (1), 46572. doi:10.1038/srep46572
Ding, D., Li, C., Zhao, T., Li, D., Yang, L., and Zhang, B. (2018). LncRNA H19/miR-29b-3p/PGRN axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on Wnt signaling. Mol. cells 41 (5), 423–435. doi:10.14348/molcells.2018.2258
Eptaminitaki, G. C., Wolff, N., Stellas, D., Sifakis, K., and Baritaki, S. (2021). Long non-coding RNAs (lncRNAs) in response and resistance to cancer immunosurveillance and immunotherapy. Cells 10 (12), 3313. doi:10.3390/cells10123313
Erdogan, F., Radu, T. B., Orlova, A., Qadree, A. K., de Araujo, E. D., Israelian, J., et al. (2022). JAK-STAT core cancer pathway: an integrative cancer interactome analysis. J. Cell Mol. Med. 26 (7), 2049–2062. doi:10.1111/jcmm.17228
Fang, Y., Zhong, Q., Wang, Y., Gu, C., Liu, S., Liu, A., et al. (2020). CPEB3 functions as a tumor suppressor in colorectal cancer via JAK/STAT signaling. Aging (Albany NY) 12 (21), 21404–21422. doi:10.18632/aging.103893
Farooqi, A. A., Mukhanbetzhanovna, A. A., Yilmaz, S., Karasholakova, L., and Yulaevna, I. M. (2021). Mechanistic role of DANCR in the choreography of signaling pathways in different cancers: spotlight on regulation of Wnt/β-catenin and JAK/STAT pathways by oncogenic long non-coding RNA. Non-coding RNA Res. 6 (1), 29–34. doi:10.1016/j.ncrna.2021.01.001
Gao, Z., Jiang, J., Hou, L., and Zhang, B. (2022). Dysregulation of MiR-144-5p/rnf187 Axis contributes to the progression of colorectal cancer. J. Transl. Int. Med. 10 (1), 65–75. doi:10.2478/jtim-2021-0043
Gotthardt, D., Putz, E. M., Grundschober, E., Prchal-Murphy, M., Straka, E., Kudweis, P., et al. (2016). STAT5 is a key regulator in NK cells and acts as a molecular switch from tumor surveillance to tumor promotion. Cancer Discov. 6 (4), 414–429. doi:10.1158/2159-8290.CD-15-0732
He, C., Yang, W., Yang, J., Ding, J., Li, S., Wu, H., et al. (2017). Long noncoding RNA MEG3 negatively regulates proliferation and angiogenesis in vascular endothelial cells. DNA Cell Biol. 36 (6), 475–481. doi:10.1089/dna.2017.3682
He, J., and Wu, W. (2023). Comprehensive landscape and future perspectives of long noncoding RNAs (lncRNAs) in colorectal cancer (CRC): based on a bibliometric analysis. Noncoding RNA Res. 8 (1), 33–52. doi:10.1016/j.ncrna.2022.10.001
Heinrich, P. C., Behrmann, I., Haan, S., Hermanns, H. M., Müller-Newen, G., and Schaper, F. (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374 (Pt 1), 1–20. doi:10.1042/BJ20030407
Hong, Y. G., Xin, C., Zheng, H., Huang, Z. P., Yang, Y., Zhou, J. D., et al. (2020). miR-365a-3p regulates ADAM10-JAK-STAT signaling to suppress the growth and metastasis of colorectal cancer cells. J. Cancer 11 (12), 3634–3644. doi:10.7150/jca.42731
Hu, Q., Ye, Y., Chan, L. C., Li, Y., Liang, K., Lin, A., et al. (2019a). Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat. Immunol. 20 (7), 835–851. doi:10.1038/s41590-019-0400-7
Hu, X., Ding, D., Zhang, J., and Cui, J. (2019b). Knockdown of lncRNA HOTAIR sensitizes breast cancer cells to ionizing radiation through activating miR-218. Biosci. Rep. 39 (4). doi:10.1042/BSR20181038
Huang, D., Chen, J., Yang, L., Ouyang, Q., Li, J., Lao, L., et al. (2018). NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat. Immunol. 19 (10), 1112–1125. doi:10.1038/s41590-018-0207-y
Huang, G., Wu, X., Li, S., Xu, X., Zhu, H., and Chen, X. (2016a). The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci. Rep. 6 (1), 26524. doi:10.1038/srep26524
Huang, G., Wu, X., Li, S., Xu, X., Zhu, H., and Chen, X. (2016b). The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci. Rep. 6, 26524. doi:10.1038/srep26524
Huang, J.-Z., Chen, M., Chen, D., Gao, X. C., Zhu, S., Huang, H., et al. (2017). A peptide encoded by a putative lncRNA HOXB-AS3 suppresses colon cancer growth. Mol. Cell 68 (1), 171–184. doi:10.1016/j.molcel.2017.09.015
Hugen, N., Brown, G., Glynne-Jones, R., de Wilt, J. H. W., and Nagtegaal, I. D. (2016). Advances in the care of patients with mucinous colorectal cancer. Nat. Rev. Clin. Oncol. 13 (6), 361–369. doi:10.1038/nrclinonc.2015.140
Jiang, L., Zhao, X. H., Mao, Y. L., Wang, J. F., Zheng, H. J., and You, Q. S. (2019). Long non-coding RNA RP11-468E2.5 curtails colorectal cancer cell proliferation and stimulates apoptosis via the JAK/STAT signaling pathway by targeting STAT5 and STAT6. J. Exp. Clin. Cancer Res. 38 (1), 465. doi:10.1186/s13046-019-1428-0
Jin, K.-T., Yao, J. Y., Fang, X. L., Di, H., and Ma, Y. Y. (2020). Roles of lncRNAs in cancer: focusing on angiogenesis. Life Sci. 252, 117647. doi:10.1016/j.lfs.2020.117647
Kang, Y. H., Biswas, A., Field, M., and Snapper, S. B. (2019). STAT1 signaling shields T cells from NK cell-mediated cytotoxicity. Nat. Commun. 10 (1), 912. doi:10.1038/s41467-019-08743-8
Khodaii, Z., Mehrabani Natanzi, M., Khalighfard, S., Ghandian Zanjan, M., Gharghi, M., Khori, V., et al. (2022). Novel targets in rectal cancer by considering lncRNA–miRNA–mRNA network in response to Lactobacillus acidophilus consumption: a randomized clinical trial. Sci. Rep. 12 (1), 9168. doi:10.1038/s41598-022-13297-9
Kogo, R., Shimamura, T., Mimori, K., Kawahara, K., Imoto, S., Sudo, T., et al. (2011). Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 71 (20), 6320–6326. doi:10.1158/0008-5472.CAN-11-1021
Li, B., Zheng, L., Ye, J., Zhang, C., Zhou, J., Huang, Q., et al. (2022b). CREB1 contributes colorectal cancer cell plasticity by regulating lncRNA CCAT1 and NF-κB pathways. Sci. China Life Sci. 65 (8), 1481–1497. doi:10.1007/s11427-022-2108-x
Li, D., and Wen, S. (2020). Silencing of lncRNA LINC00346 inhibits the proliferation and promotes the apoptosis of colorectal cancer cells through inhibiting JAK1/STAT3 signaling. Cancer Manag. Res. 12, 4605–4614. doi:10.2147/CMAR.S249491
Li, G., Kryczek, I., Nam, J., Li, X., Li, S., Li, J., et al. (2021). LIMIT is an immunogenic lncRNA in cancer immunity and immunotherapy. Nat. Cell Biol. 23 (5), 526–537. doi:10.1038/s41556-021-00672-3
Li, P., Zhang, X., Wang, L., Du, L., Yang, Y., Liu, T., et al. (2017). lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-κB/TS signaling in colorectal cancer. Mol. Therapy-Nucleic Acids 8, 356–369. doi:10.1016/j.omtn.2017.07.007
Li, Q., Johnston, N., Zheng, X., Wang, H., Zhang, X., Gao, D., et al. (2016). miR-28 modulates exhaustive differentiation of T cells through silencing programmed cell death-1 and regulating cytokine secretion. Oncotarget 7 (33), 53735–53750. doi:10.18632/oncotarget.10731
Li, S., Yao, W., Liu, R., Gao, L., Lu, Y., Zhang, H., et al. (2022a). Long non-coding RNA LINC00152 in cancer: roles, mechanisms, and chemotherapy and radiotherapy resistance. Front. Oncol. 12, 4153. doi:10.3389/fonc.2022.960193
Li, Z. Y., Yang, L., Liu, X. J., Wang, X. Z., Pan, Y. X., and Luo, J. M. (2018). The long noncoding RNA MEG3 and its target miR-147 regulate JAK/STAT pathway in advanced chronic myeloid leukemia. EBioMedicine 34, 61–75. doi:10.1016/j.ebiom.2018.07.013
Liang, C., Zhao, T., Li, H., He, F., Zhao, X., Zhang, Y., et al. (2019). Long non-coding RNA ITIH4-AS1 accelerates the proliferation and metastasis of colorectal cancer by activating JAK/STAT3 signaling. Mol. Ther. Nucleic Acids 18, 183–193. doi:10.1016/j.omtn.2019.08.009
Liang, W., Wu, J., and Qiu, X. (2021a). LINC01116 facilitates colorectal cancer cell proliferation and angiogenesis through targeting EZH2-regulated TPM1. J. Transl. Med. 19 (1), 45–13. doi:10.1186/s12967-021-02707-7
Liang, W., Wu, J., and Qiu, X. (2021b). LINC01116 facilitates colorectal cancer cell proliferation and angiogenesis through targeting EZH2-regulated TPM1. J. Transl. Med. 19 (1), 45. doi:10.1186/s12967-021-02707-7
Liang, W., Xia, B., Zhai, G., Li, M., and Zhou, J. (2020). Overexpression of LINC00691 promotes the proliferation and invasion of gastric cancer cells via the Janus kinase/signal transducer and activator of transcription signalling pathway. Int. J. Biochem. Cell Biol. 123, 105751. doi:10.1016/j.biocel.2020.105751
Liao, L., Kuang, H., Xue, J., Zhou, X., Yin, F., and Wang, Y. (2019). Up-regulated long noncoding RNA AB073614 modulates the tumor cell proliferation, invasion and migration in human colorectal cancer. Int. J. Clin. Exp. Pathol. 12 (8), 2849–2857.
Liao, Z., Nie, H., Wang, Y., Luo, J., Zhou, J., and Ou, C. (2021). The emerging landscape of long non-coding RNAs in colorectal cancer metastasis. Front. Oncol. 11, 641343. doi:10.3389/fonc.2021.641343
Lin, G., Wu, T., Gao, X., He, Z., and Nong, W. (2022). Research progress of long non-coding RNA GAS5 in malignant tumors. Front. Oncol. 12, 846497. doi:10.3389/fonc.2022.846497
Lin, X., Wu, Z., Hu, H., Luo, M. L., and Song, E. (2021). Non-coding RNAs rewire cancer metabolism networks. Seminars Cancer Biol. 75, 116–126. doi:10.1016/j.semcancer.2020.12.019
Liu, A., Jiang, B., Song, C., Zhong, Q., Mo, Y., Yang, R., et al. (2023). Isoliquiritigenin inhibits circ0030018 to suppress glioma tumorigenesis via the miR-1236/HER2 signaling pathway. MedComm 4 (3), e282. doi:10.1002/mco2.282
Liu, B., Liu, Q., Pan, S., Huang, Y., Qi, Y., Li, S., et al. (2019). The HOTAIR/miR-214/ST6GAL1 crosstalk modulates colorectal cancer procession through mediating sialylated c-Met via JAK2/STAT3 cascade. J. Exp. Clin. Cancer Res. 38 (1), 455. doi:10.1186/s13046-019-1468-5
Liu, C., Yang, Z., Deng, Z., Zhou, Y., Gong, Q., Zhao, R., et al. (2018). Upregulated lncRNA ADAMTS9-AS2 suppresses progression of lung cancer through inhibition of miR-223-3p and promotion of TGFBR3. IUBMB life 70 (6), 536–546. doi:10.1002/iub.1752
Liu, L., Wang, Q., Qiu, Z., Kang, Y., Liu, J., Ning, S., et al. (2020). Noncoding RNAs: the shot callers in tumor immune escape. Signal Transduct. Target. Ther. 5 (1), 102–124. doi:10.1038/s41392-020-0194-y
Liu, Z., Wang, Y., Yuan, S., Wen, F., Liu, J., Zou, L., et al. (2021). Regulatory role of long non-coding RNA UCA1 in signaling pathways and its clinical applications. Oncol. Lett. 21 (5), 404–414. doi:10.3892/ol.2021.12665
Lulli, M., Napoli, C., Landini, I., Mini, E., and Lapucci, A. (2022). Role of non-coding RNAs in colorectal cancer: focus on long non-coding RNAs. Int. J. Mol. Sci. 23 (21), 13431. doi:10.3390/ijms232113431
Luo, N., Formisano, L., Gonzalez-Ericsson, P. I., Sanchez, V., Dean, P. T., Opalenik, S. R., et al. (2018). Melanoma response to anti-PD-L1 immunotherapy requires JAK1 signaling, but not JAK2. Oncoimmunology 7 (6), e1438106. doi:10.1080/2162402X.2018.1438106
Ming, H., Li, B., Zhou, L., Goel, A., and Huang, C. (2021). Long non-coding RNAs and cancer metastasis: molecular basis and therapeutic implications. Biochimica Biophysica Acta (BBA)-Reviews Cancer 1875 (2), 188519. doi:10.1016/j.bbcan.2021.188519
Misale, S., Yaeger, R., Hobor, S., Scala, E., Janakiraman, M., Liska, D., et al. (2012). Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486 (7404), 532–536. doi:10.1038/nature11156
Moradi, M. T., Fallahi, H., and Rahimi, Z. (2019). Interaction of long noncoding RNA MEG3 with miRNAs: a reciprocal regulation. J. Cell. Biochem. 120 (3), 3339–3352. doi:10.1002/jcb.27604
Morris, R., Kershaw, N. J., and Babon, J. J. (2018). The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 27 (12), 1984–2009. doi:10.1002/pro.3519
Niinuma, T., Suzuki, H., Nojima, M., Nosho, K., Yamamoto, H., Takamaru, H., et al. (2012). Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 72 (5), 1126–1136. doi:10.1158/0008-5472.CAN-11-1803
Niu, L., Yang, W., Duan, L., Wang, X., Li, Y., Xu, C., et al. (2021). Biological implications and clinical potential of metastasis-related miRNA in colorectal cancer. Mol. Therapy-Nucleic Acids 23, 42–54. doi:10.1016/j.omtn.2020.10.030
O'Shea, J. J., Schwartz, D. M., Villarino, A. V., Gadina, M., McInnes, I. B., and Laurence, A. (2015). The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 66, 311–328. doi:10.1146/annurev-med-051113-024537
Ou, A., Ott, M., Fang, D., and Heimberger, A. B. (2021b). The role and therapeutic targeting of JAK/STAT signaling in glioblastoma. Cancers 13 (3), 437. doi:10.3390/cancers13030437
Ou, J., Lei, P., Yang, Z., Yang, M., Luo, L., Mo, H., et al. (2021a). LINC00152 mediates CD8+ T-cell infiltration in gastric cancer through binding to EZH2 and regulating the CXCL9, 10/CXCR3 axis. J. Mol. Histology 52 (3), 611–620. doi:10.1007/s10735-021-09967-z
Owen, K. L., Brockwell, N. K., and Parker, B. S. (2019). JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers (Basel) 11 (12), 2002. doi:10.3390/cancers11122002
Pan, S., Liu, Y., Liu, Q., Xiao, Y., Liu, B., Ren, X., et al. (2019). HOTAIR/miR-326/FUT6 axis facilitates colorectal cancer progression through regulating fucosylation of CD44 via PI3K/AKT/mTOR pathway. Biochimica Biophysica Acta (BBA)-Molecular Cell Res. 1866 (5), 750–760. doi:10.1016/j.bbamcr.2019.02.004
Pandey, P. R., Young, K. H., Kumar, D., and Jain, N. (2022). RNA-mediated immunotherapy regulating tumor immune microenvironment: next wave of cancer therapeutics. Mol. cancer 21 (1), 58–18. doi:10.1186/s12943-022-01528-6
Park, J. H., Pyun, W. Y., and Park, H. W. (2020). Cancer metabolism: phenotype, signaling and therapeutic targets. Cells 9 (10), 2308. doi:10.3390/cells9102308
Ren, N., Jiang, T., Wang, C., Xie, S., Xing, Y., Piao, D., et al. (2020). LncRNA ADAMTS9-AS2 inhibits gastric cancer (GC) development and sensitizes chemoresistant GC cells to cisplatin by regulating miR-223-3p/NLRP3 axis. Aging (Albany NY) 12 (11), 11025–11041. doi:10.18632/aging.103314
Sabaawy, A., and Zeeshan, S. (2021). Targeting the immune microenvironment during immunotherapy for solid tumors. Mol. Cell. Oncol. 8 (5), 1994327. doi:10.1080/23723556.2021.1994327
Sabaawy, H. E., Ryan, B. M., Khiabanian, H., and Pine, S. R. (2021). JAK/STAT of all trades: linking inflammation with cancer development, tumor progression and therapy resistance. Carcinogenesis 42 (12), 1411–1419. doi:10.1093/carcin/bgab075
Seif, F., Khoshmirsafa, M., Aazami, H., Mohsenzadegan, M., Sedighi, G., and Bahar, M. (2017). The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal 15 (1), 23. doi:10.1186/s12964-017-0177-y
Shaath, H., Toor, S. M., Nada, M. A., Elkord, E., and Alajez, N. M. (2021). Integrated whole transcriptome and small RNA analysis revealed multiple regulatory networks in colorectal cancer. Sci. Rep. 11 (1), 14456. doi:10.1038/s41598-021-93531-y
Shaker, O. G., Senousy, M. A., and Elbaz, E. M. (2017). Association of rs6983267 at 8q24, HULC rs7763881 polymorphisms and serum lncRNAs CCAT2 and HULC with colorectal cancer in Egyptian patients. Sci. Rep. 7 (1), 16246. doi:10.1038/s41598-017-16500-4
Shakhpazyan, N. K., Mikhaleva, L. M., Bedzhanyan, A. L., Sadykhov, N. K., Midiber, K. Y., Konyukova, A. K., et al. (2023). Long non-coding RNAs in colorectal cancer: navigating the intersections of immunity, intercellular communication, and therapeutic potential. Biomedicines 11 (9), 2411. doi:10.3390/biomedicines11092411
Shang, W., Gao, Y., Tang, Z., Zhang, Y., and Yang, R. (2019). The pseudogene olfr29-ps1 promotes the suppressive function and differentiation of monocytic MDSCs. Cancer Immunol. Res. 7 (5), 813–827. doi:10.1158/2326-6066.CIR-18-0443
Shi, L. Z., and Bonner, J. A. (2021). Bridging radiotherapy to immunotherapy: the IFN–JAK–STAT Axis. Int. J. Mol. Sci. 22 (22), 12295. doi:10.3390/ijms222212295
Sun, Z., Ou, C., Liu, J., Chen, C., Zhou, Q., Yang, S., et al. (2019). YAP1-induced MALAT1 promotes epithelial–mesenchymal transition and angiogenesis by sponging miR-126-5p in colorectal cancer. Oncogene 38 (14), 2627–2644. doi:10.1038/s41388-018-0628-y
Tabarestani, F. O., Akbari, A., Karizi, S. Z., and Sotoodehnejadnematalahi, F. (2022). Regulation of long non-coding RNAs XIST and ROR induced by homeodomain protein TGIF2LX in colorectal cancer. J. Cancer Res. Ther. 18, S359–S366. doi:10.4103/jcrt.JCRT_869_20
Takebe, N., Miele, L., Harris, P. J., Jeong, W., Bando, H., Kahn, M., et al. (2015). Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 12 (8), 445–464. doi:10.1038/nrclinonc.2015.61
Tan, X., Mao, L., Huang, C., Yang, W., Guo, J., Chen, Z., et al. (2021). Comprehensive analysis of lncRNA-miRNA-mRNA regulatory networks for microbiota-mediated colorectal cancer associated with immune cell infiltration. Bioengineered 12 (1), 3410–3425. doi:10.1080/21655979.2021.1940614
Tang, Y., He, Y., Shi, L., Yang, L., Wang, J., Lian, Y., et al. (2017). Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma. Oncotarget 8 (24), 39001–39011. doi:10.18632/oncotarget.16545
Waldmann, T. A., and Chen, J. (2017). Disorders of the JAK/STAT pathway in T cell lymphoma pathogenesis: implications for immunotherapy. Annu. Rev. Immunol. 35, 533–550. doi:10.1146/annurev-immunol-110416-120628
Wang, G., Zhang, Z. J., Jian, W. G., Liu, P. H., Xue, W., Wang, T. d., et al. (2019). Novel long noncoding RNA OTUD6B-AS1 indicates poor prognosis and inhibits clear cell renal cell carcinoma proliferation via the Wnt/β-catenin signaling pathway. Mol. cancer 18 (1), 15. doi:10.1186/s12943-019-0942-1
Wang, H., Zhu, Y., Chen, H., Yang, N., Wang, X., Li, B., et al. (2021b). Colorectal cancer risk variant rs7017386 modulates two oncogenic lncRNAs expression via ATF1-mediated long-range chromatin loop. Cancer Lett. 518, 140–151. doi:10.1016/j.canlet.2021.07.021
Wang, J., Zhang, Y., Song, H., Yin, H., Jiang, T., Xu, Y., et al. (2021a). The circular RNA circSPARC enhances the migration and proliferation of colorectal cancer by regulating the JAK/STAT pathway. Mol. Cancer 20 (1), 81. doi:10.1186/s12943-021-01375-x
Wang, K., Song, W., Shen, Y., Wang, H., and Fan, Z. (2021d). LncRNA KLK8 modulates stem cell characteristics in colon cancer. Pathology-Research Pract. 224, 153437. doi:10.1016/j.prp.2021.153437
Wang, W., Cheng, X., and Zhu, J. (2021c). Long non-coding RNA OTUD6B-AS1 overexpression inhibits the proliferation, invasion and migration of colorectal cancer cells via downregulation of microRNA-3171. Oncol. Lett. 21 (3), 193. doi:10.3892/ol.2021.12454
Wang, Y., Yan, X. L., and Tian, S. K. (2020). Downregulating long non-coding RNA CCAT5 inhibits tumor growth, invasion and metastasis in colorectal cancer through suppressing STAT3. Eur. Rev. Med. Pharmacol. Sci. 24 (19), 9770. doi:10.26355/eurrev_202010_23166
Wang, Z., Jin, H., Xu, R., Mei, Q., and Fan, D. (2009). Triptolide downregulates Rac1 and the JAK/STAT3 pathway and inhibits colitis-related colon cancer progression. Exp. Mol. Med. 41 (10), 717–727. doi:10.3858/emm.2009.41.10.078
Wei, Y., Zhou, K., Wang, C., Du, X., Xiao, Q., and Chen, C. (2020). Adsorption of miR-218 by lncRNA HOTAIR regulates PDE7A and affects glioma cell proliferation, invasion, and apoptosis. Int. J. Clin. Exp. Pathology 13 (12), 2973–2983.
Wu, J., Chen, Z., Liu, W., Zhang, Y., Feng, W., Yuan, Y., et al. (2022b). MicroRNA-188-5p targeting Forkhead Box L1 promotes colorectal cancer progression via activating Wnt/β-catenin signaling. Oncol. Res. 29 (2), 119–128. doi:10.32604/or.2022.03178
Wu, K., Xu, K., Liu, K., Huang, J., Chen, J., Zhang, J., et al. (2018). Long noncoding RNA BC200 regulates cell growth and invasion in colon cancer. Int. J. Biochem. Cell Biol. 99, 219–225. doi:10.1016/j.biocel.2018.04.001
Wu, K., Xu, T., Song, X., Shen, J., Zheng, S., Zhang, L., et al. (2021). LncRNA SLCO4A1-AS1 modulates colon cancer stem cell properties by binding to miR-150-3p and positively regulating SLCO4A1. Lab. Investig. 101 (7), 908–920. doi:10.1038/s41374-021-00577-7
Wu, M., Li, W., Huang, F., Sun, J., Li, K. P., Shi, J., et al. (2019). Comprehensive analysis of the expression profiles of long non-coding RNAs with associated ceRNA network involved in the colon cancer staging and progression. Sci. Rep. 9 (1), 16910–10. doi:10.1038/s41598-019-52883-2
Wu, W., Zhang, S., and He, J. (2022a). The mechanism of long non-coding RNA in cancer radioresistance/radiosensitivity: a systematic review. Front. Pharmacol. 13, 879704. doi:10.3389/fphar.2022.879704
Xiao, L., Gorospe, M., and Wang, J.-Y. (2019). Long noncoding RNAs in intestinal epithelium homeostasis. Am. J. Physiology-Cell Physiology 317 (1), C93-C100–C100. doi:10.1152/ajpcell.00092.2019
Xie, J., Ye, F., Deng, X., Tang, Y., Liang, J. Y., Huang, X., et al. (2023). Circular RNA, a promising new star of vaccine. J. Transl. Intern Med. AOP. doi:10.2478/jtim-2023-0122
Xie, Y., Han, J., Xie, K., and Gou, Q. (2022). LncRNAs as biomarkers for predicting radioresistance and survival in cancer: a meta-analysis. Sci. Rep. 12 (1), 18494. doi:10.1038/s41598-022-21785-1
Xu, C., Yang, M., Tian, J., Wang, X., and Li, Z. (2011). MALAT-1: a long non-coding RNA and its important 3' end functional motif in colorectal cancer metastasis. Int. J. Oncol. 39 (1), 169–175. doi:10.3892/ijo.2011.1007
Xu, J., Shi, A., Long, Z., Xu, L., Liao, G., Deng, C., et al. (2018). Capturing functional long non-coding RNAs through integrating large-scale causal relations from gene perturbation experiments. EBioMedicine 35, 369–380. doi:10.1016/j.ebiom.2018.08.050
Xu, Y., Yu, X., Zhang, M., Zheng, Q., Sun, Z., He, Y., et al. (2021). Promising advances in LINC01116 related to cancer. Front. Cell Dev. Biol. 9, 736927. doi:10.3389/fcell.2021.736927
Xue, J., Liao, L., Yin, F., Kuang, H., Zhou, X., and Wang, Y. (2018). LncRNA AB073614 induces epithelial-mesenchymal transition of colorectal cancer cells via regulating the JAK/STAT3 pathway. Cancer Biomark. 21 (4), 849–858. doi:10.3233/CBM-170780
Yamada, A., Yu, P., Lin, W., Okugawa, Y., Boland, C. R., and Goel, A. (2018). A RNA-Sequencing approach for the identification of novel long non-coding RNA biomarkers in colorectal cancer. Sci. Rep. 8 (1), 575. doi:10.1038/s41598-017-18407-6
Yan, D., Jin, F., and Lin, Y. (2020). lncRNA HAND2-AS1 inhibits liver cancer cell proliferation and migration by upregulating SOCS5 to inactivate the JAK-STAT pathway. Cancer Biother Radiopharm. 35 (2), 143–152. doi:10.1089/cbr.2019.2958
Yang, G., Lu, X., and Yuan, L. (2014). LncRNA: a link between RNA and cancer. Biochim. Biophys. Acta 1839 (11), 1097–1109. doi:10.1016/j.bbagrm.2014.08.012
Yang, S., Wang, Y., Ren, J., Zhou, X., Cai, K., Guo, L., et al. (2020). Identification of diagnostic and prognostic lncRNA biomarkers in oral squamous carcinoma by integrated analysis and machine learning. Cancer Biomarkers 29 (2), 265–275. doi:10.3233/CBM-191215
Yang, Y., Yan, X., Li, X., Ma, Y., and Goel, A. (2021). Long non-coding RNAs in colorectal cancer: novel oncogenic mechanisms and promising clinical applications. Cancer Lett. 504, 67–80. doi:10.1016/j.canlet.2021.01.009
Yao, J., Zhou, B., Zhang, J., Geng, P., Liu, K., Zhu, Y., et al. (2014). A new tumor suppressor LncRNA ADAMTS9-AS2 is regulated by DNMT1 and inhibits migration of glioma cells. Tumor Biol. 35 (8), 7935–7944. doi:10.1007/s13277-014-1949-2
Ye, J., Zhu, J., Chen, H., Qian, J., Zhang, L., Wan, Z., et al. (2020). A novel lncRNA-LINC01116 regulates tumorigenesis of glioma by targeting VEGFA. Int. J. cancer 146 (1), 248–261. doi:10.1002/ijc.32483
Yoshimura, A., Ito, M., Chikuma, S., Akanuma, T., and Nakatsukasa, H. (2018). Negative regulation of cytokine signaling in immunity. Cold Spring Harb. Perspect. Biol. 10 (7), a028571. doi:10.1101/cshperspect.a028571
Yu, H., Lee, H., Herrmann, A., Buettner, R., and Jove, R. (2014). Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer 14 (11), 736–746. doi:10.1038/nrc3818
Yu, Y., and Hann, S. S. (2019). Novel tumor suppressor lncRNA growth arrest-specific 5 (GAS5) in human cancer. OncoTargets Ther. 12, 8421–8436. doi:10.2147/OTT.S221305
Zaniani, N. R., Oroujalian, A., Valipour, A., and Peymani, M. (2021). LAMTOR5 expression level is a biomarker for colorectal cancer and lncRNA LAMTOR5-AS1 predicting miRNA sponging effect. Mol. Biol. Rep. 48 (8), 6093–6101. doi:10.1007/s11033-021-06623-3
Zhang, B., Yu, L., Han, N., Hu, Z., Wang, S., Ding, L., et al. (2018). LINC01116 targets miR-520a-3p and affects IL6R to promote the proliferation and migration of osteosarcoma cells through the Jak-stat signaling pathway. Biomed. Pharmacother. 107, 270–282. doi:10.1016/j.biopha.2018.07.119
Zhang, H., Zhang, G., Liu, H., Shan, Y., and Zhang, X. (2021a). RP11-462C24. 1 suppresses proliferation and invasion of colorectal carcinoma cells by regulating HSP70 through PI3K/AKT signaling pathway. Hum. Cell 34 (1), 132–151. doi:10.1007/s13577-020-00426-7
Zhang, L., Ye, F., Zuo, Z., Cao, D., Peng, Y., Li, Z., et al. (2021b). Long noncoding RNA TPT1-AS1 promotes the progression and metastasis of colorectal cancer by upregulating the TPT1-mediated FAK and JAK-STAT3 signalling pathways. Aging (Albany NY) 13 (3), 3779–3797. doi:10.18632/aging.202339
Zhou, B., Lin, W., Long, Y., Yang, Y., Zhang, H., Wu, K., et al. (2022). Notch signaling pathway: architecture, disease, and therapeutics. Signal Transduct. Target Ther. 7 (1), 95. doi:10.1038/s41392-022-00934-y
Zhu, Y., Wang, X., Zheng, L., Li, D., Liu, Z., and Teng, L. (2022). The lncRNA NEAT1 inhibits miRNA-216b and promotes colorectal cancer progression by indirectly activating YY1. J. Oncol. 2022, 8130132. doi:10.1155/2022/8130132
Zhu, Y.-S., and Zhu, J. (2022). Molecular and cellular functions of long non-coding RNAs in prostate and breast cancer. Adv. Clin. Chem. 106, 91–179. doi:10.1016/bs.acc.2021.09.005
Zhuo, W., Liu, Y., Li, S., Guo, D., Sun, Q., Jin, J., et al. (2019). Long noncoding RNA GMAN, up-regulated in gastric cancer tissues, is associated with metastasis in patients and promotes translation of ephrin A1 by competitively binding GMAN-AS. Gastroenterology 156 (3), 676–691. doi:10.1053/j.gastro.2018.10.054
Keywords: long non-coding RNAs, colorectal cancer, Janus kinase/signal transducer and activator of transcription, signaling pathways, regulation
Citation: Ghasemian A, Omear HA, Mansoori Y, Mansouri P, Deng X, Darbeheshti F, Zarenezhad E, Kohansal M, Pezeshki B, Wang Z and Tang H (2023) Long non-coding RNAs and JAK/STAT signaling pathway regulation in colorectal cancer development. Front. Genet. 14:1297093. doi: 10.3389/fgene.2023.1297093
Received: 19 September 2023; Accepted: 10 November 2023;
Published: 29 November 2023.
Edited by:
Bodhisattwa Banerjee, University of Vermont, United StatesReviewed by:
Sumit Mukherjee, National Cancer Institute (NIH), United StatesMehak Gupta, Vertex Pharmaceuticals, United States
Copyright © 2023 Ghasemian, Omear, Mansoori, Mansouri, Deng, Darbeheshti, Zarenezhad, Kohansal, Pezeshki, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hailin Tang, dGFuZ2hsQHN5c3VjYy5vcmcuY24=; Zhangling Wang, d2FuZ3psMUBzeXN1Y2Mub3JnLmNu
 Abdolmajid Ghasemian
Abdolmajid Ghasemian Hadeel A. Omear2
Hadeel A. Omear2 Yaser Mansoori
Yaser Mansoori Xinpei Deng
Xinpei Deng Zhangling Wang
Zhangling Wang Hailin Tang
Hailin Tang