- 1Institute of Animal Husbandry and Veterinary, Tibet Autonomous Regional Academy of Agricultural Sciences, Lhasa, China
- 2Liaocheng Research Institute of Donkey High-Efficiency Breeding and Ecological Feeding, Agricultural Science and Engineering School, Liaocheng University, Liaocheng, China
- 3Faculty of Veterinary and Animal Sciences, The University of Agriculture, Dera Ismail Khan, Pakistan
- 4Anhui Province Key Laboratory of Embryo Development and Reproduction Regulation, Anhui Province Key Laboratory of Environmental Hormone and Reproduction, School of Biological and Food Engineering, Fuyang Normal University, Fuyang, China
- 5Tibet Autonomous Region Animal Husbandry Station, Lhasa, China
- 6College of Life Sciences, Liaocheng University, Liaocheng, China
- 7State Key Laboratory of Animal Nutrition, Beijing Engineering Technology Research Center of Raw Milk Quality and Safety Control, College of Animal Science and Technology, China Agricultural University, Beijing, China
- 8Genome Analysis Laboratory of the Ministry of Agriculture, Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen, China
- 9Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway
Mammary glands are known for their ability to convert nutrients present in the blood into milk contents. In cows, milk synthesis and the proliferation of cow mammary epithelial cells (CMECs) are regulated by various factors, including nutrients such as amino acids and glucose, hormones, and environmental stress. Amino acids, in particular, play a crucial role in regulating cell proliferation and casein synthesis in mammalian epithelial cells, apart from being building blocks for protein synthesis. Studies have shown that environmental factors, particularly heat stress, can negatively impact milk production performance in dairy cattle. The mammalian target of rapamycin complex 1 (mTORC1) pathway is considered the primary signaling pathway involved in regulating cell proliferation and milk protein and fat synthesis in cow mammary epithelial cells in response to amino acids and heat stress. Given the significant role played by the mTORC signaling pathway in milk synthesis and cell proliferation, this article briefly discusses the main regulatory genes, the impact of amino acids and heat stress on milk production performance, and the regulation of mTORC signaling pathway in cow mammary epithelial cells.
1 Introduction
The mammary gland is a highly organized organ that has the ability to transform nutrients from the bloodstream into milk constituents (Bauman et al., 2006). The quality of milk is determined by its contents, and breeders are highly concerned with the milk phenotypic traits of their animals. Several factors, such as the environment, nutrition, and endocrine factors, can affect the quantity and quality of milk produced by the mammary gland (Toerien and Cant, 2007; Hayashi et al., 2009; Burgos et al., 2010a; Burgos and Cant, 2010b; Toerien et al., 2010). For example, studies have shown that milk protein synthesis in the bovine mammary gland requires an adequate supply of amino acids and adenosine triphosphate (ATP) in the animal’s body (Pszczolkowski et al., 2020). The kinase mammalian or mechanistic target of rapamycin (mTOR) is involved in integrating various signals from the environment and translating them into specific biological responses such as milk yield and protein synthesis (Gordon et al., 2014). As a master regulator of cell development and milk synthesis, mTORC1 stimulates anabolic cellular metabolism in response to growth stimuli, nutrients, and energy (Huang and Fingar, 2014). Recent studies have also reported on the role of mTORC1 signaling in the regulation of milk contents (Ma and Blenis, 2009; Salama et al., 2019; Hu et al., 2020; Zhou J et al., 2022).
The mTOR is an atypical serine/threonine kinase that is highly conserved through evolution. It plays a central role in regulating cell growth, proliferation, and metabolism in response to a variety of signals, including growth factors, nutrient availability, and stress (Sengupta et al., 2010; Jewell et al., 2013a; Jewell and Guan, 2013b). In mammalian cells, mTOR is found in two distinct protein complexes called mTORC1 and mTORC2. The activity of mTORC1 is regulated by several factors, including the intracellular energy status, external growth hormones like insulin, stress factors, and the availability of amino acids. Amino acid supplementation, environmental factors, and endocrine changes have been shown to affect the milk quantity and quality via the regulation of mTORC signaling pathway in the bovine mammary gland (Hanigan et al., 2009; Pszczolkowski et al., 2020; Zhou J et al., 2022). In this review, the underlying factors (such as genetics, environmental factors, and amino acids) that regulate the mTOR signaling pathway and how mTORC1 integrates these signals to promote milk synthesis in bovine mammary gland cells are highlighted. Figure 1 summarizes the general regulatory mechanism and biological functions performed by the mTOR signaling pathway in mammary bovine epithelial cells.
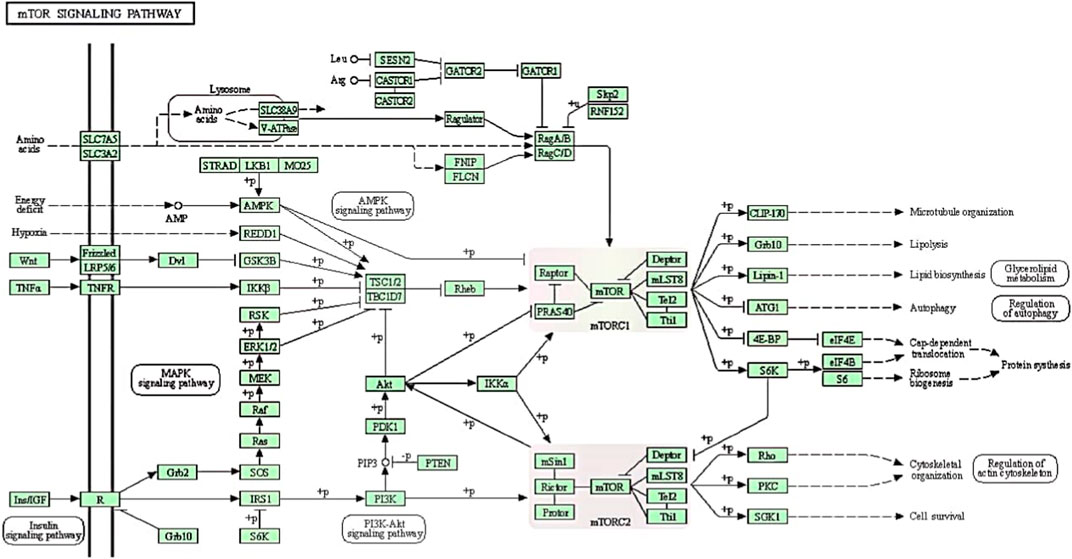
FIGURE 1. The regulatory mechanism of mTOR signaling pathways to facilitate biological functions processes such as lipid metabolism, autophagy, protein synthesis and ribosome biogenesis. mTORC1 contains mTOR, Raptor, PRAS40, Deptor, mLST8, Tel2 and Tti1. On the other hand, mTORC2, which consists of mTOR, mSin1, Rictor, Protor, Deptor, mLST8, Tel2 and Tti1, responds to growth factors and controls cytoskeletal organization, metabolism and survival. Note: +p indicates phosphorylation; −p indicates dephosphorylation; → indicates activation;  indicates indirect or unknown relationship,
indicates indirect or unknown relationship,  or
or indicates complexes,
indicates complexes,  indicates repression.
indicates repression.
Understanding the regulation of the mTOR signaling pathway and its role in milk synthesis is critical for improving animal breeding practices and ensuring a consistent supply of high-quality milk products. This knowledge can be used to develop targeted interventions that can optimize milk production and quality in livestock.
2 Genetic regulation of mTOR signaling pathway for milk fat and protein synthesis
Genetic regulation of the mTOR signaling pathway in the cow mammary gland is complex and involves multiple genes and pathways. Several genes involved in regulating the mTOR pathway have been identified in cows, including the genes encoding for the mTOR protein itself, as well as its upstream regulators such as AKT and PI3K. Besides, many others genes such as sterol regulatory element-binding protein 1 (SREBP1), peroxisome proliferator-activated receptor-gamma (PPARγ), menin, stearoylcoenzyme desaturase (SCD), and fatty acid synthase (FASN) have also been documented as key regulators of mTOR signaling to facilitate its activity in milk regulation (Li, H et al., 2017a; Liu, X et al., 2021). Furthermore, it has been reported that β-sitosterol could enhance the milk protein synthesis via Janus kinase 2/signal transducer and activator of transcription 5 (JAK2/STAT5) and mTORC1 signaling (Liu, X et al., 2021). Consistently, studies have reported that mTORC1 via RPL8, FASN, SREBP1 and SCD regulates milk fat and protein synthesis (Liu, L et al., 2016; Zhou J. et al., 2021). Previous studies have found the two main signaling pathways including peroxisome proliferator-activated receptor gamma and SREBP1 are significantly associated with milk fat synthesis (Li N et al., 2014; Osorio et al., 2016; Li S et al., 2017b; Liu X. et al., 2017; He et al., 2021). The SREBP1 pathway and the PPARγ pathway are the two main pathways for milk fat synthesis.
The SREBP1 is a transcription factor that plays a crucial role in regulating lipid metabolism in the bovine mammary gland, and mTOR (mechanistic target of rapamycin) signaling has been shown to be involved in this process (Li N et al., 2014). When there is an increase in the demand for milk production in the mammary gland, SREBP1 is activated and translocates to the nucleus, where it binds to the promoter regions of genes involved in lipid metabolism and transcriptionally upregulates their expression. This results in increased de novo synthesis of fatty acids and triglycerides in the mammary gland, which are essential components of milk. mTOR signaling is a key pathway involved in regulating cellular metabolism and growth. It is activated by various factors, including amino acids, growth factors, and insulin, and plays an important role in regulating lipid metabolism in the mammary gland. Studies have shown that mTOR signaling is involved in the regulation of SREBP1 activity in the bovine mammary gland (Che et al., 2019). The SREBP1 responds to hormones or nutrition via mTOR signaling and then stimulates milk fat synthesis by promoting the expression of genes involved in fat synthesis, such as acetyl-CoA carboxylase alpha (ACACA), FAS and SCD1 (Li N et al., 2014). Similarly, other studies have also found that the SREBP1 plays an important role in the integrated regulation of lipid synthesis in dairy cow mammary epithelial cells through the regulation of key enzymes including fatty acid synthase, acetyl-CoA carboxylase alpha and stearoyl-CoA desaturase (Ma and Corl, 2012; Oppi-Williams et al., 2013). Studies have shown that the activation of SREBP1 is regulated by various upstream factors or pathways. Consistently, it has been reported that insulin and growth factor activate SREBP1 through phosphatidylinositol 3-kinase (PI3K)-Protein Kinase B (AKT)-mTORC1 pathway (Ricoult et al., 2016) and cholesterol activates SREBP1 through the liver X receptor (LXR) (Oppi-Williams et al., 2013; Xu et al., 2019). Specifically, mTOR signaling has been shown to activate SREBP1 by phosphorylating and inhibiting its negative regulator, insulin-induced gene (INSIG) protein. This results in the translocation of SREBP1 to the nucleus and the upregulation of genes involved in lipid metabolism. Additionally, mTOR signaling has also been shown to regulate the expression of genes involved in milk protein synthesis and secretion in the mammary gland. This is achieved through the activation of various downstream effectors, including the eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and the ribosomal protein S6 kinase (S6K1), which play important roles in regulating translation initiation and elongation. The phosphorylation of S6K and 4E-BP1 by mTOR activates the translation machinery and enhances the synthesis of proteins, including milk proteins such as casein and whey proteins, as well as enzymes involved in milk lipid synthesis. In addition, mTOR signaling also increases the expression of genes involved in milk protein synthesis and lipid metabolism by activating transcription factors such as SREBP1c.
Ribosomal protein L8 (RPL8) is another key gene involved in regulating milk fat synthesis. When RPL8 is knocked down, it reduces the expression of genes involved in lipid synthesis, resulting in decreased milk fat production. RPL8 is a component of the large ribosomal subunit and regulates milk production in the mammary gland through the mTOR signaling pathway (Lou et al., 2014). Specifically, RPL8 interacts with mTOR and enhances its kinase activity, leading to increased phosphorylation of downstream targets such as S6K and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). Previous studies have experimentally proved that RPL8 is a promising candidate gene responsible for milk fat percentage trait in dairy cattle (Jiang L et al., 2010; Jiang et al., 2015; Zheng et al., 2019). Similarly it has been evaluated through genome-wide association analysis (GWAS) that RPL8 has strong association with milk fat percentage in dairy cattle (Jiang L et al., 2010; Jiang et al., 2015). In addition, RPL8 may regulates fatty acid de novo synthesis and is considered promising candidate gene for the milk fat percentage trait in dairy cattle (Liu J. et al., 2017; Zheng et al., 2019).
PPARs are a class of nuclear receptors that play an important role in regulating lipid metabolism and glucose homeostasis. Bolsoni-Lopes et al. (2015) reported that PPARs and their pathways have an important regulatory role in adipocyte differentiation and adipose metabolism. Adipocyte differentiation is the process by which pre-adipocytes develop into mature adipocytes, which are the primary cells responsible for storing excess energy as fat in adipose tissue. Adipose metabolism refers to the processes involved in the uptake, storage, and release of lipids in adipose tissue. Consistently, a study has shown that cell-death-inducing DNA fragmentation factor-α-like effector A (CIDEA) by inhibiting the activity of AMP-activated protein kinase (AMPK) which is followed by enhancement of SREBP1 and PPARs which are important regulators of lipid metabolism in the mammary gland of cows, and are involved in the synthesis of milk fat (Cheng et al., 2022). Furthermore, they investigated the role of CIDEA in regulating de novo fatty acid synthesis in bovine mammary epithelial cells (BMECs). The authors found that CIDEA promotes de novo fatty acid synthesis in BMECs by regulating the AMPK/PPARγ axis and SREBP1 (Cheng et al., 2022). Specifically, CIDEA enhances the synthesis of fatty acids, which are used to produce milk fat. This effect is achieved by inhibiting the activity of AMPK, a cellular energy sensor that inhibits fatty acid synthesis, and promoting the expression of PPARγ. PPARγ is a transcription factor that activates SREBP1, another transcription factor that upregulates the expression of genes involved in fatty acid synthesis. Overall, these findings suggest that CIDEA is an important regulator of milk production in dairy cows, and that targeting the CIDEA-AMPK/PPARγ-SREBP1 pathway could be a potential strategy for improving milk production efficiency. Specifically, inhibiting AMPK activity and promoting the expression of PPARγ and SREBP1 could lead to increased fatty acid synthesis in the mammary gland, resulting in higher milk fat production. In recent studies, it has been found that lithium chloride supplementation significantly promotes milk fat synthesis in cows by regulating the activity of PPARγ, a transcription factor involved in lipid metabolism. Specifically, Zong et al. (2023) demonstrated that lithium chloride inhibits the expression of SOCS2 and SOCS3 proteins through JAK2/STAT5, mTOR, and SREBP1 signaling pathways in mammary epithelial cells, leading to increased PPARγ activity and enhanced fatty acid synthesis. These findings suggest that lithium chloride could be a potential supplement for improving milk fat production in dairy cows.
In separate studies of buffalo and dairy cattle, Liu et al. (2016) and Zhou F. et al. (2021) investigated the role of the PPARγ pathway in regulating lipid metabolism in the mammary gland. They found that the PPARγ pathway positively regulates the gene expression of fatty acid synthase, an enzyme involved in the de novo synthesis of fatty acids from non-lipid sources such as carbohydrates and amino acids. This suggests that the PPARγ pathway may play a positive role in regulating milk fat synthesis in cows via the mTOR signaling pathway. Overall, these studies highlight the complex signaling pathways involved in regulating milk fat synthesis in cows and suggest that targeting specific pathways, such as PPARγ and mTOR, could be a potential strategy for improving milk production efficiency. However, further research is needed to fully understand the mechanisms underlying these pathways and their effects on milk composition and quality. In addition, experimental trials is also recommended to fully understand the complex regulatory mechanisms involved in milk production in dairy cows, and to identify potential therapeutic targets for improving milk production efficiency.
ATPase family AAA-domain containing protein 3A (ATAD3A) is a protein that is encoded by nuclear genes and localizes to the mitochondrial membrane. It plays a crucial role in cellular metabolism and cell growth. ATAD3A has been implicated in the regulation of milk production in dairy cattle by modulating the mTOR signaling pathway. A recent study investigated the expression of ATAD3A in the mammary gland tissue of lactating dairy cows and found a positive correlation between its expression and milk production. The researchers also observed that ATAD3A expression was upregulated in response to mTOR signaling activation, suggesting that it may play a role in regulating milk production through this pathway (Han and Zhang, 2021). A study was conducted to investigate the impact of dietary protein on milk production in dairy cows. The results revealed that an increase in dietary protein resulted in a corresponding increase in milk production. Moreover, it was observed that the rise in milk production was linked with an increase in the expression of a protein known as ATAD3A in the mammary gland tissue. This finding supports the notion that ATAD3A plays a significant role in regulating milk production, as noted by Yang et al. (2021) in their study published in 2021. Additionally, Chen et al. (2018) conducted a study that documented the effect of extracellular amino acids and hormones on ATAD3A expression. The researchers found that these external factors significantly upregulated the expression of ATAD3A, which in turn triggered the phosphorylation of mTOR, SREBP-1c, and cyclin D1. This, in turn, led to an improvement in milk biosynthesis and cell proliferation. Overall, these findings suggest that ATAD3A may play a crucial role in regulating milk production in dairy cows, and external factors such as dietary protein and extracellular amino acids and hormones can influence ATAD3A expression and milk biosynthesis. Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1 (NUCKS1) is a highly phosphorylated nuclear protein that functions as a chromatin modifier and transcriptional regulator in the mammary epithelial cells of cows. The expression of NUCKS1 is regulated by specific amino acids such as methionine and leucine, as well as hormones like estrogen and prolactin. NUCKS1 is positively associated with the activation of various signaling pathways such as the mTOR, SREBP-1c, and cyclin D1. It has been found to be critical for regulating milk protein, milk fat, and lactose synthesis, as well as cell development. A study conducted by Yuan et al. (2019) reported that NUCKS1 expression levels were positively correlated with milk synthesis and proliferation of mammary epithelial cells. Furthermore, a study conducted by Shi et al.(2020) investigated the effects of mTOR signaling inhibition on milk production in dairy cows. They found that blocking the mTOR signaling pathway led to a decrease in milk production, which was associated with a decrease in the expression of ATAD3A in mammary gland tissue. This study provided further evidence for the crucial role of NUCKS1 in regulating milk production through the mTOR pathway.
Kisspeptin-10 (KP-10) is the most effective and potent member of the Kisspeptin peptide family and its treatment increased the phosphorylation levels of mTOR and its downstream targets, including S6K1 and 4EBP1, in BMECs, indicating the activation of the mTOR pathway (Cao et al., 2021). This activation was accompanied by increased milk synthesis in BMECs. Further investigation revealed that KP-10 inhibited the expression and activity of SIRT6, a member of the sirtuin family of NAD-dependent deacetylases that can negatively regulate the mTOR pathway. The authors found that Kp-10 treatment reduced the expression of SIRT6 and increased the acetylation levels of mTOR, leading to the activation of the mTOR pathway and increased milk synthesis in BMECs. Consistently, other studies also focused on the role of KP-10 in promoting BMECs proliferation and milk synthesis through the activation of GPR54 and its downstream signaling pathways (Sun et al., 2017; Li Y et al., 2020). In the 2020 study by Li et al., the authors investigated the mechanism by which KP-10 promotes BMEC proliferation. They found that KP-10 activated GPR54 and its downstream signaling pathways, including the PI3K/Akt/mTOR pathway, leading to increased cell proliferation. Consequently, the 2017 study by Sun et al. focused on the role of KP-10 in inducing β-Casein synthesis in BMECs. The authors found that KP-10 activated GPR54 and its downstream signaling pathways, including the MAPK/ERK and PI3K/Akt/mTOR pathways, leading to increased β-Casein synthesis. Overall, these studies provide insights into the mechanisms by which KP-10 promotes BMECs proliferation and milk synthesis, and highlight the potential of KP-10 as a therapeutic target for improving milk production in dairy cows. However, further research is needed to understand the precise mechanisms involved in this process and to explore the potential side effects of using KP-10 as a milk enhancer. The summary of studies reported the genetic regulations of mTOR signaling pathway to promote milk contents in mammary gland of dairy cattle has been provided in Table 1.
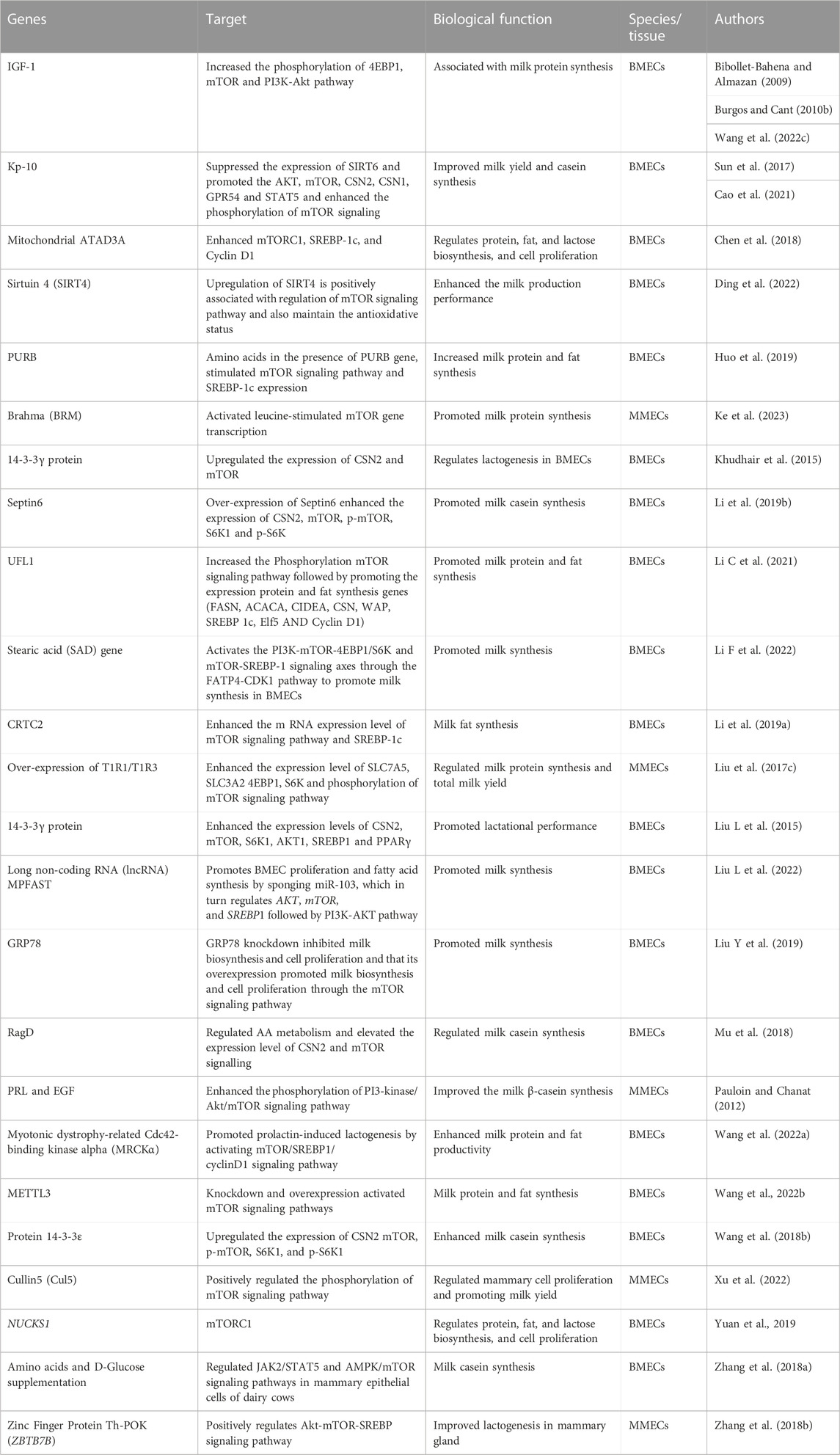
TABLE 1. Genetic regulation of mTOR signaling pathway to promote milk contents in mammary gland of dairy cattle.
3 Factors causing reduction of milk production performance via inhibition of mTOR signaling pathway in dairy cattle
3.1 Genetic factors role in the inhibition of mTOR signaling pathway and reduction of milk production performance in dairy cattle
The genetic factors involved in inhibiting the mTOR signaling pathway and reducing milk production have been summarized in Table 2. It has been found that over-expression of certain genes can suppress milk yield, milk fat, and protein synthesis by blocking the activation of the mTOR signaling pathway. Most of these genes are involved in inhibiting the stimulation of amino acids on mTOR gene transcription (Huang and Fingar, 2014; Lin G et al., 2023). For instance, ARID4B, a member of the DNA binding AT-rich interactive domain (ARID) family, has been shown to attach to the promoter region of mTOR and block the attachment of methionine, which can result in the suppression of milk protein synthesis (Lin G et al., 2023). Another study reported that ARID1A inhibits milk protein synthesis in the mammary gland by impairing the signaling from amino acids (Met and Leu) to the mTOR pathway (Qi et al., 2022).
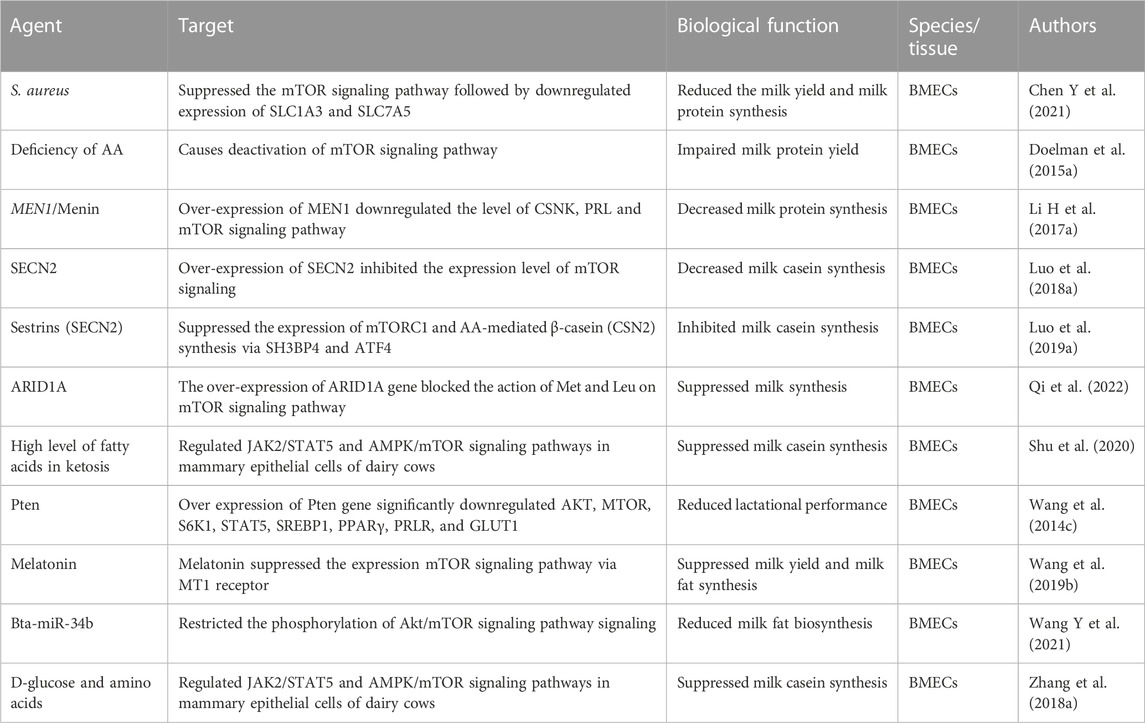
TABLE 2. Summary of factors (genetic, nutritional and environmental) causing inhibition of mTORC signaling pathways and consequent low milk production performance in dairy cattle.
MEN1 is a key gene encoding protein menin that was documented to have a role in milk protein synthesis in mammary epithelial cells (Li H et al., 2017a). Furthermore, they documented that the over expression of menin was negatively correlated with κ-casein (CSNK), prolactin (PRL) and/or insulin (INS) and followed by downregulation of mTOR signaling resulting in lower milk protein synthesis in mammary epithelial cells (Li H et al., 2017a). Similarly, another study found that melatonin significantly compromised the milk fat synthesis by inhibiting the regulation of mTOR signaling in mammary epithelial cells (Wang Y. et al., 2019). Overall, the findings revealed that menin protein interact with mTOR and its downstream signaling components in mammary epithelial cells. This interaction leads to the inhibition of mTOR signaling pathway, which is a crucial regulator of milk protein synthesis in these cells. The overexpression of menin protein in mammary epithelial cells has been associated with a decrease in the expression of κ-casein, prolactin, and insulin, which are essential factors for milk protein synthesis. This downregulation of these factors leads to a decrease in the activation of mTOR signaling, leading to a reduction in milk protein synthesis.
Melatonin has been shown to inhibit mTOR signaling in mammary epithelial cells, leading to a reduction in milk protein and fat synthesis (Wang Y. et al., 2019). This suggests that melatonin may have an inhibitory effect on milk production in dairy cows. Furthermore, the inhibitory effect of melatonin on milk fat synthesis was found to be mediated by the MT1 receptor, which is a receptor for melatonin (Wang Y. et al., 2019). One possible explanation for this effect is that melatonin regulates the expression of genes involved in milk synthesis and secretion by modulating mTOR signaling. Another possibility is that melatonin affects the secretion of other hormones, such as prolactin, which is an essential regulator of milk production in dairy cows. In addition, melatonin may reduce milk production in dairy cows by inhibiting mTOR signaling in mammary epithelial cells, which leads to a decrease in milk protein and fat synthesis. Further research is needed to fully understand the mechanisms underlying the effects of melatonin on milk production in dairy cows.
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression post-transcriptionally by binding to the 3′untranslated region (UTR) of target mRNAs. miRNAs have been shown to play important roles in regulating milk synthesis in mammary epithelial cells (Wang Y et al., 2021). One mechanism by which miRNAs can reduce milk production is through the mTOR signaling pathway. Specifically, miRNAs can inhibit the expression of key genes (PPARγ, FASN, Akt, mTOR, IGF2, Raptor, S6K1 and TNS1) which are involved in the activation of mTOR pathway. This inhibition can lead to a decrease in milk protein synthesis and a reduction in milk production (Wang Y et al., 2021). Consequently, a study by Wang M et al. (2021) investigated the effect of lipopolysaccharide (LPS) on triglyceride synthesis in dairy cow mammary epithelial cells. They found that LPS can inhibit triglyceride synthesis by upregulating microRNA-27a-3p (miR-27a-3p), which targets the peroxisome proliferator-activated receptor gamma (PPARG) gene. PPARG is a transcription factor that plays a critical role in regulating milk fat synthesis in mammary epithelial cells. Recent studies have suggested that the inhibitory effect of lipopolysaccharide (LPS) on triglyceride synthesis may be mediated by miR-27a-3p, which targets and downregulates PPARG expression (Wang M et al., 2021). This suggests that miR-27a-3p may play a crucial role in regulating milk fat synthesis under conditions of inflammation or stress. Similarly, microRNA-432 has also been identified as a regulator of milk fat synthesis in ovine mammary epithelial cells (Hao et al., 2021). The authors found that microRNA-432 inhibits milk fat synthesis by targeting SCD and lipoprotein lipase (LPL), which are essential for fatty acid synthesis and uptake, respectively. These findings suggest that microRNA-432 could be a potential target for improving milk composition in dairy animals.
Interestingly, it has been noticed that high fatty acids supplementation in dairy cattle during periparturient period also compromised the milk casein synthesis by suppressing the mTOR activity in mammary gland (Shu et al., 2020). Furthermore, they documented that high fatty acids attenuated the positive impact of methionine and prolactin on mTOR signaling which result in reduction of milk casein synthesis (Shu et al., 2020). Consistently Zhang et al. (2018a) also observed that deficiency of d-glucose and amino acids were associated with declining activity of mTOR, stat5a, 4EBP1 and S6K1 in mammary gland. Furthermore, they found that the synthesis of milk protein was significantly compromised in bovine mammary epithelial cells.
3.2 Heat stress compromise dairy cattle milk production performance via reduced phosphorylation of mTOR signaling
Hyperthermia or heat stress is a common stressor that dairy animals may experience during hot summer months, which can negatively impact their production performance. Recent studies have investigated the effects of heat stress on the mTOR signaling pathway and its link with milk synthesis in dairy animals. Heat stress has been shown to decrease the expression levels of mTOR signaling pathway-linked genes, such as ribosomal protein S6 (RPS6), AKT serine/threonine kinase 1 (AKT1), and ribosomal protein S6 kinase B1 (RPS6KB1), in bovine mammary epithelial cells (Zhou J et al., 2022). The downregulation of these genes leads to the suppression of mTOR signaling, which can have a negative impact on milk synthesis in the mammary gland of dairy animals. Furthermore, in goat mammary glands, the phosphorylation of several proteins, including mTORC1, was found to be upregulated in response to lactation-related signaling pathways such as calcium signaling and oxytocin signaling pathways (Zhu C et al., 2021). These findings suggest that these signaling pathways play a key role in the regulation of lactation in dairy animals. Fu L et al. (2021) discovered that heat stress can have a negative impact on milk production in bovine mammary epithelial cells (BMECs) by suppressing the expression of genes associated with the mTORC1 signaling pathway, including Ras homolog enriched in the brain (Rheb), AKT, eIF4E, and eEF2K. This finding was further supported by Guo Z et al. (2021). In addition; Ding Q et al. (2022) reported a significant decrease in the expression of the SIRT4 gene in response to heat stress, which was associated with increased oxidative stress and decreased phosphorylation levels of the mTORC1 signaling pathway. As a result, the reduced expression of SIRT4 gene due to oxidative stress was found to be the main factor responsible for decreased milk protein synthesis and milk yield in BMECs. In conclusion, these studies suggest that heat stress can downregulate the expression of genes associated with the mTORC1 signaling pathway, leading to poor milk production performance in BMECs (Table 2).
In summary, we concluded that not only genetic factors but both environmental and nutritional management practices can also affect mTOR signaling and enhance milk or fat synthesis. Here are some practices that can support milk or fat synthesis through mTOR signaling pathway.
Feeding high-quality protein is an essential nutrient required for milk synthesis, and feeding high-quality protein sources can stimulate mTOR signaling and enhance milk production. The amino acid leucine, in particular, is known to activate mTOR signaling and promote milk synthesis. Providing adequate energy: Adequate energy intake is crucial for milk synthesis, and underfeeding can suppress mTOR signaling and reduce milk production. Ensuring that lactating animals receive enough energy through their diet can support milk or fat synthesis.
Optimizing feeding frequency and timing (i.e., 3–4 times/day) can increase mTOR signaling and enhance milk synthesis compared to infrequent feeding (i.e., 1–2 times/day). Additionally, feeding before or after milking can affect milk production with some evidence, suggesting that feeding after milking can improve milk yield.
Providing a comfortable environment is needed because environmental stressors, such as heat or cold stress, can suppress mTOR signaling and reduce milk production. Providing a comfortable environment that minimizes stress can help support milk or fat synthesis. It is important to note that the specific management practices that enhance milk or fat synthesis through the mTOR signaling pathway may vary depending on the animal species, breed, and individual characteristics.
4 Amino acids supplementation regulates milk production via mTOR signaling pathway
The positive role of nutrition in improvement of milk production performance in ruminants has been extensively discussed in a recent study (Abdelrahman et al., 2022). Consistently it is well-established that amino acids are taken up from the bloodstream into the mammary gland cells by specific transporters. Amino acids act as a signal to activate the mTOR pathway by binding to intracellular sensors, such as the Rag GTPases and the leucine sensor Sestrin2. The activated mTOR pathway leads to the formation of the mTORC1, which is a key regulator of protein synthesis and cell growth. Furthermore, mTORC1 activation leads to the phosphorylation of downstream targets, such as the S6K and 4EBP, which in turn activate milk synthesis in mammary glands of dairy cattle.
Several studies have identified amino acids as important supplements for regulating cell proliferation, protein synthesis, and other physiological processes through the mTORC1 signaling pathway (Sancak et al., 2008; Sengupta et al., 2010; Lei et al., 2012; Wang F. et al., 2019). In the presence of sufficient amino acid supply, mTORC1 is activated and translocates to the lysosomal surface, where it triggers the phosphorylation of mTOR (Sancak et al., 2008). In vitro studies have shown that the supplementation of essential amino acids (EAA) can effectively regulate milk protein synthesis in BMECs through the activation of the mTOR signaling pathway (Toerien et al., 2010; Appuhamy et al., 2011; Appuhamy et al. 2012; Appuhamy et al. 2014). These studies demonstrated that EAA supplementation can increase the phosphorylation of mTOR, S6K1, eIF4E, 4EBP1, and insulin receptor substrate 1 (IRS1), leading to enhanced milk protein synthesis in BMECs. Furthermore, insulin treatment has also been found to enhance the phosphorylation of mTOR, Akt, S6K1, 4EBP1, and IRS1, indicating the importance of insulin signaling in regulating milk protein synthesis (Appuhamy et al., 2011; Appuhamy et al. 2012; Appuhamy et al. 2014).
The mammary gland utilizes amino acids as substrates for milk synthesis through various signaling pathways, including the mTORC1 (Appuhamy et al., 2014). Amino acids such as leucine, isoleucine, methionine, and threonine have been shown to function as signaling molecules that favorably control milk synthesis and lactation in earlier investigations on cows and mice (Trottier, 2014). As upstream activators of mTORC1, amino acid sensors, such as vacuolar H+-ATPase, SLC38A9, Sestrin2, CASTOR1 homodimer, and CASTOR1-CASTOR2, are thought to regulate milk production and lactation (Stransky and Forgac, 2015; Chantranupong et al., 2016; Gai et al., 2016). The mTOR pathway regulates its targets after activation to facilitate milk protein synthesis (Gao et al., 2015). Previous studies have demonstrated that high temperature (42°C) incubation of bovine mammary cells inhibited protein translation by decreasing the activity of the mTOR downstream pathway (Kaufman et al., 2018; Salama et al., 2019). Additionally, Nan et al. (2014) experimentally demonstrated that a 2.9:1 ratio of Lys: Met supply positively regulates the in vitro synthesis of casein by targeting mTORC1 signaling in bovine mammary epithelial cells. Similarly, another study found that methionine and arginine supply could significantly contribute to milk protein synthesis in bovine mammary epithelial cells via the mTORC1 pathway (Hu et al., 2020). They documented that an increased supply of methionine and arginine upregulated mTORC1 signaling by targeting the SLC7A1 gene. Consistently, in vitro findings have shown that amino acid supplementation enhances the phosphorylation of the mTORC1 signaling pathway, which further regulates the synthesis of milk protein in mammary gland cells (Appuhamy et al., 2011). In addition, the mammary epithelial cells were treated with a mixture of essential amino acids, and the rate of milk protein synthesis was measured. The researchers observed that amino acid treatment enhanced the phosphorylation of mTORC1 and downstream targets, such as S6K1 and 4EBP1, leading to increased milk protein synthesis (Appuhamy et al., 2011). In summary, these studies suggest that amino acids, including leucine, isoleucine, methionine, and threonine, play important roles in regulating milk protein synthesis via the mTORC1 signaling pathway in mammary epithelial cells. Additionally, amino acid sensors and gene regulators such as SLC7A1 play key roles in controlling milk production and lactation.
Studies have shown that amino acid treatment can enhance mammary protein synthesis rate and phosphorylation of mTORC1 in mammary epithelial cells. In particular, methionine and valine have been found to activate mTORC1 via the heterodimeric amino acid taste receptor TAS1R1/TAS1R3 and intracellular Ca2+ in bovine mammary epithelial cells, further facilitating milk synthesis (Zhou Y et al., 2018). In a study conducted by Zhou Y et al. (2018), it was found that knockdown of TAS1R1 and TAS1R3 significantly reduced the phosphorylation of mTORC1 and lowered the biosynthesis of β-casein in bovine mammary epithelial cells. This indicates that TAS1R1/TAS1R3 regulates the extracellular amino acids and mTORC1 pathway, resulting in elevated levels of Ca2+ concentration, β-casein, and protein in bovine mammary epithelial cells. These findings suggest that the TAS1R1/TAS1R3 receptor plays a critical role in regulating the mTORC1 pathway and milk synthesis in dairy cows. However, further research is needed to fully understand the complex interplay between amino acid receptors, the mTOR pathway, and other regulatory factors that may influence milk synthesis in mammary epithelial cells. A deficiency of amino acids and D-glucose has been found to significantly reduce the phosphorylation of mTORC1, AMP-activated protein kinase (AMPK), and the Janus kinase (Jak)-signal transducer and activator of transcription (Stat) signaling pathway in bovine mammary epithelial cells, as demonstrated in a study by Zhang et al. (2018a). For ease, the summary of studies evaluated the effect of amino acids supplementation on milk production performance in dairy cattle mammary epithelial cells by regulation mTORC signaling pathway has been highlighted in Table 3.
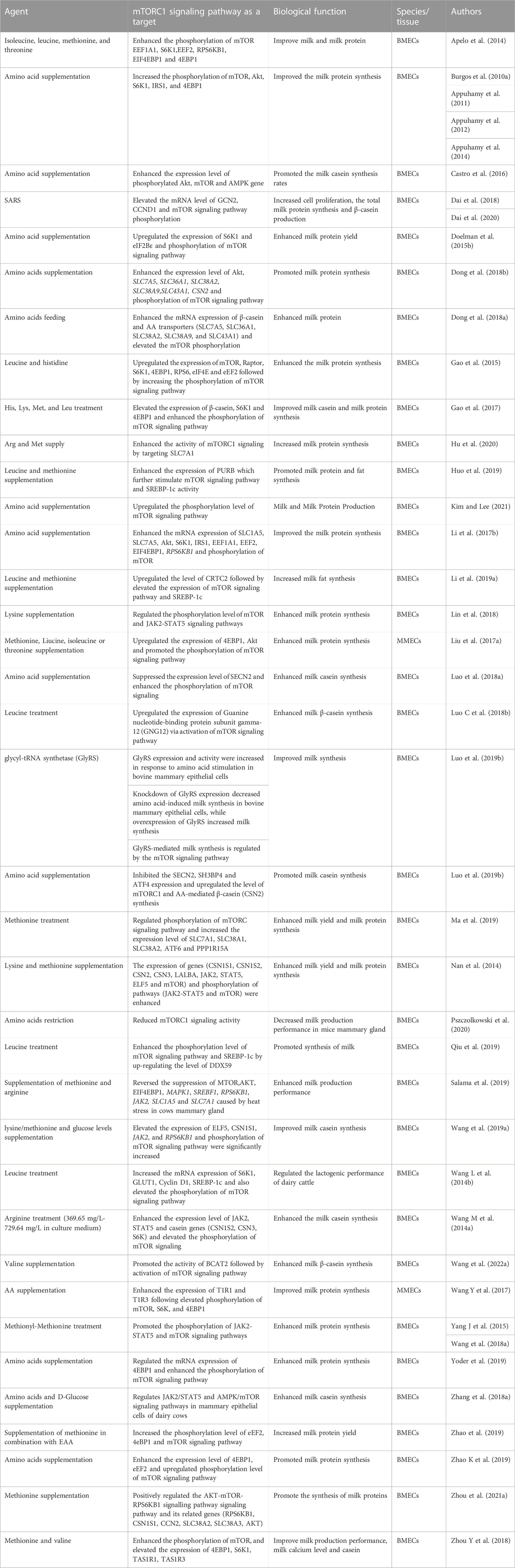
TABLE 3. Summary of studies on amino acids supplementation effect on bovine milk production performance via regulation of mTORC signaling pathway in dairy cattle.
5 Conclusion
The mTOR signaling pathway is a crucial regulatory pathway in cells that plays an essential role in controlling various cellular processes such as protein synthesis, cell growth, and proliferation. It is a conserved pathway that is activated by multiple signals such as growth factors, amino acids, energy status, and stress. The process of milk biosynthesis in cow mammary epithelial cells is dependent upon a consistent supply of energy and nutrients, specifically in the form of amino acids. As the fundamental building blocks of proteins, amino acids play a critical role in the regulation of the mTOR pathway. Through the activation of key upstream regulators including mTOR, S6K1, eIF4E, 4EBP1, FASN, Akt, IGF2, Raptor, Sestrin2, CASTOR1, CASTOR2, S6K1, TNS1, and Rheb, amino acids stimulate the mTORC1 complex, ultimately driving milk biosynthesis. Environmental factors such as heat stress inhibit the activity of mTORC1 by reducing the levels of amino acids resulting in poor milk production performance. Understanding the mechanism of mTOR regulation by amino acids and heat stress can help to develop strategies to enhance milk production in cows under heat stress conditions.
Author contributions
BL, MK, MZ, IK, and NJ designed this study. The manuscript is supervised and wrote by BL, MK, MZ, IK, and NJ. In addition, BL, Z-MC, QU, AK, BH, NZ, DW, and YM collected the data sources, review and edit the final version of the manuscript. The article is finally visualized by MZ and MK. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by Key research and development Plan Projects in Tibet Autonomous Region Project XZ202201ZY0004N.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CCND1, Seryl-tRNA synthetase (SARS), cyclin D1; 4EBP1, eukaryotic initiation factor 4E binding protein 1; eEF2, elongation factor 2; TAS1R1/TAS1R3, AA taste 1 receptor member 1/3; mTOR, mechanistic target of rapamycin; AKT, eukaryotic initiation factor 2a, serine-threonine protein kinase; EIF4EBP1, 4E binding protein 1; MMECs, Mouse mammary epithelial cells; BCAT2, branched-chain aminotransferase transaminase 2; EAA, Essential amino acid; ATAD3A, ATPase family AAA-domain containing protein 3A; NUCKS1, Nuclear ubiquitous casein and cyclin-dependent kinase substrate 1; BMECs, bovine mammary epithelial cells; (IGF)-1, insulin-like growth factor; PRL, Kisspeptin-10 (Kp-10), Prolactin; EGF, Epidermal growth factor; MMECs, mouse mammary epithelial cells; GLUT1, Zinc Finger and BTB Domain Containing 7B, glucose transporter 1; UFL1, Ufmylation; PURB, purine-rich element binding protein B; CRTC2, cAMP response element-binding protein-regulated transcription coactivator 2; METTL3, Methyltransferase-like 3; GRP78, Glucose-Regulated Protein 78; CSN2, AA-mediated β-casein; SESN2, Sestrin2; SH3BP4, SH3 domain-binding protein 4; ATF4, Activating transcription factor 4; CSNK, milk protein κ-casein; ARID1A, AT-rich interaction domain 1A.
References
Abdelrahman, M., Wang, W., Shaukat, A., Kulyar, M. F., Lv, H., Abulaiti, A., et al. (2022). Nutritional modulation, gut, and omics crosstalk in ruminants. Animals 12 (8), 997. doi:10.3390/ani12080997
Apelo, S. A., Singer, L. M., Lin, X. Y., McGilliard, M. L., St-Pierre, N. R., and Hanigan, M. D. (2014). Isoleucine, leucine, methionine, and threonine effects on mammalian target of rapamycin signaling in mammary tissue. J. Dairy Sci. 97 (2), 1047–1056. doi:10.3168/jds.2013-7348
Appuhamy, J. A., Nayananjalie, W. A., England, E. M., Gerrard, D. E., Akers, R. M., and Hanigan, M. D. (2014). Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J. Dairy Sci. 97 (1), 419–429. doi:10.3168/jds.2013-7189
Appuhamy, J. R., Bell, A. L., Nayananjalie, W. D., Escobar, J., and Hanigan, M. D. (2011). Essential amino acids regulate both initiation and elongation of mRNA translation independent of insulin in MAC-T cells and bovine mammary tissue slices. J. Nutr. 141 (6), 1209–1215. doi:10.3945/jn.110.136143
Appuhamy, J. R., Knoebel, N. A., Nayananjalie, W. D., Escobar, J., and Hanigan, M. D. (2012). Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J. Nutr. 142 (3), 484–491. doi:10.3945/jn.111.152595
Bauman, D. E., Mather, I. H., Wall, R. J., and Lock, A. L. (2006). Major advances associated with the biosynthesis of milk. J. Dairy Sci. 89 (4), 1235–1243. doi:10.3168/jds.S0022-0302(06)72192-0
Bibollet-Bahena, O., and Almazan, G. (2009). IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J. Neurochem. 109 (5), 1440–1451. doi:10.1111/j.1471-4159.2009.06071.x
Bolsoni-Lopes, A., Deshaies, Y., and Festuccia, W. T. (2015). Regulation of Brown adipose tissue recruitment, metabolism and thermogenic function by peroxisome proliferator-activated receptor γ. Temp. (Austin) 19, 476–482. doi:10.1080/23328940.2015.1011564
Burgos, S. A., and Cant, J. P. (2010b). IGF-1 stimulates protein synthesis by enhanced signaling through mTORC1 in bovine mammary epithelial cells. Domest. Anim. Endocrinol. 38 (4), 211–221. doi:10.1016/j.domaniend.2009.10.005
Burgos, S. A., Dai, M., and Cant, J. P. (2010a). Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. J. Dairy Sci. 93 (1), 153–161. doi:10.3168/jds.2009-2444
Cao, Y., Hu, G., Zhang, Q., Ma, L., Wang, J., Li, W., et al. (2021). Kisspeptin-10 maintains the activation of the mTOR signaling pathway by inhibiting SIRT6 to promote the synthesis of milk in bovine mammary epithelial cells. J. Agric. Food Chem. 69 (14), 4093–4100. doi:10.1021/acs.jafc.0c07613
Castro, J. J., Apelo, S. A., Appuhamy, J. A., and Hanigan, M. D. (2016). Development of a model describing regulation of casein synthesis by the mammalian target of rapamycin (mTOR) signaling pathway in response to insulin, amino acids, and acetate. J. Dairy Sci. 99 (8), 6714–6736. doi:10.3168/jds.2015-10591
Chantranupong, L., Scaria, S. M., Saxton, R. A., Gygi, M. P., Shen, K., Wyant, G. A., et al. (2016). The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165 (1), 153–164. doi:10.1016/j.cell.2016.02.035
Che, L., Xu, M., Gao, K., Zhu, C., Wang, L., Yang, X., et al. (2019). Valine increases milk fat synthesis in mammary gland of gilts through stimulating AKT/MTOR/SREBP1 pathway. Biol. Reproduction 101, 126–137. doi:10.1093/biolre/ioz065
Chen, D., Yuan, X., Liu, L., Zhang, M., Qu, B., Zhen, Z., et al. (2018). Dual effects of metformin on adipogenic differentiation of 3T3-L1 preadipocyte in AMPK-dependent and independent manners. Cell Biol. Int. 42 (6), 1547–1556. doi:10.3390/ijms19061547
Chen, Y., Ma, Y., Ji, Q., Yang, X., Feng, X., Yao, R., et al. (2021). Intracellular Staphylococcus aureus infection decreases milk protein synthesis by preventing amino acid uptake in bovine mammary epithelial cells. Front. Veterinary Sci. 8, 756375. doi:10.3389/fvets.2021.756375
Cheng, J., Xu, D., Chen, L., Guo, W., Hu, G., Liu, J., et al. (2022). CIDEA regulates de novo fatty acid synthesis in bovine mammary epithelial cells by targeting the AMPK/PPARγ Axis and regulating SREBP1. J. Agric. Food Chem. 70 (36), 11324–11335. doi:10.1021/acs.jafc.2c05226
Dai, W. T., White, R. R., Liu, J. X., and Liu, H. Y. (2018). Seryl-tRNA synthetase-mediated essential amino acids regulate β-casein synthesis via cell proliferation and mammalian target of rapamycin (mTOR) signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 101 (11), 10456–10468. doi:10.3168/jds.2018-14568
Dai, W., Zhao, F., Liu, J., and Liu, H. (2020). Seryl-tRNA synthetase is involved in methionine stimulation of β-casein synthesis in bovine mammary epithelial cells. Br. J. Nutr. 123 (5), 489–498. doi:10.1017/S0007114519002885
Ding, Q., Wang, Y., Xia, S-W., Zhao, F., Zhong, J-F., Wang, H-L., et al. (2022). SIRT4 expression ameliorates the detrimental effect of heat stress via AMPK/mTOR signaling pathway in BMECs. Int. J. Mol. Sci. 23 (3), 13307. doi:10.3390/ijms232113307
Doelman, J., Curtis, R. V., Carson, M., Kim, J. J., Metcalf, J. A., and Cant, J. P. (2015a). Essential amino acid infusions stimulate mammary expression of eukaryotic initiation factor 2Bε but milk protein yield is not increased during an imbalance. J. Dairy Sci. 98 (7), 4499–4508. doi:10.3168/jds.2014-9051
Doelman, J., Kim, J. J., Carson, M., Metcalf, J. A., and Cant, J. P. (2015b). Branched-chain amino acid and lysine deficiencies exert different effects on mammary translational regulation. J. Dairy Sci. 98 (11), 7846–7855. doi:10.3168/jds.2015-9819
Dong, X., Zhou, Z., Wang, L., Saremi, B., Helmbrecht, A., Wang, Z., et al. (2018a). Increasing the availability of threonine, isoleucine, valine, and leucine relative to lysine while maintaining an ideal ratio of lysine: methionine alters mammary cellular metabolites, mammalian target of rapamycin signaling, and gene transcription. J. Dairy Sci. 101 (6), 5502–5514. doi:10.3168/jds.2017-13707
Dong, X., Zhou, Z., Saremi, B., Helmbrecht, A., Wang, Z., and Loor, J. J. (2018b). Varying the ratio of Lys: met while maintaining the ratios of Thr: phe, Lys: thr, Lys: his, and Lys: val alters mammary cellular metabolites, mammalian target of rapamycin signaling, and gene transcription. J. Dairy Sci. 101 (3), 1708–1718. doi:10.3168/jds.2017-13351
Fu, L., Zhang, L., Liu, L., Yang, H., Zhou, P., Song, F., et al. (2021). Effect of heat stress on bovine mammary cellular metabolites and gene transcription related to amino acid metabolism, amino acid transportation and mammalian target of rapamycin (mTOR) signaling. Animals 11, 3153. doi:10.3390/ani11113153
Gai, Z., Wang, Q., Yang, C., Wang, L., Deng, W., and Wu, G. (2016). Structural mechanism for the arginine sensing and regulation of CASTOR1 in the mTORC1 signaling pathway. Cell Discov. 2 (1), 16051–16114. doi:10.1038/celldisc.2016.51
Gao, H. N., Zhao, S. G., Zheng, N., Zhang, Y. D., Wang, S. S., Zhou, X. Q., et al. (2017). Combination of histidine, lysine, methionine, and leucine promotes β-casein synthesis via the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 100 (9), 7696–7709. doi:10.3168/jds.2015-10729
Gao, H. N., Hu, H., Zheng, N., and Wang, J. Q. (2015). Leucine and histidine independently regulate milk protein synthesis in bovine mammary epithelial cells via mTOR signaling pathway. J. Zhejiang University-Science B 16 (7), 560–572. doi:10.1631/jzus.B1400337
Gordon, B. S., Kazi, A. A., Coleman, C. S., Dennis, M. D., Chau, V., Jefferson, L. S., et al. (2014). RhoA modulates signaling through the mechanistic target of rapamycin complex 1 (mTORC1) in mammalian cells. Cell. Signal. 26 (3), 461–467. doi:10.1016/j.cellsig.2013.11.035
Guo, Z., Gao, S., Ouyang, J., Ma, L., and Bu, D. (2021). Impacts of heat stress-induced oxidative stress on the milk protein biosynthesis of dairy cows. Animals 11, 726. doi:10.3390/ani11030726
Han, M., and Zhang, M. (2021). The regulatory mechanism of amino acids on milk protein and fat synthesis in mammary epithelial cells: a mini review. Anim. Biotechnol. 32 (4), 402–412. doi:10.1080/10495398.2021.1950743
Hanigan, M. D., France, J., Mabjeesh, S. J., McNabb, W. C., and Bequette, B. J. (2009). High rates of mammary tissue protein turnover in lactating goats are energetically costly. J. Nutr. 139, 1118–1127. doi:10.3945/jn.108.103002
Hayashi, A. A., Nones, K., Roy, N. C., McNabb, W. C., Mackenzie, D. S., Pacheco, D., et al. (2009). Initiation and elongation steps of mRNA translation are involved in the increase in milk protein yield caused by growth hormone administration during lactation. J. Dairy Sci. 92, 1889–1899. doi:10.3168/jds.2008-1334
He, Q., Luo, J., Wu, J., Li, Z., Yao, W., Zang, S., et al. (2021). ELOVL6 promoter binding sites directly targeted by sterol regulatory element binding protein 1 in fatty acid synthesis of goat mammary epithelial cells. J. Dairy Sci. 104, 6253–6266. doi:10.3168/jds.2020-19292
Hu, L., Chen, Y., Cortes, I. M., Coleman, D. N., Dai, H., Liang, Y., et al. (2020). Supply of methionine and arginine alters phosphorylation of mechanistic target of rapamycin (mTOR), circadian clock proteins, and α-s1-casein abundance in bovine mammary epithelial cells. Food Funct. 11, 883–894. doi:10.1039/c9fo02379h
Huang, K., and Fingar, D. C. (2014). Growing knowledge of the mTOR signaling network. Seminars Cell & Dev. Biol. 36, 79–90. doi:10.1016/j.semcdb.2014.09.011
Huo, N., Yu, M., Li, X., Zhou, C., Jin, X., and Gao, X. (2019). PURB is a positive regulator of amino acid-induced milk synthesis in bovine mammary epithelial cells. J. Cell. Physiology 234 (5), 6992–7003. doi:10.1002/jcp.27452
Jewell, J. L., and Guan, K. L. (2013b). Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 38 (5), 233–242. doi:10.1016/j.tibs.2013.01.004
Jewell, J. L., Russell, R. C., and Guan, K. L. (2013a). Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 14 (3), 133–139. doi:10.1038/nrm3522
Jiang, L., Liu, J., Sun, D., Ma, P., Ding, X., Yu, Y., et al. (2010). Genome wide association studies for milk production traits in Chinese Holstein population. PLoS One 5, e13661. doi:10.1371/journal.pone.0013661
Jiang, N., Hu, L., Liu, C., Gao, X., and Zheng, S. (2015). 60S ribosomal protein L35 regulates β-casein translational elongation and secretion in bovine mammary epithelial cells. Archives Biochem. Biophysics 583, 130–139. doi:10.1016/j.abb.2015.08.006
Kaufman, J. D., Kassube, K. R., Almeida, R. A., and Ríus, A. G. (2018). Short communication: high incubation temperature in bovine mammary epithelial cells reduced the activity of the mTOR signaling pathway. J. Dairy Sci. 101 (8), 7480–7486. doi:10.3168/jds.2017-13958
Ke, C., Zhao, S., Wang, L., Zhang, M., and Gao, X. (2023). Chromatin remodeler BRM is a key mediator of leucine-stimulated mTOR gene transcription in mouse mammary epithelial cells. Biochem. Biophysical Res. Commun. 643, 88–95. doi:10.1016/j.bbrc.2022.12.064
Khudhair, N., Luo, C., Khalid, A., Zhang, L., Zhang, S., Ao, J., et al. (2015). 14-3-3γ affects mTOR pathway and regulates lactogenesis in dairy cow mammary epithelial cells. In VitroCell. Dev. Biology-Animal. 51, 697–704. doi:10.1007/s11626-015-9879-x
Kim, J. E., and Lee, H. G. (2021). Amino acids supplementation for the milk and milk protein production of dairy cows. Animals 11 (7), 2118. doi:10.3390/ani11072118
Lei, J., Feng, D., Zhang, Y., Zhao, F. Q., Wu, Z., San Gabriel, A., et al. (2012). Nutritional and regulatory role of branched-chain amino acids in lactation. Front. Bioscience-Landmark 17 (7), 2725–2739. doi:10.2741/4082
Li, B., Basang, Z., Hu, L., Liu, L., and Jiang, N. (2019b). Septin6 regulates cell growth and casein synthesis in dairy cow mammary epithelial cells via mTORC1 pathway. J. Dairy Res. 86(2), 181–187. doi:10.1017/S0022029919000268
Li, C., Li, L., Ali, I., Kuang, M., Wang, X., and Wang, G. (2021). UFL1 regulates milk protein and fat synthesis–related gene expression of bovine mammary epithelial cells probably via the mTOR signaling pathway. Vitro Cell. Dev. Biology-Animal 57 (5), 550–559. doi:10.1007/s11626-021-00587-1
Li, F., Hu, G., Long, X., Cao, Y., Li, Q., Guo, W., et al. (2022). Stearic acid activates the PI3K-mTOR-4EBP1/S6K and mTOR-SREBP-1 signaling axes through FATP4-CDK1 to promote milk synthesis in primary bovine mammary epithelial cells. J. Agric. Food Chem. 70 (13), 4007–4018. doi:10.1021/acs.jafc.2c00208
Li, H., Liu, X., Wang, Z., Lin, X., Yan, Z., Cao, Q., et al. (2017a). MEN1/Menin regulates milk protein synthesis through mTOR signaling in mammary epithelial cells. Sci. Rep. 7, 5479. doi:10.1038/s41598-017-06054-w
Li, N., Zhao, F., Wei, C., Liang, M., Zhang, N., Wang, C., et al. (2014). Function of SREBP1 in the milk fat synthesis of dairy cow mammary epithelial cells. Int. J. Mol. Sci. 15, 16998–17013. doi:10.3390/ijms150916998
Li, P., Zhou, C., Li, X., Yu, M., Li, M., and Gao, X. (2019a). CRTC2 is a key mediator of amino acid-induced milk fat synthesis in mammary epithelial cells. J. Agric. Food Chem. 67 (37), 10513–10520. doi:10.1021/acs.jafc.9b04648
Li, S., Loor, J. J., Liu, H. Y., Liu, L., Hosseini, A., Zhao, W. S., et al. (2017b). Optimal ratios of essential amino acids stimulate β-casein synthesis via activation of the mammalian target of rapamycin signaling pathway in MAC-T cells and bovine mammary tissue explants. J. Dairy Sci. 100, 6676–6688. doi:10.3168/jds.2017-12681
Li, Y., Cao, Y., Wang, J., Fu, S., Cheng, J., Ma, L., et al. (2020). Kp-10 promotes bovine mammary epithelial cell proliferation by activating GPR54 and its downstream signaling pathways. J. Cell. Physiology 235 (5), 4481–4493. doi:10.1002/jcp.29325
Lin, G., Qi, H., Guo, X., Wang, W., Zhang, M., and Gao, X. (2023). ARID1B blocks methionine-stimulated mTOR activation to inhibit milk fat and protein synthesis in and proliferation of mouse mammary epithelial cells. J. Nutr. Biochem. 114, 109274. doi:10.1016/j.jnutbio.2023.109274
Lin, X., Li, S., Zou, Y., Zhao, F. Q., Liu, J., and Liu, H. (2018). Lysine stimulates protein synthesis by promoting the expression of ATB0,+ and activating the mTOR pathway in bovine mammary epithelial cells. J. Nutr. 148 (9), 1426–1433. doi:10.1093/jn/nxy140
Liu, G. M., Hanigan, M. D., Lin, X. Y., Zhao, K., Jiang, F. G., White, R. R., et al. (2017a). Methionine, leucine, isoleucine, or threonine effects on mammary cell signaling and pup growth in lactating mice. J. Dairy Sci. 100 (5), 4038–4050. doi:10.3168/jds.2016-11973
Liu, J., Wang, Y., Li, D., Wang, Y., Li, M., Chen, C., et al. (2017b). Milk protein synthesis is regulated by T1R1/T1R3, a G protein-coupled taste receptor, through the mTOR pathway in the mouse mammary gland. Mol. Nutr. Food Res. 61 (9), 1601017. doi:10.1002/mnfr.201601017
Liu, L., Lin, Y., Liu, L., Bian, Y., Zhang, L., Gao, X., et al. (2015). 14-3-3γ regulates lipopolysaccharide-induced inflammatory responses and lactation in dairy cow mammary epithelial cells by inhibiting NF-κB and MAPKs and up-regulating mTOR signaling. Int. J. Mol. Sci. 16 (7), 16622–16641. doi:10.3390/ijms160716622
Liu, L., Sun, B., Zhang, F., Zhong, Z., Zhang, Y., Li, F., et al. (2022). lncRNA MPFAST promotes proliferation and fatty acid synthesis of bovine mammary epithelial cell by sponging miR-103 regulating PI3K-AKT pathway. J. Agric. Food Chem. 70 (38), 12004–12013. doi:10.1021/acs.jafc.2c04789
Liu, L., Lin, Y., Liu, L., Wang, L., Bian, Y., Gao, X., et al. (2016). Regulation of peroxisome proliferator-activated receptor gamma on milk fat synthesis in dairy cow mammary epithelial cells. In VitroCell. Dev. Biology-Animal. 52, 1044–1059. doi:10.1007/s11626-016-0059-4
Liu, X., Shen, J., Zong, J., Liu, J., and Jin, Y. (2021). Beta-sitosterol promotes milk protein and fat syntheses-related genes in bovine mammary epithelial cells. Animals 11, 3238. doi:10.3390/ani11113238
Liu, X., Yang, J., Zhang, Q., and Jiang, L. (2017c). Regulation of DNA methylation on EEF1D and RPL8 expression in cattle. Genetica 145, 387–395. doi:10.1007/s10709-017-9974-x
Liu, Y., Wang, X., Zhen, Z., Yu, Y., Qiu, Y., and Xiang, W. (2019). GRP78 regulates milk biosynthesis and the proliferation of bovine mammary epithelial cells through the mTOR signaling pathway. Cell. Mol. Biol. Lett. 24, 57–62. doi:10.1186/s11658-019-0181-x
Lou, Q., Cao, S., Xu, W., Zhang, Y., Qin, Z., and Wei, W. (2014). Molecular characterization and mRNA expression of ribosomal protein L8 in Rana nigromaculata during development and under exposure to hormones. J. Environ. Sci. 26 (11), 2331–2339. doi:10.1016/j.jes.2014.09.018
Luo, C., Qi, H., Huang, X., Li, M., Zhang, L., Lin, Y., et al. (2019a). GlyRS is a new mediator of amino acid-induced milk synthesis in bovine mammary epithelial cells. J. Cell. Physiology 234 (3), 2973–2983. doi:10.1002/jcp.27115
Luo, C., Zhao, S., Dai, W., Zheng, N., and Wang, J. (2018a). Proteomic analyses reveal GNG12 regulates cell growth and casein synthesis by activating the Leu-mediated mTORC1 signaling pathway. Biochimica Biophysica Acta (BBA)-Proteins Proteomics 1866 (11), 1092–1101. doi:10.1016/j.bbapap.2018.08.013
Luo, C., Zhao, S., Zhang, M., Gao, Y., Wang, J., Hanigan, M. D., et al. (2018b). SESN2 negatively regulates cell proliferation and casein synthesis by inhibition of the amino acid-mediated mTORC1 pathway in cow mammary epithelial cells. Sci. Rep. 8 (1), 1–0. doi:10.1038/s41598-018-22208-w
Luo, C., Zheng, N., Zhao, S., and Wang, J. (2019b). Sestrin2 negatively regulates casein synthesis through the SH3BP4-mTORC1 pathway in response to AA depletion or supplementation in cow mammary epithelial cells. J. Agric. Food Chem. 67 (17), 4849–4859. doi:10.1021/acs.jafc.9b00716
Ma, L., and Corl, B. A. (2012). Transcriptional regulation of lipid synthesis in bovine mammary epithelial cells by sterol regulatory element binding protein-1. J. Dairy Sci. 95, 3743–3755. doi:10.3168/jds.2011-5083
Ma, X. M., and Blenis, J. (2009). Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 10 (5), 307–318. doi:10.1038/nrm2672
Ma, Y. F., Batistel, F., Xu, T. L., Han, L. Q., Bucktrout, R., Liang, Y., et al. (2019). Phosphorylation of AKT serine/threonine kinase and abundance of milk protein synthesis gene networks in mammary tissue in response to supply of methionine in periparturient Holstein cows. J. Dairy Sci. 102 (5), 4264–4274. doi:10.3168/jds.2018-15451
Mu, Y., Zheng, D., Wang, C., Yu, W., and Zhang, X. (2018). RagD regulates amino acid mediated-casein synthesis and cell proliferation via mTOR signalling in cow mammary epithelial cells. J. Dairy Res. 85 (2), 204–211. doi:10.1017/S0022029918000146
Nan, X., Bu, D., Li, X., Wang, J., Wei, H., Hu, H., et al. (2014). Ratio of lysine to methionine alters expression of genes involved in milk protein transcription and translation and mTOR phosphorylation in bovine mammary cells. Physiol. Genomics 46 (8), 268–275. doi:10.1152/physiolgenomics.00119.2013
Oppi-Williams, C., Suagee, J. K., and Corl, B. A. (2013). Regulation of lipid synthesis by liver X receptor α and sterol regulatory element-binding protein 1 in mammary epithelial cells. J. Dairy Sci. 96, 112–121. doi:10.3168/jds.2011-5276
Osorio, J. S., Lohakare, J., and Bionaz, M. (2016). Biosynthesis of milk fat, protein, and lactose: roles of transcriptional and posttranscriptional regulation. Physiol. Genomics 48, 231–256. doi:10.1152/physiolgenomics.00016.2015
Pauloin, A., and Chanat, E. (2012). Prolactin and epidermal growth factor stimulate adipophilin synthesis in HC11 mouse mammary epithelial cells via the PI3-kinase/Akt/mTOR pathway. Biochimica Biophysica Acta (BBA)-Molecular Cell Res. 1823 (5), 987–996. doi:10.1016/j.bbamcr.2012.02.016
Pszczolkowski, V. L., Halderson, S. J., Meyer, E. J., Lin, A., and Arriola Apelo, S. I. (2020). Pharmacologic inhibition of mTORC1 mimics dietary protein restriction in a mouse model of lactation. J. Animal Sci. Biotechnol. 11 (1), 67–10. doi:10.1186/s40104-020-00470-1
Qi, H., Wang, L., Zhang, M., Wang, Z., Gao, X., and Li, M. (2022). Methionine and leucine induce ARID1A degradation to promote mTOR expression and milk synthesis in mammary epithelial cells. J. Nutr. Biochem. 101, 108924. doi:10.1016/j.jnutbio.2021.108924
Qiu, Y., Qu, B., Zhen, Z., Yuan, X., Zhang, L., and Zhang, M. (2019). Leucine promotes milk synthesis in bovine mammary epithelial cells via the PI3K-DDX59 signaling. J. Agric. Food Chem. 67 (32), 8884–8895. doi:10.1021/acs.jafc.9b03574
Ricoult, S. J., Yecies, J. L., Ben-Sahra, I., and Manning, B. D. (2016). Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 35, 1250–1260. doi:10.1038/onc.2015.179
Salama, A. A. K., Duque, M., Wang, L., Shahzad, K., Olivera, M., and Loor, J. J. (2019). Enhanced supply of methionine or arginine alters mechanistic target of rapamycin signaling proteins, messenger RNA, and microRNA abundance in heat-stressed bovine mammary epithelial cells in vitro. J. Dairy Sci. 102, 2469–2480. doi:10.3168/jds.2018-15219
Sancak, Y., Peterson, T. R., Shaul, Y. D., Lindquist, R. A., Thoreen, C. C., Bar-Peled, L., et al. (2008). The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320 (5882), 1496–1501. doi:10.1126/science.1157535
Sengupta, S., Peterson, T. R., and Sabatini, D. M. (2010). Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40 (2), 310–322. doi:10.1016/j.molcel.2010.09.026
Shi, H., Yang, W., Zhang, Z., Ji, S., and Yang, Y. (2020). Inhibition of mTOR signalling reduces milk production and mammary epithelial cell proliferation in lactating dairy cows. J. Animal Physiology Animal Nutr. 104 (2), 437–444. doi:10.1111/jpn.13305
Shu, X., Fang, Z., Guan, Y., Chen, X., Loor, J. J., Jia, H., et al. (2020). High levels of fatty acids inhibit β-casein synthesis through suppression of the JAK2/STAT5 and mTOR signaling pathways in mammary epithelial cells of cows with clinical ketosis. J. Dairy Res. 87 (2), 212–219. doi:10.1017/S0022029920000175
Stransky, L. A., and Forgac, M. (2015). Amino acid availability modulates vacuolar H+-ATPase assembly. J. Biol. Chem. 290, 27360–27369. doi:10.1074/jbc.M115.659128
Sun, J., Liu, J., Huang, B., Kan, X., Chen, G., Wang, W., et al. (2017). Kisspeptin-10 induces β-Casein synthesis via GPR54 and its downstream signaling pathways in bovine mammary epithelial cells. Int. J. Mol. Sci. 18 (12), 2621. doi:10.3390/ijms18122621
Toerien, C. A., and Cant, J. P. (2007). Abundance and phosphorylation state of translation initiation factors in mammary glands of lactating and nonlactating dairy cows. J. Dairy Sci. 90, 2726–2734. doi:10.3168/jds.2006-778
Toerien, C. A., Trout, D. R., and Cant, J. P. (2010). Nutritional stimulation of milk protein yield of cows is associated with changes in phosphorylation of mammary eukaryotic initiation factor 2 and ribosomal s6 kinase 1. J. Nutr. 140, 285–292. doi:10.3945/jn.109.114033
Trottier, N. L., Bequette, B. J., and Wu, G. (2014). Linking our understanding of mammary gland metabolism to amino acid nutrition. Amino Acids 46, 2447–2462. doi:10.1007/s00726-014-1818-8
Wang, C., Zhao, F., Liu, J., and Liu, H. (2018a). Dipeptide (Methionyl-Methionine) transport and its effect on β-Casein synthesis in bovine mammary epithelial cells. Cell. Physiology Biochem. 49 (2), 479–488. doi:10.1159/000492987
Wang, F., van Baal, J., Ma, L., Gao, X., Dijkstra, J., and Bu, D. (2022a). MRCKα is a novel regulator of prolactin-induced lactogenesis in bovine mammary epithelial cells. Anim. Nutr. 1 (10), 319–328. doi:10.1016/j.aninu.2022.06.001
Wang, F., Van Baal, J., Ma, L., Loor, J. J., Wu, Z. L., Dijkstra, J., et al. (2019a). Short communication: relationship between lysine/methionine ratios and glucose levels and their effects on casein synthesis via activation of the mechanistic target of rapamycin signaling pathway in bovine mammary epithelial cells. J. Dairy Sci. 102 (9), 8127–8133. doi:10.3168/jds.2018-15916
Wang, L., Lin, Y., Bian, Y., Liu, L., Shao, L., Lin, L., et al. (2014a). Leucyl-tRNA synthetase regulates lactation and cell proliferation via mTOR signaling in dairy cow mammary epithelial cells. Int. J. Mol. Sci. 15 (4), 5952–5969. doi:10.3390/ijms15045952
Wang, L., Qi, H., Li, D., Liu, L., Chen, D., and Gao, X. (2022b). METTL3 is a key regulator of milk synthesis in mammary epithelial cells. Cell Biol. Int. 46 (3), 359–369. doi:10.1002/cbin.11733
Wang, M., Wang, Z., Yang, C., Liu, L., and Jiang, N. (2018b). Protein 14-3-3ε regulates cell proliferation and casein synthesis via PI3K-mTOR pathway in dairy cow mammary epithelial cells. J. Agric. Food Chem. 66 (45), 12000–12008. doi:10.1021/acs.jafc.8b04590
Wang, M., Xu, B., Wang, H., Bu, D., Wang, J., and Loor, J. J. (2014b). Effects of arginine concentration on the in vitro expression of casein and mTOR pathway related genes in mammary epithelial cells from dairy cattle. PLoS One 9 (5), e95985. doi:10.1371/journal.pone.0095985
Wang, X., Xu, J., Zeng, H., and Han, Z. (2022c). Enhancement of BCAT2-mediated valine catabolism stimulates β-casein synthesis via the AMPK-mTOR signaling Axis in bovine mammary epithelial cells. J. Agric. Food Chem. 70 (32), 9898–9907. doi:10.1021/acs.jafc.2c03629
Wang, Y., Guo, W., Xu, H., Tang, K., Zan, L., and Yang, W. (2019b). Melatonin suppresses milk fat synthesis by inhibiting the mTOR signaling pathway via the MT1 receptor in bovine mammary epithelial cells. J. Pineal Res. 67 (3), e12593. doi:10.1111/jpi.12593
Wang, Y., Liu, J., Wu, H., Fang, X., Chen, H., and Zhang, C. (2017). Amino acids regulate mTOR pathway and milk protein synthesis in a mouse mammary epithelial cell line is partly mediated by T1R1/T1R3. Eur. J. Nutr. 56, 2467–2474. doi:10.1007/s00394-016-1282-1
Wang, Y., Wang, X., Wang, M., Zhang, L., Zan, L., and Yang, W. (2021). Bta-miR-34b controls milk fat biosynthesis via the Akt/mTOR signaling pathway by targeting RAI14 in bovine mammary epithelial cells. J. Animal Sci. Biotechnol. 12 (1), 83–12. doi:10.1186/s40104-021-00598-8
Wang, Z., Hou, X., Qu, B., Wang, J., Gao, X., and Li, Q. (2014c). Pten regulates development and lactation in the mammary glands of dairy cows. PLoS One 9 (7), e102118. doi:10.1371/journal.pone.0102118
Xu, H. F., Luo, J., Zhang, X. Y., Li, J., and Bionaz, M. (2019). Activation of liver X receptor promotes fatty acid synthesis in goat mammary epithelial cells via modulation of SREBP1 expression. J. Dairy Sci. 102, 3544–3555. doi:10.3168/jds.2018-15538
Xu, M., Zhou, Y., Fan, S., Zhang, M., and Gao, X. (2022). Cul5 mediates taurine-stimulated mTOR mRNA expression and proliferation of mouse mammary epithelial cells. Amino Acids 30, 243–252. doi:10.1007/s00726-022-03222-9
Yang, J. X., Wang, C. H., Xu, Q. B., Zhao, F. Q., Liu, J. X., and Liu, H. Y. (2015). Methionyl-Methionine promotes α-s1 casein synthesis in bovine mammary gland explants by enhancing intracellular substrate availability and activating JAK2-STAT5 and mTOR-mediated signaling pathways. J. Nutr. 145 (8), 1748–1753. doi:10.3945/jn.114.208330
Yang, Y., Zhang, Z., Ji, S., Shi, H., and Yang, W. (2021). High dietary protein promotes milk production by increasing the expression of lactation-related genes in dairy cows. J. Dairy Res. 88 (3), 330–333. doi:10.1017/S0022029921000135
Yoder, P. S., Ruiz-Cortes, T., Castro, J. J., and Hanigan, M. D. (2019). Effects of varying extracellular amino acid profile on intracellular free amino acid concentrations and cell signaling in primary mammary epithelial cells. J. Dairy Sci. 102 (10), 8977–8985. doi:10.3168/jds.2018-16122
Yuan, X., Zhang, M., Ao, J., Zhen, Z., Gao, X., and Li, M. (2019). NUCKS1 is a novel regulator of milk synthesis in and proliferation of mammary epithelial cells via the mTOR signaling pathway. J. Cell. Physiology 234 (9), 15825–15835. doi:10.1002/jcp.28240
Zhang, M. C., Zhao, S. G., Wang, S. S., Luo, C. C., Gao, H. N., Zheng, N., et al. (2018a). d-Glucose and amino acid deficiency inhibits casein synthesis through JAK2/STAT5 and AMPK/mTOR signaling pathways in mammary epithelial cells of dairy cows. J. Dairy Sci. 101 (2), 1737–1746. doi:10.3168/jds.2017-12926
Zhang, R., Ma, H., Gao, Y., Wu, Y., Qiao, Y., Geng, A., et al. (2018b). Th-POK regulates mammary gland lactation through mTOR-SREBP pathway. PLoS Genet. 14 (2), e1007211. doi:10.1371/journal.pgen.1007211
Zhao, K., Liu, W., Lin, X., Hu, Z., Yan, Z., Wang, Y., et al. (2019). Effects of rumen-protected methionine and other essential amino acid supplementation on milk and milk component yields in lactating Holstein cows. J. Dairy Sci. 102 (9), 7936–7947. doi:10.3168/jds.2018-15703
Zheng, X. R., Jiang, L., Ning, C., Hu, Z. Z., Zhou, L., Yu, Y., et al. (2019). A novel mutation in the promoter region of RPL8 regulates milk fat traits in dairy cattle by binding transcription factor Pax6. Biochim Biophys Acta Mol. Cell Biol. Lipids 1864, 158528. doi:10.1016/j.bbalip.2019.158528
Zhou, F., Ouyang, Y., and Miao, Y. (2021b). Peroxisome proliferator-activated receptor gamma regulates genes involved in milk fat synthesis in mammary epithelial cells of water buffalo. J. Animal Sci. 92, e13537. doi:10.1111/asj.13537
Zhou, J., Yue, S., Xue, B., Wang, Z., Wang, L., Peng, Q., et al. (2022). Metabolic disorders sensitise endometrial carcinoma through endoplasmic reticulum stress. J. Animal Sci. Technol. 64 (1), 110. doi:10.1186/s11658-022-00412-x
Zhou, J., Yue, S., Xue, B., Wang, Z., Wang, L., Peng, Q., et al. (2021a). Enhanced supply of methionine regulates protein synthesis in bovine mammary epithelial cells under hyperthermia condition. J. Animal Sci. Technol. 63 (5), 1126–1141. doi:10.5187/jast.2021.e93
Zhou, Y., Zhou, Z., Peng, J., and Loor, J. J. (2018). Methionine and valine activate the mammalian target of rapamycin complex 1 pathway through heterodimeric amino acid taste receptor (TAS1R1/TAS1R3) and intracellular Ca2+ in bovine mammary epithelial cells. J. Dairy Sci. 101 (12), 11354–11363. doi:10.3168/jds.2018-14461
Zhu, C., Zhu, J., Duan, Q., Jiang, Y., Yin, H., He, Y., et al. (2021). Exploration of the lactation function of protein phosphorylation sites in goat mammary tissues by phosphoproteome analysis. BMC Genomics 22 (1), 703–717. doi:10.1186/s12864-021-07993-5
Keywords: cow mammary epithelial cells, mTORC1 signaling pathway, amino acids, environmental stress, milk production
Citation: Li B, Khan MZ, Khan IM, Ullah Q, Cisang Z-M, Zhang N, Wu D, Huang B, Ma Y, Khan A, Jiang N and Zahoor M (2023) Genetics, environmental stress, and amino acid supplementation affect lactational performance via mTOR signaling pathway in bovine mammary epithelial cells. Front. Genet. 14:1195774. doi: 10.3389/fgene.2023.1195774
Received: 28 March 2023; Accepted: 26 June 2023;
Published: 10 August 2023.
Edited by:
Tania Bobbo, National Research Council (CNR), ItalyReviewed by:
Tatiana Ruiz-Cortés, University of Antioquia, ColombiaMohamed Abdelrahman, Assiut University, Egypt
Copyright © 2023 Li, Khan, Khan, Ullah, Cisang, Zhang, Wu, Huang, Ma, Khan, Jiang and Zahoor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Zahoor Khan, emFob29ya2hhdHRhazkxQHlhaG9vLmNvbQ==; Nan Jiang, amlhbmduYW5AZGx1LmVkdS5jbg==; Muhammad Zahoor, bXVoYW1tYWQuemFob29yQG1lZGlzaW4udWlvLm5v
†These authors have contributed equally to this work
 Bin Li
Bin Li Muhammad Zahoor Khan
Muhammad Zahoor Khan Ibrar Muhammad Khan
Ibrar Muhammad Khan Qudrat Ullah
Qudrat Ullah Zhuo-Ma Cisang1
Zhuo-Ma Cisang1 Yulin Ma
Yulin Ma Muhammad Zahoor
Muhammad Zahoor