- 1Graduate School, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Integrative Oncology, China-Japan Friendship Hospital, Beijing, China
- 3Department of Dermatology, China-Japan Friendship Hospital, Beijing, China
- 4School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
- 5Oncology Department, Wangjing Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 6Oncology Department, Beijing Hospital of Traditional Chinese Medicine, Capital Medicine University, Beijing, China
Background: Colorectal cancer and Alzheimer’s disease are both common life-threatening diseases in the elderly population. Some studies suggest a possible inverse relationship between colorectal cancer and Alzheimer’s disease, but real-world research is subject to many biases. We hope to clarify the causal relationship between the two through a bidirectional two-sample Mendelian randomization study.
Methods: In our study, we used genetic summary data from large-scale genome-wide association studies to investigate the relationship between colorectal cancer and Alzheimer’s disease. Our primary analysis employed the inverse-variance weighted method and we also used complementary techniques, including MR-Egger, weighted median estimator, and Maximum likelihood. We applied simex adjustment to the MR-Egger results. We also utilized the MRlap package to detect potential sample overlap and its impact on the bias of the results. In addition, we performed several sensitivity and heterogeneity analyses, to ensure the reliability of our results.
Results: The combined effect size results of the inverse-variance weighted method indicate that colorectal cancer may decrease the incidence of Alzheimer’s disease, with an odds ratio (OR) of 0.846 (95% CI: 0.762–0.929). Similar results were observed using other methods such as MR-Egger, weighted median estimator, and Maximum likelihood. On the other hand, Alzheimer’s disease may slightly increase the incidence of colorectal cancer, with an OR of 1.014 (95% CI: 1.001–1.027). However, the results of one subgroup were not significant, and the results from MRlap indicated that sample overlap introduced bias into the results. Therefore, the results of the reverse validation are not reliable. The F-statistic for all SNPs was greater than 20. Four SNPs related to the outcome were excluded using Phenoscanner website but the adjustment did not affect the overall direction of the results. The results of these statistics were further validated by MR-PRESSO, funnel plots, leave-one-out analyses, Cochran’s Q, demonstrating the reliability of the findings.
Conclusion: According to the findings of this Mendelian randomization study, there appears to be a causal association between colorectal cancer and Alzheimer’s disease. These results could have important implications for clinical practice in terms of how colorectal cancer and Alzheimer’s disease are treated. To better understand the relationship between these two diseases, more research and screening are needed in clinical settings.
1 Introduction
Cancer is a major cause of mortality worldwide, presenting a significant obstacle to increasing life expectancy (Bray et al., 2021). Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the second leading cause of cancer-related deaths. In 2020, there were over 1.9 million new cases of CRC and 935,000 deaths globally, comprising approximately 10% of all cancer cases and deaths. It is worth noting that the incidence of CRC varies by a factor of approximately 9 between different regions of the world, with transitioned countries having an incidence rate that is approximately 4 times higher than that of transitioning countries. Among these regions, Europe has the highest incidence rate (Sung et al., 2021). Globally, approximately 50 million people, mostly elderly, are affected by dementia, with an estimated increase to 100–130 million patients between 2040 and 2050 (Taudorf et al., 2021). Alzheimer’s disease (AD) is a degenerative neurological disease that leads to neuronal and synaptic loss, brain atrophy, and ultimately death. It is prevalent among older adults and is the primary cause of dementia, which is a leading cause of mortality in this population. There appears to be no obvious connection between cancer and AD, both of which are age-related conditions. However, according to some research reports, there may be a close or even inverse relationship between the incidence and pathogenesis of cancer and central nervous system diseases, particularly AD (Catalá-López et al., 2014; Jiang et al., 2013). For example, Ferrán et al. found a sustained decrease in the overall co-occurrence of cancer in patients with neurodegenerative diseases and AD (Catalá-López et al., 2014). Similarly, a meta-analysis revealed a weak inverse correlation between AD and cancer that cannot be explained by confounding factors, diagnostic bias, or bias in competitive risks. The random-effects meta-analysis indicated that the pooled fixed-effect hazard ratio for AD in CRC survivors was 0.88 (95% CI, 0.80–0.97) compared to individuals without a history of cancer (Ospina-Romero et al., 2020). There are also many studies in the field of basic experiments. For instance, Park et al. discovered that inflammatory response is an important mechanism underlying these two phenotypically opposite diseases (Park et al., 2015). Several proteins that suppress tau and amyloid-β deposits, regulate cell cycle (Ma et al., 2012; Li et al., 2013), and undergo common epigenetic modifications (Tremolizzo et al., 2006), as well as age-related metabolic dysfunction (Driver, 2014), are all involved in the pathogenesis of cancer and neurodegeneration. In reality, a portion of patients with AD may experience a decrease in CRC diagnosis rates due to memory loss, cognitive decline, and other factors that prevent them from seeking timely medical examinations such as colonoscopy (Lv et al., 2022). Furthermore, patients diagnosed with cancer often experience emotional stress, invasive surgical treatments, cytotoxic chemotherapy, and persistent pain, which may lead to reduced cognitive function and an increased risk of AD (Aboalela et al., 2015; Hermelink et al., 2015; Whitlock et al., 2017; Ahles and Hurria, 2018). These findings are contrary to many epidemiological and basic experimental research results. In addition, the association between CRC and AD remains unclear due to the bias of reverse causation and confounding factors in traditional observational studies.
Mendelian randomization (MR) employs single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) to establish causality between an exposure and an outcome. Since offspring inherit their alleles from their parents according to Mendelian principles, MR studies resemble randomized controlled trials that occur naturally within populations. The genetic effects of genotypes are relatively stable and less influenced by environmental factors, and all genetic variations occur before disease onset, making it possible to overcome confounding factors and reverse causation (Emdin et al., 2017; Davies et al., 2018). In order to examine the causal relationship between CRC and AD, we performed bidirectional two-sample MR analyses in this study.
2 Materials and methods
2.1 Study design
To explore the potential relationship between CRC and AD, we utilized summary statistics data from genome-wide association studies (GWASs) and performed a bidirectional two-sample Mendelian randomization study. To conduct a reliable MR study, three core assumptions, namely, relevance, independence, and exclusion restriction, need to be simultaneously satisfied. Further details regarding the MR design can be found here (Davies et al., 2018; Sanderson et al., 2022). Since we are using aggregated data, which is of a de-identified nature and does not constitute human subject research, ethical approval is not required. However, it is important to note that all primary studies included in our analysis obtained ethical approval and obtained informed consent from participants.
2.2 Study samples
We utilized three GWAS datasets for MR analysis. In order to avoid population stratification, the analysis only incorporated genetic variants derived from European ancestry. The GWAS dataset related to colorectal cancer is sourced from the United Kingdom Biobank, as reported in the study by Burrows et al., in 2021, which includes 5,657 colorectal cancer patients and 372,016 healthy controls. The GWAS datasets associated with AD are sourced from two databases: The Medical Research Council Integrative Epidemiology Unit (MRC IEU) and the European Bioinformatics Institute (EBI) databases. The study by Ben Elsworth et al., in 2018 includes 19,255 cases and 380,538 controls and the study conducted by Jeremy et al., in 2021 includes 53,042 cases and 355,900 controls (Schwartzentruber et al., 2021).
2.3 Instruments selection
Firstly, SNPs (p < 5 × 10−6) closely associated with colorectal cancer (or Alzheimer’s disease in the reverse validation) were selected from the GWAS database. Typically, SNPs with p < 5 × 10−8 are considered genome-wide significant, however, selecting SNPs below this threshold yields a limited number of effective SNPs, resulting in reduced statistical power. Therefore, a lower threshold was appropriate. Secondly, the clump step was performed using the TwoSampleMR package in R 4.2.2 software to exclude SNPs in linkage disequilibrium (LD) (Hemani et al., 2018). The specific parameters were set as R2 = 0.001 and kb = 10,000, which removed SNPs with R2 > 0.001 within a 10-MB range of the most significant SNP. For missing SNPs in the outcome dataset, those with strong LD (R2 > 0.8) were used as proxies, and SNPs without alternative sites were removed. Data were then extracted and organized from both datasets to match the exposure and outcome effect values with the same effect allele. To eliminate weak instrumental variables, we introduce the F-statistic (F = beta2/se2, where beta represents the effect size of the allele and se represents the standard error) to calculate the power of each SNP. We exclude SNPs with an F-statistic less than 10 and compute the average of all F-statistics to represent the overall F-statistic of the IVs (Xie et al., 2023). Then, we remove SNPs that are associated with AD (or CRC in reverse validation) and any phenotypes that might lead to AD using the PhenoScanner website (http://www.phenoscanner.medschl.cam.ac.uk/). Following that, we utilize the MR-Egger regression model’s intercept test and the MRPRESSO method to assess genetic pleiotropy and remove outliers (Verbanck et al., 2018).
2.4 Statistical analyses
Four methods were used including inverse-variance weighted (IVW) (Yavorska and Burgess, 2017), MR-Egger (Bowden et al., 2015), weighted median estimator (WM) (Bowden et al., 2016a), and Maximum likelihood (ML) (Yavorska and Burgess, 2017), to estimate the causal association between CRC and AD. The odds ratio (OR) of the outcome variable per 1 log-odds increase in the exposure variable was used to represent the results. The scatter plot was generated using the TwoSampleMR package (Hemani et al., 2018), which includes the most commonly used five methods, including simple mode and weighted mode, to improve the accuracy of causal association assessment. The IVW method requires the regression line to pass through the origin and assumes that all IVs included in the model are valid. Therefore, if there are no pleiotropic IVs in the regression model, IVW can provide an unbiased and efficient estimate of the causal association. The MR-Egger method assumes that the pleiotropy of IVs is unrelated to their effects on exposure and does not require the regression line to pass through the origin. Thus, the intercept term represents the average estimate of genetic pleiotropy, and the slope of the regression line represents the estimate of the true causal association after correcting for genetic pleiotropy (Bowden et al., 2015). When more than 50% of the IVs are valid, the WM method can provide a more accurate estimate of the causal effect, with efficiency approaching that of the IVW method (Wang and Shen, 2020). The Maximum likelihood method is not fundamentally different from the IVW method, but it fully considers uncertainty in genetic associations with both the exposure and outcome, which is ignored in the simple weighting of the IVW method (Yavorska and Burgess, 2017). Additionally, both IVW and MR-Egger methods require the NO Measurement Error (NOME) assumption, and violation of this assumption can result in weak instrument bias. The IVW method can be tested using the F-statistic, while the MR-Egger method can be tested using the I2GX statistic. When I2GX is less than 90%, the simex approach (available in the TwoSampleMR package) should be used for adjustment (Bowden et al., 2016b; Hemani et al., 2018).
Finally, conducting sensitivity analysis to validate the reliability of the statistical results. MRlap employs cross-trait LD score regression (LDSC) to approximate overlap, enabling the evaluation and correction of biases introduced by sample overlap in Mendelian Randomization analyses. Cochran’s Q test and Funnel plots were applied to assess the heterogeneity of SNPs. Using the “leave-one-out” method to assess the magnitude of the causal association effect influenced by individual SNPs.
Analyses were done using the statistical software R (version 4.2.2) with packages TwoSampleMR (version 0.5.6) (Hemani et al., 2018), MRPRESSO (version 1.0), simex (version 1.8), MRlap (version 0.0.3). A significance level of p < 0.05 (two-tailed) was considered statistically significant.
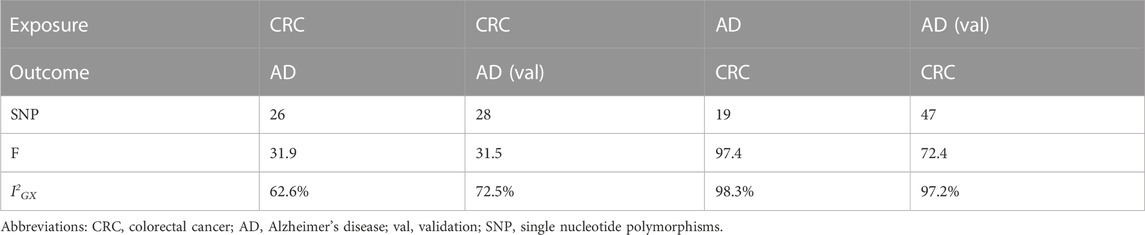
3 Results
3.1 CRC and AD related SNPs
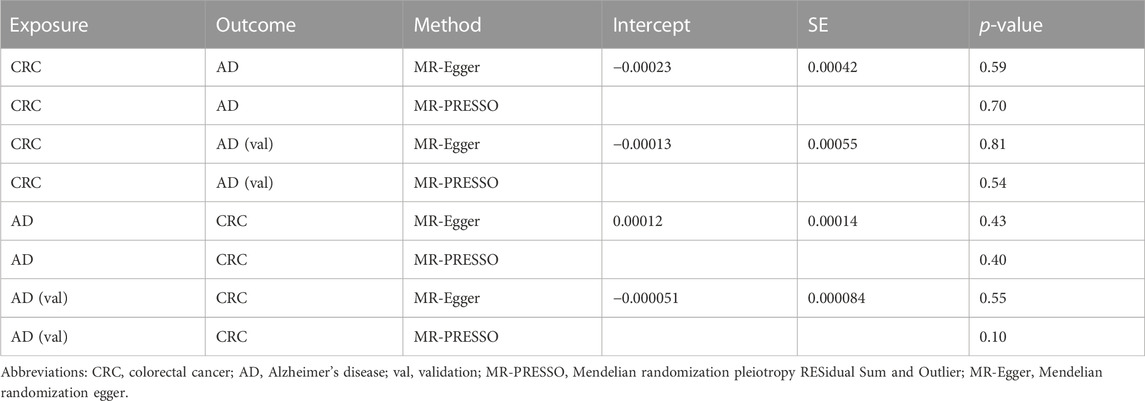
In the study examining the impact of colorectal cancer (CRC) on Alzheimer’s disease (AD) and AD (val), we initially extracted SNPs and identified rs12488768 as correlated with neuroticism score and rs143058554 as correlated with schizophrenia using PhenoScanner website. Since both of these SNPs can promote the onset of Alzheimer’s disease (Low et al., 2013; Ribe et al., 2015), they were subsequently excluded, resulting in the identification of 26 and 28 SNPs, respectively, that were associated with CRC (Table 1). Similarly, in the study examining the impact of AD and AD (val) on CRC, we initially extracted SNPs and identified rs35511257 as correlated with malignant neoplasm of anus and anal canal, and rs2760980 as correlated with inflammatory bowel disease using PhenoScanner website. These two SNPs can promote the onset of colorectal cancer (Shah and Itzkowitz, 2022), and were therefore excluded, resulting in the identification of 19 and 47 SNPs, respectively, that were associated with AD (Table 1). The detailed information of SNPs can be found in Supplementary Tables S1–S4. The intercept of the MR-Egger regression was zero, indicating the absence of genetic pleiotropy between SNPs and both CRC and AD (p-values >0.05). Results from the MRPRESSO test similarly showed no evidence of pleiotropic bias (p-values >0.05) or outliers (Table 2). All SNPs have an F-statistic greater than 20, indicating that the results are not influenced by weak instrument bias (Table 1). However, since I2GX in the study examining the impact of CRC on AD and AD (val) was less than 90%, the MR-Egger results needed to be adjusted using the simex approach (Table 1) (Bowden et al., 2016b). Nevertheless, the removal of SNPs and the adjustment of MR-Egger results with the simex approach did not significantly affect the causal associations between the two diseases.
3.2 Estimation of causal relationship between CRC and AD
3.2.1 Causal effect of CRC on AD
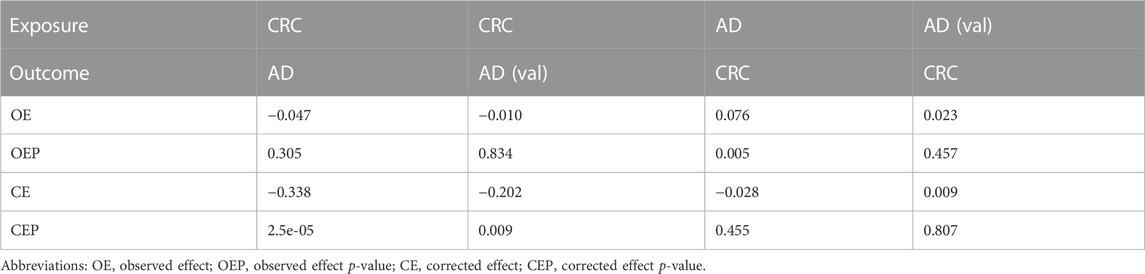
IVW, WM, MR-Egger, and ML methods were used to estimate the causal relationship between colorectal cancer (CRC) and Alzheimer’s disease (AD). In terms of the impact of CRC on AD, the two were significantly negatively correlated. The combined effect value of IVW showed that an increase of 1 log-odds in CRC led to a 15.4% reduction in the risk of AD (OR = 0.846, 95% CI: 0.762–0.929). Similar results were obtained with WM, MR-Egger regression, and ML methods, although some individual boundary values exceeded 1, they did not affect the overall results (Table 3; Figure 1). The forest plot was used to visualize the causal effect of each single SNP on the risk of Alzheimer’s disease, and the overall results were consistent with the previous findings (Supplementary Figures S1A, B). The slope of the regression line in the scatter plot also indicated a consistent causal effect direction, that is, CRC would reduce the risk of AD (Figures 2A, B).
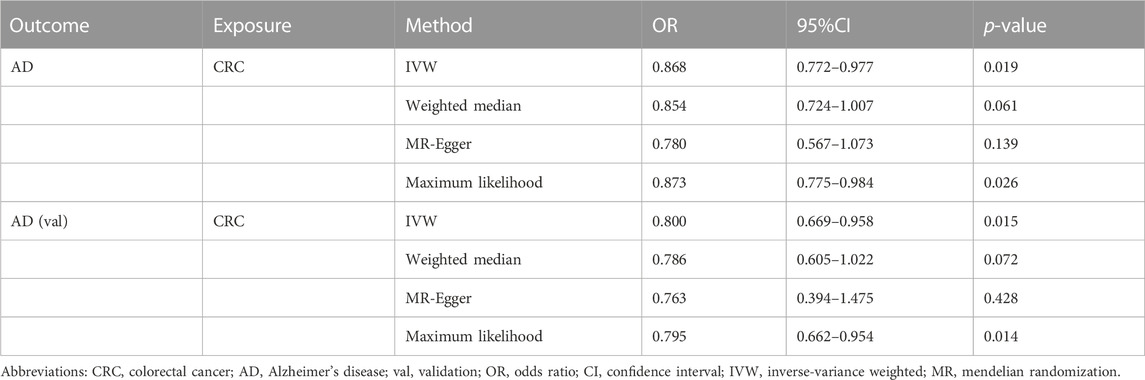
TABLE 3. Mendelian randomization estimates for the effect of colorectal cancer on Alzheimer’s disease.
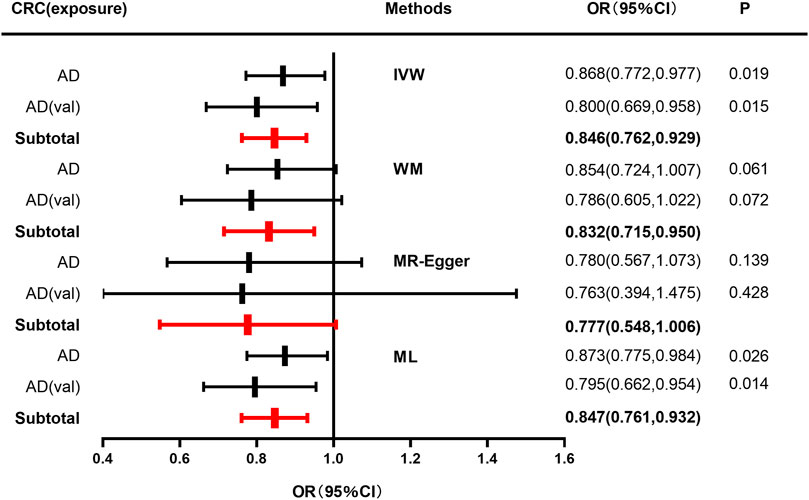
FIGURE 1. Forest plot to visualize causal effect of colorectal cancer on the risk of Alzheimer’s disease. For AD, a discovery sample (AD) and a validation sample (AD [val]) were used and the combined effect value was showed. Estimates are presented as odds ratios (ORs) and 95% CIs from four Mendelian randomization analyses methods including inverse-variance weighted (IVW), Weighted median (WM), Mendelian randomization egger (MR-Egger), Maximum likelihood (ML) and their subtotal results.) (Abbreviations: CRC, colorectal cancer; AD, Alzheimer’s disease; val, validation; OR, odds ratio; CI, confidence interval; P, p-value; IVW, inverse-variance weighted; MR-Egger, Mendelian randomization egger; ML, Maximum likelihood.).
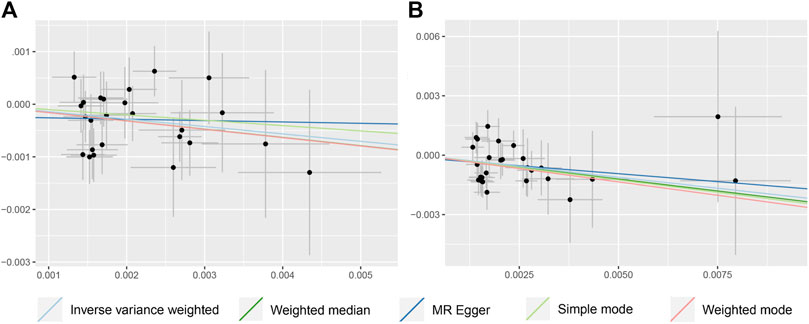
FIGURE 2. Scatter plot to visualize causal effect of colorectal cancer on the risk of Alzheimer’s disease (A, B). Figures A and B respectively represent the causal effect of CRC on AD and AD (val), both estimated using five different statistical methods. The slope of the straight line indicates the magnitude of the causal association.
3.2.2 Causal effect of AD on CRC
In terms of the impact of AD on CRC, the two were positively correlated, although the effect size was small. The combined effect value of IVW showed that an increase of 1 log-odds in AD led to a 1.4% increase in the risk of CRC (OR = 1.014, 95% CI: 1.001–1.027). Similar results were obtained with WM, MR-Egger regression, and ML methods. However, in the AD (val) subgroup, multiple statistical methods showed no significant differences (Table 4; Figure 3), which was supported by the forest plot of single SNP evaluation (Supplementary Figures S1C, D). However, the slopes of regression lines for all methods in scatter plots indicated an increased risk of CRC in both subgroups AD and AD (val) (Figures 4C, D).
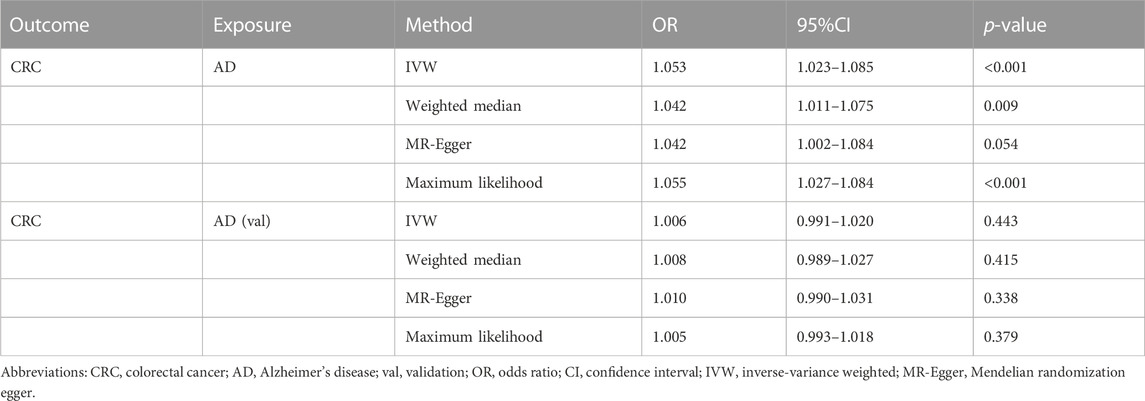
TABLE 4. Mendelian randomization estimates for the effect of Alzheimer’s disease on colorectal cancer.
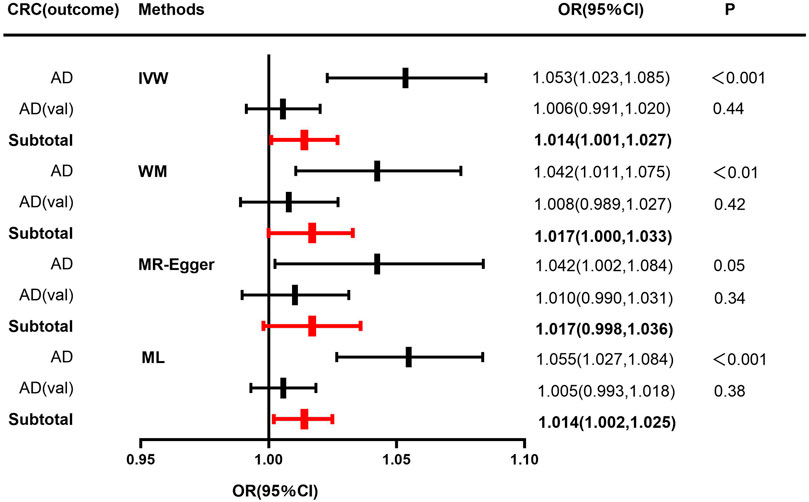
FIGURE 3. Forest plot to visualize causal effect of Alzheimer’s disease on the risk of colorectal cancer. For AD, a discovery sample (AD) and a validation sample (AD (val)) were used and the combined effect value was showed. Estimates are presented as odds ratios (ORs) and 95% CIs from four Mendelian randomization analyses methods including inverse-variance weighted (IVW), Weighted median (WM), Mendelian randomization egger (MR-Egger), Maximum likelihood (ML) and their subtotal results.) (Abbreviations: CRC, colorectal cancer; AD, Alzheimer’s disease; val, validation; OR, odds ratio; CI, confidence interval; P, p-value; IVW, inverse-variance weighted; MR-Egger, Mendelian randomization egger; ML, Maximum likelihood.).
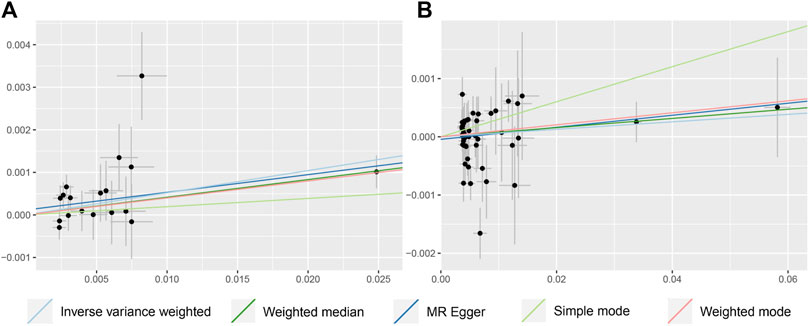
FIGURE 4. Scatter plot to visualize causal effect of colorectal cancer on the risk of Alzheimer’s disease (C, D). Figures C and D respectively represent the causal effect of AD and AD (val) on CRC, both estimated using five different statistical methods. The slope of the straight line indicates the magnitude of the causal association.
3.3 Sensitivity analyses
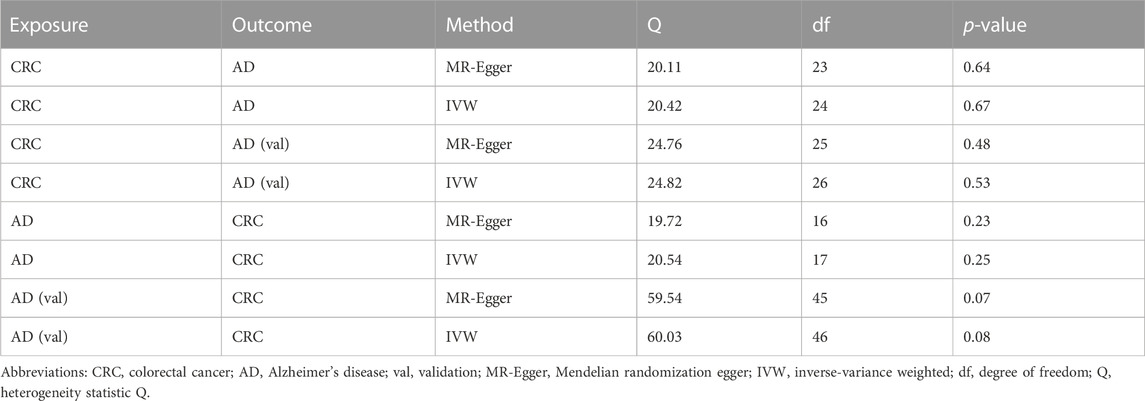
The results from MRlap indicate that the MR results of AD on CRC are influenced by sample overlap, introducing bias and rendering these findings unreliable (Table 5). Cochran’s Q test of IVW and MR-Egger regression indicates no heterogeneity among SNPs (Table 6). The funnel plot shows a symmetrical distribution of points representing causal association effects when a single SNP is used as IV, suggesting a small likelihood of potential bias (Supplementary Figure S2). Sensitivity analyses using the “leave-one-out” approach reveals that the estimates of the remaining SNPs after excluding each SNP in turn are similar to those obtained with all SNPs included, indicating no SNPs with significant influence on the estimate of causal association (Figure 5). Therefore, all estimates of causal associations are reliable.
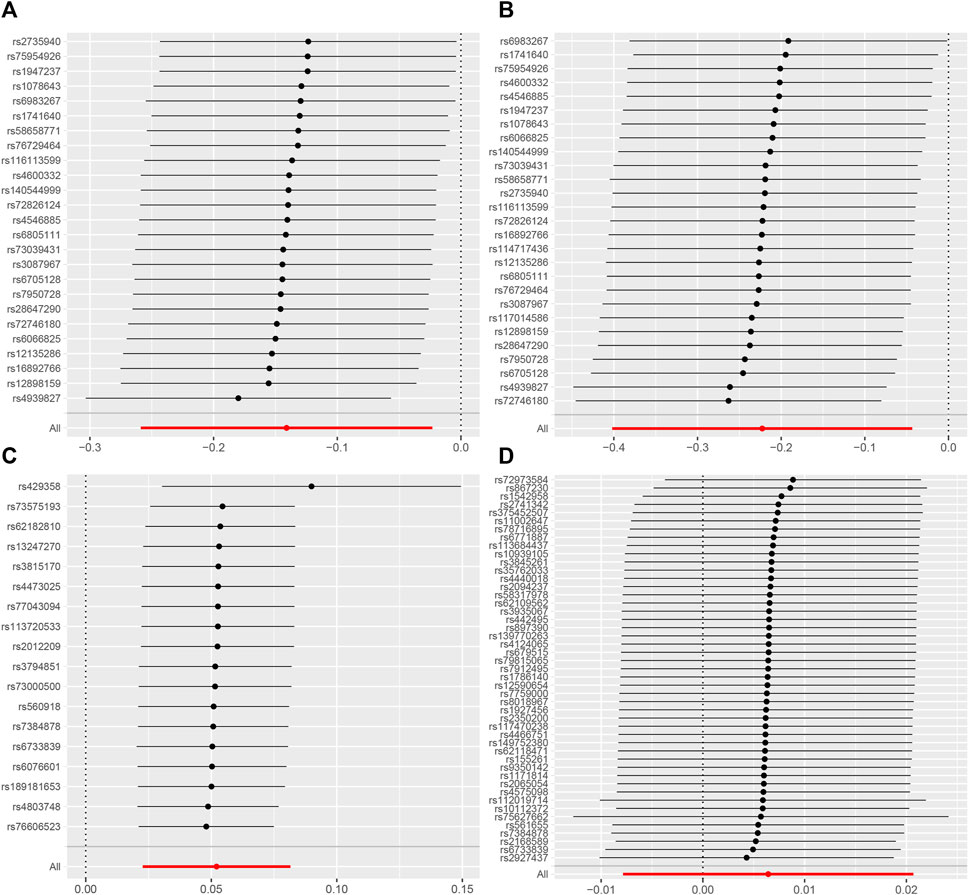
FIGURE 5. Leave-one-out plot to visualize causal effect of colorectal cancer on the risk of Alzheimer’s disease [(A) for AD subgroup and (B) for AD (val) subgroup] and vice versa (reverse direction) [(C) for AD subgroup and (D) for AD (val) subgroup] when leaving one SNP out.
4 Discussion
This study represents the first MR study to evaluate the causal relationship between CRC and AD from a genetic susceptibility perspective. As mentioned in the research design, MR analyses require meeting three key assumptions: relevance, exclusion restriction, and independence. Therefore, statistical analyses revolve around these three core assumptions. Instruments selection and weak instrument testing have ensured relevance. Using the PhenoScanner website to exclude phenotypes, performing the intercept test in the MR-Egger regression model, and employing the MRPRESSO method to remove outliers all help to eliminate genetic pleiotropy, thus ensuring the exclusion restriction assumption. In terms of independence, testing the hypothesis rigorously can be challenging due to the lack of individual-level data and the inability to account for all confounding factors. Heterogeneity testing, leave-one-out analysis, and similar methods can be helpful for validation. One of the major concerns of MR is horizontal pleiotropy because the presence of genetic pleiotropy can introduce severe bias in MR analyses results (Verbanck et al., 2018; Rao et al., 2020). Therefore, in this study, we first used methods such as the PhenoScanner website, MR-Egger, and MRPRESSO to preliminarily exclude the pleiotropy of genes before conducting further MR analyses and sensitivity analyses. Our research findings indicate that genetic variations associated with CRC may potentially reduce the risk of AD. We also found evidence of an increased risk of CRC associated with AD, but the effect size was small and subgroup analyses were not statistically significant. Most importantly, the results of the MRlap analysis indicate a significant impact of sample overlap on the MR results, introducing substantial bias and rendering the results unreliable. Therefore, further research is needed to clarify the impact of AD on CRC. These results were supported by a series of sensitivity analyses exploring genetic pleiotropy, heterogeneity, and susceptibility. In general, current MR studies suggest that there may be a causal relationship between CRC and AD, and whether it is bidirectional still requires further investigation.
The incidence rate of CRC continues to increase with advancing age. The number of cases within each age group follows a bell-shaped distribution, with a peak occurring in the 60–74 age group (GBD, 2019 Colorectal Cancer Collaborators, 2022). Similarly, the incidence rate of AD also continues to rise with increasing age, with the ages of 60 or 65 and above being the high-risk age groups. These age ranges are also the focal points of AD research in various countries (Tahami Monfared et al., 2022). Both of them are common age-related diseases, with their incidence gradually increasing in old age. Interestingly, these two diseases, one representing ‘immortality’ of cells and the other representing ‘stagnation and death’ of cells, ultimately lead to the demise of the organism, as if it were an inevitable outcome that the human body cannot escape. Therefore, research on the correlation between these two diseases is of significant importance. There have been numerous observational studies focusing on the relationship between cancer and Alzheimer’s disease or central nervous system disorders. Most studies have shown a negative correlation between the two. However, there has been little research specifically addressing the link between colorectal cancer and Alzheimer’s disease (Driver et al., 2012; Catalá-López et al., 2014; Catalá-López et al., 2017; Ospina-Romero et al., 2020). Therefore, the present study holds significant importance. A meta-analysis indicated that individuals diagnosed with AD have a 42% reduced risk of developing cancer (95% CI, 0.40–0.86; p < 0.05), while patients with a history of cancer have a 37% reduced risk of developing AD (RR = 0.63; 95% CI, 0.56–0.72; p = 0.495), and the data did not show significant bias (Ma et al., 2014). In addition, a retrospective study discovered that individuals diagnosed with Alzheimer’s disease (AD) experience a decreased age-sex standardized rate of cancer development, which is not observed in individuals with Huntington’s disease (Panegyres and Chen, 2021). Furthermore, according to a cohort study involving more than 10,000 participants, individuals with AD had a 50% reduced risk of cancer, whereas cancer patients had a 35% lower risk of developing AD (Musicco et al., 2013). A MR study investigating the association between AD and gastrointestinal diseases revealed genetic correlations between cognitive traits and various gastrointestinal disorders (Adewuyi et al., 2022). All these pieces of evidence suggest a close and primarily negative correlation between CRC and AD; however, there is also evidence indicating that the negative correlation between the two may be influenced by real-world factors. Firstly, research suggests that the lower risk of AD among cancer patients may be attributed to underdiagnosis of AD (Freedman et al., 2016). On the other hand, many AD patients, especially male patients, cannot undergo colonoscopy in a timely manner due to cognitive dysfunction, memory loss, and emotional issues, and may not be diagnosed until the disease is advanced or even until death (Yang et al., 2021; Lv et al., 2022; Morishima et al., 2023). Secondly, research has found that chemotherapy for cancer survivors can reduce the risk of AD, such as the multi-target kinase inhibitor regorafenib used to treat CRC that can inhibit the inflammatory response caused by microglia and treat AD (Han et al., 2020; Akushevich et al., 2021). And some other drugs such as aspirin, metformin, angiotensin-converting enzyme inhibitors, and melatonin have benefits for both CRC and AD (Hybiak et al., 2020; Shafabakhsh et al., 2020; Naseri et al., 2022; Bueno and Frasca, 2023). Therefore, AD or CRC patients may be suppressing the occurrence of one disease while taking these drugs. Furthermore, research findings have indicated a significant positive correlation between the two. For example, a study found that CRC patients with vascular-related diseases may promote the occurrence of AD, and from 2000 to 2016, the number of patients with CRC who died from AD increased by 180 times in the United States (Lu et al., 2021; Du et al., 2022). Additionally, CRC patients with prolonged anesthesia exposure during abdominal or pelvic surgery have been reported to be associated with an elevated risk of developing AD (Akushevich et al., 2022). Another study has found that the incidence of various cancers increases rather than decreases in individuals at high risk for AD (Valentine et al., 2022). These studies are not consistent with the conclusion that they can reduce the risk of AD in CRC patients, but support the finding that AD patients, as indicated by the MR analyses in this study, may contribute to an increased risk of CRC. However, as previously mentioned, a subgroup result of the MR analysis for the latter is not statistically significant, so the relationship between the two requires further clinical and foundational research.
There is still controversy over whether CRC and AD have common biological mechanisms, but there are already some biological theories explaining the inverse correlation between cancer and AD (Ibáñez et al., 2014; Guo et al., 2017; Nudelman et al., 2019; Lanni et al., 2021). It has been suggested that PIN1 may serve as a unique and critical regulator connecting cancer and AD. studies on brain samples from individuals with mild cognitive impairment and AD have demonstrated significantly reduced levels of PIN1 expression, while PIN1 is typically overexpressed in various human cancers, such as colorectal cancer (Lu et al., 1999; Bao et al., 2004). Moreover, the Wnt signaling pathway and TMEFF2 methylation exhibit opposite activation states or effects in CRC and AD, while other factors such as Psen1, microRNA, methylation, mitochondrial oxidative stress, and blood-brain barrier ATP-binding cassette transporter proteins also play a regulatory role in both diseases (Tremolizzo et al., 2006; Thinnes, 2012; Aliev et al., 2013; Manandhar et al., 2020; Masood et al., 2020; Ghafouri-Fard et al., 2021; Kadkhoda et al., 2022). In recent years, research on the gut-brain axis has linked gastrointestinal diseases to AD. The gut microbiome dysbiosis leads to the secretion of amyloid proteins and lipopolysaccharides (LPS). This disrupts gut permeability and the blood-brain barrier, promotes neuroinflammation, and ultimately results in neuronal death in AD through inflammatory signaling pathways and neuronal damage (Kesika et al., 2021; Kim et al., 2021; Escobar et al., 2022; Thu Thuy Nguyen and Endres, 2022). However, few studies have explored the relationship between CRC gut microbiome dysbiosis and AD, which may be a critical point in the mechanisms underlying these two diseases.
In general, multiple clinical and basic research studies have demonstrated a close relationship between CRC and AD, with the main association being inverse. However, there are many biases in the real world, such as those related to economics, drugs, cognition, lifespan, and diagnosis, which have led some studies to question this relationship. Both CRC and AD are important diseases that lead to death in the elderly. Therefore, understanding their common mechanisms is of great significance for the treatment of these two diseases as well as longevity-related research.
5 Strengths and limitations
This study provides evidence for a causal relationship between CRC and AD in European ancestry populations. The use of publicly available GWAS data saves research costs and time and does not violate ethical principles. MR simulates the random allocation process and explores the causal relationship between the two diseases from a genetic etiology perspective, reducing bias caused by various confounding factors and reverse causality. However, this study has some limitations. Firstly, MR cannot further investigate and explain the biological mechanisms by which genetic variations affect the two diseases. Secondly, due to the lack of detailed individual data, subgroup analysis by age or gender cannot be performed. Furthermore, this study only includes populations of European ancestry, and therefore may not represent other populations such as those of Asian or African ancestry well.
6 Conclusion
In summary, our MR analyses revealed a decreased risk of Alzheimer’s disease (AD) in colorectal cancer (CRC) patients, while a slightly elevated risk of CRC in AD patients was observed. But due to the impact of sample overlap, the results of the latter are not reliable. Despite various biases inherent in real-world data, our results provide additional support for the opposing mechanisms of CRC and AD. Nonetheless, given the close relationship between these two diseases, our study highlights the importance of early diagnosis and treatment for both patient groups. Further investigations are warranted to elucidate the underlying pathophysiological mechanisms and treatment strategies for both diseases.
Data availability statement
The datasets presented in this study can be found in online repositories at IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) or GWAS Catalog (https://www.ebi.ac.uk/gwas/).
Ethics statement
The studies involving humans were approved by The University of Bristol’s Faculty of Health Sciences Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CY and SL proposed the research design and drafted the manuscript. They contributed equally to this work and share first authorship. JZ and WZ searched for and evaluated the data. KY, FX, and YL analyzed the GWAS data. YG participated in proofreading and revising the manuscript. ZC critically revised the manuscript’s key content. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Beijing Municipal Natural Science Foundation (No.7212194).
Acknowledgments
We express our gratitude to the staff and researchers involved in the development of the IEU OPEN GWAS PROJECT.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1180905/full#supplementary-material
References
Aboalela, N., Lyon, D., Elswick, R. K., Kelly, D. L., Brumelle, J., Bear, H. D., et al. (2015). Perceived stress levels, chemotherapy, radiation treatment and tumor characteristics are associated with a persistent increased frequency of somatic chromosomal instability in women diagnosed with breast cancer: a one year longitudinal study. PLoS One 10 (7), e0133380. doi:10.1371/journal.pone.0133380
Adewuyi, E. O., O'Brien, E. K., Porter, T., and Laws, S. M. (2022). Relationship of cognition and Alzheimer's disease with gastrointestinal tract disorders: a large-scale genetic overlap and mendelian randomisation analysis. Int. J. Mol. Sci. 23 (24), 16199. doi:10.3390/ijms232416199
Ahles, T. A., and Hurria, A. (2018). New challenges in psycho-oncology research IV: cognition and cancer: conceptual and methodological issues and future directions. Psychooncology 27 (1), 3–9. doi:10.1002/pon.4564
Akushevich, I., Yashkin, A. P., Kravchenko, J., and Kertai, M. D. (2021). Chemotherapy and the risk of Alzheimer's disease in colorectal cancer survivors: evidence from the medicare system. JCO Oncol. Pract. 17 (11), e1649–e1659. doi:10.1200/OP.20.00729
Akushevich, I., Yashkin, A. P., Kravchenko, J., and Kertai, M. D. (2022). Extended anesthesia exposure for abdominal and pelvic procedures in older adults with colorectal cancer: associations with chart dementia diagnoses. Exp. Gerontol. 164, 111830. doi:10.1016/j.exger.2022.111830
Aliev, G., Obrenovich, M. E., Tabrez, S., Jabir, N. R., Reddy, V. P., Li, Y., et al. (2013). Link between cancer and Alzheimer disease via oxidative stress induced by nitric oxide-dependent mitochondrial DNA overproliferation and deletion. Oxid. Med. Cell Longev. 2013, 962984. doi:10.1155/2013/962984
Bao, L., Kimzey, A., Sauter, G., Sowadski, J. M., Lu, K. P., and Wang, D. G. (2004). Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 164 (5), 1727–1737. doi:10.1016/S0002-9440(10)63731-5
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44 (2), 512–525. doi:10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016a). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 (4), 304–314. doi:10.1002/gepi.21965
Bowden, J., Del Greco M, F., Minelli, C., Davey Smith, G., Sheehan, N. A., and Thompson, J. R. (2016b). Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45 (6), 1961–1974. doi:10.1093/ije/dyw220
Bray, F., Laversanne, M., Weiderpass, E., and Soerjomataram, I. (2021). The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127 (16), 3029–3030. doi:10.1002/cncr.33587
Bueno, V., and Frasca, D. (2023). Mini-review: angiotensin-converting enzyme 1 (ACE1) and the impact for diseases such as Alzheimer's disease, sarcopenia, cancer, and COVID-19. Front. Aging 4, 1117502. doi:10.3389/fragi.2023.1117502
Catalá-López, F., Hutton, B., Driver, J. A., Page, M. J., Ridao, M., Valderas, J. M., et al. (2017). Cancer and central nervous system disorders: protocol for an umbrella review of systematic reviews and updated meta-analyses of observational studies. Syst. Rev. 6 (1), 69. doi:10.1186/s13643-017-0466-y
Catalá-López, F., Suárez-Pinilla, M., Suárez-Pinilla, P., Valderas, J. M., Gómez-Beneyto, M., Martinez, S., et al. (2014). Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother. Psychosom. 83 (2), 89–105. doi:10.1159/000356498
Davies, N. M., Holmes, M. V., and Davey Smith, G. (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj 362, k601. doi:10.1136/bmj.k601
Driver, J. A. (2014). Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology 15 (6), 547–557. doi:10.1007/s10522-014-9523-2
Driver, J. A., Beiser, A., Au, R., Kreger, B. E., Splansky, G. L., Kurth, T., et al. (2012). Inverse association between cancer and Alzheimer's disease: results from the Framingham Heart Study. Bmj 344, e1442. doi:10.1136/bmj.e1442
Du, X. L., Song, L., Schulz, P. E., Xu, H., and Chan, W. (2022). Associations between vascular diseases and Alzheimer's disease or related dementias in a large cohort of men and women with colorectal cancer. J. Alzheimers Dis. 90 (1), 211–231. doi:10.3233/JAD-220548
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian randomization. Jama 318 (19), 1925–1926. doi:10.1001/jama.2017.17219
Escobar, Y. H., O'Piela, D., Wold, L. E., and Mackos, A. R. (2022). Influence of the microbiota-gut-brain Axis on cognition in Alzheimer's disease. J. Alzheimers Dis. 87 (1), 17–31. doi:10.3233/JAD-215290
Freedman, D. M., Wu, J., Chen, H., Kuncl, R. W., Enewold, L. R., Engels, E. A., et al. (2016). Associations between cancer and Alzheimer's disease in a U.S Medicare population. Cancer Med. 5 (10), 2965–2976. doi:10.1002/cam4.850
GBD 2019 Colorectal Cancer Collaborators (2022). Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 7 (7), 627–647. doi:10.1016/S2468-1253(22)00044-9
Ghafouri-Fard, S., Shoorei, H., Bahroudi, Z., Abak, A., Majidpoor, J., and Taheri, M. (2021). An update on the role of miR-124 in the pathogenesis of human disorders. Biomed. Pharmacother. 135, 111198. doi:10.1016/j.biopha.2020.111198
Guo, J., Cheng, J., North, B. J., and Wei, W. (2017). Functional analyses of major cancer-related signaling pathways in Alzheimer's disease etiology. Biochim. Biophys. Acta Rev. Cancer 1868 (2), 341–358. doi:10.1016/j.bbcan.2017.07.001
Han, K. M., Kang, R. J., Jeon, H., Lee, H. J., Lee, J. S., Park, H., et al. (2020). Regorafenib regulates AD pathology, neuroinflammation, and dendritic spinogenesis in cells and a mouse model of AD. Cells 9 (7), 1655. doi:10.3390/cells9071655
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-Base platform supports systematic causal inference across the human phenome. Elife 7, e34408. doi:10.7554/eLife.34408
Hermelink, K., Voigt, V., Kaste, J., Neufeld, F., Wuerstlein, R., Bühner, M., et al. (2015). Elucidating pretreatment cognitive impairment in breast cancer patients: the impact of cancer-related post-traumatic stress. J. Natl. Cancer Inst. 107 (7), djv099. doi:10.1093/jnci/djv099
Hybiak, J., Broniarek, I., Kiryczyński, G., Los, L. D., Rosik, J., Machaj, F., et al. (2020). Aspirin and its pleiotropic application. Eur. J. Pharmacol. 866, 172762. doi:10.1016/j.ejphar.2019.172762
Ibáñez, K., Boullosa, C., Tabarés-Seisdedos, R., Baudot, A., and Valencia, A. (2014). Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 10 (2), e1004173. doi:10.1371/journal.pgen.1004173
Jiang, T., Yu, J. T., Tian, Y., and Tan, L. (2013). Epidemiology and etiology of Alzheimer's disease: from genetic to non-genetic factors. Curr. Alzheimer Res. 10 (8), 852–867. doi:10.2174/15672050113109990155
Kadkhoda, S., Eslami, S., Mahmud Hussen, B., and Ghafouri-Fard, S. (2022). A review on the importance of miRNA-135 in human diseases. Front. Genet. 13, 973585. doi:10.3389/fgene.2022.973585
Kesika, P., Suganthy, N., Sivamaruthi, B. S., and Chaiyasut, C. (2021). Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer's disease. Life Sci. 264, 118627. doi:10.1016/j.lfs.2020.118627
Kim, N., Jeon, S. H., Ju, I. G., Gee, M. S., Do, J., Oh, M. S., et al. (2021). Transplantation of gut microbiota derived from Alzheimer's disease mouse model impairs memory function and neurogenesis in C57BL/6 mice. Brain Behav. Immun. 98, 357–365. doi:10.1016/j.bbi.2021.09.002
Lanni, C., Masi, M., Racchi, M., and Govoni, S. (2021). Cancer and Alzheimer's disease inverse relationship: an age-associated diverging derailment of shared pathways. Mol. Psychiatry 26 (1), 280–295. doi:10.1038/s41380-020-0760-2
Li, Q., Dong, Z., Lin, Y., Jia, X., and Jiang, H. (2013). The rs2233678 polymorphism in PIN1 promoter region reduced cancer risk: a meta-analysis. PLoS One 8 (7), e68148. doi:10.1371/journal.pone.0068148
Low, L. F., Harrison, F., and Lackersteen, S. M. (2013). Does personality affect risk for dementia? A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 21 (8), 713–728. doi:10.1016/j.jagp.2012.08.004
Lu, L., Ma, L., Zhang, X., Susanne Mullins, C., and Linnebacher, M. (2021). Analyzing non-cancer causes of death of colorectal carcinoma patients in the US population for the years 2000-2016. Cancer Med. 10 (8), 2740–2751. doi:10.1002/cam4.3673
Lu, P. J., Zhou, X. Z., Shen, M., and Lu, K. P. (1999). Function of WW domains as phosphoserine- or phosphothreonine-binding modules. Science 283 (5406), 1325–1328. doi:10.1126/science.283.5406.1325
Lv, G., Wang, X., Jiang, X., and Lu, K. (2022). Impact of Alzheimer's disease and related dementias on colorectal cancer screening utilization, knowledge, and associated health disparities. Front. Pharmacol. 13, 872702. doi:10.3389/fphar.2022.872702
Ma, L. L., Yu, J. T., Wang, H. F., Meng, X. F., Tan, C. C., Wang, C., et al. (2014). Association between cancer and Alzheimer's disease: systematic review and meta-analysis. J. Alzheimers Dis. 42 (2), 565–573. doi:10.3233/JAD-140168
Ma, S. L., Tang, N. L. S., Tam, C. W. C., Lui, V. W. C., Lam, L. C. W., Chiu, H. F. K., et al. (2012). A PIN1 polymorphism that prevents its suppression by AP4 associates with delayed onset of Alzheimer's disease. Neurobiol. Aging 33 (4), 804–813. doi:10.1016/j.neurobiolaging.2010.05.018
Manandhar, S., Kabekkodu, S. P., and Pai, K. S. R. (2020). Aberrant canonical Wnt signaling: phytochemical based modulation. Phytomedicine 76, 153243. doi:10.1016/j.phymed.2020.153243
Masood, M., Grimm, S., El-Bahrawy, M., and Yagüe, E. (2020). TMEFF2: a transmembrane proteoglycan with multifaceted actions in cancer and disease. Cancers (Basel) 12 (12), 3862. doi:10.3390/cancers12123862
Morishima, T., Kuwabara, Y., Saito, M. K., Odani, S., Kudo, H., Kato, M., et al. (2023). Patterns of staging, treatment, and mortality in gastric, colorectal, and lung cancer among older adults with and without preexisting dementia: a Japanese multicentre cohort study. BMC Cancer 23 (1), 67. doi:10.1186/s12885-022-10411-y
Musicco, M., Adorni, F., Di Santo, S., Prinelli, F., Pettenati, C., Caltagirone, C., et al. (2013). Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology 81 (4), 322–328. doi:10.1212/WNL.0b013e31829c5ec1
Naseri, A., Sanaie, S., Hamzehzadeh, S., Seyedi-Sahebari, S., Hosseini, M. S., Gholipour-Khalili, E., et al. (2022). Metformin: new applications for an old drug. J. Basic Clin. Physiol. Pharmacol. 34, 151–160. doi:10.1515/jbcpp-2022-0252
Nudelman, K. N. H., McDonald, B. C., Lahiri, D. K., and Saykin, A. J. (2019). Biological hallmarks of cancer in Alzheimer's disease. Mol. Neurobiol. 56 (10), 7173–7187. doi:10.1007/s12035-019-1591-5
Ospina-Romero, M., Glymour, M. M., Hayes-Larson, E., Mayeda, E. R., Graff, R. E., Brenowitz, W. D., et al. (2020). Association between alzheimer disease and cancer with evaluation of study biases: a systematic review and meta-analysis. JAMA Netw. Open 3 (11), e2025515. doi:10.1001/jamanetworkopen.2020.25515
Panegyres, P. K., and Chen, H. Y. (2021). Alzheimer's disease, Huntington's disease and cancer. J. Clin. Neurosci. 93, 103–105. doi:10.1016/j.jocn.2021.09.012
Park, S., Yu, S. J., Cho, Y., Balch, C., Lee, J., Kim, Y. H., et al. (2015). Network comparison of inflammation in colorectal cancer and Alzheimer's disease. Biomed. Res. Int. 2015, 205247. doi:10.1155/2015/205247
Rao, S., Lau, A., and So, H. C. (2020). Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care 43 (7), 1416–1426. doi:10.2337/dc20-0643
Ribe, A. R., Laursen, T. M., Charles, M., Katon, W., Fenger-Grøn, M., Davydow, D., et al. (2015). Long-term risk of dementia in persons with schizophrenia: a Danish population-based cohort study. JAMA Psychiatry 72 (11), 1095–1101. doi:10.1001/jamapsychiatry.2015.1546
Sanderson, E., Glymour, M. M., Holmes, M. V., Kang, H., Morrison, J., Munafò, M. R., et al. (2022). Mendelian randomization. Nat. Rev. Methods Prim. 2 (1), 6. doi:10.1038/s43586-021-00092-5
Schwartzentruber, J., Cooper, S., Liu, J. Z., Barrio-Hernandez, I., Bello, E., Kumasaka, N., et al. (2021). Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer's disease risk genes. Nat. Genet. 53 (3), 392–402. doi:10.1038/s41588-020-00776-w
Shafabakhsh, R., Mirzaei, H., and Asemi, Z. (2020). Melatonin: a promising agent targeting leukemia. J. Cell Biochem. 121 (4), 2730–2738. doi:10.1002/jcb.29495
Shah, S. C., and Itzkowitz, S. H. (2022). Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology 162 (3), 715–730.e3. doi:10.1053/j.gastro.2021.10.035
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tahami Monfared, A. A., Byrnes, M. J., White, L. A., and Zhang, Q. (2022). Alzheimer's disease: Epidemiology and clinical progression. Neurol. Ther. 11 (2), 553–569. doi:10.1007/s40120-022-00338-8
Taudorf, L., Nørgaard, A., Waldemar, G., and Laursen, T. M. (2021). Mortality in dementia from 1996 to 2015: a national registry-based cohort study. J. Alzheimers Dis. 79 (1), 289–300. doi:10.3233/JAD-200823
Thinnes, F. P. (2012). Why cancer survivors have a lower risk of Alzheimer disease. Mol. Genet. Metab. 107 (3), 630–631. doi:10.1016/j.ymgme.2012.06.016
Thu Thuy Nguyen, V., and Endres, K. (2022). Targeting gut microbiota to alleviate neuroinflammation in Alzheimer's disease. Adv. Drug Deliv. Rev. 188, 114418. doi:10.1016/j.addr.2022.114418
Tremolizzo, L., Rodriguez-Menendez, V., Brighina, L., and Ferrarese, C. (2006). Is the inverse association between Alzheimer's disease and cancer the result of a different propensity to methylate DNA? Med. Hypotheses 66 (6), 1251–1252. doi:10.1016/j.mehy.2005.12.022
Valentine, D., Teerlink, C. C., Farnham, J. M., Rowe, K., Kaddas, H., Tschanz, J., et al. (2022). Comorbidity and cancer disease rates among those at high-risk for Alzheimer's disease: a population database analysis. Int. J. Environ. Res. Public Health 19 (24), 16419. doi:10.3390/ijerph192416419
Verbanck, M., Chen, C. Y., and Neale, B. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50 (5), 693–698. doi:10.1038/s41588-018-0099-7
Wang, Y. Z., and Shen, H. B. (2020). Challenges and factors that influencing causal inference and interpretation, based on Mendelian randomization studies. Zhonghua Liu Xing Bing Xue Za Zhi 41 (8), 1231–1236. doi:10.3760/cma.j.cn112338-20200521-00749
Whitlock, E. L., Diaz-Ramirez, L. G., Glymour, M. M., Boscardin, W. J., Covinsky, K. E., and Smith, A. K. (2017). Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med. 177 (8), 1146–1153. doi:10.1001/jamainternmed.2017.1622
Xie, J., Huang, H., Liu, Z., Yu, C., Xu, L., et al. (2023). The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology 77 (3), 949–964. doi:10.1002/hep.32728
Yang, S., Bian, J., George, T. J., Daily, K., Zhang, D., Braithwaite, D., et al. (2021). The association between cognitive impairment and breast and colorectal cancer screening utilization. BMC Cancer 21 (1), 539. doi:10.1186/s12885-021-08321-6
Keywords: colorectal cancer, Alzheimer’s disease, two-sample Mendelian randomization, genome-wide association study, causal effect
Citation: Yuan C, Liu S, Yang K, Xie F, Li Y, Guo Y, Zhao W, Zhang J and Cheng Z (2024) Causal association between colorectal cancer and Alzheimer’s disease: a bidirectional two-sample mendelian randomization study. Front. Genet. 14:1180905. doi: 10.3389/fgene.2023.1180905
Received: 06 March 2023; Accepted: 11 December 2023;
Published: 05 January 2024.
Edited by:
Shitao Rao, Fujian Medical University, ChinaCopyright © 2024 Yuan, Liu, Yang, Xie, Li, Guo, Zhao, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiqiang Cheng, emhpcWlhbmdjaGVuZ0AxNjMuY29t
†These authors have contributed equally to this work
 Chunsheng Yuan
Chunsheng Yuan Saisai Liu1,3†
Saisai Liu1,3† Kezhen Yang
Kezhen Yang Yinan Li
Yinan Li