- 1 Department of Statistics, Harvard University, Cambridge, MA, United States
- 2 Department of Statistics, Carnegie Mellon University, Pittsburgh, PA, United States
Subject clustering (i.e., the use of measured features to cluster subjects, such as patients or cells, into multiple groups) is a problem of significant interest. In recent years, many approaches have been proposed, among which unsupervised deep learning (UDL) has received much attention. Two interesting questions are 1) how to combine the strengths of UDL and other approaches and 2) how these approaches compare to each other. We combine the variational auto-encoder (VAE), a popular UDL approach, with the recent idea of influential feature-principal component analysis (IF-PCA) and propose IF-VAE as a new method for subject clustering. We study IF-VAE and compare it with several other methods (including IF-PCA, VAE, Seurat, and SC3) on 10 gene microarray data sets and eight single-cell RNA-seq data sets. We find that IF-VAE shows significant improvement over VAE, but still underperforms compared to IF-PCA. We also find that IF-PCA is quite competitive, slightly outperforming Seurat and SC3 over the eight single-cell data sets. IF-PCA is conceptually simple and permits delicate analysis. We demonstrate that IF-PCA is capable of achieving phase transition in a rare/weak model. Comparatively, Seurat and SC3 are more complex and theoretically difficult to analyze (for these reasons, their optimality remains unclear).
1 Introduction
We are interested in the problem of high-dimensional clustering or subject clustering. Suppose we have a group of n subjects (e.g., patients or cells) measured on the same set of p features (e.g., genes). The subjects come from K different classes or groups (e.g., normal group and diseased group), but unfortunately, the class labels are unknown. In such a case, we say the data are unlabeled. For 1 ≤ i ≤ n, we denote the class label of subject i by Y i and denote the p-dimensional measured feature vector of subject i by X i . It is important to note that Y i takes values from {1, 2, …, K}. The class labels are unknown, and the goal is to predict them using the measured features X 1, X 2, …, X n .
High-dimensional clustering is an unsupervised learning problem. It is especially interesting in the big data era: although the volume of available scientific data increases rapidly, a significant fraction of them are unlabeled. In some cases, it is simply hard to label each individual sample [e.g., action unit recognition (Wu et al., 2015)]. In some other cases, labeling each individual sample is not hard, but due to the large sample size, it takes a substantial amount of time and effort to label the whole data set [e.g., ImageNet (Deng et al., 2009)]. In other instances (e.g., cancer diagnosis), we may have a preliminary opinion on how to label the data, but we are unsure of the labels’ accuracy, so we would like a second, preferably independent, opinion. In all these cases, we seek an effective and user-friendly clustering method.
In recent years, the area of high-dimensional clustering has witnessed exciting advancements in several directions. First, many new types of data sets (e.g., single-cell data) have emerged and become increasingly more accessible. Second, remarkable successes have been made in non-linear modeling for high-dimensional data, and several unsupervised deep learning (UDL) approaches have been proposed (Fan et al., 2021), including, but not limited to, the variational auto-encoder (VAE) and generative adversarial network (GAN). Finally, several clustering methods for single-cell data [e.g., Seurat (Satija et al., 2015) and SC3 (Kiselev et al., 2017)] have been proposed and become popular.
In this paper, we are primarily interested in influential feature-principal component analysis (IF-PCA), a clustering algorithm proposed by Jin and Wang (2016). As in many recent works in high-dimensional data analysis (e.g., Abramovich et al., 2006; Paul, 2007), we assume
• p ≫ n ≫ 1
• Out of all p measured features, only a small fraction of them are relevant to the clustering decision.
IF-PCA is easy-to-use and does not have tuning parameters. It is conceptually simple and (on a high level) contains two steps as follows:
• IF step. A feature selection step that selects a small fraction of measured features, which we believe to be influential or significant to the clustering decision.
• Clustering step. A clustering step in which PCA (as a spectral clustering approach) is applied to all retained features.Instead of viewing IF-PCA as a specific clustering algorithm, we can view it as a generic two-step clustering approach: for each of the two steps, we can choose methods that may vary from occasion to occasion in order to best suit the nature of the data. We anticipate that IF-PCA will adapt and develop over time as new data sets and tasks emerge.
Jin and Wang (2016) compared IF-PCA to a number of clustering algorithms [including the classical k-means (MacQueen, 1967), k-means++ (Arthur and Vassilvitskii, 2007), Spectral-Gem (Lee et al., 2010b), hierarchical clustering (Hastie et al., 2009), and sparse PCA (Zou et al., 2006)] using 10 microarray data sets. They found that IF-PCA was competitive in clustering accuracy. Later, Jin et al. (2017) developed a theoretical framework for clustering and showed that IF-PCA is optimal in the rare/weak signal model [a frequently used model in high-dimensional data analysis (Donoho and Jin, 2004; Donoho and Jin, 2015)].
These appealing properties of IF-PCA motivate a revisit of this method. Specifically, we are interested in the two questions listed below.
• There are many recent clustering algorithms specifically designed for single-cell data, such as Seurat (Satija et al., 2015), SC3 (Kiselev et al., 2017), RaceID (Grün et al., 2015), ActioNet (Mohammadi et al., 2020), Monocle 3 (Trapnell et al., 2014), and SINCERA (Guo et al., 2015). In addition, many UDL algorithms have been proposed and become well-known in recent years. An interesting question is how IF-PCA compares with these popular algorithms.
Jin and Wang (2016) only examined IF-PCA with gene microarray data. The single-cell RNA-seq data are similar to gene microarray data in some aspects but also have some distinguished characteristics [e.g., single-cell RNA-sequencing provides an unbiased view of all transcripts and is, therefore, reliable for accurately measuring gene expression level changes (Zhao et al., 2014)]. How IF-PCA compares to other popular methods for subject clustering with single-cell data is an intriguing question.
• The PCA employed in the clustering step of IF-PCA is a linear method. Although we believe that the associations between class labels and measured features may be non-linear, the significance of the non-linear effects is unclear. To investigate this, we may consider a variant of IF-PCA, in which PCA is replaced by some non-linear UDL methods in the clustering step. An interesting question is how this variant compares to IF-PCA and standard UDL methods (which has no IF step). It helps us understand how significant the non-linear effects are.
To answer these questions, first, we propose a new approach, IF-VAE, which combines the main idea of IF-PCA with the VAE (Kingma and Welling, 2013) (one of the most popular UDL approaches in recent literature).
Second, we compare the IF-VAE with several methods, including VAE, IF-PCA, Spectral-Gem (Lee et al., 2010b), and classical k-means, using the 10 microarray data sets in Jin and Wang (2016). We find that
• Somewhat surprisingly, the VAE underperforms compared to most other methods, including the classical k-means.
• IF-VAE, which combines the VAE with the IF step of IF-PCA, significantly outperforms the VAE.
• The performance of IF-PCA and IF-VAE is comparable for approximately half of the data sets, whereas IF-VAE significantly underperforms compared to IF-PCA for the remaining half of the data sets.
These results suggest the following:
• (a) The idea of combining the IF step in the IF-PCA with the VAE is valuable.
• (b) Deep neural network methods do not appear to have a clear advantage for this type of data set.
For (b), one possible reason is that the associations between class labels and measured features are not highly non-linear. Another possible reason is that existing deep neural network approaches need further improvements in order to perform satisfactorily on these data sets. Since IF-PCA and IF-VAE use the same IF step, the unsatisfactory performance of IF-VAE is largely attributable to the VAE step, and not the IF step. To see this, we observe that Spectral-Gem is essentially the classical PCA clustering method (see Section 2.2). The VAE does not show an advantage over Spectral-Gem, explaining why IF-VAE cannot outperform IF-PCA.
Last, we compare the IF-VAE with IF-PCA, Seurat, and SC3 on eight single-cell RNA-seq data sets. We observe that
• IF-VAE continues to underperform compared to other methods on the eight single-cell data sets, but similar as previously mentioned; the unsatisfactory performance is largely attributable to the VAE step and not the IF step.
• IF-PCA outperforms SC3 slightly and outperforms Seurat more significantly.
At the same time, we observe that
• Seurat has four tuning parameters and is the method that has the shortest execution time.
• The idea of SC3 is quite similar to that of IF-PCA, except that SC3 has a “consensus voting” step that aggregates the strengths of many clustering results. With consensus voting, SC3 may empirically perform more satisfactorily, but it is also more complex internally. Regarding the computational cost, it runs much slower than IF-PCA due to the consensus voting step.
Moreover, IF-PCA is conceptually simple and permits fine-grained analysis. In Section 4, we develop a theoretical framework and show that IF-PCA achieves the optimal phase transition in a rare/weak signal setting. Especially, we show in the region of interest (where successful subject clustering is possible),
• if the signals are less sparse, signals may be individually weak. In this case, PCA is optimal (and IF-PCA reduces to PCA if we choose the IF step properly).
• If the signals are sparser, the signals need to be relatively strong (so successful clustering is possible). In this case, feature selection is necessary and IF-PCA is optimal. However, PCA may be non-optimal as it does not use a feature selection step.
In comparison, other popular methods are difficult to analyze theoretically; hence, their optimality is unclear. We note that hard-to-analyze methods will also be hard to improve in the future.
In conclusion, IF-PCA is quite competitive compared to the recently popular subject clustering methods, both for gene microarray data and single-cell data. It is worthwhile to study IF-PCA both theoretically and in (a variety of) applications. IF-VAE is a significant improvement over VAE, but it is still inferior to other prevalent methods in this area (the underperformance is largely due to the VAE step, not the IF step). It is desirable to further improve IF-VAE (especially the VAE step) to make it more competitive.
2 Models and methods
As before, suppose we have measurements on the same set of p features for n samples. We denote the data matrix by
where
In Section 2.1, we introduce two models for X; then in Sections 2.2, 2.3, 2.4, 2.5, 2.6, we describe the clustering methods considered in this paper, some of which (e.g., IF-VAE, IF-VAE(X), and IF-PCA(X)) are new.
2.1 Two models
A reasonable model is as follows. We encode the class label Y
i
as a K-dimensional vector π
i
, where π
i
= e
k
if and only if sample i belongs to class k, and e
k
is the kth standard Euclidean basis vector of
Let
In addition, it is reasonable to assume that out of many measured features, only a small fraction of them are useful in the clustering decision. Therefore, letting
It follows that the n × p signal matrix
We recall that W is the normalized data matrix. Similar to (5), we may decompose W as the sum of a signal matrix and a noise matrix. However, due to the normalization, the rank of the signal matrix is reduced to (K − 1).
In Models (3)–(5),
The recent idea of neural network modeling provides a wide class of non-linear models, which may be useful for our setting. As an alternative to Models (3)–(5), we may consider a neural network model as follows. In this model, we assume
where f (x, θ) belongs to a class of non-linear functions. For example, we may assume f (x, θ) belongs to the class of functions (without loss of generality, x always includes a constant feature):
where A 1, A 2, … , A L are matrices of certain sizes and s 1, s 2, … , s L are some nonlinear functions. Similar to Models (3)–(5), we can impose some sparsity conditions on Model (6). See Fan et al. (2021) for example.
2.2 The PCA clustering approach and Spectral-Gem
PCA is a classical spectral clustering approach, which is especially appropriate for linear models like those in (3–5) when the relevant features are non-sparse (see the following text for discussions on the case when the relevant features are sparse). The PCA clustering approach contains two simple steps as follows. Input: normalized data matrix X and number of clusters K. Output: predicted class label vector
• We obtain the n × K matrix
• We cluster the n rows of
From time to time, we choose to apply the PCA clustering approach to the normalized data matrix W. As explained before, we can similarly write W as the sum of a “signal” matrix and a “noise” matrix as in (5), but due to the normalization, the rank of the “signal” matrix under Model (3) is reduced from K to (K − 1). In such a case, we replace the n × K matrix
where, similarly,
The PCA clustering approach has many modern variants, including, but not limited to, Spectral-Gem (Lee et al., 2010b) and SCORE (Jin, 2015; Ke and Jin, 2023). In this paper, we consider Spectral-Gem but skip the discussion on SCORE (SCORE was motivated by unsupervised learning in network and text data and shown to be effective on those types of data; it is unclear whether SCORE is also effective for genetic and genomic data). Instead of applying PCA clustering to the data matrix X (or W) directly, Spectral-Gem constructs an n × n symmetric matrix M, where M(i, j) can be viewed as a similarity metric between subject i and subject j. The remaining part of the algorithm has many small steps, but the essence is to apply the PCA clustering approach to the Laplacian normalized graph induced by M.
The PCA spectral clustering approach is based on two important assumptions.
• The signal matrix
• It is hard to exploit sparsity in the data: either the data are non-sparse (such as the classical setting of p ≪ n) or how the sparsity can be exploited is unclear.
In many modern settings, these assumptions are not satisfied: the relationship between the signal matrix
2.3 The variational autoencoder and VAE(X) clustering approaches
Given an n × p data matrix X and an integer d ≤ rank(X), the essence of the PCA spectral clustering approach is to obtain a rank-d approximation of X to use singular value decomposition (SVD),
Here, σ k is the kth smallest singular value of X, and u k and v k are the corresponding left and right singular vectors of X, respectively. The VAE can be viewed as an extension of SVD, which obtains a rank-d approximation of X from training a neural network. The classical SVD is a linear method, but the neural network approach can be highly non-linear.
The VAE was first introduced by Kingma and Welling (2013) and has been successfully applied to many areas [e.g., image processing (Razavi et al., 2019), computer vision (Goodfellow et al., 2020), and text mining (Serban et al., 2017)]. The VAE consists of an encoder, a decoder, and a loss function. Given a data matrix
A popular way to use the VAE for subject clustering is as follows (Wang and Gu, 2018). Input: normalized data matrix
• (Dimension reduction by the VAE). We train the VAE and use the trained encoder to obtain an n × d matrix
• (Clustering). We cluster all n subjects into K classes by applying k-means to the rows of
Except for using a non-linear approach to dimension reduction, the VAE is similar to the PCA approach in clustering. We can apply the VAE either to the normalized data matrix W or the unnormalized data matrix X. We call them VAE(W) and VAE(X), respectively. In the context of using these notations, it is unnecessary to use (W) and (X) at the same time, so we write VAE(W) as VAE for short (and to avoid confusion, we still write VAE(X) as VAE(X)).
2.4 The orthodox IF-PCA and its variant IF-PCA(X)
For many genomic and genetic data, Models (3)–(5) are already reasonable models. We recall that under these models, the normalized data matrix can be approximately written as
where, approximately,
and is sparse (in the sense that only a small fraction of the columns of Q have a large ℓ 2-norm; the ℓ 2-norm of other columns is small or 0). In such a setting, it is appropriate to conduct feature selection, which removes a substantial amount of noise while keeping most non-zero columns of Q.
Such observations motivate the (orthodox) IF-PCA. The IF-PCA was first proposed in Jin and Wang (2016) and has been shown to have appealing clustering results with 10 gene microarray data sets. In Jin et al. (2017), it was shown that IF-PCA is optimal in high-dimensional clustering. IF-PCA contains an IF step and a PCA step, and the IF step contains two important components introduced as follows.
The first component of the IF step is the use of the Kolmogorov–Smirnov (KS) test for feature selection. Suppose we have n (univariate) samples z 1, z 2, … , z n from a cumulative distribution function (CDF) denoted by F. We introduce the empirical CDF by
Let z = (z 1, z 2, … , z n ). The KS testing score is then
In the IF-PCA given as follows, we take F to be the theoretical CDF of
The second component of the IF step is the higher criticism threshold (HCT). Higher criticism (HC) was initially introduced by Donoho and Jin (2004) (see also Jager and Wellner, 2007; Hall and Jin, 2010; Donoho and Jin, 2015; Verzelen and Arias-Castro, 2017) as a method for global testing. It has been recently applied to genetic data (e.g., Barnett et al., 2017). HCT adapts HC to a data-driven threshold choice (Jin and Wang, 2016). It takes as input p marginal p-values, one for a feature, and outputs a threshold for feature selection. Suppose we have p-values π 1, π 2, … , π p . We sort them in the ascending order:
We define the feature-wise HC score by
IF-PCA runs as follows. Input: normalized feature vectors
• (IF step). For each 1 ≤ j ≤ p, we compute a KS score for feature j by applying (7, 8) with z = w
j
. We denote the KS scores by ϕ
n
(w
1), …, ϕ
n
(w
p
) and let μ* and σ* be their empirical mean and standard deviation, respectively. Let
• (Clustering step). Let W
IF
be the n × m sub-matrix of W consisting of columns of W corresponding to the retained features only [m is the number of retained features in (a)]. For any 1 ≤ k ≤ min{m, n}, let
In the IF step, the normalization of
In the (orthodox) IF-PCA, we apply both the IF step and the clustering step to the normalized data matrix W. Seemingly, for the IF step, applying the algorithm to W instead of the un-normalized data matrix X is preferred. However, for the clustering step, whether we should apply the algorithm to W or X remains unclear. We propose a small variant of IF-PCA by applying the IF step and the clustering step to W and X, respectively.
• (IF step). We apply exactly the same IF step to W as in the (orthodox) IF-PCA previously mentioned.
• (Clustering step). Let X
IF
be the n × m sub-matrix of X consisting of columns of X corresponding to the retained features in the IF step only. For any 1 ≤ k ≤ min{m, n}, let
To differentiate from the (orthodox) IF-PCA (which we call IF-PCA as follows), we call the aforementioned variant IF-PCA(X). See Table 1 in Section 2.7. The new variant was never proposed or studied before. It outperforms the (orthodox) IF-PCA in several data sets (e.g., see Section 3).
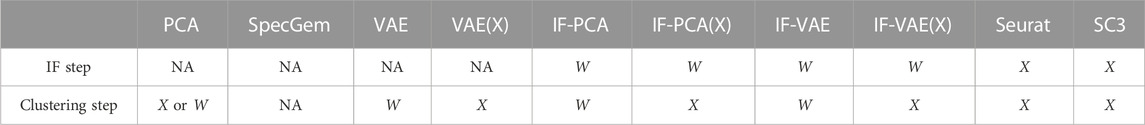
TABLE 1. Summary of all methods discussed in this section. This table clarifies the small differences between similar methods. Take the column IF-PCA(X) for example: “W” on row 2 means that the IF step of this method is applied to the normalized data matrix W defined in (2), and “X” on row 3 means the clustering step is applied to the un-normalized data matrix X (NA: not applicable).
2.5 IF-VAE and IF-VAE(X)
Near the end of Section 2.2, we mention that the classical PCA has two disadvantages: not exploiting sparsity in feature vectors and not accounting for possible non-linear relationships between the signal matrix and class labels. In Sections 2.3, 2.4, we have seen that the VAE aims to exploit non-linear relationships and IF-PCA aims to exploit sparsity. We may combine the VAE with the IF step of IF-PCA for simultaneously exploiting sparsity and non-linearity. To this end, we propose a new algorithm called IF-VAE.
The IF-VAE contains an IF step and a clustering step and runs as follows. Input: normalized data matrix
• (IF step). We run the same IF step as in Section 2.4, and let
• (Clustering step). We apply the VAE with
In the clustering step, we apply the VAE to the normalized data matrix W. Similarly, as in Section 2.4, if we apply the VAE to the un-normalized data matrix X, then we have a variant of IF-VAE, which we denote by IF-VAE(X). See Table 1 in Section 2.7.
2.6 Seurat and SC3
We now introduce Seurat and SC3, two recent algorithms that are especially popular for subject clustering with single-cell RNA-seq data. We discuss them separately.
Seurat was proposed in Satija et al. (2015). On a high level, Seurat is quite similar to IF-PCA, and we can view it as having only two main steps: a feature selection step and a clustering step. However, different from IF-PCA, Seurat uses a different feature selection step and a much more complicated clustering step (which combines several methods including PCA, k-nearest neighborhood algorithm, and modularity optimization). Seurat needs four tuning parameters: m, N, k 0, δ, where m is the number of selected features in the feature selection step, and N, k 0, δ are the clustering step, corresponding to the PCA part, the k-nearest neighborhood algorithm part, and the modularity optimization part, respectively.
A high-level sketch for Seurat is mentioned as follows (see Satija et al., 2015 for a more detailed description). Input: un-normalized n × p data matrix X, number of clusters K, and tuning parameters m, N, k
0, δ. Output: predicted class label vectors
• (IF step). We select the m features that are mostly variable. We obtain the n × m post-selection data matrix.
• (Clustering step). We normalize the post-selection data matrix and obtain the first N left singular vectors. For each pair of subjects, we compute how many neighbors (for each subject, we only count the k 0 nearest neighbors) they share with each other and use the results to construct a shared nearest neighborhood (SNN) graph. We cluster the class labels by applying a modularity optimization algorithm to the SNN graph, where we need a resolution parameter δ.
An apparent limitation of Seurat is that it needs four tuning parameters. Following the recommendations by Hao et al. (2021), we may take (N, k 0) = (50, 20), but it remains unclear how to select (m, δ).
SC3 was first presented by Kiselev et al. (2017). To be consistent with many other methods we discuss in this paper, we may view SC3 as containing two main steps: a gene-filtering step and a clustering step. Similar to Seurat, the clustering step of SC3 is much more complicated than that of IF-PCA, where the main idea is to apply PCA many times (each for a different number of leading singular vectors) and use the results to construct a matrix of consensus. We then cluster all subjects into K groups by applying the classical hierarchical clustering method to the consensus matrix. SC3 uses one tuning parameter x 0 in the gene-filtering step and two tuning parameters, d 0 and k 0, in the clustering step, corresponding to the PCA part and the hierarchical clustering part, respectively.
A high-level sketch for SC3 is given as follows (see Kiselev et al., 2017 for a more detailed description). Input: un-normalized n × p data matrix X, true number of clusters K, and tuning parameters x
0, d
0, k
0. Output: predicted class label vectors
• (Gene-filtering step). Removes genes/transcripts that are either expressed (expression value is more than 2) in less than x 0% of cells or expressed (expression value is more than 0) in at least (100 − x 0)% of cells. This step may reduce a significant fraction of features, and we consider it to be more like a feature selection step than a preprocessing step.
• (Clustering step). First, we take a log-transformation of the post-filtering data matrix and construct an n × n matrix M, where M(i, j) is some kind of distance (e.g., Euclidean, Pearson, and Spearman) between subject i and j. Second, let
Following the recommendation by Kiselev et al. (2017), we set (x 0, d 0) = (6, 15) and take k 0 to be the true number of clusters K. Such a tuning parameter choice may work effectively in some cases, but for more general cases, we may (as partially mentioned in Kiselev et al. (2017)] need more complicated tuning.
In summary, on a high level, we can view both Seurat and SC3 as two-stage algorithms, which consist of a feature selection step and a clustering step, just as in IF-PCA. However, these methods use more complicated clustering steps, where the key is combining many different clustering results to reach a consensus; it is important to note that the SNN in Seurat can be viewed as a type of consensus matrix. Such additional steps taken in Seurat and SC3 may not only help reduce the clustering error rates but also make the algorithms conceptually more complex, computationally more expensive, and theoretically more difficult to analyze.
2.7 A brief summary of all the methods
We have introduced approximately 10 different methods, some of which (e.g., IF-PCA(X), IF-VAE, and IF-VAE(X)) have never never proposed before. Among these methods, the VAE is a popular UDL approach, Seurat and SC3 are especially popular in clustering with single-cell data, and IF-PCA is a conceptually simple method that has been shown to be effective in clustering with gene microarray data. It is important to note that some of the methods are conceptually similar to each other, with some small differences (though it is unclear how different their empirical performances are). For example, many of these methods are two-stage methods, containing an IF step and a clustering step. In the IF step, we usually use the normalized data matrix W. In the clustering step, we may use either W or the unnormalized data matrix X. To summarize all these methods and especially to clarify the small differences between similar methods, we have prepared a table given as follows; see Table 1 for details.
3 Results
Our study consists of two parts. In Section 3.1, we compare the IF-VAE with several other methods using 10 microarray data sets. In Section 3.2, we compare the IF-VAE with several other methods, including the popular approaches of Seurat and SC3, using eight single-cell data sets. In all these data sets, the class labels are given. However, we do not use the class labels in any of the clustering approaches; we only use them when we evaluate the error rates. The code for numerical results in this section can be found at https://github.com/ZhengTracyKe/IFPCA. The 10 microarray data sets can be downloaded at https://data.mendeley.com/datasets/cdsz2ddv3t, and the eight single-cell RNA-seq data sets can be downloaded at https://data.mendeley.com/drafts/nv2x6kf5rd.
3.1 Comparison of clustering approaches with 10 microarray data sets
Table 2 tabulates 10 gene microarray data sets (alphabetically) studied in Jin and Wang (2016). Here, data sets 1, 3, 4, 7, 8, and 9 were analyzed and cleaned in Dettling (2004) and data sets 2, 6, and 10 were analyzed and grouped into two classes in Yousefi et al. (2010), among which data set 10 was cleaned by Jin and Wang (2016) in the same way as Dettling (2004). Data set 5 is obtained from Gordon et al. (2002).
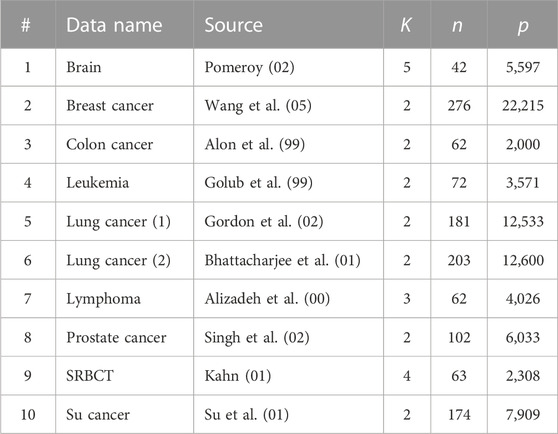
TABLE 2. Ten gene microarray data sets analyzed in Section 3.1 (n: number of subjects; p: number of genes; K: number of clusters).
First, we compare the IF-VAE approach introduced in Section 2.5 with four existing clustering methods: 1) the classical k-means; 2) Spectral-GEM (SpecGem) (Lee et al., 2010a), which is essentially classical PCA combined with a Laplacian normalization; 3) the orthodox IF-PCA (Jin and Wang, 2016), which adds a feature selection step prior to spectral clustering (see Section 2.4 for details); and 4) the VAE approach, which uses the VAE for dimension reduction and then runs k-means clustering (see Section 2.3 for details). Among these methods, SpecGem and VAE involve dimension reduction, and IF-PCA and IF-VAE use both dimension reduction and feature selection. For IF-PCA, VAE, and IF-VAE, we can implement the PCA step and the VAE step to either the original data matrix X or the normalized data matrix W. The version of IF-PCA associated with X is called IF-PCA(X), and the version associated with W is still called IF-PCA; similar rules apply to the VAE and IF-VAE. A total of eight different algorithms were obtained.
Table 3 shows the numbers of clustering errors (i.e., number of incorrectly clustered samples, subject to a permutation of K clusters) of these methods. The results of SpecGem and IF-PCA are copied from Jin and Wang (2016). We implemented k-means using the Python library sklearn, wrote the MATLAB code for IF-PCA(X), and wrote the Python code for the remaining four methods. The IF step of IF-VAE needs no tuning. In the VAE step of IF-VAE, we fix the latent dimension as d = 25 and use a traditional architecture in which both the encoder and decoder have one hidden layer; the encoder uses the ReLU activation, and the decoder uses the sigmoid activation; when training the encoder and decoder, we use a mini-batch stochastic gradient descent with 50 batches, 100 epochs, and a learning rate of 0.0005. The same neural network architecture and tuning parameters are applied to the VAE. We note that the outputs of these methods may have randomness due to the initialization in the k-means step or in the VAE step. For the VAE, IF-VAE, and IF-VAE(X), we repeat the algorithm 10 times and report the average clustering error. For k-means, we repeat it five times (because the results are more stable); for IF-PCA(X), we repeat it 20 times. We use the clustering errors to rank all eight methods for each data set; in the presence of ties, we assign ranks in a way such that the total rank sum is 36 (e.g., if two methods have the smallest error rate, we rank both of them as 1.5 and rank the second-best method as 3; other cases are similar). The average rank of a method is a metric of its overall performance across multiple data sets. In addition to ranks, we also compute regrets: for each data set, the regret of a method is defined to be r = (e − e min )/(e max − e min ), where e is the clustering error of this method and e max and e min are the maximum and minimum clustering error, respectively, among all the methods. The average regret also measures the overall performance of a method (the smaller, the better).
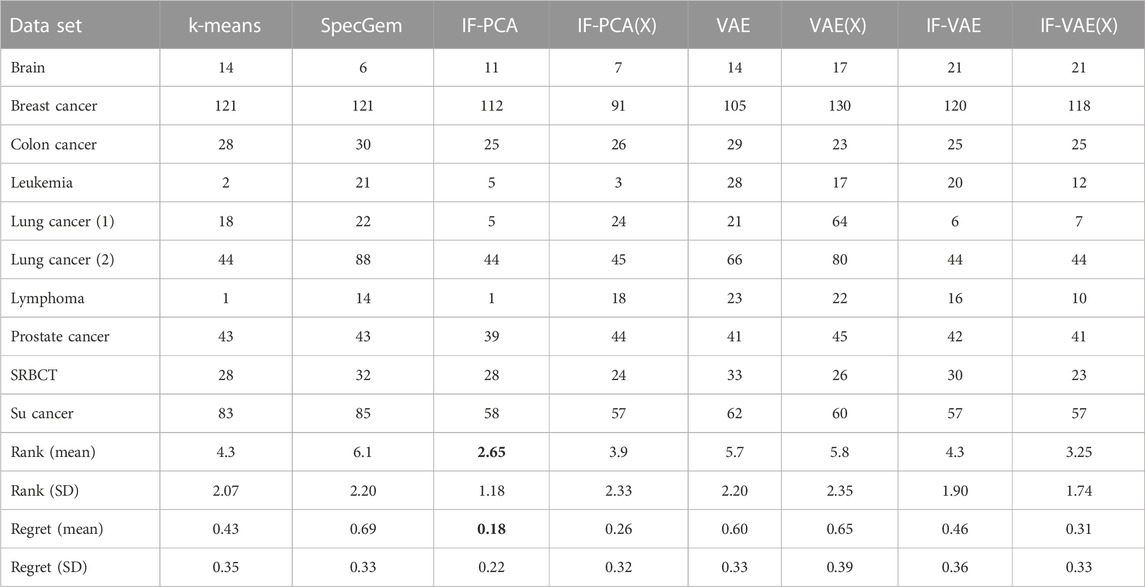
TABLE 3. Comparison of clustering errors of different methods with the 10 microarray data sets in Table 2. IF-PCA has the smallest average rank and average regret (boldface) and is regarded as the best on average.
There are several notable observations. First, somewhat surprisingly, the simple and tuning-free method, IF-PCA, has the best overall performance. It has the lowest average rank among all eight methods and achieves the smallest number of clustering errors in four out of 10 data sets. We recall that the key idea of IF-PCA is to add a tuning-free feature selection step prior to dimension reduction. The results in Table 2 confirm that this idea is highly effective with microarray data and hard to surpass by other methods. Second, the VAE (either on W or on X), which combines k-means with non-linear dimension reduction, significantly improves k-means on some “difficult” data sets, such as BreastCancer, ColonCancer, and SuCancer. However, for those “easy” datasets, such as Leukemia and Lymphoma, the VAE significantly underperforms compared to k-means. It suggests that the non-linear dimension reduction is useful mainly on “difficult” datasets. Third, the IF-VAE (either on W or on X) improves the VAE in the majority of data sets. In some datasets, such as LungCancer (1), the error rate of the IF-VAE is much lower than that of the VAE. This observation confirms that the IF step plays a key role in reducing the clustering errors. Jin and Wang (2016) made a similar observation by combining the IF step with linear dimension reduction by PCA. Our results suggest that the IF step continues to be effective when it is combined with non-linear dimension reduction by the VAE. Last, IF-VAE(X) achieves the lowest error rate in three out of 10 data sets, and it has the second lowest average rank among all eight methods. Compared with IF-PCA (the method with the lowest average rank), IF-VAE(X) has an advantage in three data sets (BreastCancer, SRBCT, and SuCancer) but has a similar or worse performance in the other data sets. These two methods share the same IF step; hence, the results imply that the non-linear dimension reduction by the VAE has an advantage over the linear dimension reduction by PCA only on “difficult” data sets.
Next, we study IF-VAE(X) more carefully with the LungCancer (1) data set. We recall that the IF step ranks all the features using KS statistics and selects the number of features by a tuning-free procedure. We use the same feature ranking but manually change the number of retained features. For each m, we select the m top-ranked features, perform VAE on the unnormalized data matrix X restricted to these m features, and report the average number of clustering errors over five repetitions of the VAE. Figure 1 displays the number of clustering errors as a function of m. An interesting observation is that as m increases, the clustering error first decreases and then increases (for a good visualization; Figure 1 only shows the results for m between 1 and 0.1p; we also tried larger values of m and found that the number of clustering errors continued to increase; especially, the number of errors increased quickly when m > 4,000). A possible explanation is as follows: when m is too small, some influential features are missed, resulting in weak signals in the VAE step; when m is too large, too many non-influential features are selected, resulting in large noise in the VAE step. There is a sweet spot between 200 and 400, and the tuning-free procedure in the IF step selects m = 251. Figure 1 explains why the IF step benefits the subsequent VAE step. A similar phenomenon was discovered by Jin and Wang (2016), but it is for PCA instead of VAE.
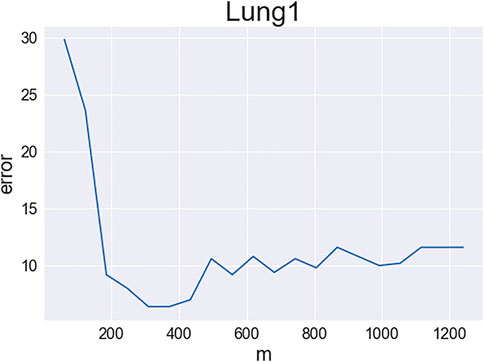
FIGURE 1. Clustering errors of IF-VAE(X) as a function of the number of selected features in the IF step (data set: LungCancer (1); y-axis: number of clustering errors; x-axis: number of selected features).
Remark 1 (comparison with other clustering methods for microarray): Jin and Wang (2016) reported the clustering errors of several classical methods on these 10 microarray data sets. We only include k-means and SpecGem in Table 3 because k-means is the most widely used generic clustering method and SpecGem is specially designed for microarray data. Table 4 shows the clustering errors of other methods reported by Jin and Wang (2016), including k-means++ (a variant of k-means with a particular initialization) and hierarchical clustering. It suggests that these methods significantly underperform compared to IF-PCA.
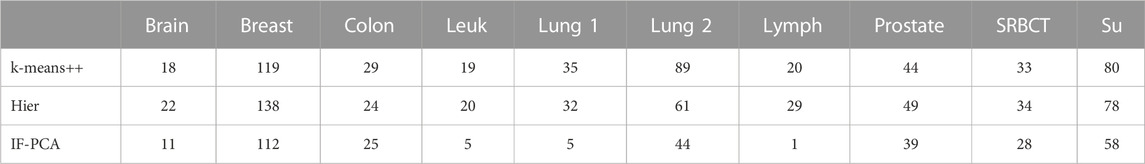
TABLE 4. Clustering errors of k-means++ and hierarchical clustering with the 10 microarray data sets (the clustering errors of IF-PCA are listed for reference).
3.2 Comparison of clustering approaches with eight single-cell RNA-seq datasets
Table 5 tabulates eight single-cell RNA-seq datasets. The data were downloaded from the Hemberg Group at the Sanger Institute (https://hemberg-lab.github.io/scRNA.seq.datasets). It contains scRNA-seq datasets from Human and Mouse. Among them, we selected eight datasets that have a sample size between 100 and 2,000 and can be successfully downloaded and pre-processed using the code provided by the Hemberg Group under the column ‘Scripts’. The datasets Camp1, Camp2, Darmanis, Li, and Patel come from Human, and the data sets Deng, Goolam, and Grun come from Mouse. Each data matrix contains the log-counts of the RNA-seq reads of different genes (features) in different cells (samples). The cell types are used as the true cluster labels to evaluate the performances of clustering methods. We first pre-processed all the data using the code provided by the Hemberg Group, then features (genes) with fractions of nonzero entries
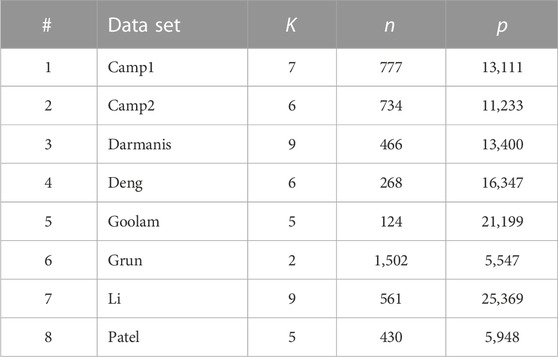
TABLE 5. Single-cell RNA-seq datasets investigated in this paper. (n: number of cells; p: number of genes; K: number of cell types).
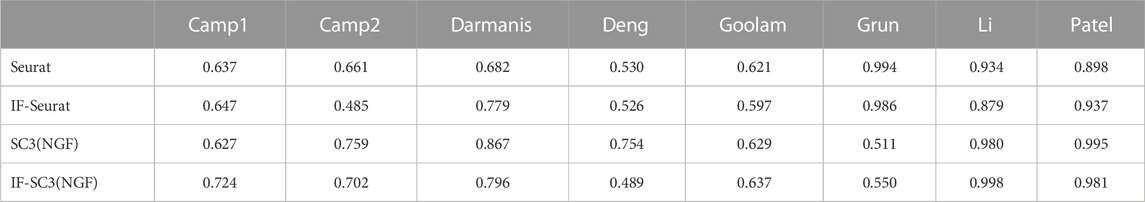
We compare the IF-VAE with three other existing methods: 1) the orthodox IF-PCA (Jin and Wang, 2016), 2) Seurat (Satija et al., 2015), and 3) SC3 (Kiselev et al., 2017). The orthodox IF-PCA was proposed for subject clustering with microarray data. It is the first time this method has been applied to single-cell data. Seurat and SC3 are two popular methods clustering single-cell RNA-seq data (see Sections 2.6 for details). As discussed in Section 2.6, Seurat and SC3 implicitly use some feature selection ideas and some dimension reduction ideas, but they are much more complicated than IF-PCA and have several tuning parameters. Seurat has four tuning parameters, where m is the number of selected features, N is the number of principal components in use, k 0 is the number of clusters in k-nearest neighbors, and δ is a ‘resolution’ parameter. We fix (m, N, k 0) = (1,000, 50, 20) for all datasets (the values of (N, k 0) are the default ones; the default value of m is 2,000, but we found that m = 1,000 gives the same results with the eight data sets and is faster to compute). We choose a separate value of δ for each dataset in a way such that the resulting number of clusters from a modularity optimization is exactly K [details can be found in Waltman and Van Eck (2013)]. Seurat is implemented by the R package Seurat (Hao et al., 2021). SC3 has three tuning parameters, where x 0% is a threshold of the cell fraction used in the gene-filtering step, d 0 is the number of eigenvectors in use, and k 0 is the level of hierarchy in the hierarchical clustering step. We fix (x 0, d 0) = (10, 15) and set k 0 as the number of true clusters in each dataset. SC3 is implemented using the R package SC3 (Kiselev et al., 2017). We observed that SC3 outputs an NA value on the Patel dataset because the gene filtering step removed all of the genes. To resolve this issue, we introduced a variant of SC3 by skipping the gene-filtering step. This variant is called SC3(NGF), where NGF stands for ‘no gene filtering.’ Seurat, SC3, and SC3(NGF) can only be applied to the unnormalized data matrix X. These methods also have randomness in the output, but the standard deviation of the clustering error is quite small; hence, we only run one repetition for each of them. The implementation of IF-PCA, IF-PCA(X), IF-VAE, and IF-VAE(X) is the same as shown in Section 3.1.
Table 6 contains the clustering accuracies (number of correctly clustered cells divided by the total number of cells) of different methods. For each dataset, we rank all six methods (excluding SC3) by their clustering accuracies (the higher the accuracy, the lower the rank). SC3 is excluded in rank calculation because it outputs NA on the Patel data set. Instead, we include SC3 (NGF), a version of SC3 that resolves this issue on Patel and has better performances in most other data sets; this gives more favor to SC3 in the comparison. For each data set, we also compute the regret of each method (the same as in Section 3.1). Similarly, we exclude SC3 but include SC3(NGF) in the regret calculation. Each method has a rank and a regret on each data set. The last four rows of Table 6 show the mean and standard deviation of the eight ranks of each method, as well as the mean and standard deviation of the eight regrets of each method.
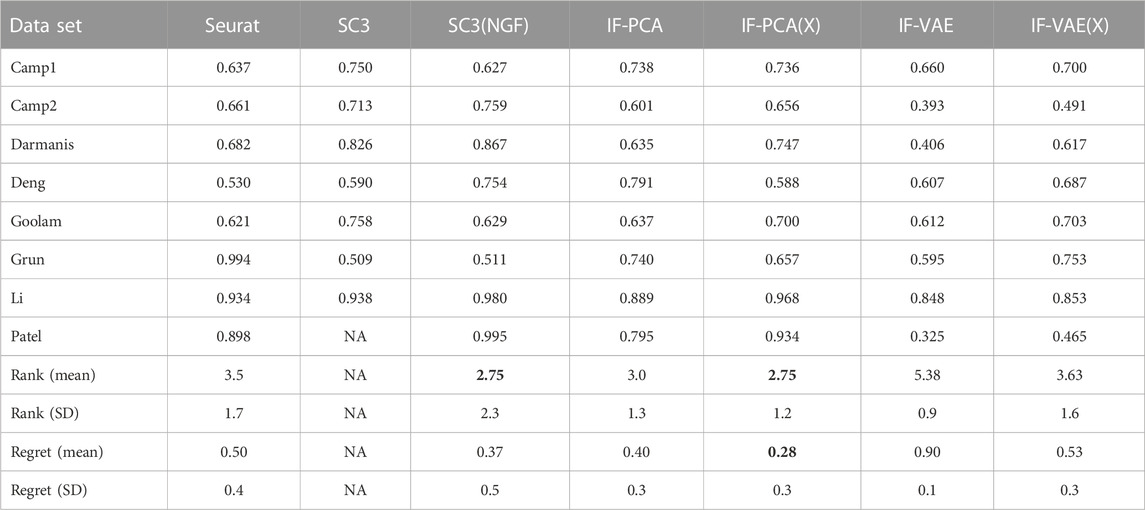
TABLE 6. Comparison of the clustering accuracies with the eight single-cell RNA-seq datasets shown in Table 5. The result for SC3 on Patel is NA because all genes are removed in the gene-filtering step; for this reason, we exclude SC3 when calculating the rank and the regret. To resolve this issue, we also introduce a variant of SC3 by skipping the gene-filtering step. This variant is called SC3(NGF), where ‘NGF’ stands for no gene filtering. It performs better than the original SC3. It is important to note that IF-PCA(X) is regarded as the best on average: it has the smallest average regret (boldface) and average rank (boldface). It is also important to note that the standard deviation (SD) of its rank is only approximately 50% of that of SC3 (NGF).
We make a few comments. First, if we measure the overall performance with eight data sets using the average rank, then IF-PCA(X) and SC3(NGF) are the best. If we use the average regret as the performance metric, then IF-PCA(X) is the best method. Second, a closer look at SC3(NGF) and IF-PCA(X) suggests that their performances have different patterns. SC3(NGF) is ranked 1 in some data sets (e.g., Camp2 and Darmanis) but has low ranks in some other data sets (e.g., Goolam and Grun). In contrast, IF-PCA(X) is ranked 2 in almost all data sets. Consequently, IF-PCA(X) has a smaller rank standard deviation, even though the two methods have the same average rank. One possible explanation is that SC3 is a complicated method with several tuning parameters. For some data sets, the current tuning parameters are appropriate, and so SC3 can achieve an extremely good accuracy; for some other data sets, the current tuning parameters are probably inappropriate, resulting in an unsatisfactory performance. In comparison, IF-PCA is a simple and tuning-free method and has more stable performances across multiple data sets. Third, IF-VAE(X) is uniformly better than the IF-VAE; hence, we recommend applying the IF-VAE to the unnormalized data matrix instead of the normalized one. Last, IF-VAE(X) significantly improves IF-PCA(X) on Deng and Grun. This suggests that the non-linear dimension reduction by the VAE is potentially useful with these two data sets. In the other data sets, IF-VAE(X) either underperforms compared to IF-PCA(X) or performs similarly.
In terms of computational costs, Seurat is the fastest, followed by IF-PCA. VAE and SC3 are more time-consuming, where the main cost of VAE arises from training the neural network, and the main cost of SC3 arises from computing the n × n similarity matrix among subjects. For a direct comparison, we report the running time of different methods with the Camp1 data set (n = 777 and p = 13,111). IF-PCA is implemented in MATLAB and takes approximately 1.7 min. The VAE and IF-VAE are implemented in Python, where the VAE steps are conducted using the Python library keras. The running time of the VAE is 2.7 min, and the running time of the IF-VAE is 1.4 min. SC3 is implemented via the package SC3 of Bioconductor in R, and it takes 3 min. Seurat is implemented using the R package Seurat and takes only 6 s.
Remark 2 (using ARI as the performance metric): The Adjusted Rand Index (ARI) is another commonly used metric for clustering performance. As shown in Table 7, we report the ARI of different methods and recalculate the ranks and regrets. The results are quite similar to those in Table 6.
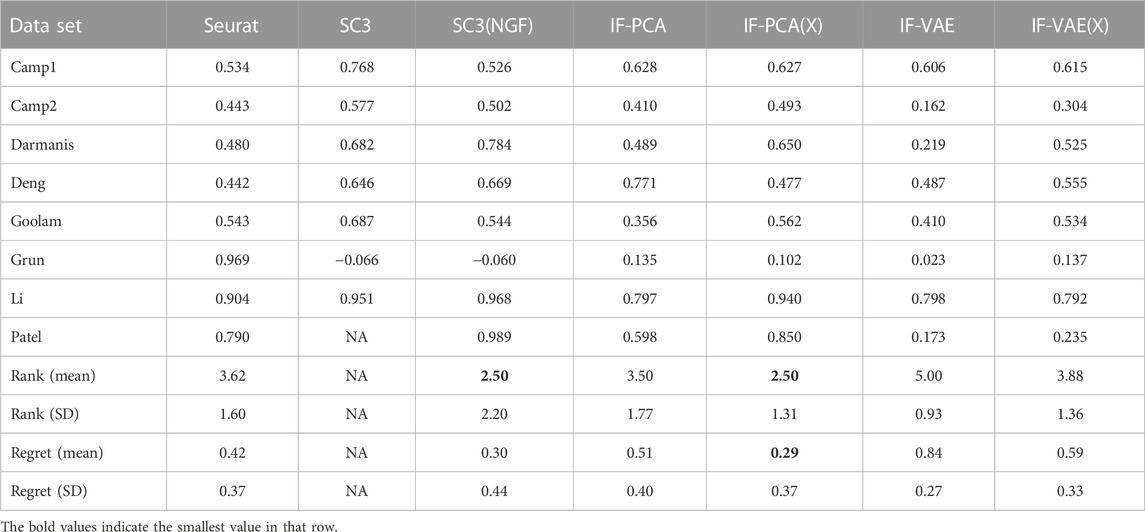
TABLE 7. Values of the Adjusted Rand Index (ARI) for the same data sets and methods as shown in Table 6. Similarly, the average rank and regret of SC3 are denoted as NA, for it generated NA with the Patel data set.
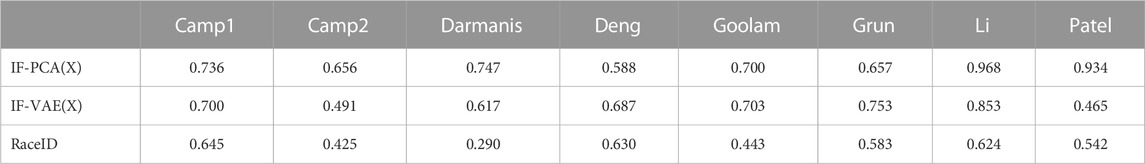
Remark 3 (comparison with RaceID): In addition to Seurat and SC3, there are many other clustering methods for single-cell data (e.g., see Yu et al. (2022) for a survey). RaceID (Grün et al., 2015) is a recent method. It runs an initial clustering, followed by an outlier identification; and the outlier identification is based on a background model of combined technical and biological variability in single-cell RNA-seq measurements. We now compare IF-PCA(X) and IF-VAE(X) with RaceID (we used the R package RaceID and set all tuning parameters to be at default values in this package). The results are shown in Table 8. We observe that IF-PCA(X) and IF-VAE(X) outperform RaceID with most data sets. One possible reason is that the outlier identification step in RaceID is probably more suitable for applications with a large number of cells (e.g., tens of thousands of cells).
Remark 4 (combining the IF step with Seurat and SC3): We investigate whether the IF step of IF-PCA can be used to conduct feature selection for other clustering methods. To this end, we introduce IF-Seurat and IF-SC3(NGF), in which Seurat and SC3(NGF) are applied, respectively, to the post-selection unnormalized data matrix from the IF step of IF-PCA. Table 9 compares these two methods with their original versions. For Seurat, the IF step improves the clustering accuracies on Camp1, Darmanis, and Patel; yields similar performances on Deng, Goolam Grun, and Li; and deteriorates the performances significantly on Camp2. For SC3, the IF step sometimes yields a significant improvement (e.g., Camp1) and sometimes a significant deterioration (e.g., Deng). It is an interesting theoretical question when the current IF step is suitable to combine with clustering methods other than PCA.
4 Phase transition for PCA and IF-PCA
Compared with VAE, Seurat, and SC3, an advantage of IF-PCA is that it is conceptually much simpler and thus comparably easier to analyze. In this section, we present some theoretical results and show that IF-PCA is optimal in a rare/weak signal setting.
We are interested in several intertwined questions.
• When the IF step of the IF-PCA is really necessary. As IF-PCA reduces to classical PCA when we omit the IF step, an equivalent question is when IF-PCA really has an advantage over PCA.
• When IF-PCA is optimal in a minimax decision framework.
To facilitate the analysis, we consider a high-dimensional clustering setting where K = 2, so we only have two classes. We assume the two classes are equally likely so that the class labels satisfy
extension to the case where we replace the Bernoulli parameter 1/2 by a δ ∈ (0, 1), which is comparably straightforward. We also assume that the p-dimensional data vectors X i ’s are standardized so that for a contrast mean vector μ ∈ R p (I p standards for the p × p identity matrix),
As mentioned before, we write
For any 1 ≤ j ≤ p, we call feature j an “influential feature” or “useless feature” if μ(j) ≠ 0 and a “noise” or “useless feature” otherwise. We adopt a rare/weak model setting where (ν a stands for point mass at a)
For fixed parameters 0 < θ, β, α < 1,
From time to time, we drop the subscript of n p and write n = n p . For later use, let
It is seen that |S p (μ)|∼Bernoulli (p, ϵ p ) and |S p (μ)|/s p ∼ 1. Models (10)–(13) model a scenario where 1 ≪ n ≪ p and
• (Signals are sparse/rare). The fraction of the influential feature is p −β , which → 0 rapidly as p → ∞.
• (Signals are individually weak). The signal strength of each influential feature may be much smaller than n −1/4, and the signals are individually weak; it is non-trivial to separate the useful features from the useless ones.
• (No free lunch). Summing over X either across rows (samples) or across columns (feature) would not provide any useful information for clustering decisions.
The model is frequently used if we want to study the fundamental limits and phase transition associated with a high-dimensional statistical decision problem (e.g., classification, clustering, and global testing). Despite the seeming simplicity, the RW model is actually very delicate to study, for it models a setting where the signals (i.e., useful features) are both rare and weak. See Donoho and Jin (2004); Jager and Wellner (2007); Hall and Jin (2010); Xie et al. (2011); Donoho and Jin (2015); Verzelen and Arias-Castro (2017) for example.
Compared with the model in Jin and Wang (2016) (which only considers one-sided signals, where all non-zero μ(j) are positive), our model allows the two-sided signal and so is different. In particular, in our model, summing over X either across rows or columns would not provide any useful information for clustering decisions. As a result, the phase transition we derive as follows is different from that in Jin and Wang( 2016).
We consider a clustering procedure and let
where the probability measure is with respect to the randomness of (μ, Y, Z).
4.1 A slightly simplified version of PCA and IF-PCA
To facilitate analysis for Models (10)–(13), we consider a slightly more idealized version of PCA and IF-PCA, where the main changes are 1) we skip the normalization step (as we assume the model is for data that are already normalized); 2) we replace feature selection by Kolmogorov–Smirnov statistics in IF-PCA by feature selection by the χ 2 statistics; 3) we remove Efron’s correction in IF-PCA (Efron’s correction is especially useful for analyzing gene microarray data, but is not necessary for the current model); and 4) we skip the HCT choice (the study on HCT is quite relevant for our model, but technically it is very long, so we skip it). It is also important to note that the rank of the signal matrix Yμ′ is 1 in Models (10)–(13), so in both PCA and the clustering step of IF-PCA, we should apply k-means clustering to the first singular vector of X only. Despite these simplifications, the essences of original PCA and IF-PCA are retained. A more detailed description of the (simplified) PCA and IF-PCA is as follows.
In detail, to use PCA for Models (10)–(13), we run the following.
• We obtain the first singular vector of X and denote it by ξ (this is simpler than
• We cluster by letting
To be differentiable from PCA in Section 2.2, we may call the approach the slightly simplified PCA.
In addition, to use IF-PCA for Models (1)–(4), we introduce the normalized χ 2-testing scores for feature j by
By elementary statistics,
Fix a threshold
The IF-PCA runs as follows.
• (IF step). We select feature j if and only if
• (Clustering step). Let
and let
Similarly, to differentiate from the IF-PCA in Section 2.4, we call this the slightly simplified IF-PCA.
4.2 The computational lower bound
We first discuss the computational lower bound (CLB). The notion of CLB is an extension of the classical information lower bound (LB) (e.g., the Cramer–Rao lower bound), and in comparison,
• Classical information LB usually claims a certain goal is not achievable for any method (which includes methods that are computationally NP hard).
• CLB usually claims a certain goal is not achievable for any method with a polynomial computational time.
From a computational perspective, we highly prefer to have algorithms with a polynomial computation time. Therefore, compared with classical information LB, CLB is practically more relevant.
Let s p = pϵ p . It is important to note that in our model, the number of signals is Bernoulli (p, ϵ p ), which concentrates at s p . We recall that in our calibrations, n = p θ and s p = p 1−β , and the strength of individual signals is τ p . We introduce the critical signal strength by
We have the following theorem.
Theorem 4.1. [Computational lower bound)]. We fix (θ, β) ∈ (0,1)2 and consider the clustering problem for Models (10)–(13). As p → ∞, if
4.3 A useful lemma on classical PCA
Suppose we have a data matrix
In such a setting, we investigate when the PCA approach in Section 4.1 is successful. We recall that ξ is the first singular vector of X. By basic algebra, it is the first eigenvector of the N × N matrix XX′, or equivalently, the first eigenvector of XX′ − mI N . We write
In order for the PCA approach to be successful, the spectral norm of ‖μ‖2
YY′ should be much larger than that of (ZZ′ − mI
N
). It is important to note that ‖μ‖2
YY′ is a rank-1 matrix, where the spectral norm is N‖μ‖2. In addition, by random matrix theory (Vershynin, 2012), the spectral norm of (ZZ′ − mI
N
) concentrates at
We have the following lemma.
Lemma 4.1. Here, we consider Model (8) where condition (9) holds and that ‖μ‖2 ≫ log(N + m). Let ξ be the first left singular vector of X. When min{N, m} → ∞, with probability 1 − o(m −3),
Therefore, if we let
This says that the PCA approach can fully recover the true class labels.
4.4 Achievability of classical PCA and IF-PCA
We now come back to Models (10)–(13) and study the behavior of classical PCA and IF-PCA in our setting. The computational limits of clustering have received extensive interest [e.g., (Luo and Zhang, 2022)]. By the CLB (Jin et al., 2017), successful clustering by a computable algorithm is impossible when
We first discuss when feature selection by χ 2-test is feasible. As mentioned before, let
be the feature-wise χ 2-testing scores and recall that approximately,
We can view
• (Less sparse case of β < 1/2). In this case, the number of useful features s
p
is much larger than
• (More sparse case of β > 1/2). In this case, the number of useful features s
p
is much smaller than
Consider the first case and suppose we apply the PCA approach in Section 4.1 directly to matrix X. Applying Lemma 4.1 with (N, m) = (n, p) and noting that in this setting,
the PCA approach is successful if
Comparing this with the definition of
We have the following theorem.
Theorem 4.2. (Possibility Region for PCA). We fix (θ, β) ∈ (0,1)2 and consider the clustering problem for Models (1)–(4). Let
then
which is slightly stronger than that of
We recall that S(μ) is the true support of μ and
is the set of selected features in the IF step of IF-PCA. We recall that
By (20, 21), for any useful feature j, the SNR is
By elementary statistics, we have that approximately,
Therefore, except for a negligible probability,
where similar as before, μ S is the sub-vector of μ with all entries restricted to S, and X S and Z S are the sub-matrices of X and Z respectively, with columns restricted to S. Therefore, in the clustering step of IF-PCA, we are, in effect, applying the PCA approach of Section 4.1 to X S , where we recall |S|/s p ≈ 1. Applying Lemma 4.1 with (N, m) = (n, |S|) and noting that
it follows that in order for the clustering step of IF-PCA to be successful, we need
Combining this with (22) and recalling that in the current case,
Comparing this with the definition of
which is slightly stronger than that of
Theorem 4.3. (Possibility Region for IF-PCA). We fix (θ, β) ∈ (0,1)2 and consider the clustering problem for Models (10)–(13). Let
then, in the IF step of IF-PCA,
Moreover,
4.5 Phase transition
We recall that s p = pϵ p and that in Models (10)–(13),
It follows
We fix 0 < θ < 1 and consider the two-dimensional space where the two axes are β and α, respectively. Combining Theorems 4.2, 4.3, the curve α = α*(β, θ) partitions the region {(α, β): 0 < β < 1, α > 0} into two regions.
• Region of Impossibility {(α, β): α > α*(β, θ), 0 < β < 1}. In this region, the Hamming clustering error rate of any method with polynomial computation time is bounded away from 0.
• Region of Possibility {(α, β): α < α*(β, θ), 0 < β < 1}. The region further partitions into two parts: β < 1/2 (left) and β > 1/2 (right).
• The left is the less sparse case where the number of useful features
• The right is the more sparse case, where the number of useful features
• In particular, for any fixed parameters in the region {1/2 < β < 1, (1 − θ − 2β) < α < (1 − β)/2} (shaded green region of Figure 2), the Hamming clustering error rate of IF-PCA is o (1), but that of PCA is bounded away from 0. Therefore, PCA is non-optimal in this particular region.
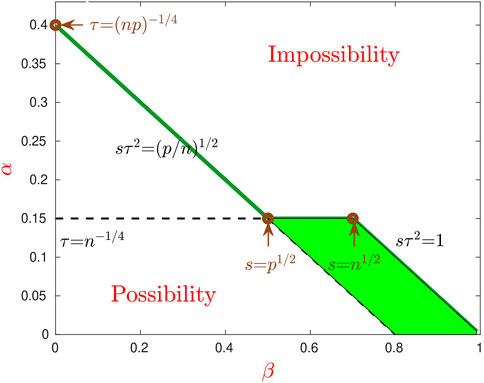
FIGURE 2. Phase transition for PCA and IF-PCA (θ = 0.6). The (three-segment) solid green line is α = α*(β, θ), which separates the whole region into the Region of Impossibility (top) and Region of Possibility (bottom). In the part of Region of Possibility (β < 1/2), feature selection is infeasible, PCA is optimal, and IF-PCA reduces to PCA with an appropriate threshold. In the right part (β > 1/2), it is desirable to conduct feature selection, and IF-PCA is optimal. However, PCA is non-optimal for parameters in the shaded green region.
See Figure 2 for details.
5 Discussion
IF-PCA is a simple and tuning-free approach to unsupervised clustering of high-dimensional data. The main idea of IF-PCA is a proper combination of feature selection and dimension reduction by PCA. In this study, we make several contributions. First, we extend IF-PCA to IF-VAE, by replacing PCA with the VAE, a popular UDL algorithm. Second, we study the theoretical properties of IF-PCA in a simple clustering model and derive the phase transitions. Our results reveal how the feature sparsity and the feature strength affect the performance of IF-PCA and explain why IF-PCA can significantly improve the classical PCA. Third, we investigate the performances of IF-PCA and IF-VAE on two applications, the subject clustering with gene microarray data and the cell clustering with single-cell RNA-seq data, and compare them with those of some other popular methods.
We discover that IF-PCA performs quite well in the aforementioned applications. Its success with microarray data was reported by Jin and Wang (2016), but it has never been applied to single-cell data. To use IF-PCA with single-cell data, we recommend a mild modification of the original procedure called IF-PCA(X), which performs the PCA step on the unnormalized data matrix X instead of the normalized data matrix W. On the eight single-cell RNA-seq data sets considered in this paper, IF-PCA(X) has the second-best accuracy in almost all the data sets, showing a stable performance across multiple data sets. We think IF-PCA has significant potential for single-cell clustering, as the method is simple, transparent, and tuning-free. Although the current IF-PCA(X) still underperforms compared to the state-of-the-art methods (e.g., SC3) in some data sets, it is hopeful that a variant of IF-PCA (say, by borrowing the consensus voting in SC3 or replacing PCA with some other embedding methods [Cai and Ma, 2022; Ma et al., 2023]) can outperform them.
We also find that UDL algorithms do not immediately yield improvements over classical methods with the microarray data and the single-cell data. The IF-VAE underperforms compared to IF-PCA in most data sets; there are only a few data sets in which the IF-VAE slightly outperforms IF-PCA. The reason can be either that non-linear dimension reduction has no significant advantage over linear dimension reduction in these data sets or IF-VAE is not optimally tuned. How to tune the deep learning algorithms in unsupervised settings is an interesting future research direction. Moreover, the theory on the VAE remains largely unknown (Fan et al., 2021). A theoretical investigation of the VAE requires an understanding of both the deep neural network structures and the variational inference procedure. We also leave this to future work.
The framework of IF-PCA only assumes feature sparsity but no other particular structures on the features. It is possible that the features are grouped (Chang et al., 2017) or have some tree structures (Li et al., 2021). How to adapt IF-PCA to this setting is an interesting yet open research direction.
In the real data analysis, we assume that the number of clusters, K, is given. When K is unknown, how to estimate K is a problem of independent interest. One approach is to use the scree plot. For example, Ke et al. (2023) proposed a method that first computes a threshold from the bulk eigenvalues in the scree plot and then applies this threshold to the top eigenvalues to estimate K. Another approach is based on global testing. Given a candidate K, we may first apply a clustering method with this given K and then apply the global testing methods in Jin et al. (2017) to test whether each estimated cluster has no sub-clusters;
Data availability statement
Publicly available data sets were analyzed in this study. These data can be found here: https://data.mendeley.com/datasets/cdsz2ddv3t. https://data.mendeley.com/drafts/nv2x6kf5rd.
Author contributions
JJ conceived the research idea, designed the experiments, and oversaw the project. ZK contributed to the research design, data collection, and interpretation. DC designed and performed the experiments, collected and analyzed the data, and implemented the algorithm. DC wrote the initial draft of the manuscript, JJ contributed to the revision and editing of the Introduction and Model and method sections. ZK revised and edited the Result and Discussion sections. All authors contributed to the article and approved the submitted version.
Funding
The research of JJ is supported in part by NSF Grant DMS-2015469, and the research of ZK is supported in part by NSF Grant DMS-1943902.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2023.1166404/full#supplementary-material
References
Abbe, E., Fan, J., Wang, K., and Zhong, Y. (2020). Entrywise eigenvector analysis of random matrices with low expected rank. Ann. statistics 48, 1452–1474. doi:10.1214/19-aos1854
Abramovich, F., Benjamini, Y., Donoho, D., and Johnstone, I. (2006). Adapting to unknown sparsity by controlling the false discovery rate. Ann. Statistics 34, 584–653. doi:10.1214/009053606000000074
Arthur, D., and Vassilvitskii, S. (2007). “k-means++: The advantages of careful seeding,” in Proceedings of the eighteenth annual ACM-SIAM symposium on Discrete algorithms, New Orleans, Louisiana, USA, January 7-9, 2007, 1027–1035.
Barnett, I., Mukherjee, R., and Lin, X. (2017). The generalized higher criticism for testing snp-set effects in genetic association studies. J. Am. Stat. Assoc. 112, 64–76. doi:10.1080/01621459.2016.1192039
Cai, T. T., and Ma, R. (2022). Theoretical foundations of t-sne for visualizing high-dimensional clustered data. J. Mach. Learn. Resarch 23, 1–54.
Chang, J., Zhou, W., Zhou, W.-X., and Wang, L. (2017). Comparing large covariance matrices under weak conditions on the dependence structure and its application to gene clustering. Biometrics 73, 31–41. doi:10.1111/biom.12552
Deng, J., Dong, W., Socher, R., Li, L.-J., Li, K., and Fei-Fei, L. (2009). “Imagenet: A large-scale hierarchical image database,” in Proceedings of the 2009 IEEE conference on computer vision and pattern recognition, Miami, FL, USA, June 2009 (IEEE), 248–255.
Dettling, M. (2004). Bagboosting for tumor classification with gene expression data. Bioinformatics 20, 3583–3593. doi:10.1093/bioinformatics/bth447
Donoho, D., and Jin, J. (2004). Higher criticism for detecting sparse heterogeneous mixtures. Ann. Statistics 32, 962–994. doi:10.1214/009053604000000265
Donoho, D., and Jin, J. (2015). Higher criticism for large-scale inference, especially for rare and weak effects. Statical Sci. 30, 1–25. doi:10.1214/14-sts506
Efron, B. (2004). Large-scale simultaneous hypothesis testing. J. Am. Stat. Assoc. 99, 96–104. doi:10.1198/016214504000000089
Fan, J., Ma, C., and Zhong, Y. (2021). A selective overview of deep learning. Stat. Sci. a Rev. J. Inst. Math. Statistics 36, 264–290. doi:10.1214/20-sts783
Fan, J., Fan, Y., Han, X., and Lv, J. (2022). Asymptotic theory of eigenvectors for random matrices with diverging spikes. J. Am. Stat. Assoc. 117, 996–1009. doi:10.1080/01621459.2020.1840990
Goodfellow, I., Pouget-Abadie, J., Mirza, M., Xu, B., Warde-Farley, D., Ozair, S., et al. (2020). Generative adversarial networks. Commun. ACM 63, 139–144. doi:10.1145/3422622
Gordon, G. J., Jensen, R. V., Hsiao, L.-L., Gullans, S. R., Blumenstock, J. E., Ramaswamy, S., et al. (2002). Translation of microarray data into clinically relevant cancer diagnostic tests using gene expression ratios in lung cancer and mesothelioma. Cancer Res. 62, 4963–4967.
Grün, D., Lyubimova, A., Kester, L., Wiebrands, K., Basak, O., Sasaki, N., et al. (2015). Single-cell messenger rna sequencing reveals rare intestinal cell types. Nature 525, 251–255. doi:10.1038/nature14966
Guo, M., Wang, H., Potter, S. S., Whitsett, J. A., and Xu, Y. (2015). Sincera: A pipeline for single-cell RNA-seq profiling analysis. PLoS Comput. Biol. 11, e1004575. doi:10.1371/journal.pcbi.1004575
Hall, P., and Jin, J. (2010). Innovated higher criticism for detecting sparse signals in correlated noise. Ann. Statistics 38, 1686–1732. doi:10.1214/09-aos764
Hao, Y., Hao, S., Andersen-Nissen, E., William, M. M., Zheng, S., Butler, A., et al. (2021). Integrated analysis of multimodal single-cell data. Cell. 184, 3573–3587.e29. doi:10.1016/j.cell.2021.04.048
Hastie, T., Tibshirani, R., and Friedman, J. (2009). The elements of statistical learning. 2nd edn. Springer.
Jager, L., and Wellner, J. (2007). Goodness-of-fit tests via phi-divergence. Ann. Statistics 35, 2018–2053. doi:10.1214/0009053607000000244
Jin, J., and Wang, W. (2016). Influential features PCA for high dimensional clustering. Ann. Statistics 44, 2323–2359. doi:10.1214/15-aos1423
Jin, J., Ke, Z. T., and Wang, W. (2015). Optimal spectral clustering by higher criticism thresholding. Manuscript.
Jin, J., Ke, Z. T., and Wang, W. (2017). Phase transitions for high dimensional clustering and related problems. Ann. Statistics 45. doi:10.1214/16-aos1522
Jin, J. (2015). Fast community detection by score. Ann. Statistics 43, 57–89. doi:10.1214/14-aos1265
Ke, Z. T., and Jin, J. (2023). Special invited paper: The SCORE normalization, especially for heterogeneous network and text data. Stat 12, e545. doi:10.1002/sta4.545
Ke, Z. T., Ma, Y., and Lin, X. (2023). Estimation of the number of spiked eigenvalues in a covariance matrix by bulk eigenvalue matching analysis. J. Am. Stat. Assoc. 118, 374–392. doi:10.1080/01621459.2021.1933497
Kingma, D. P., and Welling, M. (2013). Auto-encoding variational bayes. arXiv preprint arXiv:1312.6114.
Kiselev, V. Y., Kirschner, K., Schaub, M. T., Andrews, T., Yiu, A., Chandra, T., et al. (2017). SC3: Consensus clustering of single-cell RNA-seq data. Nat. methods 14, 483–486. doi:10.1038/nmeth.4236
Lee, A. B., Luca, D., Klei, L., Devlin, B., and Roeder, K. (2010a). Discovering genetic ancestry using spectral graph theory. Genet. Epidemiol. Official Publ. Int. Genet. Epidemiol. Soc. 34, 51–59. doi:10.1002/gepi.20434
Lee, A. B., Luca, D., and Roeder, K. (2010b). A spectral graph approach to discovering genetic ancestry. Ann. Appl. Statistics 4, 179–202. doi:10.1214/09-AOAS281
Li, X., Sung, A., and Xie, J. (2021). Distance assisted recursive testing. arXiv preprint arXiv:2103.11085.
Luo, Y., and Zhang, A. R. (2022). Tensor clustering with planted structures: Statistical optimality and computational limits. Ann. Statistics 50, 584–613. doi:10.1214/21-aos2123
Ma, R., Sun, E. D., and Zou, J. (2023). A spectral method for assessing and combining multiple data visualizations. Nat. Commun. 14, 780. doi:10.1038/s41467-023-36492-2
MacQueen, J. (1967). “Classification and analysis of multivariate observations,” in 5th berkeley symp. Math. Statist. Probability (University of California Los Angeles LA USA), 281–297.
Mohammadi, S., Davila-Velderrain, J., and Kellis, M. (2020). A multiresolution framework to characterize single-cell state landscapes. Nat. Commun. 11, 5399. doi:10.1038/s41467-020-18416-6
Paul, D. (2007). Asymptotics of sample eigenstructure for a large dimensional spiked covariance model. Stat. Sin. 17, 1617.
Razavi, A., Van den Oord, A., and Vinyals, O. (2019). Generating diverse high-fidelity images with vq-vae-2. Adv. Neural Inf. Process. Syst. 32.
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F., and Regev, A. (2015). Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502. doi:10.1038/nbt.3192
Serban, I., Sordoni, A., Lowe, R., Charlin, L., Pineau, J., Courville, A., et al. (2017). A hierarchical latent variable encoder-decoder model for generating dialogues. Proc. AAAI Conf. Artif. Intell. 31, 1. doi:10.1609/aaai.v31i1.10983
Strehl, A., and Ghosh, J. (2002). Cluster ensembles—A knowledge reuse framework for combining multiple partitions. J. Mach. Learn. Res. 3, 583–617.
Trapnell, C., Cacchiarelli, D., Grimsby, J., Pokharel, P., Li, S., Morse, M., et al. (2014). The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386. doi:10.1038/nbt.2859
Vershynin, R. (2012). Introduction to the non-asymptotic analysis of random matrices. Compress. Sens., 210–268. doi:10.1017/cbo9780511794308.006
Verzelen, N., and Arias-Castro, E. (2017). Detection and feature selection in sparse mixture models. Ann. Statistics 45, 1920–1950. doi:10.1214/16-aos1513
Waltman, L., and Van Eck, N. J. (2013). A smart local moving algorithm for large-scale modularity-based community detection. Eur. Phys. J. B 86, 1–14. doi:10.1140/epjb/e2013-40829-0
Wang, D., and Gu, J. (2018). Vasc: Dimension reduction and visualization of single-cell RNA-seq data by deep variational autoencoder. Genomics, proteomics Bioinforma. 16, 320–331. doi:10.1016/j.gpb.2018.08.003
Wu, B., Lyu, S., Hu, B.-G., and Ji, Q. (2015). Multi-label learning with missing labels for image annotation and facial action unit recognition. Pattern Recognit. 48, 2279–2289. doi:10.1016/j.patcog.2015.01.022
Xie, J., Cai, T., and Li, H. (2011). Sample size and power analysis for sparse signal recovery in genome-wide association studies. Biometrika 98, 273–290. doi:10.1093/biomet/asr003
Yousefi, M. R., Hua, J., Sima, C., and Dougherty, E. R. (2010). Reporting bias when using real data sets to analyze classification performance. Bioinformatics 26, 68–76. doi:10.1093/bioinformatics/btp605
Yu, L., Cao, Y., Yang, J. Y., and Yang, P. (2022). Benchmarking clustering algorithms on estimating the number of cell types from single-cell rna-sequencing data. Genome Biol. 23, 49–21. doi:10.1186/s13059-022-02622-0
Zhao, S., Fung-Leung, W.-P., Bittner, A., Ngo, K., and Liu, X. (2014). Comparison of rna-seq and microarray in transcriptome profiling of activated t cells. PloS one 9, e78644. doi:10.1371/journal.pone.0078644
Keywords: gene microarray, feature selection, higher criticism threshold, PCA, ScRNA-seq, sparsity, subject clustering, variational
Citation: Chen D, Jin J and Ke ZT (2023) Subject clustering by IF-PCA and several recent methods. Front. Genet. 14:1166404. doi: 10.3389/fgene.2023.1166404
Received: 15 February 2023; Accepted: 03 May 2023;
Published: 23 May 2023.
Edited by:
Jichun Xie, Duke University, United StatesReviewed by:
Yuping Zhang, University of Connecticut, United StatesWei Vivian Li, University of California, Riverside, United States
Copyright © 2023 Chen, Jin and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiashun Jin, amlhc2h1bmpAYW5kcmV3LmNtdS5lZHU=
 Dieyi Chen
Dieyi Chen Jiashun Jin2*
Jiashun Jin2* Zheng Tracy Ke
Zheng Tracy Ke