
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 14 June 2023
Sec. Applied Genetic Epidemiology
Volume 14 - 2023 | https://doi.org/10.3389/fgene.2023.1136483
In various cross-sectional and longitudinal studies, exercise has been associated with cardiometabolic outcomes, including high-density lipoprotein (HDL) cholesterol. Exercise-induced changes in HDL cholesterol seem to be affected by genetic polymorphisms. In this study, we examined whether variant APOE rs7412 is involved in the association between HDL cholesterol and exercise. From adults assessed in Taiwan Biobank (TWB) between 2008 and 2019, we analyzed data from 57,638 normolipidemic subjects. To examine the association between exercise, APOE rs7412, and HDL cholesterol, a multiple linear regression model was used. A higher HDL was associated with both aerobic exercise (regression coefficient [mg/dL] beta- (β), 1.112; 95% confidence interval (CI); 0.903–1.322) and resistance exercise (β, 2.530; 95% CI, 2.093–2.966). In comparison with the APOE rs7412-CC genotype, the β was 2.589 (95% CI, 2.329–2.848) among those with the CT + TT genotype. Compared to adults who had the CC genotype and did not exercise (the CC/no exercise group), the β-coefficient determined for the different genotype and exercise groups was 1.135 (95% CI, 0.911–1.359) for the CC genotype and aerobic exercise group, 2.753 (95% CI, 2.283–3.322) for the CC genotype and resistance exercise group, 2.705 (95% CI, 2.390–3.020) for the CT + TT genotype and no exercise group, 3.682 (95% CI, 3.218–4.146) for the CT + TT genotype and aerobic exercise group, and 3.855 (95% CI, 2.727–4.982) for the CT + TT genotype and resistance exercise group, respectively. This study demonstrates that self-reported aerobic and resistance exercise both raised HDL levels, yet resistance exercise was associated with a greater increase, particularly among Taiwanese subjects carrying the APOE rs7412-CT+TT genotype.
There is evidence that high HDL cholesterol plays a vital role in predicting cardiovascular events, and can be used as a therapeutic target in disease management (Barter et al., 2007). Previous studies have indicated that an increase in HDL cholesterol by just 1 mg/dL may reduce CHD risk by approximately 2%–3% (Sharrett et al., 2001; Ashen and Blumenthal, 2005). Research efforts have recently been focused on understanding the various physical activities associated with HDL cholesterol (Woudberg et al., 2018).
Exercise plays an instrumental role in managing lipoprotein levels associated with heart diseases (Stefanick et al., 1998). Numerous cross-sectional and longitudinal studies have demonstrated an association between cardiometabolic outcomes, such as HDL cholesterol and physical activity (Pattyn et al., 2013; Lin et al., 2015; Lemes et al., 2018). In a previous study, an 8-week aerobic exercise program was associated with an increase in HDL cholesterol levels, from 47.5 mg/dL to 52.5 mg/dL (Vazzana et al., 2013). Meanwhile, findings from another study (Lemes et al., 2016) have demonstrated that exercise, whether aerobic or resistance, does not result in clinically significant changes in HDL cholesterol.
The concentration of HDL cholesterol in our body reflects a combination of several factors, including environmental and lifestyle factors, as well as genetic factors, which account for approximately 50% of the changes (Heller et al., 1993; Cuchel and Rader, 2003). According to a previous review (Brunham and Hayden, 2015), about 62%–77% of the variance in HDL-C levels has been attributed to genetic factors even though the biological roles of the genes involved are still to be fully understood. Some of the genes linked to HDL cholesterol include the apolipoprotein E (APOE), apolipoprotein A-I (apoA-I), ATP binding cassette transporter, sub-family A, member 1 (ABCA1), lecithin cholesterol acyltransferase (LCAT), Cholesterol ester transfer protein (CETP), lipoprotein lipase (encoded by the LPL gene), hepatic lipase (encoded by LIPC), endothelial lipase (encoded by LIPG), phospholipid transfer protein (PLTP), the scavenger receptor class B member I (SR-BI, encoded by the SCARB1 gene), and others (Brunham and Hayden, 2015). Several variants of these genes have been associated with HDL cholesterol (Haase et al., 2010; Haase et al., 2012; Voight et al., 2012).
An important protein component of HDL cholesterol is APOE which is secreted by the liver and is involved in the hepatic uptake of triglyceride-rich lipoprotein. APOE rs7412 is one of the variants known to influence plasma lipid levels and other metabolic disorders (Bennet et al., 2007; Phillips, 2014). As far as we are aware, its influence on lipid metabolism has been widely studied, with the majority of studies focusing on low-density lipoprotein cholesterol (LDL-C) levels. Recently, Taiwanese researchers identified this variant as one of the most potent genetic factors influencing HDL cholesterol levels in Taiwan (Yeh et al., 2022). To gain a deeper understanding of this variant, we investigated whether its association with HDL cholesterol is related to exercise, which has been shown to improve lipoprotein levels and cholesterol efflux capacity (Stanton et al., 2022).
Data were available for 132,720 subjects within the TWB. These individuals were between the ages of 30 and 70 years old without a previous diagnosis of cancer. Their assessment in biobank centers took place between 2008 and 2019. Individuals participating in TWB are normally required to sign written informed consent before being assessed. Among the total subjects, 73,196 had genetic data. Our analysis, however, excluded those with hyperlipidemia (n = 5,496), as well as those with incomplete or missing data (n = 10,062). The final analysis models included 57,638 subjects. Each individual signed a consent form before undergoing assessments at biobank centers across the country. This study was approved by the Institutional Review Board of Lanseed International Hospital (IRB-206-B1).
Sociodemographic (age, sex, ethnicity), lifestyle (exercise, alcohol consumption, cigarette smoking) and disease (hypertension and diabetes) data were gathered from the TWB questionnaires. Using colorimetric assays (Hitachi LST008, Automatic Clinical Chemistry Analyzer, Hitachi, Naka, Japan), individual lipid levels, including HDL cholesterol, triglycerides (TG), and non-HDL cholesterol were measured from blood samples collected at TWB assessment centers.
In this study, exercise habits were divided into three categories: no exercise, aerobic exercise, and resistance exercise. Subjects who regularly exercised for at least 30 min a day, three times a week, were considered regular exercisers. Ball games and weight training were included in the study as forms of resistance exercise. Aerobic exercise included Chinese martial arts, brisk walking, Taijiquan, hiking, jogging, basketball, rope jumping, gymnastics, yoga, qigong, swimming, biking, table tennis, tennis, soccer, badminton, aerobic dance, hula hooping, golf, and ballroom dance.
Our study focused on variant rs7412 of the apolipoprotein E gene, which was chosen based on literature searches. The reason for analyzing the variant was that it represents one of those variants that are known to affect plasma lipid levels as mentioned above. To our knowledge, only a few studies have used TWB to examine this variant in the context of TG, Alzheimer’s, hypertension, C-reactive protein (CRP), and diabetic neuropathy.
The Chi-square test and Student’s t-test were used to examine differences in the distribution of study variables (categorical and continuous variables) based on CC, CT + TT genotypes of the variant rs7412. To examine the association between exercise, APOE rs7412, and HDL cholesterol, we used multiple linear regression models that controlled for age, sex, cigarette smoking, alcohol consumption, body mass index (BMI), triglycerides (TG), non-HDL, diabetes, hypertension, and serum glutamate pyruvate transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and gamma-glutamyl transferase (GGT). We tested for a possible interaction between APOE rs7412 and exercise using a multiple linear regression model, followed by stratified analyses of these variables. Covariates included in the interaction model included age, sex, cigarette smoking, alcohol consumption, BMI, TG, non-HDL, diabetes, and hypertension, SGPT, SGOT and GGT. The significance levels were based on a p-value less than 0.05. Besides Plink version 2.0, SAS 9.4 software (SAS Institute, Cary, NC, United States) was used for data analysis.
A total of 57,638 non-hyperlipidemic subjects (39,144 women and 18,494 men) were included in this study (Table 1). The mean (SE) HDL cholesterol level was 54.794 (0.060) mg/dL in rs7412-CC subjects and 56.549 (0.155) mg/dL in rs7412-CC + TT subjects. APOE rs7412-CC subjects made up 49,395 of the sample, while APOE rs7412-CT+TT subjects made up 8,251. There were two types of exercise in the multiple regression model: aerobic and resistance (Table 2). In comparison with the no exercise group, both aerobic and resistance exercise were associated with increased HDL cholesterol: The β-coefficient (95% CI) was 1.112 (0.903–1.322) and 2.530 (2.093–2.966), respectively. In comparison with the rs7412-CC genotype, the β-coefficient was 2.589 (95% CI 2.329–2.848) among those with the CT + TT genotype. With normal weight representing the reference group, the β-coefficients (95% CI) were 6.128 (5.634–6.621), −4.323 (−4.547–−4.100), −6.519 (−6.777 to −6.261) for the underweight, overweight, and obese categories, respectively. Men compared to women had significantly lower levels of HDL cholesterol (β = −7.066, 95% CI -7.295 to −6.838).
In addition, there was evidence of an interaction between APOE rs7412 and exercise (p for interaction = 0.049). Further stratification by the SNP genotypes (Table 3) showed β-values (95% CI) of 1.111 (0.887–1.335) and 2.722 (2.256–3.189) for aerobic exercise and resistance exercise among CC genotype individuals, and 1.112 (0.533–1.691) and 1.374 (0.150–2.598), respectively among those with the CT + TT genotype.
In a multiple regression model combining rs7412 genotypes and exercise types (Table 4) and using the CC genotype and no exercise group as a reference group, the β-coefficients (95% CI) were 1.135 (0.911–1.359) for the CC genotype and aerobic exercise group, 2.753 (2.283–3.222) for the CC genotype and resistance exercise group, 2.705 (2.390–3.020) for the CT + TT genotype and no exercise group, 3.682 (3.218–4.146) for the CT + TT genotype and aerobic exercise group, and 3.855 (2.727–4.982) for the CT + TT genotype and resistance exercise group, respectively.
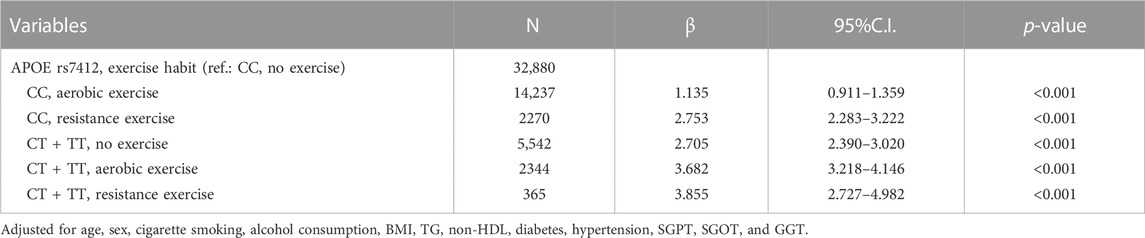
TABLE 4. HDL cholesterol levels based on a combination of exercise modalities and APOE rs7412 genotypes.
The apolipoprotein, APOE plays an instrumental role in regulating HDL cholesterol levels. It has also been shown in previous research that the function and composition of HDL are influenced by lifestyle factors during midlife (Nasr et al., 2022). Researchers have also indicated that exercise-induced changes in HDL cholesterol are modified by genetic polymorphisms (Blazek et al., 2013). Using data from Taiwan Biobank subjects aged 30–70 years who were recruited between 2008 and 2019, we examined whether the association between HDL cholesterol and exercise is related to the APOE variant. To test this hypothesis, specifically, we used the variant APOE rs7412, well-known for its association with HDL cholesterol levels. As part of our analysis, we looked at exercise categories such as aerobics and resistance exercises, and SNP genotypes, CT + TT and CC, respectively. We found that self-reported resistance and aerobic exercise were associated with higher HDL cholesterol but the increase was more pronounced with resistance exercise. The trend was maintained regardless of SNP genotypes added to the analysis models. For instance, CT + TT individuals who engaged in resistance exercise demonstrated elevated HDL cholesterol levels [β = 4.032 (95% CI 3.249 to 4.181)], surpassing those who engaged in aerobic exercise [β = 3.715 (95% CI 3.249 to 4.181)], in comparison to individuals with the CC genotype, as determined by multivariate regression analysis. Despite these, further research is needed to explore the possible mechanisms involved.
Researchers have previously proposed that a reduction in cardiovascular disease risk may also be associated with exercise-induced increases in HDL function (Stanton et al., 2022). Our study suggests that these increases may occur more in APOE rs7412-CT+TT individuals with the APOE gene in Taiwan. It has been proposed that apolipoprotein E together with exercise training can greatly affect lipoprotein metabolism (Thompson et al., 2004). Currently, genetic variations linked to HDL cholesterol and exercise training have not been extensively explored, especially in Taiwan. Consequently, the results of the current study could serve as a reference point for future epidemiological studies.
HDL cholesterol levels can be improved in later life by keeping a healthier body mass index, according to a previous study (Nasr et al., 2022). In the current study, we found significant associations between HDL cholesterol and BMI. That is, compared with normal BMI, lower BMI (underweight category) was associated with a greater increase in HDL cholesterol, whereas higher BMI (overweight and obese categories) was associated with lower HDL cholesterol.
We found that several factors were inversely related to HDL cholesterol, including smoking, diabetes, and hypertension. Earlier research indicated that smoking adversely affects both HDL quantity and function, thereby increasing cardiovascular disease risk (He et al., 2013). To our knowledge, positive and negative findings have been reported regarding the relationship between alcohol consumption and HDL cholesterol. There has been an indication, however, that the type of alcohol consumed affects HDL cholesterol levels (Huang et al., 2017).
Overall, the study contributes to our understanding of the interplay between genetics, exercise, and HDL cholesterol levels, providing valuable insights that can inform future research, clinical practice, and public health strategies aimed at promoting cardiovascular health. It is important, however, to acknowledge the limitations of our study. Data used in the current study may not have sufficient scope or outcomes to accurately explain the relationship of HDL cholesterol with genetic and lifestyle factors. Given that exercise intensity is emerging as the most potent prescriptive factor in disease modification and improving metabolic outcomes, the absence of this crucial descriptor does make it hard to appropriately determine the true relationship between physical exercise and HDL cholesterol. Lastly, our study did not examine the possible impact of diet on HDL cholesterol levels.
This study observed that the association between HDL cholesterol and APOE rs7412 varied according to the type of exercise performed. Specifically, individuals with the T-allele (CT and TT) exhibited a more notable increase in HDL cholesterol compared to those with the C-allele (CC), particularly in the context of engaging in resistance training. These results have implications for clinical practice and public health initiatives. Promoting regular exercise, especially resistance exercise, may be a useful strategy for improving HDL cholesterol levels and reducing the risk of cardiovascular diseases in the general population. Genetic screening for APOE rs7412 genotype could be considered to identify individuals who may benefit the most from specific exercise interventions.
The data analyzed in this study is subject to the following licenses/restrictions: data used in this study are held by the Taiwan Biobank (https://www.twbiobank.org.tw/new_web/) and are available from the authors upon reasonable request or directly from the Taiwan Biobank (YmlvYmFua0BnYXRlLnNpbmljYS5lZHUudHc=). requests to access these datasets should be directed to https://www.twbiobank.org.tw/new_web/.
Conceptualization, H-CC, ON, PH-C, C-CH, and Y-PL; data curation, C-CH; formal analysis, ON, PH-C, and Y-PL; methodology, H-CC, ON, and Y-PL; supervision, Y-PL; writing–original draft, H-CC; writing–review and editing, ON, PH-C, C-CH, and Y-PL. All authors contributed to the article and approved the submitted version.
This study was funded by Landseed International Hospital (LSH-CSMU-108-01) and partially by the Ministry of Science and Technology (MOST 111-2121-M040-002, MOST 111-2811-M-040-001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ashen, M. D., and Blumenthal, R. S. (2005). Clinical practice. Low HDL cholesterol levels. N. Engl. J. Med. 353 (12), 1252–1260. doi:10.1056/NEJMcp044370
Barter, P., Gotto, A. M., LaRosa, J. C., Maroni, J., Szarek, M., Grundy, S. M., et al. (2007). HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357 (13), 1301–1310. doi:10.1056/NEJMoa064278
Bennet, A. M., Di Angelantonio, E., Ye, Z., Wensley, F., Dahlin, A., Ahlbom, A., et al. (2007). Association of apolipoprotein E genotypes with lipid levels and coronary risk. Jama 298 (11), 1300–1311. doi:10.1001/jama.298.11.1300
Blazek, A., Rutsky, J., Osei, K., Maiseyeu, A., and Rajagopalan, S. (2013). Exercise-mediated changes in high-density lipoprotein: Impact on form and function. Am. heart J. 166 (3), 392–400. doi:10.1016/j.ahj.2013.05.021
Brunham, L. R., and Hayden, M. R. (2015). Human genetics of HDL: Insight into particle metabolism and function. Prog. lipid Res. 58, 14–25. doi:10.1016/j.plipres.2015.01.001
Cuchel, M., and Rader, D. J. (2003). Genetics of increased HDL cholesterol levels: Insights into the relationship between HDL metabolism and atherosclerosis. Am. Heart Assoc. 23, 1710–1712. doi:10.1161/01.ATV.0000092947.15939.93
Haase, C. L., Tybjærg-Hansen, A., Ali Qayyum, A., Schou, J., Nordestgaard, B. G., and Frikke-Schmidt, R. (2012). LCAT, HDL cholesterol and ischemic cardiovascular disease: A mendelian randomization study of HDL cholesterol in 54,500 individuals. J. Clin. Endocrinol. Metabolism 97 (2), E248–E256. doi:10.1210/jc.2011-1846
Haase, C. L., Tybjærg-Hansen, A., Grande, P., and Frikke-Schmidt, R. (2010). Genetically elevated apolipoprotein AI, high-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J. Clin. Endocrinol. Metabolism 95 (12), E500–E510. doi:10.1210/jc.2010-0450
He, B. M., Zhao, S. P., and Peng, Z. Y. (2013). Effects of cigarette smoking on HDL quantity and function: Implications for atherosclerosis. J. Cell. Biochem. 114 (11), 2431–2436. doi:10.1002/jcb.24581
Heller, D. A., de Faire, U., Pedersen, N. L., Dahlen, G., and McClearn, G. E. (1993). Genetic and environmental influences on serum lipid levels in twins. N. Engl. J. Med. 328 (16), 1150–1156. doi:10.1056/NEJM199304223281603
Huang, S., Li, J., Shearer, G. C., Lichtenstein, A. H., Zheng, X., Wu, Y., et al. (2017). Longitudinal study of alcohol consumption and HDL concentrations: A community-based study. Am. J. Clin. Nutr. 105 (4), 905–912. doi:10.3945/ajcn.116.144832
Lemes, Í. R., Ferreira, P. H., Linares, S. N., Machado, A. F., Pastre, C. M., and Netto, J. (2016). Resistance training reduces systolic blood pressure in metabolic syndrome: A systematic review and meta-analysis of randomised controlled trials. Br. J. sports Med. 50 (23), 1438–1442. doi:10.1136/bjsports-2015-094715
Lemes, Í. R., Turi-Lynch, B. C., Cavero-Redondo, I., Linares, S. N., and Monteiro, H. L. (2018). Aerobic training reduces blood pressure and waist circumference and increases HDL-c in metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. J. Am. Soc. Hypertens. 12 (8), 580–588. doi:10.1016/j.jash.2018.06.007
Lin, X., Zhang, X., Guo, J., Roberts, C. K., McKenzie, S., Wu, W. C., et al. (2015). Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 4 (7), e002014. doi:10.1161/JAHA.115.002014
Nasr, A., Matthews, K. A., Brooks, M. M., Barinas-Mitchell, E., Orchard, T., Billheimer, J., et al. (2022). Early midlife cardiovascular health influences future HDL metrics in women: The SWAN HDL study. J. Am. Heart Assoc. 11 (21), e026243. doi:10.1161/JAHA.122.026243
Pattyn, N., Cornelissen, V. A., Eshghi, S. R. T., and Vanhees, L. (2013). The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: A meta-analysis of controlled trials. Sports Med. 43 (2), 121–133. doi:10.1007/s40279-012-0003-z
Phillips, M. C. (2014). Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB life 66 (9), 616–623. doi:10.1002/iub.1314
Sharrett, A. R., Ballantyne, C., Coady, S., Heiss, G., Sorlie, P., Catellier, D., et al. (2001). Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein (a), apolipoproteins AI and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 104 (10), 1108–1113. doi:10.1161/hc3501.095214
Stanton, K. M., Kienzle, V., Dinnes, D. L. M., Kotchetkov, I., Jessup, W., Kritharides, L., et al. (2022). Moderate-and high-intensity exercise improves lipoprotein profile and cholesterol efflux capacity in healthy young men. J. Am. Heart Assoc. 11 (12), e023386. doi:10.1161/JAHA.121.023386
Stefanick, M. L., Mackey, S., Sheehan, M., Ellsworth, N., Haskell, W. L., and Wood, P. D. (1998). Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N. Engl. J. Med. 339 (1), 12–20. doi:10.1056/nejm199807023390103
Thompson, P. D., Tsongalis, G. J., Seip, R. L., Bilbie, C., Miles, M., Zoeller, R., et al. (2004). Apolipoprotein E genotype and changes in serum lipids and maximal oxygen uptake with exercise training. Metabolism 53 (2), 193–202. doi:10.1016/j.metabol.2003.09.010
Vazzana, N., Ganci, A., Cefalù, A. B., Lattanzio, S., Noto, D., Santoro, N., et al. (2013). Enhanced lipid peroxidation and platelet activation as potential contributors to increased cardiovascular risk in the low-HDL phenotype. J. Am. Heart Assoc. 2 (2), e000063. doi:10.1161/JAHA.113.000063
Voight, B. F., Peloso, G. M., Orho-Melander, M., Frikke-Schmidt, R., Barbalic, M., Jensen, M. K., et al. (2012). Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 380 (9841), 572–580. doi:10.1016/S0140-6736(12)60312-2
Woudberg, N. J., Pedretti, S., Lecour, S., Schulz, R., Vuilleumier, N., James, R. W., et al. (2018). Pharmacological intervention to modulate HDL: What do we target? Front. Pharmacol. 8, 989. doi:10.3389/fphar.2017.00989
Keywords: exercise, HDL cholesterol, genetic predisposition, cardiometabolic factors, epidemiology
Citation: Chang H-C, Nfor ON, Ho C-C, Chen P-H and Liaw Y-P (2023) Variations in high density cholesterol levels based on apolipoprotein E variant and exercise type. Front. Genet. 14:1136483. doi: 10.3389/fgene.2023.1136483
Received: 03 January 2023; Accepted: 02 June 2023;
Published: 14 June 2023.
Edited by:
Chao-Qiang Lai, Tufts University, United StatesReviewed by:
Martha Guevara-Cruz, National Institute of Medical Sciences and Nutrition Salvador Zubirán, MexicoCopyright © 2023 Chang, Nfor, Ho, Chen and Liaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yung-Po Liaw, bGlhd3lwQGNzbXUuZWR1LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.