- 1Department of Breast and Thyroid Surgery, The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang, China
- 2Department of Surgery, Yinchuan Maternal and Child Health Hospital, Yinchuan, China
Background: We aimed to explore prognostic risk factors in patients with malignant phyllodes tumors (PTs) of the breast and construct a survival prediction model.
Methods: The Surveillance, Epidemiology, and End Results database was used to collect information on patients with malignant breast PTs from 2004 to 2015. The patients were randomly divided into training and validation groups using R software. Univariate and multivariate Cox regression analyses were used to screen out independent risk factors. Then, a nomogram model was developed in the training group and validated in the validation group, and the prediction performance and concordance were evaluated.
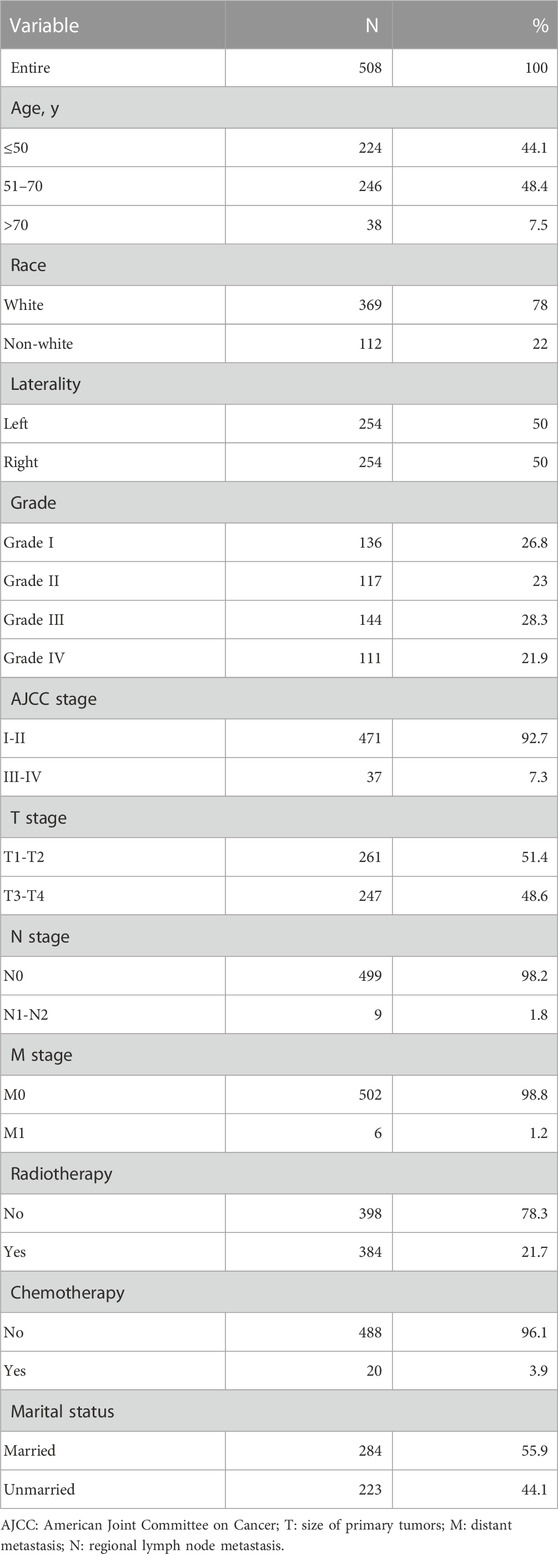
Results: The study included 508 patients with malignant PTs of the breast, including 356 in the training group and 152 in the validation group. Univariate and multivariate Cox proportional hazard regression analyses showed that age, tumor size, tumor stage, regional lymph node metastasis (N), distant metastasis (M) and tumor grade were independent risk factors for the 5-year survival rate of patients with breast PTs in the training group (p < 0.05). These factors were used to construct the nomogram prediction model. The results showed that the C-indices of the training and validation groups were 0.845 (95% confidence interval [CI] 0.802–0.888) and 0.784 (95% CI 0.688–0.880), respectively. The calibration curves of the two groups were close to the ideal 45° reference line and showed good performance and concordance. Receiver operating characteristic and decision curve analysis curves showed that the nomogram has better predictive accuracy than other clinical factors.
Conclusion: The nomogram prediction model constructed in this study has good predictive value. It can effectively assess the survival rates of patients with malignant breast PTs, which will aid in the personalized management and treatment of clinical patients.
1 Introduction
Breast phyllodes tumors (PTs) are rare fibroepithelial neoplasms that account for less than 1% of all breast tumors (Siegel et al., 2019). The World Health Organization classifies breast PTs as benign, marginal, or malignant based on their performance, prognosis, and treatment approaches. Malignant PTs are diagnosed when marked stromal hypercellularity, atypia, increased mitoses of ≥10/10 high-power fields (HPFs), permeative tumor borders, and stromal overgrowth are observed (Tan et al., 2016). The development of malignant PTs may be related to the loss of stromal dependency on the epithelium (Sawhney et al., 1992). Previous studies report that approximately 10%–15% of PTs are malignant, with poor prognostication and high recurrence and metastasis rates (Cimino-Mathews et al., 2013). For instance, about 15%–40% of malignant PTs recur locally, whereas 9%–27% metastasize to distant organs (Lissidini et al., 2022). Surgery is the primary treatment for PTs, and chemoradiotherapy is recommended for patients with recurring or metastatic tumors. However, increasing evidence shows that chemoradiotherapy is ineffective in PT patients with metastases and that these patients usually die within 3 year of treatment (Macdonald et al., 2006). Therefore, there is an urgent need to distinguish patients prone to recurrence/metastasis and provide effective treatment for patients with malignant PTs.
Nomogram is a visual and individualized predictive tool that provides a simple graphical representation of statistical predictive models to quantify personalized risk for a clinical event by incorporating a variety of risk factors (Balachandran et al., 2015). Nomograms are usually constructed for prognostic prediction and are widely used in many types of cancer because they can accurately predict patient survival time (Iasonos et al., 2008; Wang et al., 2013; Liang et al., 2015; Tan et al., 2018; Wu et al., 2018; Zhang et al., 2019). If the survival rate and severity of patients with malignant PTs can be accurately assessed by conventional clinical pathological characteristics, it can provide a high reference value for patients’ families and clinicians (Kim and Kim, 2017). Histological classification and tumor size are common risk factors for malignant PTs (Neron et al., 2020; Li et al., 2021; Choi et al., 2022). In addition, a study of 605 PT cases developed a nomogram based on histological criteria and surgical margins, in which stromal atypia, mitoses, overgrowth, and surgical margins (the AMOS criteria) were of independent significance in predicting the malignant behavior of PTs and the surgical margin status was considered the most important (Tan et al., 2012). However, determining which malignant PTs will undergo distant metastases remains difficult in clinical practice, and there are only a few studies on the prognostic prediction of malignant PTs.
In this study, based on the Surveillance, Epidemiology, and End Results (SEER) database, we developed a nomogram model to predict the overall survival of patients with malignant breast PTs, providing a basis for individualized diagnosis and treatment.
2 Methods
2.1 The source of the data
The data were published in the publicly available SEER database; therefore, all patient-identifying information was removed, and there was no need for approval from the Ethics Committee or informed consent from the patients. All data were approved by the National Institutes of Health before being obtained (approval number: 10934-Nov 2017). In this study, postoperative patient data on malignant PT-like breast tumors from 2004 to 2015 were obtained from the SEER database using the SEER-Stat 8.3.6 software.
2.2 Patient inclusion and information extraction
The study included the patients according to the following inclusion and exclusion criteria. The inclusion criteria were 1) malignant breast PTs; 2) breast cancer as the first primary malignant tumor; and 3) a follow-up duration of >1 month. The exclusion criteria were 1) unknown tumor grade; 2) lack of follow-up data; 3) unknown information about tumor-node-metastasis (TNM) stage; 4) unknown tumor size; 5) unknown race; and 6) presence of multiple primary tumors. The following information was extracted from 508 eligible patients: pathologically proven mammary phyllodes malignancies (ICD-O-3 9020/3), single-sided breast cancer, American Joint Committee on Cancer (AJCC) stage, patients diagnosed from 2004 to 2015, size of primary tumors (T), tumor grade, race, age, primary lesions, regional lymph node metastasis (N), distant metastasis (M), radiation therapy, and marital status. Although all 508 patients who met the group criteria underwent surgery, the specific details of the interventions were not provided in the SEER database; therefore, surgery could not be included as an independent prognostic variable in the model study.
In the retrieved information, low-level tumors had high and moderate differentiation grades (ICD-O-3 1 and 2 levels), whereas high-level tumors had low or undifferentiated grades (ICD-O-3 3 and 4 levels). The critical values for diagnostic age and tumor size were determined using the X-tile software (Yale University, New Haven, CT, United States) (Camp et al., 2004), which has previously been shown to determine the critical point of the optimal tumor variable. The X-tile software was initially developed to determine the optimal threshold for breast cancer variables. The optimal threshold values for tumors were small (<70 mm), medium (70–160 mm), and large (>160 mm) (Figure 1). Because the optimal age limit was between 57 and 67 years (Figure 1), the patients were divided into three age groups (18–57, 57–67, or >67 years), as well as white, black, or other ethnic groups depending on race. Radiotherapy was divided into radiotherapy and non-radiotherapy. However, details such as radiotherapy type, site, and strength could not be obtained from the SEER database. Chemotherapy was divided into recipients and non-recipients.
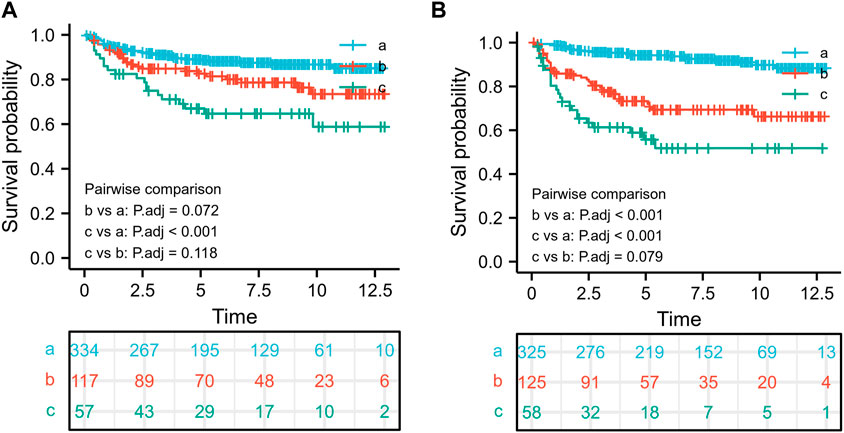
FIGURE 1. The optimal threshold for age and tumor size and the associated survival rate. The optimal threshold for age using the survival rate (A),18–57 years old (a), 57–67 years old (b), and >67 years old (c); The optimal threshold for tumor size using the survival rate (B),<70 mm (a), 70–160 mm (b), and >160 mm (c).
2.3 Statistical analysis
Statistical analysis was conducted using the SPSS 25.0 software (IBM Corp., Armonk, NY, United States) and R language software (version 3.6.1, company, state, country). Patients were randomly divided into training (n = 356 cases) and validation (n = 152 cases) groups using R with a random sampling function. The univariate Cox regression analysis was performed to identify prognostic factors in the training group, and statistically significant variables (p < 0.05) were incorporated into a multivariate Cox risk regression model. Furthermore, the nomogram graph was constructed, with the C-index mapping the subject’s working receiver operating characteristic (ROC) curve and correction curve for decision curve analysis (DCA). In the correction curve, the closer the curve is to the ideal 45° guide, the closer the prediction is to the actual observation. The C-index and the area under the ROC curve (AUC) were used to evaluate the predicted value of the nomogram graph; the maximum C-index was 1 (representing 100% prediction accuracy), and the minimum value was 0.5 α. The closer the C-index was to 1, the better the accuracy of the prediction model. Using the DCA method, we could effectively determine the certainty and benefit of the prediction model. An α level of 0.05 was used.
3 Results
3.1 Patient baseline information
According to the inclusion and exclusion criteria, 508 patients with malignant breast PTs between 2004 and 2015 were screened out of the SEER database. Table 1 shows the key characteristics of patients with PTs. The patients were randomly divided into training (n = 356) and validation (n = 152) groups. The training group was used to construct the nomogram model, and the validation group was used to validate the model.
3.2 Optimal tumor size and age truncation value
For further stratified analysis of age and tumor size, the X-tile graph determined the critical values of age to be 57 and 67 years, whereas the critical value of tumor size was determined to be 70 and 160 mm. To explore the effect of age-size thresholds on overall survival, we first divided the patients into three risk groups using 57 and 67 truncation values: 18–57 years old(a), 57–67 years old(b), and >67 years old(c). Furthermore, we found that age was an important prognostic factor in patients with malignant breast PTs; the worst prognostic factor for patients with malignant PTs was an age of >67 years, whereas patients aged <57 years had better overall survival rates than other groups (Figure 1A). Additionally, patients with a tumor size of >160 mm(c) had the worst prognosis, whereas those with a tumor size of <70 mm(a) had a better prognosis than the other groups (Figure 1B).
3.3 Prognostic factors for overall survival in patients with malignant PTs
To determine the independent prognostic factors for overall survival, we first performed univariate Cox regression analysis in the training group (n = 356). Factors such as older age, larger tumor size, race, tumor grade, chemotherapy, advanced AJCC stage, T stage, N stage, and distant metastasis (M) were significantly associated with poor overall survival (Table 2). These nine factors were further included and selected by the multivariate Cox proportional hazard regression analysis. These results showed that age at diagnosis, tumor grade, tumor size, N stage, and distant metastasis (M) were significant and independent prognostic factors for overall survival.
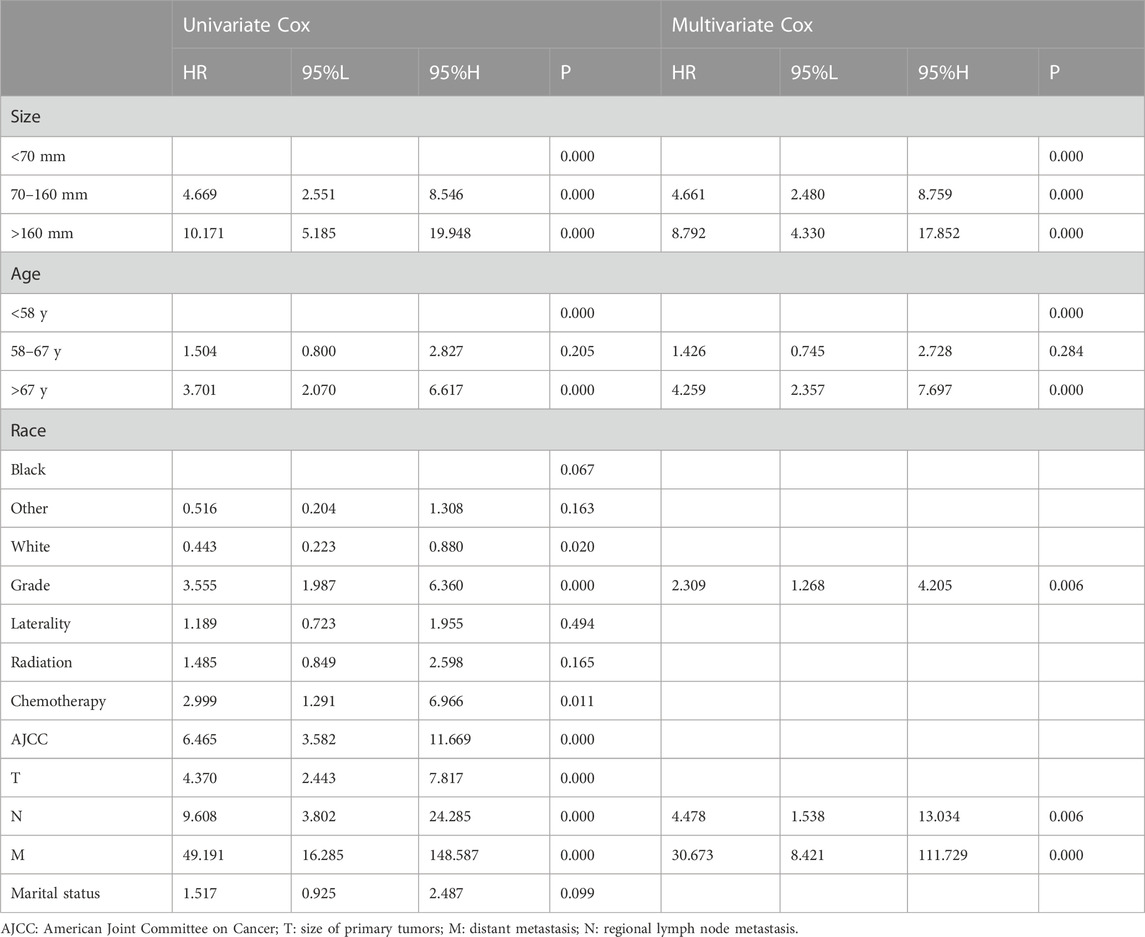
TABLE 2. Univariate and multivariate Cox regression analyses for overall survival in patients with malignant phyllode tumors of the breast.
3.4 Construction and validation of the prognostic model for overall survival
We first combined age, tumor size, N stage, distant metastasis (M), and tumor grade to construct a prognostic nomogram model for predicting the 1-, 3-, and 5-year overall survival in patients with malignant breast PTs (Figure 2). The model can score each prognostication factor on the score scale. By combining these scores with the total score of the lowest score, the 1-, 3-, and 5-year overall survival predictions for patients with malignant PTs of the breast can be calculated. The predictive accuracy of the final prognostication model was evaluated by the C-index. The C-index was 0.845 (95% confidence interval [CI], 0.802–0.888) and 0.784 (95% CI, 0.688–0.880) in the training and validation groups, respectively. The calibration plots revealed perfect consistency between the model-predicted and actual survival values (Figures 3A, C). Surgeons can easily use these prognostic models to determine the prognosis of patients with malignant PTs: age, tumor size, N stage, distant metastasis (M), and tumor grade.
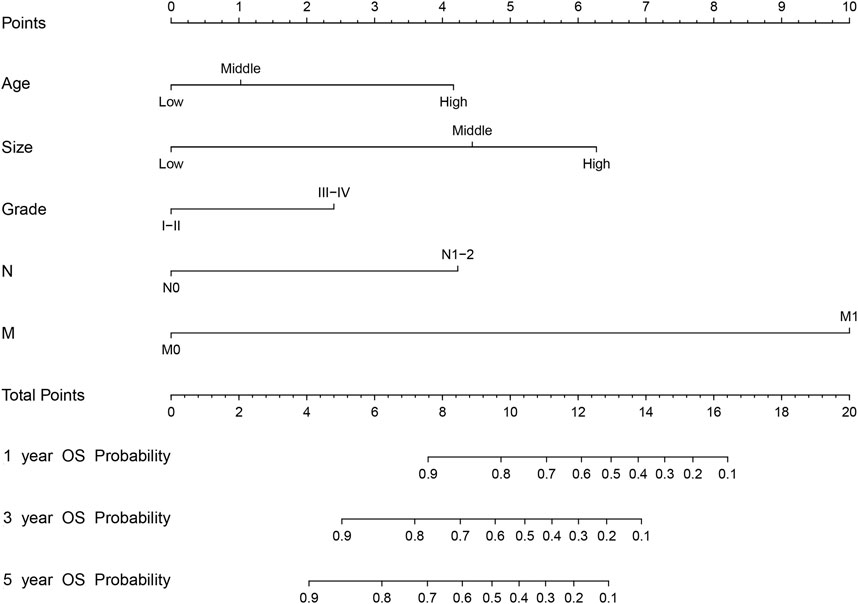
FIGURE 2. The nomogram model for predicting 1-, 3-, and 5-year OS (Overall Survival) rates of malignant breast PTs based on risk factors such as age, size, tumor grade, N stage, and distant metastasis (M). (Age-Age, Size-Size, and Grade-Level).
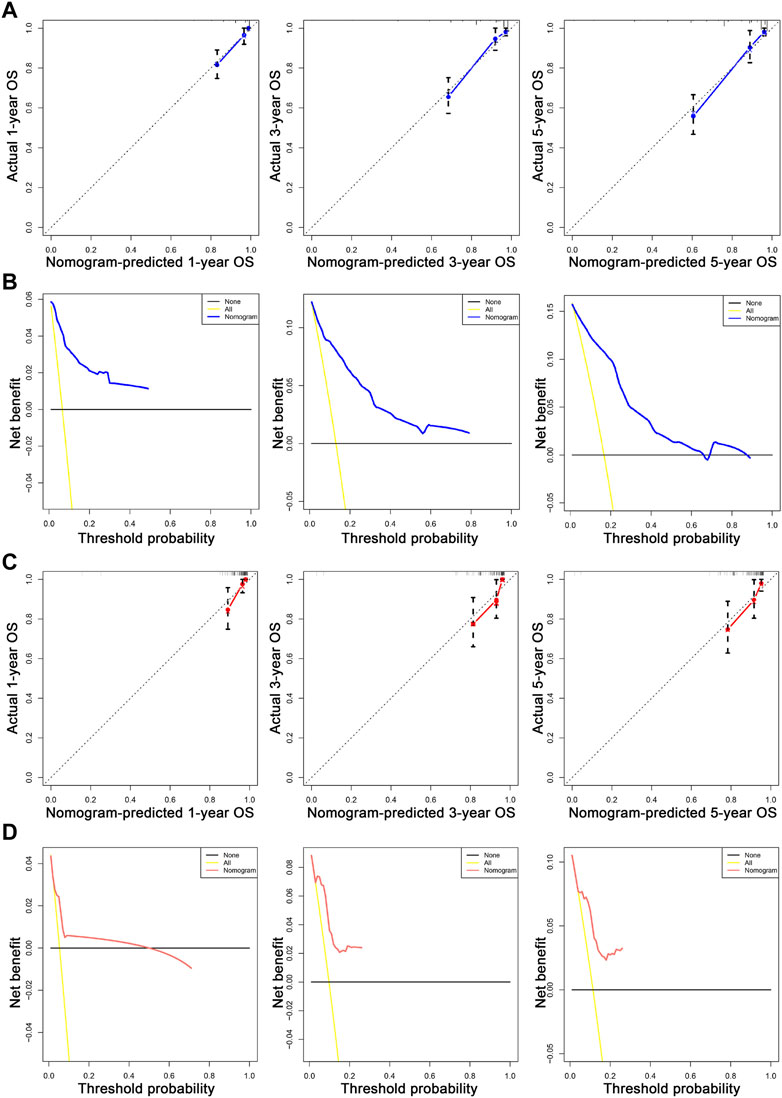
FIGURE 3. The calibration and DCA plots of the nomogram for predicting 1-, 3-, and 5-year OS. (A, C) The calibration plots for predicting 1-, 3-, and 5-year OS in the training and validation sets, respectively. (B, D) show the DCA curves evaluating the model’s clinical applicability in the training and validation sets, respectively. In the calibration curve, the X-axis is the OS predicted by the model. The actual OS is drawn on the Y-axis. The ideal calibration model is presented, in which the predicted probability corresponds to the actual survival outcome. In the decision curve, the X-axis represents a percentage of the threshold probability. In contrast, the Y-axis represents a net gain calculated by subtracting the true-positive number from the false-positive number.
Then, the predictive ability of the nomogram model was evaluated using the AUC (an AUC value of 0.5 indicates that the nomogram model has no predictive effect, and an AUC value of 1 indicates that the nomogram model has an excellent predictive effect). Patients with different survival rates were well distinguished (the higher the value between 0.5 and 1 is, the better the nomogram’s ability to distinguish), and other prognostic factors could be compared to further validate their superiority and determine which factor has the most superior predictive accuracy. In the training group, the advantages of the differentiation for overall survival shown in the nomogram were demonstrated compared to the other clinical information (1-year AUC: 0.908; 3-year AUC: 0.881; 5-year AUC: 0.879; Figures 4A–C). In addition, in the validation group, the AUC values for the 1-, 3-, and 5-year survival rates were 0.895, 0.843, and 0.807, respectively.
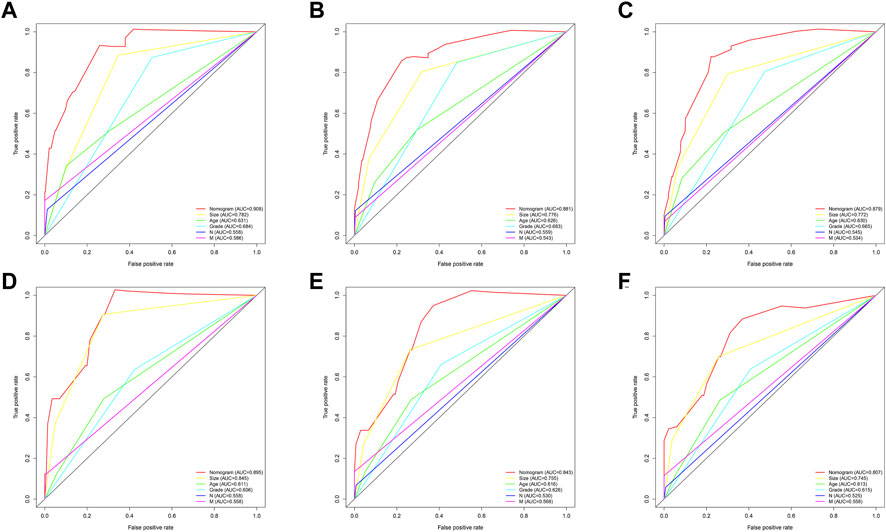
FIGURE 4. ROC curves for comparison of the sensitivity and specificity of the nomogram and clinical risk factors. The AUC comparison of the nomogram and clinical risk factors for predicting total annual survival rates at 1 (A), 3 (B), and 5 (C) years in the training group and (D–F) in the validation set, respectively.
The nomogram offers a considerable positive net benefit from the risk of mortality in both the training and validation groups (Figures 3B, D). It can predict the 1-, 3-, and 5-year overall survival rates and has good value in clinical application. Using the nomogram model (Figure 2), we can predict a patient’s survival probability based on his/her personalized information. For example, in a middle-aged woman diagnosed with breast PTs with a medium-sized primary lesion and tumor grade III, the patient’s overall survival score was 10.5 according to the model. As a result, the patient’s 1-, 3-, and 5-year overall survival rates were estimated to be 90%, 82%, and 80%, respectively.
4 Discussion
Compared with previous studies, we developed a nomogram mainly for patients with malignant PTs in our study (Ma et al., 2021; Choi et al., 2022; Bedi et al., 2022; Zhou et al., 2018b). Our nomogram performed well in terms of discrimination and calibration, and surgeons can use it to evaluate prognosis using patient-specific clinical information.
A few clinical factors may affect the survival of patients with malignant breast PTs, but previous studies have not fully integrated clinical factors (Asoglu et al., 2004; Majeski and Stroud, 2012; Wang et al., 2015; Zhang and Kleer, 2016; Strode et al., 2017; Co et al., 2018). A prognostic nomogram model is a graphical representation of several variables that combines many risk factors to accurately assess survival probability at a given period (Jin et al., 2020; Wen-Tao et al., 2021). Prognostic assessment models have been established for certain cancers, and they have proven to be more accurate than traditional tools for prognostic prediction (Zhou et al., 2018a; Dong et al., 2018; Ye et al., 2018; Zheng et al., 2018). In recent years, few studies have focused on the prognostic prediction of malignant breast PTs. Previous studies have found that surgical methods and tumor boundaries are independent prognostic factors for recurrence in marginal and malignant PTs (Neron et al., 2020; Li et al., 2021; Choi et al., 2022).
Breast PTs are rare fibroepithelial neoplasms. A study demonstrated that metastasis was predicted in malignant PTs based on tumor size and the presence of malignant heterologous elements (Koh et al., 2018). A meta-analysis of 54 studies with 9234 cases suggested that mitoses, tumor boundary, stromal cellularity, stromal atypia, stromal overgrowth, tumor necrosis, surgery type, and surgical margin status are risk factors for local recurrence (LR) (Lu et al., 2019). However, given the low incidence of malignant breast PTs, no collaborative model for predicting prognosis was available. Singaporean scholars are also attempting to predict the clinical behavior of breast PTs. Line charts based on histological criteria and surgical cut edges are used to evaluate the prognostication of PTs (Asoglu et al., 2004; Majeski and Stroud, 2012; Zhang and Kleer, 2016; Strode et al., 2017).
This study used multivariate Cox proportional hazard regression analysis to determine the independent prognostic factors for OS, including patient age, tumor grade, N stage, presence of distant metastasis (M), and tumor size. Tumor grade, N stage, and distant metastasis (M) are widely accepted as significant risk factors for LR. Patients with LR and metastases have a worse prognosis. Among patients with malignant PTs, older patients had poor OS. Prior research on the effect of tumor size on prognosis yielded conflicting findings. Kapiris et al. (2001) reported that tumor size and excision are associated with LR of malignant breast PTs. Asoglu et al. (2004) demonstrated that excessive substrate growth, tumor size, and the excision cut edge are essential factors for LR. However, it is unclear whether tumor size is a predictor of LR, as some studies have found that tumor size is not associated with LR (Salvadori et al., 1989) and that LR does not affect survival, which are inconsistent with our findings. Our study found a significant correlation between larger tumor size and shorter OS in the multivariate analysis, possibly because a tumor’s biological characteristics are more aggressive and its state tends to be worse as it becomes larger. It has also been suggested that radiation therapy (RT) can improve patient survival by reducing the risk of LR. RT was excluded from our study because of limited data. Nevertheless, the validity of the study must be examined because one of the study events was disease-free survival rather than LR (Zeng et al., 2015).
This study has several limitations. Because the insignificance of Her-2 has only been documented in the SEER database since 2010, we avoided molecular subtype analysis of breast cancer and used AJCC stage and TNM stage analyses to reduce case loss. The findings will help with the external validation cohort in the future. Clinical data and prospective studies are required to validate the value of the nomogram.
5 Conclusion
Patient age, tumor grade, N stage, distant metastasis (M), and tumor size were found to be independent factors for overall survival in malignant PTs of the breast. Compared with prognostic factors alone, these integrated factors and nomograms, can be used as a valuable and convenient assessment tool to help surgeons personalize survival assessments and identify mortality risk in patients with malignant PTs of the breast.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
WL and HC designed the project. KF, JC, JD, and DL performed data collection and analysis. WL and KF wrote the paper. WL has rigorously revised the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by The Guiding Project of Clinical Medical Technology Innovation in Hunan Province (2020SK51706), Clinical Research Center for Breast & Thyroid Disease Prevention in Hunan Province (2018SK4001) and General guidance project of Hunan Provincial Health Commission (202204013843).
Acknowledgments
We acknowledge the SEER database for providing data. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Asoglu, O., Ugurlu, M. M., Blanchard, K., Grant, C. S., Reynolds, C., Cha, S. S., et al. (2004). Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann. Surg. Oncol. 11, 1011–1017. doi:10.1245/ASO.2004.02.001
Balachandran, V. P., Gonen, M., Smith, J. J., and DeMatteo, R. P. (2015). Nomograms in oncology: More than meets the eye. Lancet Oncol. 16, e173–e180. doi:10.1016/S1470-2045(14)71116-7
Camp, R. L., Dolled-Filhart, M., and Rimm, D. L. (2004). X-Tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 10, 7252–7259. doi:10.1158/1078-0432.CCR-04-0713
Choi, J. E., Kang, S. H., Tan, P. H., and Bae, Y. K. (2022). Recurrence prediction for breast phyllodes tumours: Validation of the Singapore nomogram in Korean women. J. Clin. Pathol. 75, 159–163. doi:10.1136/jclinpath-2020-207093
Cimino-Mathews, A., Hicks, J. L., Sharma, R., Vang, R., Illei, P. B., De Marzo, A., et al. (2013). A subset of malignant phyllodes tumors harbors alterations in the Rb/p16 pathway. Hum. Pathol. 44, 2494–2500. doi:10.1016/j.humpath.2013.06.009
Co, M., Chen, C., Tsang, J. Y., Tse, G., and Kwong, A. (2018). Mammary phyllodes tumour: A 15-year multicentre clinical review. J. Clin. Pathol. 71, 493–497. doi:10.1136/jclinpath-2017-204827
Dong, F., Shen, Y., Gao, F., Shi, X., Xu, T., Wang, X., et al. (2018). Nomograms to predict individual prognosis of patients with primary small cell carcinoma of the bladder. J. Cancer 9, 1152–1164. doi:10.7150/jca.23344
Iasonos, A., Schrag, D., Raj, G. V., and Panageas, K. S. (2008). How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 26, 1364–1370. doi:10.1200/JCO.2007.12.9791
Jin, Y., Li, Y., Liu, Q., Li, L., Feng, A., Wang, T., et al. (2020). Brief introduction of medical database and data mining technology in big data era. J. Evid. Based Med. 13, 57–69. doi:10.1111/jebm.12373
Kapiris, I., Nasiri, N., A'Hern, R., Healy, V., and Gui, G. P. (2001). Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur. J. Surg. Oncol. 27, 723–730. doi:10.1053/ejso.2001.1207
Kim, Y. J., and Kim, K. (2017). Radiation therapy for malignant phyllodes tumor of the breast: An analysis of SEER data. Breast 32, 26–32. doi:10.1016/j.breast.2016.12.006
Koh, V. C. Y., Thike, A. A., Nasir, N. D. M., Yip, G. W. C., Bay, B. H., and Tan, P. H. (2018). Size and heterologous elements predict metastases in malignant phyllodes tumours of the breast. J. .Virchows Arch. 472, 615–621. doi:10.1007/s00428-017-2257-1
Li, Y., Song, Y., Lang, R., Shi, L., Gao, S., Liu, H., et al. (2021). Retrospective study of malignant phyllodes tumors of the breast: Younger age, prior fibroadenoma surgery, malignant heterologous elements and surgical margins may predict recurrence. Breast 57, 62–70. doi:10.1016/j.breast.2021.03.001
Liang, W., Zhang, L., Jiang, G., Wang, Q., Liu, L., Liu, D., et al. (2015). Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J. Clin. Oncol. 33, 861–869. doi:10.1200/JCO.2014.56.6661
Lissidini, G., Mule, A., Santoro, A., Papa, G., Nicosia, L., Cassano, E., et al. (2022). Malignant phyllodes tumor of the breast: A systematic review. Pathologica 114, 111–120. doi:10.32074/1591-951X-754
Lu, Y., Chen, Y., Zhu, L., Cartwright, P., Song, E., Jacobs, L., et al. (2019). Local recurrence of benign, borderline, and malignant phyllodes tumors of the breast: A systematic review and meta-analysis. Ann. Surg. Oncol. 26, 1263–1275. doi:10.1245/s10434-018-07134-5
Macdonald, O. K., Lee, C. M., Tward, J. D., Chappel, C. D., and Gaffney, D. K. (2006). Malignant phyllodes tumor of the female breast: Association of primary therapy with cause-specific survival from the surveillance, Epidemiology, and End results (SEER) program. Cancer 107, 2127–2133. doi:10.1002/cncr.22228
Majeski, J., and Stroud, J. (2012). Malignant phyllodes tumors of the breast: A study in clinical practice. Int. Surg. 97, 95–98. doi:10.9738/CC79.1
Neron, M., Sajour, C., Thezenas, S., Piperno-Neumann, S., Reyal, F., Laé, M., et al. (2020). Surgical margins and adjuvant therapies in malignant phyllodes tumors of the breast: A multicenter retrospective study. Ann. Surg. Oncol. 27, 1818–1827. doi:10.1245/s10434-020-08217-y
Salvadori, B., Cusumano, F., Del Bo, R., Delledonne, V., Grassi, M., Rovini, D., et al. (1989). Surgical treatment of phyllodes tumors of the breast. Cancer 63, 2532–2536. doi:10.1002/1097-0142(19890615)63:12<2532::aid-cncr2820631229>3.0.co;2-q
Sawhney, N., Garrahan, N., Douglas-Jones, A. G., and Williams, E. D. (1992). Epithelial–stromal interactions in tumors. A morphologic study of fibroepithelial tumors of the breast. Cancer 70, 2115–2120. doi:10.1002/1097-0142(19921015)70:8<2115::aid-cncr2820700818>3.0.co;2-k
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34. doi:10.3322/caac.21551
Strode, M., Khoury, T., Mangieri, C., and Takabe, K. (2017). Update on the diagnosis and management of malignant phyllodes tumors of the breast. Breast 33, 91–96. doi:10.1016/j.breast.2017.03.001
Tan, B. Y., Acs, G., Apple, S. K., Badve, S., Bleiweiss, I. J., Brogi, E., et al. (2016). Phyllodes tumours of the breast: A consensus review. Histopathology 68, 5–21. doi:10.1111/his.12876
Tan, P. H., Thike, A. A., Tan, W. J., Thu, M. M. M., Busmanis, I., Li, H., et al. (2012). Predicting clinical behaviour of breast phyllodes tumours: A nomogram based on histological criteria and surgical margins. J. Clin. Pathol. 65, 69–76. doi:10.1136/jclinpath-2011-200368
Tan, X. R., Li, Y. Q., Liang, S. B., Jiang, W., Liu, F., Ge, W. X., et al. (2018). Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: A retrospective, multicentre, cohort study. Lancet Oncol. 19, 382–393. doi:10.1016/S1470-2045(18)30080-9
Wang, F., Jia, Y., and Tong, Z. (2015). Comparison of the clinical and prognostic features of primary breast sarcomas and malignant phyllodes tumor. Jpn. J. Clin. Oncol. 45, 146–152. doi:10.1093/jjco/hyu177
Wang, Y., Li, J., Xia, Y., Gong, R., Wang, K., Yan, Z., et al. (2013). Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 31, 1188–1195. doi:10.1200/JCO.2012.41.5984
Wen-Tao, W., Yuan-Jie, L., Ao-Zi, F., Li, L., Huang, T., Xu, A. D., et al. (2021). Data mining in clinical big data: The frequently used databases, steps, and methodological models. Mil. Med. Res. 8, 44. doi:10.1186/s40779-021-00338-z
Wu, S., Chen, J. N., Zhang, Q. W., Tang, C. T., Zhang, X. T., Tang, M. Y., et al. (2018). A new metastatic lymph node classification-based survival predicting model in patients with small bowel adenocarcinoma: A derivation and validation study. EBioMedicine 32, 134–141. doi:10.1016/j.ebiom.2018.05.022
Ye, F. G., Xia, C., Ma, D., Lin, P. Y., Hu, X., and Shao, Z. M. (2018). Nomogram for predicting preoperative lymph node involvement in patients with invasive micropapillary carcinoma of breast: A SEER population-based study. BMC Cancer 18, 1085. doi:10.1186/s12885-018-4982-5
Zeng, S., Zhang, X., Yang, D., Wang, X., and Ren, G. (2015). Effects of adjuvant radiotherapy on borderline and malignant phyllodes tumors: A systematic review and meta-analysis. Mol. Clin. Oncol. 3, 663–671. doi:10.3892/mco.2015.503
Zhang, L., Dong, D., Li, H., Tian, J., Ouyang, F., Mo, X., et al. (2019). Development and validation of a magnetic resonance imaging-based model for the prediction of distant metastasis before initial treatment of nasopharyngeal carcinoma: A retrospective cohort study. EBioMedicine 40, 327–335. doi:10.1016/j.ebiom.2019.01.013
Zhang, Y., and Kleer, C. G. (2016). Phyllodes tumor of the breast: Histopathologic features, differential diagnosis, and molecular/genetic updates. Arch. Pathol. Lab. Med. 140, 665–671. doi:10.5858/arpa.2016-0042-RA
Zheng, W., Huang, Y., Chen, H., Wang, N., Xiao, W., Liang, Y., et al. (2018). Nomogram application to predict overall and cancer-specific survival in osteosarcoma. Cancer Manag. Res. 10, 5439–5450. doi:10.2147/CMAR.S177945
Zhou, H., Zhang, Y., Qiu, Z., Chen, G., Hong, S., Chen, X., et al. (2018). Nomogram to predict cause-specific mortality in patients with surgically resected stage I non-small-cell lung cancer: A competing risk analysis. Clin. Lung Cancer 19, e195–e203. doi:10.1016/j.cllc.2017.10.016
Keywords: nomogram, SEER, prognosis, malignant phyllodes tumors, breast cancer
Citation: Li W, Fang K, Chen J, Deng J, Li D and Cao H (2023) The application of clinical variable-based nomogram in predicting overall survival in malignant phyllodes tumors of the breast. Front. Genet. 14:1133495. doi: 10.3389/fgene.2023.1133495
Received: 29 December 2022; Accepted: 09 May 2023;
Published: 30 May 2023.
Edited by:
Jason Yongsheng Chan, National Cancer Centre Singapore, SingaporeReviewed by:
Jun Lyu, First Affiliated Hospital of Jinan University, ChinaXinhua Xie, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2023 Li, Fang, Chen, Deng, Li and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Cao, Y2FvaG9uZzk5NzBAMTYzLmNvbQ==; Wei Li, bGl3ZWkwNzEyQHVzYy5lZHUuY24=
†These authors have contributed equally to this work
 Wei Li
Wei Li Kun Fang
Kun Fang Jiaren Chen1
Jiaren Chen1 Hong Cao
Hong Cao