A Commentary on
Case report: Optic atrophy and nephropathy with m.13513G>A/MT-ND5 mtDNA pathogenic variant
by Barone V, La Morgia C, Caporali L, Fiorini C, Carbonelli M, Gramegna LL, Bartiromo F, Tonon C, Morandi L, Liguori R, Petrini A, Brugnano R, Del Sordo R, Covarelli C, Morroni M, Lodi R and Carelli V (2022). Front. Genet. 13:887696. doi: 10.3389/fgene.2022.887696
Letter to the editor
We read with interest the article by Barone et al. about a 48 years old male with a multisystem mitochondrial disorder (MID) due to the variant m.13513G>A in ND5 (Barone et al., 2022). Phenotypically, the patient manifested with subcortical cerebellar atrophy, mineralisation of the globus pallidus, Leber’s hereditary optic neuropathy (LHON), optic atrophy, hearing loss, and nephropathy (Barone et al., 2022). The study is appealing but raises concerns that should be discussed.
Many MIDs manifest phenotypically with cardiac disease (Lioncino et al., 2022). This is also the case with patients carrying the m.13513G>A variant (Ruiter et al., 2007). Cardiac manifestations in m.13513G>A carriers include cardiomyopathy and Wolff-Parkinson-White (WPW) syndrome. Therefore, it would be highly valuable to know the results of the clinical cardiologic examination, ECG, transthoracic echocardiography, the cardiac stress test, and eventually cardiac MRI.
Like many other MIDs, conditions due to the variant m.13513G>A may also manifest in the skeletal muscles as myopathy, ptosis, or ophthalmoparesis (Wei et al., 2021). Therefore, it would be interesting to know the results of the clinical neurologic examination, serum creatine-kinase, lactate-dehydrogenase, aldolase, myoglobin and lactate levels, and needle electromyography studies, and eventually muscle biopsy. This will definitely enhance the quality and clarity of case presentation.
According to a recent review of 50 patients carrying the m.13513G>A variant, the spectrum of the m.13513G>A variant is much broader than anticipated, and also includes fatal neonatal acidosis, renal dysplasia, cardiomyopathy, WPW syndrome, strabism, progressive external ophthalmoplegia, seizures, stroke-like episodes (SLEs), ataxia, tremor, and psychosis (Yahata et al., 2017) (Table 1). Therefore, it would be highly valuable to know if electroencephalography was normal and if any of these features were present in the index patient.
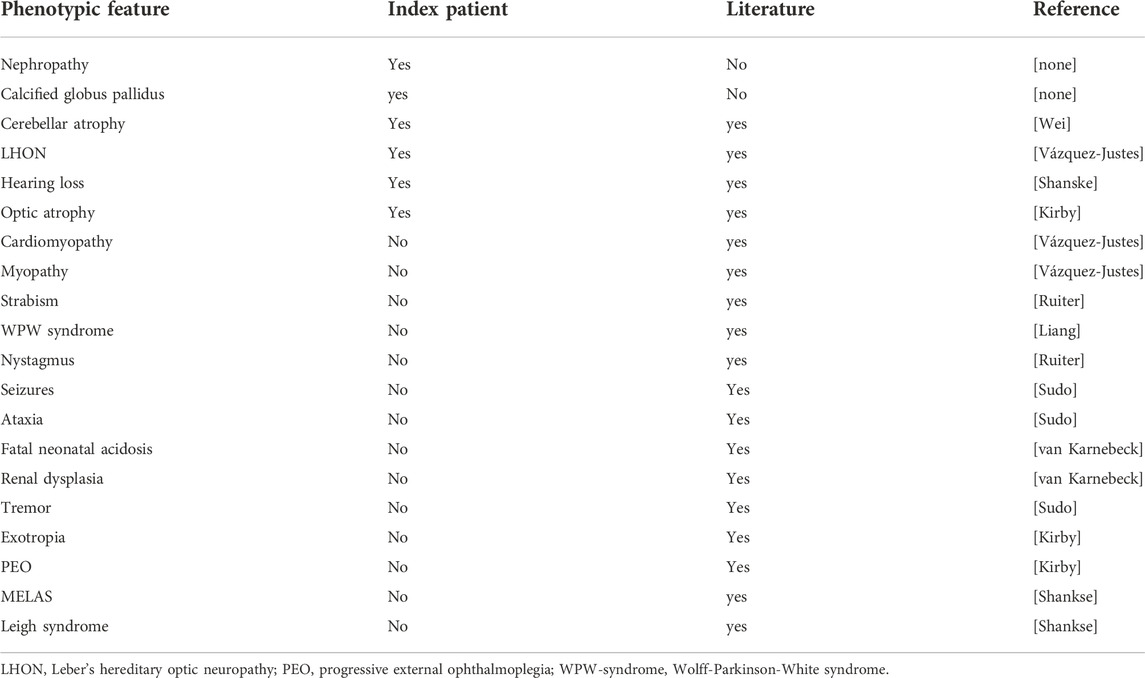
TABLE 1. Phenotypic manifestations of the variant m.13513G>A in the index patient and the literature so far reported.
Though the combination of renal insufficiency, cerebral atrophy, LHON, and hypoacusis, may have been undescribed prior to the index case, the single individual features are all well-known from m.13513G>A carriers (Table 1). LHON is a common feature of m.13513G>A carriers, as well as hypoacusis, and cerebral atrophy. Only renal failure has been found only in a few patients with the m.13513G>A variant (Yahata et al., 2017).
We disagree with the consideration that the reduced MMSE could be due to visual impairment (Barone et al., 2022). Because m.13513G>A carriers frequently manifest with cerebral atrophy, like the index patient, and with psychiatric disease, the MMSE score of 28 is more likely attributable to cerebral than ophthalmologic involvement. Furthermore, MR-spectroscopy showed cerebral lactic acidosis, suggesting that cognitive impairment may also derive from cerebral acidosis.
Bilateral T2-hyperintensities in the globus pallidus, which were hypointense on susceptibility weighted imaging (SWI) were interpreted as mineralisation (Barone et al., 2022). It would be highly valuable to know whether the cerebral CT scan showed basal ganglia calcifications or if these lesions were due to increased iron deposition.
Overall, the interesting study has some limitations that call the results and their interpretation into question. Clarifying these weaknesses would strengthen the conclusions and could improve the study. The phenotypic spectrum of m.13513G>A carriers is broader than anticipated.
Author contributions
JF was responsible for all issues.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barone, V., La Morgia, C., Caporali, L., Fiorini, C., Carbonelli, M., Gramegna, L. L., et al. (2022). Case report: Optic atrophy and nephropathy with m.13513G>A/MT-ND5 mtDNA pathogenic variant. Front. Genet. 13, 887696. doi:10.3389/fgene.2022.887696
Lioncino, M., Monda, E., Caiazza, M., Fusco, A., Cirillo, A., Dongiglio, F., et al. (2022). Cardiovascular involvement in mtDNA disease: Diagnosis, management, and therapeutic options. Heart Fail Clin. 18 (1), 51–60. doi:10.1016/j.hfc.2021.07.003
Ruiter, E. M., Siers, M. H., van den Elzen, C., van Engelen, B. G., Smeitink, J. A., Rodenburg, R. J., et al. (2007). The mitochondrial 13513G > A mutation is most frequent in Leigh syndrome combined with reduced complex I activity, optic atrophy and/or Wolff-Parkinson-White. Eur. J. Hum. Genet. 15 (2), 155–161. doi:10.1038/sj.ejhg.5201735
Wei, Y., Huang, Y., Yang, Y., and Qian, M. (2021). MELAS/LS overlap syndrome associated with mitochondrial DNA mutations: Clinical, genetic, and radiological studies. Front. Neurol. 12, 648740. doi:10.3389/fneur.2021.648740
Keywords: mtDNA, complex-I, LHON, multisystem, heteroplasmy, m.13513G>A
Citation: Finsterer J (2022) Commentary: Case report: Optic atrophy and nephropathy with m.13513G>A/MT-ND5 mtDNA pathogenic variant. Front. Genet. 13:988122. doi: 10.3389/fgene.2022.988122
Received: 06 July 2022; Accepted: 02 November 2022;
Published: 28 November 2022.
Edited by:
Jordi Pérez-Tur, Spanish National Research Council (CSIC), SpainReviewed by:
Parisa Gazerani, Oslo Metropolitan University, NorwayCopyright © 2022 Finsterer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josef Finsterer, ZmlmaWdzMUB5YWhvby5kZQ==
 Josef Finsterer
Josef Finsterer