- 1Department of Medical Genetics and Prenatal Diagnosis, Huizhou First Maternal and Child Health Care Hospital, Huizhou, Guangdong, China
- 2Berry Genomics Corporation, Beijing, China
Background: Thalassemia was the most common monogenic diseases worldwide, which was caused by mutations, deletions or duplications in human globin genes which disturbed the synthesis balance between α- and β-globin chains of hemoglobin. There were many classics methods to diagnose thalassemia, but all of them had limitations. Although variations in the human β-globin gene cluster were mainly point mutations, novel large deletions had been described in recent years along with the development of DNA sequencing technology.
Case report: We present a case of 32-year-old male with abnormal hematological results. However, 23 genotypes of the most common thalassemia were not detected by two independent conventional platforms. Finally, using multiplex ligation-dependent probe amplification (MLPA), third-generation sequencing (TGS) and Gap PCR detection methods, we first confirmed the case with a novel 7.2 Kb deletion (Chr11:5222800-5230034, hg38) located at HBB gene.
Conclusion: Our results showed that TGS technology was a powerful tool for thalassemia breakpoint detection, had promising potentiality in genetic screening of novel thalassemia, especially for the novel deletions in globin genes.
Introduction
Thalassemia was among the most common monogenic diseases worldwide (Taher et al., 2018). The estimated carrier rate for β-thalassemia was about 5.2% in the whole world. β-thalassemia was characterized by insufficient or missing synthesis of the β-globin chain of hemoglobin, up to now about 280 β-thalassemia mutations had been recognised, occurring in a wide range of ethnic groups, including at least 230 point mutations and at last 18 large deletions (Bain, 2020). Due to the limitations of routine molecular techniques for detecting β-globin gene variants, less than 30 deletions in HBB gene were reported as genotypes of β-thalassemia to date (Chen et al., 2022). Third-generation sequencing (TGS) was a new type of DNA high-throughput sequencing, based on long-read single-molecular real-time sequencing technology (SMRT) (Xu et al., 2020). Due to its long-read length and high detection accuracy, it was hypothesized that this technology could be applied to thalassemia carrier screening (Xiao and Zhou, 2020). In this study, we first discovered a novel 7.2 Kb deletion in the β-globin gene, which was identified precisely by the TGS technology.
Case presentation
We present a 32-year-old male with abnormal hematological results, who was admitted to the outpatient for genetic screening of thalassemia during pregnancy examination. Hb electrophoresis and the routine hematological indices were determined during our screening program. Hematological examinations were measured by automatic hematological analyzer (BC-5180, China), which showed that he had a microcytic hypochromic anemia (Hb level of 119.0 g/L), mean cell hemoglobin (MCH) of 16.5 pg and a mean cell volume (MCV) of 63.8 fl (Table 1). The Hb electrophoresis was performed and quantified by automatic capillary electrophoresis (Sebia, France), which showed that the high HbA2 (7.0%), high HbF (3.0%), and the HbA was 90.0% (Table 1), iron deficiency was excluded. His wife had normal Hb and normal MCV and MCH (Table 1). The study was approved by the Medical Ethics Committee of Huizhou First Maternal and Child Health Care Hospital (2021030) and all individuals provided informed written consent.
Genomic DNA was extracted from the whole blood sample using automatic nucleic acid extraction instrument (Lab-Aid 824, Zhishan Biotechnology Co., Ltd., Xiamen, China). Thalassemia genotyping analysis were used two independent platforms: suspension-array system (Yin et al., 2014) and a reverse dot blot (RDB) assay thalassemia gene chip combined with flow-through hybridization technology platform (Chaozhou Hybribio Limited Corporation, China) (Lin et al., 2012). All this two assays were performed according to the manufacturer’s protocol. 23 genotypes of most common thalassemia had been detected, including 3 types of α-globin (HBA) genes deletions (–SEA, -α3.7, -α4.2), 3 types of point mutations in α-globin genes (αCSα, αQSα and αWSα) and 17 types of point mutations β-globin (HBB) genes (41–42M/N, 654M/N, -28M/N, 71–72M/N, 17M/N, βEM/N, 43M/N, −29M/N, 31M/N, −32M/N, IVS-I-1M, 27/28M, -30M, 14–15M, CAPM, IntM and IVS-I-5M). However, no variants had been detected of the above 23 genotypes of thalassemia in this couple (Figure 1). Considering the obvious abnormality of hematological phenotype in the husband, multiplex ligation-dependent probe amplification (MLPA, MRCHolland, Amsterdam, Netherlands) was utilized to test deletions and duplications within the β-globin genes. MLPA detected a deletion in the β-globin gene cluster (deletion probes involved 10 probes between hg38 loc. 11p15.4: 5224905-5229846, including HBB-up, HBB-1, HBB-1 (WT) c., HBB-Intr.1, HBB-Intr.2, HBB-3, and HBB-down regions, Figure 2A). However, the exact breakpoint location was not determined. Therefore TGS was adopted to characterize the deletion. Then, consistent with the MLPA result, TGS analysis confirmed a novel 7.2 Kb deletion (Chr11:5222800-5230034, hg38) at β-globin gene, and this deletion removed all of the HBB gene (Chr11:5225464-5227197, hg38) (Figure 2B). Ultimately, this novel deletion was further verificated by Gap PCR and Sanger sequencing (Figure 3).
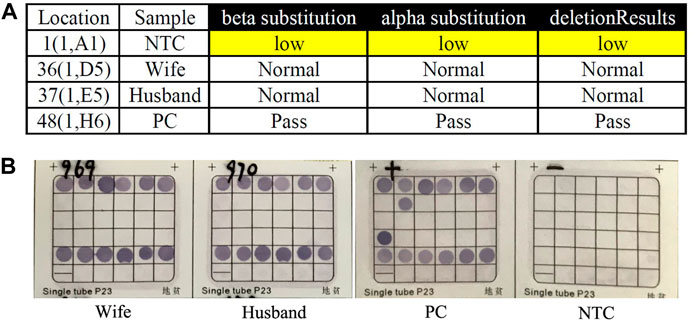
FIGURE 1. Routine genetic assays for 23 types of variations in HBA and HBB genes. (A) Auspension-array system was performed to detect 23 types of common variations in thalassemia. (B) Reverse dot blot (RDB) assay was used to detect 23 types of common variations in thalassemia (PC was β17M/N and α–SEA/N). No routine variants in thalassemia had been detected in this couple by this two independent platforms (NTC: No Template Control; PC: Positive Control; Pass: Quality Control Passed).
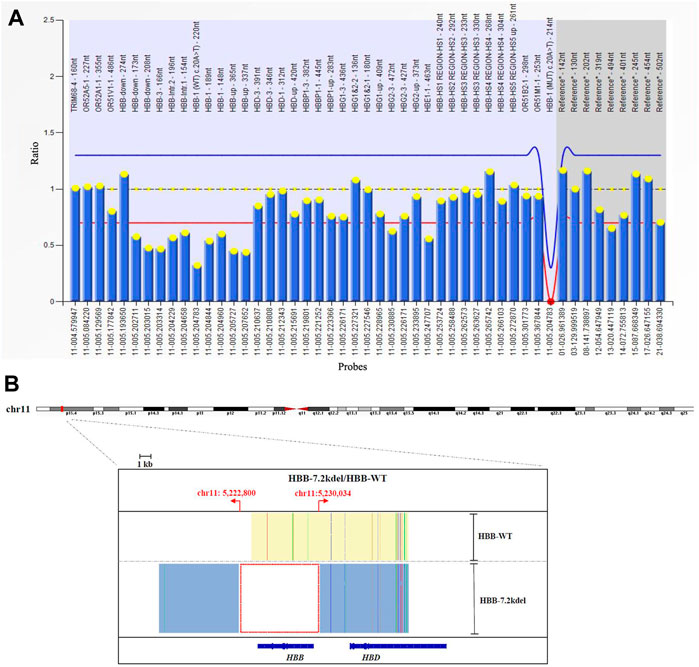
FIGURE 2. MLPA and TGS results. (A) MLPA result showed the deletion across 10 probes targeting HBB gene. (deletion probes involved 10 probes between hg38 loc. 11p15.4: 5224905-5229846, including HBB-up, HBB-1, HBB-1 (WT) c., HBB-Intr.1, HBB-Intr.2, HBB-3 and HBB-down regions). (B) TGS result showed the exact length of the deletion was 7.2 Kb (Chr11:5222800-5230034, hg38) located at HBB gene.
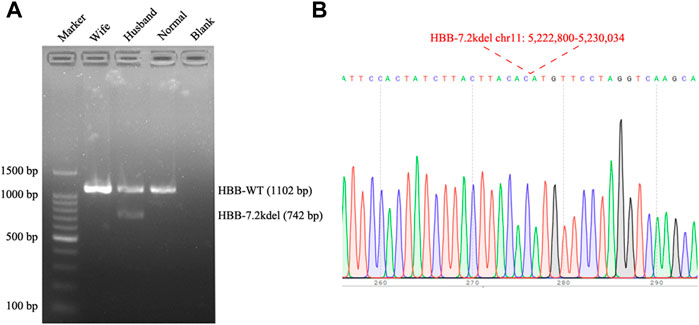
FIGURE 3. Gap PCR and Sanger sequencing results. (A) Gap PCR and agarose gel electrophoresis were performed to detect the deletion breakpoint. With the primers (7.2 Kb deletion forward primer: 5′-ATTTCTCCCTTGATAGTTTCTACTTTGGGTT-3′, Wild Type forward primer: 5′-TACCATCATCCTGGCTTCAAGGC-3′ and the common reverse primer: 5′-ATCTGAAGACTGTACCTCTGCTCTCC-3′), an unique fragment about 742 bp in length was only amplified in the husband (Normal: Normal individual; Blank: Water). (B) The fragment was then sequenced, the breakpoint was accordant with the TGS result.
Discussion and conclusion
Although variations in the β-thalassemia was mainly due to point mutations, several large deletions had been described in recent years (Zhu et al., 2019). Due to the technical limitations, many breakpoints of large deletions in the β-globin gene cluster were not confirmed (Rooks et al., 2012). Research had shown that there were multiple cis-elements located in the β-globin gene cluster that could regulate the expression of the globin genes (Thein, 2013; Zhu et al., 2019). Therefore, ascertaining the breakpoints of deletions was important for hemoglobinopathy research (Hu et al., 2016). Furthermore, it was difficult to studies of molecular structure of these large deletions especially the accurate breakpoints, until the invention of TGS technology which could relatively accurately estimate the deletion range. TGS conducting on the PacBio sequencing platform, had shown significant advantages such as extra-long reads (80 Kb) and no requirement for PCR, had been used for thalassemia carrier screening in the last 2 years (Xiao and Zhou, 2020; Liang et al., 2021; Luo et al., 2022).
In this study, different methods were used for thalassemia carriers screening. First, routine hematological phenotypes of thalassemia were detected (MCV, MCH, and Hb%). Subsequently, genetic genotypes for 23 genotypes of common thalassemia variations, MLPA, TGS, and Gap PCR analyses for known and unknown genotypes were carried out. MLPA found a deletion in the β-globin gene cluster but it could not make clear the exact location of the breakpoint. Finally, a novel 7.2 Kb heterozygous deletion in β-globin gene cluster (Chr11:5222800-5230034, hg38) was confirmed by TGS and Gap PCR, which was consistent with the MLPA study. This novel 7.2 Kb heterozygous deletion in β-globin gene included all the functional HBB gene, corresponding to high Hb A2 level (7.0%), high Hb F level (3.0%) and low MCV(63.8 fl).
In conclusion, our studies showed that TGS technology was a powerful tool for thalassemia breakpoint detection, had promising potentiality in genetic screening of novel HBA1/2 and HBB variants that might be outside the scope or difficult to be accurately detected by traditional analysis methods, especially for the novel deletions in globin genes. Furthermore, MLPA combining with TGS analysis should be considered to perform an accurate diagnosis in uncertain thalassemia cases, which could also improve the accuracy of genetic counseling.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Huizhou First Maternal and Child Health Care Hospital (2021030). The patients/participants provided their written informed consent to participate in this study
Author contributions
GZ designed the study, collected and analyzed the data, and drafted the manuscript. ZZ collected and analyzed the data and drafted the manuscript. ZG, DC, and ZW performed the experiments. KY and DC collected and analyzed the data. YL and RX provided the technical support. JC designed the study, wrote the manuscript and provided research funds.
Funding
This study was supported by the Guangdong Medical Research Fund (A2017534).
Acknowledgments
The authors would like to thank RX and Juyan Zhang in Berry Genomics Corporation for the third-generation sequencing technology support.
Conflict of interest
RX and YL were employed by Berry Genomics Corporation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bain, B. J. (2020). Haemoglobinopathy diagnosis. Third Edition. Oxford: Blackwell Publishing (Holdings) Ltd.
Chen, X., Luo, M., Pan, L., Huang, Y., Yan, Z., Shen, K., et al. (2022). A novel 4.9 Kb deletion at beta-globin gene is identified by the third-generation sequencing: Case report from Baoan, China. Clin. Chim. Acta. 529, 10–16. doi:10.1016/j.cca.2022.01.024
Hu, L., Shang, X., Yi, S., Cai, R., Li, Z., Liu, C., et al. (2016). Two novel copy number variations involving the alpha-globin gene cluster on chromosome 16 cause thalassemia in two Chinese families. Mol. Genet. Genomics 291 (3), 1443–1450. doi:10.1007/s00438-016-1193-0
Liang, Q., Gu, W., Chen, P., Li, Y., Liu, Y., Tian, M., et al. (2021). A more universal approach to comprehensive analysis of thalassemia alleles (CATSA). J. Mol. Diagn. 23 (9), 1195–1204. doi:10.1016/j.jmoldx.2021.06.008
Lin, M., Zhu, J. J., Wang, Q., Xie, L. X., Lu, M., Wang, J. L., et al. (2012). Development and evaluation of a reverse dot blot assay for the simultaneous detection of common alpha and beta thalassemia in Chinese. Blood Cells Mol. Dis. 48 (2), 86–90. doi:10.1016/j.bcmd.2011.12.001
Luo, S., Chen, X., Zeng, D., Tang, N., Yuan, D., Zhong, Q., et al. (2022). The value of single-molecule real-time technology in the diagnosis of rare thalassemia variants and analysis of phenotype-genotype correlation. J. Hum. Genet. 67 (4), 183–195. doi:10.1038/s10038-021-00983-1
Rooks, H., Clark, B., Best, S., Rushton, P., Oakley, M., Thein, O. S., et al. (2012). A novel 506kb deletion causing εγδβ thalassemia. Blood Cells Mol. Dis. 49 (3-4), 121–127. doi:10.1016/j.bcmd.2012.05.010
Taher, A. T., Weatherall, D. J., and Cappellini, M. D. (2018). Thalassaemia. Lancet 391 (10116), 155–167. doi:10.1016/S0140-6736(17)31822-6
Thein, S. L. (2013). The molecular basis of beta-thalassemia. Cold Spring Harb. Perspect. Med. 3 (5), a011700. doi:10.1101/cshperspect.a011700
Xiao, T., and Zhou, W. (2020). The third generation sequencing: The advanced approach to genetic diseases. Transl. Pediatr. 9 (2), 163–173. doi:10.21037/tp.2020.03.06
Xu, L., Mao, A., Liu, H., Gui, B., Choy, K. W., Huang, H., et al. (2020). Long-Molecule sequencing: A new approach for identification of clinically significant DNA variants in alpha-thalassemia and beta-thalassemia carriers. J. Mol. Diagn. 22 (8), 1087–1095. doi:10.1016/j.jmoldx.2020.05.004
Yin, A., Li, B., Luo, M., Xu, L., Wu, L., Zhang, L., et al. (2014). The prevalence and molecular spectrum of alpha- and beta-globin gene mutations in 14, 332 families of Guangdong Province, China. PLoS One 9 (2), e89855. doi:10.1371/journal.pone.0089855
Keywords: thalassemia, deletion, β-globin gene, breakpoint, third-generation sequencing
Citation: Zhong G, Zhong Z, Guan Z, Chen D, Wu Z, Yang K, Chen D, Liu Y, Xu R and Chen J (2022) Case Report: The third-generation sequencing confirmed a novel 7.2 Kb deletion at β-globin gene in a patient with rare β-thalassemia. Front. Genet. 13:984996. doi: 10.3389/fgene.2022.984996
Received: 03 July 2022; Accepted: 23 August 2022;
Published: 12 September 2022.
Edited by:
Ahmed Rebai, Centre of Biotechnology of Sfax, TunisiaReviewed by:
Hajkhelil Amel, University of Monastir, TunisiaDuran Canatan, Akdenz Kan Hastaliklari Vakfi, Turkey
Copyright © 2022 Zhong, Zhong, Guan, Chen, Wu, Yang, Chen, Liu, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhong Chen, Y2pob25nMjAwNEBob3RtYWlsLmNvbQ==
 Guoxing Zhong
Guoxing Zhong Zeyan Zhong
Zeyan Zhong Zhiyang Guan1
Zhiyang Guan1 Yinyin Liu
Yinyin Liu Jianhong Chen
Jianhong Chen