
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 19 August 2022
Sec. Cancer Genetics and Oncogenomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.959684
This article is part of the Research Topic The Involvement of Systemic Homeostasis in Tumour Biology View all 21 articles
This study analyzed PSAT1-targeted miRNAs as a prognostic predictor for gastric cancer. The relationship between the clinical manifestations of gastric cancer in patients and phosphoserine aminotransferase 1 (PSAT1) was analyzed using correlation analysis. PSAT1 was highly expressed in gastric cancer, and its low expression was associated with a poor prognosis. By pan-cancer analysis, PSAT1 could affect the tumor immune microenvironment by immune infiltration analysis. Nine microRNAs targeting PSAT1 and associated with gastric cancer were screened by miRwalk and microRNA expression in TCGA tumor tissues. Six microRNAs were obtained by survival curve analysis, including hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p, and hsa-miR-497-5p. Based on the above six microRNAs, a model for bone metastasis prediction in gastric cancer prediction was constructed. An analysis of a decision curve was performed based on the microRNAs obtained to predict bone metastasis from gastric cancer. It had a positive area under the curve (AUC) value of 0.746, and the decision curve analysis (DCA) indicated that it was clinically significant. Dual-luciferase reporter genes indicated that hsa-miR-497-5p and PSAT1 were targeted, and qRT-PCR results confirmed that hsa-miR-497-5p could down-regulate PSAT1 expression. MicroRNAs targeting the regulation of PSAT1 expression can well predict the prognosis of gastric cancer.
Cancer of the gastric mucosa arises from the epithelium of the mucosa, and most of its clinical manifestations are indigestion, abdominal pain, early satiety, or anorexia, but it may also present as reflux, dysphagia, and gastrointestinal bleeding (Machlowska et al., 2020). Gastric cancer is a heterogeneous and multifactorial disease caused by a combination of environmental and genetic factors, with a complex pathogenesis (Oliveira et al., 2015). Infections with H. pylori, nitrate- and nitrite-rich diets, smoking and drinking alcohol are all risk factors for gastric cancer (Machlowska et al., 2020; Joshi and Badgwell, 2021). Some genetic syndromes, such as CDH1, Lynch, and Peutz-Jegher syndromes, are also associated with gastric cancer (Hansford et al., 2015; Lott and Carvajal-Carmona, 2018). There were approximately 1.089 million new cases of gastric cancer worldwide in 2020, accounting for 5.6% of all cancers, making it the fifth-largest malignant tumor in the world after breast, lung, colorectal, and prostate cancers. (Sung et al., 2021). In addition, since gastric cancer exhibits insidious symptoms in the early stages, metastases are often detected at the time of diagnosis, making the prognosis poor and the mortality rate high. Globally, approximately 769,000 people will die from stomach cancer in 2020, accounting for 7.7% of all cancer-related deaths. The most common sites of metastasis for gastric cancer are the peritoneum, liver, lung, and lymph nodes, while bone metastases are rare, occurring in less than 5% of patients (Park et al., 2013; Turkoz et al., 2014). However, studies have shown that bone metastases from gastric cancer are an independent poor prognostic factor for gastric cancer and are significantly associated with overall patient survival. Bone metastases from gastric cancer have a significantly lower 5-year survival rate than non-bone metastases, with a median survival time of only about four months (Lee et al., 2007; Xiaobin et al., 2022). Because most bone metastases do not show significant clinical symptoms, the actual incidence of bone metastasis may be higher than reported. There is an urgent need for biomolecular markers that can determine the risk factors for bone metastasis in gastric cancer, assess their risk in patients, and detect early and accurately.
PSAT1 encodes the catalytic enzyme phosphoserine aminotransferase, which is associated with cell proliferation and serine anabolism (Hart et al., 2007; Montrose et al., 2021). Researchers have found that PSAT1 is aberrantly expressed in various tumor cells and promotes proliferation, metastasis, invasion, and drug resistance in a variety of malignancies, including breast, lung, and colorectal cancer (Metcalf et al., 2020; Biyik-Sit et al., 2021; Montrose et al., 2021). In addition, PSAT1 was found to promote extracellular vesicle (EV) secretion via the serine-ceramide synthesis pathway in multiple cancer types, affecting the tumor microenvironment. By activating osteoclasts, it could also promote bone metastasis. EVs are a collective term for vesicular structures encased in lipid bilayers released by various cells, including exosomes and particles. Exo is a signaling vesicle involved in normal homeostatic processes or pathological exchanges of nucleic acids, proteins, and other components between cells (Kowal et al., 2016). Exosomes not only play a role in regulating normal physiological processes, such as immune response and cell differentiation, but can also be involved in the pathophysiology of diseases, such as cancer development, progression, and metastasis (Yao et al., 2021). Tumor cells can interact with cells in the bone microenvironment through the secretion of exosomes and transfer tumor-specific contents, such as miRNA, to the bone microenvironment through exosomes, thus promoting tumor bone metastasis (Rossi et al., 2018; Tiedemann et al., 2019). Furthermore, breast cancer cells has been suggested to promote the development of breast cancer bone metastases by releasing exosomes containing miRNA-19a and IBSP (Wu et al., 2021a). However, it remains to be seen whether exosomes can regulate PSAT1.
In this study, we analyzed the clinical and tumor microenvironment of gastric cancer patients to investigate the relationship between PSAT1 and prognosis. We screened the PSAT1-targeting microRNAs with miRWalk, and then analyzed their expression in gastric cancer. Diagnosis and treatment strategies can be provided by screening marker proteins associated with gastric cancer prognosis.
We downloaded PSAT1 pan-cancer data from the UCSC Xena database for a total of 18 cancer types, including bladder uroepithelial carcinoma (BLCA), breast invasive carcinoma (BRCA), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), esophageal carcinoma (ESCA), glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous carcinoma (LUSC), prostate adenocarcinoma (PRAD), rectal adenocarcinoma (READ), stomach adenocarcinoma (STAD), thyroid carcinoma (THCA), and uterine corpus endometrial carcinoma (UCEC). From the Cancer Genome Atlas (TCGA) database (https://www.cancer.gov/about-nci/organization/ccg/research), raw RNA sequencing data and clinical information were downloaded from gastric cancer patients (Wang et al., 2016; Goldman et al., 2020). Information about survival time, survival status, age, sex, tumor grade, clinical stage, pathological stage, TNM stage, OS, DSS, and PFI were collected from the patients.
Patients’ clinical information included age, gender, clinical stage, and TMN stage. To investigate the relationship between PSAT1 expression and clinical characteristics, we selected clinical, pathological, and TNM stages as representative outcomes with significant differences. The gastric cancer samples were then divided into high and low expression groups based on their median PSAT1 expression values. Kaplan-Meier survival curves were plotted using this method. We analyzed the relationship between PSAT1 expression and prognostic DSS (disease-specific survival) in gastric cancer taking into account the possibility of non-tumor death during follow-up. The relationship between PSAT1 expression and PFI (progression-free interval) was also examined.
CIBERSORT deconvolution algorithm is a computational method for identifying 22 types of immune cells in tissues (Bindea et al., 2013; Hänzelmann et al., 2013; Wu et al., 2021b). With R software, the CIBERSORT deconvolution algorithm was used to simulate the transcriptional features matrix of 22 immune cells, including B cells, plasma cells, T cells, natural killer cells, monocytes, macrophages, dendritic cells, mast cells, eosinophils, and neutrophils. Calculations were set at 100, and data with p < 0.05 were analyzed. In order to analyze the correlation between PSAT1 and immune cells, R software calculated correlation coefficients between immune cells and PSAT1. Additionally, the ESTIMATE algorithm in the R language estimation package was used to estimate the ratio of immune to stromal components in tumor microenvironments. Three types of scores were presented: immune, stromal, and ESTIMATE. Based on the correlation between PSAT1 and these three scores, we were able to analyze the correlation between PSAT1 and the tumor microenvironment. The relationships between PSAT1 expression, immune cell infiltration score, and tumor microenvironment were assessed by Spearman correlation analysis.
All microRNAs that may target PSAT1 were predicted using the online miRNA target gene prediction tool miRWalk2.0 as previous researches (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2) (Dweep et al., 2014; Sticht et al., 2018; Feng et al., 2021; Chen et al., 2022; Zhao and Jiang, 2022). MiRWalk integrated with several different miRNA target gene prediction tools, including miRanda, RNA22, miRDB, Targetscan, etc., which can perform multiple databases of miRNA co-screening and find the common target genes among them, maximizing the prediction confidence. Then, we conducted correlation analysis of the miRNAs that correlated negatively with PSAT1.
The microRNAs obtained above were calculated as the expression between gastric cancer tissues and normal tissues, and the microRNAs with statistically significant differences in expression were screened by the p < 0.05. Survival curves were plotted by Kaplan-Meier method based on the median expression value of each microRNA as previous researches (Rich et al., 2010; Lacny et al., 2015; Sun et al., 2022; Xuan et al., 2022).
Using the Akaike information criterion (AIC), the optimal logistic nomogram model was constructed. We evaluated the expressiveness of the model using ROC curves and calibration curves. We also performed the Hosmer-Lemeshow goodness-of-fit test. Decision curve analysis (DCA) was used to examine the effect of the model on net clinical benefit rates at different positive thresholds. Threshold probability is the horizontal coordinate of DCA. When the nomogram model assessment value reached a certain value, bone metastasis probability was denoted as p.
Chinese Academy of Sciences, Shanghai, provided 293T and SGC-7901 cells, which were cultured at 37 °C in a constant humidity CO2 incubator with DMEM + 10% FBS + 1% double antibody. Afterwards, we transfected 293T cells with 100 pmol hsa-miR-497-5p, purchased from Bioindustries, for 48 h before RNA extraction.
SGC-7901 cells were treated with Trizol (Sangon, China) according to Trizol’s guidelines. In order to measure mRNA expression levels, RNA was reverse-transcribed into cDNA using Promega’s Reverse Transcription Kit (GoScriptTM Reverse Transcription Kit), followed by qRT-PCR analysis using Biotech’s qRT-PCR reagents (2X SG Fast qPCR Master Mix). As an internal reference, GAPDH was used and 2−ΔΔCt was used to calculate mRNA expression levels.
PmirGLO-PSAT1-3' UTR-WT and pmirGLO-PSAT1-3' UTR-MUT vector plasmids were purchased from Shanghai Sangon Biotech. 500 ng of vector plasmid and 100 pmol of hsa-miR-497-5p mimics were transfected into 293T cells and the fluorescence situation was determined 24 h after transfection using Dual Luciferase Assay Kit (Promega).
Statistical analysis was performed using the R software package (version 3.6.3). The Spearman correlation test was used to determine whether the two variables were correlated. Differences with p < 0.05 were considered statistically significant.
From the TCGA database, clinical information was downloaded for 375 gastric cancer patients. PSAT1 expression was divided into low and high groups based on median expression. Their median ages were 65.5 and 69, respectively, statistically significantly different (p < 0.05). In addition, there was a statistically significant difference in pathological types between low and high expression groups (p = 0.028) (Table 1). PSAT1 expression was statistically significantly different between pathological Stage IV, TNMF Stage IV, and clinical Stage IV based on patient’s clinical data [HR = 1.70 (1.09–2.68), p = 0.021]. There was a difference in PSAT1 expression at pathological stage IV. However, there were no significant differences in the other clinical-pathological symptoms.
UCSC database was used to analyze PSAT1 mRNA expression levels in tumor and normal tissue samples (Figure 1A). The results disclosed that PSAT1 was highly expressed in ten cancer types relative to normal tissues, including BLCA, COAD, ESCA, HNSC, LUAD, LUSC, PRAD, READ, STAD, and UCEC. In contrast, PSAT1 expression was low in BRCA, CHOL, KIRC, KIRP, LIHC, and THCA. PSAT1 expression was not significant in GBM and KICH.
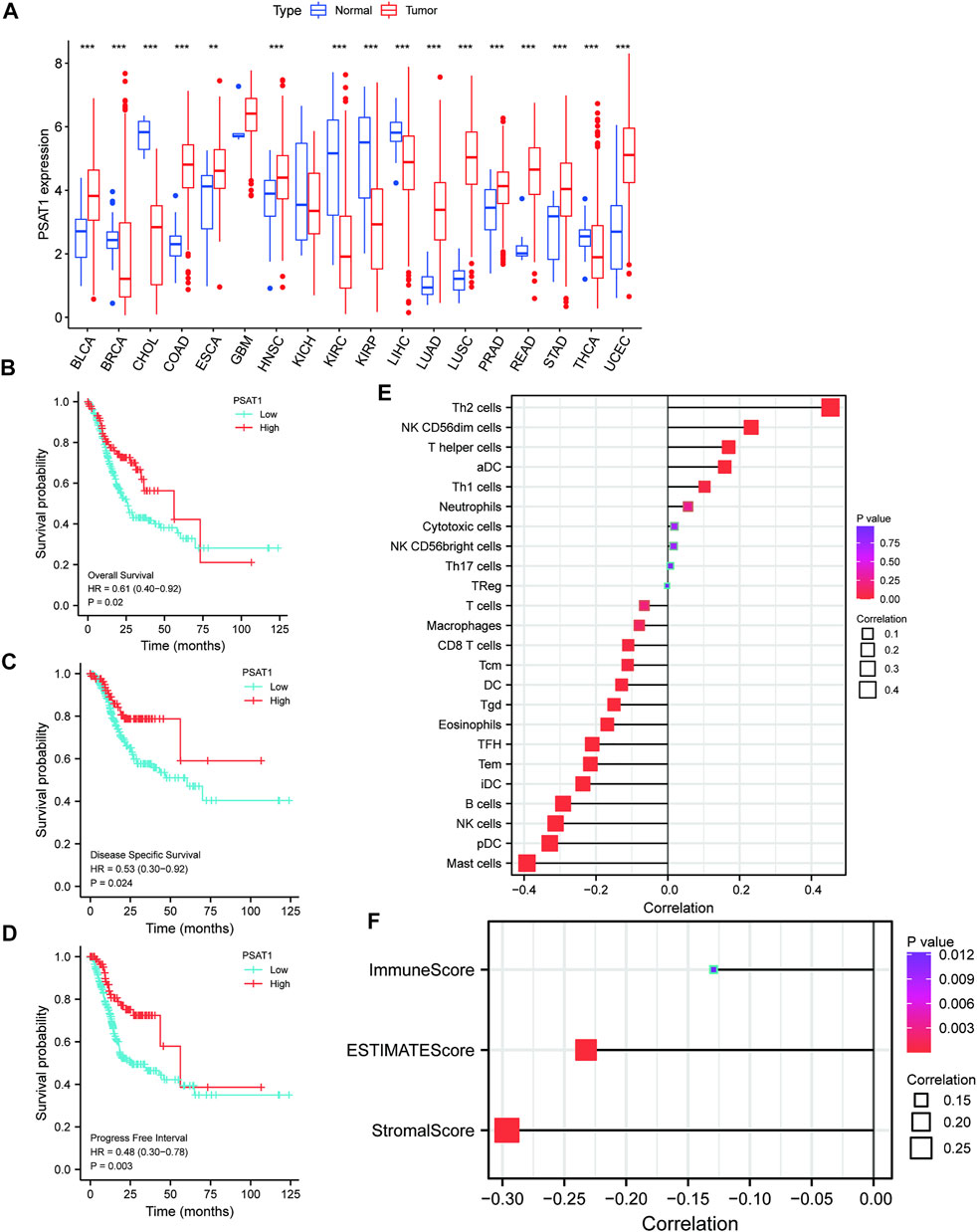
FIGURE 1. A correlation between PSAT1 expression levels and cancer patient prognosis. (A). Box plot showing the level of PSAT1 mRNA expression in different cancer tissues and normal tissues; data from UCSC database; ***p < 0.001, **p < 0.01; (B). Survival analysis demonstrating the overall survival of gastric cancer patients with high PSAT1 expression (OS, HR = 0.61, 95% CI: 0.40–0.92, p = 0.02); (C). Survival analysis demonstrating the relationship between PSAT1 expression levels and DSS of gastric cancer patients (DSS, HR= 0.53, 95% CI: 0.30–0.92, p = 0.024); (D). Survival analysis demonstrating the relationship between PSAT1 expression levels and progress-free interval of gastric cancer patients (PFI, HR = 0.48, 95% CI: 0.30–0.78, p = 0.003); (E). Correlation analysis of PSAT1 expression with immune cell infiltration displaying that TH2 cells and NK cells are positively correlated with PSAT1, whereas plasmacytoid dendritic cells and mast cells are negatively correlated with PSAT1; (F). PSAT1 correlation analysis with ImmuneScore, StromalScore, and ESTIMATEScore.
Patients with high PSAT1 expression had significantly longer overall survival (OS, HR= 0.61, 95% CI: 0.40–0.92, p = 0.02), disease-specific survival (DSS, HR= 0.53, 95% CI: 0.30–0.92, p = 0.024), and progression-free interval (PFI, HR= 0.48, 95% CI: 0.30–0.78, p = 0.003) (Figures 1B–D). In gastric cancer, low expression of PSAT1 was associated with worse OS, DSS, and PFI. Therefore, patients with gastric cancer with low expression of PSAT1 had a poor prognosis.
The percentage of immune cell infiltration was calculated by Cibersort software, and samples that met the requirements were screened according to the p < 0.05 criterion. According to our analysis, each immune cell shows a positive correlation with PSAT1 expression, while plasmacytoid dendritic cells and mast cells have a negative correlation with PSAT1 (Figure 1E). Furthermore, ImmuneScore, StromalScore, and ESTIMATEScore were calculated using the ESTIMATE algorithm in R language estimate package. PSAT1 expression and these three scores were negatively correlated (Figure 1F). PSAT1 expression levels can influence the immune activity of tumor microenvironments, according to these results.
MiRNAs targeting and regulating PSAT1 gene expression were identified using miRwalk database and visualized using Cytoscope (Figure 2A). We calculated the expression of the above 116 miRNAs in TCGA in gastric cancer. By analyzing the correlation between their expression and PSAT1 expression, we found that the following were negatively correlated with PSAT1 expression: hsa-miR-1-3p (r = −0.3, p < 0.05), hsa-miR-29c-3p (r = −0.25, p < 0.05), hsa-miR-101 -3p (r = −0.25, p < 0.05), hsa-miR-129-5p (r = −0.34, p < 0.05), hsa-miR-139-5p (r = −0.29, p < 0.05), hsa-miR-145- 5p (r = −0.29, p < 0.05), hsa-miR-195-5p (r = −0.34, p < 0.05), hsa-miR-497-5p (r = −0.36, p < 0.05), and hsa-miR-218-5p (r = −0.36, p < 0.05) (Figure 2B). Eight miRNAs were found to have low expression and be significantly different between tumors and normal tissue, namely: hsa-miR-1-3p, hsa-miR-29c-3p, hsa-miR-129-5p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-497-5 (Figure 2C). We calculated the p-value for each miRNA in relation to patient survival using KM survival curves of the eight differential miRNAs listed above. Six miRNAs were found to have significant correlations with prognosis, including hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p (Figure 3A). Then, we constructed a regulatory relationship map targeting PSAT1 using cytoscope software (Figure 3B). There was a negative correlation between these nine miRNAs (Figure 3C). Therefore, these six miRNAs (hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p, and hsa-miR-497-5p) may be associated with (hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p, and hsa-miR-497-5p) the expression of PSAT1 and the prognosis of patients.
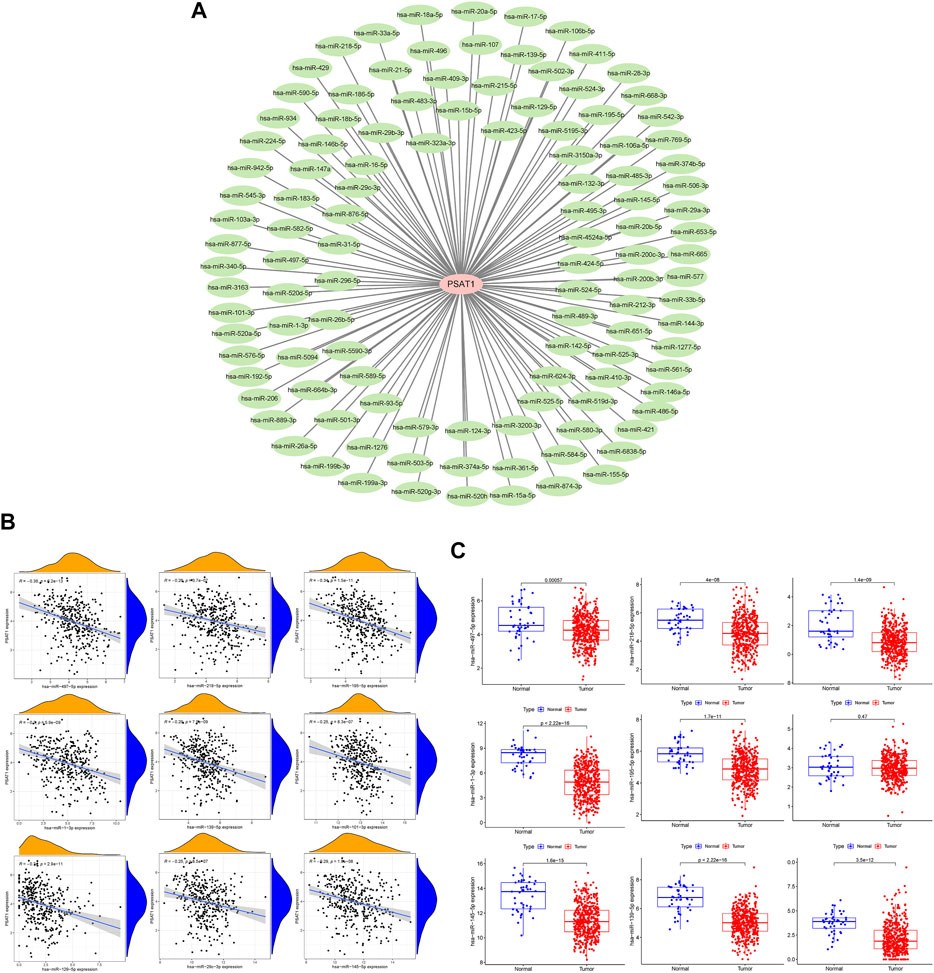
FIGURE 2. The microRNAs associated with gastric cancer expression of PSAT1. (A). The miRwalk database and correlation analysis identified microRNAs that could target PSAT1 in gastric cancer; (B). MicroRNAs negatively correlated with PSAT1 expression; (C). Gastric tumor tissues and normal tissue samples showed negative correlations between miRNA levels and PSAT1 expression levels.
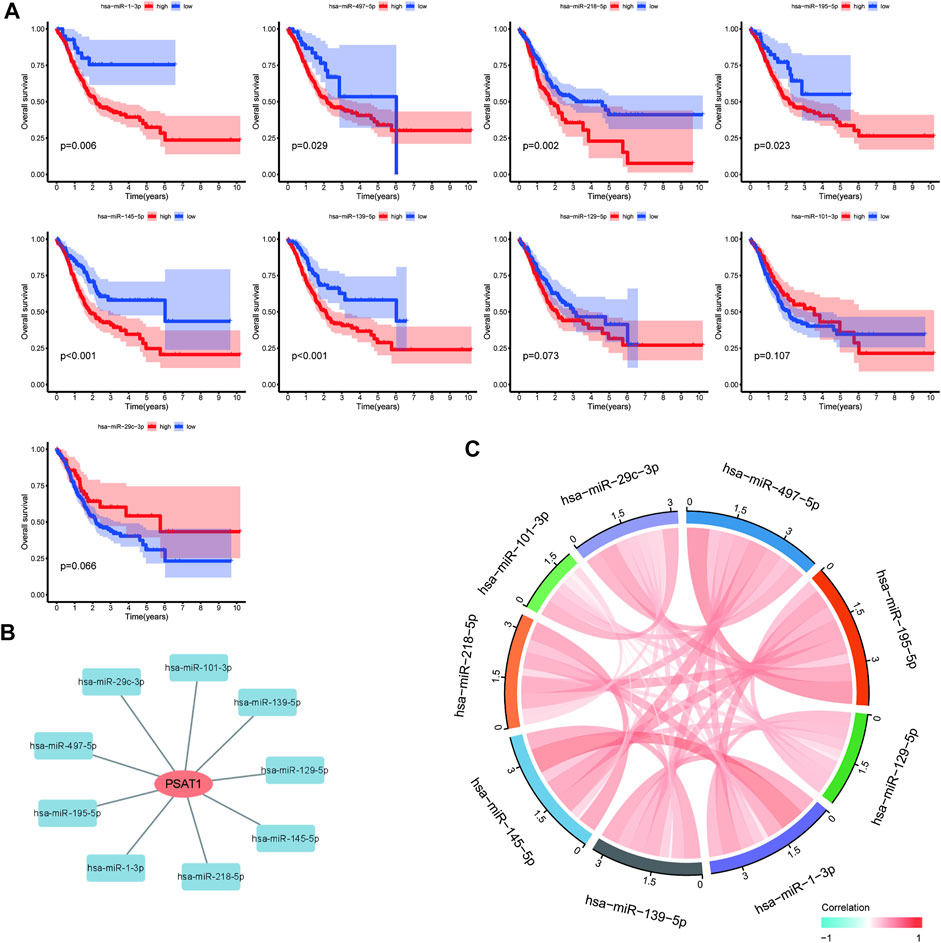
FIGURE 3. Prognostic analysis of microRNAs negatively associated with PSAT1. (A). Survival analysis of miRNAs (hsa-miR-218-5p, hsa-miR-129-5p, hsa-miR-145-5p, hsa-miR-139-5p, hsa-miR-195-5p, hsa-miR-1-3p, hsa-miR-29c-3p, and hsa-miR-497-5p) significantly associated with tumor prognosis; (B). PSAT1-related microRNA regulatory network; (C). Correlation analysis circle diagram between PSAT1 and its negatively associated microRNAs.
The six miRNAs (hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p, and hsa-miR-497-5p) were built into a nomogram model (Figure 4A). Each miRNA’s corresponding scale was determined based on its actual situation in the patient. By projecting upward to the top scale points, each miRNA’s score was calculated, and the scores were summed. The risk probability of bone metastasis in the patient was calculated by projecting downward based on the total score. Validating the model, we found that it had a good AUC value (AUC = 0.746), calibration, and goodness of fit, suggesting that it could predict the risk of bone metastasis in gastric cancer (Figures 4B,C). DCA illustrating the benefits of using the miRNA nomogram model (Figure 4D). Overall, we established a predictive model for gastric cancer bone metastasis.
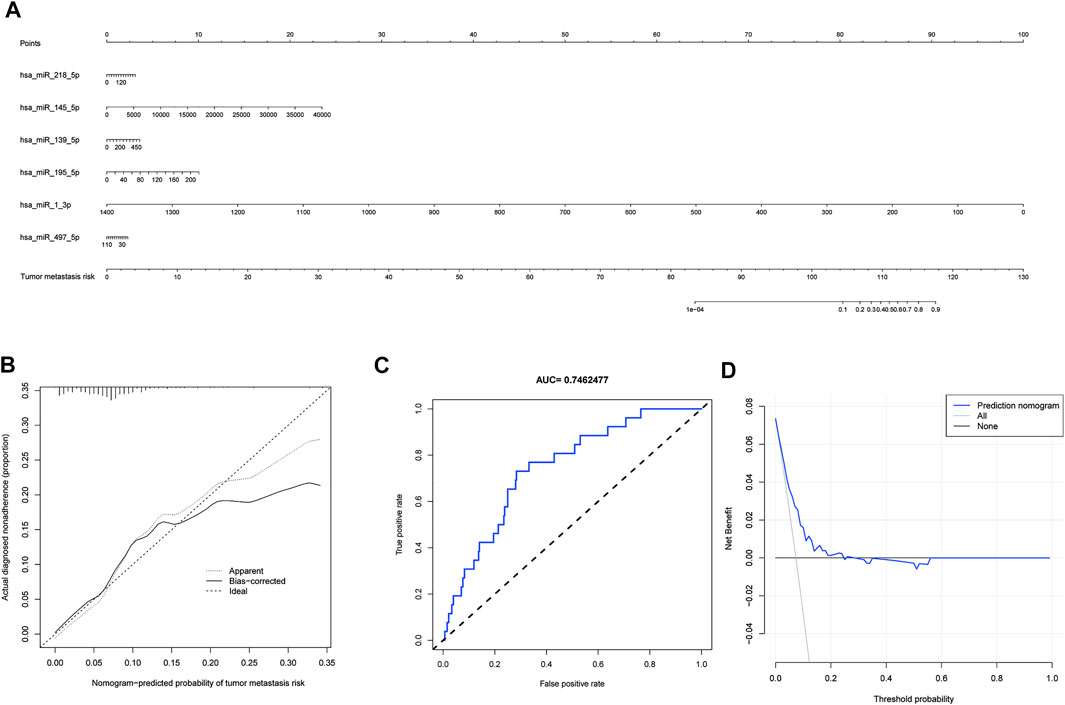
FIGURE 4. A predictive model for gastric cancer metastasis risk based on microRNAs associated with downregulated PSAT1 expression. (A). Nomogram models based on six miRNAs (hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p, and hsa-miR-497-5p); (B). Calibration curve for nomogram model; (C). ROC curve of nomogram model (AUC = 0.746); (D). Clinical decision curves illustrating the benefits of using the miRNA nomogram model.
Among the six miRNAs targeted by PSAT1, hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p, and hsa-miR-497-5p were most closely related to gastric cancer prognosis. PSAT1 and hsa-miR-497-5p were detected using a dual-luciferase reporter gene system and qRT-PCR. Figure 5A shows that hsa-miR-497-5p mimics reduced the fluorescence ratio compared to the control, demonstrating a targeting relationship between hsa-miR-497-5p and PSAT1. PSAT1 expression was reduced by hsa-miR-497-5p in further qRT-PCR experiments (Figure 5B). As a result of the above findings, hsa-miR-497-5p appears to be capable of targeting PSAT1 expression in order to affect gastric cancer prognosis.
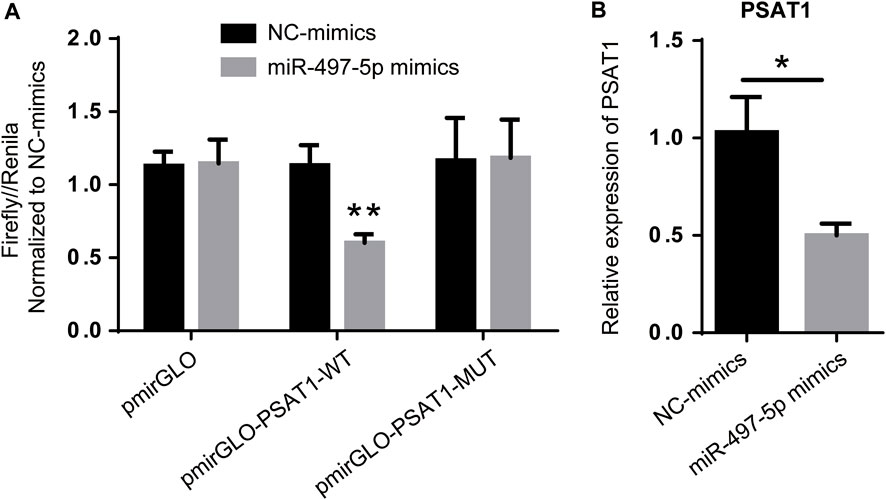
FIGURE 5. PSAT1 expression is regulated by has-miR-497-5p. (A). PSAT1 and has-miR-497-5p targeting relationship revealed by dual-luciferase reporter gene system. (B). The expression of PSAT1 was lower in has-miR-497-5p-treated gastric cancer cell lines compared to control cells. *p < 0.05, **p < 0.01.
The rapid development of precision medicine has improved the survival rate of gastric cancer by combining surgery with targeted therapy and chemotherapy, but the prognosis remains poor (Li et al., 2022). Early symptoms of gastric cancer are atypical, so early diagnosis is mainly based on endoscopic biopsy, which is a limited method. Distant metastases are often diagnosed in most patients (Dohi et al., 2017). Recently, with the continuous improvement of imaging technology, related studies have found that the percentage of patients with bone metastases from gastric cancer detected by bone scan screening can be as high as 25–45.3% (Choi et al., 1995). Several studies have demonstrated that bone metastasis, as an independent risk factor for gastric cancer, often indicated rapid deterioration of the clinical course, which seriously affected the treatment outcome and prognosis of patients (Ahn et al., 2011; Qiu et al., 2018). Moreover, patients with bone metastases may suffer from complications such as bone pain, pathological fracture, and spinal cord compression, which seriously affect their quality of life (Mikami et al., 2017). Bone metastases from gastric cancer were found to be lower than the actual rate because there were often no obvious clinical symptoms in the early stages, and skeletal screening for gastric cancer patients was not routine (Clézardin, 2017). Therefore, a predictive risk model should be developed to help detect and diagnose gastric cancer bone metastases early, enabling effective treatment plans to be developed.
PSAT1 regulates serine anabolism, playing an important role in cell proliferation, and is also essential for osteoclastogenesis (de KONING et al., 2003; Ogawa et al., 2006). Furthermore, it was found that high PSAT1 expression was closely associated with bone metastasis in malignant tumors. High expression of PSAT1 has been suggested to regulate serine anabolism, promotes osteoclast differentiation and enhances their activity, regulates the tumor microenvironment, and thus promotes bone metastasis in breast cancer (Pollari et al., 2011). PSAT1 expression was highly correlated with poor prognosis in gastric cancer in this study based on pan-cancer analysis. According to immune infiltration analysis, PSAT1 affects the tumor immune microenvironment, which indicates that PSAT1 plays a critical role in invasion and gastric cancer prognosis. Furthermore, we identified miRNAs targeting PSAT1 in TCGA tumor tissues and associated them with gastric cancer. We found that multiple miRNAs regulated PSAT1 expression (Figure 2B). The survival curve analysis identified six microRNAs, including hsa-miR-1-3p, hsa-miR-139-5p, hsa-miR-145-5p, hsa-miR-195-5p, hsa-miR-218-5p, and hsa-miR-497-5p (Figure 3A). Based on the above six microRNAs, we constructed the prognostic prediction model for gastric cancer.
MicroRNAs (miRNAs) belong to a family of non-coding RNAs of 20–24 nucleotides in length. MiRNAs are significantly associated with tumor development and metastasis (Liu et al., 2019; Wang et al., 2019; Chen et al., 2021; Zhang and Liu, 2021; Cao et al., 2022). Compared with normal tissues, miRNA expression is down-regulated in various cancers. They are widely involved in tumor metastasis and invasion by suppressing target genes and have an important role in tumor diagnosis and prognosis assessment (Daoud et al., 2019). Previous studies found that all six microRNAs used to construct predictive models were associated with malignant tumorigenesis, invasion, or metastasis. Hsa-miR-139-5p expression was low in various tumor tissues, including gastric cancer, liver cancer, and thyroid cancer (Yang et al., 2013; Montero‐Conde et al., 2020; Chi et al., 2021). Bioinformatics analysis revealed that hsa-miR-139-5p was closely associated with gastric cancer prognosis (Wang et al., 2022). Furthermore, hsa-miR-139-5p/MYB axis has been suggested to promote the proliferation, invasion, and metastasis of gastric cancer (Xie et al., 2021). The expression of hsa-miR-145-5p was down-regulated in various tumor cells, including gastric cancer, and the down-regulation of hsa-miR-145-5p expression was associated with lymph node metastasis and distant metastasis in gastric cancer, suggesting a poor prognosis (Hang et al., 2018). In addition, it was found that the exosomes secreted by ovarian cancer cells also contained hsa-miR-145-5p, and its abnormal expression was associated with distant metastasis of cancer cells (Hang et al., 2018). Hsa-miR-195-5p is also a suppressor of multiple tumor types, and its dysregulated expression is involved in the development of multiple tumors and is associated with poor tumor prognosis and drug resistance (Jin et al., 2018; Rezaei et al., 2019). It has been suggested that hsa-miR-195-5p was involved in regulating the invasion and metastasis of gastric cancer cells by binding to PD-L1 and regulating E-calmodulin expression, which was closely associated with poor prognosis for patients with gastric cancer (Zou et al., 2019; Liu et al., 2020; Liu et al., 2021). Expression of hsa-miR-218-5p is downregulated in various malignancies, including gastric, prostate, and cervical cancers, and is associated with tumor invasion and migration (Gao et al., 2009). Researchers found that hsa-miR-218-5p regulated KIT protein expression and inhibited proliferation and invasion of gastrointestinal mesenchymal tumors (Fan et al., 2014). Upregulation of hsa-miR-218-5p expression inhibits cancer progression in cervical and bladder cancer, by reducing cell migration and invasion (Chiyomaru et al., 2012; Yamamoto et al., 2013). Hsa-miR-497-5p expression is down-regulated in gastric, hepatocellular, and colorectal cancers, and is also associated with tumorigenesis, invasion, and poor prognosis (Falzone et al., 2018; Liu et al., 2021; Tian et al., 2021). In addition, abnormal expression of hsa-miR-1-3p is associated with poor prognosis of malignant tumors, such as breast cancer and small cell lung cancer (Li et al., 2020; Yan et al., 2021). Gene expression levels are closely connected to tumor metastasis according to the prediction model obtained in this study, which implicates genes involved in cancer development and metastasis. Furthermore, we developed and validated a prognostic prediction model using nine microRNAs for gastric cancer and found that AUC value with good calibration and good fit was 0.746 (Figures 4B,C). A good prediction of the risk of bone metastases in patients with gastric cancer could be obtained through this model, and it could have some application in the assessment of gastric cancer prognoses.
Gastric cancers expressed high levels of PSAT1, and low levels were associated with poor prognoses. Furthermore, microRNAs targeting PSAT1 can predict gastric cancer prognosis and bone metastasis risk. Based on the results of this study, it can be concluded that the prediction model provides good predictive value in the risk assessment of bone metastasis in gastric cancer, in addition to showing some clinical application in the prognosis evaluation of gastric cancer. The study has, however, some limitations. To validate the findings of this study, a larger sample size is required. It is also necessary to conduct further experimental studies in order to clarify the specific mechanisms involved in regulation.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
JM, MZ, XY, BW, and NZ performed the data curation and analysis. JM, MZ, XY, BW, TW, MM, TL, and NZ analyzed and interpreted the results. JM and NZ drafted and reviewed the manuscript. JM and NZ provided funding. All authors read and approved the final manuscript.
The language touch-up services provided by Home for Researchers [www.home-for-researchers.com] are greatly appreciated.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahn, J. B., Ha, T. K., and Kwon, S. J. (2011). Bone metastasis in gastric cancer patients. J. Gastric Cancer 11, 38–45. doi:10.5230/jgc.2011.11.1.38
Bindea, G., Mlecnik, B., Tosolini, M., Kirilovsky, A., Waldner, M., Obenauf, A. C., et al. (2013). Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795. doi:10.1016/j.immuni.2013.10.003
Biyik-Sit, R., Kruer, T., Dougherty, S., Bradley, J. A., Wilkey, D. W., Merchant, M. L., et al. (2021). Nuclear pyruvate kinase M2 (PKM2) contributes to phosphoserine aminotransferase 1 (PSAT1)-Mediated cell migration in EGFR-activated lung cancer cells. Cancers 13, 3938. doi:10.3390/cancers13163938
Cao, T., Hong, J., Qi, F., Zheng, B., Chen, G., Yu, B., et al. (2022). A hyperglycemic microenvironment inhibits tendon-to-bone healing through the let-7b-5p/CFTR pathway. Comput. Math. Methods Med. 2022, 82680671–82680710. doi:10.1155/2022/8268067
Chen, C., Zhao, J., Liu, J., and Sun, C. (2021). Mechanism and role of the neuropeptide LGI1 receptor ADAM23 in regulating biomarkers of ferroptosis and progression of esophageal cancer. Dis. Markers 9227897, 1–15. doi:10.1155/2021/9227897
Chen, Y., Sun, Y., Luo, Z., Lin, J., Qi, B., Kang, X., et al. (2022). Potential mechanism underlying exercise upregulated circulating blood exosome miR-215-5p to prevent necroptosis of neuronal cells and a model for early diagnosis of alzheimer’s disease. Front. Aging Neurosci. 14, 860364. doi:10.3389/fnagi.2022.860364
Chi, F., Cao, Y., and Chen, Y. (2021). Analysis and validation of circRNA-miRNA network in regulating m6A RNA methylation modulators reveals CircMAP2K4/miR-139-5p/YTHDF1 Axis involving the proliferation of hepatocellular carcinoma. Front. Oncol. 11, 560506. doi:10.3389/fonc.2021.560506
Chiyomaru, T., Enokida, H., Kawakami, K., Tatarano, S., Uchida, Y., Kawahara, K., et al. (2012). Functional role of LASP1 in cell viability and its regulation by microRNAs in bladder cancer. Urol. Oncol. 30, 434–443. doi:10.1016/j.urolonc.2010.05.008
Choi, C. W., Lee, D. S., Chung, J-K., Lee, M. C., Kim, N. K., Choi, K. W., et al. (1995). Evaluation of bone metastases by Tc-99m MDP imaging in patients with stomach cancer. Clin. Nucl. Med. 20, 310–314. doi:10.1097/00003072-199504000-00005
Clézardin, P. (2017). Pathophysiology of bone metastases from solid malignancies. Jt. Bone Spine 84, 677–684. doi:10.1016/j.jbspin.2017.05.006
Daoud, A. Z., Mulholland, E. J., Cole, G., and McCarthy, H. O. (2019). MicroRNAs in pancreatic cancer: Biomarkers, prognostic, and therapeutic modulators. BMC Cancer 19, 1130. doi:10.1186/s12885-019-6284-y
de Koning, T. J., Snell, K., Duran, M., Berger, R., Poll-The, B-T., and Surtees, R. (2003). l-Serine in disease and development. Biochem. J. 371, 653–661. doi:10.1042/bj20021785
Dohi, O., Yagi, N., Majima, A., Horii, Y., Kitaichi, T., Onozawa, Y., et al. (2017). Diagnostic ability of magnifying endoscopy with blue laser imaging for early gastric cancer: A prospective study. Gastric Cancer 20, 297–303. doi:10.1007/s10120-016-0620-6
Dweep, H., Gretz, N., and Sticht, C. (2014). “miRWalk database for miRNA–target interactions,” in RNA mapping. Methods in molecular biology. Editors M. L. Alvarez, and M. Nourbakhsh (New York, NY: Springer), 289–305. doi:10.1007/978-1-4939-1062-5_25
Falzone, L., Scola, L., Zanghì, A., Biondi, A., Di Cataldo, A., Libra, M., et al. (2018). Integrated analysis of colorectal cancer microRNA datasets: Identification of microRNAs associated with tumor development. Aging 10, 1000–1014. doi:10.18632/aging.101444
Fan, R., Zhong, J., Zheng, S., Wang, Z., Xu, Y., Li, S., et al. (2014). MicroRNA-218 inhibits gastrointestinal stromal tumor cell and invasion by targeting KIT. Tumour Biol. 35, 4209–4217. doi:10.1007/s13277-013-1551-z
Feng, W., Yang, J., Song, W., and Xue, Y. (2021). Crosstalk between heart failure and cognitive impairment via hsa-miR-933/RELB/CCL21 pathway. Biomed. Res. Int. 2021, 2291899–2291916. doi:10.1155/2021/2291899
Gao, C., Zhang, Z., Liu, W., Xiao, S., Gu, W., and Lu, H. (2009). Reduced microRNA-218 expression is associated with high nuclear factor kappa B activation in gastric cancer. Cancer 116 (1), 41–49. doi:10.1002/cncr.24743
Goldman, M. J., Craft, B., Hastie, M., Repečka, K., McDade, F., Kamath, A., et al. (2020). Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678. doi:10.1038/s41587-020-0546-8
Hang, W., Feng, Y., Sang, Z., Yang, Y., Zhu, Y., Huang, Q., et al. (2018). Downregulation of miR-145-5p in cancer cells and their derived exosomes may contribute to the development of ovarian cancer by targeting CT. Int. J. Mol. Med. 43 (1), 256–266. doi:10.3892/ijmm.2018.3958
Hansford, S., Kaurah, P., Li-Chang, H., Woo, M., Senz, J., Pinheiro, H., et al. (2015). Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 1, 23–32. doi:10.1001/jamaoncol.2014.168
Hänzelmann, S., Castelo, R., and Guinney, J. (2013). Gsva: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinforma. 14, 7. doi:10.1186/1471-2105-14-7
Hart, C. E., Race, V., Achouri, Y., Wiame, E., Sharrard, M., Olpin, S. E., et al. (2007). Phosphoserine aminotransferase deficiency: A novel disorder of the serine biosynthesis pathway. Am. J. Hum. Genet. 80, 931–937. doi:10.1086/517888
Jin, Y., Wang, M., Hu, H., Huang, Q., Chen, Y., and Wang, G. (2018). Overcoming stemness and chemoresistance in colorectal cancer through miR-195-5p-modulated inhibition of notch signaling. Int. J. Biol. Macromol. 117, 445–453. doi:10.1016/j.ijbiomac.2018.05.151
Joshi, S. S., and Badgwell, B. D. (2021). Current treatment and recent progress in gastric cancer. Ca. Cancer J. Clin. 71, 264–279. doi:10.3322/caac.21657
Kowal, J., Arras, G., Colombo, M., Jouve, M., Morath, J. P., Primdal-Bengtson, B., et al. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U. S. A. 113, E968–E977. doi:10.1073/pnas.1521230113
Lacny, S., Wilson, T., Clement, F., Roberts, D. J., Faris, P. D., Ghali, W. A., et al. (2015). Kaplan-meier survival analysis overestimates the risk of revision arthroplasty: A meta-analysis. Clin. Orthop. Relat. Res. 473, 3431–3442. doi:10.1007/s11999-015-4235-8
Lee, J., Lim, T., Uhm, J. E., Park, K. W., Park, S. H., Lee, S. C., et al. (2007). Prognostic model to predict survival following first-line chemotherapy in patients with metastatic gastric adenocarcinoma. Ann. Oncol. 18, 886–891. doi:10.1093/annonc/mdl501
Li, X., Ma, C., Luo, H., Zhang, J., Wang, J., and Guo, H. (2020). Identification of the differential expression of genes and upstream microRNAs in small cell lung cancer compared with normal lung based on bioinformatics analysis. Medicine 99, e19086. doi:10.1097/MD.0000000000019086
Li, Y., Feng, A., Zheng, S., Chen, C., and Lyu, J. (2022). Recent estimates and predictions of 5-year survival in patients with gastric cancer: A model-based period analysis. Cancer Control. 29, 10732748221099227. doi:10.1177/10732748221099227
Liu, F., Qiu, F., Fu, M., Chen, H., and Wang, H. (2020). Propofol reduces epithelial to mesenchymal transition, invasion and migration of gastric cancer cells through the MicroRNA-195-5p/snail Axis. Med. Sci. Monit. 6, e920981. doi:10.12659/MSM.920981
Liu, J., Wang, P., Zhang, P., Zhang, X., Du, H., Liu, Q., et al. (2019). An integrative bioinformatics analysis identified miR-375 as a candidate key regulator of malignant breast cancer. J. Appl. Genet. 60, 335–346. doi:10.1007/s13353-019-00507-w
Liu, W., Chen, H., and Wang, D. (2021). Protective role of astragaloside IV in gastric cancer through regulation of microRNA-195-5p-mediated PD-L1. Immunopharmacol. Immunotoxicol. 43, 443–451. doi:10.1080/08923973.2021.1936013
Lott, P. C., and Carvajal-Carmona, L. G. (2018). Resolving gastric cancer aetiology: An update in genetic predisposition. Lancet. Gastroenterol. Hepatol. 3, 874–883. doi:10.1016/S2468-1253(18)30237-1
Machlowska, J., Baj, J., Sitarz, M., Maciejewski, R., and Sitarz, R. (2020). Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci. 21, E4012. doi:10.3390/ijms21114012
Metcalf, S., Dougherty, S., Kruer, T., Hasan, N., Biyik-Sit, R., Reynolds, L., et al. (2020). Selective loss of phosphoserine aminotransferase 1 (PSAT1) suppresses migration, invasion, and experimental metastasis in triple negative breast cancer. Clin. Exp. Metastasis 37, 187–197. doi:10.1007/s10585-019-10000-7
Mikami, J., Kimura, Y., Makari, Y., Fujita, J., Kishimoto, T., Sawada, G., et al. (2017). Clinical outcomes and prognostic factors for gastric cancer patients with bone metastasis. World J. Surg. Oncol. 15, 8. doi:10.1186/s12957-016-1091-2
Montero-Conde, C., Graña-Castro, O., Martín-Serrano, G., Martínez-Montes, Á. M., Zarzuela, E., Muñoz, J., et al. (2020). Hsa‐miR‐139‐5p is a prognostic thyroid cancer marker involved in HNRNPF-mediated alternative splicing. Int. J. Cancer 146, 521–530. doi:10.1002/ijc.32622
Montrose, D. C., Saha, S., Foronda, M., McNally, E. M., Chen, J., Zhou, X. K., et al. (2021). Exogenous and endogenous sources of serine contribute to colon cancer metabolism, growth, and resistance to 5-fluorouracil. Cancer Res. 81, 2275–2288. doi:10.1158/0008-5472.CAN-20-1541
Ogawa, T., Ishida-Kitagawa, N., Tanaka, A., Matsumoto, T., Hirouchi, T., Akimaru, M., et al. (2006). A novel role of l-serine (l-Ser) for the expression of nuclear factor of activated T cells (NFAT)2 in receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclastogenesis in vitro. J. Bone Min. Metab. 24, 373–379. doi:10.1007/s00774-006-0705-0
Oliveira, C., Pinheiro, H., Figueiredo, J., Seruca, R., and Carneiro, F. (2015). Familial gastric cancer: Genetic susceptibility, pathology, and implications for management. Lancet. Oncol. 16, e60–e70. doi:10.1016/S1470-2045(14)71016-2
Park, J. M., Song, K. Y., O, J. H., Kim, W. C., Choi, M-G., and Park, C. H. (2013). Bone recurrence after curative resection of gastric cancer. Gastric Cancer 16, 362–369. doi:10.1007/s10120-012-0193-y
Pollari, S., Käkönen, S-M., Edgren, H., Wolf, M., Kohonen, P., Sara, H., et al. (2011). Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125, 421–430. doi:10.1007/s10549-010-0848-5
Qiu, M-Z., Shi, S-M., Chen, Z-H., Yu, H-E., Sheng, H., Jin, Y., et al. (2018). Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med. 7, 3662–3672. doi:10.1002/cam4.1661
Rezaei, Z., Sebzari, A., Kordi-Tamandani, D. M., and Dastjerdi, K. (2019). Involvement of the dysregulation of miR-23b-3p, miR-195-5p, miR-656-5p, and miR-340-5p in trastuzumab resistance of HER2-positive breast cancer cells and system biology approach to predict their targets involved in resistance. DNA Cell Biol. 38, 184–192. doi:10.1089/dna.2018.4427
Rich, J. T., Neely, J. G., Paniello, R. C., Voelker, C. C. J., Nussenbaum, B., and Wang, E. W. (2010). A practical guide to understanding Kaplan-Meier curves. Otolaryngol. Head. Neck Surg. 143, 331–336. doi:10.1016/j.otohns.2010.05.007
Rossi, M., Battafarano, G., D’Agostini, M., and Del Fattore, A. (2018). The role of extracellular vesicles in bone metastasis. Int. J. Mol. Sci. 19, 1136. doi:10.3390/ijms19041136
Sticht, C., De La Torre, C., Parveen, A., and Gretz, N. (2018). miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 13, e0206239. doi:10.1371/journal.pone.0206239
Sun, C., Ma, S., Chen, Y., Kim, N. H., Kailas, S., Wang, Y., et al. (2022). Diagnostic value, prognostic value, and immune infiltration of LOX family members in liver cancer: Bioinformatic analysis. Front. Oncol. 12, 843880. doi:10.3389/fonc.2022.843880
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Tian, L-L., Qian, B., Jiang, X-H., Liu, Y-S., Chen, T., Jia, C-Y., et al. (2021). MicroRNA-497-5p is downregulated in hepatocellular carcinoma and associated with tumorigenesis and poor prognosis in patients. Int. J. Genomics 2021, 6670390–6670416. doi:10.1155/2021/6670390
Tiedemann, K., Sadvakassova, G., Mikolajewicz, N., Juhas, M., Sabirova, Z., Tabariès, S., et al. (2019). Exosomal release of L-plastin by breast cancer cells facilitates metastatic bone osteolysis. Transl. Oncol. 12, 462–474. doi:10.1016/j.tranon.2018.11.014
Turkoz, F. P., Solak, M., Kilickap, S., Ulas, A., Esbah, O., Oksuzoglu, B., et al. (2014). Bone metastasis from gastric cancer: The incidence, clinicopathological features, and influence on survival. J. Gastric Cancer 14, 164–172. doi:10.5230/jgc.2014.14.3.164
Wang, W., Zhou, R., Wu, Y., Liu, Y., Su, W., Xiong, W., et al. (2019). PVT1 promotes cancer progression via MicroRNAs. Front. Oncol. 9, 609. doi:10.3389/fonc.2019.00609
Wang, Y., Wei, Y., Fan, X., Xu, F., Dong, Z., Cheng, S., et al. (2022). Construction of a miRNA signature using support vector machine to identify microsatellite instability status and prognosis in gastric cancer. J. Oncol. 6586354, 1–13. doi:10.1155/2022/6586354
Wang, Z., Jensen, M. A., and Zenklusen, J. C. (2016). “A practical guide to the cancer Genome Atlas (TCGA),” in Statistical genomics. Methods in molecular biology. Editors E. Mathé, and S. Davis (New York, NY: Springer), 111–141. doi:10.1007/978-1-4939-3578-9_6
Wu, J-Y., Qin, J., Li, L., Zhang, K-D., Chen, Y-S., Li, Y., et al. (2021). Roles of the immune/methylation/autophagy landscape on single-cell genotypes and stroke risk in breast cancer microenvironment. Oxid. Med. Cell. Longev. 2021, 5633514. doi:10.1155/2021/5633514
Wu, K., Feng, J., Lyu, F., Xing, F., Sharma, S., Liu, Y., et al. (2021). Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 12, 5196. doi:10.1038/s41467-021-25473-y
Xiaobin, C., Zhaojun, X., Tao, L., Tianzeng, D., Xuemei, H., Fan, Z., et al. (2022). Analysis of related risk factors and prognostic factors of gastric cancer with bone metastasis: A SEER-based study. J. Immunol. Res. 2022, 3251051. doi:10.1155/2022/3251051
Xie, Y., Rong, L., He, M., Jiang, Y., Li, H., Mai, L., et al. (2021). LncRNA SNHG3 promotes gastric cancer cell proliferation and metastasis by regulating the miR-139-5p/MYB axis. Aging 13, 25138–25152. doi:10.18632/aging.203732
Xuan, Z., Ma, T., Qin, Y., and Guo, Y. (2022). Role of ultrasound imaging in the prediction of TRIM67 in brain metastases from breast cancer. Front. Neurol. 13, 889106. doi:10.3389/fneur.2022.889106
Yamamoto, N., Kinoshita, T., Nohata, N., Itesako, T., Yoshino, H., Enokida, H., et al. (2013). Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion by targeting focal adhesion pathways in cervical squamous cell carcinoma. Int. J. Oncol. 42, 1523–1532. doi:10.3892/ijo.2013.1851
Yan, L., Wang, A., Lv, Z., Yuan, Y., and Xu, Q. (2021). Mitochondria-related core genes and TF-miRNA-hub mrDEGs network in breast cancer. Biosci. Rep. 41, BSR20203481. doi:10.1042/BSR20203481
Yang, M., Liu, R., Sheng, J., Liao, J., Wang, Y., Pan, E., et al. (2013). Differential expression profiles of microRNAs as potential biomarkers for the early diagnosis of esophageal squamous cell carcinoma. Oncol. Rep. 29, 169–176. doi:10.3892/or.2012.2105
Yao, X., Mao, Y., Wu, D., Zhu, Y., Lu, J., Huang, Y., et al. (2021). Exosomal circ_0030167 derived from BM-MSCs inhibits the invasion, migration, proliferation and stemness of pancreatic cancer cells by sponging miR-338-5p and targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 512, 38–50. doi:10.1016/j.canlet.2021.04.030
Zhang, P., and Liu, B. (2021). Integrative bioinformatics analysis reveals that infarct-mediated overexpression of potential miR-662/CREB1 pathway-induced neuropeptide VIP is associated with the risk of atrial fibrillation: A correlation analysis between myocardial electrophysiology and neuroendocrine. Dis. Markers 2021, 81166331–81166417. doi:10.1155/2021/8116633
Zhao, B., and Jiang, X. (2022). hsa-miR-518-5p/hsa-miR-3135b regulates the REL/SOD2 pathway in ischemic cerebral infarction. Front. Neurol. 13, 852013. doi:10.3389/fneur.2022.852013
Keywords: PSAT1, exosome, microRNA, gastric cancer, prognosis
Citation: Ma J, Zhu M, Ye X, Wu B, Wang T, Ma M, Li T and Zhang N (2022) Prognostic microRNAs associated with phosphoserine aminotransferase 1 in gastric cancer as markers of bone metastasis. Front. Genet. 13:959684. doi: 10.3389/fgene.2022.959684
Received: 02 June 2022; Accepted: 25 July 2022;
Published: 19 August 2022.
Edited by:
Qian Wang, Tai’an City Central Hospital, ChinaReviewed by:
Qiancheng Luo, Shanghai Pudong New Area Gongli Hospital, ChinaCopyright © 2022 Ma, Zhu, Ye, Wu, Wang, Ma, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Zhang, ejc3NTUzNjYwNTQ3NjRAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.