
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Genet. , 22 August 2022
Sec. Genetics of Common and Rare Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.954288
 Lili Pan1†‡
Lili Pan1†‡ Zhuoguang Li1†‡
Zhuoguang Li1†‡ Zhe Su1*†
Zhe Su1*† Wei Su1
Wei Su1 Rongfei Zheng1
Rongfei Zheng1 Weiyan Chen2
Weiyan Chen2 Xuezhi He3
Xuezhi He3 Jianming Song4
Jianming Song4 Shoulin Li5
Shoulin Li5 Pengqiang Wen6
Pengqiang Wen6Background: Desert hedgehog (DHH), as a member of the Hedgehog (HH) family, is mainly involved in testicular development and peripheral nerve sheath formation. A DHH variant has been identified in patients with 46, XY gonadal dysgenesis (46, XY GD) with or without neuropathy, but few reports mention the involvement of other complications.
Case presentation: Here, we report a Chinese female patient who was hospitalized at 14.3 years old due to slow breast development for more than 1 year. She had a female genitalia phenotype and breast development started at 13 years old but progressed slowly. She was not yet menarche on admission, and she had intermittent muscle cramps in her hands and feet. Her karyotype analysis was 46, XY and the SRY gene was positive. Surgical exploration revealed no uterus or ovaries, and the pathology of bilateral gonads was dysplastic testis tissue, which was consistent with partial gonadal dysgenesis (PGD). Genetic analysis identified a homozygous pathogenic variant in DHH exon 3 (c.1027T>C, p. Cys343Arg). During the 6-year follow-up, she received estrogen replacement therapy, resulting in breast development progression without gender dysphoria. However, her peripheral neuropathy became more obvious, and a nerve conduction study (NCS) indicated decreased nerve conduction velocity and action potential. In addition, she also suffered complications such as obesity, insulin resistance, fatty liver, and gastric ulcers.
Conclusion: In the present study, we reported a case of 46, XY GD with minifascicular neuropathy caused by a DHH homozygous variant, and we summarized the reported cases worldwide. For the first time in such patients, we showed a comparison of NCS changes with age as well as the presence of multiple complications not previously reported.
Desert hedgehog (DHH, OMIM 605423), a member of the Hedgehog (HH) family, is homologous to the Sonic HH and Indian HH families in mammals. The HH signaling pathway regulates embryonic development, postnatal cell proliferation, and differentiation, which are related to the development and tissue homeostasis of nerves, bones, gonads, gastrointestinal, liver, and other organs. Among them, DHH is mainly involved in testicular development and peripheral nerve sheath formation (Bitgood et al., 1996; Boso et al., 2020; Qi et al., 2021). In 2000, Umehara (Umehara et al., 2000) first identified the DHH gene variant in patients with 46, XY gonadal dysgenesis (46, XY GD), also known as 46, XY disorder of sex development (46, XY DSD). As an extremely rare autosomal recessive disease, only 25 cases in detail have been reported worldwide so far, and the clinical manifestations mainly include delayed development of secondary sexual characteristics, amenorrhea, and abnormal external genitalia with or without peripheral neuropathy (Sato et al., 2017). There are fewer cases of the disease in childhood, and long-term follow-up and other systemic involvement are rarely reported. Here, we reported a Chinese child with a homozygous missense variant in the DHH gene, and during the 6-year follow-up, in addition to the description of the gonads, we showed a comparison of pre- and post-NCS changes with age as well as the presence of multiple complications not previously reported. Our report will expand the phenotypic spectrum of diseases caused by the DHH variant, and we suggest that these patients require long-term follow-up and comprehensive management.
Our patient was a 14.3-year-old female (social gender) of Han nationality from Hainan, China. She was admitted to the Department of Endocrinology, Shenzhen Childrens Hospital due to slow breast development for more than 1 year. At 13 years old, breast development started but progressed slowly, accompanied by the growth of axillary and pubic hair, and she had a height increase of approximately 10 cm/year. In addition, she had intermittent muscle spasms in the hands and feet, which were typically mild and short-lived at first, and they were gradually relieved without intervention. She performed poorly in sports, such as throwing, running, and jumping, but she had good academic performance and normal intellectual development.
Personal history and family history: She was born to a G1P1 healthy mother via vaginal delivery at full-term. At 13 years old, she went to the psychiatry department due to depression; she was diagnosed with children’s psychological behavior disorder and her mood improved after escitalopram oxalate administration. She denied a family history of gonadal dysgenesis, and her parents were from two unrelated families. Her father’s height was 173 cm and her mother’s height was 156 cm, which indicated that her genetic potential height was 158 cm (−0.4 SD).
Her height, weight, and BMI were 165.0 cm (+1.1 SD), 55.9 kg (+0.9 SD), and 18.9 kg/m2 (+0.1 SD), respectively. She had a normal appearance without special features. Her bilateral breasts were in Tanner stage B3 with normal areola color, and her bilateral axillary hair was in stage A2. No mass was palpable in the inguinal area, and her pubic hair was in stage PH3. She had a typical female vulva without clitoral hypertrophy, and two urogenital sinus openings were visible. The rest of the physical examination was basically normal.
Sexual development-related hormone examination: A GnRH stimulation test suggested hypergonadotropic hypogonadism (basal luteinizing hormone (LH) 40.94 IU/L, peak LH>250 IU/L; basal follicle-stimulating hormone (FSH) 121.94 IU/L, peak FSH>208 IU/L), and the HCG stimulation test suggested primary hypogonadism (no testosterone elevation: 2.97 to 2.91 nmol/L). The anti-Müllerian hormone (AMH) level was 1.0 μg/L, and the inhibin B level was 17.52 ng/L. Her karyotype analysis showed 46, XY and the SRY gene was positive. Other laboratory investigations revealed that there were no abnormalities in the hepatic/renal function, serum glucose, serum lipids, thyroid function, and tumor markers.
Pelvic ultrasonography revealed heterogeneous hypoechoic masses in the upper segment of the bilateral inguinal canals with sizes of 1.2 × 0.7 × 0.6 cm and 2.3 × 0.9 × 1.0 cm, and no uterine or ovarian echoes were observed. According to the GP method, her bone age was 13.5 years old and the height for bone age was +1.6 SD. NCS (at 15.4 years old) showed that the motor and sensory nerve conduction velocity of the detected nerves, and the sensory nerve action potential (SNAP) of the sural nerves were slightly decreased, and the F–M latency of the tibial nerves were mildly prolonged (see Table 1).
Gender dysphoria questionnaire (female): She was assessed using the adolescent gender dysphoria questionnaire, but she did not meet the corresponding symptoms, suggesting that she didn't have gender dysphoria.
Genetic analysis: After obtaining informed consent, genetic analysis of the proband and her parents was performed by Guangzhou KingMed Medical Diagnostics Center. Next-generation sequencing was utilized to sequence the exon coding regions of 64 DSD-related genes (see Supplementary Table S1). The candidate gene was also verified by Sanger sequencing and parental sequencing. Finally, we identified a homozygous variant in the DHH exon 3 (c.1027T>C, p. Cys343Arg), and a heterozygous variant was found at this site in both her parents (see Supplementary Table S1). According to the 2015 ACMG guidelines, this missense variant was pathogenic.
Laparoscopic exploration: The bilateral gonads were located in the abdominal cavity, and no uterus, ovaries, or fallopian tubes were found in the pelvic cavity. Cystoscopy showed that there was a blind-end vagina approximately 3 cm from the opening. The bilateral gonads were removed laparoscopically, and the pathology of the bilateral gonads was dysplastic testis tissue (see Figure 1), consistent with PGD, and no tumor cells were found.
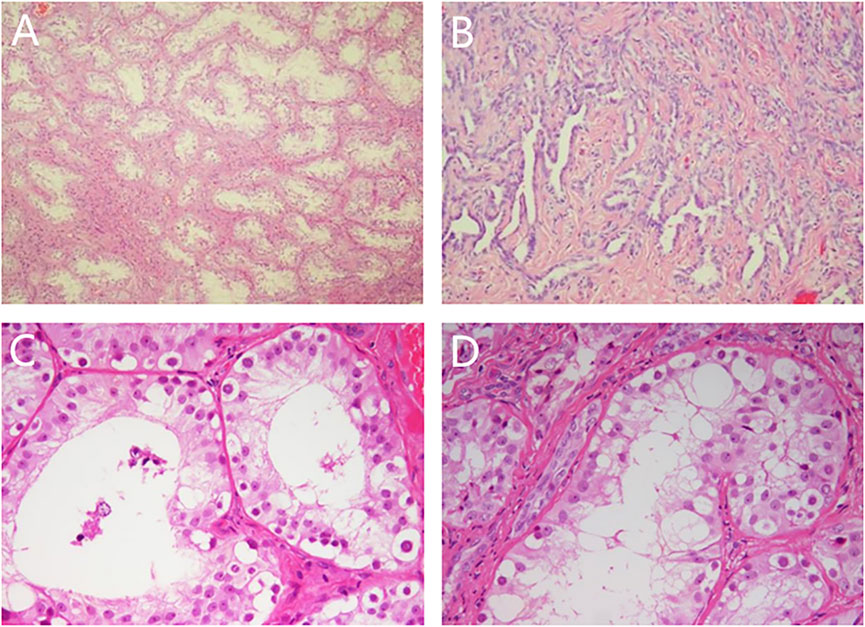
FIGURE 1. Pathological results of bilateral gonads. Note: (A) (HE × 50) and (C) (HE × 200): left gonad, spermatogonia could be seen in seminiferous tubules; (B) (HE × 100) and (D) (HE × 200): right gonad, adenoid hyperplasia of testicular hilar cells could be seen.
During the 6-year follow-up, her personality as a female was more obvious and she loved to dress-up without gender dysphoria. She continued to receive low-dose estrogen replacement therapy and the dose was gradually increased, and her breast development progressed from Tanner stage B3 to B5. However, the muscle spasm in her hands and feet were worse than than before, and there was an episode in her cheeks, which was more obvious after tiredness and stress. At a follow-up 3 years ago, she suffered upper abdominal discomfort, and a gastroscopic examination revealed gastric ulcers. After 2 weeks of oral medication, her symptoms improved but recurred after she discontinued the medication.
During the last examination in our hospital (at 18.4 years old), her height, weight, and BMI were 175.5 cm (+2.8 SD), 76.8 kg (+2.7 SD), and 24.9 kg/m2 (24-28 kg/m2 for overweight), respectively. Her bilateral breasts, axillary hair, and pubic hair were in stage B5, stage A3, and stage PH4, respectively. Evaluation of her glucose metabolism indicated fasting serum glucose, fasting insulin, and serum C-peptide and HOMA-IR levels of 4.84 mmol/L, 18.5 mIU/L, 2.84 μg/L, and 3.98, respectively, suggesting insulin resistance. Although no abnormality was found in her hepatic/renal function and serum lipids, liver ultrasonography indicated the presence of nonalcoholic fatty liver disease. A body composition examination was performed with the following results: percentage of body fat of 42.5% (reference range 18%–28%), the muscle index of 31% (reference range 33%–43%), and the area of visceral fat of 171.3%. No abnormal discharge waves were found in an electroencephalogram (EEG) examination. Re-examination of NCS showed that nerve conduction velocities and action potential were decreased, and that the FM wave latency was longer than before (see Table 1). High-resolution neurosonography showed changes in peripheral nerves, which were manifested as increased echogenicity of the median, tibial, and superficial peroneal nerve (see Figure 2). The gender dysphoria questionnaire indicated no gender dysphoria.
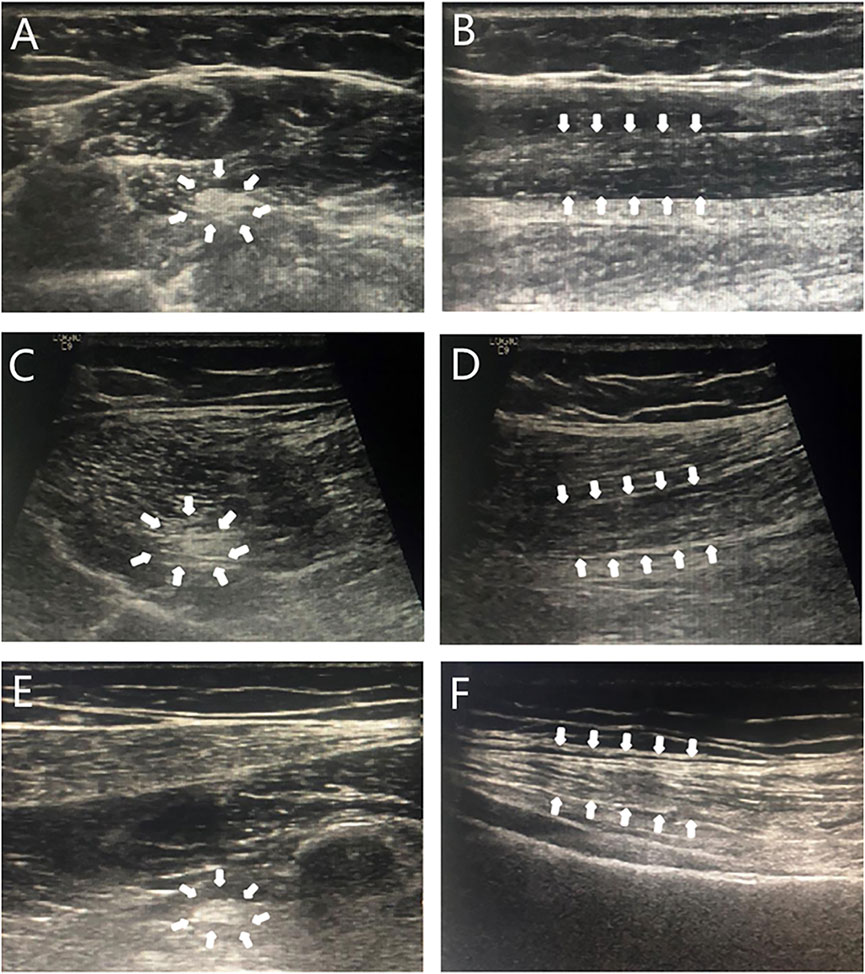
FIGURE 2. Sonogram of neurosonography. Note: Transverse sections (A,C,E): punctate hyperechoic; longitudinal sections (B,D,F): diffuse hyperechoic, less clear texture. A and B: median nerve; C and D: tibial nerve; E and F: peroneal nerve; arrows: the area shown was an abnormal signal on neurosonography.
Therapy adjustment: Vitamin B was recommended for peripheral nerve nutrition, and metformin treatment and health education were provided for obesity as well as insulin resistance. According to a recent telephone follow-up (20 years old), her weight gain was under control, but her muscle cramps were still obvious and had not improved by vitamin B therapy.
Relevant studies were searched from PubMed, Web of Science, HGMD, and some Chinese databases, such as CNKI, Wanfang, and VIP Database, up to May 2022. Our searches were based on combinations of the following index terms: DHH, 46, XY gonadal dysplasia, 46, XY gonadal dysgenesis, 46, XY disorder of sex development, neuropathy, Hedgehog signaling pathway and the corresponding terms in Chinese. A total of 25 cases of DHH variants causing 46, XY GD in detail have been reported worldwide so far (Umehara et al., 2000; Canto et al., 2004; Das et al., 2011; Werner et al., 2015; Paris et al., 2017; Sato et al., 2017; Baldinotti et al., 2018; Rothacker et al., 2018; Tajouri et al., 2018; Ayers et al., 2019; Buonocore et al., 2019; Neocleous et al., 2019; Akter et al., 2021; Kalinchenko et al., 2021; Mehta et al., 2021) (see Table 2), including 24 different variant types of missense, deletion, duplication, or transversion, and both homozygous and compound heterozygous variants have been reported. The reported ages ranged from 2.8 to 55 years. Moreover, 23 cases were raised as females and two cases were raised as males. These patients were mainly referred for delayed development of secondary sexual characteristics, amenorrhea, or abnormal external genitalia. There were 19 patients with homozygous variants, and their gender was female with a complete female phenotype. The gonadal pathology could be complete gonadal dysgenesis (CGD) or PGD. Among these patients, nine cases had neuropathy, except for three patients with incomplete information, and the incidence of neuropathy was 56% (9/16). There were six patients with compound heterozygous variants, including four females and two males of social gender, and none of them had neuropathy. The pathological results of gonads were reported in 19 cases, including nine cases of PGD and 10 cases of CGD, and 25% (4/16, three cases not mentioned) cases of them had gonad tumors. Obesity, insulin resistance, fatty liver, and gastric ulcers were not reported in the above literature.
DHH gene variants have been identified in patients with 46, XY GD, manifesting as a female phenotype or incomplete masculinization. More than half of the patients with homozygous variants suffer peripheral neuropathy, which is the so-called 46, XY GDMN (OMIM 607080). Neuropathy in these patients may gradually worsen with age, and even lead to digital ulcers and amputation in severe cases. The gonadal pathology of patients with homozygous variants may be CGD or PDG, with the former being predominant. The phenotype of patients with compound heterozygous variants is relatively mild, and in addition to the absence of peripheral neuropathy, the difference is also manifested in gonadal function; these patients may still have a certain degree of virilization and their gonadal pathology is PGD (Tajouri et al., 2018; Ayers et al., 2019; Buonocore et al., 2019). Ayers and Elzaiat (Ayers et al., 2019; Elzaiat et al., 2020) suggested that the DHH homozygous variant affects the overall conformation of the protein, thereby perturbing its self-cleavage, resulting in a significant loss (1%) of functional activity compared to the wild type, and they reported that compound heterozygotes show a significant decrease in activity (2% and 46%) and that single heterozygotes have nearly wild-type activity (90% and 98%). Similar to this case, patients with homozygous variants of DHH are more severe than those with compound heterozygous variants.
DHH belongs to the highly conserved HH protein family. The HH cell signaling pathway molecule was first discovered in Drosophila in 1980; it is involved in cell proliferation, cell differentiation, and maintenance of tissue homeostasis (Chai et al., 2021). DHH is an important regulator of gonadal development and is specifically expressed in the testis, but not in the ovary. The dhh−/− male animal model has a female phenotype with hypogonadism, decreased Leydig cell number, lack of mature spermatogenesis, incomplete testicular descent, and the existence of a blind-end vagina, suggesting that DHH is involved in the development, descent, and spermatogenesis of the testis (Bitgood et al., 1996; Yao et al., 2002; Kawai et al., 2011; Qi et al., 2021). After the expression of SRY in the early embryonic stage, DHH is secreted by Sertoli cells and acts on fetal Leydig cells (FLCs) and peritubular myoid cells in a paracrine manner. The functions of FLCs involve the synthesis and secretion of androgen, thereby promoting the development of the male reproductive system, while peritubular myoid cells directly or indirectly regulate the function of Leydig cells through paracrine factors of Sertoli cells (Bitgood et al., 1996; Martin, 2016; Chen et al., 2017). A DHH variant inhibits FLC proliferation and differentiation as well as affects the differentiation of peritubular myoid cells and formation of the testicular cord, thus causing testicular dysplasia. In addition, DHH can also act on Sertoli cells in an autocrine manner. The absence of uteruses in DHH-mutated cases with incomplete masculinization (Ayers et al., 2019; Buonocore et al., 2019) has suggested that Sertoli cells in early embryos may still secrete AMH, thereby inhibiting the development of the Müllerian duct structure; however, due to the persistent abnormality of gonad position and autocrine function, the function of Sertoli cells is reduced to varying degrees causing spermatogenesis disorders in patients. In contrast, naive uteruses have been observed in patients with the female phenotype, suggesting severely impaired AMH secretion in early embryonic stages. In the present case, the basal FSH was significantly increased, and testosterone did not respond after hCG stimulation. Moreover, the pathology showed testicular dysplasia, while AMH was low (a small amount of secretion could still be detected). Thus, Sertoli cells were relatively less dysfunctional than significantly increased FSH levels and severely dysfunctional Leydig cells, indicating that Sertoli cells developed better than Leydig cells during the embryonic stage of DHH variant patients. In some patients, CGD or PGD due to a DHH variant increases the risk of germ cell tumors, which develop into seminoma, dysgerminoma, and gonadoblastoma (Canto et al., 2004; Werner et al., 2015), with an incidence of 25%. Therefore, patients with a DHH variant should be closely monitored, and their dysplastic gonads should be removed if necessary.
DHH is also highly expressed in Schwann cells and participates in the formation of nerve sheaths, which is necessary for the integrity and normal function of peripheral nerves. Electrophysiological studies in dhh−/− mice have shown that nerve fiber conduction velocity is significantly decreased, which is related to axon reduction, increased nerve sheath–vascular permeability, and nerve fiber degeneration (Sharghi–Namini et al., 2006; Sato et al., 2017; Boso et al., 2020). Peripheral neuropathy in more than 50% of patients with DHH homozygous variants may cause sensory and motor nerve disorders, mainly the former, manifesting as spasticity, numbness, or weakness of hands and feet. Electrophysiological examination has shown decreased conduction velocity and prolonged F–M latency, and high-resolution neurosonography has shown diffuse hyperechoic in the transverse and longitudinal sections of the nerve bundle (Werner et al., 2015; Boso et al., 2020). GDMN usually appears in adult patients aged 20–30 years, but some children with GDMN have been reported in recent years (Sato et al., 2017; Kalinchenko et al., 2021). The present patient had atypical onset symptoms, which may have been related to the young age, but the comparison of her previous and recent NCS examinations showed that it was worsening after only 6 years. Because the symptoms of peripheral neuropathy worsen with age, long-term follow-up and monitoring should be performed for early detection and prevention.
In addition, some studies (Pospisilik et al., 2010; Matz-Soja et al., 2016; Braune et al., 2017; Guillen–Sacoto et al., 2017) have suggested that with the weakening of HH pathway signaling, there may be increased adipose tissue inflammation, impaired glucose tolerance, induction of hepatic steatosis, and impaired gastric cell regeneration and homeostasis. The HH molecules have various functions as follows: DHH and IHH regulate glucose metabolism and adipose tissue differentiation, SHH and IHH are involved in hepatocyte repair and lipid metabolism, and SHH is related to gastric cell regeneration (Kang et al., 2009). A previous study on an obese population (Braune et al., 2017) has reported that DHH and IHH are expressed in visceral and subcutaneous adipose tissue. With the increase in BMI, the ligands of the HH signaling pathway decrease, and the expression of DHH is significantly negatively correlated with obesity-related indicators (such as body weight, waist circumference, hip circumference, body mass percentage, and fat area). Knockout of the SMO receptor in the HH signaling pathway increases body weight, increases adipose tissue inflammation, and impairs glucose tolerance. The BMI of the patient in our study was only 24.9 kg/m2, but the percentages of body mass and fat area were significantly increased, which may be related to the abnormal expression of the DHH protein affecting the normal conduction of the HH signaling pathway. During the follow-up, she gradually developed obesity, insulin resistance, fatty liver, and gastric ulcers, which have been rarely reported in previous studies. Her symptoms may be related to the weakening of HH signaling caused by the DHH variant. However, these findings need to be further confirmed by long-term follow-up of more cases and further basic research.
In summary, we reported a case of 46, XY GD with a DHH homozygous variant resulting in a complete female phenotype, characterized by amenorrhea with neuropathy. The symptoms of peripheral neuropathy were not typical at first but worsened during our follow-up. For the first time in such patients, we showed a comparison of NCS changes with age as well as the presence of multiple complications. Our report expanded the phenotypic spectrum of diseases caused by DHH variants, and our long-term follow-up and summary of the literature would contribute to the future diagnosis and treatment of individuals with DHH variants.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shenzhen Children’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
ZS conceptualized and designed the study. LP and ZL supervised the data collection, reviewed the analyses, and wrote all versions of the manuscript. WS, RZ, WC, XH, JS, SL, and PW coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
This work was supported by Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (SZGSP012) and Shenzhen Fundamental Research Program (KCXFZ20201221173400002).
The authors would like to thank the patient and her parents for their cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.954288/full#supplementary-material
Akter, H., Hossain, M. S., Dity, N. J., Rahaman, M. A., Furkan Uddin, K. M., Nassir, N., et al. (2021). Whole exome sequencing uncovered highly penetrant recessive mutations for a spectrum of rare genetic pediatric diseases in Bangladesh. NPJ Genom. Med. 6 (1), 14. doi:10.1038/s41525-021-00173-0
Ayers, K., van den Bergen, J., Robevska, G., Listyasari, N., Raza, J., Atta, I., et al. (2019). Functional analysis of novel desert hedgehog gene variants improves the clinical interpretation of genomic data and provides a more accurate diagnosis for patients with 46, XY differences of sex development. J. Med. Genet. 56 (7), 434–443. doi:10.1136/jmedgenet-2018-105893
Baldinotti, F., Cavallaro, T., Dati, E., Baroncelli, G. I., Bertini, V., Valetto, A., et al. (2018). Novel familial variant of the Desert hedgehog gene: Clinical findings in two sisters with 46, XY gonadal dysgenesis or 46, XX karyotype and literature review. Horm. Res. Paediatr. 89 (3), 141–149. doi:10.1159/000485507
Bitgood, M. J., Shen, L., and McMahon, A. P. (1996). Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 6 (3), 298–304. doi:10.1016/s0960-9822(02)00480-3
Boso, F., Zanette, G., Baldinotti, F., Bertelloni, S., Taioli, F., Monaco, S., et al. (2020). Convergent pathological and ultrasound features in hereditary syndromic and non-syndromic minifascicular neuropathy related to DHH. J. Peripher. Nerv. Syst. 25 (4), 423–428. doi:10.1111/jns.12417
Braune, J., Weyer, U., Matz-Soja, M., Hobusch, C., Kern, M., Kunath, A., et al. (2017). Hedgehog signalling in myeloid cells impacts on body weight, adipose tissue inflammation and glucose metabolism. Diabetologia 60 (5), 889–899. doi:10.1007/s00125-017-4223-5
Buonocore, F., Clifford-Mobley, O., King, T. F. J., Striglioni, N., Man, E., Suntharalingham, J. P., et al. (2019). Next-generation sequencing reveals novel genetic variants (SRY, DMRT1, NR5A1, DHH, DHX37) in adults with 46, XY DSD. J. Endocr. Soc. 3 (12), 2341–2360. doi:10.1210/js.2019-00306
Canto, P., Soderlund, D., Reyes, E., and Mendez, J. P. (2004). Mutations in the desert hedgehog (DHH) gene in patients with 46, XY complete pure gonadal dysgenesis. J. Clin. Endocrinol. Metab. 89 (9), 4480–4483. doi:10.1210/jc.2004-0863
Chai, S., Tian, R., Yang, Y., Yang, G., Xu, S., Ren, W., et al. (2021). Enhanced negative regulation of the DHH signaling pathway as a potential mechanism of ascrotal testes in laurasiatherians. Evol. Biol. 48 (3), 335–345. doi:10.1007/s11692-021-09542-0
Chen, H., Wang, Y., Ge, R., and Zirkin, B. R. (2017). Leydig cell stem cells: Identification, proliferation and differentiation. Mol. Cell. Endocrinol. 445, 65–73. doi:10.1016/j.mce.2016.10.010
Das, D. K., Sanghavi, D., Gawde, H., Idicula-Thomas, S., and Vasudevan, L. (2011). Novel homozygous mutations in Desert hedgehog gene in patients with 46, XY complete gonadal dysgenesis and prediction of its structural and functional implications by computational methods. Eur. J. Med. Genet. 54 (6), e529–534. doi:10.1016/j.ejmg.2011.04.010
Elzaiat, M., Flatters, D., Sierra-Diaz, D. C., Legois, B., Laissue, P., Veitia, R. A., et al. (2020). DHH pathogenic variants involved in 46, XY disorders of sex development differentially impact protein self-cleavage and structural conformation. Hum. Genet. 139 (11), 1455–1470. doi:10.1007/s00439-020-02189-5
Guillen-Sacoto, M. J., Martinez, A. F., Abe, Y., Kruszka, P., Weiss, K., Everson, J. L., et al. (2017). Human germline hedgehog pathway mutations predispose to fatty liver. J. Hepatol. 67 (4), 809–817. doi:10.1016/j.jhep.2017.06.008
Kalinchenko, N. Y., Batyrova, Z. K., Kostrova, I. B., Kolodkina, A. A., Uvarova, E. N., Kumykova, Z. K., et al. (2021). Clinical Findings in Two patients with DSD 46XY caused by new variant of the Desert Hedgehog Gene and review of the literature of the role of DHH signaling pathway in sex development. Probl. Endokrinol. 67 (3), 73–77. doi:10.14341/probl12757
Kang, D. H., Han, M. E., Song, M. H., Lee, Y. S., Kim, E. H., Kim, H. J., et al. (2009). The role of hedgehog signaling during gastric regeneration. J. Gastroenterol. 44 (5), 372–379. doi:10.1007/s00535-009-0006-1
Kawai, Y., Noguchi, J., Akiyama, K., Takeno, Y., Fujiwara, Y., Kajita, S., et al. (2011). A missense mutation of the Dhh gene is associated with male pseudohermaphroditic rats showing impaired Leydig cell development. Reproduction 141 (2), 217–225. doi:10.1530/REP-10-0006
Martin, L. J. (2016). Cell interactions and genetic regulation that contribute to testicular Leydig cell development and differentiation. Mol. Reprod. Dev. 83 (6), 470–487. doi:10.1002/mrd.22648
Matz-Soja, M., Rennert, C., Schonefeld, K., Aleithe, S., Boettger, J., Schmidt-Heck, W., et al. (2016). Hedgehog signaling is a potent regulator of liver lipid metabolism and reveals a GLI-code associated with steatosis. Elife 5, e13308. doi:10.7554/eLife.13308
Mehta, P., Singh, P., Gupta, N. J., Sankhwar, S. N., Chakravarty, B., Thangaraj, K., et al. (2021). Mutations in the desert hedgehog (DHH) gene in the disorders of sexual differentiation and male infertility. J. Assist. Reprod. Genet. 38 (7), 1871–1878. doi:10.1007/s10815-021-02140-1
Neocleous, V., Fanis, P., Cinarli, F., Kokotsis, V., Oulas, A., Toumba, M., et al. (2019). 46, XY complete gonadal dysgenesis in a familial case with a rare mutation in the desert hedgehog (DHH) gene. Horm. (Athens) 18 (3), 315–320. doi:10.1007/s42000-019-00116-6
Paris, F., Flatters, D., Caburet, S., Legois, B., Servant, N., Lefebvre, H., et al. (2017). A novel variant of DHH in a familial case of 46, XY disorder of sex development: Insights from molecular dynamics simulations. Clin. Endocrinol. 87 (5), 539–544. doi:10.1111/cen.13420
Pospisilik, J. A., Schramek, D., Schnidar, H., Cronin, S. J., Nehme, N. T., Zhang, X., et al. (2010). Drosophila genome-wide obesity screen reveals hedgehog as a determinant of Brown versus white adipose cell fate. Cell 140 (1), 148–160. doi:10.1016/j.cell.2009.12.027
Qi, Q., Dong, Z., Zhang, N., Wang, L., Shao, C., Xu, W., et al. (2021). Cloning, expression and functional analysis of the desert hedgehog (dhh) gene in Chinese tongue sole (Cynoglossus semilaevis). Gene Expr. Patterns. 39, 119163. doi:10.1016/j.gep.2020.119163
Rothacker, K. M., Ayers, K. L., Tang, D., Joshi, K., van den Bergen, J. A., Robevska, G., et al. (2018). A novel, homozygous mutation in desert hedgehog (DHH) in a 46, XY patient with dysgenetic testes presenting with primary amenorrhoea: a case report. Int. J. Pediatr. Endocrinol. 2018, 2. doi:10.1186/s13633-018-0056-3
Sato, N. S., Maekawa, R., Ishiura, H., Mitsui, J., Naruse, H., Tokushige, S.-i., et al. (2017). Partial duplication of DHH causes minifascicular neuropathy: A novel mutation detection of DHH. Ann. Clin. Transl. Neurol. 4 (6), 415–421. doi:10.1002/acn3.417
Sharghi-Namini, S., Turmaine, M., Meier, C., Sahni, V., Umehara, F., Jessen, K. R., et al. (2006). The structural and functional integrity of peripheral nerves depends on the glial-derived signal desert hedgehog. J. Neurosci. 26 (23), 6364–6376. doi:10.1523/JNEUROSCI.0157-06.2006
Tajouri, A., Kharrat, M., Hizem, S., Zaghdoudi, H., M'Rad, R., Simic-Schleicher, G., et al. (2018). In vitro functional characterization of the novel DHH mutations associated with gonadal dysgenesis. Hum. Mutat. 39 (12), 2097–2109. doi:10.1002/humu.23664
Umehara, F., Tate, G., Itoh, K., Yamaguchi, N., Douchi, T., Mitsuya, T., et al. (2000). A novel mutation of desert hedgehog in a patient with 46, XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Hum. Genet. 67 (5), 1302–1305. doi:10.1016/S0002-9297(07)62958-9
Werner, R., Merz, H., Birnbaum, W., Marshall, L., Schroder, T., Reiz, B., et al. (2015). 46, XY gonadal dysgenesis due to a homozygous mutation in Desert hedgehog (DHH) identified by exome sequencing. J. Clin. Endocrinol. Metab. 100 (7), E1022–E1029. doi:10.1210/jc.2015-1314
Keywords: DHH, Hedgehog signaling pathway, 46, XY gonadal dysgenesis, neuropathy, complications
Citation: Pan L, Li Z, Su Z, Su W, Zheng R, Chen W, He X, Song J, Li S and Wen P (2022) Case Report: Long-term follow-up of desert hedgehog variant caused 46, XY gonadal dysgenesis with multiple complications in a Chinese child. Front. Genet. 13:954288. doi: 10.3389/fgene.2022.954288
Received: 27 May 2022; Accepted: 04 July 2022;
Published: 22 August 2022.
Edited by:
Jun Mitsui, The University of Tokyo, JapanReviewed by:
Singh Rajender, Central Drug Research Institute (CSIR), IndiaCopyright © 2022 Pan, Li, Su, Su, Zheng, Chen, He, Song, Li and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Su, c3VfemhlQDEyNi5jb20=
†ORCID: Lili Pan, orcid.org/0000-0001-9840-2689; Zhuguang Li, orcid.org/0000-0003-1634-6295; Zhe Su, orcid.org/0000-0003-4101-8429
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.