- 1Department of Otorhinolaryngology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Otorhinolaryngology, Wuhan First Hospital/Wuhan Hospital of Traditional Chinese and Western Medicine, Wuhan, China
- 3Department of Otorhinolaryngology, The Central Hospital of Wuhan, Wuhan, China
Background: β-Catenin has been recently identified as a promising novel therapeutic target and prognostic marker in different types of cancer. Here, we conduct a meta-analysis to better clarify the correlation between β-Catenin expression and survival outcomes in nasopharyngeal carcinoma (NPC) patients.
Patients/methods: Following the Preferred Reporting Items or Systematic Reviews Meta Analyses (PRISMA) 2020 guidelines, the PubMed, Embase, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases were systematically searched for relevant studies to explore the prognostic significance of β-Catenin in NPC. Pooled hazards ratios (HRs) and 95% confidence intervals (CIs) were used to estimate the association of β-Catenin expression with survival outcomes in NPC patients. Odd ratios (ORs) and 95% CIs for clinicopathological characteristics were also statistically analyzed.
Results: Eight studies involving 1,179 patients with NPC were ultimately included in the meta-analysis. Pooled analysis indicated that elevated β-Catenin expression was significantly associated with poor OS (HR = 2.45, 95% CIs: 1.45–4.16, p = 0.001) and poor DFS/PFS (HR 1.79, 95% CIs: 1.29–2.49, p = 0.000). Furthermore, β-cadherin was signifcantly associated with higher TMN stages (OR = 5.10, 95% CIs 2.93–8.86, p = 0.000), clinical stages (OR = 5.10, 95% CIs 2.93–8.86, p = 0.000) and lymph node metastasis (LNM) (OR = 5.01, 95% CIs 2.40–10.44, p = 0.000).
Conclusions: This study demonstrated that for NPC, patients with elevated β-Catenin expression are more likely to have poor survival.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common types of head and neck tumors and shows remarkable differences in geographic and racial distribution (Stransky et al., 2011). NPC is prevalent in Southeast Asia, especially in Southern China, the Arctic region and North Africa (Chang and Adami, 2006). Risk factors for NPC include male sex, EBV infection, Cantonese ethnicity, salt-preserved fish consumption, low fresh fruit and vegetable intake, and smoking, among others. Irrespective of the progress in radiation therapy and potent chemotherapy, approximately 5%–15% local recurrence and 15%–30% distant metastasis rates remain the main causes of failure after NPC treatment (Lee et al., 2015). Clinical staging is essential for the prognosis of NPC; however, patients at the same clinical stage may have different prognoses. In general, the current staging system is inadequate to predict survival due to variations in treatment outcomes. Hence, it is necessary to identify more reliable prognostic factors to improve the prognosis of NPC.
β-Catenin was first characterized as a family of cell-cell adhesion molecules dependent on Ca2+ that are present in most cell types, and it was also shown to have more detailed specificity with regard to cell-cell aggregation patterns and segregation during development (Takeichi, 1990). β-Catenin is one of the hallmarks of the epithelial-mesenchymal transition, which is important for early tumor metastasis and invasion (Thiery and Sleeman, 2006). It also plays a crucial role in the Wnt/β-Catenin signaling pathway, which is one of the most important signaling pathways involved in many human malignancies, and might participate in the development of various cancers and tumors (Anastas and Moon, 2013). Indeed, aberrant activation of Wnt/β-Catenin signaling is found in various types of human cancer, including osteosarcoma, lung cancer, colorectal cancer, renal cell carcinoma, breast cancer, and hepatocellular cancer, among others (Kim et al., 2002; Hoang et al., 2004; Arai et al., 2014; Jang et al., 2015; Fu et al., 2016).
Numerous studies have focused on the identification of new prognostic markers that can be used for cancer monitoring and detection. An association between β-Catenin expression and survival has been shown in NPC (Wang et al., 2009; Luo et al., 2012; Xu et al., 2013; Pang et al., 2019). Although many studies have reported an association between β-Catenin expression and NPC patient survival, the results are still controversial and ambiguous. For example, Jin et al. (2019), Sun et al. (2017), Wang et al. (2017) found that β-Catenin is highly expressed in NPC and is a potential risk factor that leads to an unfavorable survival prognosis in these patients. However, contradictory results were reported by Hao et al. (2014), Galera-Ruiz et al. (2011), who found no association between β-Catenin and survival in NPC patients compared with normal controls. In this study, we conducted a meta-analysis based on PubMed, Embase, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases to statistically assess the association between β-Catenin and the prognosis of NPC patients.
Methods
Search strategy
Following the Preferred Reporting Items or Systematic Reviews Meta Analyses (PRISMA) 2020 guidelines, electronic searches for relevant studies were performed in the PubMed, Web of Science, EMBASE, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI) and Wanfang database until 1 March 2022 (Page et al., 2021). The search terms of PubMed were “((((((((((((((((((((Nasopharyngeal Neoplasm) OR (Neoplasm, Nasopharyngeal)) OR (Neoplasms, Nasopharyngeal)) OR (Nasopharynx Neoplasms)) OR (Nasopharynx Neoplasm)) OR (Neoplasm, Nasopharynx)) OR (Neoplasms, Nasopharynx)) OR (Cancer of Nasopharynx)) OR (Nasopharynx Cancers)) OR (Nasopharyngeal Cancer)) OR (Cancer, Nasopharyngeal)) OR (Cancers, Nasopharyngeal)) OR (Nasopharyngeal Cancers)) OR (Nasopharynx Cancer)) OR (Cancer, Nasopharynx)) OR (Cancers, Nasopharynx)))) OR (Cancer of the Nasopharynx)) AND ((((((prognosis) OR (outcome)) OR (recurrence)) OR (survival)) OR (mortality)) OR (progression))) AND ((Catenin, beta) OR (beta-Catenin)) ” The EMTREE terms were as follows “(‘nasopharynx cancer'/exp OR rhinopharyngioma OR ‘cancer, nasopharynx’ OR ‘epipharynx cancer’ OR ‘nasopharyngeal cancer’ OR ‘rhinopharynx cancer’) AND (prognosis OR outcome OR recurrence OR survival OR mortality OR progression) AND (‘beta catenin’/exp OR “catenin beta”).” Furthermore, the reference lists of retrieved articles were manually searched for additional articles. If several publications reported the same patient populations, the most complete study was enrolled to avoid duplication.
Selection criteria
This meta-analysis was limited to publications about the association between NPC and β-Catenin. The inclusion criteria of the meta-analysis were as follows: 1) all patients diagnosed with NPC; 2) β-Catenin was evaluated in both samples of NPC and normal controls; 3) the study revealed the association between β-Catenin and survival of NPC; 4) sufficient statistical analysis, including hazard ratios (HRs), odds ratios (ORs) and their 95% confidence intervals (95% CIs) were reported. The exclusion criteria were as follows: 1) studies without sufficient data for meta-analysis; 2) abstracts, reviews, letters, expert opinions; 3) studies about cell lines, in vivo/vitro studies, and human xenografts. If several studies reported the same cohort, we used the most recent one in our meta-analysis.
Data extraction
First, we inspected duplicates and removed repeated papers. Then, we carefully perused the titles and abstracts of the papers. Finally, full articles were selected to include appropriate studies. Two researchers independently evaluated the literature using the inclusion and exclusion criteria (LQ Zhou and Y Hu). Any discrepancy in assessment was resolved by consulting with a third researcher (HJ Xiao). The authors of the studies were contacted by e-mail to request data or additional information for meta-analysis calculations. Eligible studies were reviewed by two reviewers (LQ Zhou and Y Hu) independently. The Newcastle–Ottawa Scale (NOS) (Peterson et al., 2011) was included to assess the quality of the included publications, and a star system (maximum is nine stars) was adopted to evaluate a study in three domains: comparability of study groups, selection of participants and ascertainment of outcomes of interest. Scores of NOS ≥6 indicated high-quality studies. Reporting recommendations for tumor marker prognostic studies (REMARK) were also applied to evaluate study quality in cancer-related meta-analyses (Sauerbrei et al., 2018).
The following information was extracted from each publication: 1) first author’s name, year, cancer type, country of the population, patient age, sample size, publication journal; 2) survival data including overall survival (OS), disease-free survival (DFS) and progression-free survival (PFS) (OS was detected from the date of medical treatment to the date of the last follow-up or death of patient; PFS was detected from the date of treatment to the date of death or recurrence tumor from any cause; DFS was detected from the date of diagnosis to the date of relapse, progression, death, or last follow-up visit and similarly censored at last follow-up visit); 3) The number of patients in each group was divided according to the TMN stages, clinical stages, the presence or absence of lymph node metastasis (LNM), gender and the number of patients with high or low β-catenin expression in each group. 4) Methods and cut-off value (Table 1).
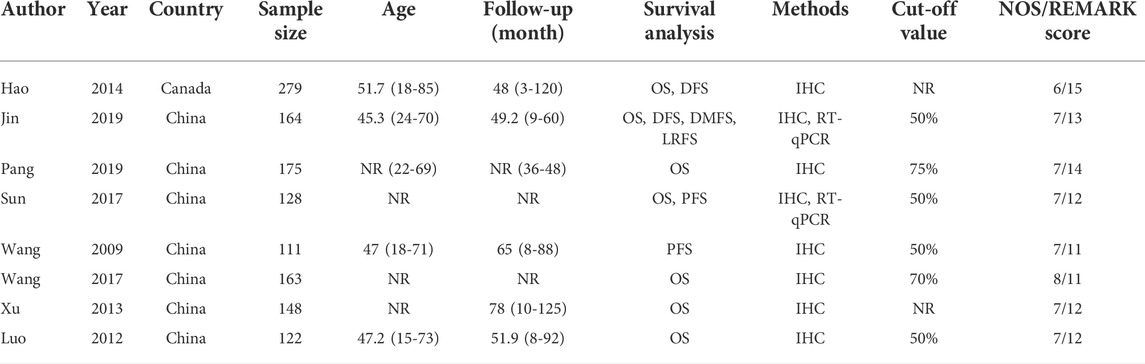
TABLE 1. Characteristics of the studies examined in the meta-analysis. NR, not reported; IHC, Immunohistochemistry; RT-qPCR, Reverse transcription-quantitative polymerase chain reaction.
Statistical analysis
Pooled HRs, ORs and their 95% CIs were directly obtained or estimated by p values and other published data following Parmer’s methods from the primary studies (Ambrosio et al., 2014). Statistical heterogeneity among the included studies was assessed by the χ2-based Q test and I2 test (Higgins et al., 2003). The fixed-effect model was used for analysis in the absence of significant heterogeneity between studies (p > 0.10, I2 = 0%); we adopted the random-effects model if significant heterogeneity was present. We also performed sensitivity analysis to investigate the influence of each individual study on the overall pooled results. Begg’s test and Egger’s test were applied to detect publication bias (p > 0.05 indicated no publication bias). All statistical analyses were performed using STATA statistical software version 12.0 (STATA, College Station, TX).
Results
Study selection and characteristics
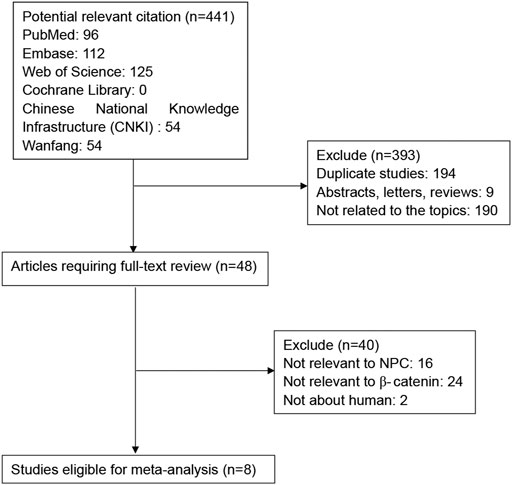
As shown in Figure 1, a total of 312 potential publications were initially identified by searching the PubMed, Web of Science, EMBASE, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI) and Wanfang databases. Following exclusion of duplicates (n = 194), abstracts, letters and reviews (n = 9), and studies not related to the topics (n = 190), the remaining potentially relevant studies (n = 48) were further identified by reading their full texts. 40 studies did not provide specific data regarding NPC or β-Catenin and therefore were excluded. Finally, eight studies between 2009 and 2020 with a total of 1179 NPC patients were included in our meta-analysis.
The study characteristics are summarized in Table 1. All of the eight publications involved >100 patients. Seven studies including 1,068 patients reported OS, 2 studies including 443 patients reported DFS, and 2 studies including 239 patients reported PFS. All HRs, ORs and 95%CIs values were directly reported in the original study. NOS scores for all publications were above 6, and REMARK scores were between 11-15.
Association between β-Catenin and survival in nasopharyngeal carcinoma patients
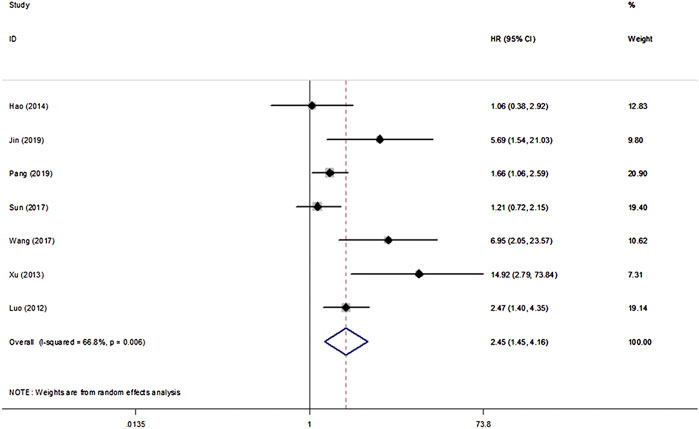
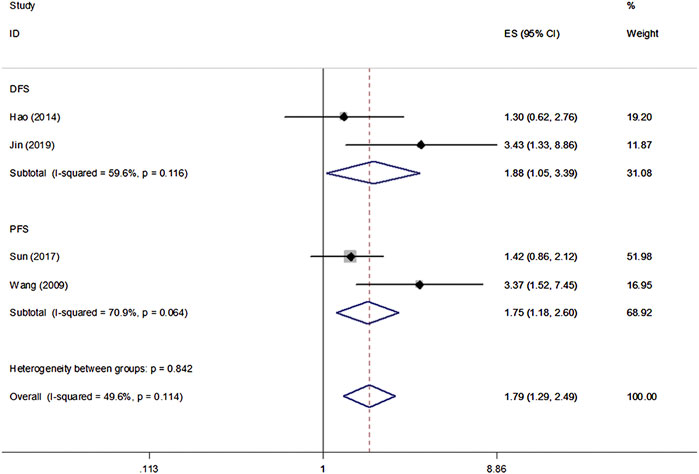
All eight studies in our analysis reported the association between β-Catenin and the OS, DFS and PFS of patients with NPC. Heterogeneity among the publications was significant based on the Q test (p < 0.1). Hence, the random-effect model was adopted and showed that β-Catenin was significantly associated with shorter OS in NPC (HR = 2.45, 95% CIs: 1.45–4.16, p = 0.001). Medium heterogeneity was noted between β-Catenin expression and OS (I2 = 66.8%, Pheterogeneity = 0.006) (Figure 2). Furthermore, two studies including 433 patients reported DFS, and two studies including 239 patients reported PFS. A significant correlation between β-Catenin and shorter DFS/PFS (HR = 1.79, 95% CIs: 1.29–2.49, p = 0.000) was observed, with low heterogeneity (I2 = 49.6%, Pheterogeneity = 0.114) (Figure 3).
Association between β-cadherin and nasopharyngeal carcinoma patients outcomes
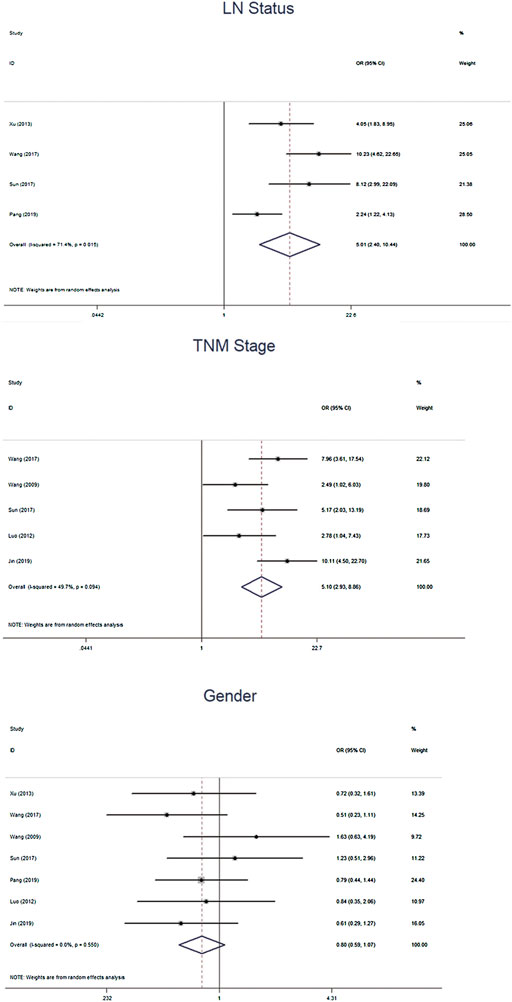
We further calculated the pooled ORs and the 95% CIs to evaluate the association between β-catenin and NPC outcomes: gender (female vs male), TMN stage (T3–4 vs T1–2), clinical stage (T3–4 vs T1–2) and lymph node (LN) status (LNM vs No LNM). The pooled analysis showed that β-cadherin was signifcantly associated with higher TMN stages (OR = 5.10, 95% CIs 2.93–8.86, p = 0.000) and LNM (OR = 5.01, 95% CIs 2.40–10.44, p = 0.000). However, β-cadherin was not signifcantly correlated with gender (OR = 0.80, 95% CIs 0.59–1.07, p = 0.135) (Figure 4).
Sensitivity analysis
Sensitivity analysis was conducted to evaluate the impact of each single study on the overall effect. As depicted in Figure 5, the analysis did not detect a single study that significantly altered the combined results. Overall, the pooled effect size of our meta-analytic results was stable and reliable.
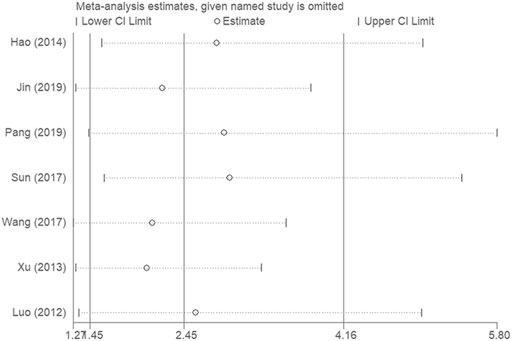
FIGURE 5. Sensitivity analyses were conducted to evaluate the impact of each single study on the overall effect.
Publication bias
Publication bias was assessed by using Begg’s funnel plots and Egger’s test. The results were quite symmetric, with those based on Begg’s funnel plot (p = 0.077) and Egger’s test (p = 0.077) revealing no publication bias among the studies (Figure 6).
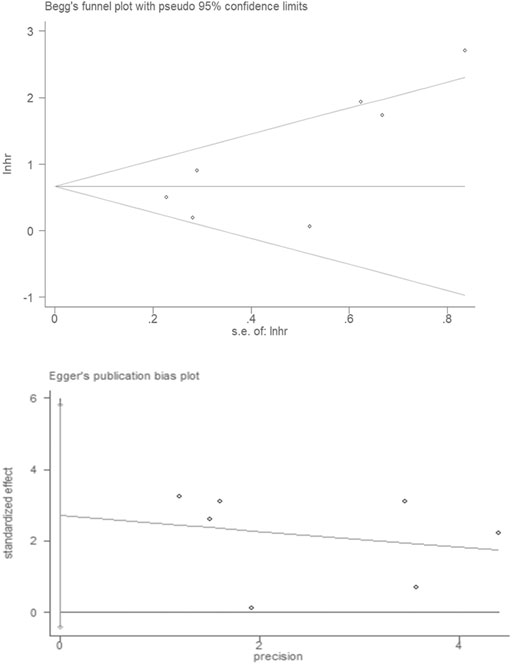
FIGURE 6. Publication bias in the enrolled studies. Publication bias was assessed using Begg’s funnel plots and Egger’s test.
Meta-regression analysis
Medium heterogeneity was noted between β-Catenin expression and OS (I2 = 66.8%, Pheterogeneity = 0.006). Hence, the meta regression analyses were used to explain statistical heterogeneity. The HR was not modified by the year of publication, female ratio (%), area, sample size, or quality score, this result does not fully explain the medium level of heterogeneity observed.
Discussion
The present study is the first meta-analysis including eight published studies with 1,179 patients to provide useful information for clinical decision-making in NPC. β-Catenin was significantly associated with shorter OS in NPC patients, with HR values of 2.45. Significant correlation between β-Catenin and shorter DFS/PFS (HR 1.79) was also observed. Furthermore, our results also demostrated that β-cadherin was signifcantly associated with higher TMN stages, clinical stages and LNM. These results confirm the clinical value of β-Catenin in NPC. NPC tumor cells invade adjacent tissues or metastasize to regional lymph nodes at an early stage of NPC development (Wei and Mok, 2007), though the exact mechanism underlying the process remains unknown. It has been reported that cell–cell adhesion molecules, cytokines and the matrix metalloproteinase family may be involved in adjacent invasion and distant metastasis. β-Catenin is a key mediator in the cadherin-Catenin complex, which is essential for connecting the actin filaments of cells to the cell-cell interface at adherent junctions (Anastas and Moon, 2013); it is also a key mediator of canonical signaling in the Wnt/β-Catenin pathway. β-Catenin can accumulate in both the cytoplasm and nucleus (Khramtsov et al., 2010), and it helps to promote the progression of tumors by suppressing T-cell responses (Hong et al., 2015). Gene mutations or aberrant activation of Wnt receptors activate Wnt/β-Catenin signaling and trigger tumorigenesis in the skin, colon, liver, bone marrow, breast, and possibly other tissues (Fodde and Brabletz, 2007; Monga, 2015). In addition, β-Catenin plays roles in maintaining the stemness of normal intestinal cells, and high-level nuclear localization and cytoplasmic expression promote cancer cell proliferation and survival (Valkenburg et al., 2011). β-Catenin is high expressed when Wnt/β-catenin signal is aberrantly activated, it activates numerous Wnt pathway downstream proliferation signals, including c-Myc and cyclin D1 and finally accelerates cell cycle, facilitates cell proliferation and migration, which induced to poor diagnosis of NPC (Alamoud and Kukuruzinska, 2018).
Targeted therapies have produced striking benefits for cancer patients. The Wnt/β-Catenin pathway has been proven to play a key role in various kinds of carcinomas (Sanchez-Vega et al., 2018). Therefore, this signaling pathway is a preferable target for fighting cancer. Although there are no drugs specifically inhibiting this signaling pathway approved for the clinic, intensive efforts have been made in signaling pathway development. Wnt/β-Catenin pathway inhibitors can be classified into five categories according to their properties: peptides, small molecules, antibodies, natural compounds and RNA interference (Cui et al., 2018). There are already some ongoing phase 1/2 trials with Wnt/β-Catenin pathway inhibitors in metastatic colorecta, head and neck cancers, breast cancers and some other cancers (Krishnamurthy and Kurzrock, 2018). These trials provide proof that in certain patients, cancer can be treated with Wnt/β-Catenin inhibitors. According to our results, Wnt/β-Catenin inhibitors may constitute therapeutics against NPC.
Nevertheless, the present meta-analysis contains several limitations. First, significant heterogeneity was noted in the association between β-Catenin and the OS of patients with NPC. The heterogeneity of the population was most likely due to differences in the baseline characteristics of the included patients (age, race, and tumor stage), the duration of follow-up, the method of mutation detection, and other parameters. A random-effects model was employed to minimize the effects of these differences. Second, the number of articles used for assessing the association between β-Catenin and the prognosis of NPC patients was limited in the present meta-analysis. Therefore, additional studies are required to produce accurate conclusions. Finally, our results may overestimate the prognostic significance of β-Catenin to some extent because the majority of the included studies reported positive results.
In summary, we searched electronic databases, and a total of 1,179 patients in eight studies were enrolled for meta-analysis, demonstrating that patients with elevated β-Catenin expression are more likely to have poorer prognosis. Taken together, our meta-analysis results suggest that β-Catenin has prognostic value for NPC. However, studies with larger sample sizes are needed to obtain more representative and precise results.
Author Contributions
YH, CL, and L-QZ collected and analyzed the data, wrote the paper. L-QZ, YH, JS, and H-JX analyzed the data and wrote the paper. LZ and H-JX conceived and designed this study, analyzed the data, wrote the paper. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (81500792 and 81771005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.953739/full#supplementary-material
Supplementary Table S1 | Quality assessment based on the Recommendations for Tumor Marker Prognostic Studies (REMARK).
Supplementary Table S2 | PRISMA_2020_checklist.
Supplementary Table S3 | PRISMA_2015_checklist.
Supplementary Table S4 | Quality assessment based on the Newcastle-Ottawa Scale (NOS).
References
Alamoud, K. A., and Kukuruzinska, M. A. (2018). Emerging insights into wnt/β-catenin signaling in head and neck cancer. J. Dent. Res. 97 (6), 665–673. doi:10.1177/0022034518771923
Ambrosio, M. R., De Falco, G., Gozzetti, A., Rocca, B. J., Amato, T., Mourmouras, V., et al. (2014). Plasmablastic transformation of a pre-existing plasmacytoma: A possible role for reactivation of epstein barr virus infection. Haematologica 99 (11), e235–7. doi:10.3324/haematol.2014.111872
Anastas, J. N., and Moon, R. T. (2013). WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 13 (1), 11–26. doi:10.1038/nrc3419
Arai, E., Sakamoto, H., Ichikawa, H., Totsuka, H., Chiku, S., Gotoh, M., et al. (2014). Multilayer-omics analysis of renal cell carcinoma, including the whole exome, methylome and transcriptome. Int. J. Cancer 135 (6), 1330–1342. doi:10.1002/ijc.28768
Chang, E. T., and Adami, H.-O. (2006). The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomarkers Prev. 15 (10), 1765–1777. doi:10.1158/1055-9965.EPI-06-0353
Cui, C., Zhou, X., Zhang, W., Qu, Y., and Ke, X. (2018). Is β-catenin a druggable target for cancer therapy? Trends biochem. Sci. 43 (8), 623–634. doi:10.1016/j.tibs.2018.06.003
Fodde, R., and Brabletz, T. (2007). Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr. Opin. Cell Biol. 19 (2), 150–158. doi:10.1016/j.ceb.2007.02.007
Fu, X., Li, H., Liu, C., Hu, B., Li, T., and Wang, Y. (2016). Long noncoding RNA AK126698 inhibits proliferation and migration of non-small cell lung cancer cells by targeting Frizzled-8 and suppressing Wnt/β-catenin signaling pathway. Onco. Targets. Ther. 9, 3815–3827. doi:10.2147/OTT.S100633
Galera-Ruiz, H., Rios, M. J., Gonzalez-Campora, R., de Miguel, M., Carmona, M. I., Moreno, A. M., et al. (2011). The cadherin-catenin complex in nasopharyngeal carcinoma. Eur. Arch. Otorhinolaryngol. 268 (9), 1335–1341. doi:10.1007/s00405-010-1464-z
Hao, D., Phan, T., Jagdis, A., Siever, J. E., Klimowicz, A. C., Laskin, J. J., et al. (2014). Evaluation of E-cadherin, β-catenin and vimentin protein expression using quantitative immunohistochemistry in nasopharyngeal carcinoma patients. Clin. Invest. Med. 37 (5), E320–E330. doi:10.25011/cim.v37i5.22012
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hoang, B. H., Kubo, T., Healey, J. H., Sowers, R., Mazza, B., Yang, R., et al. (2004). Expression of LDL receptor related protein 5 (LRP5) as a novel marker for disease progression in high‐grade osteosarcoma. Int. J. Cancer 109 (1), 106–111. doi:10.1002/ijc.11677
Hong, Y., Manoharan, I., Suryawanshi, A., Majumdar, T., Angus-Hill, M. L., Koni, P. A., et al. (2015). β-catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res. 75 (4), 656–665. doi:10.1158/0008-5472.CAN-14-2377
Jang, G.-B., Kim, J. Y., Cho, S. D., Park, K. S., Jung, J. Y., Lee, H. Y., et al. (2015). Blockade of Wnt/β-catenin signaling suppresses breast cancer metastasis by inhibiting CSC-like phenotype. Sci. Rep. 5, 12465. doi:10.1038/srep12465
Jin, P.-Y., Zheng, Z. H., Lu, H. J., Yan, J., Zheng, G. H., Zheng, Y. L., et al. (2019). Roles of β-catenin, TCF-4, and survivin in nasopharyngeal carcinoma: Correlation with clinicopathological features and prognostic significance. Cancer Cell Int. 19 (1), 48. doi:10.1186/s12935-019-0764-7
Khramtsov, A. I., Khramtsova, G. F., Tretiakova, M., Huo, D., Olopade, O. I., and Goss, K. H. (2010). Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 176 (6), 2911–2920. doi:10.2353/ajpath.2010.091125
Kim, H. C., Kim, H. J., and Kim, J. C. (2002). Reduced E-cadherin expression as a cause of distinctive signet-ring cell variant in colorectal carcinoma. J. Korean Med. Sci. 17 (1), 23–28. doi:10.3346/jkms.2002.17.1.23
Krishnamurthy, N., and Kurzrock, R. (2018). Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat. Rev. 62, 50–60. doi:10.1016/j.ctrv.2017.11.002
Lee, A., Ma, B. B. Y., Ng, W. T., and Chan, A. T. C. (2015). Management of nasopharyngeal carcinoma: Current practice and future perspective. J. Clin. Oncol. 33 (29), 3356–3364. doi:10.1200/JCO.2015.60.9347
Luo, W., Fang, W., Li, S., and Yao, K. (2012). Aberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinoma. Int. J. Cancer 131 (8), 1863–1873. doi:10.1002/ijc.27467
Monga, S. P. (2015). β-Catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 148 (7), 1294–1310. doi:10.1053/j.gastro.2015.02.056
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906. doi:10.1016/j.ijsu.2021.105906
Pang, Q., Hu, W., Zhang, X., and Pang, M. (2019). Wnt/β-Catenin signaling pathway-related proteins (DKK-3, β-catenin, and c-MYC) are involved in prognosis of nasopharyngeal carcinoma. Cancer biother. Radiopharm. 34 (7), 436–443. doi:10.1089/cbr.2019.2771
Peterson, J., Shea, B. J., O’Connell, A. M., Peterson, J., Welch, V., Losos, M., et al. (2011). The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute 2.1, 1–12.
Sanchez-Vega, F., Mina, M., Armenia, J., Chatila, W. K., Luna, A., La, K. C., et al. (2018). Oncogenic signaling pathways in the cancer genome atlas. Cell 173 (2), 321–337. doi:10.1016/j.cell.2018.03.035e10
Sauerbrei, W., Taube, S. E., McShane, L. M., Cavenagh, M. M., and Altman, D. G. (2018). Reporting recommendations for tumor marker prognostic studies (REMARK): An abridged explanation and elaboration. J. Natl. Cancer Inst. 110 (8), 803–811. doi:10.1093/jnci/djy088
Stransky, N., Egloff, A. M., Tward, A. D., Kostic, A. D., Cibulskis, K., Sivachenko, A., et al. (2011). The mutational landscape of head and neck squamous cell carcinoma. Science 333 (6046), 1157–1160. doi:10.1126/science.1208130
Sun, H., Liu, M., Wu, X., Yang, C., Zhang, Y., Xu, Z., et al. (2017). Overexpression of N-cadherin and β-catenin correlates with poor prognosis in patients with nasopharyngeal carcinoma. Oncol. Lett. 13 (3), 1725–1730. doi:10.3892/ol.2017.5645
Takeichi, M. (1990). Cadherins: A molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 59 (1), 237–252. doi:10.1146/annurev.bi.59.070190.001321
Thiery, J. P., and Sleeman, J. P. (2006). Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7 (2), 131–142. doi:10.1038/nrm1835
Valkenburg, K. C., Graveel, C. R., Zylstra-Diegel, C. R., Zhong, Z., and Williams, B. O. (2011). Wnt/β-catenin signaling in normal and cancer stem cells. Cancers (Basel) 3 (2), 2050–2079. doi:10.3390/cancers3022050
Wang, F. L., Guo, X., Yuan, T. Z., Cao, S. M., Rao, H. L., Hou, J. H., et al. (2009). Expression and clinical significance of Wnt-1 and beta-catenin in nasopharyngeal carcinoma. Ai Zheng 28 (1), 72–75.
Wang, W., Wen, Q., Luo, J., Chu, S., Chen, L., Xu, L., et al. (2017). Suppression of β-catenin nuclear translocation by CGP57380 decelerates poor progression and potentiates radiation-induced apoptosis in nasopharyngeal carcinoma. Theranostics 7 (7), 2134–2149. doi:10.7150/thno.17665
Wei, W. I., and Mok, V. W. (2007). The management of neck metastases in nasopharyngeal cancer. Curr. Opin. Otolaryngol. Head. Neck Surg. 15 (2), 99–102. doi:10.1097/MOO.0b013e3280148a06
Keywords: β-catenin, nasopharyngeal carcinoma, prognosis, meta-analysis, os
Citation: Zhou L-Q, Shen J-X, Zhou T, Li C-L, Hu Y and Xiao H-J (2022) The prognostic significance of β-Catenin expression in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Front. Genet. 13:953739. doi: 10.3389/fgene.2022.953739
Received: 26 May 2022; Accepted: 30 June 2022;
Published: 09 August 2022.
Edited by:
Yilin Zhang, The University of Chicago, United StatesReviewed by:
Edmund Ui-Hang Sim, Universiti Malaysia Sarawak, MalaysiaHongru Li, Xiangya Hospital, Central South University, China
Copyright © 2022 Zhou, Shen, Zhou, Li, Hu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Li Li, bGljaHVubGkyMDAwQHFxLmNvbQ==; Yao Hu, aHV5YW8xMjVAc2luYS5jb20=; Hong-Jun Xiao, eGhqZW50X3doeGhAaHVzdC5lZHUuY24=
†These authors have contributed equally to this work
 Liu-Qing Zhou
Liu-Qing Zhou Jin-Xiong Shen1†
Jin-Xiong Shen1† Hong-Jun Xiao
Hong-Jun Xiao