- 1Birth defect prevention and Control Technology Research Center, Medical Innovation Research Division, Chinese PLA General Hospital, Beijing, China
- 2Department of Pediatrics, Maternity and children’s Hospital of Tibet Autonomous Region, Tibet, China
- 3Department of pediatrics, The second people’s hospital of Tibet Autonomous Region, Tibet, China
- 4Department of Pediatrics, Chinese PLA General Hospital, Beijing, China
- 5Department of Women and children, Health commission of Tibet autonomous region, Tibet, China
- 6Department of cardiology, Beijing hospital, Beijing, China
Background: The purpose of the study was to investigate the levels of amino acids and acylcarnitines in newborns of the Tibet Autonomous Region for the first time and to provide an experimental basis for the diagnosis of genetic metabolic diseases.
Methods: We detected concentrations of 43 kinds of amino acids, acylcarnitines and succinylacetone in the dried blood spots of 18482 newborns using liquid chromatography tandem mass spectrometry and diagnose the case by gene sequencing. We compared the indexes between Tibet and our lab, where most data come from an inland area and Han Chinese people. Then we compared amino acid and acylcarnitine levels of seven regions in Tibet and explored their impact factors.
Results: We described the levels of amino acids and acylcarnitines in Tibet newborns using 95% confidence intervals. The distribution of amino acid and acylcarnitines were different in Tibet.
Conclusion: This study has contributed to filling in the blanks of Tibet newborn screening, which should be considered in the newborn metabolic disease screening in this area.
Introduction
Inherited metabolic diseases (IMD) are caused by genetic mutations that interfere with typical metabolism. This genetic mutation tends to result in a deficiency of an enzyme defect, leading to a lack of the enzyme’s products as well as an accumulation of the enzyme’s substrates, which then causes a series of clinical symptoms (Sahai and Marsden, 2009). At present, more than 1,000 types of IMD have been diagnosed (Ferreira and van Karnebeek, 2019). While individual metabolic disorders are rare, collectively, their incidence is approximately 1 in 1,000 (Hendriksz, 2009).
Owing to the high morbidity, mortality and strong risk of recurrence in affected families, neonatal IMD screening is an important element of modern preventive medicine as it allows the identification of both potential and asymptomatic infants as early as possible. Meanwhile, neonatal screening programs are an effective measure to reduce birth defects and improve the health of a population.
At present, the technique liquid chromatography-tandem mass chromatography (LC-MS/MS) has become the ideal analysis technique for IMD screening, due to its high sensitivity, signal-to-noise ratio, high specificity and high selectivity (Rashed et al., 1997) (Tu et al., 2010). In China, Shanghai and Zhejiang were the first to apply LC-MS/MS technology to newborn screening, and it is now being gradually introduced into the public health care network system in China (Gu et al., 2002) (Maitusong et al., 2012).
In 2020, the Chinese National Center for Clinical Laboratories (NCCL) launched a nationwide study to investigate the dynamic pattern of 35 MS/MS NBS biomarkers and establish accurate and robust reference intervals, in which 4,714,089 Chinese neonates were tested in participating centers/laboratories (He et al., 2021). However, there is little data on neonatal IMD screening in the Tibet Autonomous Region. This region is located in the southwest of the Qinghai-Tibet Plateau. With a mean elevation of more than 4,000 m, it is known as the ‘roof of the world’. The region covers an area of 122.84 million square kilometers, which accounts for about one eighth of the total area of China. The Tibet Autonomous Region has seven prefecture-level cities and had a population of approximately 3,656,000, a birth rate of 14.6‰ and around 53,400 newborns in 2020. Statistics from the population and family planning commission of the region in 2018 showed that the incidence of birth defects in Tibet was 10.2%, which was significantly higher than the national average 5.6% (Cui et al., 2015) (Zhuoma Solang, 2018). Among the permanent residents in Tibet Autonomous Region, 90.48% are Tibetan, 1.35% are other ethnic minorities, and 8.17% are Han nationality. Due to the special geographical environment and nomadic life style in Tibet, a special diet culture of Tibetan people has been formed, mainly highland barley and beef and mutton, with a single structure and few vegetables. At the same time, the newborn diet has special customs, including eating highland barley wine and yak milk after birth, and the Han people mainly use breast milk or milk powder. Therefore, the metabonomics of newborns in this area is relatively special. The ‘birth defect intervention project’ was started in this region in November 2015, meaning that neonatal IMD screening was carried out there for the first time.
We performed the detection and statistics of amino acid and acylcarnitine levels in neonates from the Tibet Autonomous, with the aim of providing a preliminary reference data in this region.
Materials and methods
Patients and samples collection
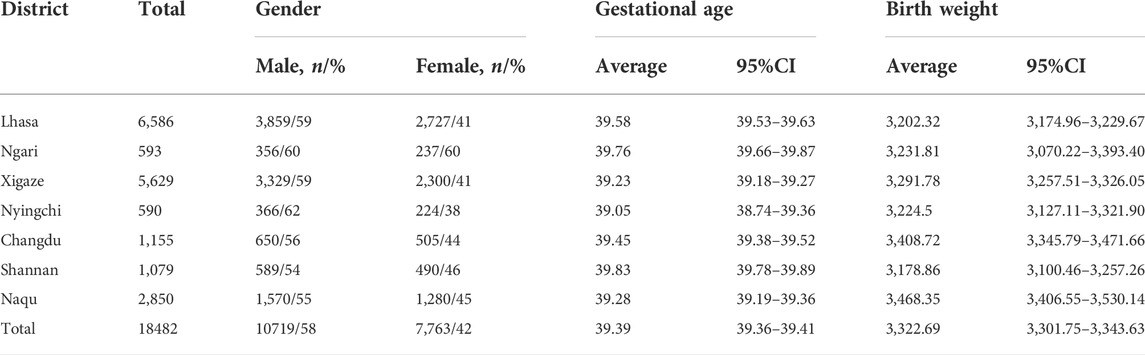
Samples were taken from 18482 live born infants from seven provinces of Tibet Autonomous Region between October 2015 and February 2021. Heel blood was collected after 72 h after the infants were born and fully breastfed, and then dropped onto filter paper (Whatman 903). Blood spot samples were placed in the shade to dry and were sealed and stored at -4°C prior to detection.
LC-MS/MS detection
Small molecule metabolites of dry blood spots were extracted using underivatized amino acids, carnitine and succinylacetone assay kits (PerkinElmer, Finland) according to the manual. Concentrations of 43 types of amino acid, carnitine and succinylacetone were detected by liquid chromatography and tandem mass spectrometry (Xevo TQ Detector, Waters, Milford, United States) and then analyzed by the software Masslynx (Waters Corporation, Milford, United States) and Neolynx. (Waters Corporation, Milford, United States).
Disease diagnose
The diseases were diagnosed by combining with clinical manifestation, laboratory examination and gene sequencing. Genomic DNA of peripheral blood leukocytes from patients and their parents was extracted using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). The genes of patients were sequencing by next-generation sequencing technology (Illumina Exome Panel, Illumina, United States), then the mutations of the parents were confirmed by Sanger sequencing. The data was analyzed and reported by software TGex (tgex.genecards.org).
Statistical analysis
Between-group comparisons were performed by independent-samples t-tests and ANOVA with Bonferroni posthoc multiple comparisons test for normally distributed data or Mann-Whitney U test for non-parametric sample distribution. All statistical analysis was performed using SPSS (version 21.0), and two-tail p < 0.05 was considered statistically significant. Graphs were generated using GraphPad Prism software (version 6.0).
Ethics
This study was approved by the Ethics Committee of the Chinese PLA General Hospital (No. S2018-025-01) and this trial has been verified by Chinese Clinical Trial Registry (No. ChiCTR1800016903). All participants provided informed written consent for sample collection, as well as permission for the samples’ use in research.
Results
The distribution of neonates from the tibet autonomous region
A total of 18482 neonatal screenings were conducted between 2015 and 2021, including 10719 males and 7,763 females. Some 99.67% of these babies (18421/18482) were Tibetan. The cases were distributed in seven prefecture-level cities in the Tibet Autonomous Region, including 6,586 in Lhasa, 2,850 in Naqu, 1,155 in Changdu, 1,079 in Shannan, 5,629 in Xigaze, 593 in Ngari and 590 in Nyingchi. The distribution of neonates from the Tibet Autonomous Region is depicted in Table 1.
Comparison to the accumulated laboratory results
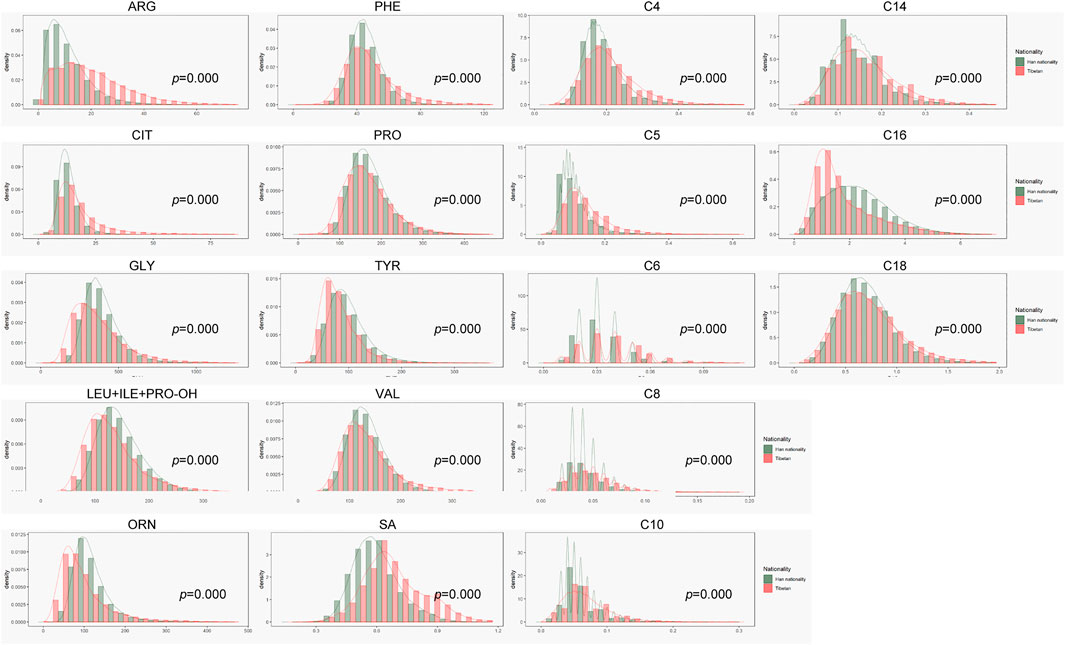
Before this study, there was little data on neonatal screening from the Tibet region and Tibetan people. As shown in Figure 1, concentration of many indexes in the Tibet region were different from our lab, where most data come from inland areas and Han Chinese people. Amino acids, ketones indexes, Arg, Phe, Cit and SA were higher, while Orn, Pro, Tyr, Gly, Leu + Ile + Pro-OH and Val were lower in Tibet than in inland areas. The concentration of medium and long chain acylcarnitine C6-C18 were higher than in inland areas. At the initial stage, we used the original cut-off value for screening, and the recall rate in Tibet was close to 6%. Obviously, the inland reference range was not very suitable for screening in Tibet, which would cause a higher false positive rate.
Description of levels of amino acids and acylcarnitines in tibet neonates
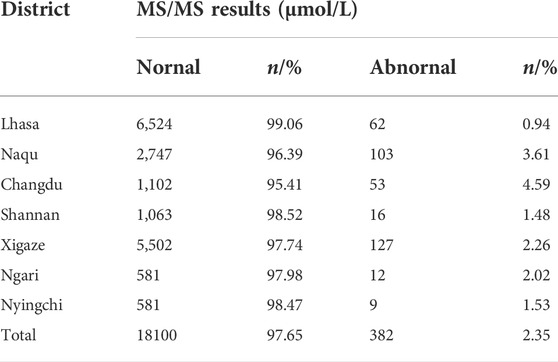
The levels of amino acids and acylcarnitines in neonates from the Tibet Autonomous Region were described based on the P0.5%∼P99.5% of the values (Table 2 and Supplemental Table S1). If we recall refer to these values, the recall rate of statistical screening was significantly reduced to 2.35%. These results, which can be seen in Table 3, are approximate to the recall rates of other regions. Based on the results of metabolite and gene detection, we diagnosed four cases with phenylketonuria (PKU) (Table 4). Among of the four PKU cases, case 1 and case 2 belong to the same family.
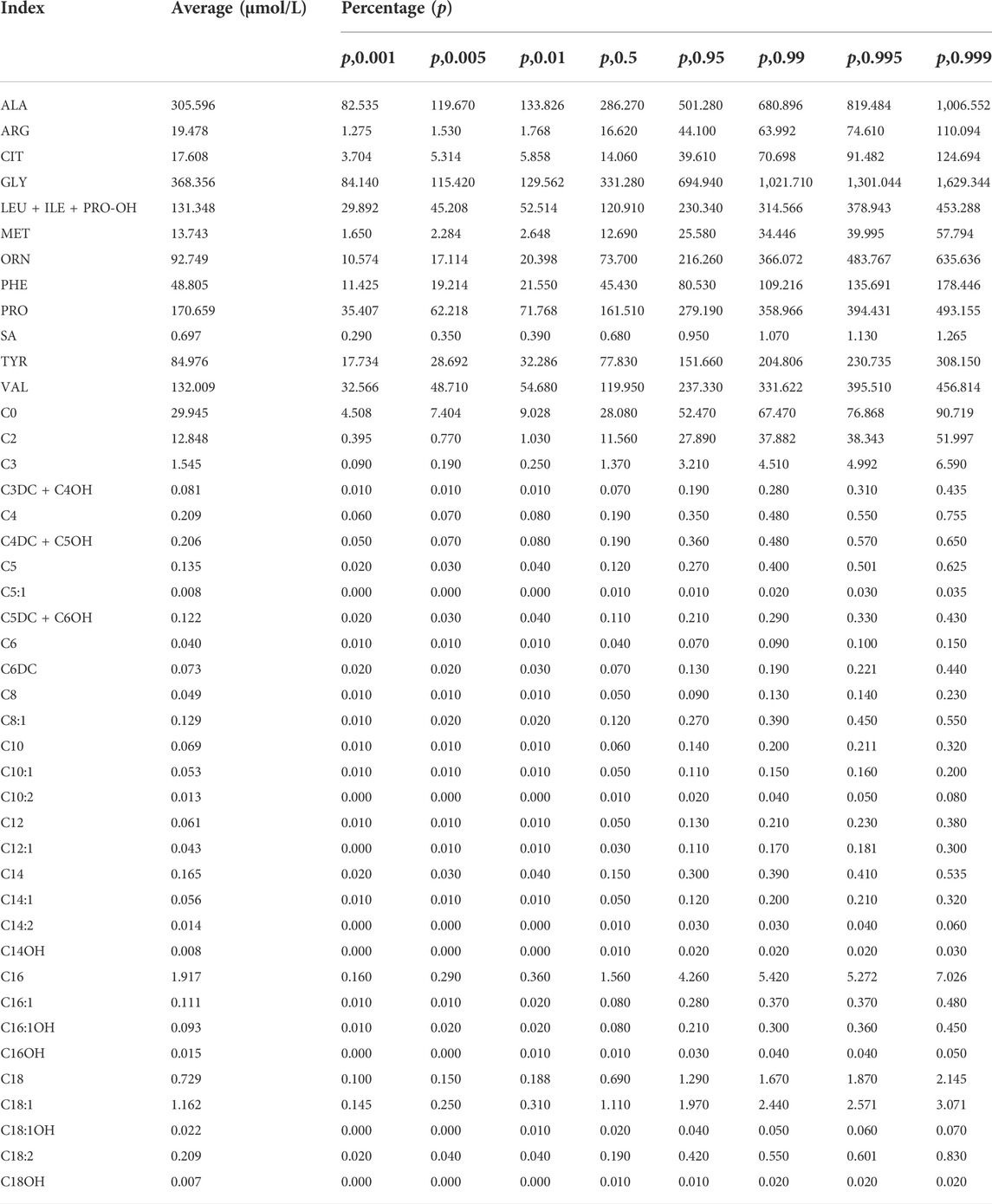
TABLE 2. Statistical results of amino acids and acylcarnitines of neonates from the Tibet Autonomous Region.
The effects of diet and altitude on small molecule metabolites
The screening area covers seven prefecture-level cities. A total of 99.67% of the babies (18421/18482) were Tibetan. Due to local limited medical conditions and nomadic customs, the mean blood collection time was 30 days after birth. Most babies had been given supplemental food from the birth, according to the local custom, which caused differences of small metabolites molecules between this area and inland areas. For example, babies in Xigaze region were fed by breast milk and zanba (highland barley) and were also given a small amount of barley wine and buttered tea. Therefore, we initially explored the impact of the special dietary habits in different areas of Tibet on the small molecule metabolites indicators.
As shown in Figure 2, many amino acids were significantly higher in Changdu and Naqu, where babies were fed with breast milk, cow (yak) milk and yoghurt, than in Lhasa, Xigaze and Shannan, where babies were fed with breast milk, zanba and yak butter tea. These results were replicated for lipometabolism levels. Although there was no significant difference in free carnitine C0 in the different regions, levels of short-chain acylcarnitine C2-C5 were higher in Changdu and Naqu and were lower in Ngari, Xigaze and Shannan. Levels of medium and long chain acylcarnitine C6-C18 were significantly higher in Lhasa, Changdu, Nyingchi and Naqu than in Ngari, Xigaze and Shannan.
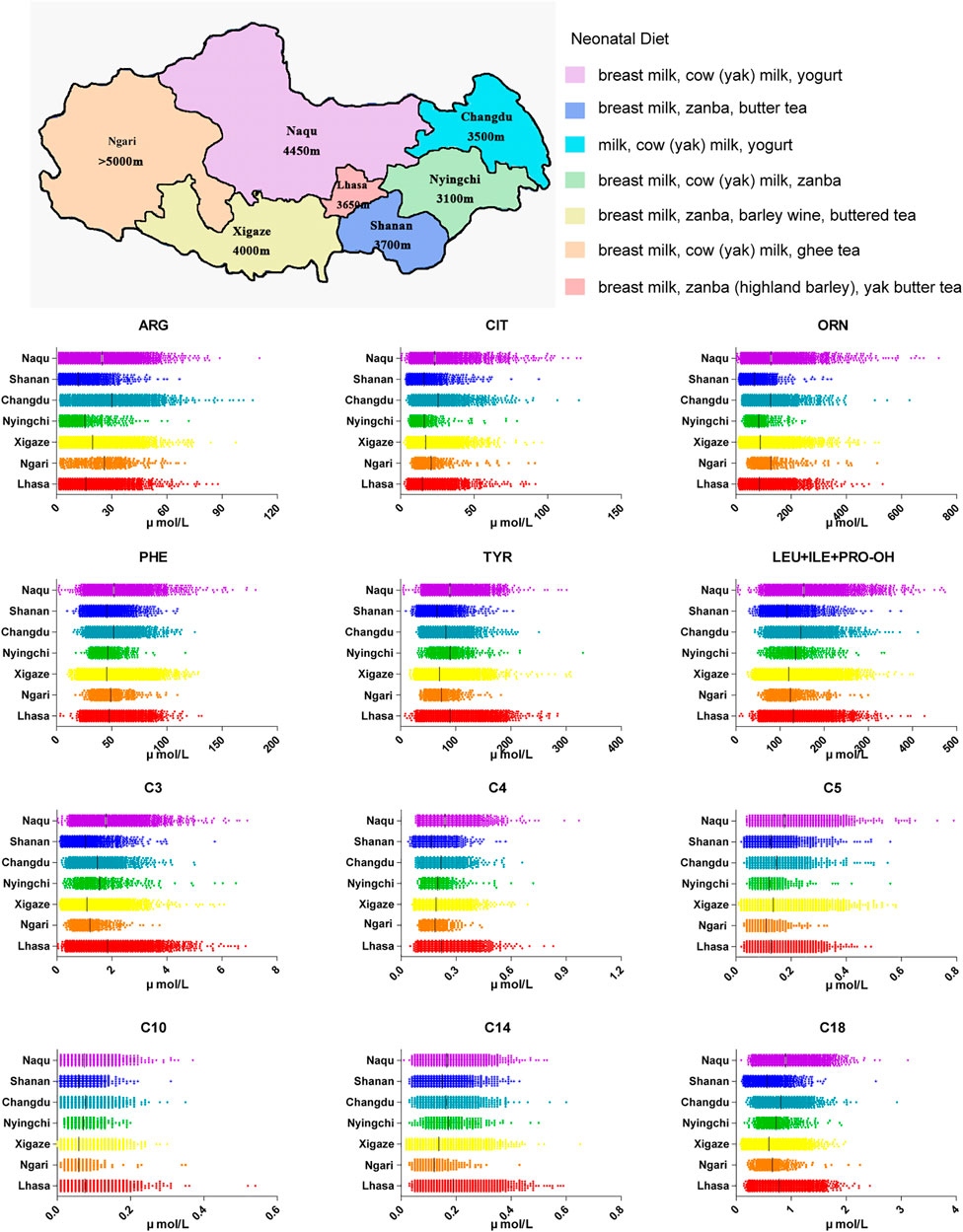
FIGURE 2. Neonatal diet in Tibet Autonomous Region as well as amino acids and acylcarnitine in different areas of Tibet.
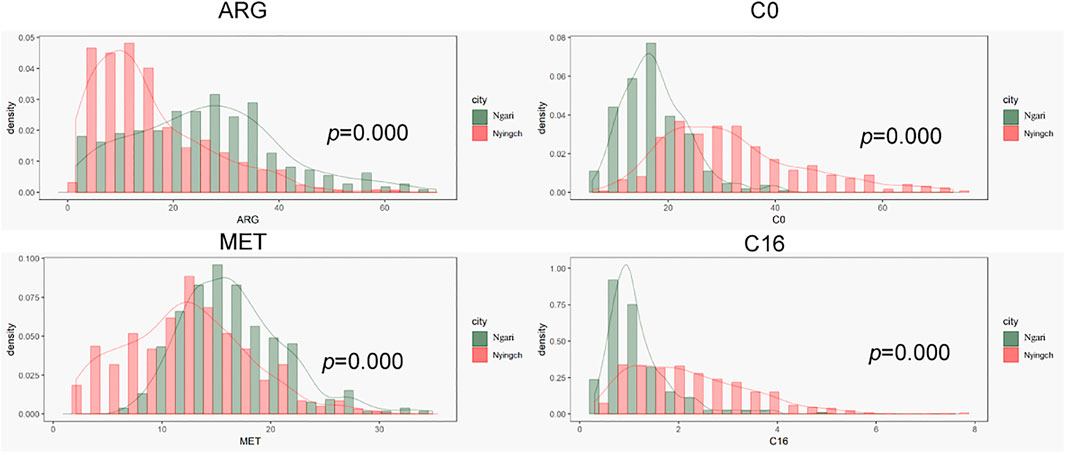
In order to explore effects of climate and altitude on tandem results, we compared the results of Nyingchi and Ngari, which have similar diets. Ngari is located in the southwest border of China and has a mean altitude of more than 5,000 m. Due to its high altitude, the climate is cold and dry, annual rainfall is relatively low, the difference between day and night temperature is large, and winter is long and cold. In contrast, Nyingchi, which is located in the southeast of Tibet, has a mean altitude of 3,100 m, while its lowest point is only 900 m, lower than other areas of Tibet. Due to the Indian ocean current, the climate is warm and comfortable. Annual rainfall is approximately 650 mm, the average annual temperature is 8.7°C, average annual sunshine is 2022.2 h, and the frost-free period is 180 days. Therefore, while the two regions have similar population composition and eating habits, their altitudes, climates and other factors are different.
As shown in Figure 3, amino acids that easily degraded were lower in Nyingchi and higher in Ngari region, such as Arg and Met. These results were consistent with previous reports that Arg and Met would fluctuate regularly, with low concentration in summer and high concentration in winter. Most acylcarnitine levels in the Ngari region were lower in Ngari, which may be related to the low basal metabolic rate in Ngari region.
Discussion
In the past 10 years, MS/MS was widely used for neonatal hereditary metabolic disease screening in China, improving the ability and efficiency of newborn screening (Huang et al., 2017). The NCCL initiated a large-scale NBS survey where 114 newborn screening laboratories/centers distributed in 29 provinces or municipalities participated in 2020, and 59 of the 114 (50.9%) MS/MS NBS facilities developed their own reference intervals based on their local samples (He et al., 2021). Until now, there was little data relating to the screening, diagnosis and monitoring of neonatal diseases in the Tibet Autonomous Region. This was for many reasons, such as nomadic and technological factors. Statistics from the population and family planning commission of the region in 2013 showed that incidences of birth defects in Tibet were significantly higher than the national mean. Therefore, early screening as well as timely and effective treatment are crucial for protecting the healthy growth of newborns from this area. Small molecular metabolites reflect the conditions of newborns and mothers, while regional differences in dietary habits, ethnic groups, climate and incidence rates lead to regional differences in indicators. Therefore, it is necessary to establish a reference range of the local population, as this can reduce the false positive rate and improve accuracy.
Here we investigated the amino acid and acylcarnitine levels in 18482 neonates from the Tibet Autonomous Region, providing preliminary data in this region for the first. There were some unsatisfactory factors, such as the time of blood collection and follow up. According to the technical specifications for neonatal disease screening (2010 version), blood sampling time is 72 h after birth, within 7 days, and full breastfeeding. However, in the actual situation, due to the inconvenient transportation, limited medical conditions, nomadic habits, special eating habits, and weak awareness of medical screening, the blood collection time in Tibet could not meet the requirements of the standard, with an average of 30 days. Therefore, the sampling time was one factor that affect the levels of small molecule metabolites. Considering the particularity of Tibet and the blank of data, we think it is very necessary to set up a new cut-off value according to the actual situation.
Our data were mostly obtained from domestic provinces, including Beijing, Guizhou, Sichuan, Hubei and so on. Compared with inland newborn, the levels of Arg, Cit were higher and the level of Orn was lower in Tibetan newborn. The three amino indicators are related to urea cycle disorder and citrin deficiency. In the urea cycle, Cit reacts with Asp to form arginosuccinate, which is decomposed to Arg; Arg is hydrolyzed to Orn and urea under the action of arginase (Ali et al., 2018) (Kiykim et al., 2018). The upstream and downstream metabolites of Arg were Cit and Orn, respectively. In the analysis of individual data, the three indicators did have similar trends, and the gene detection results for the cases with1.5∼2 times higher levels were negative. Therefore, these results were caused by metabolism in vivo and reflected the metabolic level in Tibetan newborns, which may be caused by special diet during newborn, such as high-protein food, like yak milk. Interestingly, in the metabolism pathway of Phe and Tyr, Tibetan healthy newborn showed a higher level of Phe, and lower level of Tyr. Phe, one of special indexes for diagnosing hyperphenylalaninemia, had a maximum value (95%CI) of 130 μmol/L in Tibet, which is higher than our regular cutoff 120 μmol/L (Li et al., 2015) (Liu et al., 2017). The results in Table 4 showed that the level of Phe in patients with PKU were much higher than the normal reference range. The phenomenon maybe related to folate metabolism. It has been reported that a folate-increasing allele of the SNP rs1801133 at the MTHFR locus has an increased frequency in the Tibetan population, more than in Han population, which is possibly a consequence of adaptation to high UV radiation (Yang et al., 2017). To illuminate the phenomenon, further research needed to be done. In terms of lipid metabolism, we observed that most acylcarnitine levels, especially medium and long chain acylcarnitine C6-C18, were higher than in inland area. C0 levels in babies from Tibet babies were lower than others. Newborns from the inland area may have a single diet source (milk or milk powder), but newborns in Tibet, due to local customs, have a varied diet in the first 3 days. The distribution of amino acid and acylcarnitine was different in Tibet and reflect the difference of human metabolism activity in different regions.
We detected accuracy and inaccuracy before we performed the study to ensure that results were reliable. Our laboratory reported results of the neonatal genetic metabolic disease screening (tandem mass spectrometry technology) to the National Center for Examination and took part in interventricular quality assessment twice every year, which ensures that results are valid. The preliminary reference range is only applicable to the current neonatal disease screening in Tibet. After the popularization of the new screening education, the improvement of medical conditions, more standardized blood collection and the expansion of sample size, more suitable cutoff value is required in the future.
Conclusion
By examining data from more than 18,000 newborns, this study investigated the amino acid and acylcarnitine levels in neonates of the Tibet Autonomous for the first time. Data reflect the metabolic level of newborns in Tibet and fill in gaps in the region. This study suggested an independent cut-off value of neonatal screening should be established in different regions, especially in high altitude areas, which will greatly improve the diagnostic efficiency for neonatal screening.
Data availability statement
The data presented in the study are deposited in the National Population Health Science Data Center repository (https://www.ncmi.cn/), doi:10.12213/11.A005Y.202109.1871.V1.0.
Ethics statement
This study was approved by the Ethics Committee of the Chinese PLA General Hospital (No. S2018-025-01). Written informed consent to participate in this study was provided by the participants’ legal guardian.
Author contributions
The authors declare that all of them have participated actively in the study and all meet the requirements the authorship. CZ, DD, and YT designed the study. DD, YM, DT, DN, PD, and JL took charge of organizing nurse to recruit the patients and collect the samples. TJ and YS preformed the clinical test. YM and JC were responsible for clinical recall and diagnosis. CZ and YT analyzed the data and wrote the manuscript.
Funding
This research was supported by the National Key Research and Development Plan (SQ2020YFF0426581, 2017YFC1001700), National Natural Science Foundation of China (81501821) and Beijing Nova program (Z181100006218038).
Acknowledgments
Authors thank the support and salvage from the March of Dimes Birth Defects Foundation of China. The authors are also very grateful to the health committee of Nagqu district, Xigaze district, Shannan district, Qamdo district, Ngari district, Nyingchi district for their energetic support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.941938/full#supplementary-material
References
Ali, E. Z., Zakaria, Y., Mohd Radzi, M. A., Ngu, L. H., and Jusoh, S. A. (2018). Mutation study of Malaysian patients with ornithine transcarbamylase deficiency: Clinical, molecular, and bioinformatics analyses of two novel missense mutations of the OTC gene. Biomed. Res. Int. 2018, 4320831. doi:10.1155/2018/4320831
Cui, Z., Wu, F., Li, Y., Xiao, D., and Chen, R. (2015). Survey on birth defects related knowledge among women of childbearing age in Tibetan autonomous region. Zhonghua Yu Fang. Yi Xue Za Zhi 49, 576–578.
Ferreira, C. R., and van Karnebeek, C. D. M. (2019). Inborn errors of metabolism. Handb. Clin. Neurol. 162, 449–481. doi:10.1016/B978-0-444-64029-1.00022-9
Gu, X., Zhou, J., and Ye, J. (2002). Neonatal screening for congenital adrenal hyperplasia in Shanghai areas. Zhonghua Yu Fang. Yi Xue Za Zhi 36, 16–18.
He, F., Yang, R., Huang, X., Tian, Y., Pei, X., Bohn, M. K., et al. (2021). Reference standards for newborn screening of metabolic disorders by tandem mass spectrometry: A nationwide study on millions of Chinese neonatal populations. Front. Mol. Biosci. 8, 719866. doi:10.3389/fmolb.2021.719866
Hendriksz, C. J. (2009). Inborn errors of metabolism for the diagnostic radiologist. Pediatr. Radiol. 39, 211–220. doi:10.1007/s00247-008-1072-x
Huang, X., Zhang, Y., Hong, F., Zheng, J., Yang, J., Tong, F., et al. (2017). Screening for amino acid metabolic disorders of newborns in Zhejiang province:prevalence, outcome and follow-up. Zhejiang Da Xue Xue Bao Yi Xue Ban. 46, 233–239.
Kiykim, E., Zubarioglu, T., Cansever, M. S., Celkan, T., Haberle, J., and Aktuglu Zeybek, A. C. (2018). Coagulation disturbances in patients with argininemia. Acta Haematol. 140, 221–225. doi:10.1159/000493678
Li, N., Jia, H., Liu, Z., Tao, J., Chen, S., Li, X., et al. (2015). Molecular characterisation of phenylketonuria in a Chinese mainland population using next-generation sequencing. Sci. Rep. 5, 15769. doi:10.1038/srep15769
Liu, N., Huang, Q., Li, Q., Zhao, D., Li, X., Cui, L., et al. (2017). Spectrum of PAH gene variants among a population of Han Chinese patients with phenylketonuria from northern China. BMC Med. Genet. 18, 108. doi:10.1186/s12881-017-0467-7
Maitusong, R., Japaer, R., Zhao, Z. Y., Yang, R. L., Huang, X. L., and Mao, H. Q. (2012). Newborn screening in Zhejiang, China. Chin. Med. J. 125, 702–704. doi:10.3760/cma.j.issn.0366-6999.2012.04.026
Rashed, M. S., Bucknall, M. P., Little, D., Awad, A., Jacob, M., Alamoudi, M., et al. (1997). Screening blood spots for inborn errors of metabolism by electrospray tandem mass spectrometry with a microplate batch process and a computer algorithm for automated flagging of abnormal profiles. Clin. Chem. 43, 1129–1141. doi:10.1093/clinchem/43.7.1129
Sahai, I., and Marsden, D. (2009). Newborn screening. Crit. Rev. Clin. Lab. Sci. 46, 55–82. doi:10.1080/10408360802485305
Tu, W., Song, X., Dai, F., and Ho, J. J. (2010). Application of liquid chromatography-tandem mass spectrometry (LC-MS/MS) in screening of high risk children with inherited metabolic diseases in northern China. J. Pediatr. Endocrinol. Metab. 23, 1245–1252. doi:10.1515/jpem.2010.198
Yang, J., Jin, Z. B., Chen, J., Huang, X. F., Li, X. M., Liang, Y. B., et al. (2017). Genetic signatures of high-altitude adaptation in Tibetans. Proc. Natl. Acad. Sci. U. S. A. 114, 4189–4194. doi:10.1073/pnas.1617042114
Zhuoma Solang, M. D. (2018). Status of 322 Tibeatan newborns with birth defects in Lhasa. Sci. Technol. Tibet 09, 36–37.
Glossary
IMD Inherited metabolic diseases
Ala Alanine
Arg Arginine
Cit Citrulline
Gly glycine
Leu Leucine
Ile isoleucine
Pro-OH hydroxyprolin
Met methionine
Orn ornithine
Phe phenylalanine
Pro proline
Asp aspartate
SA Succinyl acetone
C0 free carnitine
C2 Acetyl carnitine
C3 Propionyl carnitine
C3-DC Malonyl carnitine
C4 Butyryl/isobutyryl carnitine
C4-DC Methylmalonyl/succinyl carnitine
C5:1 Tiglyl carnitine;
C5 Isovaleryl carnitine;
C5-DC Glutaryl carnitine;
C5-OH 3-Hydroxy-isovaleryl carnitine
C6 Hexanoyl carnitine
C6-DC Adipoyl carnitine
C8:1 Octenoyl carnitine
C8 Octanoyl carnitine
C10:1 Decenoyl carnitine
C10 Decanoyl carnitine
C12:1 Dodecenoyl carnitine
C12 Dodecanoyl carnitine
C12-OH 3-Hydroxydodecanoyl carnitine
C14:2 Tetradecadienoyl carnitine
C14:1 Tetradecenoyl carnitine
C14 Tetradecanoyl carnitine
C14:1-OH 3-Hydroxy-tetradecenoyl carnitine
C14-OH 3-Hydroxy-tetradecanoyl carnitine
C16:1 Palmitoleoyl carnitine
C16 Palmitoyl carnitine
C16:1-OH 3-Hydroxy-palmitoleoyl carnitine
C16-OH 3-Hydroxy-palmitoyl carnitine
C18:2 Linoleyl carnitine
C18:1 Oleoyl carnitine
C18 Stearoyl carnitine
C18:2-OH three- Hydroxy-linoleyl carnitine
C18:1-OH 3-Hydroxy-oleoyl carnitine
Keywords: amino acid, acyl carnitine, newborn screening, Tibet region, reference interval, tandem mass spectrometry
Citation: Zhang C, Dha D, Cheng Y, Ma Y, Meng Y, Tse D, Ngawang D, Dekyi P, Jiang T, Shu Y, Cui J, Li J and Tian Y (2022) A preliminary investigation of amino acid and acylcarnitine levels in neonates from the Tibet autonomous. Front. Genet. 13:941938. doi: 10.3389/fgene.2022.941938
Received: 14 July 2022; Accepted: 06 September 2022;
Published: 26 September 2022.
Edited by:
María L. Couce, Complejo Hospitalario Universitario de Santiago, SpainReviewed by:
Jianxiang Liao, Shenzhen Children’s Hospital, ChinaJose A. Cocho, Hospital Universitario de Santiago, Spain
Copyright © 2022 Zhang, Dha, Cheng, Ma, Meng, Tse, Ngawang, Dekyi, Jiang, Shu, Cui, Li and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yaping Tian, VGlhbnlwQDMwMWhvc3BpdGFsLmNvbS5jbg==;
†ORCID: Chunyan Zhang, orcid.org/0000-0002-7582-2207; Yaping Tian, orcid.org/0000-0002-8245-3745
 Chunyan Zhang
Chunyan Zhang Drun Dha2
Drun Dha2 Yaping Tian
Yaping Tian