- 1Department of Dermatology, Peking University Shenzhen Hospital, Shenzhen, Guangdong, China
- 2Shenzhen Key Laboratory for Translational Medicine of Dermatology, Shenzhen Peking University—The Hong Kong University of Science and Technology Medical Center, Shenzhen, Guangdong, China
- 3School of Clinical Medicine, Health Science Center, Shenzhen University, Shenzhen, Guangdong, China
- 4Department of Dermatology, The Second Affiliated Hospital, Anhui Medical University, Hefei, Anhui, China
- 5Anhui Provincial Institute of Translational Medicine, Hefei, Anhui, China
- 6Marcus Institute for Aging Research at Hebrew SeniorLife, Boston, MA, United States
- 7Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
- 8Department of Internal Medicine, University of New Mexico Health Sciences Center, Albuquerque, NM, United States
- 9Department of Dermatology, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
Psoriasis is an immune-mediated chronic inflammatory skin disease caused by a combination of environmental incentives, polygenic genetic control, and immune regulation. The inflammation-related gene absent in melanoma 2 (AIM2) was identified as a susceptibility gene for psoriasis. AIM2 inflammasome formed from the combination of AIM2, PYD-linked apoptosis-associated speck-like protein (ASC) and Caspase-1 promotes the maturation and release of inflammatory cytokines such as IL-1β and IL-18, and triggers an inflammatory response. Studies showed the genetic and epigenetic associations between AIM2 gene and psoriasis. AIM2 gene has an essential role in the occurrence and development of psoriasis, and the inhibitors of AIM2 inflammasome will be new therapeutic targets for psoriasis. In this review, we summarized the roles of the AIM2 gene and AIM2 inflammasome in pathogenesis and treatment of psoriasis, hopefully providing a better understanding and new insight into the roles of AIM2 gene and AIM2 inflammasome in psoriasis.
1 Introduction
Psoriasis is an inflammatory skin disease caused by a combination of environmental incentives, polygenic genetic control, and immune regulation (Branisteanu et al., 2022). The worldwide average prevalence of psoriasis is 2% (Samotij et al., 2020), and the disease is often characterized by recurrence and incurability. It is often accompanied by complex comorbidities such as cardiovascular and autoimmune diseases (Gao et al., 2021), bringing great physical and mental harm to patients, and causing a substantial social–economic burden. The pathogenesis and novel therapies for psoriasis are hot research topics. In the past two decades, many susceptibility genes/loci and epigenetic modification factors of psoriasis have been identified (Nedoszytko et al., 2020). The inflammation-related gene absent in melanoma 2 (AIM2) was identified as a susceptibility gene for psoriasis (Zuo et al., 2015), and the function of AIM2 gene and its role in psoriasis were explored (Ciążyńska et al., 2021; Liang et al., 2021). In this review, we systemically summarized genetic and epigenetic associations between AIM2 gene and psoriasis, discussed the roles of AIM2 gene and AIM2 inflammasome in the pathogenesis of psoriasis, and provided a better understanding of AIM2 inflammasome as a promising therapeutic target for psoriasis.
2 AIM2 Gene and AIM2 Inflammasome
2.1 AIM2 Gene
DeYoung et al. (1997) reported a novel gene, AIM2, also known as PYHIN4, located on chromosome 1q23.1-q23.2. The protein AIM2, encoded by the AIM2 gene is a member of the IFI20X/IFI16 family, consisting of a C-terminal HIN domain and an N-terminal pyrin domain (PYD). RNA sequencing (RNA-seq) of 27 different human tissues showed that the expression level of AIM2 gene in descending order are: lymph nodes, appendix, and spleen. AIM2 gene is also expressed at a relatively low level in the skin compared with the top three tissues (Fagerberg et al., 2014). The expression of AIM2 gene can be promoted by interferon-gamma (IFN-γ) (Tang H. et al., 2021). AIM2 gene with various functions in the occurrence and development of diseases, the most common function is to initiate the assembly process of the AIM2 inflammasome. AIM2 response to dsDNA and then induce AIM2-dependent release of IL-18 and IL-1β, which plays a critical role as a trigger of autoimmune diseases, including psoriasis (Dombrowski et al., 2011), systemic lupus erythematosus (Shin et al., 2019), primary Sjogren’s syndrome (Vakrakou et al., 2020). AIM2 gene plays two-sided roles in tumorigenesis or anti-tumorigenesis in different tumors (Choubey et al., 2000). AIM2 gene with the tumor-promoting effects in nonsmall-cell lung cancer (NSCLC) via the inflammasome-dependent manner and regulation of mitochondrial dynamics (Qi et al., 2020). On the contrary, AIM2 gene is required to restrain the progression of colon cancer through an inflammasome-independent manner, proliferation control of intestinal stem cells, and the regulation of gut microbiota, suggesting that AIM2 gene plays a protective role in colorectal cancer (Wilson et al., 2015; Zhang Z. et al., 2017).
2.2 AIM2 Inflammasome
In 2009, the composition of the AIM2 inflammasome was reported (Fernandes-Alnemri et al., 2009; Hornung et al., 2009). AIM2 can recognize double-stranded DNA (dsDNA) and interacts with N-terminal PYD. PYD-linked apoptosis-associated speck-like protein (ASC) induces the recruitment of Caspase-1 by the caspase recruitment domain (CARD) of ASC to form the AIM2 inflammasome. AIM2 inflammasome can induce the maturation and release of inflammatory factors such as IL-1β and IL-18, triggering an inflammatory response (Wang et al., 2019). Studies over the past decades have shown that AIM2 inflammasome plays a vital role in many kinds of inflammatory diseases, immune diseases, and cancers (Man et al., 2016; Kumari et al., 2020; Zhu H. et al., 2021).
The assembly and activation of AIM2 inflammasome and subsequent inflammatory response was shown in Figure 1. The primary role of AIM2 is to initiate the assembly process of the inflammasome. ASC acts as an inflammasome adaptor protein, connecting upstream AIM2 and downstream Caspase-1. Caspase-1 is the effector protein of AIM2 inflammasome, and the activated caspase-1 leads to proteolytic cleavage of IL-1β and IL-18. The massive release of downstream inflammatory cytokines (IL-1β and IL-18) directly affects the host’s innate immune regulation to infection and injury, induces acute and chronic inflammatory responses, and participates in the occurrence and development of these diseases, such as skin diseases, chronic kidney disease, cardiovascular diseases, neuronal diseases, and diabetes mellitus (Sharma et al., 2019).
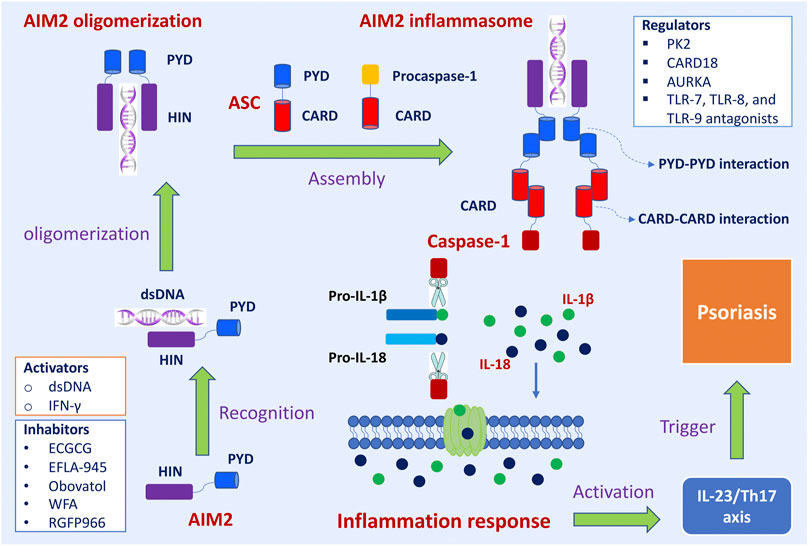
FIGURE 1. The assembly and activation of AIM2 inflammasome and subsequently regulatory and trigger pathways in psoriasis. Upon sensing viral DNA, self-derived dsDNA, and cytosolic bacterium, the HIN domain of AIM2 directly recognizes dsDNA in a sequence-independent manner, which triggers the assembly of the AIM2 oligomerization. The PYD domain of AIM2 interacts with the PYD of a recruiting adapter protein ASC, resulting in a high polymer complex AIM2 inflammasome. Inactive procaspases-1 are recruited into AIM2 inflammasome via the CARD-CARD interaction. When the main components of the inflammasome are connecting and the active inflammasome is formed, it directly recruits and cleaves pro-caspase1 into active caspase-1, which proteolytically activates the pro-inflammatory cytokines IL-1β and IL-18. These inflammatory cytokines directly induce inflammatory responses and participate in the occurrence and development of diseases. The active IL-1β and IL-18 involve in IL23/Th17 pathway and then induce many kinds of chemokines and inflammatory cytokines, which trigger the development of psoriasis. Interferon-gamma (IFN-γ) promoted the expression of the AIM2 gene. Epigallocatechin gallate (EGCG), EFLA-945, obovatol, withaferin A (WFA), and RGFP966 are inhibitory effects on AIM2. Prokineticin 2 (PK2), caspase recruitment domain family member 18 (CARD18), aurora kinase A (AURKA), TLR-7, TLR-8, and TLR-9 antagonists are regulators of AIM2 inflammasome signaling pathway.
3 Genetic and Epigenetic Associations Between AIM2 Gene and Psoriasis
3.1 Genetic Associations Between AIM2 Gene and Psoriasis
In the early stage, sequencing results showed that the coding region of AIM2 gene with a high frequency of frameshift and missense mutations in primary high-level microsatellite instability (MSI-H) colon cancers and cell lines (Woerner et al., 2007). In 2015, an exome-wide association study in large-scale individuals was performed to investigate the coding variants in psoriasis. AIM2 gene was firstly identified as a susceptibility gene for psoriasis at the genome-wide level, this gene locates at an important topological position in the gene–gene interaction network. In addition, Zuo et al. (2015) predicted that a variant (rs2276405) in AIM2 gene affects AIM2 protein structure (Glu32Lys). The chemical properties of Glu (the chemical nature of Glu residue is acidic) and Lys (the chemical nature of Lys residue is alkaline) are completely opposite, which may destabilize the alpha-helix motif. Researchers investigated the correlation between the genetic pattern of AIM2 gene polymorphism and the psoriasis phenotype (Li et al., 2016). Genotype and allele distribution of AIM2 gene showed that allele A was the minor allele and G was the risk allele. Genetic pattern analysis showed that the dominant pattern was the best genetic pattern for AIM2 gene polymorphism loci. Compared with the controls, the distribution of the dominant inheritance pattern was higher in psoriasis area and severity index (PASI) score ≤20 than that of PASI score >20, and family history of negative patients is more significantly different statistically than positive family history.
3.2 Epigenetic Associations Between AIM2 Gene and Psoriasis
Epigenetic associations between AIM2 gene and diseases have also been studied. Hypermethylation of the AIM2 promoter conferred insensitivity to IFN-γ-induced AIM2 expression of MSI-H colon cancer cell lines, demonstrating the inactivation of AIM2 was regulated by epigenetic factors (Woerner et al., 2007). In 2016, an epigenome association study (EWAS) of psoriasis was conducted in the Chinese Han population, and the results showed that three CpG sites (cg17217296, cg17515347, and cg07195224) in the promoter region of AIM2 gene were significantly associated with psoriasis (Li et al., 2016). Assay for transposase-accessible chromatin using sequencing (ATAC-seq) was used to explore the landscape of chromatin accessibility of psoriatic skin tissue (PP) and nonpsoriatic skin tissue (PN) from patients with psoriasis and normal skin tissue (NN) from healthy individuals, the results show that AIM2 gene promoter region was specifically more accessible in PP, and contained a CpG site (cg07195224), which was previously reported to be significantly hypomethylated in psoriasis (Tang L. et al., 2021). In addition, it was observed that the intensity of the promoter-associated peak of AIM2 was negatively correlated with the methylation level of cg07195224 but positively correlated with AIM2 mRNA expression level. Meanwhile, the methylation level of cg07195224 was strongly and negatively associated with AIM2 mRNA expression level (Tang L. et al., 2021). Studies will be needed to explore the correlation between epigenetic regulation and AIM2 gene expression in the future and to further clarify its regulating effect in the pathogenesis of psoriasis.
4 Roles of AIM2 Gene and AIM2 Inflammasome in the Pathogenesis of Psoriasis
4.1 AIM2 Inflammasome Mediated Inflammatory Response Involved in Psoriasis
The roles of AIM2 gene in the pathogenesis of psoriasis have been studied (Table 1). AIM2 inflammasome activity may represent a potential trigger for the occurrence and development of inflammatory diseases. Kopfnagel et al. (2011) found that the AIM2 inflammasome is active in human keratinocytes, triggering IL-1β secretion with an important role in inflammatory processes. In cultured keratinocytes, the expression of AIM2 gene was induced by INF-γ; cytoplasmic DNA can trigger the release of IL-1β through the AIM2 inflammasome (Dombrowski et al., 2011). In CD14+ and CD16+ monocyte subsets in the blood of patients with psoriasis, the AIM2 gene expression level was significantly higher than that of the control group, indicating that AIM2 inflammasome exists in the immune cell subsets in the peripheral blood of psoriasis patients in a stimulated state (Verma et al., 2021).
The expression of AIM2 gene in lesional skin from psoriasis patients was explored in several studies (Dombrowski et al., 2011; de Koning et al., 2012; Šahmatova et al., 2017; Yuan et al., 2020). Compared with skin tissues from healthy individuals, abundant cytoplasmic DNA and increased AIM2 gene expression were detected in psoriatic skin lesions (Dombrowski et al., 2011). Šahmatova et al. (2017) also identified that the AIM2 gene was expressed at an increased level in psoriatic skin lesions. Yuan et al. (2020) found that the formation of neutrophil extracellular trapping net (NET) structure and the high expression of AIM2 gene can be detected in the epidermis of skin lesions from psoriasis patients. NET may promote the expression of AIM2 gene by activating keratinocytes, and the secretion of IL-1β accelerates the inflammatory process of psoriasis. In addition, the expression of AIM2 gene is also significantly upregulated in several inflammatory skin disorders, including contact dermatitis, venous ulcers, atopic dermatitis, and experimental wounds (de Koning et al., 2012). The expression of AIM2 gene was dynamic in human tissues and primary cells, with restricted expression in Langerhans cell and melanocytes of the normal epidermis, but a strong upregulation in subpopulations of epidermal keratinocytes under inflammatory conditions (de Koning et al., 2012).
The IL-23/Th17 axis plays a central role in the development of psoriasis (Hawkes et al., 2017), some biologics (IL-17 antagonists: Secukinumab, Ixekizumab, and Brodalumab; IL-23 antagonists: Risankizumab, Guselkumab, and Tildrakizumab) focus on this signaling pathway have been used for the treatment of moderate-severe psoriasis (Sharma et al., 2022). AIM2 induces AIM2-dependent release of IL-18 and IL-1β; furthermore, the active IL-1β and IL-18 involve in IL23/Th17 pathway and then induce many kinds of chemokines and inflammatory cytokines (Ciążyńska et al., 2021). Taken together, the aforementioned studies indicate that the activation of the AIM2 inflammasome may be a potential trigger for the development of psoriasis, and the inflammatory response mediated by the AIM2 inflammasome plays an important role in the initial onset and persistence of psoriasis.
4.2 Factors Regulate AIM2 Inflammasome Signaling Pathway in Psoriasis
Recent studies have found that certain factors regulate the AIM2 inflammasome signaling pathway and participate in the pathogenesis of psoriasis, including prokineticin 2 (PK2), caspase recruitment domain family member 18 (CARD18), aurora kinase A (AURKA), TLR-7, TLR-8, and TLR-9 antagonists.
4.2.1 PK2
PK2 is a psoriasis-specific factor that is highly expressed in mouse and human psoriatic skins, but is not significantly expressed in other autoimmune diseases, such as inflammatory bowel diseases, atherosclerosis, and diabetes; PK2 significantly increases the expression of Caspase-1, and also strongly up-regulates the AIM2 inflammasome signaling pathway in monocytic THP-1 cells, which is engaged by AIM2 to promote the synthesis and secretion of proinflammatory cytokine IL-1β (He et al., 2016). Thus, PK2 regulates the AIM2 inflammasome signaling pathway involved in the pathogenesis of psoriasis.
4.2.2 CARD18
CARD18 is highly expressed in psoriatic noninvolved epidermis compared to healthy skin epidermis (Szabó et al., 2014). CARD18 involves AIM2 inflammasome-mediated keratinocyte functions and modifies the inflammatory process in keratinocytes. Silencing of CARD18 in keratinocyte cells treated with poly (dA:dT) resulted in a significant decrease in AIM2 gene expression and significantly reduced Caspase-1 mRNA expression (Göblös et al., 2016), which indicates that CARD18 may contribute to the fine-tuning of innate immune processes.
4.2.3 AURKA
AURKA is a member of the serine/threonine kinases family, which plays a critical role in the suppression of autophagy (Zhang S. et al., 2017), and is elevated in lesional psoriatic tissue (Liu et al., 2011). A recent study found that AURKA promotes the occurrence and development of psoriatic inflammation by blocking autophagy-mediated suppression of the AIM2 inflammasome (Tang H. et al., 2021).
4.2.4 TLR-7, TLR-8, and TLR-9 Antagonists
Immune modulatory oligonucleotides and small molecular weight compounds, IMO-3100, IMO-8400, and IMO-9200 target TLR-7, TLR-8, and TLR-9, respectively. These are under clinical investigation for their effectiveness in the treatment of psoriasis. Chemical compounds, such as AS-2444697, PF-05387252, PF-05388169, PF-06650833, ML120B, and PHA-408, can inhibit TLR signaling (Gao et al., 2017). Jiang et al. (2013) evaluated an antagonist of TLR-7, TLR-8, and TLR-9 as a therapeutic agent in an IL-23-induced psoriasis model in C57BL/6 mice. Treatment with an antagonist reduced the expression of inflammasome components (including NLRP3, AIM2, and antimicrobial peptides) in the dermis, which indicated that targeting TLR-7, TLR-8, and TLR-9 may provide a method for neutralizing the multiple inflammatory pathways that are involved in psoriasis.
4.3 How Fra-1 Regulates AIM2 and What’s the Regulatory Effect in Psoriasis?
Recently, bioinformatics prediction and luciferase reporter assay showed that Fra-1 targeted binding to 363 bp and 57 bp upstream of the AIM2 gene transcription start site (Tang L et al., 2021). Fra-1 is encoded by the FOSL1 gene and plays a role in cell proliferation and differentiation, gene expression and regulation, and the occurrence and progression of psoriasis (Talotta et al., 2020). FOSL1 knockdown inhibited IL-22-induced proliferation and enhanced keratinocyte apoptosis, whereas IL-22 stimulation and FOSL1 overexpression further enhanced keratinocyte proliferation (Meng et al., 2021). The expression of FOSL1 was significantly increased in lesional psoriatic skin and positively correlated with the PASI score (Sobolev et al., 2011). The aforementioned findings show that the high expression of FOSL1 in lesional psoriatic skin is one of the markers of the pathological activity of psoriasis and that Fra-1 plays an important role in the pathogenesis of psoriasis. However, Fra-1 needs to be further investigated on how it regulates the expression of AIM2 in psoriasis, which is still unclear.
4.4 The Expression of AIM2 Gene Can Be a Biomarker to Distinguish Different Subtypes of Psoriasis?
The expression level of AIM2 gene in CD4+ tissue-resident memory T (CD4+ Trm) cells was measured in the patients with acute cutaneous lupus erythematosus (ACLE), subacute CLE (SCLE), localized discoid lupus erythematosus (localized DLE), psoriasis, and other inflammatory skin diseases (Zhao et al., 2022). The results showed that AIM2 expression in CD4+ Trm cells was significantly lower in patients with ACLE than in localized DLE and SCLE. In psoriasis patients, CD4+ Trm cells were mainly located in the epidermis of skin lesions. The AIM2 total fluorescence intensity in CD4+ Trm cells in patients with SCLE and localized DLE were higher than in patients with psoriasis. Compared to ACLE with localized DLE and/or SCLE, the receiver operating characteristic curve for AIM2 expression in CD4+ Trm cells had different sensitivities and specificities at different cutoff values. Therefore, AIM2 expression in skin CD4+ Trm cells can be a significant indicator of distinguishing patients with ACLE from those patients with SCLE and localized DLE. Psoriasis is also divided into subtypes with different clinical manifestations, including psoriasis vulgaris, erythrodermic psoriasis, pustular psoriasis, and psoriatic arthritis (Zhu C. et al., 2021). It is worthy to further investigate whether AIM2 expression can be a biomarker to distinguish different subtypes of psoriasis. A valuable study will be carried out in the future to explore this scientific question.
5 AIM2 Inflammasome Is a Promising Therapeutic Target for Psoriasis
5.1 AIM2 Correlates With Therapeutic Efficacy of Psoriasis
Recent studies have shown that the expression of AIM2 gene can be a biomarker to predict the benefit of therapy in patients with epithelial ovarian cancer (Hsu et al., 2021), melanoma (Fukuda et al., 2021), systemic lupus erythematosus (Yang et al., 2021) and heart failure (Onódi et al., 2021). These studies implicate its potential utility in predicting clinical treatment outcomes. Gong et al. calculated innate immune cell proportion in psoriatic skin by utilizing microarray data, results show that AIM2 gene was negatively associated with resting mast cells but positively associated with activated dendritic cells (Gong and Wang, 2021). Brodalumab and Ustekinumab are used to treat moderate to severe plaque psoriasis, and most patients can get better treatment effects. Interestingly, AIM2 gene was positively associated with the therapeutic efficacy of Brodalumab and negatively associated with Ustekinumab treatment response (Gong and Wang, 2021), which provide novel clues for clinical decisions on treatment for psoriasis. In addition, KEGG analysis shows pathways involving RIG-I-like receptor signaling, NOD-like receptor signaling, toll-like receptor signaling, and cytosolic DNA-sensing pathways, these pathways were significantly enriched and positively correlated with AIM2 gene (Gong and Wang, 2021).
5.2 LL-37 Neutralizes Inflammation Mediated by AIM2 Inflammasome
The antimicrobial peptide LL-37 (also known as cathelicidin antimicrobial peptide, CAMP) is overexpressed in psoriatic lesions and acts as the critical factor that mediates plasmacytoid dendritic cells (pDCs) activation in psoriasis (Lande et al., 2007). AIM2 inflammasomes activate pro-inflammatory processes and trigger IL-1β secretion in psoriatic lesions. However, IL-1β secretion was completely abolished when LL-37 and DNA were delivered together into keratinocytes. This may suggest that LL-37 can translocate into the cytosol of psoriatic keratinocytes and specifically neutralizes cytosolic DNA, thus acting as an inhibitor of AIM2 inflammasome activation (Dombrowski and Schauber, 2012). Topical treatment with vitamin D analogs decreases inflammation and pro-inflammatory cytokines, but strongly increases cathelicidin’s expression (Lebwohl et al., 2004; Peric et al., 2009). However, repeated treatments with narrowband-UVB (NB-UVB) decreased skin inflammation in patients with psoriasis but increased vitamin D serum levels and the expression of cutaneous cathelicidin (Vähävihu et al., 2010). Thus, established therapies targeting the vitamin D pathway reduce inflammatory responses while increasing epidermal antimicrobial peptide expression in psoriatic lesions, the anti-inflammatory effect of LL-37 on the AIM2 inflammasome pathway may account for these observed effects (Reinholz et al., 2012). LL-37 can modulate the pathogenic response to nucleic acids and be helpful for the development of anti-inflammatory therapies.
5.3 Potential AIM2 Inflammasome Inhibitors for Psoriasis Treatment
At present, there are some AIM2 inflammasome inhibitors that have been explored to treat inflammatory diseases, including epigallocatechin gallate (EGCG), EFLA-945, obovatol, withaferin A (WFA), and RGFP966.
5.3.1 EGCG
EGCG is the most abundant main polyphenol component of green tea, and the alloy moiety of catechins possesses the most biological activities, including angiogenesis and anti-inflammatory effects (Kondo et al., 2002). The structure of EGCG and related information are shown in Supplementary Figure S1. EGCG inhibits the transfection of NF-κB and AP-1 to downregulate the expression of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and decreases the production of inflammatory factors (Nagai et al., 2002). Studies have shown that EGCG attenuates AIM2-induced IL-1β secretion by inhibiting inflammasome IFN-γ secretion and dA:dT-induced ASC oligomerization in neonatal human epidermal keratinocytes (Yun et al., 2015).
5.3.2 EFLA-945
EFLA-945 is an extract from red grapevine leaf in traditional medicine in Japan (Rabe et al., 2011) and over-the-counter drugs in Europe and Japan (Hoshino et al., 2018) for its anti-inflammatory properties and circulatory benefits. Resveratrol (Supplementary Figure S2) and peonidin 3-O-glucoside (Supplementary Figure S3) are the major phytochemicals of EFLA 945. Study results showed that EFLA-945 could limit the entry of DNA into THP-1-derived macrophages, thereby inhibiting cytoplasmic DNA-dependent ASC and activation of the AIM2 inflammasome. Resveratrol and peonidin 3-O-glucoside are two major phytochemicals of EFL-945 that mediate this inhibition (Chung et al., 2020). Furthermore, EFLA-945 attenuated the associated pro-inflammatory response in localized skin lesions of an imiquimod-induced psoriasis-like mouse model, indicating that EFLA-945 may be beneficial for the treatment of psoriasis.
5.3.3 Obovatol
Obovatol (Supplementary Figure S4), a bisphenol chemical originating from Magnolia obovata, affects the AIM2 inflammasome by inhibiting the formation of ASC pyroptosome and the generation of mitochondrial ROS. Furthermore, obovatol has been used as a traditional treatment for inflammatory diseases (Kim et al., 2019) and neuroinflammation (Ock et al., 2010). Furthermore, obovatol disrupted inflammasome activation’s initiation step, inhibited the transcription of inflammatory cytokines, and decreased serum IL-1β elevation in response to sodium urate crystals in mice (Kim et al., 2019). Whether obovatol has a therapeutic effect on psoriasis will be further studied.
5.3.4 WFA
WFA is an extract from the medicinal plant Withania somnifera and has various biological activities, including acting as an anti-inflammatory, angiogenesis, and anticancer (Mohan et al., 2004). The structure of EGCG and related information are shown in Supplementary Figure S5. Studies show that WFA regulates AIM2 inflammasome and Caspase-1 in THP-1 polarized macrophages; however, WFA treatment of M2 macrophages inhibits TGF-β compared with M1 secretion (Ngoungoure and Owona, 2019). Currently, further research is needed to determine whether WFA can affect the occurrence and progression of psoriasis by inhabiting the AIM2 inflammasome pathway.
5.3.5 RGFP966
Histone deacetylases 3 (HDAC3) modulates the acetylation of histone and non-histone proteins. RGFP966 (Supplementary Figure S6) is a selective inhibitor of HDAC3 (Zhang et al., 2020). Specifically, RGFP966 regulates the inflammatory process in stroke (Chen et al., 2012) and brain damage (Zhang et al., 2020). Lipopolysaccharide (LPS) stimulation caused time-dependent increases of HDAC3 and AIM2 inflammasome in primary cultured microglia. AIM2 gene was spatiotemporally regulated by RGFP966, which was confirmed in an experimental mouse stroke model. RGFP966 can enhance STAT1 acetylation and decrease STAT1 phosphorylation, which may partially explain the negative regulatory effect of AIM2 gene by RGFP966 (Zhang et al., 2020). This study indicated that RGFP966 alleviated the inflammatory process by regulating the AIM2 inflammasome. The further therapeutic effects of RGFP966 on psoriasis by regulating the AIM2 inflammasome will be investigated in the future.
6 The Disadvantages of Targeting AIM2 Gene as a Potential Therapeutic Target
AIM2 gene act as a double-edged sword in the pathogenesis of some autoimmune diseases and cancers. The increased and decreased expression of AIM2 gene with different roles in the development of different diseases. AIM2 gene plays the tumor-suppressive role in HPV-infected cervical cancer (So et al., 2018), breast cancer (Yoon et al., 2015), squamous cell carcinoma (Farshchian et al., 2017); while AIM2 gene is regarded as a protective factor in melanoma (DeYoung et al., 1997; de Koning et al., 2014) and colorectal cancer (Wilson et al., 2015; Zhang Z. et al., 2017). Intriguingly, AIM2 gene with two contrasting roles in different models or different disease stages of hepatocellular carcinoma (Martínez-Cardona et al., 2018; Shi et al., 2019). AIM2 gene as a therapeutic target may with different effects on different diseases, therefore we should consider the advantages and disadvantages of targeting AIM2 gene. For instance, when we use AIM2 inflammasome inhibitors or drugs to treat psoriatic patients also with cancer, in the future, we need to pay attention to its impact on cancer. Taken together, the advantages and disadvantages of targeting AIM2 gene should be considered at the same time.
7 Conclusion and Perspectives
In this review, we provided an overview of the research progress on the effect and role of AIM2 gene and AIM2 inflammasome in psoriasis. We hope this provides further potential therapeutic targets for psoriasis. Although some potential inhibitors of the AIM2 inflammasome were explored, no AIM2 inflammasome target drug has been used in the clinical treatment of psoriasis. Therefore, further researches need to focus on the development of new drugs for psoriasis.
Author Contributions
CS, BY, and YZ designed this study; JW, CS, JG, CH, WZ, CC, and XS collected and summarized the data from the literature search; JW, JG, and CS prepared the manuscript; JW, JG, CS, SJ, RK, CH, CC, WZ, YZ, and BY revised this manuscript. All authors contributed to the article and approved the submitted version.
Fundings
This study was supported by grants from the Research Foundation of Peking University Shenzhen Hospital (No: JCYJ2020004), Shenzhen Sanming Project (No: SZSM201812059), Shenzhen Key Medical Discipline Construction Fund (No: SZXK040), Guangdong Basic and Applied Basic Research Foundation (No: 2021A1515110097), National Natural Science Foundation of China (No: 82103726), Natural Science Foundation of Anhui Province (No: 2008085QH427), and Research Fund of Anhui Institute of Translational Medicine (No: 2021ZHYX-C48).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.929162/full#supplementary-material
References
Branisteanu, D., Cojocaru, C., Diaconu, R., Porumb, E., Alexa, A., Nicolescu, A., et al. (2022). Update on the Etiopathogenesis of Psoriasis (Review). Exp. Ther. Med. 23, 201. doi:10.3892/etm.2022.11124
Chen, Y.T., Zang, X.F., Pan, J., Zhu, X.L., Chen, F., Chen, Z.B., et al. (2012). Expression Patterns of Histone Deacetylases in Experimental Stroke and Potential Targets for Neuroprotection. Clin. Exp. Pharmacol. Physiol. 39, 751–758. doi:10.1111/j.1440-1681.2012.05729.x
Choubey, D., Walter, S., Geng, Y., and Xin, H. (2000). Cytoplasmic Localization of the Interferon-Inducible Protein that Is Encoded by theAIM2(absent in Melanoma) Gene from the 200-gene Family. FEBS Lett. 474, 38–42. doi:10.1016/s0014-5793(00)01571-4
Chung, I.C., Yuan, S.N., OuYang, C.N., Hu, S.I., Lin, H.C., Huang, K.Y., et al. (2020). EFLA 945 Restricts AIM2 Inflammasome Activation by Preventing DNA Entry for Psoriasis Treatment. Cytokine 127, 154951. doi:10.1016/j.cyto.2019.154951
Ciążyńska, M., Olejniczak-Staruch, I., Sobolewska-Sztychny, D., Narbutt, J., Skibińska, M., and Lesiak, A. (2021). The Role of NLRP1, NLRP3, and AIM2 Inflammasomes in Psoriasis: Review. Int. J. Mol. Sci. 22, 5898. doi:10.3390/ijms22115898
de Koning, H. D., Bergboer, J. G. M., van den Bogaard, E. H., van Vlijmen-Willems, I. M. J. J., Rodijk-Olthuis, D., Simon, A., et al. (2012). Strong Induction of AIM2 Expression in Human Epidermis in Acute and Chronic Inflammatory Skin Conditions. Exp. Dermatol. 21, 961–964. doi:10.1111/exd.12037
de Koning, H. D., van Vlijmen-Willems, I. M. J. J., Zeeuwen, P. L. J. M., Blokx, W. A. M., and Schalkwijk, J. (2014). Absent in Melanoma 2 Is Predominantly Present in Primary Melanoma and Primary Squamous Cell Carcinoma, but Largely Absent in Metastases of Both Tumors. J. Am. Acad. Dermatol. 71, 1012–1015. doi:10.1016/j.jaad.2014.06.012
DeYoung, K. L., Ray, M. E., Su, Y. A., Anzick, S. L., Johnstone, R. W., Trapani, J. A., et al. (1997). Cloning a Novel Member of the Human Interferon-Inducible Gene Family Associated with Control of Tumorigenicity in a Model of Human Melanoma. Oncogene 15, 453–457. doi:10.1038/sj.onc.1201206
Dombrowski, Y., Peric, M., Koglin, S., Kammerbauer, C., Göß, C., Anz, D., et al. (2011). Cytosolic DNA Triggers Inflammasome Activation in Keratinocytes in Psoriatic Lesions. Sci. Transl. Med. 3, 82ra38. doi:10.1126/scitranslmed.3002001
Dombrowski, Y., and Schauber, J. (2012). Cathelicidin LL-37: a Defense Molecule with a Potential Role in Psoriasis Pathogenesis. Exp. Dermatol. 21, 327–330. doi:10.1111/j.1600-0625.2012.01459.x
Fagerberg, L., Hallström, B. M., Oksvold, P., Kampf, C., Djureinovic, D., Odeberg, J., et al. (2014). Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-Based Proteomics. Mol. Cell. Proteomics 13, 397–406. doi:10.1074/mcp.M113.035600
Farshchian, M., Nissinen, L., Siljamäki, E., Riihilä, P., Piipponen, M., Kivisaari, A., et al. (2017). Tumor Cell-specific AIM2 Regulates Growth and Invasion of Cutaneous Squamous Cell Carcinoma. Oncotarget 8, 45825–45836. doi:10.18632/oncotarget.17573
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J., and Alnemri, E. S. (2009). AIM2 Activates the Inflammasome and Cell Death in Response to Cytoplasmic DNA. Nature 458, 509–513. doi:10.1038/nature07710
Fukuda, K., Okamura, K., Riding, R. L., Fan, X., Afshari, K., Haddadi, N.-S., et al. (2021). AIM2 Regulates Anti-tumor Immunity and Is a Viable Therapeutic Target for Melanoma. J. Exp. Med. 218, e20200962. doi:10.1084/jem.20200962
Gao, J., Shen, X., Ko, R., Huang, C., and Shen, C. (2021). Cognitive Process of Psoriasis and its Comorbidities: From Epidemiology to Genetics. Front. Genet. 12, 735124. doi:10.3389/fgene.2021.735124
Gao, W., Xiong, Y., Li, Q., and Yang, H. (2017). Inhibition of Toll-like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front. Physiol. 8, 508. doi:10.3389/fphys.2017.00508
Göblös, A., Danis, J., Vas, K., Bata-Csörgő, Z., Kemény, L., and Széll, M. (2016). Keratinocytes Express Functional CARD18, a Negative Regulator of Inflammasome Activation, and its Altered Expression in Psoriasis May Contribute to Disease Pathogenesis. Mol. Immunol. 73, 10–18. doi:10.1016/j.molimm.2016.03.009
Gong, X., and Wang, W. (2021). Profiles of Innate Immune Cell Infiltration and Related Core Genes in Psoriasis. BioMed Res. Int. 2021, 6656622. doi:10.1155/2021/6656622
Hawkes, J. E., Chan, T. C., and Krueger, J. G. (2017). Psoriasis Pathogenesis and the Development of Novel Targeted Immune Therapies. J. Allergy Clin. Immunol. 140, 645–653. doi:10.1016/j.jaci.2017.07.004
He, X., Shen, C., Lu, Q., Li, J., Wei, Y., He, L., et al. (2016). Prokineticin 2 Plays a Pivotal Role in Psoriasis. EBioMedicine 13, 248–261. doi:10.1016/j.ebiom.2016.10.022
Hornung, V., Ablasser, A., Charrel-Dennis, M., Bauernfeind, F., Horvath, G., Caffrey, D. R., et al. (2009). AIM2 Recognizes Cytosolic dsDNA and Forms a Caspase-1-Activating Inflammasome with ASC. Nature 458, 514–518. doi:10.1038/nature07725
Hoshino, T., Muto, N., Tsukada, S., Nakamura, T., and Maegawa, H. (2018). European Ethnopharmaceuticals for Self-Medication in Japan: Review Experience of Vitis vinifera L., Folium Extract and Vitex Agnus-Castus L., Fructus Extract as OTC Drugs. Medicines 5, 3. doi:10.3390/medicines5010003
Hsu, P.C., Chao, T.K., Chou, Y.C., Yu, M.H., Wang, Y.C., Lin, Y.H., et al. (2021). AIM2 Inflammasome in Tumor Cells as a Biomarker for Predicting the Treatment Response to Antiangiogenic Therapy in Epithelial Ovarian Cancer Patients. J. Clin. Med. 10, 4529. doi:10.3390/jcm10194529
Jiang, W., Zhu, F.G., Bhagat, L., Yu, D., Tang, J. X., Kandimalla, E. R., et al. (2013). A Toll-like Receptor 7, 8, and 9 Antagonist Inhibits Th1 and Th17 Responses and Inflammasome Activation in a Model of IL-23-induced Psoriasis. J. Invest. Dermatol. 133, 1777–1784. doi:10.1038/jid.2013.57
Kim, J., Ahn, H., Han, B. C., Shin, H., Kim, J.C., Jung, E.M., et al. (2019). Obovatol Inhibits NLRP3, AIM2, and Non-canonical Inflammasome Activation. Phytomedicine 63, 153019. doi:10.1016/j.phymed.2019.153019
Kondo, T., Ohta, T., Igura, K., Hara, Y., and Kaji, K. (2002). Tea Catechins Inhibit Angiogenesis In Vitro, Measured by Human Endothelial Cell Growth, Migration and Tube Formation, through Inhibition of VEGF Receptor Binding. Cancer Lett. 180, 139–144. doi:10.1016/S0304-3835(02)00007-1
Kopfnagel, V., Wittmann, M., and Werfel, T. (2011). Human Keratinocytes Express AIM2 and Respond to dsDNA with IL-1β Secretion. Exp. Dermatol. 20, 1027–1029. doi:10.1111/j.1600-0625.2011.01382.x
Kumari, P., Russo, A. J., Shivcharan, S., and Rathinam, V. A. (2020). AIM2 in Health and Disease: Inflammasome and beyond. Immunol. Rev. 297, 83–95. doi:10.1111/imr.12903
Lande, R., Gregorio, J., Facchinetti, V., Chatterjee, B., Wang, Y.H., Homey, B., et al. (2007). Plasmacytoid Dendritic Cells Sense Self-DNA Coupled with Antimicrobial Peptide. Nature 449, 564–569. doi:10.1038/nature06116
Lebwohl, M., Menter, A., Koo, J., and Feldman, S. R. (2004). Combination Therapy to Treat Moderate to Severe Psoriasis. J. Am. Acad. Dermatol. 50, 416–430. doi:10.1016/j.jaad.2002.12.002
Li, G., Tao, L., Xu, Y., Dou, J., Wang, W., Zheng, X., et al. (2016). Genetic Model Analysis of AIM2 Single Nucleotide Polymorphism in Han Chinese Patients with Psoriasis Vulgaris. Acta Univ. Med. Anhui 51, 1486-1490. doi:10.19405/j.cnki.issn1000-1492.2016.10.020
Liang, D. W., Wang, L. Y., and Zhu, X. F. (2021). Research Advances in Correlation between Absent in Melanoma 2 and Psoriasis. J. Clin. Med. Pract. 25, 122-124. doi:10.7619/jcmp.20200520
Liu, Y., Luo, W., and Chen, S. (2011). Comparison of Gene Expression Profiles Reveals Aberrant Expression of FOXO1, Aurora A/B and EZH2 in Lesional Psoriatic Skins. Mol. Biol. Rep. 38, 4219–4224. doi:10.1007/s11033-010-0544-x
Man, S. M., Karki, R., and Kanneganti, T.D. (2016). AIM2 Inflammasome in Infection, Cancer, and Autoimmunity: Role in DNA Sensing, Inflammation, and Innate Immunity. Eur. J. Immunol. 46, 269–280. doi:10.1002/eji.201545839
Martínez-Cardona, C., Lozano-Ruiz, B., Bachiller, V., Peiró, G., Algaba-Chueca, F., Gómez-Hurtado, I., et al. (2018). AIM2 Deficiency Reduces the Development of Hepatocellular Carcinoma in Mice. Int. J. Cancer 143, 2997–3007. doi:10.1002/ijc.31827
Meng, J., Chen, F.R., Yan, W.J., and Lin, Y.K. (2021). MiR-15a-5p Targets FOSL1 to Inhibit Proliferation and Promote Apoptosis of Keratinocytes via MAPK/ERK Pathway. J. Tissue Viability 30, 544–551. doi:10.1016/j.jtv.2021.08.006
Mohan, R., Hammers, H., Bargagna-mohan, P., Zhan, X., Herbstritt, C., Ruiz, A., et al. (2004). Withaferin A Is a Potent Inhibitor of Angiogenesis. Angiogenesis 7, 115–122. doi:10.1007/s10456-004-1026-3
Nagai, K., Jiang, M. H., Hada, J., Nagata, T., Yajima, Y., Yamamoto, S., et al. (2002). (−)-Epigallocatechin Gallate Protects against NO Stress-Induced Neuronal Damage after Ischemia by Acting as an Anti-oxidant. Brain Res. 956, 319–322. doi:10.1016/S0006-8993(02)03564-3
Nedoszytko, B., Szczerkowska-Dobosz, A., Stawczyk-Macieja, M., Owczarczyk-Saczonek, A., Reich, A., Bartosińska, J., et al. (2020). Pathogenesis of Psoriasis in the “omic” Era. Part II. Genetic, Genomic and Epigenetic Changes in Psoriasis. Postepy Dermatol. Alergol. 37, 283–298. doi:10.5114/ada.2020.96243
Ngoungoure, F. P., and Owona, B. A. (2019). Withaferin A Modulates AIM2 Inflammasome and Caspase-1 Expression in THP-1 Polarized Macrophages. Exp. Cell Res. 383, 111564. doi:10.1016/j.yexcr.2019.111564
Ock, J., Han, H. S., Hong, S. H., Lee, S. Y., Han, Y.-M., Kwon, B.M., et al. (2010). Obovatol Attenuates Microglia-Mediated Neuroinflammation by Modulating Redox Regulation. Br. J. Pharmacol. 159, 1646–1662. doi:10.1111/j.1476-5381.2010.00659.x
Onódi, Z., Ruppert, M., Kucsera, D., Sayour, A. A., Tóth, V. E., Koncsos, G., et al. (2021). AIM2-driven Inflammasome Activation in Heart Failure. Cardiovasc. Res. 117, 2639–2651. doi:10.1093/cvr/cvab202
Peric, M., Koglin, S., Dombrowski, Y., Gross, K., Bradac, E., Büchau, A., et al. (2009). Vitamin D Analogs Differentially Control Antimicrobial Peptide/“Alarmin” Expression in Psoriasis. PloS One 4, e6340. doi:10.1371/journal.pone.0006340
Qi, M., Dai, D., Liu, J., Li, Z., Liang, P., Wang, Y., et al. (2020). AIM2 Promotes the Development of Non-small Cell Lung Cancer by Modulating Mitochondrial Dynamics. Oncogene 39, 2707–2723. doi:10.1038/s41388-020-1176-9
Rabe, E., Stücker, M., Esperester, A., Schäfer, E., and Ottillinger, B. (2011). Efficacy and Tolerability of a Red-Vine-Leaf Extract in Patients Suffering from Chronic Venous Insufficiency - Results of a Double-Blind Placebo-Controlled Study. Eur. J. Vasc. Endovascular Surg. 41, 540–547. doi:10.1016/j.ejvs.2010.12.003
Reinholz, M., Ruzicka, T., and Schauber, J. (2012). Cathelicidin LL-37: an Antimicrobial Peptide with a Role in Inflammatory Skin Disease. Ann. Dermatol. 24, 126–135. doi:10.5021/ad.2012.24.2.126
Šahmatova, L., Sügis, E., Šunina, M., Hermann, H., Prans, E., Pihlap, M., et al. (2017). Signs of Innate Immune Activation and Premature Immunosenescence in Psoriasis Patients. Sci. Rep. 7, 7553. doi:10.1038/s41598-017-07975-2
Samotij, D., Nedoszytko, B., Bartosińska, J., Batycka-Baran, A., Czajkowski, R., Dobrucki, I., et al. (2020). Pathogenesis of Psoriasis in the "omic" Era. Part I. Epidemiology, Clinical Manifestation, Immunological and Neuroendocrine Disturbances. Postepy Dermatol. Alergol. 37, 135–153. doi:10.5114/ada.2020.94832
Sharma, A., Upadhyay, D. K., Gupta, G. D., Narang, R. K., and Rai, V. K. (2022). IL-23/Th17 Axis: A Potential Therapeutic Target of Psoriasis. Curr. Drug. Res. Rev. 14, 24–36. doi:10.2174/2589977513666210707114520
Sharma, B. R., Karki, R., and Kanneganti, T. D. (2019). Role of AIM2 Inflammasome in Inflammatory Diseases, Cancer and Infection. Eur. J. Immunol. 49, 1998–2011. doi:10.1002/eji.201848070
Shi, X., Wang, L., Ren, L., Li, J., Li, S., Cui, Q., et al. (2019). Dihydroartemisinin, an Antimalarial Drug, Induces Absent in Melanoma 2 Inflammasome Activation and Autophagy in Human Hepatocellular Carcinoma HepG2215 Cells. Phytotherapy Res. 33, 1413–1425. doi:10.1002/ptr.6332
Shin, J. I., Lee, K. H., Joo, Y. H., Lee, J. M., Jeon, J., Jung, H. J., et al. (2019). Inflammasomes and Autoimmune and Rheumatic Diseases: A Comprehensive Review. J. Autoimmun. 103, 102299. doi:10.1016/j.jaut.2019.06.010
So, D., Shin, H. W., Kim, J., Lee, M., Myeong, J., Chun, Y. S., et al. (2018). Cervical Cancer Is Addicted to SIRT1 Disarming the AIM2 Antiviral Defense. Oncogene 37, 5191–5204. doi:10.1038/s41388-018-0339-4
Sobolev, V. V., Zolotorenko, A. D., Soboleva, A. G., Elkin, A. M., Il’ina, S. A., Serov, D. N., et al. (2011). Effects of Expression of Transcriptional Factor AP-1 FOSL1 Gene on Psoriatic Process. Bull. Exp. Biol. Med. 150, 632–634. doi:10.1007/s10517-011-1208-0
Szabó, K., Bata-Csörgő, Z., Dallos, A., Bebes, A., Francziszti, L., Dobozy, A., et al. (2014). Regulatory Networks Contributing to Psoriasis Susceptibility. Acta Derm. Venerol. 94, 380–385. doi:10.2340/00015555-1708
Talotta, F., Casalino, L., and Verde, P. (2020). The Nuclear Oncoprotein Fra-1: a Transcription Factor Knocking on Therapeutic Applications' Door. Oncogene 39, 4491–4506. doi:10.1038/s41388-020-1306-4
Tang, H., Tang, X., Guo, Z., Cheng, H., Zheng, X., Chen, G., et al. (2021). AURKA Facilitates the Psoriasis-Related Inflammation by Impeding Autophagy-Mediated AIM2 Inflammasome Suppression. Immunol. Lett. 240, 98–105. doi:10.1016/j.imlet.2021.10.004
Tang, L., Wang, M., Shen, C., Wen, L., Li, M., Wang, D., et al. (2021). Assay for Transposase-Accessible Chromatin Using Sequencing Analysis Reveals a Widespread Increase in Chromatin Accessibility in Psoriasis. J. Invest. Dermatol. 141, 1745–1753. doi:10.1016/j.jid.2020.12.031
Vähävihu, K., Ala-Houhala, M., Peric, M., Karisola, P., Kautiainen, H., Hasan, T., et al. (2010). Narrowband Ultraviolet B Treatment Improves Vitamin D Balance and Alters Antimicrobial Peptide Expression in Skin Lesions of Psoriasis and Atopic Dermatitis. Br. J. Dermatol. 163, 321–328. doi:10.1111/j.1365-2133.2010.09767.x
Vakrakou, A. G., Svolaki, I. P., Evangelou, K., Gorgoulis, V. G., and Manoussakis, M. N. (2020). Cell-autonomous Epithelial Activation of AIM2 (Absent in Melanoma-2) Inflammasome by Cytoplasmic DNA Accumulations in Primary Sjögren's Syndrome. J. Autoimmun. 108, 102381. doi:10.1016/j.jaut.2019.102381
Verma, D., Fekri, S. Z., Sigurdardottir, G., Bivik Eding, C., Sandin, C., and Enerbäck, C. (2021). Enhanced Inflammasome Activity in Patients with Psoriasis Promotes Systemic Inflammation. J. Invest. Dermatol. 141, 586–595. doi:10.1016/j.jid.2020.07.012
Wang, B., Tian, Y., and Yin, Q. (2019). AIM2 Inflammasome Assembly and Signaling. Adv. Exp. Med. Biol. 1172, 143–155. doi:10.1007/978-981-13-9367-9_7
Wilson, J. E., Petrucelli, A. S., Chen, L., Koblansky, A. A., Truax, A. D., Oyama, Y., et al. (2015). Inflammasome-independent Role of AIM2 in Suppressing Colon Tumorigenesis via DNA-PK and Akt. Nat. Med. 21, 906–913. doi:10.1038/nm.3908
Woerner, S. M., Kloor, M., Schwitalle, Y., Youmans, H., Doeberitz, M. v. K., Gebert, J., et al. (2007). The Putative Tumor suppressorAIM2is Frequently Affected by Different Genetic Alterations in Microsatellite Unstable Colon Cancers. Genes Chromosom. Cancer 46, 1080–1089. doi:10.1002/gcc.20493
Yang, M., Long, D., Hu, L., Zhao, Z., Li, Q., Guo, Y., et al. (2021). AIM2 Deficiency in B Cells Ameliorates Systemic Lupus Erythematosus by Regulating Blimp-1-Bcl-6 Axis-Mediated B-Cell Differentiation. Sig Transduct. Target Ther. 6, 341. doi:10.1038/s41392-021-00725-x
Yoon, N., Park, M. S., Peltier, G. C., and Lee, R. H. (2015). Pre-activated Human Mesenchymal Stromal Cells in Combination with Doxorubicin Synergistically Enhance Tumor-Suppressive Activity in Mice. Cytotherapy 17, 1332–1341. doi:10.1016/j.jcyt.2015.06.009
Yuan, X., Shao, S., and Wang, G. (2020). Neutrophil Extracellular Traps Contribute to the Occurrence and Development of Psoriasis via Activating AIM2 Inflammasomes in Keratinocytes. Chin. J. Dermatol. 53, 324-329. doi:10.35541/cjd.20190897
Yun, M., Seo, G., Lee, J. Y., Chae, G. T., and Lee, S. B. (2015). Epigallocatechin-3-gallate Attenuates the AIM2-Induced Secretion of IL-1β in Human Epidermal Keratinocytes. Biochem. Biophysical Res. Commun. 467, 723–729. doi:10.1016/j.bbrc.2015.10.075
Zhang, M. J., Zhao, Q. C., Xia, M. X., Chen, J., Chen, Y. T., Cao, X., et al. (2020). The HDAC3 Inhibitor RGFP966 Ameliorated Ischemic Brain Damage by Downregulating the AIM2 Inflammasome. FASEB J. 34, 648–662. doi:10.1096/fj.201900394RRR
Zhang, S., Li, J., Zhou, G., Mu, D., Yan, J., Xing, J., et al. (2017). Aurora-A Regulates Autophagy through the Akt Pathway in Human Prostate Cancer. Cancer Biomark 19, 27–34. doi:10.3233/cbm-160238
Zhang, Z., Dong, X., Yang, X., Wan, D., Sun, L., Gu, M., et al. (2017). Expression and Clinical Significance of Absent in Melanoma 2 in Colorectal Cancer. Biomed. Pharmacother. 94, 843–849. doi:10.1016/j.biopha.2017.07.161
Zhao, Z., Zhu, H., Li, Q., Liao, W., Chen, K., Yang, M., et al. (2022). Skin CD4+ Trm Cells Distinguish Acute Cutaneous Lupus Erythematosus from Localized Discoid Lupus Erythematosus/subacute Cutaneous Lupus Erythematosus and Other Skin Diseases. J. Autoimmun. 128, 102811. doi:10.1016/j.jaut.2022.102811
Zhu, C., Fei, W., Wang, W., Tang, L., Gao, J., and Zhou, F. (2021). Copy Number Variation Analysis of IL22 and LCE3C in Different Subtypes of Psoriasis in a Chinese Han Population. Med. Sci. Monit. 27, e934927. doi:10.12659/msm.934927
Zhu, H., Zhao, M., Chang, C., Chan, V., Lu, Q., and Wu, H. (2021). The Complex Role of AIM2 in Autoimmune Diseases and Cancers. Immun. Inflamm. Dis. 9, 649–665. doi:10.1002/iid3.443
Keywords: psoriasis, AIM2, AIM2 inflammasome, pathogenesis, treatment
Citation: Wang J, Gao J, Huang C, Jeong S, Ko R, Shen X, Chen C, Zhong W, Zou Y, Yu B and Shen C (2022) Roles of AIM2 Gene and AIM2 Inflammasome in the Pathogenesis and Treatment of Psoriasis. Front. Genet. 13:929162. doi: 10.3389/fgene.2022.929162
Received: 26 April 2022; Accepted: 22 June 2022;
Published: 02 September 2022.
Edited by:
Yonghu Sun, Shandong Provincial Hospital of Dermatology, ChinaReviewed by:
Rui-qun Qi, The First Hospital of China Medical University, ChinaXiaojing Yu, Qilu Hospital, Shandong University, China
Copyright © 2022 Wang, Gao, Huang, Jeong, Ko, Shen, Chen, Zhong, Zou, Yu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changbing Shen, Y2FtYnJpZGdlMjAwOEAxMjYuY29t
†These authors have contributed equally to this work
 Jieyi Wang
Jieyi Wang Jing Gao
Jing Gao Cong Huang
Cong Huang Sohyun Jeong
Sohyun Jeong Randy Ko
Randy Ko Xue Shen
Xue Shen Chaofeng Chen
Chaofeng Chen Weilong Zhong
Weilong Zhong Yanfen Zou1,2
Yanfen Zou1,2 Changbing Shen
Changbing Shen