- 1Birth Defect Group, Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, Shanghai, China
- 2Department of Regenerative Medicine, School of Medicine, Tongji University, Shanghai, China
- 3National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
- 4University of Chinese Academy of Sciences, Beijing, China
- 5Department of Medical Genetics, School of Medicine, Tongji University, Shanghai, China
- 6Research Center for Translational Medicine, Shanghai East Hospital, School of Medicine, Tongji University, Shanghai, China
- 7Key Laboratory of Spine and Spinal Cord Injury Repair and Regeneration of Ministry of Education, Stem Cell Translational Research Center of Tongji Hospital, School of Medicine, Tongji University, Shanghai, China
OCA (oculocutaneous albinism) refers to a group of heterogeneous congenital disorders of which the common manifestations are variable degrees of cutaneous hypopigmentation and significant visual impairment, including poor visual acuity, photophobia, and nystagmus. Molecular analysis may elucidate its pathogenesis and be in favor of accurate diagnosis. High-throughput sequencing and Sanger sequencing were performed to detect mutational alleles and in silico analysis was performed for prediction of variant pathogenicity. Ten TYR-related and two OCA2-related patients were identified with 16 different variants with potential pathogenicity. Two novel missense variants [TYR: c.623T > G, p(Leu208Arg) and OCA2: c.1325A > G, p(Asn442Ser)] are identified in this study, and three OCA cases are reported for the first time in Chinese population based on their associated variants. Analysis of crystal structures of TYR ortholog and its paralog TYRP1 suggests that the substitution of Leu208 may have an impact on protein stability. This study may facilitate OCA diagnosis by expanding the mutational spectrum of TYR and OCA2 as well as further basic studies about these two genes.
Introduction
OCA (oculocutaneous albinism) is a group of congenital pigmentary disorders which result from the deficiency of melanin predominantly responsible for skin, hair, and iris color and providing us thermoregulation and photoprotection. Such patients clinically present a series of hallmark manifestations including variable degrees of cutaneous hypopigmentation, poor visual acuity, nystagmus, and photophobia. It is inherited in the autosomal recessive pattern and has a prevalence of about 1:18,000 in the Chinese population (Gong et al., 1994; Federico and Krishnamurthy, 2021). OCA can be either nonsyndromic or syndromic, and molecular testing is helpful in its accurate diagnosis and health care (Federico and Krishnamurthy, 2021; Pennamen et al., 2021).
Tyrosinase (E.C. 1.14.18.1) encoded by TYR is the rate-limiting enzyme critical for melanin synthesis, and the optimal pH condition of TYR activity is neutral (Fitzpatrick et al., 1950). The complete or partial loss of tyrosinase or its activity can lead to melanin deficiency. OCA1 (MIM#203100w), caused by variants of TYR (NM_000372.5) encoding a 529aa (amino acids) protein, has been reported as the most prevalent OCA in China, America, and Europe (Hutton and Spritz, 2008; Wei et al., 2010; Lasseaux et al., 2018; Marti et al., 2018; Zhong et al., 2019). TYR has five exons, and six pairs of primers are enough to screen its coding regions plus flanking splicing sites (Zhong et al., 2019). Thus, we used direct sequencing TYR helpful when considering its costeffectiveness. Tyrosinase is conserved across a broad variety of species. Crystal structures of tyrosinase from germs and its paralog TYRP1 (tyrosinase-related protein have been resolved and contribute to the prediction of variant pathogenicity. Another important protein in melanin synthesis is melanosomal transmembrane p protein involved in the regulation of melanosomal pH critical to TYR activity (Rinchik et al., 1993; Bellono et al., 2014). OCA2 caused by variants of OCA2 (NM_000275.3), encoding an 838aa-sized glycoprotein, is the second prevalent type in the Chinese cohort and in the European set, while being the most common worldwide due to high prevalence of 1/3,900 in sub-Saharan Africa (Lasseaux et al., 2018; Marti et al., 2018; Zhong et al., 2019; Federico and Krishnamurthy, 2021). OCA2 spans 334 kb in genomic DNA, has 25 exons, and no mutational hotspot of this gene has been observed for OCA cases (Lasseaux et al., 2018; Zhong et al., 2019; Federico and Krishnamurthy, 2021). In addition to these two genes, there are more than ten known causative OCA genes (TYRP1, SLC45A2, SLC24A5, LRMDA, MITF, HPS1, AP3B1, HPS3, HPS4, BLOC1S3, HPS5, HPS6, DTNBP1, BLOC1S6, AP3D, BLOC1S5, GPR143, LYST, MY5A, RAB27A, and MLPH) (Federico and Krishnamurthy, 2021), although their related OCA is relatively rare. Therefore, high-throughput sequencing is more effective for detection of variants in these genes. There is hitherto no resolved crystal structure of p protein in any species, but recently a few reports about its function may be helpful as a clue to prediction of variant pathogenicity.
In this study, all exons of TYR and their flanking regions were directly sequenced in all 12 patients, and then high-throughput sequencing of known OCA genes was performed on the patients without likely pathogenic variants in TYR. In silico resources about the genes and cases were reviewed to predict the pathogenicity of novel variants and speculate the possible role of the residues where novel variants change.
Materials and Methods
Study Cases
This study was approved by the institutional review board at Shanghai Fourth Peoples Hospital, School of Medicine, Tongji University, China. All procedures in this study were performed according to the principles of the Declaration of Helsinki. Twelve unrelated patients were recruited for this study. All the patients present obvious hypopigmentation in skin and hair and have no complaints of disorders in other organs. About 2 ml of peripheral blood was collected from the participants or their guardians who signed informed consent.
Variants Detection and In Silico Analysis
Touchdown PCR amplification was carried out with the procedures of an annealing temperature of 60–57°C for all primers used in this study. Sanger sequencing was performed on total genomic DNA from all the patients with six pairs of primers previously described to amplify TYR exons plus their flanking regions which are mutational hotspots in Chinese OCA (Zhong et al., 2019). For the remaining unsolved patients with the abovementioned primers, TES (target exome sequencing) was carried out. About 3 micrograms of genomic DNA were quantified with a NanoDrop Spectrophotometer. Libraries were prepared according to Illumina standard protocol and were loaded on the NextSeq500 platform. Known OCA genes were included in the TES panel. SOAPaligner was used to align the reads to reference sequences of humans. The following workflow was used to filter out candidate variants. Variants in known OCA genes→ rare or absent in healthy controls→ homozygous or compound heterozygous variants. The pathogenicity of variants was predicted by in silico tools SIFT, Polyphen-2, and MutationTaster (Kumar et al., 2009; Adzhubei et al., 2010; Schwarz et al., 2010). Sanger sequencing was performed to verify candidate variants or for cosegregation analysis if DNA from family members was available. In addition, as for variants closed together, amplicons containing the variants were cloned into vectors and the vectors were transformed into Escherichia coli for amplification. Then, at least five single clones were sequenced by Sanger sequencing with the primers (M13F: 5′-GTAAAACGACGGCCAGT-3′ and M13R: 5′-CAGGAAACAGCTATGAC-3′) to assess genetic phase of neighboring heterozygous variants. For literature review, keywords such as Chinese, albinism, tyrosinase, OCA2, or their aliases were selected for combination and used to search in PubMed, and then duplicates were removed. All extracted data were double-checked for accuracy. All Chinese TYR-related or OCA2-related cases from the published literature (till 31 October 2021) at PubMed and its linked journal websites were reviewed to evaluate the impact of the variants identified in this study sharing on proband counts. The frequency of the variants in healthy controls of gnomAD (genome Aggregation Database) is shown in Supplementary Table S1, and the recurrence of variants in Chinese OCA cases is shown in Table S2. Evidence of pathogenicity can be categorized into very strong (PVS), strong (PS), moderate (PM), and supporting (PP) based on the guidelines of the American College of Medical Genetics and Genomics (Richards et al., 2015). The crystal structures of Streptomyces castaneoglobisporus tyrosinase (PDB: 1WX2) and human TYRP1 (PDB: 5M8L) were available (Matoba et al., 2006; Lai et al., 2017) and human p protein (PDB: AF-Q04671-F1-model_v2) was obtained from AlphaFold, and those structures were analyzed by Pymol. In addition to the abovementioned protein sequences, the following sequences Homo sapiens (NP_000363.1), Bos taurus (NP_851344.1), Canis lupus familiaris (NP_001002941.1), Gallus gallus (NP_989491.1), Rattus norvegicus (NP_001101005.1), Mus musculus (NP_035791.1), Xenopus tropicalis (NP_001096518.1), Danio rerio (NP_571088.3), Homo sapiens (NP_000266.2), Sus scrofa (NP_999259.2), Rattus norvegicus (NP_001258422.1), and Mus musculus (NP_068679.1) were used for amino acid alignment.
Result
Clinical Manifestation
In this study, 12 patients were from unrelated families. All of them have poor visual acuity, conspicuous hypopigmentation in skin and hair, and no complaint of other disorders. Their parents were non-consanguineous. Nine patients (P1–P9) exhibit stark white skin and white hair at analysis substantially without change in later age (Supplementary Table S2). The other three patients (P10–P12) present creamy white skin, and the patient P12 has freckles on the face. P10 manifests blond hair at analysis (Figure 1) and nearly no change in cutaneous color from birth to adulthood. P11 presents golden hair at analysis and light yellow hair at birth. In P12, his hair is grayish white at birth and flaxen hair at analysis (Figure 1).
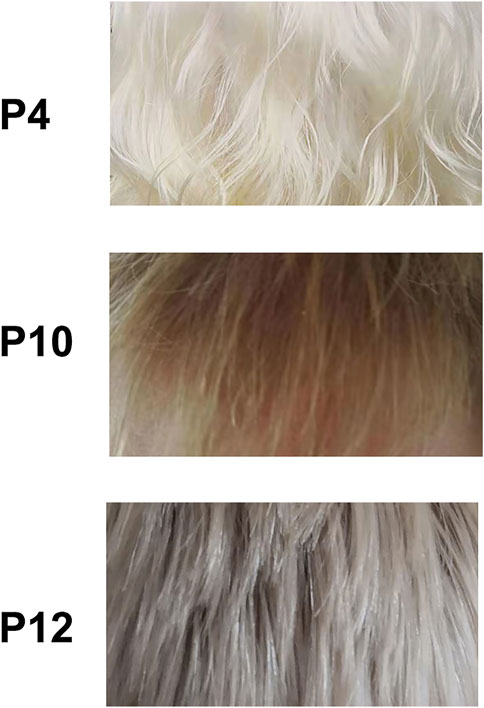
FIGURE 1. Hair phenotype of OCA cases. p, patient. P4 had homozygous variant TYR: c.623T > G, p (Leu208Arg). P10 had compound variants TYR: c.929dupC, p (Arg311Lysfs*7) and TYR: c.1037–7A > T plus c.1037–10_11delTT. P12 had compound variants OCA2: c.1325A > G, p (Asn442Ser) and OCA2: c.2180T > C, p(Leu727Pro).
TYR Variants in OCA Patients
Twelve different variants of TYR were identified in ten OCA patients by Sanger sequencing including a homozygous variant c.623T > G in P4 (Figure 2). Nine patients had compound heterozygous variants, including P2 with c.230G > A and c.230_232dupGGG confirmed to locate on different alleles by M13 sequencing of the clone (Supplementary Figure S1). In the healthy control database, all of these variants were rare or absent, and their allele and genotype frequency data were absent (Supplementary Table S1). Moreover, c.623T > G is novel, c.1200G > T was previously reported in a Chinese patient, and other eight variants in this study have been reported in no less than four different OCA cases according to the published literature (Supplementary Table S2 and Figure 3). Except for a novel missense variant c.623T > G and the recurrent variants including the missense variant c.1200G > T, and the splicing variant c.1037–7A > T plus c.1037–10_11delTT closed together on the same allele confirmed by M13 clone sequencing, other variants have been assigned pathogenic or likely pathogenic in ClinVar, including two nonsense variants (c.346C > T and c.832C > T), a frameshift insertion c.929dupC, an in-frame insertion c.230_232dupGGG, and three missense variants (c.895C > A, c.895C > T and c.896G > A) (Table 1). The variants c.623T > G and c.1200G > T were predicted deleterious by in silico tools (Table 1). The variant c.1200G > T may bring about cysteine for Trp400 where either c.1198T > A or c.1199G > T change recorded pathogenic or likely pathogenic in ClinVar (Table 1), and thus it can be determined to be likely pathogenic (PS1, PM2, pP3). The novel variant c.623T > G may lead to substitution for Leu208 located in the mutational hotspot (exon1 and exon2) and in the region where the methadone tolerance landscape of TYR demonstrated that nearly no tolerant region is located (Wiel et al., 2019) (Figure 4A). Also, so we considered the variant c.623T > G in P4 as VUS (a variant of uncertain significance) (PM1, PM2, and pP3), and this patient was not detected to have candidate variants in other known OCA genes by TES. For the splicing variant, the published study has confirmed the mutational allele c.1037–7A > T plus c.1037–10_11delTT as a pathological splicing site by in vitro splicing assay (Goto et al., 2004). Therefore, the variant c.1037–7A > T plus c.1037–10_11delTT can be considered likely pathogenic (PS3, PM2, and pP5).
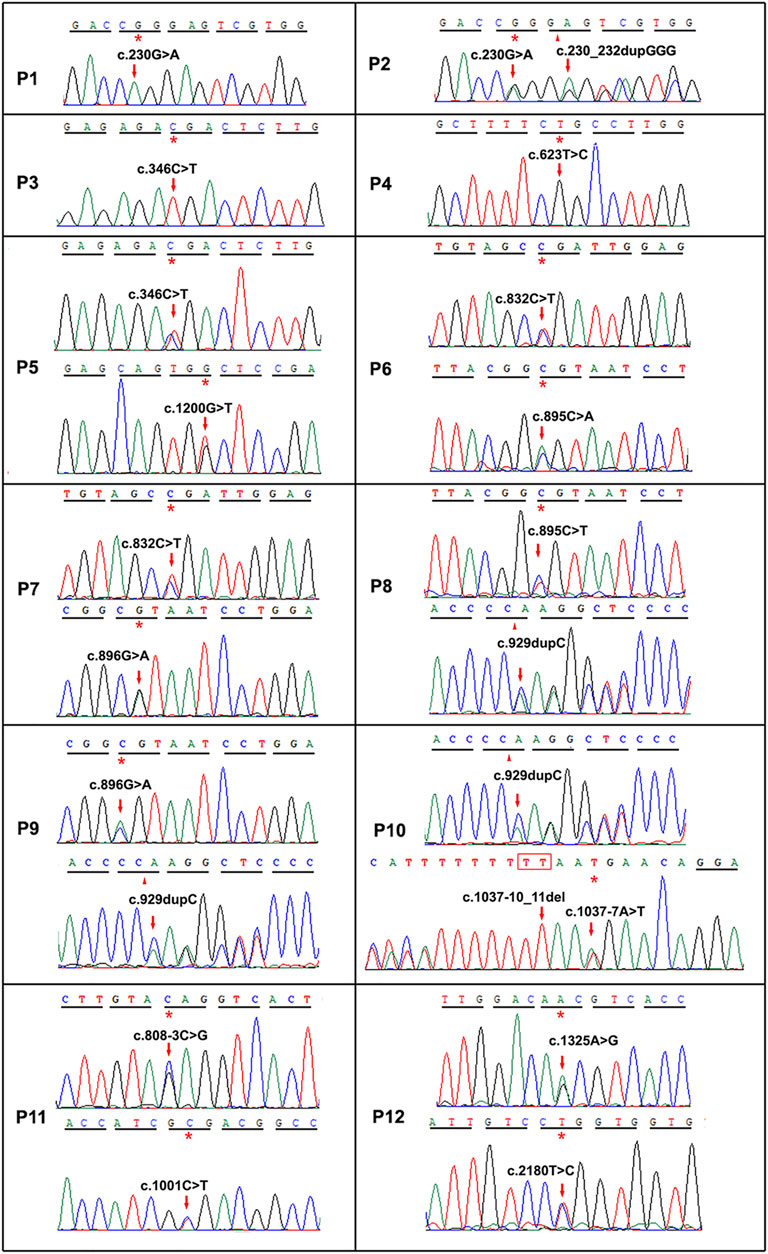
FIGURE 2. Sequence chromatograms of TYR or OCA2 variants of twelve OCA cases in this study. The nucleotide changes were pointed by red arrows. p, patient. The first ten cases P1–P10 related to TYR variants, and the last two cases P11–P12 related to OCA2.
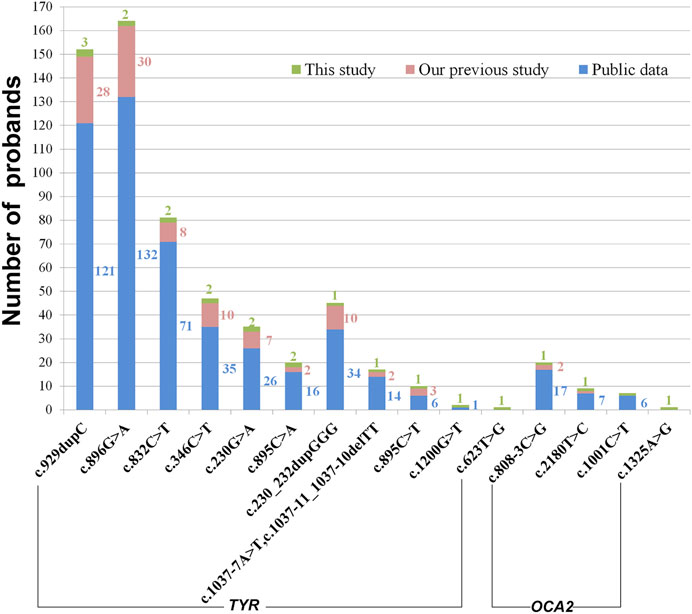
FIGURE 3. Variant sharing on proband counts. The number of probands was in the same color as that of bars. Publicly available data were collected from the published literature about Chinese OCA cases.

TABLE 1. Pathogenicity prediction of OCA-related variants in this study and their relevant variants.
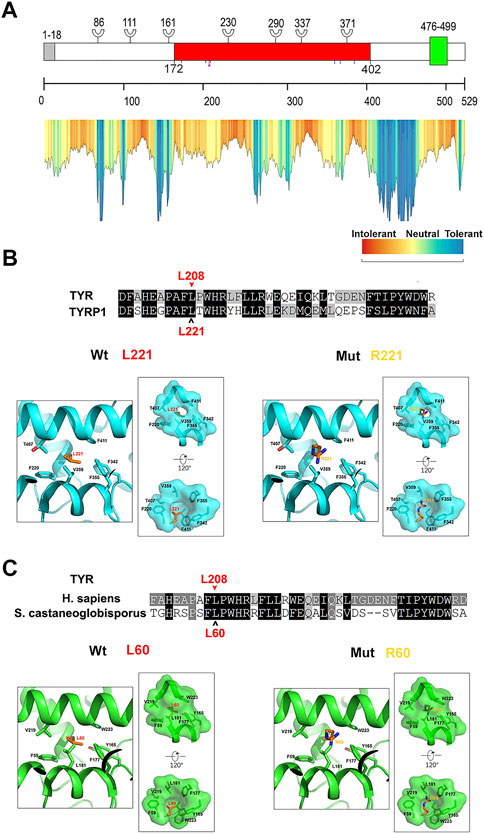
FIGURE 4. Computational analysis of the residue where a novel variant changes in TYR (A) Schematic representation and tolerance landscape of TYR. The signal peptide (1–18aa) was colored in gray; the common central domain of tyrosinase (172–402aa) in red; transmembrane domain (476–499aa) in green. Branch symbols indicate putative N-linked glycosylation sites. Blue lines indicate the positions of copper-coordinating histidine. The red line plus a purple blob indicate the novel variant in this study (B) Counterpart of TYR L208 in human TYRP1 and the effect of substitution of L208R. The counterpart of TYR L208 is L221 in human TYRP1. The alignment of amino acids of human TYR and its paralog TYRP1 shows that they are homologs to each other and the residue where a variant changes is located in the conserved domain. In the structure model of human TYRP1, the residue in red is the counterpart of L208 in human TYR, and other residues associated with it are in black. Left: structure model of wildtype TYRP1. The left square shows that L221 is buried in the hydrophobic pocket formed by hydrophobic side-chains of surrounding residues. Residues provided by the hydrophobic surface are highlighted, and right rectangle, the hydrophobic surface is shown in surface mode. Right: destabilization effect of leucine to arginine substitution structural analyses show that L221R causes steric hindrance and introduces changes that are incompatible with the hydrophobic environment (C) Counterpart of TYR L208 in tyrosinase from Streptomyces castaneoglobisporus and the effect of substitution L208R. The counterpart of TYR L208 is L60 in tyrosinase from Streptomyces castaneoglobisporus. Based on the alignment of amino acid sequence, the residue in TYR where a novel variant changes is in red and pointed by a red arrowhead, and its counterpart is in red and pointed by a black open arrowhead. Left: structure model of wildtype tyrosinase from Streptomyces castaneoglobisporus. The left square shows that L60 is buried in the hydrophobic pocket formed by hydrophobic side-chains of surrounding residues. Residues providing the hydrophobic surface are highlighted, and right rectangle, the hydrophobic surface is shown in surface mode. Right: destabilization effect of the substitution of leucine to arginine structural analyses show that L60R cause steric hindrance and introduces charges that are incompatible with a hydrophobic environment.
OCA2 Variants in OCA Patients
Four OCA2 variants were detected by TES in two OCA patients (P11 and P12) and confirmed by Sanger sequencing (Figure 2). The frequency of the variants was rare or absent, and their homozygosity has not been observed in the healthy control database (Supplementary Table S1). All three missense variants (c.1001C > T, c.1325A > G, and c.2180T > C) were predicted deleterious by in silico analysis (Table 1). c.1001C > T has been assigned pathogenic or likely pathogenic in ClinVar but no information about other variants is realized (Table 1). The splicing variant c.808–3C > G has been confirmed pathogenic via in vitro splicing assay (Zhong et al., 2019). Furthermore, checking the literature reported about Chinese OCA, we found that c.1325A > G is novel and the other three variants have been reported in at least five different OCA cases in Chinese (Supplementary Table S2 and Figure 3). c.2180T > C may lead to substitution for Leu727 located in the permease domain (342–775aa) and in the region where the methadone tolerance landscape of p protein demonstrated that nearly no tolerant region locates (Wiel et al., 2019) (Supplementary Figure S3A). Therefore, c.808–3C > G can be considered likely pathogenic (PS3, PM2, and pP5), c.2180T > C considered likely pathogenic (PM1, PM2, and pP3, pP5), and c.1325A > G classified uncertain significance (PM2 and pP3).
In Silico Analysis of the Impact of Novel Variants
Two novel variants NM_000372.5: c.623T > G, p.(Leu208Arg) and NM_000275.3: c.1325A > G, p.(Asn442Ser) have been found in this study. TYR sequon 202_212HEAPAFLPWHR in which Leu208 locates is in high homology to its paralog TYRP1 (Figure 4B), and His202 and His211 form a copper center within the catalytically active domain (Noh et al., 2020). The counterpart of Leu208 is Leu60 in tyr of Streptomyces castaneoglobisporus and Leu221 in human TYRP1. As shown by the crystal structures, Leu60 is inserted into the hydrophobic core formed by Phe59, Tyr165, Phe177, Leu181, Val219, and Trp223 in Tyr (PDB: 1WX2), and Leu221 is inserted into the hydrophobic core formed by Phe220, Phe342, Phe355, Val359, Thr407, and Phe411 in TYRP1 (PDB: 5M8L) to stabilize the protein structure (Figures 4B,C). Leu60Arg in Tyr of Streptomyces castaneoglobisporus and Leu221Arg in human TYRP1 may cause steric hindrance in the proteins and introduce charges that are incompatible with the hydrophobic environment (Figures 4B,C). Alignment analysis showed that the counterparts of Phe59, Tyr165, Phe177, Leu181, Val219, and Trp223 in tyr of Streptomyces castaneoglobisporus were correspondingly Phe207, Tyr327, Phe340, Leu344, Val393, and Phe397 in human TYR (Supplementary Figure S2A). The counterparts of Phe220, Phe342, Phe355, Val359, Thr407, and Phe411 in TYRP1 were correspondingly Phe207, Phe328, Phe340, Leu344, Val393, and Phe397 in human TYR (Supplementary Figure S2B). Therefore, we speculated that in human TYR, Leu208 may be inserted into the hydrophobic core which may be formed by Phe207, Tyr327, Phe328, Phe340, Leu344, Val393, and Phe397. It is possible that the substitution of leucine at position 208, which has a hydrophobic side chain, by arginine, which is hydrophilic and has a positively charged side chain, may destroy hydrophobicity and affect TYR stability. In OCA2, the novel variant c.1325A > G identified in this study may result in the substitution of serine for Asn442. Asn442 was conserved among the orthologs of p protein (Supplementary Figure S3B). Structure analysis of p protein in AlphaFold revealed that multiple polar interactions may be formed between the side-chain of Asn442 and nearby residues Val443, Asn476, and Asp486. These interactions are likely abrogated in the p.N442S mutant due to removal of the asparagine side-chain (Supplementary Figure S3C). Moreover, the residue Asn442 in sequon 442_444Asn-Val-Thr-Thr bearing canonical consensus (Asn-X-Ser/Thr-X, X≠Pro) of N-linked glycosylation is an essential posttranslational modification of secretory and membrane protein in eukaryotic cells. Moreover, the same amino acid Asn442 change resulting from the variant c.1324_1326del has been determined to be likely pathogenic in ClinVar (Table 1). However, further studies are necessary to verify the impact of two novel variants.
Discussion
In this study, in total, sixteen variants were detected in twelve OCA cases including fourteen recurrent variants and two novel variants. Among the recurrent variants identified in this study, some variants such as c.929dupC and c.230_232dupGGG in TYR and c.808–3C > G in OCA2 were common in OCA cases from East Asian regions, while these variants have seldom been reported in OCA cases from the Western population, even in a large cohort of 990 index patients with albinism (Lasseaux et al., 2018). On the other hand, their heterozygosity was absent in the Western population based on healthy controls of gnomAD (genome Aggregation Database) (Supplementary Table S1). In addition, a pathogenic variant TYR: c.896G > A is most common in Chinese OCA cases, while it is just in three of 161 TYR-related cases identified out of 990 index patients from France (Lasseaux et al., 2018). In gnomAD, we noticed that heterozygous variant c.896G > A presented in East Asians, Latino/Admixed American, and European non-Finnish and was absent in other populations (Supplementary Table S1). Hence, the abovementioned observations hinted at the possibility that the occurrence of rare variants with potential pathogenicity in Mendelian OCA genes may vary by ethnicity. In addition, three cases (P1, P3, and P4) have homozygous variants, although their parents have no consanguineous marriage. Two unrelated individuals have the same heterozygous variant due to its higher frequency in the heterozygous state, and then the offspring segregate these variants from both parents thus we speculated that a relatively higher proportion of individuals might be the carriers with heterozygous variants TYR: c.230G > A, TYR: c.346C > T or TYR: c.623T > C in Chinese population.
Twelve OCA cases have been identified in this study, including ten TYR-related and two OCA2-related patients. In TYR-related patients, eight patients have candidate variants in TYR within its first two exons that are mutational hotspots, while two patients (P5 and P10) have variants out of mutational hotspot. The patient P10 manifesting blond hair has been clinically diagnosed as OCA2 at first (Figure 1), and then identification of compound variants c.929dupC and c.1037–7A > T plus c.1037–10_11delTT in TYR supported to be affected by OCA1B, which is caused by the partial loss of TYR or its activity. Additionally, P12 was the adult with flaxen hair and was finally diagnosed as OCA2 due to identification of the compound variants c.2180T > C and c.1325A > G in the OCA2 gene, while most OCA2 cases have hair color from light yellow to blond or to brown. Based on the cutaneous phenotype of the cases in this study and the cases in previous reports, homozygosity or compound heterozygosity of ten variants in TYR (c.230G > A, c.230_232dupGGG, c.346C > T, c.623T > G, c.832C > T, c.895C > A, c.895C > T, c.896G > A, c.929dupC and c.1200G > T) may be related to stark white skin and white hair and no change in later age due to complete loss of melanin. Moreover, TYR: c.1037–7A > T plus c.1037–10_11delTT on the same allele identified in P10 may be related to creamy white skin and yellow or blond or brown hair due to residual TYR function since another allele is c.929dupC, which may bring about complete loss of TYR function. Therefore, molecular testing is conducive to a definitive diagnosis of OCA, and the identification of variants may further our understanding of the genes.
Two novel variants (TYR: c.623T > G and OCA2: c.1325A > G) were identified in this study. TYR: c.623T > G may lead to substitution of arginine for Leu208 whose counterpart is Leu60 in Streptomyces, and Leu221 in human TYRP1 may be crucial to protein stabilization as demonstrated in crystal structures (Figure 4). In addition, His38, His54, and His63 in Streptomyces tyr (His180, His202, His211 in human TYR) coordinated a copper ion, and the sequon between His38 and His63, including Leu60, may be involved in the formation of the binuclear copper center as part of catalytic center (Matoba et al., 2006). The residue Leu208 has been mentioned for its proximity to the active site of tyrosinase and its potential role in maintaining catalytic activity (Schweikardt et al., 2007). Arginine having a positively charged side chain substituted at 208th residue for Leucine having an uncharged nonpolar side may disrupt the binuclear copper center. Also, milky skin and white hair were observed in P4 patients being a homozygosity of the variant. Hence, we speculate that Leu208 may also be the key residue of TYR activity. Another novel variant OCA2:c.1325A > G may lead to substitution of serine for Asn442 in p protein. p protein Structure from AlphaFold can be used for analysis, although there is no resolved crystal structure available for analysis. Although the interactions between Asn442 and nearby residues Val443, Asn476, and Asp486 in p protein may be abrogated in the p.N442S mutant (Supplementary Figure S3C), the overall structure of the mutant might be unchanged since both Asn and Ser are polar amino acids. In addition, OCA2 has been suggested in a recent study to play a key role in melanosome-specific anion channel, and the putative luminal loop 440–449aa is critical to OCA2-medicated Cl− current regulating melanosomal pH (Rinchik et al., 1993; Bellono et al., 2014). Sequence analysis showed that c.1325A > G in OCA2 may disrupt a putative N-linked glycosylation site Asn442 that has also been predicted as a glycosylation site on the website https://www.uniprot.org/uniprot/Q04671. N-linked glycosylation is important in quality control, stability, and function of glycoproteins such as trafficking or gating kinetics of channel protein, and the sequon Asn-X-Thr/Ser-X is a key determinant in the efficiency of glycosylation (Esmail and Manolson, 2021). The variant at Val443 adjacent to Asn442 has recurred in European OCA (Hutton and Spritz, 2008; Lasseaux et al., 2018; Marti et al., 2018) and is frequently reported in Chinese OCA (Figure 3). The substitution Val443Ile near Asn442 has been experimentally demonstrated to reduce current amplitudes required in melanogenesis but has intact subcellular localization (Bellono et al., 2014), from which we inferred that the substitution Asn442Ser might result in dysregulation of the anion channel and impair optimal pH for TYR activity in the melanosome. The impact of these two novel variants here may deserve further study.
In summary, we reported sixteen different variants in twelve OCA cases, including two novel OCA-related variants and three cases reported for the first time based on their associated variants, which can be in favor of OCA diagnosis by expanding the phenotypic and mutational spectrum. Meanwhile, we present hair phenotypes related to two novel variants. In addition, the analysis of available crystal structures implies that Leu208, affected by the novel variant TYR: c.623T > G, may play an important role in the stability of TYR.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board at Shanghai Fourth People’s Hospital, School of Medicine, Tongji University, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, ZZ and JC; methodology, ZZ and ZZ; formal analysis, ZZ and JC; investigation, ZZ and ZZ; resources, JC; writing—original draft preparation, ZZ; writing—review and editing, JC and JZ. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology of China (2020YFA0112500 and 2021YFA1101300) and the National Natural Science Foundation of China (81371062).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciated all the volunteers for participating in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.926511/full#supplementary-material
References
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P., et al. (2010). A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 7 (4), 248–249. doi:10.1038/nmeth0410-248
Bellono, N. W., Escobar, I. E., Lefkovith, A. J., Marks, M. S., and Oancea, E. (2014). An Intracellular Anion Channel Critical for Pigmentation. Elife 3, e04543. doi:10.7554/eLife.04543
Esmail, S., and Manolson, M. F. (2021). Advances in Understanding N-Glycosylation Structure, Function, and Regulation in Health and Disease. Eur. J. Cell Biol. 100 (7-8), 151186. doi:10.1016/j.ejcb.2021.151186
Federico, J. R., and Krishnamurthy, K. (2021). “Albinism,” in StatPearls (Treasure Island (FL): StatPearls Publishing).
Fitzpatrick, T. B., Becker, S. W., Lerner, A. B., and Montgomery, H. (1950). Tyrosinase in Human Skin: Demonstration of its Presence and of its Role in Human Melanin Formation. Science 112 (2904), 223–225. doi:10.1126/science.112.2904.223
Gong, Y., Shao, C., Zheng, H., Chen, B., and Guo, Y. (1994). Study on Genetic Epidemiology of Albinism. Yi Chuan Xue Bao 21 (3), 169–172. doi:10.1007/bf00944078
Goto, M., Sato-Matsumura, K. C., Sawamura, D., Yokota, K., Nakamura, H., and Shimizu, H. (2004). Tyrosinase Gene Analysis in Japanese Patients with Oculocutaneous Albinism. J. Dermatological Sci. 35 (3), 215–220. doi:10.1016/j.jdermsci.2004.06.007
Hutton, S. M., and Spritz, R. A. (2008). A Comprehensive Genetic Study of Autosomal Recessive Ocular Albinism in Caucasian Patients. Invest.. Ophthalmol. Vis. Sci. 49 (3), 868–872. doi:10.1167/iovs.07-0791
Kumar, P., Henikoff, S., and Ng, P. C. (2009). Predicting the Effects of Coding Non-synonymous Variants on Protein Function Using the SIFT Algorithm. Nat. Protoc. 4 (7), 1073–1081. doi:10.1038/nprot.2009.86
Lai, X., Wichers, H. J., Soler‐Lopez, M., and Dijkstra, B. W. (2017). Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc Active Site Important for Melanogenesis. Angew. Chem. Int. Ed. 56 (33), 9812–9815. doi:10.1002/anie.201704616
Lasseaux, E., Plaisant, C., Michaud, V., Pennamen, P., Trimouille, A., Gaston, L., et al. (2018). Molecular Characterization of a Series of 990 Index Patients with Albinism. Pigment. Cell Melanoma Res. 31 (4), 466–474. doi:10.1111/pcmr.12688
Marti, A., Lasseaux, E., Ezzedine, K., Léauté-Labrèze, C., Boralevi, F., Paya, C., et al. (2018). Lessons of a Day Hospital: Comprehensive Assessment of Patients with Albinism in a European Setting. Pigment. Cell Melanoma Res. 31 (2), 318–329. doi:10.1111/pcmr.12651
Matoba, Y., Kumagai, T., Yamamoto, A., Yoshitsu, H., and Sugiyama, M. (2006). Crystallographic Evidence that the Dinuclear Copper Center of Tyrosinase Is Flexible during Catalysis. J. Biol. Chem. 281 (13), 8981–8990. doi:10.1074/jbc.M509785200
Noh, H., Lee, S. J., Jo, H.-J., Choi, H. W., Hong, S., and Kong, K.-H. (2020). Histidine Residues at the Copper-Binding Site in Human Tyrosinase Are Essential for its Catalytic Activities. J. Enzyme Inhibition Med. Chem. 35 (1), 726–732. doi:10.1080/14756366.2020.1740691
Pennamen, P., Tingaud-Sequeira, A., Gazova, I., Keighren, M., McKie, L., Marlin, S., et al. (2021). Dopachrome Tautomerase Variants in Patients with Oculocutaneous Albinism. Genet. Med. 23 (3), 479–487. doi:10.1038/s41436-020-00997-8
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and Guidelines for the Interpretation of Sequence Variants: a Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17 (5), 405–424. doi:10.1038/gim.2015.30
Rinchik, E. M., Bultman, S. J., Horsthemke, B., Lee, S.-T., Strunk, K. M., Spritz, R. A., et al. (1993). A Gene for the Mouse Pink-Eyed Dilution Locus and for Human Type II Oculocutaneous Albinism. Nature 361 (6407), 72–76. doi:10.1038/361072a0
Schwarz, J. M., Rödelsperger, C., Schuelke, M., and Seelow, D. (2010). MutationTaster Evaluates Disease-Causing Potential of Sequence Alterations. Nat. Methods 7 (8), 575–576. doi:10.1038/nmeth0810-575
Schweikardt, T., Olivares, C., Solano, F., Jaenicke, E., García-Borrón, J. C., and Decker, H. (2007). A Three-Dimensional Model of Mammalian Tyrosinase Active Site Accounting for Loss of Function Mutations. Pigment. Cell Res. 20 (5), 394–401. doi:10.1111/j.1600-0749.2007.00405.x
Wei, A., Wang, Y., Long, Y., Wang, Y., Guo, X., Zhou, Z., et al. (2010). A Comprehensive Analysis Reveals Mutational Spectra and Common Alleles in Chinese Patients with Oculocutaneous Albinism. J. Investigative Dermatology 130 (3), 716–724. doi:10.1038/jid.2009.339
Wiel, L., Baakman, C., Gilissen, D., Veltman, J. A., Vriend, G., and Gilissen, C. (2019). MetaDome: Pathogenicity Analysis of Genetic Variants through Aggregation of Homologous Human Protein Domains. Hum. Mutat. 40 (8), 1030–1038. doi:10.1002/humu.23798
Keywords: albinism, TYR, OCA2, variant, structure
Citation: Zhong Z, Zhou Z, Chen J and Zhang J (2022) Identification of 12 OCA Cases in Chinese Population and Two Novel Variants. Front. Genet. 13:926511. doi: 10.3389/fgene.2022.926511
Received: 22 April 2022; Accepted: 09 June 2022;
Published: 12 July 2022.
Edited by:
Rituraj Purohit, Institute of Himalayan Bioresource Technology (CSIR), IndiaReviewed by:
Qingnan Liang, Baylor College of Medicine, United StatesYang Ying, Xi’an Children’s Hospital, China
Asmat Ullah, University of Copenhagen, Denmark
Roberto Rosati, Pelé Pequeno Príncipe Research Institute, Brazil
Copyright © 2022 Zhong, Zhou, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Chen, Y2hlbmppYW5qdW5AdG9uZ2ppLmVkdS5jbg== Jun Zhang, anVuemhhbmdAdG9uZ2ppLmVkdS5jbg==
 Zilin Zhong
Zilin Zhong Zheng Zhou
Zheng Zhou Jianjun Chen
Jianjun Chen Jun Zhang6,7*
Jun Zhang6,7*