- 1ICAR-Indian Institute of Wheat and Barley Research, Karnal, India
- 2CCS Haryana Agricultural University, Hisar, India
Annually, the cost of insect pest control in agriculture crosses billions of dollars around the world. Until recently, broad-spectrum synthetic pesticides were considered as the most effective means of pest control in agriculture. However, over the years, the overreliance on pesticides has caused adverse effects on beneficial insects, human health and the environment, and has led to the development of pesticide resistant insects. There is a critical need for the development of alternative pest management strategies aiming for minimum use of pesticides and conservation of natural enemies for maintaining the ecological balance of the environment. Host plant resistance plays a vital role in integrated pest management but the development of insect-resistant varieties through conventional ways of host plant resistance takes time, and is challenging as it involves many quantitative traits positioned at various loci. Biotechnological approaches such as gene editing, gene transformation, marker-assisted selection etc. in this direction have recently opened up a new era of insect control options. These could contribute towards about exploring a much wider array of novel insecticidal genes that would otherwise be beyond the scope of conventional breeding. Biotechnological interventions can alter the gene expression level and pattern as well as the development of transgenic varieties with insecticidal genes and can improve pest management by providing access to novel molecules. This review will discuss the emerging biotechnological tools available to develop insect-resistant engineered crop genotypes with a better ability to resist the attack of insect pests.
1 Introduction
Under the changing climate scenario, the world’s population is estimated to increase by 2 billion in the next 30 years, rising from the current 7.7 billion population to 10 billion by 2050 (Zsögön et al., 2022). In this context, there is a continuous need for an increment of food production to fulfill the need of the rising worldwide population. Additionally, it is assessed that total global food demand will increase from 35% in 2010 to 56% in 2050 (van Dijk et al., 2021). To meet these goals it is critical to increase crop yield and reduce pre- and post-harvest losses. Also despite of operating control measures, a large portion of the economically significant harvests experiences a wide range of yield losses. Population extension, depletion of natural resources, environmental change, and developing insect pests are the key limitations that contrarily affect overall agricultural production and productivity (Alemu, 2020). Amongst these production constraints, biotic factors address one of the foremost constraints to crop productivity. Amongst the biotic factors, the insect pests are assessed to cause 25%–30% losses to agricultural production (Joshi et al., 2020; Mateos Fernandez et al., 2021). This suggests that insect pests pose a severe danger to food security and sustainable development, necessitating the development of effective plant protection technologies to prevent and control pest-related crop losses (Oerke et al., 2006). Chemical pesticides provide the first line of defense for farmers against insect pests, and their widespread use has resulted in a number of issues, including the killing of the beneficial insects, environmental pollution (Pedigo and Rice, 2006; Stevens et al., 2012), human and animal health problems and; imparting resistance in pests (Nderitu et al., 2020). To meet these challenges, it is necessary to move towards more sustainable and modern agricultural practices. Moreover, these detrimental non-target effects have motivated researchers around the world to develop novel and environment-friendly, alternative insect pest management strategies. Therefore, host plant resistance can form the backbone of pest management in different agro-ecosystems (Sharma, 2007).
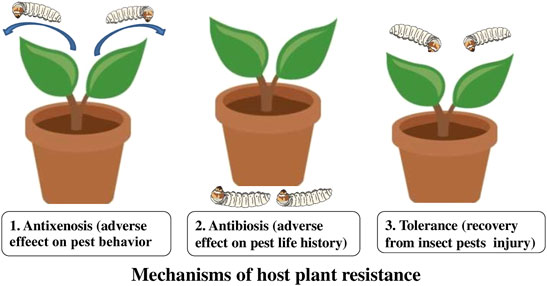
Host plant resistance is the key component of pest management and one of the most appreciated control tactics in advanced agriculture (Horgan et al., 2020; El-Dessouki et al., 2022). It is the consequences of heritable plant characteristics that make a plant to be less damaged than a plant lacking these qualities. Insect-resistant crop varieties reduce the number of insect pests by increasing their tolerance for injury. Three types of resistance determine the relationship between the insect and the plant, e.g. antibiosis, antixenosis (non-preference), and tolerance (Koch et al., 2016; Iqbal et al., 2018) (Figure 1). Antibiosis resistance influences the biology of the pest to diminish its population and subsequent damage, resulting in higher mortality or reduced longevity and reproduction of the insect. Antixenosis resistance is defined as non-preference of the pest for a resistant plant and influences the behavioral traits of a pest (Painter, 1951; Smith, 2005). Tolerance is a resistance where a plant can resist or recover from damage caused by the pest population (Smith, 2005).
The development of insect-resistant plants began in 1782 when Havens published an article on a Hessian fly-resistant wheat cultivar. Since that time, several insect-resistant cultivars have been developed by the international and national research centers, the private sector using conventional or biotechnological tools (Jaiswal et al., 2018). The major biochemical principles underlying such resistance and the genes included have been distinguished for their coordinated utilization through biotechnological advancements in the course of the most recent 30 years (Joshi et al., 2020). Furthermore, for global food security and agricultural sustainability, contemporary agriculture’s primary goal is to enhance yields using existing land and resources. Therefore, innovative technologies have to be exploited to control pests and ensure adequate food availability in the future. Different plant protection innovations have been created to control, prevent and manage these pests with the trend of emphasizing/concentrating on the use of newer and more advance biotechnological approaches that are proven to be most efficient and provide results in a very short time as compared to conventional methods. These approaches can act as the backbone of crop protection against a broad spectrum of insect pests. In this due consideration, modern biotechnology put forward the best possible alternatives for diversifying agricultural production by accelerating the development of new insect resistant varieties of cereals, horticultural and even underutilized crops (Abah et al., 2010). Therefore, the main objective of this review is to assess the opportunities provided by these new biotechnological tools in developing various crop plants that are resistant to a wide range of insect pests.
2 Biotechnological Approaches in Insect Pest Management
Biotechnology can be broadly defined as “a method of creating or modifying a product, improving plants or animals, or developing microorganisms for specific purposes by utilising biological systems, living organisms, or derivatives thereof” (Persley, 2000). However, it can be described as the regulated and deliberate manipulation of biological processes to accomplish efficient insect pest control. From insect resistance breeding to transgenic introgression of novel genes, biotechnological interventions in insect pest management to protect crop yield have been enormous. The different biotechnological approaches include gene transformation, genome editing, RNA interference, marker-assisted selection, anther culture, embryo culture, protoplast fusion, somaclonal variations etc. (Talakayala et al., 2020). These approaches are described in detail below.
2.1 Gene Transformation
Gene transformation or genetic engineering of crops for insect resistance involves incorporation of specific DNA segment or gene into crop plants to provide resistance against insect pests. The DNA segment which get introduced usually encode a protein with insecticidal activity. Resistance is plant is conferred against specific insect pest through the expression of an insecticidal protein present in the introduced DNA segment (Gatehouse, 2013). This technology has been tested against a wide range of insect pests belonging to orders; Lepidoptera, Coleoptera, and Diptera (Birkett and Pickett, 2014). Genetically modified crops producing insecticidal proteins from Bacillus thuringiensis, a soil bacterium, have been widely used in agriculture globally since their introduction in 1996 (Abbas, 2018). The cry gene transformation technology involves the transfer of specified DNA sequences or genes into crop plants via Agrobacterium-mediated transformation or particle bombardment (Juturu et al., 2015). Apart from this, other strategies to protect plant from insects attack have also been investigated. Lectins, usually found in a number of plants, bind to carbohydrates in the midguts of phytophagous insects disrupting the digestive system (Vandenborre et al., 2011). Transgenic techniques have also been used to deploy protease inhibitors, which are designed to prevent insects from digesting their food (Singh et al., 2020). Similarly, transgenic plants expressing alpha-amylase inhibitors have been produced which are resistant to Lepidopterans, Coleoptrans, Dipterans and Hemipterans insects. Also, insect chitinase and chitinase-like proteins play a significant role in degrading chitin in the exoskeletal and gut linings of insects. These have been successfully cloned into plants and show insecticidal properties. Each of these strategies along with their role in insect pest management is discussed in detail below:
2.1.1 Cry Genes
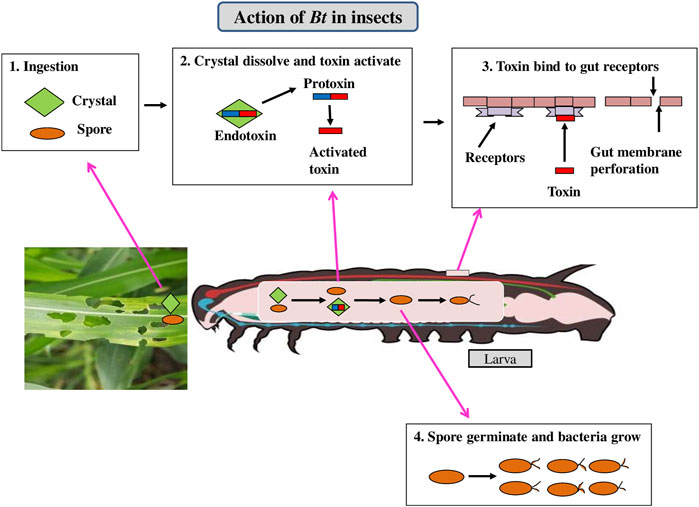
Bacillus thuringiensis (Bt) is a Gram-positive soil bacterium expressing insecticidal crystalline proteins (ICPs) that are exceptionally toxic to specific classes of pests (Panwar et al., 2018). Insecticidal activity in insect-resistant Bt crops is expressed by the genes coding for parasporal crystal protoxins (Palma et al., 2014). ICPs produced by transgenic plants have had a significant impact on the successful evolution of insect resistance. The crystal involves a protoxin protein which get solubilized in the larval midgut due to alkaline pH and subsequently cleaved enzymatically to an active toxin. The toxin diffuses through the peritrophic membrane covering the gut and binds to receptors present in the midgut epithelium (Paul and Das, 2020) making pores in the midgut epithelium. The gut gets paralyzed and then the pest stops feeding and dies within 2–3 days (Figure 2).
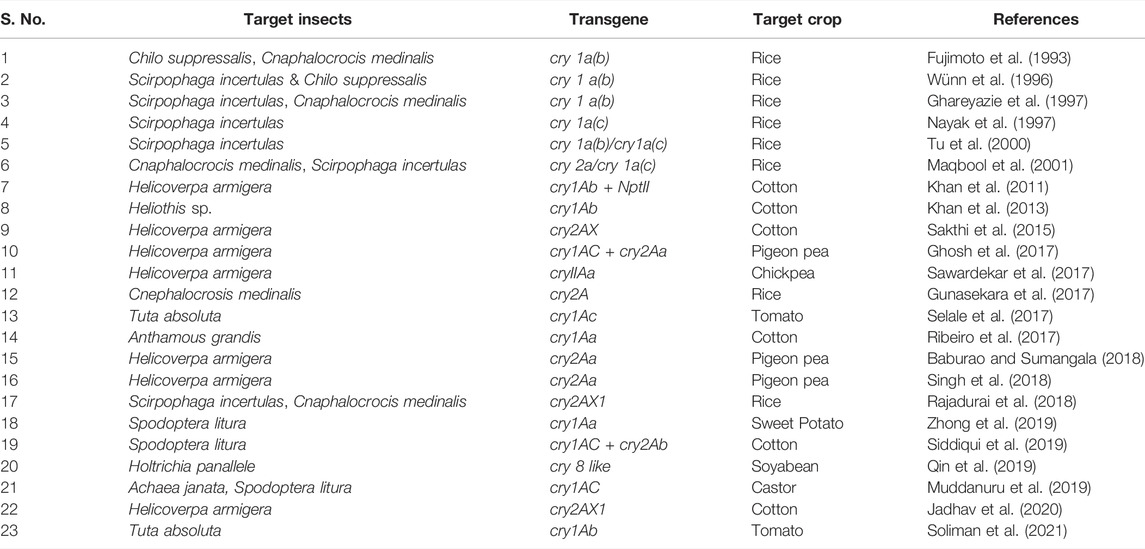
The first-generation Bt cotton, Bollgard I (BG I) expressingcry1Ac was commercialized and released in 2002 to control the dominant bollworms including Pectinophora gossypiella, Earias vittella, and Helicoverpa armigera in cotton-cultivating areas of India. After thatBollgard II (BG II) was launched in 2006 as a second-generation Bt cotton with pyramided traits expressing cry1Ac and cry2Ab (MON15985 event), which is now cultivated in 95% of the total India’s cotton sowing area. In comparison to BG I containing cry 1Ac alone, BG II having multiple toxins, cry1Ac and cry2Ab have greater ability for pest management (Carrière et al., 2015). Another transgenic cotton i.e., wide strike cotton expressing cry1Ac + cry1F was approved in the USA in the year 2004 by Dow Agro Sciences which improved both the crop yield as well as farmer’s income. It is observed that both BG II and wide-strike cotton expressing pyramided traits have a higher potential to suppress a wide range of Lepidopteran, Coleopteran and Dipteran insects than BG I. Another transgenic cotton containing cry10Aa exhibited strong resistance to cotton boll weevil (Anthonomous grandis) with 100% mortality observed through bioassay testing when the larvae of T1 generation consumed the leaves of transgenic cotton (Ribeiro et al., 2017). Agrobacterium-mediated transformation was employed to produce transgenic cotton expressing pyramided traits, cry1Ac and cry2Ab cloned in the T-DNA, and the resulting plant demonstrated resistance to S. litura with 93 percent larval mortality (Siddiqui et al., 2019). Transgenic rice lines constructed through the expression of the cry2AX1 gene showed resistance to rice leaf folder (C. medinalis) and rice yellow stem borer (S. incertulas) (Rajadurai et al., 2018).
Transgenic cotton and brinjal cultivars having resistance to borers were permitted for commercial usage in Bangladesh and in Latin America, insect-resistant Bt soybean expressing cry1Ac + cry1Ab were allowed for production during 2014 (Koch et al., 2015). Another study found that a synthetic cry1Ab gene introduced into tomato conferred resistance to the tomato leaf miner, T. absoluta, with 100 percent insect mortality at T0 generation within 4–5 days (Soliman et al., 2021). Rice lines (var. Bg94–1) produced by transferring the insecticidal protein cry2A cause mortality in rice leaf folders in 80% of cases (Gunasekara et al., 2017). Similarly, transgenic pigeon pea lines constructed using a combination of cry1Ac and cry2Aa exhibited resistance to H. armigera, resulting in 80%–100% larval mortality (Ghosh et al., 2017). Cry1Aa gene expression in sweet potatoes conferred resistance to Lepidopteran insect i. e, Spodoptera litura (Zhong et al., 2019). Expression of cry2AX gene in transgenic cotton event CH12 showed 88% mortality in H. armigera at T0 generation (Sakthi et al., 2015). et alAn industrially important non-edible castor developed by transferring the cry1Aa gene using Agrobacterium transformation technique, exhibited strong resistance against two lepidopteran pests, i.e., Achaea janata (semi-looper) and S. litura (Muddanuru et al., 2019). Transgenic soybean, expressing cry8-like gene from B. thuringenesis conferred resistance to Holotrichia parallela, a Coleopteran pest (Qin et al., 2019). The transgenic cotton event MNH93 carrying cry1Ab demonstrated 40–60 percent larval mortality against H. armigera with displaying 0.26 percent transformation frequency (Khan et al., 2011). In H. armigera, expression of the cry2AXI gene in the T3 generation of the cotton event CH12 resulted in 90% death (Jadhav et al., 2020). Other toxins or proteins, such as cyt2Aa, which provides aphid resistance (Chougule et al., 2013) and cry51Aa2, increased Lygus species mortality in cotton (Gowda et al., 2016). The expression of cry gene has been studied in different crop species and is depicted in Table 1. Despite the successful deployment of cry gene technologies in crops to achieve resistance against several insect pests, some of the agricultural pests often develop resistance to insecticidal toxins and devastate the crop production. Other problems that limit the usefulness of transgenic crops for insect control include secondary pest outbreak, evolution of new biotypes, effects on non-target organisms, environmental influences on gene expression, biosafety of food from transgenic crops, and socio-economic and ethical issues. Also the research groups should take up the challenges of understanding plant insect interactions to understand the mechanism of resistance development in insects against cry genes.
2.1.2 Vegetative Insecticidal Proteins Genes
The Bacillus thuringiensis (Bt) bacteria found in a variety of ecological habitats, has natural entomo-pesticidal properties against a variety of economically important crop pests due to the secretion of various proteins during different growth phases. One of the best known families of Bt proteins is Vip, which are produced during the vegetative growth phase of the plant and are recognized as an excellent toxic candidate due to the sequence homology and receptor sites differences from cry proteins. There are three subfamilies of Vip proteins. Vip1 and Vip2 heterodimer toxins, effective against pests belonging to Hemiptera and Coleoptera orders, whereas Vip3, the most extensively studied family of Vip toxins have toxicity toward Lepidopterans et al. Vip proteins are also known as second-generation insecticidal proteins, that can be used either alone or in combination with cry proteins to control a number of insect-pests (Gupta et al., 2021). In terms of toxicity potential against susceptible insects, these Vip3 proteins are comparable to cry proteins. They are reported to be toxic toward pests, which can’t be controlled with cry proteins. They reduce the insect pest’s population by osmotic lysis which causes swelling and interruption of the midgut epithelial cells. The Vip3 proteins have been successfully pyramided along with cry proteins in transgenic crops such as maize and cotton, to overcome resistant pest populations and delay the evolution of resistance (Syed et al., 2020).
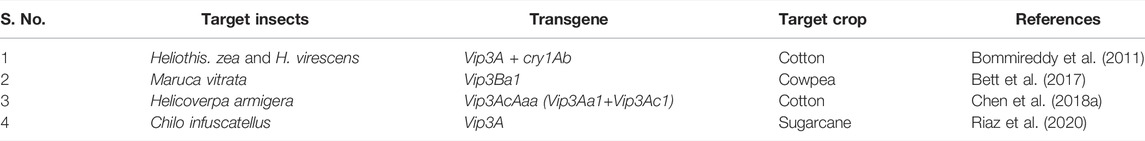
Vip genes exhibiting greater resistance against cotton bollworm (H. armigera) and tobacco budworm (Heliothis virescens) showing that these genes are an excellent option for these insect control. The transgenic cotton containing Vip3A alone and another multitoxin line expressing Vip3A and cry1Ab (VipCot) had greater resistance to both the insects, H. zea and H. virescens (Bommireddy et al., 2011). Transgenic cotton lines expressing Vip3AcAaa demonstrated greater insect resistance, showing that the Vip3AcAaa protein is highly effective in insect control (Chen et al., 2018a). The cowpea lines containing Vip3Ba1 exhibited greater suppression of larval growth and showed further resistance against the pod borer (Bett et al., 2017). A toxin Vip3A transferred in sugarcane showed superior resistance against sugarcane stem borer (Chilo infuscatellus) with 100% mortality (Riaz et al., 2020). The effect of the Vip gene in different crop species is depicted in Table 2. These proteins are promising candidates for further development of insect resistant plants and show extended ranges of toxicity particularly toward lepidopteran pests. Efforts are underway to use these proteins to induce insect pest resistance.
2.1.3 Lectins
Carbohydrate-binding proteins known as lectins, are entomotoxic proteins with insecticidal properties and are found in many plant species. They prevent predation by being detrimental to a variety of insects and animals that eat plants. They are mostly found in plants belonging to the families Solanaceae, Fabaceae and Poaceae; especially some of leguminous seeds contain a high concentration of lectins. Plant lectins act as storage proteins and are involved in defense mechanisms against phytophagus insects. Various plants lectins from different sources have already been reported to be toxic towards important members of insects belonging to Lepidoptera (Czapla and Lang, 1990), Coleoptera (Gatehouse et al., 1984; Czapla and Lang, 1990) and Homoptera orders (Powell et al., 1993; Sauvion et al., 1996). The first lectin discovered and commercially available was Concanavalin A; which is now the most extensively studied lectin for insect pest control. The adverse impact of lectins on biological parameters of insects includes, feeding inhibition, reduction in larval weight delays in adult emergence, retardation in total developmental period and increased mortality and reduced fecundity in the first and second generation (Powell et al., 1993).
Insect-resistant plants have emerged in recent years, paving the way for the use of plant lectins in pest management strategies. The use of lectins in transgenic plants has yielded promising results, particularly for crops expressing Bacillus thuringiensis (Bt) Cry toxins, which show resistance to sap-sucking insects. Furthermore, lectins in artificial diets and their expression in transgenic plants have been shown to reduce performance in insects of various orders, including Lepidoptera, Coleoptera, and Hemiptera.
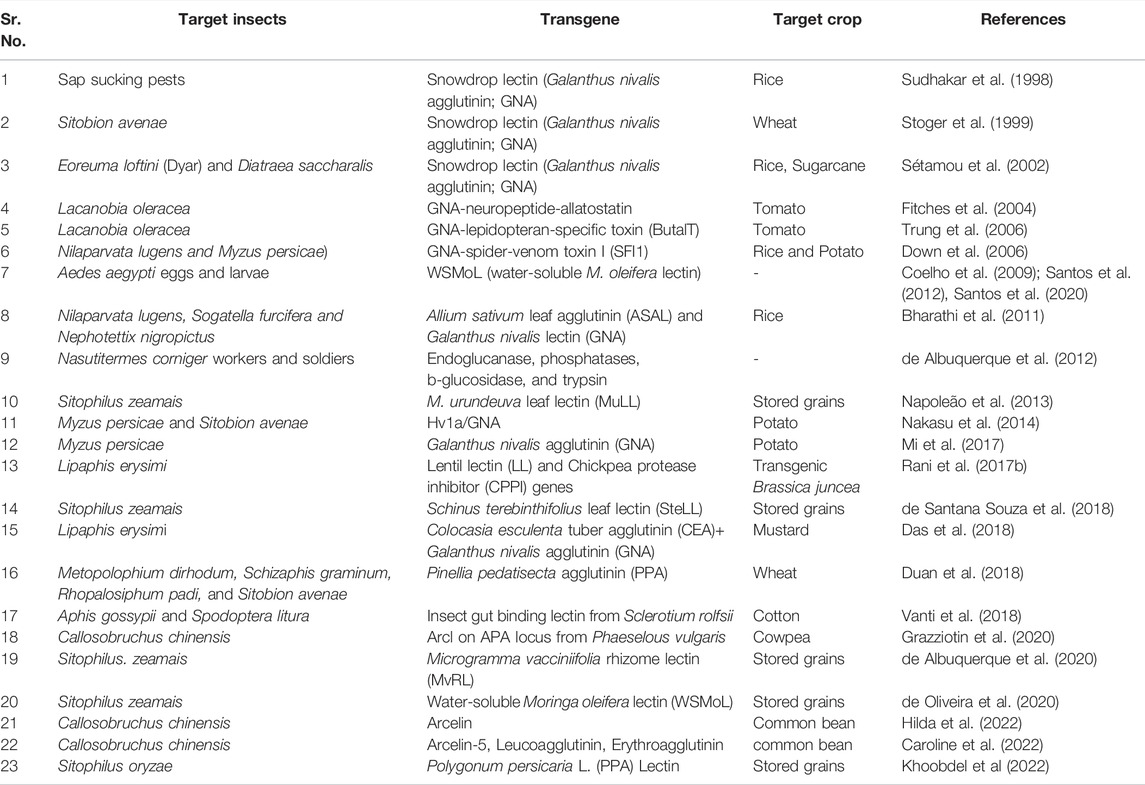
Plant lectins are carbohydrate-binding proteins that have greater affinity for certain sugar components found in glycoproteins and glycolipids in the cell membrane (Camaroti et al., 2017). Transgenic rice carrying Allium sativum leaf agglutinin (ASAL) and Galanthus nivalis lectin (GNA) conferred resistance to major sap-sucking insects like brown planthoppers (BPH), white-backed planthoppers (WBPH), and green leafhoppers (GLH) (Bharathi et al., 2011). Transgenic rice (Oryza sativa L.) plants expressing an insecticidal protein (the snowdrop lectin, GNA) produced through particle bombardment exhibited the significant levels of resistance against sap-sucking pests (Sudhakar et al., 1998). Enhanced toxicity of GNA-spider-venom toxin I (SFI1) fusion protein to larvae of the tomato moth (L. oleracea), rice brown planthopper (N. lugens), and the peach potato aphid (M. persicae) reported by Fitches et al. (2004) and Down et al. (2006). In an another study, the harmful effects of snowdrop lectin (GNA) expressed in transgenic sugarcane on the life cycle of Mexican rice borer Eoreuma loftini (Dyar) and sugarcane borer Diatraea saccharalis (F.) was reported by Sétamou et al. (2002). Expression of Galanthus nivalis lectin (GNA) gene showed resistance to aphids in potato (Mi et al., 2017). Transgenic Arabidopsis expressing recombinant fusion protein (Hv1a/GNA) exhibited resistance to peach potato aphids and grain aphids (Nakasu et al., 2014). A GNA-neuropeptide-allatostatin fusion protein was found to inhibit the feeding and growth of the tomato moth (L. oleracea). (Fitches et al., 2004). The larvae of the tomato moth were found to be more toxic to a fusion protein containing a GNA-lepidopteran-specific toxin (ButalT) from the South Indian red scorpion (Mesobuthus tamulus). (Trung et al., 2006). The transgenic cotton lines containing insect gut binding lectin demonstrated significant level of resistance to sucking and chewing insects at T1 generation (Vanti et al., 2018). The lentil lectin (LL) and chickpea protease inhibitor (CPPI) genes transferred to Brassica juncea lines and the resulting transgenic plants showed resistance to sap-sucking pests such as aphids (Rani et al., 2017b). Mannose-binding lectin expressed through Agrobacterium-mediated transformation, exhibited resistance to wheat aphid in BE104 (Duan et al., 2018). In a bioassay study it was found that the transgenic wheat plants produced through the particle bombardment method expressing the gene encoding snowdrop lectin (Galanthus nivalis agglutinin; GNA) showed reduced fecundity of grain aphids Sitobion avenae (Stoger et al., 1999). At T1 and T2 generations, transgenic B. juncea expressed a new lectin gene, Colocasia esculenta tuber agglutinin (CEA), as well as a GNA, exhibited enhanced resistance against mustard aphid (Lipaphis erysimi) (Das et al., 2018). Transgenic cowpea expressing arcelin 1 gene from Phaseolus vulgaris L. conferred greater resistance to bruchids like Zabrotes subfasciatus and Callosobruchus maculatus. In addition, against both bruchid insects, the percentage of eggs laid, hatching, adult emergence, and grain mass loss was much lower in transgenic cowpea than in control (Grazziotin et al., 2020). Hilda et al. (2022) demonstrated that arcelin found in the wild accession of common bean is an insecticidal protein that can prevent the bruchid beetle (Bruchidae: Coleoptera) from digesting it. Aside from focusing on coleopteran insects, arcelin was reported to be highly effective against specific insects that belong to Lepidoptera and Hemiptera insect order (Oriani and Lara, 2000; Malaikozhundan et al., 2003). The binding of lectin molecules to glycosylated proteins in the midgut of larvae reduces the efficiency of nutrient uptake and diet utilisation, resulting in a drop in total larval mass and decline in the average survival rate. (Paiva et al., 2012). Caroline et al. (2022) reported that Erythroagglutinin, Arcelin-5, Leucoagglutinin, and a hypothetical seed storage protein are responsible for bruchid resistance which is among the most devastating insect pest of the common bean. Findings of Khoobdel et al. (2022) indicated that a lectin derived from Polygonum persicaria L. (PPA), causes oxidative stress in Sitophilus oryzae in addition to causing digestive disorders,. The leaf lectin (SteLL) from Schinus terebinthifolius did not cause death in S. zeamais adults, according to Camaroti et al. (2018) and de Santana Souza et al. (2018), but it did inhibit protease activity and promote amylase activity in the digestive system. MvRL was found to affect the activity of gut endoglucanase, phosphatases, b-glucosidase, and trypsin-like enzymes in Nasutitermes corniger workers and soldiers, reported by de Albuquerque et al. (2012).
Plant lectins have been found to be biologically active against a variety of insects. Chitin-binding lectins derived from Microgramma vacciniifolia rhizome lectin (MvRL) showed anti-nutritional effects on survival, feeding, and nutrition of Sitophilus zeamais adults (de Albuquerque et al., 2020). Moringa oleifera seeds containing a water-soluble lectin (WSMoL) negatively impacted the physiology of the pest Sitophilus zeamais, which could have long-term consequences (de Oliveira et al., 2020). According to Napoleão et al. (2013), the leaf lectin (MuLL) derived from M. urundeuva adversely affected the activity of digestive enzymes in the stomachs of S. zeamais adults, inhibiting digestive processes. Seeds containing the lectin WSMoL (water-soluble M. oleifera lectin) have been shown to be insecticidal against Aedes aegypti eggs and larvae. (Coelho et al., 2009; Santos et al., 2012, Santos et al., 2020). The survival and nutritional parameters of S. zeamais adults are negatively affected by the ingestion of an artificial diet containing a saline concentrate from Schinus terebinthifolius Raddi leaves (LE) or its lectin (SteLL, S. terebinthifolius leaf lectin). (Camaroti et al., 2018). However, progress in lectin research has been hampered due to concerns about the effects of ingesting snowdrop lectin on higher animals,, although no adverse effect was seen in rats fed for 90 days on transgenic rice containing GNA was seen (Poulsen et al., 2007). Insect resistance was demonstrated by the expression of lectin genes in various crop plants (Table 3). Although, lectins have been found to have negative effects on insect pests of various orders and stages of development, preventing growth, survival, nutrition, development, and reproduction (Napoleão et al., 2019). However, because of their known toxicity to mammals and humans, caution should be exercised in their use in transgenic plants.
2.1.4. Fusion Proteins
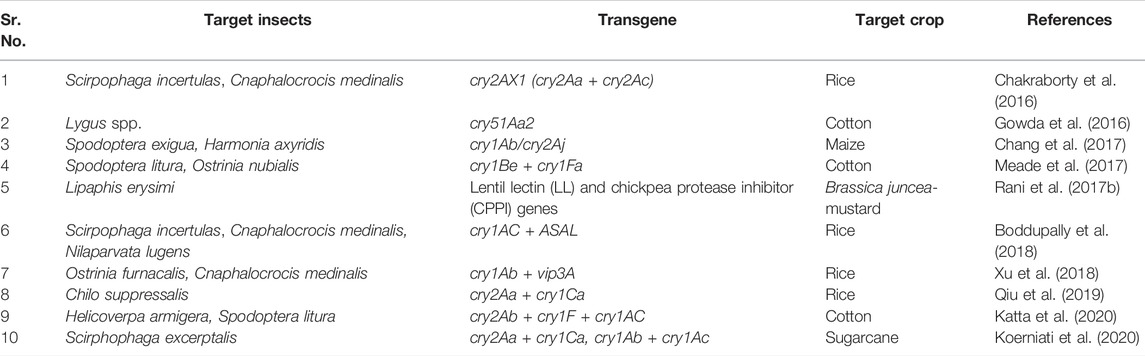
Bt insecticides are widely used, with Bt toxins accounting for up to 90% of microbiological insect control products. As a result, there’s a possibility that insects will become resistant to Bt toxins. (Tabashnik, 1994; Ferré et al., 1995). The diamondback moth (Plutella xylostella) has evolved resistance in some open field populations in response to repeated exposure to foliar sprays containing Bt proteins. (Perez and Shelton, 1997), whereas recessive mutant alleles can confer resistance to multiple Bt toxins in laboratory selection experiments with other insect pests (Tabashnik et al., 1996). The stacking or pyramiding of multiple transgenes in the same transgenic plant and the use of hybrid toxins against insect pests are two recent strategies to address potential limitations in conventional transgenic insect pest control. et alThus fusion proteins are formed by joining together different insecticidal proteins. In the host plant system after transcription and translation these proteins form a single polypeptide units which are more effective against phytophagous insects. Dow Agro Sciences’ transgenic cotton lines carrying a hybrid fusion protein, cry1Be + cry1Fa, demonstrated increased resistance to S. litura and O. nubilalis insects (Meade et al., 2017). Transgenic rice containing the cry2AX1 (derivative of cry2Aa and cry2Ac) gene was viewed as resistant to lepidopteran insects, as indicated by Chakraborty et al. (2016). Koerniati et al. (2020) reported that the synthetic sugarcane expressing cry1Ab + cry1Ac fusion protein showed resistance against shoot borer. Agrobacterium-mediated transformation of transgenic B. juncea expressing a fusion protein derived from lectin and a protease inhibitor resulted in resistance to phytophagous aphids. (Rani et al., 2017a). In another case study, Chang et al. (2017) demonstrated that transgenic maize containing cry1Ab/cry2Aj fusion protein in kernel conferred higher mortality in S. exigua, a lepidopteran pest and Harmonia axyridis, a coleopteran pest. Transgenic rice line expressing cry1Ac + ASAL conferred durable and enhanced resistance to major insects such as yellow stem borer, leaf folder,, and brown plant hopper (Boddupally et al., 2018). Pyramiding of cry1Ab + vip3A showed resistance against the rice leaf folder and Asiatic rice borer (Xu et al., 2018). A significant level of protein expression was observed in transgenic rice lines carrying cry2Aa + cry1Ca protein lethal to Asiatic rice borer, Chilo suppressalis (Qiu et al., 2019). Katta et al. (2020) investigated that the transgenic plant developed by transferring a triple gene construct containing cry2Ab + cry1F + cry1Ac genes into an elite cotton variety (Narasimha) conferred a significant level of mortality to H. armigera and S. litura at T2 generation. An account of studies utilization of the fusion proteins used for insect resistance in crop plants for pest management is depicted in Table 4. Pyramiding of two or more genes is a sustainable strategy for achieving good management of lepidopteran, coleopteran and hemipteran insects pests. Furthermore, these innovations could pave the way for development of insect resistant crops by delaying the phenonmenon of resistance development in insects.
2.1.5 Protease Inhibitors
Protease inhibitors (PIs) are plant-derived inhibitors that prevent insect pests from digesting their food by inhibiting the activity of digestive proteases (Haq et al., 2004; Macedo and Freire, 2011; Zhu-Salzman and Zeng, 2015). Insect digestive proteases are known to be inhibited by PIs, through preventing proteolysis and results in decreased fecundity, increased mortality and longer developmental period due to the deficiencies of essential amino acids. The most investigated plant PIs against pests are serpins and cystatins. Serpins, with a molecular mass of approximately 39–43 kDa, are irreversible serious inhibitors of serine proteases. Serine proteases have been discovered in insect orders like, Diptera (flies), Lepidoptera (moths and butterflies), Orthoptera (grasshoppers, locusts), Coleoptera (beetles) and Hymenoptera (bees and wasps) (Irving et al., 2002). Cystatins, a PIs protein with a molecular mass of 12–16 kDa, inhibit the activity of cysteine proteases, which are the primary digesting proteases in Coleopterans and Hemipterans. Several studies have also reported that volatile compounds such as methyl jasmonate, one of the key regulators of plants’ defensive response to insect herbivores, inhibit gut protease after wounding. (Singh et al., 2016), cause neighboring unwounded plants to produce proteinase inhibitors, effectively prearming the local population against insect attack (Stevens et al., 2012). Legume trypsin inhibitors inhibit a wide spectrum of proteases and have an insecticidal activity against a variety of key insects (Macedo et al., 2004; Sharma, 2015). Protease inhibitor genes were incorporated in rice cultivars (Duan et al., 1996; Xu et al., 1996) to improve protection against stem borers, and wheat (Altpeter et al., 1999) to protect them against foliage-feeding and storage pests. Protease inhibitors when fed to insect pests either through artificial diet or transgenic plants resulted into increased insect mortality (de Pg Gomes et al., 2005; Gatehouse, 2011) and adversely affected the growth and development of insect larvae from different insect orders (de Pg Gomes et al., 2005; A,; Gatehouse, 2011) (Burgess et al., 1994; De Leo et al., 2001; Outchkourov et al., 2004; Ribeiro et al., 2006; Tamhane et al., 2007; Dunse et al., 2010; Schneider et al., 2017). Figure 3 depicts the success and failure of protease inhibitors in a variety of insect pests. Protease inhibitors may enlighten a new dimension in insect pest management. However, due to lack of understanding of insect physiology and biochemistry, it suffered great failure in recent past. Also PIs turned worthless due to immense adaptive potential of insect pests and its long coevolutionary relationship with host plant. Solving these issues could pave the way for future research.
2.1.6 α-Amylase Inhibitors
α-amylase is a digestive enzyme present in insects for digestion of carbohydrates. An α-amylase inhibitor, affect digestion in insects by inhibiting the activity of α-Amylase enzyme in insects. Various types of α-amylase inhibitors, present in seeds and vegetative organs of plants, was found to control a numbers of phytophagous insects (Chrispeels et al., 1998). Seeds of Phaseolus vulgaris expressing an α-amylase inhibitor negatively affected the growth and development of cowpea weevil Callosobruchus maculatus and Azuki bean weevil Callosobruchus chinensis (Ishimoto and Kitamura, 1989; Shade et al., 1994). Morton et al. (2000) reported that transgenic pea and azuki bean seeds expressing the inhibitor, aAI-1, exhibited enhanced resistance against bruchids, the pea weevil (Bruchus pisorum), the cowpea weevil (Callosobruchus maculatus) and the azuki bean weevil (Callosobruchus chinensis). Kaur et al. (2022) investigated that higher activity of α-amylase inhibitors in the central whorl leaves and stems of maize genotypes might be responsible for inducing resistance against Chilo partellus infestation. The multiplication and damage of Rhyzopertha dominica, a major pests of stored wheat grains can be effectively controlled by inhibiting the α-amylase enzyme through wheat α-amylase inhibitors (Priya et al., 2010). A gene named aAI-Pc1, encoding an α-amylase inhibitor was isolated from cotyledons of Phaseolus coccineus and introduced into coffee plants, confers resistance to coffee berry borer, Hypothenemus hampei (de Azevedo Pereira et al., 2006). Recently, a wheat gene encoding an α-amylase inhibitor was expressed in tobacco, resulting in increased resistance to Spodaptera spp. and Agrotis spp (Jaiswal et al., 2018). Isolation of a novel alpha-amylase inhibitor from papaya seeds (Carica papaya) showed increased larval mortality, decreased insect fecundity and adult longevity of cowpea weevil (Callosobruchus maculates) (Farias et al., 2007). Therefore these studies indicates the successful utilization of α-amylase inhibitors in insect pests management.
2.1.6.1 Insect Chitinase
Insect chitinases are the hydrolytic enzymes having potential to inhibit or degrade the chitin. In insects, chitin is the main component of the exoskeleton and peritrophic membrane. It provide protection from harsh environmental conditions, external mechanical disruption and natural enemies (Chen et al., 2018b). The hydrolysis of chitin is essential for ecdysis (periodic shedding of the old cuticle). Chitinases are expressed in various organisms including those that lack chitin such as plants to recognize and degrade the chitin in chitin containing insects ((Oyeleye and Normi, 2018). Transgenic expression of chitinase enzyme has been proposed as a crop protection strategy. Role of chitinase enzyme in insect pests management has been studied in several insects such as silkworm B. mori, rice brown planthopper N. lugens, cotton mealybug P. solenopsis and rice striped stem borer C. suppressalis (Pan et al., 2012; Xi et al., 2015; Su et al., 2016; Omar et al., 2019). Transgenic maize plants expressing a chitinase gene showed enhanced resistance against corn borer (Sesamia cretica) (Osman et al., 2016). Insect chitinases have been established as biopesticides and transgenes in crop protection due to the inhibitory effects on the growth and development of insects. Not much research on insect chitinase has been done and lack of structural information on some insect chitinase has hampered the development of potential agrochemicals targeting insect chitinase. Better understanding of their structure and biochemistry will accelerate their usage in biotechnological processes.
2.2 Genome Editing
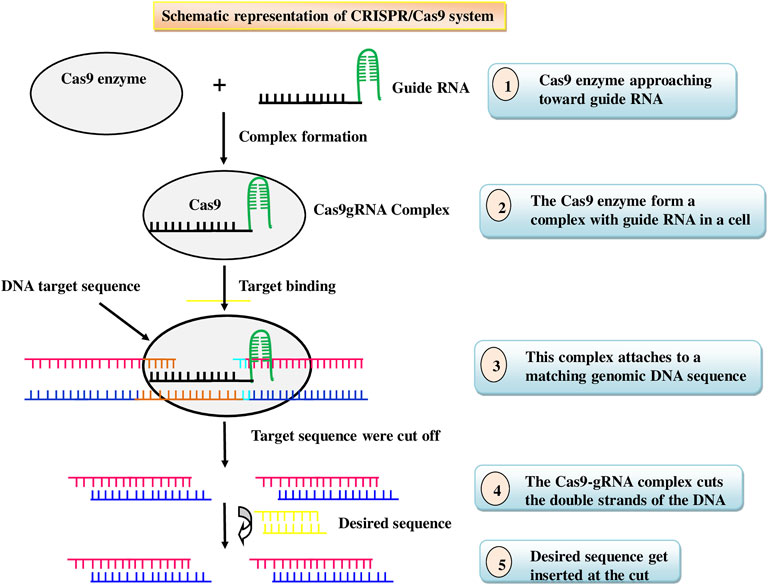
Insects acquiring resistance to the Bt traits has posed a threat to agricultural productivity, prompting researchers to seek out novel, cost-effective, and environment friendly techniques to insect pest management, as well as ways to combat insect resistance. Nowadays, insect pest management tactic has shifted to gene editing, which is a newer and more advanced method (Anastacia Books, 2019). Gene editing, also called genome editing, is a technique that involves inserting, deleting, or replacing DNA bases in a specific target DNA sequence of the genome for effectively altering the function of a gene by using the cell’s natural mechanisms (Bortesi and Fischer 2015). It is one of the most widely used technologies in present-day science that empowers researchers to change/alter a living being’s DNA (Belfort and Bonocora, 2014). It is an emerging opportunity increasingly being used in insect pest management through expanding its possibilities and opportunities to enhance plant resistance to insect pests. Nucleases are used in these technologies to cut certain genomic target sequences. Two types of genome editing tools, comprising transcriptional activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9, are accessible and are frequently applied. Cas9 protein and single-guide RNAs (sgRNAs) which can be easily designed are the two main parts of the CRISPR/Cas9 system, while TALEN requires to be redesigned to target different loci each time. Cas9-mediated genome editing is achieved by a process: DNA cleavage followed by DNA repair (Figure 4). Furthermore, the CRISPR-Cas9 technology allows researchers to add, remove, replace, or regulate genes in a variety of animals, resulting in heritable, targeted modifications that were previously difficult to create (Ricroch et al., 2017). One of the most important practical advantages of CRISPR-Cas9 technology is multiplexing, or the introduction of double-stranded breaks (DSBs) at many locations in the genome that can be used to edit multiple genes simultaneously (Li et al., 2013). Thus, in recent times, CRISPR/Cas9 has emerged as a technically simple, newest, most effective, and an effective tool for developing insect pest resistance. It has been successfully used to prevent the accumulation of specific gene products in a variety of crops by either deleting the gene or inducing missense mutations in the target gene (Gao, 2021). Most polyphagous insects use the plant’s own volatiles, gustatory signs, visual appearance, oviposition sites, and collaborations to recognise host plants. (Larsson et al., 2004). Genome editing can be utilized to change plant volatile mixtures, which could be an alternative pest management strategy. However, caution should be taken to ensure that the alteration has no negative consequences for the beneficial insect population. In a study, the overproduction of anthocyanin pigmentation caused the transgenic tobacco plant’s leaves to turn red which deterred both the herbivores Spodoptera litura and Helicoverpa armigera due to change in leaf color (Malone et al., 2009). In an anotherstudy, CRISPR/Cas9 was usedto target six loci associated with tomato yield and efficiency in wild tomato S. pimpinellifolium (Zsögön et al., 2018). Although this wild tomato shows resistance to a numberof arthropod insects, including spider mites, and produces modest yields (Rakha et al., 2017). Ming et al. (2021) demonstrated that the CRISPR/Cas9 genome editing system is an effective tool for studying the function of SfABCC2, a Cry1F gene receptor that confers resistance to S. frugiperda. Insect-resistant rice plants with mutations in the cytochrome P450 gene CYP71A, which catalyses the conversion of tryptamine to serotonin, accumulated high levels of salicylic acid but lacked serotonin. (Lu et al., 2018). This could make genome editing more appealing than transgene stacking for the production of next-generation insect-resistant crops. Although CRISPR gene editing is an effective tool to combat insect pest problems as it has the capacity to alter the specific gene of interest. However, commercial use of CRISPR/Cas9 in insect pest management is still in its early stages. It has been extensively reformed for various applications in model animals, which may reveal potential insect applications. The list of insect pests engineered for pest management using CRISPR/Cas9 are given in Table 5.
2.3 RNA Interference for Plant Resistance to Insect
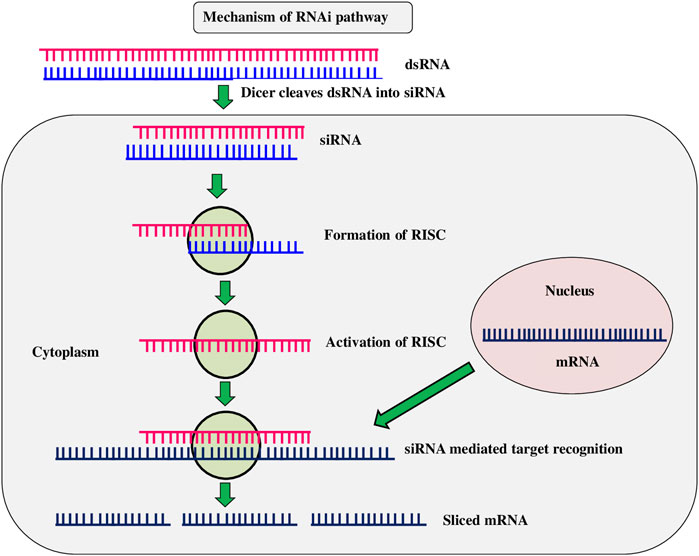
RNAi is a method of suppressing gene expression by suppressing specific sequences and is known by various names, including co-concealment, post-transcriptional gene silencing (PTGS), and suppressing. It is an advancement of novel gene silencing mechanisms triggered by double-stranded RNA at the cellular level. (Figure 5). When a double-stranded RNA (dsRNA) is injected into a cell, it makes undesirable genes to be repressed (Kamthan et al., 2015). The RNAi strategy for pest control is based on ingestion of double-stranded RNA (dsRNA) into the target pest system. After ingestion, dsRNA expresses either through hairpin or by other different means and spread throughout the insect system (Katoch et al., 2013). Transgenic Bt toxins are mostly effective against Lepidoptera and Coleoptera larvae, by acting in the mid-gut of susceptible target insects, leaving other insect orders unmanaged. The RNAi technique was expected to be able to control a wider range of insects, especially sap-sucking insects, which transgenic crops had failed to control. It also opens up new possibilities for eco-friendly insect pest control in agricultural crop plants (Mamta and Rajam 2017). dsRNAs are often utilized in plants to interfere with specific gene silence in order to develop disease resistance through genetic changes. Resistance to C. suppressalis was provided by rice knockdown lines TT51 (cryAb and cry1Ac) and T1C-19 (cry1Ac) with two aminopeptidase N genes (APN1 and APN2) (Qiu et al., 2017). Western corn rootworm (D. virgifera) fertility and larval feeding were reduced when the dvvgr and dvbol genes were silenced in maize (Niu et al., 2017). The use of a dsRNA/nano carrier formulation to target the TREH, ATPD, ATPE, and CHSI genes resulted in a greater proportion of soybean aphid (Aphis glycines) mortality (Yan et al., 2020). Transgenic cotton lines generated by combining Bt toxin with RNAi caused inhibition of juvenile hormone methyl transferase (JHMT) in H. armigera. (Ni et al., 2017). Knockdown of acetylcholine esterase gene (AChE) in rice lines resulted in reduced larval length and weight of yellow stem borer within 15 days (Kola et al., 2019). Ingestion of double-stranded (ds) RNAs in an artificial diet causes RNA interference in several coleopteran species, including the western corn rootworm (WCR) Diabrotica virgifera virgifera, which results in larval stunting and mortality. (Baum et al., 2007). The RNAi mechanism was tested by the spraying of dsRNAs in maize, resulting in gene knockdown and increased insect mortality rates in piercing, sucking, and stem borer insects (Li et al., 2015). Mamta et al. (2016) found that HI-RNAi produced induced death and developmental abnormalities in H. armigera larval, pupal, and adult stages when the chitinase gene (HaCHI) was silenced to establish resistance in tobacco and tomato. Tomato plants with a dsRNA targeting a gene encoding a phenolic glucoside malonyltransferase, which detoxifies phenolic glycosides, were recently found to be completely resistant to the tobacco whitefly, Bemisia tabaci (Xia et al., 2021). The Colorado potato beetle (Zhang et al., 2015), tobacco whitefly and tobacco hornworm, Manduca sexta (Burke et al., 2019), were all killed by chloroplast-expressed dsRNAs (Dong et al., 2020). Di Lelio et al. (2022) reported that when Spodoptera littoralis larvae eat tobacco plants expressing a dsRNA targeting the Sl 102 immune gene, the gene get silenced (et al.). RNAi technology is effective in knocking down target genes in a variety of insect orders, including Diabrotica v. virgifera, maize rootworm larvae (Khajuria et al., 2015). These findings suggest that to generate insect-resistant plants, RNAi is one of the most effective methods. However, the technology is currently being investigated, and its existing limitations make it less viable as an insect pest management strategy (Anastacia Books, 2019).
The most challenging task of this technology is allowing for efficient dsRNA uptake by the insect. The generation and transport of dsRNAs have been proven in two ways. The first is HIGS (host induced gene silencing), which involves the transgenic expression of dsRNAs derived from the crop genome. In this method, insects supposed to feed on the crop will eat the dsRNA. In the second strategy, dsRNAs are synthesized in high concentrations and applied to insect-infested crops as a foliar spray. Spray-induced gene silencing is the name for this method (SIGS). The target genes will be silenced in both approaches in the target species (Christiaens et al., 2020a). Many challenges are still there. The inherent ability of RNA, while ensuring that dsRNAs don’t persist in the environment, is destructive in unfavorable environmental conditions and restricts opportunities for SIGS approaches (Bramlett et al., 2020). Similarly, while some insects quickly take up dsRNA, resulting in high death rates, other species have low dsRNA take-up and nuclease degradation, resulting in inefficient outcomes. (Christiaens et al., 2020b; Shaffer, 2020). Success is also dependent on whether sufficient amount of dsRNA accumulate in the tissues on which the insects feed. The development of novel spray formulations, many of which use nanomaterials, is being used to address dsRNA stability and uptake in ongoing research. (Christiaens et al., 2020b). Several studies on insect pest management using the RNAi tool are shown in Table 6.
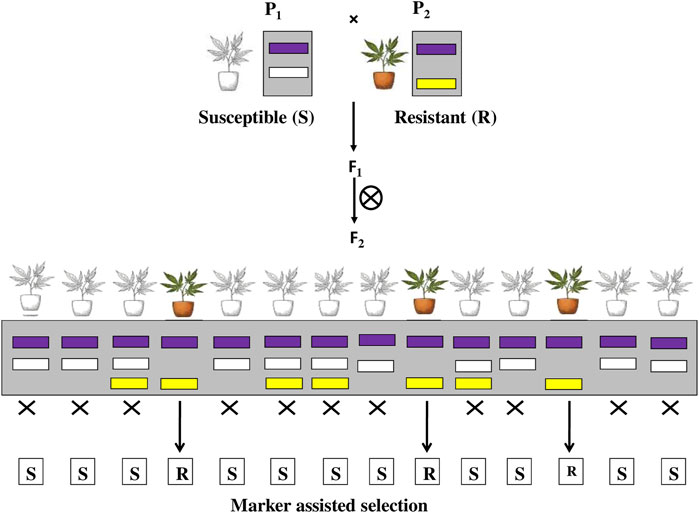
2.4 Marker-Assisted Selection
The use of molecular markers to assist phenotypic selections in crop improvement is known as marker-assisted selection (MAS). It involves selecting individuals based on their marker pattern (genotype) rather than their observable traits (phenotype) as shown in Figure 6. There are various types of molecular markers, such as single nucleotide polymorphism (SNP), have been recognised and have shown great promise in enhancing the efficiency and accuracy of conventional plant breeding. Molecular marker techniques are the most advanced method for transferring desired genes into desired crop plants in the required combination. It is the most widely used molecular techniques, and their application is a novel opportunity for increasing the yield of crop (Das et al., 2017). MAS studies showed introgression of Bph14 and Bph15 through molecular marker-assisted selection (MAS) to enhance the resistance in Minghui 63 and its derived hybrids against BPH (Hu et al., 2012). Resistance to bacterial blight (BB) and brown planthopper (BPH) was achieved in Yuehui9113 and F1 hybrids by pyramiding one BB resistance gene (Xa21) and two BPH resistance genes (Bph14 and Bph15) in Yuehui9113 using a marker-assisted backcrossing (MABC) strategy combined with phenotypic selection (He et al., 2019). Rice line, ASD7 expressing a BPH resistance gene bph2 when crossed to a susceptible cultivar C418, a japonica restorer line and evaluated through marker-assisted selection (MAS) exhibited significantly higher resistance against brown plant hopper Nilaparvata lugens, one of the most destructive pests of rice crop (Li-Hong et al., 2006). Liu et al. (2016) investigated that the pyramiding of two brown plant hopper resistance genes Bph3 and Bph27 (t), into elite rice cultivars through marker-assisted pyramiding showed significantly enhanced resistance against BPH and reduction in the yield loss caused by BPH. Shabanimofred et al. (2015) developed rice cultivars through marker-assisted selection (MAS) that provided resistance in rice against biotypes 2 and 3 of brown planthopper (BPH). Sharma et al. (2004) used marker-assisted pyramiding to successfully construct the Bph1 and Bph2 resistance genes on rice chromosome 12 to provide resistance against rice BPH. et al. As a result, using MAS to improve pest resistance would be very beneficial. There are various advantages of using MAS to enhance selection efficiency of insect resistant plants 1) It can be performed on seedling material, 2) less affected by environmental conditions, 3) MAS may be cost effective and faster than conventional phenotypic assays, 4) multiple markers can be evaluated using the same DNA sample etc. But the potential drawbacks of MAS are 1) Recombination between the marker and the gene of interest may occur, leading to inaccurate results 2) Incorrect estimates of QTL locations and effects may result in slower progress than expected, 3) Markers developed for MAS in one population may not be transferrable to other populations.
2.5 Anther Culture
Anther culture is a technique by which immature pollen is allowed to divide and grow into tissue (either callus or embryonic tissue) with the intention of generating haploids (plants with a N chromosome number). In this process, pollen-containing anthers are separated from a flower and placed in a suitable growing medium. An artificial medium could be used to culture anther or pollen grain in vitro. Anther can produce a callus, shoot, root, and eventually the entire plant in an artificial medium. All of the plants that are grown are haploid. It is the most viable and effective way for rapidly producing homozygous haploid plants. This method can hasten the development of a homozygous population of insect-resistant plants. Rice anther culture lines, 952836, 953508, 953509, 953510, 953511, 953527 and 953541 showed moderate level of tolerance to the rice water weevil, Lissorhoptrus oryzophilus Kuschel (N’guessan et al., 1994). Park et al. (2014) develop multi-resistant rice lines using anther culture for providing resistance against bacterial blight, rice stripe virus and brown planthopper.
2.6 Embryo Culture
Embryo culture is a technique in which immature or mature zygotic embryo is recovered without injury which normally aborts. These embroys are further cultured on the artificial nutrient media under an aseptic environment to get a vigor and viable plant through a successful ontogeny process. The standardization of the nutrient medium is required for induction of embryogenesis and seedling development. Generally wild species are often more resistant to insect pests. Wide hybridization has been used to transfer genes conferring insect-resistant from wild species to cultivated plants. It has been observed that such hybridization leads to the production of abnormal inter-specific hybrid embryos, which can be rescued using embryo culture technique. Jaiswal et al. (2018) has reported studies of insect resistant genes transfer from wild to cultivated species in wheat, rice, peanut, lettuce and cotton using embryo culture technique.
2.7 Protoplast Fusion
Protoplasts are plant cells of which cell walls are taken out and the cytoplasmic membrane is the peripheral layer. Protoplast can be isolated by digesting the cell wall with specific lytic enzymes. Protoplast fusion is a physical phenomenon, wherein at least two protoplasts come together and stick with each other either spontaneously or in presence of fusion-inducing agents. By protoplast fusion, it is feasible to transfer a few desirable genes from one species to another. For example, pest resistance characteristics may be present in one of two species that cannot be sexually hybridized. In this situation, protoplast fusion may result in the formation of a hybrid between two species. Protoplasts can be cultivated in an artificial medium, and some of them will grow into full-fledged plants. Thus the plants produced may be carrying the resistant traits. Also, it is the only means of combining two cytoplasmically inherited characteristics in a single genotype. Protoplast derived clones produced by Mexican wild species and cultivated potato species using protoplast fusion system expresses a significant level of resistance to both Colorado potato beetle and potato late blight (Chen et al., 2008).
2.8 Somaclonal Variation
Insect-resistant varieties can be selected through somaclonal variation. These can be chosen using the procedures below. 1) High-yielding varieties’ calli or cell suspensions were cultured for numerous or long-term cycles, 2) long-term cell lines were regenerated into plants, and 3) the regenerated plants were tested against target insects. In field plots, about 2000 sugarcane seedlings were tested for resistance to the sugarcane borer under artificial and natural infestations and it was found that some somaclones were reported to be resistant to sugarcane borer. The same process was utilized to develop sorghum somaclones, resistant to the autumn armyworm. Diawara et al. (1996) reported that somaclonal lines K-26 [1], K-180 [3]2 and K-128 exhibited improved resistant to celery major insect, Spodoptera exigua.
Although the above-mentioned plant tissue culture techniques like anther culture, embryo culture, protoplast fusion, and somaclonal variations proved to be more efficient in the development of plants resistant to various insect pests. However, these techniques are not widely used due to some potential drawbacks, such as high costs, the production of harmful secondary metabolites that kill the desired insect-resistant plants, the medium required for growth is not known, and so on. Also, due to advancements in technology, which are more efficient, quick, and reliable, these methods are no longer used.
3 Conclusion and Future Outlooks
Insects are the major concern for declining the agricultural production. To cope with the problem of insect pests, farmers are more inclined to the use of chemical insecticides as these provide a quick solution to the problem. The rapidly increasing awareness of the human and animal health issues as well as environmental impacts, of indiscriminate use of pesticide has offered new incentive to the potential alternative pest-control methods. In this perspective, host plant resistance is an environmentally friendly control method that is an important part of IPM (Integrated pest management) programmes. The development of insect-resistant varieties offers a stable and cumulative effect on the pests’ population and has no harmful effect on the environment. The identification of insect pest resistant sources in various crops has made significant progress. However, development of insect resistant crop varieties through conventional methods is slow and difficult to attain due to the entanglement of quantitative traits at multiple loci. New opportunities in the form of newer biotechnological tools have opened new ways of pest control and offers great opportunities to develop a sustainable, multi-mechanistic resistance to insect pests. Biotechnological approaches are now being used to develop novel plant resistance characteristics that provided excellent protection against invasive and destructive crop pests in a variety of crops by utilization of novel molecules, exploiting insecticidal genes and changing the level and pattern of expression of genes. Many insect-resistant plants have been developed as a result of biotechnology like corn, rice, cotton, canola, soybean, tobacco, apple, potato etc. With the advent of several tools of biotechnology such as genome editing, genetic transformation, anther culture, embryo culture, protoplast fusion, somaclonal variation, and marker-assisted selection will accelerate the development of insect-resistant crops now and in the future. By expressing bacterial delta-endotoxins, vegetative insecticidal proteins, and other plant qualities like lectins, protease inhibitors, etc., hereditary designing will guide towards the development of insect-resistant crops at much faster rate. Furthermore, RNA interference and genome editing by CRISPR/Cas9 offer novel approach to the production of insect-resistant crops. Therefore, biotechnology have come as a boon in tackling global pest problem, contributing to the development of noval insect resistant crop plants that have proven to be cost effective, pesticide-resistant, and environmentally safe. Despite the utilization of modern technology in crops to achieve resistance to a variety of insect pests, some agricultural pests frequently develop resistance to insecticidal toxins, wreaking havoc on crop productivity. The obstacles of understanding plant-insect interactions should be addressed by the research groups. To develop plants resistant to insects advances like RNAi and CRISPR techniques can be used to silence/edit sensitive or negative regulatory alleles of plant immune genes. New advancements that give more viable solutions for arising pests can improve and supplement the perseverance of plant-resistant elements. However, before the commercialization of an insect pest resistant transgenic crop variety, it is pertinent to study the potential impacts on environment specifically on non-target organisms. Also, the benefits and hazards associated with the adoption of insect-resistant crops, particularly for developing nations and resource-poor smallholder farmers, should be considered prior to carry out such initiatives. No doubt, biotechnology has opened the door to a plethora of novel ways for controlling insect pests, many of these products will necessitate regulatory frameworks that may not currently exist for certain products or in some places. They will also require the support of producers and consumers, which will necessitate open conversations, including the potential for new technology to make a significant contribution to societal change. In short it can be concluded that biotechnology exhibits unique applications of science that can be used for the welfare of society through the development of crops with improved nutritional quality, resistance to pests and diseases, and low cost of production. Biotechnology, in this context, is an aspect of science that, if used with caution and ethics, has the potential to offer substantial benefits.
Author Contributions
PK and DK—Collected and compiled the literature. PJ: Conceived the idea and wrote the manuscript. PK, SaK, CM, SuK, and GS: Assisted in manuscript writing.
Funding
Funds will be given by ICAR.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.914029/full#supplementary-material
References
Abah, J., Ishaq, M. N., and Wada, A. C. (2010). The Role of Biotechnology in Ensuring Food Security and Sustainable Agriculture. Afr. J. Biotechnol. 9 (52), 8896–8900.
Abbas, M. S. T. (2018). Genetically Engineered (Modified) Crops (Bacillus Thuringiensis Crops) and the World Controversy on Their Safety. Egypt. J. Biol. Pest Control 28 (1), 1–12. doi:10.1186/s41938-018-0051-2
Albuquerque, L. P. D., Procópio, T. F., Guedes, C. C. D. S., Pontual, E. V., Paiva, P. M. G., and Napoleão, T. H. (2020). Antinutritional Effects of the Chitin-Binding Lectin from Microgramma Vacciniifolia Rhizome (MvRL) on Sitophilus Zeamais. J. Stored Prod. Res. 88, 101652. doi:10.1016/j.jspr.2020.101652
Alemu, M. (2020). Trend of Biotechnology Applications in Pest Management: a Review. Int. J. Appl. Sci. Biotechnol. 8 (2), 108–131. doi:10.3126/ijasbt.v8i2.28326
Altpeter, F., Diaz, I., McAuslane, H., Gaddour, K., Carbonero, P., and Vasil, I. K. (1999). Increased Insect Resistance in Transgenic Wheat Stably Expressing Trypsin Inhibitor CMe. Mol. Breed. 5 (1), 53–63. doi:10.1023/a:1009659911798
Anastacia Books (2019). New Biotechnological Approaches to Insect Pest Management and Crop Protection; Gene Editing Approach (CRISPR-Cas System). Lincoln: University of Nebraska.
Baburao, T. M., and Sumangala, B. (2018). Development and Molecular Characterization of Transgenic Pigeon Pea Carrying cry2Aa for Pod Borer Resistance. J. Pharm. Phytochem. 75, 1581–1585.
Baum, J. A., Bogaert, T., Clinton, W., Heck, G. R., Feldmann, P., Ilagan, O., et al. (2007). Control of Coleopteran Insect Pests through RNA Interference. Nat. Biotechnol. 25, 1322–1326. doi:10.1038/nbt1359
Belfort, M., and Bonocora, R. P. (2014). Homing Endonucleases: from Genetic Anomalies to Programmable Genomic Clippers. Homing Endonucleases 1, 1–26. doi:10.1007/978-1-62703-968-0_1
Bett, B., Gollasch, S., Moore, A., James, W., Armstrong, J., Walsh, T., et al. (2017). Transgenic Cowpeas (Vigna Unguiculata L. Walp) Expressing Bacillus Thuringiensis Vip3Ba Protein Are Protected against the Maruca Pod Borer (Maruca Vitrata). Plant Cell Tiss. Organ Cult. 131 (2), 335–345. doi:10.1007/s11240-017-1287-3
Bharathi, Y., Vijaya Kumar, S., Pasalu, I. C., Balachandran, S. M., Reddy, V. D., and Rao, K. V. (2011). Pyramided Rice Lines Harbouring Allium Sativum (Asal) and Galanthus Nivalis (Gna) Lectin Genes Impart Enhanced Resistance against Major Sap-Sucking Pests. J. Biotechnol. 152 (3), 63–71. doi:10.1016/j.jbiotec.2011.01.021
Bi, H.-L., Xu, J., Tan, A.-J., and Huang, Y.-P. (2016). CRISPR/Cas9-mediated Targeted Gene Mutagenesis inSpodoptera Litura. Insect Sci. 23 (3), 469–477. doi:10.1111/1744-7917.12341
Birkett, M. A., and Pickett, J. A. (2014). Prospects of Genetic Engineering for Robust Insect Resistance. Curr. Opin. Plant Biol. 19, 59–67. doi:10.1016/j.pbi.2014.03.009
Boddupally, D., Tamirisa, S., Gundra, S. R., Vudem, D. R., and Khareedu, V. R. (2018). Expression of Hybrid Fusion Protein (Cry1Ac: ASAL) in Transgenic Rice Plants Imparts Resistance against Multiple Insect Pests. Sci. Rep. 8 (1), 8458. doi:10.1038/s41598-018-26881-9
Bommireddy, P. L., Leonard, B. R., Temple, J., Price, P., Emfinger, K., Cook, D., et al. (2011). Field Performance and Seasonal Efficacy Profiles of Transgenic Cotton Lines Expressing Vip3A and VipCot against Helicoverpa Zea (Boddie) and Heliothis virescens (F.). J. Cotton Sci. 15, 251–259.
Bortesi, L., and Fischer, R. (2015). The CRISPR/Cas9 System for Plant Genome Editing and beyond. Biotechnol. Adv. 33 (1), 41–52. doi:10.1016/j.biotechadv.2014.12.006
Bramlett, M., Plaetinck, G., and Maienfisch, P. (2020). RNA-based Biocontrols-A New Paradigm in Crop Protection. Engineering 6 (5), 522–527. doi:10.1016/j.eng.2019.09.008
Burgess, E. P. J., Main, C. A., Stevens, P. S., Christeller, J. T., Gatehouse, A. M. R., and Laing, W. A. (1994). Effects of Protease Inhibitor Concentration and Combinations on the Survival, Growth and Gut Enzyme Activities of the Black Field Cricket, Teleogryllus Commodus. J. Insect Physiology 40 (9), 803–811. doi:10.1016/0022-1910(94)90010-8
Burke, W. G., Kaplanoglu, E., Kolotilin, I., Menassa, R., and Donly, C. (2019). RNA Interference in the Tobacco Hornworm, Manduca Sexta, Using Plastid-Encoded Long Double-Stranded RNA. Front. Plant Sci. 10, 313. doi:10.3389/fpls.2019.00313
Camaroti, J. R. S. L., de Almeida, W. A., do Rego Belmonte, B., de Oliveira, A. P. S., de Albuquerque Lima, T., Ferreira, M. R. A., et al. (2018). Sitophilus Zeamais Adults Have Survival and Nutrition Affected by Schinus Terebinthifolius Leaf Extract and its Lectin (SteLL). Industrial Crops Prod. 116, 81–89. doi:10.1016/j.indcrop.2018.02.065
Camaroti, J. R. S. L., Oliveira, A. P. S., Paiva, P. M. G., Pontual, E. V., and Napoleao, T. H. (20172017). “Phytoinsecticides for Controlling Pests and Mosquito Vectors of Diseases,” in Biocontrol Agents: Types, Applications and Research Insights. Editor V. Green (New York: Nova Science Publishers), 147e188.
Caroline, N. h. M., Deogracious, P. M., George, M. T., James, R. M., Joel, W. D., and Paul, M. K. (2022). Identification of Potential Seed Storage Protein Responsible for Bruchid Resistance in Common Bean Landraces from Tanzania and Malawi. Afr. J. Biotechnol. 21 (1), 35–45. doi:10.5897/ajb2021.17354
Carrière, Y., Crickmore, N., and Tabashnik, B. E. (2015). Optimizing Pyramided Transgenic Bt Crops for Sustainable Pest Management. Nat. Biotechnol. 33 (2), 161–168. doi:10.1038/nbt.3099
Chakraborty, M., Reddy, P. S., Mustafa, G., Rajesh, G., Narasu, V. M. L., Udayasuriyan, V., et al. (2016). Transgenic Rice Expressing the cry2AX1 Gene Confers Resistance to Multiple Lepidopteran Pests. Transgenic Res. 25 (5), 665–678. doi:10.1007/s11248-016-9954-4
Chang, X., Lu, Z., Shen, Z., Peng, Y., and Ye, G. (2017). Bitrophic and Tritrophic Effects of Transgenic cry1Ab/cry2Aj Maize on the Beneficial, Nontarget Harmonia axyridis (Coleoptera: Coccinellidae). Environ. Entomol. 46 (5), 1171–1176. doi:10.1093/ee/nvx113
Chen, B., Hu, J., Almeida, R., Liu, H., Balakrishnan, S., Covill-Cooke, C., et al. (2016). Expanding the CRISPR Imaging Toolset with Staphylococcus aureus Cas9 for Simultaneous Imaging of Multiple Genomic Loci. Nucleic Acids Res. 44 (8), e75. doi:10.1093/nar/gkv1533
Chen, Q., Li, H. Y., Shi, Y. Z., Beasley, D., Bizimungu, B., and Goettel, M. S. (2008). Development of an Effective Protoplast Fusion System for Production of New Potatoes with Disease and Insect Resistance Using Mexican Wild Potato Species as Gene Pools. Can. J. Plant Sci. 88 (4), 611–619. doi:10.4141/cjps07045
Chen, W., Liu, C., Lu, G., Cheng, H., Shen, Z., and Wu, K. (2018a). Effects of Vip3AcAa+ Cry1Ac Cotton on Midgut Tissue in Helicoverpa Armigera (Lepidoptera: Noctuidae). J. Insect Sci. 18 (4), 13. doi:10.1093/jisesa/iey075
Chen, W., Qu, M., Zhou, Y., and Yang, Q. (2018b). Structural Analysis of Group II Chitinase (ChtII) Catalysis Completes the Puzzle of Chitin Hydrolysis in Insects. J. Biol. Chem. 293 (8), 2652–2660. doi:10.1074/jbc.ra117.000119
Chougule, N. P., Li, H., Liu, S., Linz, L. B., Narva, K. E., Meade, T., et al. (2013). Retargeting of the Bacillus Thuringiensis Toxin Cyt2Aa against Hemipteran Insect Pests. Proc. Natl. Acad. Sci. U.S.A. 110 (21), 8465–8470. doi:10.1073/pnas.1222144110
Chrispeels, M. J., Fatima Grossi de Sa, M., and Higgins, T. J. V. (1998). Genetic Engineering with α-amylase Inhibitors Makes Seeds Resistant to Bruchids. Seed Sci. Res. 8 (2), 257–264. doi:10.1017/s0960258500004153
Christiaens, O., Petek, M., Smagghe, G., and Taning, C. N. T. (2020a). “The Use of Nanocarriers to Improve the Efficiency of RNAi-Based Pesticides in Agriculture,” in Nanopesticides (Cham: Springer), 49–68. doi:10.1007/978-3-030-44873-8_3
Christiaens, O., Whyard, S., Vélez, A. M., and Smagghe, G. (2020b). Double-stranded RNA Technology to Control Insect Pests: Current Status and Challenges. Front. Plant Sci. 11, 451. doi:10.3389/fpls.2020.00451
Coelho, J. S., Santos, N. D. L., Napoleão, T. H., Gomes, F. S., Ferreira, R. S., Zingali, R. B., et al. (2009). Effect of Moringa Oleifera Lectin on Development and Mortality of Aedes aegypti Larvae. Chemosphere 77 (7), 934–938. doi:10.1016/j.chemosphere.2009.08.022
Czapla, T. H., and Lang, B. A. (1990). Effect of Plant Lectins on the Larval Development of European Corn Borer (Lepidoptera: Pyralidae) and Southern Corn Rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 83 (6), 2480–2485. doi:10.1093/jee/83.6.2480
Das, A., Ghosh, P., and Das, S. (2018). Expression of Colocasia Esculenta Tuber Agglutinin in Indian Mustard Provides Resistance against Lipaphis Erysimi and the Expressed Protein Is Non-allergenic. Plant Cell Rep. 37 (6), 849–863. doi:10.1007/s00299-018-2273-x
Das, G., Patra, J. K., and Baek, K.-H. (2017). Insight into MAS: a Molecular Tool for Development of Stress Resistant and Quality of Rice through Gene Stacking. Front. Plant Sci. 8, 985. doi:10.3389/fpls.2017.00985
de Azevedo Pereira, R., Nogueira Batista, J. A., Mattar da Silva, M. C., Brilhante de Oliveira Neto, O., Zangrando Figueira, E. L., Valencia Jiménez, A., et al. (2006). An Alpha-Amylase Inhibitor Gene from Phaseolus Coccineus Encodes a Protein with Potential for Control of Coffee Berry Borer (Hypothenemus Hampei). Phytochemistry 67, 2009–2016. doi:10.1016/j.phytochem.2006.06.029
De Leo, F., Bonadé-Bottino, M., Ceci, L. R., Gallerani, R., and Jouanin, L. (2001). Effects of a Mustard Trypsin Inhibitor Expressed in Different Plants on Three Lepidopteran Pests. Insect Biochem. Mol. Biol. 31 (6-7), 593–602. doi:10.1016/s0965-1748(00)00164-8
de Oliveira, A. P. S., Agra-Neto, A. C., Pontual, E. V., de Albuquerque Lima, T., Cruz, K. C. V., de Melo, K. R., et al. (2020). Evaluation of the Insecticidal Activity of Moringa Oleifera Seed Extract and Lectin (WSMoL) against Sitophilus Zeamais. J. stored Prod. Res. 87, 101615. doi:10.1016/j.jspr.2020.101615
de Santana Souza, C., Procópio, T. F., do Rego Belmonte, B., Paiva, P. M. G., de Albuquerque, L. P., Pontual, E. V., et al. (2018). Effects of Opuntia Ficus-indica Lectin on Feeding, Survival, and Gut Enzymes of Maize Weevil, Sitophilus Zeamais. Appl. Biol. Chem. 61 (3), 337–343. doi:10.1007/s13765-018-0363-7
Di Lelio, I., Barra, E., Coppola, M., Corrado, G., Rao, R., and Caccia, S. (2022). Transgenic Plants Expressing Immunosuppressive dsRNA Improve Entomopathogen Efficacy against Spodoptera Littoralis Larvae. J. Pest Sci. 95, 1413–1428. doi:10.1007/s10340-021-01467-z
Diawara, M. M., Trumble, J. T., Lacy, M. L., White, K. K., and Carson, W. G. (1996). Potential of Somaclonal Celeries for Use in Integrated Pest Management. J. Econ. Entomology 89 (1), 218–223. doi:10.1093/jee/89.1.218
Dong, Y., Yang, Y., Wang, Z., Wu, M., Fu, J., Guo, J., et al. (2020). Inaccessibility to Double‐stranded RNAs in Plastids Restricts RNA Interference in Bemisia Tabaci (Whitefly). Pest Manag. Sci. 76 (9), 3168–3176. doi:10.1002/ps.5871
Douris, V., Steinbach, D., Panteleri, R., Livadaras, I., Pickett, J. A., Van Leeuwen, T., et al. (2016). Resistance Mutation Conserved between Insects and Mites Unravels the Benzoylurea Insecticide Mode of Action on Chitin Biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 113 (51), 14692–14697. doi:10.1073/pnas.1618258113
Down, R. E., Fitches, E. C., Wiles, D. P., Corti, P., Bell, H. A., Gatehouse, J. A., et al. (2006). Insecticidal Spider Venom Toxin Fused to Snowdrop Lectin Is Toxic to the Peach-Potato Aphid, Myzus persicae (Hemiptera: Aphididae) and the Rice Brown Planthopper, Nilaparvata Lugens (Hemiptera: Delphacidae). Pest. Manag. Sci. 62 (1), 77–85. doi:10.1002/ps.1119
Duan, X., Hou, Q., Liu, G., Pang, X., Niu, Z., Wang, X., et al. (2018). Expression of Pinellia Pedatisecta Lectin Gene in Transgenic Wheat Enhances Resistance to Wheat Aphids. Molecules 23 (4), 748. doi:10.3390/molecules23040748
Duan, X., Li, X., Xue, Q., Abo-Ei-Saad, M., Xu, D., and Wu, R. (1996). Transgenic Rice Plants Harboring an Introduced Potato Proteinase Inhibitor II Gene Are Insect Resistant. Nat. Biotechnol. 14 (4), 494–498. doi:10.1038/nbt0496-494
Dunse, K. M., Stevens, J. A., Lay, F. T., Gaspar, Y. M., Heath, R. L., and Anderson, M. A. (2010). Coexpression of Potato Type I and II Proteinase Inhibitors Gives Cotton Plants Protection against Insect Damage in the Field. Proc. Natl. Acad. Sci. U.S.A. 107 (34), 15011–15015. doi:10.1073/pnas.1009241107
El-Dessouki, W. A., Mansour, M. R. K., and Eryan, N. L. (2022). Effects of Certain Weather, Biotic Factors and Chemical Components on the Population of Aphids in Egyptian Wheat Fields. Egypt. Acad. J. Biol. Sci. A, Entomology 15 (1), 1–13. doi:10.21608/eajbsa.2022.212703
Farias, L. R., Costa, F. T., Souza, L. A., Pelegrini, P. B., Grossi-de-Sá, M. F., Neto, S. M., et al. (2007). Isolation of a Novel Carica Papaya α-amylase Inhibitor with Deleterious Activity toward Callosobruchus Maculatus. Pesticide Biochem. Physiology 87, 255–260. doi:10.1016/j.pestbp.2006.08.004
Ferré, J., Escriche, B., Bel, Y., and Van Rie, J. (1995). Biochemistry and Genetics of Insect Resistance to Bacillus Thuringiensis Insecticidal Crystal Proteins. FEMS Microbiol. Lett. 132 (1-2), 1–7.
Fitches, E., Wilkinson, H., Bell, H., Bown, D. P., Gatehouse, J. A., and Edwards, J. P. (2004). Cloning, Expression and Functional Characterisation of Chitinase from Larvae of Tomato Moth (Lacanobia Oleracea): a Demonstration of the Insecticidal Activity of Insect Chitinase. Insect Biochem. Mol. Biol. 34 (10), 1037–1050. doi:10.1016/j.ibmb.2004.06.012
Fujimoto, H., Itoh, K., Yamamoto, M., Kyozuka, J., and Shimamoto, K. (1993). Insect Resistant Rice Generated by Introduction of a Modified δ-endotoxin Gene of Bacillus Thuringiensis. Nat. Biotechnol. 11 (10), 1151–1155. doi:10.1038/nbt1093-1151
Gao, C. (2021). Genome Engineering for Crop Improvement and Future Agriculture. Cell 184 (6), 1621–1635. doi:10.1016/j.cell.2021.01.005
Gatehouse, A. M. R., Dewey, F. M., Dove, J., Fenton, K. A., and Pusztai, A. (1984). Effect of Seed Lectins fromPhaseolus Vulgaris on the Development of Larvae ofCallosobruchus Maculatus; Mechanism of Toxicity. J. Sci. Food Agric. 35 (4), 373–380. doi:10.1002/jsfa.2740350402
Gatehouse, J. A. (2013). “Genetic Engineering of Crops for Insect Resistance,” in Sustainable Food Production. Editors P. Christou, R. Savin, B. A. Costa-Pierce, I. Misztal, and C. B. A. Whitelaw (New York: Springer).
Gatehouse, J. A. (2011). Prospects for Using Proteinase Inhibitors to Protect Transgenic Plants against Attack by Herbivorous Insects. Curr. Protein Pept. Sci. 12 (5), 409–416. doi:10.2174/138920311796391142
Ghareyazie, B., Alinia, F., Menguito, C. A., Rubia, L. G., de Palma, J. M., Liwanag, E. A., et al. (1997). Enhanced Resistance to Two Stem Borers in an Aromatic Rice Containing a Synthetic cryIA (B) Gene. Mol. Breed. 3 (5), 401–414. doi:10.1023/a:1009695324100
Ghosh, G., Ganguly, S., Purohit, A., Chaudhuri, R. K., Das, S., and Chakraborti, D. (2017). Transgenic Pigeonpea Events Expressing Cry1Ac and Cry2Aa Exhibit Resistance to Helicoverpa Armigera. Plant Cell Rep. 36 (7), 1037–1051. doi:10.1007/s00299-017-2133-0
Gilles, A. F., Schinko, J. B., and Averof, M. (2015). Efficient CRISPR-Mediated Gene Targeting and Transgene Replacement in the beetleTribolium Castaneum. Development 142 (16), 2832–2839. doi:10.1242/dev.125054
Gomes, A. D. P. G., Dias, S. C., Bloch, C., Melo, F. R., Furtado, J. R., Monnerat, R. G., et al. (2005). Toxicity to Cotton Boll Weevil Anthonomus grandis of a Trypsin Inhibitor from Chickpea Seeds. Comp. Biochem. Physiology Part B Biochem. Mol. Biol. 140 (2), 313–319. doi:10.1016/j.cbpc.2004.10.013
Gowda, A., Rydel, T. J., Wollacott, A. M., Brown, R. S., Akbar, W., Clark, T. L., et al. (2016). A Transgenic Approach for Controlling Lygus in Cotton. Nat. Commun. 7 (1), 12213. doi:10.1038/ncomms12213
Grazziotin, M. A. G. D., Cabral, G. B., Ibrahim, A. B., Machado, R. B., and Aragão, F. J. L. (2020). Expression of the Arcelin 1 Gene from Phaseolus vulgaris L. In Cowpea Seeds (Vigna Unguiculata L.) Confers Bruchid Resistance. Ann. Appl. Biol. 176, 268–274. doi:10.1111/aab.12568
Gunasekara, J., Jayasekera, G., Perera, K., and Wickramasuriya, A. (2017). Development of a Sri Lankan Rice Variety Bg 94-1 Harbouring cry2A Gene of Bacillus Thuringiensis Resistant to Rice Leaffolder [Cnaphalocrocis Medinalis (Guenée)]. J. Natn. Sci. Found. Sri Lanka 45 (2), 143. doi:10.4038/jnsfsr.v45i2.8180
Guo, Z., Li, Y., and Ding, S.-W. (2019). Small RNA-Based Antimicrobial Immunity. Nat. Rev. Immunol. 19 (1), 31–44. doi:10.1038/s41577-018-0071-x
Gupta, M., Kumar, H., and Kaur, S. (2021). Vegetative Insecticidal Protein (Vip): A Potential Contender from Bacillus Thuringiensis for Efficient Management of Various Detrimental Agricultural Pests. Front. Microbiol. 12, 1139. doi:10.3389/fmicb.2021.659736
Haq, S. K., Atif, S. M., and Khan, R. H. (2004). Protein Proteinase Inhibitor Genes in Combat against Insects, Pests, and Pathogens: Natural and Engineered Phytoprotection. Archives Biochem. Biophysics 431 (1), 145–159. doi:10.1016/j.abb.2004.07.022
He, C., Xiao, Y., Yu, J., Li, J., Meng, Q., Qing, X., et al. (2019). Pyramiding Xa21, Bph14, and Bph15 Genes into the Elite Restorer Line Yuehui9113 Increases Resistance to Bacterial Blight and the Brown Planthopper in Rice. Crop Prot. 115, 31–39. doi:10.1016/j.cropro.2018.09.001
Hilda, K., Bhuvaragavan, S., Kamatchi, R., Meenakumari, M., and Janarthanan, S. (2022). Cloning, Expression and Characterization of Arcelin and its Impact on Digestive Enzymes of the Stored Product Insect Pest, Callosobruchus Maculatus (F.). Pesticide Biochem. Physiology 180, 104982. doi:10.1016/j.pestbp.2021.104982
Horgan, F. G., Garcia, C. P. F., Haverkort, F., de Jong, P. W., and Ferrater, J. B. (2020). Changes in Insecticide Resistance and Host Range Performance of Planthoppers Artificially Selected to Feed on Resistant Rice. Crop Prot. 127, 104963. doi:10.1016/j.cropro.2019.104963
Hu, J., Li, X., Wu, C., Yang, C., Hua, H., Gao, G., et al. (2012). Pyramiding and Evaluation of the Brown Planthopper Resistance Genes Bph14 and Bph15 in Hybrid Rice. Mol. Breed. 29 (1), 61–69. doi:10.1007/s11032-010-9526-x
Huang, Y., Chen, Y., Zeng, B., Wang, Y., James, A. A., Gurr, G. M., et al. (2016). CRISPR/Cas9 Mediated Knockout of the Abdominal-A Homeotic Gene in the Global Pest, Diamondback Moth (Plutella Xylostella). Insect Biochem. Mol. Biol. 75, 98–106. doi:10.1016/j.ibmb.2016.06.004
Hussain, T., Aksoy, E., Çalışkan, M. E., and Bakhsh, A. (2019). Transgenic Potato Lines Expressing Hairpin RNAi Construct of Molting-Associated EcR Gene Exhibit Enhanced Resistance against Colorado Potato Beetle (Leptinotarsa decemlineata, Say). Transgenic Res. 28 (1), 151–164. doi:10.1007/s11248-018-0109-7
Iqbal, M. A., Hassan, M. W., Hafiz, M. U. R., Muhammad, A., and Moazzam, J. (2018). Evaluation of Different Wheat Varieties for Resistance against Aphid, Schizaphis graminum R. (Homoptera: Aphididae) under Laboratory Conditions. Asian J. Agric. Biol. 6, 549–555.
Irving, J. A., Pike, R. N., Dai, W., Brömme, D., Worrall, D. M., Silverman, G. A., et al. (2002). Evidence that Serpin Architecture Intrinsically Supports Papain-like Cysteine Protease Inhibition: Engineering α1-antitrypsin to Inhibit Cathepsin Proteases. Biochemistry 41 (15), 4998–5004. doi:10.1021/bi0159985
Ishimoto, M., and Kitamura, K. (1989). Growth Inhibitory Effects of an α-Amylase Inhibitor from the Kidney Bean, Phaseolus vulgaris (L.) on Three Species of Bruchids (Coleoptera: Bruchidae). Appl. Entomol. Zool. 24 (3), 281–286. doi:10.1303/aez.24.281
Jadhav, M. S., Rathnasamy, S. A., Natarajan, B., Duraialagaraja, S., and Varatharajalu, U. (2020). Study of Expression of Indigenous Bt Cry2ax1 Gene in T3 Progeny of Cotton and its Efficacy against Helicoverpa Armigera (Hubner). Braz. Archieves Biol. Technol. 63 (2), 2020. doi:10.1590/1678-4324-2020180428
Jaiswal, D. K., Raju, S. V. S., Kumar, G. S., Sharma, K. R., Singh, D. K., and Vennela, P. R. (2018). Biotechnology in Plant Resistance to Insects. Indian J. Agric. Allied Sci. 4, 7–18.
Jin, L., Wang, J., Guan, F., Zhang, J., Yu, S., Liu, S., et al. (2018). Dominant Point Mutation in a Tetraspanin Gene Associated with Field-Evolved Resistance of Cotton Bollworm to Transgenic Bt Cotton. Proc. Natl. Acad. Sci. U.S.A. 115 (46), 11760–11765. doi:10.1073/pnas.1812138115
Joshi, M. J., Prithiv Raj, V., and Solanki, C. B. (2020). Role of Biotechnology in Insect-Pests Management. Agric. Food e-Newsletter 2, 574–576.
Juturu, V. N., Mekala, G. K., and Kirti, P. B. (2015). Current Status of Tissue Culture and Genetic Transformation Research in Cotton (Gossypium spp.). Plant Cell Tiss. Organ Cult. 120 (3), 813–839. doi:10.1007/s11240-014-0640-z
Kamthan, A., Chaudhuri, A., Kamthan, M., and Datta, A. (2015). Small RNAs in Plants: Recent Development and Application for Crop Improvement. Front. Plant Sci. 06, 208. doi:10.3389/fpls.2015.00208
Katoch, R., Sethi, A., Thakur, N., and Murdock, L. L. (2013). RNAi for Insect Control: Current Perspective and Future Challenges. Appl. Biochem. Biotechnol. 171 (4), 847–873. doi:10.1007/s12010-013-0399-4
Katta, S., Talakayala, A., Reddy, M. K., Addepally, U., and Garladinne, M. (2020). Development of Transgenic Cotton (Narasimha) Using Triple Gene Cry2Ab-Cry1F-Cry1Ac Construct Conferring Resistance to Lepidopteran Pest. J. Biosci. 45 (1), 1–11. doi:10.1007/s12038-020-0006-0
Kaur, S., Kaur, K., and Jindal, J. (2022). Status of Phenolic Metabolism and α-amylase Inhibitor in Maize under Chilo Partellus Infestation. Cereal Res. Commun., 1–9. doi:10.1007/s42976-021-00230-5
Khajuria, C., Vélez, A. M., Rangasamy, M., Wang, H., Fishilevich, E., Frey, M. L. F., et al. (2015). Parental RNA Interference of Genes Involved in Embryonic Development of the Western Corn Rootworm, Diabrotica Virgifera Virgifera LeConte. Insect Biochem. Mol. Biol. 63, 54–62. doi:10.1016/j.ibmb.2015.05.011
Khan, G. A., Bakhsh, A., Ghazanfar, M., Riazuddin, S., and Husnain, T. (2013). Development of Transgenic Cotton Lines Harboring a Pesticidal Gene (cry1Ab). Emir. J. Food Agric. 25 (6), 434. doi:10.9755/ejfa.v25i6.13133
Khan, G. A., Bakhsh, A., Riazuddin, S., and Husnain, T. (2011). Introduction of cry1Ab Gene into Cotton (Gossypium Hirsutum) Enhances Resistance against Lepidopteran Pest (Helicoverpa Armigera). Span. J. Agric. Res. 9, 296–302. doi:10.5424/sjar/20110901-136-10
Khoobdel, M., Rahimi, V., Ebadollahi, A., and Krutmuang, P. (2022). Evaluation of the Potential of a Lectin Extracted from Polygonumpersicaria L. As a Biorational Agent against Sitophilusoryzae L. Molecules 27 (3), 793. doi:10.3390/molecules27030793
Kim, S.-Y., Bengtsson, T., Olsson, N., Hot, V., Zhu, L.-H., and Åhman, I. (2020). Mutations in Two Aphid-Regulated β-1,3-Glucanase Genes by CRISPR/Cas9 Do Not Increase Barley Resistance to Rhopalosiphum Padi L. Front. Plant Sci. 11, 1043. doi:10.3389/fpls.2020.01043
Koch, K. G., Chapman, K., Louis, J., Heng-Moss, T., and Sarath, G. (2016). Plant Tolerance: a Unique Approach to Control Hemipteran Pests. Front. Plant Sci. 7, 1363. doi:10.3389/fpls.2016.01363
Koch, M. S., Ward, J. M., Levine, S. L., Baum, J. A., Vicini, J. L., and Hammond, B. G. (2015). The Food and Environmental Safety of Bt Crops. Front. Plant Sci. 06, 283. doi:10.3389/fpls.2015.00283
Koerniati, S., Sukmadjaja, D., and Samudra, I. M. (2020). C Synthetic Gene of CryIAb-CryIAc Fusion to Generate Resistant Sugarcane to Shoot or Stem Borer. IOP Conf. Ser. Earth Environ. Sci. 418 (1), 012069. doi:10.1088/1755-1315/418/1/012069
Kola, V. S. R., Pichili, R., Padmakumari, A. P., Mangrauthia, S. K., Balachandran, S. M., and Madhav, M. S. (2019). Knockdown of Acetylcholinesterase (AChE) Gene in Rice Yellow Stem Borer, Scirpophaga Incertulas (Walker) through RNA Interference. Agri Gene 11, 100081. doi:10.1016/j.aggene.2019.100081
Koutroumpa, F. A., Monsempes, C., François, M. C., de Cian, A., Royer, C., Concordet, J. P., et al. (2016). Heritable Genome Editing with CRISPR/Cas9 Induces Anosmia in a Crop Pest Moth. Sci. Rep. 6 (1), 29620–29629. doi:10.1038/srep29620
Larsson, M. C., Domingos, A. I., Jones, W. D., Chiappe, M. E., Amrein, H., and Vosshall, L. B. (2004). Or83b Encodes a Broadly Expressed Odorant Receptor Essential for Drosophila Olfaction. Neuron 43 (5), 703–714. doi:10.1016/j.neuron.2004.08.019
Li-Hong, S. U. N., Chun-Ming, W. A. N. G., Chang-Chao, S. U., Yu-Qiang, L. I. U., Hu-Qu, Z. H. A. I., and Jian-Min, W. A. N. (2006). Mapping and Marker-Assisted Selection of a Brown Planthopper Resistance Gene bph2 in rice (Oryza sativa L.). Acta Genetica Sinica 33 (8), 717–723.
Li, H., Guan, R., Guo, H., and Miao, X. (2015). New Insights into an RNAi Approach for Plant Defence against Piercing-Sucking and Stem-Borer Insect Pests. Plant Cell Environ. 38 (11), 2277–2285. doi:10.1111/pce.12546
Li, J.-F., Norville, J. E., Aach, J., McCormack, M., Zhang, D., Bush, J., et al. (2013). Multiplex and Homologous Recombination-Mediated Genome Editing in Arabidopsis and Nicotiana Benthamiana Using Guide RNA and Cas9. Nat. Biotechnol. 31 (8), 688–691. doi:10.1038/nbt.2654
Li, Y., Zhang, J., Chen, D., Yang, P., Jiang, F., Wang, X., et al. (2016). CRISPR/Cas9 in Locusts: Successful Establishment of an Olfactory Deficiency Line by Targeting the Mutagenesis of an Odorant Receptor Co-receptor (Orco). Insect Biochem. Mol. Biol. 79, 27–35. doi:10.1016/j.ibmb.2016.10.003
Liu, Y., Chen, L., Liu, Y., Dai, H., He, J., Kang, H., et al. (2016). Marker Assisted Pyramiding of Two Brown Planthopper Resistance Genes, Bph3 and Bph27 (T), into Elite Rice Cultivars. Rice 9 (1), 1–7. doi:10.1186/s12284-016-0096-3
Lu, H.-P., Luo, T., Fu, H.-W., Wang, L., Tan, Y.-Y., Huang, J.-z., et al. (2018). Resistance of Rice to Insect Pests Mediated by Suppression of Serotonin Biosynthesis. Nat. Plants 4 (6), 338–344. doi:10.1038/s41477-018-0152-7
Macedo, M. L., de Sá, C. M., Freire, M. D., and Parra, J. R. (2004). A Kunitz-type Inhibitor of Coleopteran Proteases, Isolated from Adenanthera pavonina L. Seeds and its Effect on Callosobruchus Maculatus. J. Agric. Food Chem. 52 (9), 2533–2540. doi:10.1021/jf035389z
Macedo, M. R., and Freire, M. D. G. (2011). Insect Digestive Enzymes as a Target for Pest Control. Invertebr. Surviv. J. 8 (2), 190–198.
Malaikozhundan, B., Suresh, P., Seshadri, S., and Janarthanan, S. (2003). Toxicity Assessment of Wild Bean Seed Protein-Arcelin on Asian Armyworm, Spodoptera Litura (Fabricius). Indian J. Exp. Biol. 41 (12), 1463–1465.
Malone, L. A., Barraclough, E. I., Lin-Wang, K., Stevenson, D. E., and Allan, A. C. (2009). Effects of Red-Leaved Transgenic Tobacco Expressing a MYB Transcription Factor on Two Herbivorous insects,Spodoptera lituraandHelicoverpa Armigera. Entomologia Exp. Appl. Entomol. Exp. Appl 133 (2), 117–127. doi:10.1111/j.1570-7458.2009.00910.x
Mamta, B., and Rajam, M. V. (2017). RNAi Technology: a New Platform for Crop Pest Control. Physiol. Mol. Biol. Plants 23 (3), 487–501. doi:10.1007/s12298-017-0443-x
Mamta, Reddy, K. R. K., and Rajam, M. V. (2016). Targeting Chitinase Gene of Helicoverpa Armigera by Host-Induced RNA Interference Confers Insect Resistance in Tobacco and Tomato. Plant Mol. Biol. 90 (3), 281–292. doi:10.1007/s11103-015-0414-y
Maqbool, S. B., Riazuddin, S., Loc, N. T., Gatehouse, A. M. R., Gatehouse, J. A., and Christou, P. (2001). Expression of Multiple Insecticidal Genes Confers Broad Resistance against a Range of Different Rice Pests. Mol. Breed. 7 (1), 85–93. doi:10.1023/a:1009644712157
Mateos Fernández, R., Petek, M., Gerasymenko, I., Juteršek, M., Baebler, Š., Kallam, K., et al. (2021). Insect Pest Management in the Age of Synthetic Biology. Plant Biotechnol. J. 20 (1), 25–36. doi:10.1111/pbi.13685
Meade, T., Narva, K., Storer, N. P., Sheets, J. J., Burton, S. L., and Woosley, A. T. (2017). U.S. Patent No. 9,556,453. Washington, DC: U.S. Patent and Trademark Office.
Meccariello, A., Monti, S. M., Romanelli, A., Colonna, R., Primo, P., Inghilterra, M. G., et al. (2017). Highly Efficient DNA-free Gene Disruption in the Agricultural Pest Ceratitis Capitata by CRISPR-Cas9 Ribonucleoprotein Complexes. Sci. Rep. 7, 10061. doi:10.1038/s41598-017-10347-5
Meng, F., Li, Y., Zang, Z., Li, N., Ran, R., Cao, Y., et al. (2017). Expression of the Double-Stranded RNA of the Soybean Pod Borer Leguminivora Glycinivorella (Lepidoptera: Tortricidae) Ribosomal Protein Po Gene Enhances the Resistance of Transgenic Soybean Plants. Pest. Manag. Sci. 73 (12), 2447–2455. doi:10.1002/ps.4637
Mi, X., Liu, X., Yan, H., Liang, L., Zhou, X., Yang, J., et al. (2017). Expression of the Galanthus Nivalis Agglutinin (GNA) Gene in Transgenic Potato Plants Confers Resistance to Aphids. Comptes Rendus Biol. 340 (1), 7–12. doi:10.1016/j.crvi.2016.10.003
Ming, H., Li, B., Zhou, L., Goel, A., and Huang, C. (2021). Long Non-coding RNAs and Cancer Metastasis: Molecular Basis and Therapeutic Implications. Biochimica Biophysica Acta (BBA) - Rev. Cancer 1875 (2), 188519. doi:10.1016/j.bbcan.2021.188519
Morton, R. L., Schroeder, H. E., Bateman, K. S., Chrispeels, M. J., Armstrong, E., and Higgins, T. J. V. (2000). Bean α-amylase Inhibitor 1 in Transgenic Peas (Pisum Sativum) Provides Complete Protection from Pea Weevil (Bruchus Pisorum) under Field Conditions. Proc. Natl. Acad. Sci. U.S.A. 97 (8), 3820–3825. doi:10.1073/pnas.070054597
Muddanuru, T., Polumetla, A. K., Maddukuri, L., and Mulpuri, S. (2019). Development and Evaluation of Transgenic castor (Ricinus communis L.) Expressing the Insecticidal Protein Cry1Aa of Bacillus Thuringiensis against Lepidopteran Insect Pests. Crop Prot. 119, 113–125. doi:10.1016/j.cropro.2019.01.016
N'guessan, F. K., Quisenberry, S. S., and Croughan, T. P. (1994). Evaluation of Rice Anther Culture Lines for Tolerance to the Rice Water Weevil (Coleoptera: Curculionidae). Environ. Entomol. 23 (2), 331–336. doi:10.1093/ee/23.2.331
Nakasu, E. Y. T., Edwards, M. G., Fitches, E., Gatehouse, J. A., and Gatehouse, A. M. R. (2014). Transgenic Plants Expressing ω-ACTX-Hv1a and Snowdrop Lectin (GNA) Fusion Protein Show Enhanced Resistance to Aphids. Front. Plant Sci. 5, 673. doi:10.3389/fpls.2014.00673
Napoleão, T. H., Albuquerque, L. P., Santos, N. D., Nova, I. C., Lima, T. A., Paiva, P. M., et al. (2019). Insect Midgut Structures and Molecules as Targets of Plant‐derived Protease Inhibitors and Lectins. Pest. Manag. Sci. 75 (5), 1212–1222. doi:10.1002/ps.5233
Napoleão, T. H., Belmonte, B. D. R., Pontual, E. V., de Albuquerque, L. P., Sá, R. A., Paiva, L. M., et al. (2013). Deleterious Effects of Myracrodruon Urundeuva Leaf Extract and Lectin on the Maize Weevil, Sitophilus Zeamais (Coleoptera, Curculionidae). J. stored Prod. Res. 54, 26–33. doi:10.1016/j.jspr.2013.04.002
Nayak, P., Basu, D., Das, S., Basu, A., Ghosh, D., Ramakrishnan, N. A., et al. (1997). Transgenic Elite Indica Rice Plants Expressing CryIAc∂-Endotoxin of Bacillus Thuringiensis Are Resistant against Yellow Stem Borer (Scirpophaga Incertulas). Proc. Natl. Acad. Sci. U.S.A. 94 (6), 2111–2116. doi:10.1073/pnas.94.6.2111
Nderitu, P. W., Jonsson, M., Arunga, E., Otieno, M., Muturi, J. J., and Wafula, G. O. (2020). Combining Host Plant Resistance, Selective Insecticides, and Biological Control Agents for Integrated Management of Tuta Absoluta. Adv. Agric. 2020, 1–8. doi:10.1155/2020/6239491
Ni, M., Ma, W., Wang, X., Gao, M., Dai, Y., Wei, X., et al. (2017). Next-generation Transgenic Cotton: Pyramiding RNAi and Bt Counters Insect Resistance. Plant Biotechnol. J. 15 (9), 1204–1213. doi:10.1111/pbi.12709
Niu, X., Kassa, A., Hu, X., Robeson, J., McMahon, M., Richtman, N. M., et al. (2017). Control of Western Corn Rootworm (Diabrotica Virgifera Virgifera) Reproduction through Plant-Mediated RNA Interference. Sci. Rep. 7 (1), 1–13. doi:10.1038/s41598-017-12638-3
Oerke, E. C., Dehne, H. W., Schönbeck, F., and Weber, A. (2006). Crop Production and Crop Protection: Estimated Losses in Major Food and Cash Crops. Netherlands. Elsevier Science B.V. doi:10.1017/S0021859605005708
Omar, M. A. A., Ao, Y., Li, M., He, K., Xu, L., Tong, H., et al. (2019). The Functional Difference of Eight Chitinase Genes between Male and Female of the Cotton Mealybug, Phenacoccus solenopsis. Insect Mol. Biol. 28 (4), 550–567. doi:10.1111/imb.12572
Oriani, M. A. D. G., and Lara, F. M. (2000). Antibiosis Effects of Wild Bean Lines Containing Arcelin on Bemisia Tabaci (Genn.) Biotype B (Homoptera: Aleyrodidae). An. Soc. Entomol. Bras. 29 (3), 573–582. doi:10.1590/S0301-80592000000300020
Osman, G. H., Assem, S. K., Alreedy, R. M., El-Ghareeb, D. K., Basry, M. A., Rastogi, A., et al. (2016). Erratum: Development of Insect Resistant Maize Plants Expressing a Chitinase Gene from the Cotton Leaf Worm, Spodoptera Littoralis. Sci. Rep. 6, 20449. doi:10.1038/srep20449
Outchkourov, N. S., De Kogel, W. J., Schuurman-de Bruin, A., Abrahamson, M., and Jongsma, M. A. (2004). Specific Cysteine Protease Inhibitors Act as Deterrents of Western Flower Thrips, Frankliniella Occidentalis (Pergande), in Transgenic Potato. J. Plant Biotechnol. 2 (5), 439–448. doi:10.1111/j.1467-7652.2004.00088.x
Oyeleye, A., and Normi, Y. M. (2018). Chitinase: Diversity, Limitations, and Trends in Engineering for Suitable Applications. Biosci. Rep. 38 (4), 1–21. doi:10.1042/BSR20180323
Painter, R. H. (1951). Insect Resistance in Crop Plants. Soil Sci. 72 (6), 481. LWW. doi:10.1097/00010694-195112000-00015
Paiva, P. M., Pontual, E. V., Napoleão, T. H., and Coelho, L. C. B. B. (2012). “Effects of Plant Lectins and Trypsin Inhibitors on Development, Morphology and Biochemistry of Insect Larvae,” in Larvae: Morphology, Biology and Life Cycle (New York, NY: Science Publishers), 3, 37–55.
Palma, L., Muñoz, D., Berry, C., Murillo, J., and Caballero, P. (2014). Bacillus Thuringiensis Toxins: an Overview of Their Biocidal Activity. Toxins 6 (12), 3296–3325. doi:10.3390/toxins6123296
Pan, Y., Lü, P., Wang, Y., Yin, L., Ma, H., Ma, G., et al. (2012). In silicoIdentification of Novel Chitinase-like Proteins in the Silkworm,Bombyx mori, Genome. J. Insect Sci. 12, 1–14. doi:10.1673/031.012.15001
Panwar, B. S., Ram, C., Narula, R. K., and Kaur, S. (2018). Pool Deconvolution Approach for High-Throughput Gene Mining from Bacillus Thuringiensis. Appl. Microbiol. Biotechnol. 102 (3), 1467–1482. doi:10.1007/s00253-017-8633-6
Park, H.-S., Baek, S.-H., Kim, W.-J., Jeung, J.-U., Lee, J.-H., Ha, K.-Y., et al. (2014). Development of Multi-Resistant Lines to Brown Planthopper, Bacterial Blight, and Rice Stripe Virus Using Anther Culture in Rice. Korean J. Breed. Sci. 46 (1), 78–89. doi:10.9787/kjbs.2014.46.1.078
Paul, S., and Das, S. (2020). Natural Insecticidal Proteins, the Promising Bio-Control Compounds for Future Crop Protection. Nucleus 64 (1), 7–20. doi:10.1007/s13237-020-00316-1
Pedigo, L. P., and Rice, M. E. (2006). Economic Decision Levels for Pest Populations. Columbus, OH: Entomology Pest Management Pearson Prentice Hall, 253–284.
Pereira de Albuquerque, L., Maria de Sá Santana, G., Pontual, E. V., Napoleão, T. H., Breitenbach Barroso Coelho, L. C., and Paiva, P. M. G. (2012). Effect of Microgramma Vaccinifolia Rhizome Lectin on Survival and Digestive Enzymes of Nasutitermes Corniger (Isoptera, Termitidae). Int. Biodeterior. Biodegrad. 75, 158–166. doi:10.1016/j.ibiod.2012.06.030
Perez, C. J., and Shelton, A. M. (1997). Resistance of Plutella Xylostella (Lepidoptera: Plutellidae) to Bacillus Thuringiensis Berliner in Central America. J. Econ. Entomology 90 (1), 87–93. doi:10.1093/jee/90.1.87
Persley, G. J. (2000). Agricultural Biotechnology and the Poor: Promethean Science. Washington, D.C.: Consultative Group on International Agricultural Research (CGIAR), 3–21.
Poulsen, M., Kroghsbo, S., Schrøder, M., Wilcks, A., Jacobsen, H., Miller, A., et al. (2007). A 90-day Safety Study in Wistar Rats Fed Genetically Modified Rice Expressing Snowdrop Lectin Galanthus Nivalis (GNA). Food Chem. Toxicol. 45 (3), 350–363. doi:10.1016/j.fct.2006.09.002
Powell, K. S., Gatehouse, A. M. R., Hilder, V. A., and Gatehouse, J. A. (1993). Antimetabolic Effects of Plant Lectins and Plant and Fungal Enzymes on the Nymphal Stages of Two Important Rice Pests, Nilaparvata Lugens and Nephotettix Cinciteps. Entomologia Exp. Appl. 66 (2), 119–126. doi:10.1111/j.1570-7458.1993.tb00699.x
Priya, S., Kaur, N., and Gupta, A. K. (2010). Purification, Characterization and Inhibition Studies of α-amylase of Rhyzopertha dominica. Pesticide Biochem. Physiology 98 (2), 231–237. doi:10.1016/j.pestbp.2010.06.012
Qin, D., Liu, X.-Y., Miceli, C., Zhang, Q., and Wang, P.-w. (2019). Soybean Plants Expressing the Bacillus Thuringiensis Cry8-like Gene Show Resistance to Holotrichia Parallela. BMC Biotechnol. 19 (1), 1–12. doi:10.1186/s12896-019-0563-1
Qiu, L., Fan, J., Zhang, B., Liu, L., Wang, X., Lei, C., et al. (2017). RNA Interference Knockdown of Aminopeptidase N Genes Decrease the Susceptibility of Chilo Suppressalis Larvae to Cry1Ab/Cry1Ac and Cry1Ca-Expressing Transgenic Rice. J. Invertebr. Pathology 145, 9–12. doi:10.1016/j.jip.2017.03.001
Qiu, L., Sun, Y., Jiang, Z., Yang, P., Liu, H., Zhou, H., et al. (2019). The Midgut V‐ATPase Subunit A Gene Is Associated with Toxicity to Crystal 2Aa and Crystal 1Ca ‐expressing Transgenic Rice in Chilo Suppressalis. Insect Mol. Biol. 28 (4), 520–527. doi:10.1111/imb.12570
Rajadurai, G., Kalaivani, A., Varanavasiyappan, S., Balakrishnan, N., Udayasuriyan, V., Sudhakar, D., et al. (2018). Generation of Insect Resistant Marker-free Transgenic Rice with a Novel cry2AX1 Gene. Electron. Journ. Plan. Breed. 9 (2), 723–732. doi:10.5958/0975-928x.2018.00086.8
Rakha, M., Bouba, N., Ramasamy, S., Regnard, J.-L., and Hanson, P. (2017). Evaluation of Wild Tomato Accessions (Solanum spp.) for Resistance to Two-Spotted Spider Mite (Tetranychus Urticae Koch) Based on Trichome Type and Acylsugar Content. Genet. Resour. Crop Evol. 64 (5), 1011–1022. doi:10.1007/s10722-016-0421-0
Rani, S., Sharma, V., Hada, A., Bhattacharya, R. C., and Koundal, K. R. (2017b). Fusion Gene Construct Preparation with Lectin and Protease Inhibitor Genes against Aphids and Efficient Genetic Transformation of Brassica Juncea Using Cotyledons Explants. Acta Physiol. Plant 39 (5), 1–13. doi:10.1007/s11738-017-2415-8
Rani, S., Sharma, V., Hada, A., and Koundal, K. R. (2017a). Efficient Genetic Transformation of Brassica Juncea with Lectin Using Cotyledons Explants. Int. J. Adv. Biotechnol. 7, 1–12.
Riaz, S., Nasir, I. A., Bhatti, M. U., Adeyinka, O. S., Toufiq, N., Yousaf, I., et al. (2020). Resistance to Chilo Infuscatellus (Lepidoptera: Pyraloidea) in Transgenic Lines of Sugarcane Expressing Bacillus Thuringiensis Derived Vip3A Protein. Mol. Biol. Rep. 47 (4), 2649–2658. doi:10.1007/s11033-020-05355-0
Ribeiro, A. P. O., Pereira, E. J. G., Galvan, T. L., Picanco, M. C., Picoli, E. A. T., Silva, D. J. H., et al. (2006). Effect of Eggplant Transformed with Oryzacystatin Gene on Myzus persicae and Macrosiphum euphorbiae. J. Appl. Entomol. 130 (2), 84–90. doi:10.1111/j.1439-0418.2005.01021.x
Ribeiro, T. P., Arraes, F. B. M., Lourenço-Tessutti, I. T., Silva, M. S., Lisei-de-Sá, M. E., Lucena, W. A., et al. (2017). Transgenic Cotton Expressing Cry10Aa Toxin Confers High Resistance to the Cotton Boll Weevil. Plant Biotechnol. J. 15 (8), 997–1009. doi:10.1111/pbi.12694
Ricroch, A., Clairand, P., and Harwood, W. (2017). Use of CRISPR Systems in Plant Genome Editing: toward New Opportunities in Agriculture. Emerg. Top. Life Sci. 1 (2), 169–182. doi:10.1042/etls20170085
Sakthi, A. R., Naveenkumar, A., Deepikha, P. S., Balakrishnan, N., Kumar, K. K., Devi, E. K., et al. (2015). Expression and Inheritance of Chimeric cry2AX1 Gene in Transgenic Cotton Plants Generated through Somatic Embryogenesis. Vitro Cell.Dev.Biol.-Plant 51 (4), 379–389. doi:10.1007/s11627-015-9695-8
Santos, N. D. D. L., de Moura, K. S., Napoleão, T. H., Santos, G. K. N., Coelho, L. C. B. B., Navarro, D. M. D. A. F., et al. (2012). Oviposition-stimulant and Ovicidal Activities of Moringa Oleifera Lectin on Aedes aegypti. PLoS ONE 7, e44840. doi:10.1371/journal.pone.0044840
Santos, N. D. L., Napoleão, T. H., Benevides, C. A., Albuquerque, L. P., Pontual, E. V., Oliveira, A. P. S., et al. (2020). Effect of Gamma Irradiation of Moringa Oleifera Seed Lectin on its Larvicidal, Ovicidal, and Oviposition-Stimulant Activities against Aedes aegypti. South Afr. J. Bot. 129, 3–8. doi:10.1016/j.sajb.2018.05.001
Sauvion, N., Rahbé, Y., Peumans, W. J., Damme, E. J. M., Gatehouse, J. A., and Gatehouse, A. M. R. (1996). Effects of GNA and Other Mannose Binding Lectins on Development and Fecundity of the Peach-Potato Aphid Myzus persicae. Entomologia Exp. Appl. 79 (3), 285–293. doi:10.1111/j.1570-7458.1996.tb00836.x
Sawardekar, S. V., Katageri, I. S., Salimath, P. M., Kumar, P. A., and Kelkar, V. G. (2017). Standardization of In-Vitro Genetic Transformation Technique in Chickpea (Cicer Arietinum L.) for Pod-Borer Resistance. Adv. Agric. Res. Technol. 1 (2), 198.
Schneider, V. K., Soares-Costa, A., Chakravarthi, M., Ribeiro, C., Chabregas, S. M., Falco, M. C., et al. (2017). Transgenic Sugarcane Overexpressing CaneCPI-1 Negatively Affects the Growth and Development of the Sugarcane Weevil Sphenophorus Levis. Plant Cell Rep. 36 (1), 193–201. doi:10.1007/s00299-016-2071-2
Selale, H., Dağlı, F., Mutlu, N., Doğanlar, S., and Frary, A. (2017). Cry1Ac-mediated Resistance to Tomato Leaf Miner (Tuta Absoluta) in Tomato. Plant Cell Tiss. Organ Cult. 131 (1), 65–73. doi:10.1007/s11240-017-1262-z
Sétamou, M., Bernal, J. S., Legaspi, J. C., Mirkov, T. E., and Legaspi, B. C. (2002). Evaluation of Lectin-Expressing Transgenic Sugarcane against Stalkborers (Lepidoptera: Pyralidae): Effects on Life History Parameters. J. Econ. Entomol. 95 (2), 469–477. doi:10.1603/0022-0493-95.2.469
Shabanimofrad, M., Yusop, M. R., Ashkani, S., Musa, M. H., Adam, N. A., Haifa, I., et al. (2015). Marker-assisted Selection for Rice Brown Planthopper (Nilaparvata Lugens) Resistance Using Linked SSR Markers. Turk J. Biol. 39 (5), 666–673. doi:10.3906/biy-1406-78
Shade, R. E., Schroeder, H. E., Pueyo, J. J., Tabe, L. M., Murdock, L. L., Higgins, T. J. V., et al. (1994). Transgenic Pea Seeds Expressing the α-Amylase Inhibitor of the Common Bean Are Resistant to Bruchid Beetles. Nat. Biotechnol. 12 (8), 793–796. doi:10.1038/nbt0894-793
Shaffer, L. (2020). Inner Workings: RNA-Based Pesticides Aim to Get Around Resistance Problems. Proc. Natl. Acad. Sci. U.S.A. 117 (52), 32823–32826. doi:10.1073/pnas.2024033117
Sharma, H. C. (2007). Host Plant Resistance to Insects: Modern Approaches and Limitations. Industrial J. Plant Prot. 35 (2), 179–184.
Sharma, K. (2015). Protease Inhibitors in Crop Protection from Insects. Int. J. Curr. Res. Acad. Rev. 3, 55–70. doi:10.12983/ijsres-2015-p0411-0419
Sharma, P. N., Torii, A., Takumi, S., Mori, N., and Nakamura, C. (2004). Marker-assisted Pyramiding of Brown Planthopper (Nilaparvata Lugens Stål) Resistance Genes Bph1 and Bph2 on Rice Chromosome 12. Hereditas 140 (1), 61–69. doi:10.1111/j.1601-5223.2004.01726.x
Siddiqui, H. A., Asif, M., Asad, S., Naqvi, R. Z., Ajaz, S., Umer, N., et al. (2019). Development and Evaluation of Double Gene Transgenic Cotton Lines Expressing Cry Toxins for Protection against Chewing Insect Pests. Sci. Rep. 9 (1), 1–7. doi:10.1038/s41598-019-48188-z
Singh, A., Singh, S., and Singh, I. K. (2016). Recent Insights into the Molecular Mechanism of Jasmonate Signaling during Insect-Plant Interaction. Australas. Plant Pathol. 45 (2), 123–133. doi:10.1007/s13313-015-0392-1
Singh, S., Kumar, N. R., Maniraj, R., Lakshmikanth, R., Rao, K. Y. S., Muralimohan, N., et al. (2018). Expression of Cry2Aa, a Bacillus Thuringiensis Insecticidal Protein in Transgenic Pigeon Pea Confers Resistance to Gram Pod Borer, Helicoverpa Armigera. Sci. Rep. 8 (1), 1–12. doi:10.1038/s41598-018-26358-9
Singh, S., Singh, A., Kumar, S., Mittal, P., and Singh, I. K. (2020). Protease Inhibitors: Recent Advancement in its Usage as a Potential Biocontrol Agent for Insect Pest Management. Insect Sci. 27 (2), 186–201. doi:10.1111/1744-7917.12641
Smith, C. M. (Editor) (2005). Plant Resistance to Arthropods: Molecular and Conventional Approaches (Dordrecht: Springer Netherlands).
Soliman, H. I. A., Abo-El-Hasan, F. M., El-Seedy, A. S., and Mabrouk, Y. M. (2021). Agrobacterium-mediated Transformation of Tomato (Lycopersicon esculentum mill.) Using a Synthetic Cry1ab Gene for Enhanced Resistance against Tuta Absoluta (Meyrick). J. Microbiol. Biotechnol. Food Sci. 7, 67–74. doi:10.15414/jmbfs.2017.7.1.67-74
Stevens, J., Dunse, K., Fox, J., Evans, S., and Anderso, M. (2012). “Biotechnological Approaches for the Control of Insect Pests in Crop Plants,” in Pesticides- Advances in Chemical and Botanical Pesticides. Editor R. P. Soundararajan (InTech Open), 269–308. doi:10.5772/46233
Stoger, E., Williams, S., Christou, P., Down, R. E., and Gatehouse, J. A. (1999). Expression of the Insecticidal Lectin from Snowdrop (Galanthus Nivalis Agglutinin; GNA) in Transgenic Wheat Plants: Effects on Predation by the Grain Aphid Sitobion Avenae. Mol. Breed. 5 (1), 65–73. doi:10.1023/a:1009616413886
Su, C., Tu, G., Huang, S., Yang, Q., Shahzad, M. F., and Li, F. (2016). Genome-wide Analysis of Chitinase Genes and Their Varied Functions in Larval Moult, Pupation and Eclosion in the Rice Striped Stem borer,Chilo Suppressalis. Insect Mol. Biol. 25 (4), 401–412. doi:10.1111/imb.12227
Sudhakar, D., Fu, X., Stoger, E., Williams, S., Spence, J., Brown, D. P., et al. (1998). Expression and Immunolocalisation of the Snowdrop Lectin, GNA in Transgenic Rice Plants. Transgenic Res. 7 (5), 371–378. doi:10.1023/a:1008856703464
Syed, T., Askari, M., Meng, Z., Li, Y., Abid, M. A., Wei, Y., et al. (2020). Current Insights on Vegetative Insecticidal Proteins (Vip) as Next Generation Pest Killers. Toxins (Basel) 12 (8), 522. doi:10.3390/toxins12080522
Tabashnik, B. E. (1994). Evolution of Resistance to Bacillus Thuringiensis. Annu. Rev. Entomol. 39 (1), 47–79. doi:10.1146/annurev.en.39.010194.000403
Tabashnik, B. E., Malvar, T., Liu, Y. B., Finson, N., Borthakur, D., Shin, B. S., et al. (1996). Cross-resistance of the Diamondback Moth Indicates Altered Interactions with Domain II of Bacillus Thuringiensis Toxins. Appl. Environ. Microbiol. 62 (8), 2839–2844. doi:10.1128/aem.62.8.2839-2844.1996
Talakayala, A., Katta, S., and Garladinne, M. (2020). Genetic Engineering of Crops for Insect Resistance: An Overview. J. Biosci. 45 (1), 1–12. doi:10.1007/s12038-020-00081-y
Tamhane, V. A., Giri, A. P., Sainani, M. N., and Gupta, V. S. (2007). Diverse Forms of Pin-II Family Proteinase Inhibitors from Capsicum Annuum Adversely Affect the Growth and Development of Helicoverpa Armigera. Gene 403 (1-2), 29–38. doi:10.1016/j.gene.2007.07.024
Trung, N. P., Fitches, E., and Gatehouse, J. A. (2006). A Fusion Protein Containing a Lepidopteran-specific Toxin from the South Indian Red Scorpion (Mesobuthus Tamulus) and Snowdrop Lectin Shows Oral Toxicity to Target Insects. BMC Biotechnol. 6 (1), 1–12. doi:10.1186/1472-6750-6-18
Tu, J., Zhang, G., Datta, K., Xu, C., He, Y., Zhang, Q., et al. (2000). Field Performance of Transgenic Elite Commercial Hybrid Rice Expressing Bacillus Thuringiensis δ-endotoxin. Nat. Biotechnol. 18 (10), 1101–1104. doi:10.1038/80310
van Dijk, M., Morley, T., Rau, M. L., and Saghai, Y. (2021). A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010-2050. Nat. Food 2 (7), 494–501. doi:10.1038/s43016-021-00322-9
Vandenborre, G., Smagghe, G., and Van Damme, E. J. M. (2011). Plant Lectins as Defense Proteins against Phytophagous Insects. Phytochemistry 72 (13), 1538–1550. doi:10.1016/j.phytochem.2011.02.024
Vanti, G. L., Katageri, I. S., Inamdar, S. R., Hiremathada, V., and Swamy, B. M. (2018). Potent Insect Gut Binding Lectin from Sclerotium Rolfsii Impart Resistance to Sucking and Chewing Type Insects in Cotton. J. Biotechnol. 278, 20–27. doi:10.1016/j.jbiotec.2018.04.018
Wang, J., Zhang, H., Wang, H., Zhao, S., Zuo, Y., Yang, Y., et al. (2016). Functional Validation of Cadherin as a Receptor of Bt Toxin Cry1Ac in Helicoverpa Armigera Utilizing the CRISPR/Cas9 System. Insect Biochem. Mol. Biol. 76, 11–17. doi:10.1016/j.ibmb.2016.06.008
Wang, J., Ma, H., Zuo, Y., Yang, Y., and Wu, Y. (2020a). CRISPR ‐mediated Gene Knockout Reveals Nicotinic Acetylcholine Receptor (nAChR) Subunit α6 as a Target of Spinosyns in Helicoverpa Armigera. Pest Manag. Sci. 76 (9), 2925–2931. doi:10.1002/ps.5889
Wang, X., Xu, Y., Huang, J., Jin, W., Yang, Y., and Wu, Y. (2020b). CRISPR-mediated Knockout of the ABCC2 Gene in Ostrinia Furnacalis Confers High-Level Resistance to the Bacillus Thuringiensis Cry1Fa Toxin. Toxins 12 (4), 246. doi:10.3390/toxins12040246
Wünn, J., Klöti, A., Burkhardt, P. K., Biswas, G. C. G., Launis, K., Iglesias, V. A., et al. (1996). Transgenic Indica Rice Breeding Line IR58 Expressing a Synthetic crylA (B) Gene from Bacillus Thuringiensis Provides Effective Insect Pest Control. Bio/technology 14 (2), 171–176.
Xi, Y., Pan, P.-L., Ye, Y.-X., Yu, B., Xu, H.-J., and Zhang, C.-X. (2015). Chitinase-like Gene Family in the Brown planthopper,Nilaparvata Lugens. Insect Mol. Biol. 24 (1), 29–40. doi:10.1111/imb.12133
Xia, J., Guo, Z., Yang, Z., Han, H., Wang, S., Xu, H., et al. (2021). Whitefly Hijacks a Plant Detoxification Gene that Neutralizes Plant Toxins. Cell 184 (7), 1693–1705. doi:10.1016/j.cell.2021.02.014
Xu, C., Cheng, J., Lin, H., Lin, C., Gao, J., and Shen, Z. (2018). Characterization of Transgenic Rice Expressing Fusion Protein Cry1Ab/Vip3A for Insect Resistance. Sci. Rep. 8 (1), 1–8. doi:10.1038/s41598-018-34104-4
Xu, D., Xue, Q., McElroy, D., Mawal, Y., Hilder, V. A., and Wu, R. (1996). Constitutive Expression of a Cowpea Trypsin Inhibitor Gene, CpTi, in Transgenic Rice Plants Confers Resistance to Two Major Rice Insect Pests. Mol. Breed. 2 (2), 167–173. doi:10.1007/bf00441431
Yan, S., Qian, J., Cai, C., Ma, Z., Li, J., Yin, M., et al. (2020). Spray Method Application of Transdermal dsRNA Delivery System for Efficient Gene Silencing and Pest Control on Soybean Aphid Aphis Glycines. J. Pest Sci. 93 (1), 449–459. doi:10.1007/s10340-019-01157-x
Zhang, J., Khan, S. A., Hasse, C., Ruf, S., Heckel, D. G., and Bock, R. (2015). Full Crop Protection from an Insect Pest by Expression of Long Double-Stranded RNAs in Plastids. Science 347 (6225), 991–994. doi:10.1126/science.1261680
Zhong, Y., Ahmed, S., Deng, G., Fan, W., Zhang, P., and Wang, H. (2019). Improved Insect Resistance against Spodoptera Litura in Transgenic Sweetpotato by Overexpressing Cry1Aa Toxin. Plant Cell Rep. 38 (11), 1439–1448. doi:10.1007/s00299-019-02460-8
Zhu-Salzman, K., and Zeng, R. (2015). Insect Response to Plant Defensive Protease Inhibitors. Annu. Rev. Entomol. 60, 233–252. doi:10.1146/annurev-ento-010814-020816
Zsögön, A., Peres, L. E. P., Xiao, Y., Yan, J., and Fernie, A. R. (2022). Enhancing Crop Diversity for Food Security in the Face of Climate Uncertainty. Plant J. 109 (2), 402–414. doi:10.1111/tpj.15626
Zsögön, A., Čermák, T., Naves, E. R., Notini, M. M., Edel, K. H., Weinl, S., et al. (2018). De Novo domestication of Wild Tomato Using Genome Editing. Nat. Biotechnol. 36 (12), 1211–1216. doi:10.1038/nbt.4272
Zuo, Y., Wang, H., Xu, Y., Huang, J., Wu, S., Wu, Y., et al. (2017). CRISPR/Cas9 Mediated G4946E Substitution in the Ryanodine Receptor of Spodoptera Exigua Confers High Levels of Resistance to Diamide Insecticides. Insect Biochem. Mol. Biol. 89, 79–85. doi:10.1016/j.ibmb.2017.09.005
Keywords: biotic stress, insect pests, insecticide, biotechnology, resistant variety
Citation: Kumari P, Jasrotia P, Kumar D, Kashyap PL, Kumar S, Mishra CN, Kumar S and Singh GP (2022) Biotechnological Approaches for Host Plant Resistance to Insect Pests. Front. Genet. 13:914029. doi: 10.3389/fgene.2022.914029
Received: 06 April 2022; Accepted: 16 May 2022;
Published: 02 June 2022.
Edited by:
Reyazul Rouf Mir, Sher-e-Kashmir University of Agricultural Sciences and Technology, IndiaReviewed by:
Jiban Shrestha, Nepal Agricultural Research Council, NepalJasdeep Chatrath Padaria, Indian Council of Agricultural Research, India
Mohammad Monobrulla, ICAR-Research Complex for Eastern Region, India
Mahadevan Raghuraman, Banaras Hindu University, India
Copyright © 2022 Kumari, Jasrotia, Kumar, Kashyap, Kumar, Mishra, Kumar and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Poonam Jasrotia, poonam.jasrotia@icar.gov.in, poonamjasrotia@gmail.com
 Pritam Kumari
Pritam Kumari Poonam Jasrotia
Poonam Jasrotia Deepak Kumar
Deepak Kumar Prem Lal Kashyap
Prem Lal Kashyap Satish Kumar
Satish Kumar Chandra Nath Mishra
Chandra Nath Mishra Sudheer Kumar
Sudheer Kumar Gyanendra Pratap Singh
Gyanendra Pratap Singh