
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Genet., 11 July 2022
Sec. Pharmacogenetics and Pharmacogenomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.912478
This article is part of the Research TopicPharmacogenomics in Asians: Differences and Similarities with other Human PopulationsView all 6 articles
We investigated the genetic predisposition for the pathogenesis of Stevens–Johnson syndrome/epidermal necrolysis with severe ocular complications (SJS/TEN with SOC). Cold medicines (CMs) including multi-ingredient cold-medications and non-steroidal anti-inflammatory drugs (NSAIDs) were implicated in the development of SJS/TEN with SOC. Studies on the association between HLA genotypes and CM-related SJS/TEN with SOC (CM-SJS/TEN with SOC) revealed an association with HLA-A*02:06 in the Japanese; it may be a marker in Koreans. HLA-B*44:03 was associated with the Japanese, Thais, and Indians; in Brazilians of European ancestry, it may be a positive marker. PTGER3 is a susceptibility gene; HLA-A*02:06 and PTGER3 polymorphisms exerted additive effects in Japanese and Korean patients. A genome-wide association study showed that IKZF1 was associated with the Japanese. A meta-analysis including Japanese, Koreans, Indians, and Brazilians also revealed an association between CM-SJS/TEN with SOC and IKZF1. The upregulation of hsa-miR-628-3p in the plasma of SJS/TEN with SOC patients may suppress the expression of TLR3 and innate immune-related genes. Not only CMs but also the interaction of TLR3, PTGER3, IKZF1, and HLA and maybe some microbial infections are necessary for the onset of SJS/TEN with SOC.
Stevens–Johnson syndrome (SJS) and its severe phenotype, toxic epidermal necrolysis (TEN), are acute inflammatory vesiculobullous reactions of the skin, mucosa of the ocular surface, oral cavity, and genitals. About half of SJS/TEN patients in the acute stage, who were diagnosed in burn units and/or by dermatologists, have had severe ocular complications (SOC) such as severe conjunctivitis with both ocular surface epithelial defects and pseudomembrane (Sotozono et al., 2015).
Burn unit physicians and dermatologists usually see SJS/TEN patients only in the acute stage since the skin lesions have healed after the acute stage (Yamane et al., 2007). On the other hand, since some SJS/TEN patients present with ocular sequelae such as severe dry eye and corneal opacity with vision disturbance (Sotozono et al., 2007), ophthalmologists tend to see these patients not only in the acute but also in the chronic stage.
In the acute stage, the ocular surface of SJS/TEN with SOC patients manifests severe conjunctivitis with both epithelial defects and pseudomembrane (Sotozono et al., 2009). In the chronic stage, many SJS/TEN with SOC patients suffer serious ocular sequelae such as vision disturbance due to severe dry eye and conjunctival invasion into the cornea (Sotozono et al., 2007).
SJS and TEN with SOC tend to be reported as “SJS” in ophthalmology (Ueta and Kinoshita, 2012), because it can be difficult for ophthalmologists to make a differential diagnosis of SJS or TEN in the chronic stage since the vesiculobullous skin lesions observed in the acute stage have healed, and ophthalmologists tend to diagnose SJS/TEN in their chronic stage based on a confirmed history of acute-onset high fever, serious mucocutaneous illness with skin eruptions, and the involvement of at least two mucosal sites including the ocular surface (Ueta et al., 2007a; Ueta et al., 2007b; Ueta et al., 2010a; Ueta et al., 2015a; Ueta et al., 2017a).
For more than 15 years, we focused on the genetic predisposition for and the pathogenesis of SJS/TEN with SOC. We found that cold medicines (CMs) including multi-ingredient cold medications and non-steroidal anti-inflammatory drugs (NSAIDs) such as acetaminophen and dipyrone are major causative drugs for SJS/TEN with SOC (Ueta et al., 2010a; Lee et al., 2017; Wakamatsu et al., 2017; Jongkhajornpong et al., 2018; Jongkhajornpong et al., 2020; Ma et al., 2021; Wakamatsu et al., 2021), although dermatologists and others reported that allopurinol (a uric acid-lowering drug) (Hung et al., 2005; Tohkin et al., 2013) and anticonvulsants such as carbamazepine (Chung et al., 2004; Kaniwa et al., 2010; McCormack et al., 2011; Mockenhaupt et al., 2019) are the main SJS/TEN-inciting drugs.
We have reported that about 80% of SJS/TEN with SOC patients seen at the Kyoto Prefectural University of Medicine developed SJS/TEN within several days after taking cold medicines (CMs) (Ueta et al., 2010a; Ueta et al., 2014a; Ueta et al., 2015a). Our Brazilian collaborators also found that 53% of their SJS/TEN with SOC patients had taken cold medicines (Wakamatsu et al., 2017) as had 69% of Thai (Jongkhajornpong et al., 2018) and 50% of Taiwanese patients (Ma et al., 2021). Our Korean collaborators suspected that NSAIDs and CMs were associated with SOC in their SJS/TEN patients (Lee et al., 2017). Thus, in patients of different ethnicities, ophthalmologists reported that CMs appear to be major causative drugs for SJS/TEN with SOC.
Because for SJS/TEN with SOC, the purpose of taking cold medicines including multi-ingredient cold medications and NSAIDs before their onset might be to combat the common cold, we also suspect that the onset of CM-related SJS/TEN with SOC was associated not only with cold medicines but also with putative microbial infection (Ueta and Kinoshita, 2012; Ueta, 2018; Ueta, 2021a).
Moreover, the associated HLA types vary among different causative drugs; for example, HLA-B* 58:01 for allopurinol (Hung et al., 2005; Tohkin et al., 2013), HLA-B*15:02 (Chung et al., 2004; Kaniwa et al., 2008; Kaniwa et al., 2013), HLA-A*31:01 (McCormack et al., 2011; Mockenhaupt et al., 2019), HLA-B*57:01 (Mockenhaupt et al., 2019) for carbamazepine, and HLA-A*02:06 and HLA-B*44:03 for CM-related SJS/TEN with SOC (Ueta et al., 2014a; Ueta et al., 2014b). Therefore, it is likely that pathogenesis is different among different causative drugs or between SJS/TEN with and without SOC (Ueta et al., 2014a). Here, we focus on CM-related SJS/TEN with SOC.
We have analyzed the association between CM-related SJS/TEN with SOC and HLA genotypes, and found that CM-related SJS/TEN with SOC was significantly associated with HLA-A*02:06 [151 patients, 639 normal controls; odds ratio (OR) = 5.6, p = 2.7 × 10–20] and with HLA-B*44:03 in the Japanese (151 patients, 639 normal controls; OR = 2.0, p = 1.3 × 10–3) (Ueta et al., 2014a). HLA-A*02:06 and HLA-B*44:03 were not associated with CM-related SJS/TEN without SOC, suggesting that a different HLA genotype plays a role in the development of SJS/TEN with and without SOC (Ueta et al., 2014a). Moreover, these HLA genotypes are not associated with CM-unrelated, that is, other medicine-related SJS/TEN with SOC (Ueta et al., 2014a).
We have suspected that the pathogenesis of SJS/TEN with SOC is different from the pathogenesis of SJS/TEN without SOC (Ueta and Kinoshita, 2012), since major causative drugs for SJS/TEN with SOC were different from those for SJS/TEN without SOC, and the HLA association with SJS/TEN with SOC was different from SJS/TEN without SOC.
For further investigation of the genetic predisposition for SJS/TEN with SOC, we have engaged in an international collaboration that included participants from Korea, Brazil, Thailand, Taiwan, India, and Japan.
Our Korean collaborators identified HLA-A*02:06 (40 patients, 120 controls; OR = 3.0, p = 0.0083) as potential positive markers for CM-related SJS/TEN with SOC in Korea as same as in Japan. They also reported that HLA-C*03:04 (40 patients, 120 controls; OR = 3.5, p = 0.010) might be a potential positive marker for CM-related SJS/TEN with SOC, and HLA-C*03:03 (40 patients, 120 controls; OR = 0.10, p = 0.0056) might be a possible indicator of the protection against CM-related SJS/TEN with SOC in Korea (Jun et al., 2019).
Our Brazilian collaborators reported HLA-A*66:01 as a potential marker for CM-related SJS/TEN with SOC in Brazilians (39 patients, 133 controls; OR = 24.0, p < 0.001) of both Pardo (19 patients, 66 controls; OR = 12.2, p = 0.03) and European ancestry (16 patients, 61 controls; OR = 21.2, p = 0.04), HLA-B*44:03 (16 patients, 61 controls; OR = 5.50, p = 0.01) and HLA-C*12:03 (16 patients, 61 controls; OR = 8.79, p = 0.008) may be markers only in individuals of European ancestry, and HLA-A*11:01 (39 patients, 133 controls; OR = 0.074, p = 0.008) may be a marker of resistance to CM-related SJS/TEN with SOC in the Brazilian population (Wakamatsu et al., 2017).
Our Thai collaborators reported that HLA-B*44:03 (49 patients, 159 controls; OR = 7.2, p < 0.0001) and HLA-C*07:01 (49 patients, 159 controls; OR = 6.1, p < 0.0001) were significantly associated with Thai CM-related SJS/TEN with SOC, and identified that the HLA-B*44:03 - HLA-C*07:01 haplotypes were a potential risk factor for CM-related SJS/TEN with SOC in their population (Jongkhajornpong et al., 2018).
Our Taiwanese collaborators reported that HLA-A*02:07 (13 patients, 98 controls; OR = 5.6, p = 0.016) was associated with Han Chinese CM-related SJS/TEN with SOC patients (Ma et al., 2021). As HLA-A*02:06 and HLA-A*02:07 are very similar peptides—they differ in only a single amino acid residue substitution—it is possible that the expression of HLA-A*02:07 but not of HLA-A*02:06 was associated with CM-related SJS/TEN with SOC in the Han Chinese population (Ma et al., 2021).
Our Indian collaborators reported that it was difficult to obtain a detailed history of disease onset from their SJS/TEN with SOC patients and in many patients; they could not identify causative drugs. However, an HLA analysis showed that HLA-A*33:03 (80 patients, 50 controls; OR = 3.4, p = 2.7 × 10–3), HLA-B*44:03 (80 patients, 50 controls; OR = 12.2, p = 7.3 × 10–9), and HLA-C*07:01 (80 patients, 50 controls; OR = 6.5, p = 4.4 × 10–6) were risk alleles, and haplotypes comprising HLA-B*44:03 and HLA-C*07:01 were strongly associated with SJS/TEN with SOC in the Indian population (80 patients, 50 controls; OR = 11.0, p = 1.1 × 10–7) (Kannabiran et al., 2017). We also reported that HLA-B*44:03 was strongly associated with CM-related SJS/TEN with SOC in the Indian population (20 patients, 55 controls; OR = 12.3, p = 1.1 × 10–5) (Ueta et al., 2014b).
In summary, HLA-B*44:03 was significantly associated with CM-related SJS/TEN with SOC in the Japanese (Ueta et al., 2014a), Brazilians (particularly in Caucasian Brazilians) (Ueta et al., 2014b; Wakamatsu et al., 2017), Indian patients (Ueta et al., 2014b; Kannabiran et al., 2017), and Thais (Jongkhajornpong et al., 2018; Jongkhajornpong et al., 2020). HLA-A*02:06 was significantly associated with CM-related SJS/TEN with SOC in the Japanese (Ueta et al., 2014a) and Koreans (Ueta et al., 2014b; Jun et al., 2019) (Figure 1).
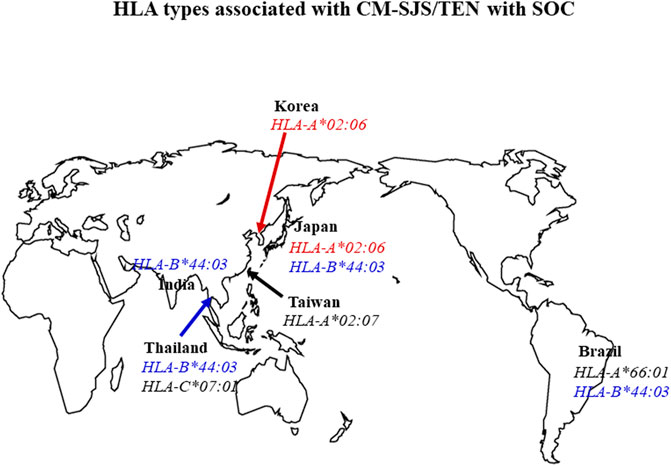
FIGURE 1. Summary of the association between HLA types and CM-related SJS/TEN with SOC. HLA-B*44:03 was significantly associated with CM-related SJS/TEN with SOC in Japanese, Brazilian, Indian, and Thai populations. HLA-A*02:06 was significantly associated with CM-related SJS/TEN with SOC in Japanese and Korean patients (OR: odds ratio OR).
CMs which combat the common cold include multi-ingredient CMs and NSAIDs such as ibuprofen and acetaminophen. We found that acetaminophen, present in various CMs, was the most frequently implicated causative drug in Japan (Ueta et al., 2014a; Ueta et al., 2019) and that HLA-A*02:06 was also strongly associated with acetaminophen-related SJS/TEN with SOC (80 patients, 113 controls; OR = 5.4, p = 8.0 × 10–7) (Ueta et al., 2019).
Similar to the United States and the United Kingdom, CMs, particularly acetaminophen (paracetamol), are widely used over-the-counter drugs in Thailand. Therefore, our Thai collaborators also investigated the HLA types in Thai patients with acetaminophen-related SJS/TEN with SOC. They found a significant association with HLA-A*33:03 (20 patients, 60 controls; OR = 5.4, p = 0.0030), HLA-B*44:03 (20 patients, 60 controls; OR = 9.0, p = 0.0004), HLA-C*07:01 (20 patients, 60 controls; OR = 9.3, p = 0.0002), and the HLA-B*44:03–HLA-C*07:01 haplotype (20 patients, 60 controls; OR = 9.0, p < 0.001) (Jongkhajornpong et al., 2020). This suggests that these HLA types play a role in the pathogenesis of SOC in acetaminophen-related SJS/TEN.
Our Brazilian collaborators found that among CMs, dipyrone, classified as an anti-inflammatory drug and widely used, was the main drug responsible for inciting SJS/TEN with SOC. They identified HLA-B*44:03 and HLA-DQB1*04:02 as potential risk factors for dipyrone-related SJS/TEN with SOC in the Brazilian population of European ancestry, and HLA-C*05:01 as a potential risk factor for dipyrone-related SJS/TEN with SOC in the Pardo Brazilian population (Wakamatsu et al., 2021).
We have suspected that a common function of CMs such as acetaminophen and dipyrone is highly implicated in the onset of SJS/TEN with SOC (Ueta et al., 2010a; Ueta, 2016; Ueta, 2018; Ueta, 2020; Ueta, 2021a; Ueta, 2021b).
The common function of CMs is the suppression of prostaglandin E2 (PGE2) production. We have suggested that the common function of CMs might be important for the onset of CM-related SJS/TEN with SOC (Ueta et al., 2010a; Ueta, 2016; Ueta, 2018; Ueta, 2020; Ueta, 2021a; Ueta, 2021b) because PGE2 suppresses mucocutaneous inflammation (Kunikata et al., 2005; Ueta et al., 2009a; Honda et al., 2009); PGE2 acts on EP3 (PGE2 receptor 3) in the epidermis (Honda et al., 2009) and the mucosal epithelium such as the conjunctival (Ueta et al., 2009a) and tracheal epithelium (Kunikata et al., 2005), and it negatively regulates mucocutaneous inflammation (Kunikata et al., 2005; Ueta et al., 2009a; Honda et al., 2009). We have suspected that CMs including acetaminophen and NSAIDs could upregulate inflammatory responses by suppressing the production of PGE2 which suppresses mucocutaneous inflammation, that they augment abnormal immune responses, and that they elicit the induction of SJS/TEN with SOC (Ueta et al., 2010a; Ueta, 2016; Ueta, 2018; Ueta, 2020; Ueta, 2021a; Ueta, 2021b).
PTGER3 is the gene of EP3. We also found that PTGER3 is a susceptibility gene for CM-related SJS/TEN with SOC (Ueta et al., 2010a) and that HLA-A*02:06 and PTGER3 polymorphisms exerted additive effects in Japanese and Korean patients with CM-related SJS/TEN with SOC (OR = 10.8 and 14.2, respectively) (Ueta et al., 2015b).
Our investigation of EP3 protein expression on the human ocular surface showed that the EP3 protein level was much lower in the conjunctival epithelium of patients with SJS/TEN with SOC than in the controls, that is, patients with conjunctival chalasis or chemical burns (Ueta et al., 2010a; Ueta et al., 2011a). This suggests that EP3 expression might be strongly downregulated on the ocular surface of patients with SJS/TEN with SOC and that the downregulation of EP3 protein expression might contribute to ocular surface inflammation in these patients (Ueta et al., 2010a; Ueta et al., 2011a; Ueta and Kinoshita, 2012; Ueta, 2021a).
We also have studied other susceptibility genes for CM-related SJS/TEN with SOC using a genome-wide association study (GWAS) with Affymetrix Axiom Genome-Wide ASI 1 Array. Our study with 117 Japanese patients with CM-related SJS/TEN with SOC and 691 controls showed that the IKZF1 gene was strongly associated with CM-related SJS/TEN with SOC in Japanese individuals (Ueta et al., 2015a). We found that a meta-analysis using samples from Japanese, Korean, Indian, and Brazilian patients revealed a significant genome-wide association between CM-related SJS/TEN with SOC and IKZF1 [rs4917014 (G vs. T), OR = 0.5, p = 8.5 × 10–11] (Ueta et al., 2015a). We also analyzed the association between IKZF1 single-nucleotide polymorphisms (SNPs) and Thai patients with CM-related SJS/TEN with SOC, and found that the IKZF1 SNP rs4917014 (G vs. T) was also significantly associated with Thai patients with CM-related SJS/TEN with SOC (Chantaren et al., 2019). These findings suggest IKZF1 is a universal marker for susceptibility to CM-related SJS/TEN with SOC (Ueta et al., 2015a; Chantaren et al., 2019).
Because our functional analysis of IKZF1 SNPs suggested the enhancement of the function of the IKZF1 gene in CM-related SJS/TEN with SOC (Ueta et al., 2015a), we produced K5-Ikzf1-EGFP transgenic (Ikzf1Tg) mice by introducing the Ik1 isoform into their cells expressing keratin 5, which is expressed in the epithelial tissues of, for example, the epidermis and conjunctiva. We found that mucocutaneous inflammation was exacerbated in Ikzf1Tg mice (Ueta et al., 2018), in which keratinocyte and mucosal epithelium including conjunctiva strongly expressed IKAROS, the protein of the IKZF1 gene. They developed not only dermatitis but also blepharoconjunctivitis. SJS/TEN with SOC in the acute stage shows not only skin and ocular surface inflammation but also oral mucosal erosion and paronychia. Our histology studies on Ikzf1Tg mice also showed not only dermatitis but also inflammation of their tongue tissue, blepharoconjunctiva, and paronychia, similar patients with SJS/TEN with SOC in the acute stage (Ueta et al., 2018). Thus, we concluded that IKZF1 plays a critical role in maintaining mucocutaneous homeostasis (Ueta et al., 2018). The association between IKZF1 SNPs and CM-related SJS/TEN with SOC suggests that IKZF1 could strongly contribute to the pathogenesis of CM-related SJS/TEN with SOC (Ueta, 2018; Ueta, 2020; Ueta, 2021a).
Among TLR1–TLR10, TLR3 is expressed most strongly in the ocular surface epithelium such as conjunctiva and cornea, which is more intense than that in mononuclear cells (Ueta et al., 2005; Ueta and Kinoshita, 2010). TLR3 recognizes dsRNA, a component of the life-cycle of most viruses, and is a member of the toll-like receptor family that is important for innate immunity, and could induce pro-inflammatory cytokines and IFN-β on the ocular surface (Ueta et al., 2005; Ueta et al., 2010b; Ueta and Kinoshita, 2010). Using the candidate-gene approach, we analyzed TLR3 gene polymorphisms and found that several TLR3 SNPs were significantly associated with CM-related SJS/TEN with SOC (Ueta et al., 2007a; Ueta et al., 2012a; Ueta et al., 2012b). Our investigations of TLR3 gene functions using TLR3 transgenic (TLR3Tg) and TLR3 knock-out (KO) mice showed that the rate of ocular surface inflammation was significantly increased in TLR3Tg and significantly decreased in TLR3-KO mice (Ueta et al., 2009b), suggesting that TLR3 positively regulates ocular surface inflammation (Ueta et al., 2009b). TLR3 is expressed in the epidermis of the skin and it positively regulates skin inflammation (Nakamura et al., 2015; Yasuike et al., 2017), and it is possible that innate immunity such as TLR3 might contribute to mucocutaneous inflammation seen in SJS/TEN with SOC (Ueta and Kinoshita, 2012; Ueta, 2016; Ueta, 2018; Ueta, 2021a).
In patients with SJS/TEN with SOC, the plasma level of miR-628-3p miRNA was significantly elevated and this miRNA could silence the mRNA expressions of pathogen-associated molecular patterns (PAMPs) of TLR3, RIG-I, MDA5, and other innate immune-related molecules, such as IFI44L, CXCL11, TNFSF10, RSAD2, CXCL10, and CCL8 (Ueta et al., 2021). Consequently, the upregulation of hsa-miR-628-3p in the plasma of SJS/TEN with SOC patients may suppress TLR3 gene expression and the expression of innate immune-related genes. On the other hand, we also found that in the conjunctival epithelium of SJS/TEN with SOC patients, hsa-miR-628-3p was downregulated, suggesting that its systemic (plasma) upregulation may compensate for its local (ocular surface) downregulation (Ueta et al., 2021). Because hsa-miR628p can regulate innate immunity, the upregulation of hsa-miR-628-3p in the plasma of SJS/TEN with SOC patients supports our hypothesis that abnormal innate immunity was observed in SJS/TEN patients with SOC.
The examination of tear cytokines of patients with SJS/TEN with SOC in the chronic stage showed that CXCL10 was significantly downregulated (Ueta et al., 2017b). As CXCL10 is highly induced by the TLR3 ligand poly(I:C) in human corneal and conjunctival epithelial cells (Ueta et al., 2010b), it is possible that abnormal innate immunity is involved in the presence of TLR3 on the ocular surface of SJS/TEN with SOC (Ueta and Kinoshita, 2012; Ueta, 2016; Ueta, 2018; Ueta, 2021a).
The susceptibility genes for CM-related SJS/TEN with SOC, PTGER3, and IKZF1 also have functional interactions with TLR3; EP3 (PTGER3) negatively regulated TLR3-dependent ocular surface inflammation (Ueta et al., 2011b; Ueta et al., 2012a; Ueta et al., 2012c); and IKZF1 mRNA was upregulated by TLR3 in human epidermal keratinocytes and conjunctival epithelial cells (Ueta et al., 2018).
These combined findings suggest that abnormal innate immunity could strongly contribute to the etiology of SJS/TEN with SOC (Ueta and Kinoshita, 2012; Ueta, 2016; Ueta, 2018; Ueta, 2020; Ueta, 2021a).
Although there are some reports on oligoclonal T cell populations showing HLA restriction and drug reactivity for HLA-B*58:01 restricted allopurinol-SJS/TEN (Lin et al., 2015) and HLA-B*15:02 restricted carbamazepine-SJS/TEN (Wei et al., 2012), there are few reports on T cell-mediated mechanisms in CM-related SJS/TEN with SOC, suggesting that more investigations are required to elucidate the pathogenic mechanisms of CM-related SJS/TEN with SOC.
Since CM-related SJS/TEN with SOC developed in patients after taking CMs for the common cold due to some viral or mycoplasma infection, we suspected that not only CMs, miRNAs such as hsa-miR-628-3p, and the interaction of susceptibility genes such as TLR3, PTGER3 (which ligand, PGE2 is downregulated by cold medicines such as NSAIDs, acetaminophen, and dipyrone), and IKZF1 but also some microbial infections are important and necessary for triggering the onset of SJS/TEN with SOC (Ueta and Kinoshita, 2012; Ueta, 2016; Ueta, 2018; Ueta, 2020; Ueta, 2021a) (Figure 2).
Despite the genetic diversity of CM-related SJS/TEN with SOC among different ethnic groups, we need to continue to identify the genetic predisposition for SJS/TEN with SOC to prevent its onset and to reduce the incidence of blindness due to SJS/TEN with SOC.
Since CM-related SJS/TEN with SOC is a rare condition with a complex genetic background, it is reasonable to posit the presence of multiplicative interactions of HLA and susceptibility genes such as HLA-A and TLR3 (Ueta et al., 2012a), HLA-A and REC14 32, and HLA-A and PTGER3 (Ueta et al., 2015b), and it is possible that multiple susceptibility genes for CM-related SJS/TEN with SOC are involved in forming functional networks. An imbalance in these genes may trigger mucocutaneous inflammation seen in patients with CM-related SJS/TEN with SOC.
As our investigations identified HLA and several SNP sets with a high OR, their use may help alert the possibility of SJS/TEN with SOC onset.
MU wrote this mini review.
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese government, and also partly by grants-in-aids for scientific research from the Japanese Ministry of Health, Labor and Welfare, research grants from the Kyoto Foundation for the Promotion of Medical Science, and the Intramural Research Fund of Kyoto Prefectural University of Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Chantaren, P., Jongkhajornpong, P., Ueta, M., Puangsricharern, V., Lekhanont, K., Pisuchpen, P., et al. (2019). Association of IKZF1 SNPs in Cold Medicine-Related Stevens-Johnson Syndrome in Thailand. Clin. Transl. Allergy 9, 61. doi:10.1186/s13601-019-0300-9
Chung, W.-H., Hung, S.-I., Hong, H.-S., Hsih, M.-S., Yang, L.-C., Ho, H.-C., et al. (2004). A Marker for Stevens-Johnson Syndrome. Nature 428, 486. doi:10.1038/428486a
Honda, T., Matsuoka, T., Ueta, M., Kabashima, K., Miyachi, Y., and Narumiya, S. (2009). Prostaglandin E2-EP3 Signaling Suppresses Skin Inflammation in Murine Contact Hypersensitivity. J. Allergy Clin. Immunol. 124, 809–818. doi:10.1016/j.jaci.2009.04.029
Hung, S.-I., Chung, W.-H., Liou, L.-B., Chu, C.-C., Lin, M., Huang, H.-P., et al. (2005). HLA-B*5801 Allele as a Genetic Marker for Severe Cutaneous Adverse Reactions Caused by Allopurinol. Proc. Natl. Acad. Sci. U.S.A. 102, 4134–4139. doi:10.1073/pnas.0409500102
Jongkhajornpong, P., Ueta, M., Lekhanont, K., Puangsricharern, V., Prabhasawat, P., Chantaren, P., et al. (2020). Association of HLA Polymorphisms and Acetaminophen-Related Steven-Johnson Syndrome with Severe Ocular Complications in Thai Population. Br. J. Ophthalmol. 106, 317315. doi:10.1136/bjophthalmol-2020-317315
Jongkhajornpong, P., Lekhanont, K., Pisuchpen, P., Chantaren, P., Puangsricharern, V., Prabhasawat, P., et al. (2018). Association between HLA-B*44:03-HLA-C*07:01 Haplotype and Cold Medicine-Related Stevens-Johnson Syndrome with Severe Ocular Complications in Thailand. Br. J. Ophthalmol. 102, 1303–1307. doi:10.1136/bjophthalmol-2017-311823
Jun, I., Rim, J. H., Kim, M. K., Yoon, K.-C., Joo, C.-K., Kinoshita, S., et al. (2019). Association of Human Antigen Class I Genes with Cold Medicine-Related Stevens-Johnson Syndrome with Severe Ocular Complications in a Korean Population. Br. J. Ophthalmol. 103, 573–576. doi:10.1136/bjophthalmol-2018-313263
Kaniwa, N., Saito, Y., Aihara, M., Matsunaga, K., Tohkin, M., Kurose, K., et al. (2010). HLA-B*1511 Is a Risk Factor for Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japanese Patients. Epilepsia 51, 2461–2465. doi:10.1111/j.1528-1167.2010.02766.x
Kaniwa, N., Saito, Y., Aihara, M., Matsunaga, K., Tohkin, M., Kurose, K., et al. (2008). HLA-B Locus in Japanese Patients with Anti-epileptics and Allopurinol-Related Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Pharmacogenomics 9, 1617–1622. doi:10.2217/14622416.9.11.1617
Kaniwa, N., Sugiyama, E., Saito, Y., Kurose, K., Maekawa, K., Hasegawa, R., et al. (2013). Specific HLA Types Are Associated with Antiepileptic Drug-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japanese Subjects. Pharmacogenomics 14, 1821–1831. doi:10.2217/pgs.13.180
Kannabiran, C., Ueta, M., Sangwan, V., Rathi, V., Basu, S., Tokunaga, K., et al. (2017). Association of Human Leukocyte Antigen Class 1 Genes with Stevens Johnson Syndrome with Severe Ocular Complications in an Indian Population. Sci. Rep. 7, 15960. doi:10.1038/s41598-017-15965-7
Kunikata, T., Yamane, H., Segi, E., Matsuoka, T., Sugimoto, Y., Tanaka, S., et al. (2005). Suppression of Allergic Inflammation by the Prostaglandin E Receptor Subtype EP3. Nat. Immunol. 6, 524–531. doi:10.1038/ni1188
Lee, H. K., Yoon, K. C., Seo, K. Y., Ueta, M., and Kim, M. K. (2017). Chronic Ocular Complications of Stevens-Johnson Syndrome Associated with Causative Medications in Korea. J. Allergy Clin. Immunol. Pract. 6, 700–702.e2. doi:10.1016/j.jaip.2017.09.001
Lin, C.-H., Chen, J.-K., Ko, T.-M., Wei, C.-Y., Wu, J.-Y., Chung, W.-H., et al. (2015). Immunologic Basis for Allopurinol-Induced Severe Cutaneous Adverse Reactions: HLA-B*58:01-restricted Activation of Drug-specific T Cells and Molecular Interaction. J. Allergy Clin. Immunol. 135, 1063–1065. doi:10.1016/j.jaci.2014.09.041
Ma, K. S., Chung, W. H., Hsueh, Y. J., Chen, S. Y., Tokunaga, K., Kinoshita, S., et al. (2021). Human Leucocyte Antigen Association of Patients with Stevens-Johnson Syndrome/toxic Epidermal Necrolysis with Severe Ocular Complications in Han Chinese. Br. J. Ophthalmol. 106, 610–615. doi:10.1136/bjophthalmol-2020-317105
McCormack, M., Alfirevic, A., Bourgeois, S., Farrell, J. J., Kasperavičiūtė, D., Carrington, M., et al. (2011). HLA-A*3101 and Carbamazepine-Induced Hypersensitivity Reactions in Europeans. N. Engl. J. Med. 364, 1134–1143. doi:10.1056/nejmoa1013297
Mockenhaupt, M., Wang, C. W., Hung, S. I., Sekula, P., Schmidt, A. H., Pan, R. Y., et al. (2019). HLA‐B*57:01 Confers Genetic Susceptibility to Carbamazepine‐induced SJS/TEN in Europeans. Allergy 74, 2227–2230. doi:10.1111/all.13821
Nakamura, N., Tamagawa-Mineoka, R., Ueta, M., Kinoshita, S., and Katoh, N. (2015). Toll-like Receptor 3 Increases Allergic and Irritant Contact Dermatitis. J. Investigative Dermatology 135, 411–417. doi:10.1038/jid.2014.402
Sotozono, C., Ang, L. P. K., Koizumi, N., Higashihara, H., Ueta, M., Inatomi, T., et al. (2007). New Grading System for the Evaluation of Chronic Ocular Manifestations in Patients with Stevens-Johnson Syndrome. Ophthalmology 114, 1294–1302. doi:10.1016/j.ophtha.2006.10.029
Sotozono, C., Ueta, M., Koizumi, N., Inatomi, T., Shirakata, Y., Ikezawa, Z., et al. (2009). Diagnosis and Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis with Ocular Complications. Ophthalmology 116, 685–690. doi:10.1016/j.ophtha.2008.12.048
Sotozono, C., Ueta, M., Nakatani, E., Kitami, A., Watanabe, H., Sueki, H., et al. (2015). Predictive Factors Associated With Acute Ocular Involvement in Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. Am. J. Ophthalmol. 160, 228–237. doi:10.1016/j.ajo.2015.05.002
Tohkin, M., Kaniwa, N., Saito, Y., Sugiyama, E., Kurose, K., Nishikawa, J., et al. (2013). A Whole-Genome Association Study of Major Determinants for Allopurinol-Related Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japanese Patients. Pharmacogenomics J. 13, 60–69. doi:10.1038/tpj.2011.41
Ueta, M. (2016). Cold Medicine-Related Stevens-Johnson Syndrome/toxic Epidermal Necrolysis with Severe Ocular Complications-Phenotypes and Genetic Predispositions. Taiwan J. Ophthalmol. 6, 108–118. doi:10.1016/j.tjo.2016.06.001
Ueta, M. (2021). Findings by an International Collaboration on SJS/TEN With Severe Ocular Complications. Front. Med. 8, 649661. doi:10.3389/fmed.2021.649661
Ueta, M., Hamuro, J., Kiyono, H., and Kinoshita, S. (2005). Triggering of TLR3 by polyI:C in Human Corneal Epithelial Cells to Induce Inflammatory Cytokines. Biochem. Biophysical Res. Commun. 331, 285–294. doi:10.1016/j.bbrc.2005.02.196
Ueta, M., Hamuro, J., Nishigaki, H., Nakamura, N., Shinomiya, K., Mizushima, K., et al. (2018). Mucocutaneous Inflammation in the Ikaros Family Zinc Finger 1-keratin 5-specific Transgenic Mice. Allergy 73, 395–404. doi:10.1111/all.13308
Ueta, M., Kaniwa, N., Sotozono, C., Tokunaga, K., Saito, Y., Sawai, H., et al. (2014). Independent Strong Association of HLA-A*02:06 and HLA-B*44:03 with Cold Medicine-Related Stevens-Johnson Syndrome with Severe Mucosal Involvement. Sci. Rep. 4, 4862. doi:10.1038/srep04862
Ueta, M., Kannabiran, C., Wakamatsu, T. H., Kim, M. K., Yoon, K.-C., Seo, K. Y., et al. (2014). Trans-ethnic Study Confirmed Independent Associations of HLA-A*02:06 and HLA-B*44:03 with Cold Medicine-Related Stevens-Johnson Syndrome with Severe Ocular Surface Complications. Sci. Rep. 4, 5981. doi:10.1038/srep05981
Ueta, M., and Kinoshita, S. (2010). Innate Immunity of the Ocular Surface. Brain Res. Bull. 81, 219–228. doi:10.1016/j.brainresbull.2009.10.001
Ueta, M., and Kinoshita, S. (2012). Ocular Surface Inflammation Is Regulated by Innate Immunity. Prog. Retin. Eye Res. 31, 551–575. doi:10.1016/j.preteyeres.2012.05.003
Ueta, M., Matsuoka, T., Narumiya, S., and Kinoshita, S. (2009). Prostaglandin E Receptor Subtype EP3 in Conjunctival Epithelium Regulates Late-phase Reaction of Experimental Allergic Conjunctivitis. J. Allergy Clin. Immunol. 123, 466–471. doi:10.1016/j.jaci.2008.09.044
Ueta, M., Matsuoka, T., Sotozono, C., and Kinoshita, S. (2012). Prostaglandin E2 Suppresses Poly I. Cornea 31, 1294–1298. doi:10.1097/ico.0b013e318242fd7c
Ueta, M., Matsuoka, T., Yokoi, N., and Kinoshita, S. (2011). Prostaglandin E2 Suppresses Polyinosine-Polycytidylic Acid (polyI:C)-stimulated Cytokine Production via Prostaglandin E2 Receptor (EP) 2 and 3 in Human Conjunctival Epithelial Cells. Br. J. Ophthalmol. 95, 859–863. doi:10.1136/bjo.2010.199679
Ueta, M., Mizushima, K., Yokoi, N., Naito, Y., and Kinoshita, S. (2010). Gene-expression Analysis of polyI:C-Stimulated Primary Human Conjunctival Epithelial Cells. Br. J. Ophthalmol. 94, 1528–1532. doi:10.1136/bjo.2010.180554
Ueta, M., Nakamura, R., Saito, Y., Tokunaga, K., Sotozono, C., Yabe, T., et al. (2019). Association of HLA Class I and II Gene Polymorphisms with Acetaminophen-Related Stevens-Johnson Syndrome with Severe Ocular Complications in Japanese Individuals. Hum. Genome Var. 6, 50. doi:10.1038/s41439-019-0082-6
Ueta, M., Nishigaki, H., Mizushima, K., Naito, Y., Sotozono, C., and Kinoshita, S. (2021). Regulation of Innate Immune Response by miR-628-3p Upregulated in the Plasma of Stevens-Johnson Syndrome Patients. Ocular Surf. 21, 174–177. doi:10.1016/j.jtos.2021.05.008
Ueta, M., Nishigaki, H., Sotozono, C., and Kinoshita, S. (2017). Downregulation of Interferon-γ-Induced Protein 10 in the Tears of Patients with Stevens-Johnson Syndrome with Severe Ocular Complications in the Chronic Stage. BMJ Open Ophth 1, e000073. doi:10.1136/bmjophth-2017-000073
Ueta, M. (2021). Pathogenesis of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis With Severe Ocular Complications. Front. Med. 8, 651247. doi:10.3389/fmed.2021.651247
Ueta, M. (2018). Results of Detailed Investigations into Stevens-Johnson Syndrome With Severe Ocular Complications. Invest. Ophthalmol. Vis. Sci. 59, DES183–DES91. doi:10.1167/iovs.17-23537
Ueta, M., Sawai, H., Shingaki, R., Kawai, Y., Sotozono, C., Kojima, K., et al. (2017). Genome-wide Association Study Using the Ethnicity-specific Japonica Array: Identification of New Susceptibility Loci for Cold Medicine-Related Stevens-Johnson Syndrome with Severe Ocular Complications. J. Hum. Genet. 62, 485–489. doi:10.1038/jhg.2016.160
Ueta, M., Sawai, H., Sotozono, C., Hitomi, Y., Kaniwa, N., Kim, M. K., et al. (2015). IKZF1, a New Susceptibility Gene for Cold Medicine-Related Stevens-Johnson Syndrome/toxic Epidermal Necrolysis with Severe Mucosal Involvement. J. Allergy Clin. Immunol. 135, 1538–1545. doi:10.1016/j.jaci.2014.12.1916
Ueta, M., Sotozono, C., Inatomi, T., Kojima, K., Tashiro, K., Hamuro, J., et al. (2007). Toll-like Receptor 3 Gene Polymorphisms in Japanese Patients with Stevens-Johnson Syndrome. Br. J. Ophthalmol. 91, 962–965. doi:10.1136/bjo.2006.113449
Ueta, M., Sotozono, C., Nakano, M., Taniguchi, T., Yagi, T., Tokuda, Y., et al. (2010). Association between Prostaglandin E Receptor 3 Polymorphisms and Stevens-Johnson Syndrome Identified by Means of a Genome-wide Association Study. J. Allergy Clin. Immunol. 126, 1218–1225. doi:10.1016/j.jaci.2010.08.007
Ueta, M., Sotozono, C., Tokunaga, K., Yabe, T., and Kinoshita, S. (2007). Strong Association Between HLA-A*0206 and Stevens-Johnson Syndrome in the Japanese. Am. J. Ophthalmol. 143, 367–368. doi:10.1016/j.ajo.2006.09.029
Ueta, M., Sotozono, C., Yokoi, N., Inatomi, T., and Kinoshita, S. (2011). Prostaglandin E Receptor Subtype EP3 Expression in Human Conjunctival Epithelium and its Changes in Various Ocular Surface Disorders. PLoS One 6, e25209. doi:10.1371/journal.pone.0025209
Ueta, M. (2020). Stevens-Johnson Syndrome/toxic Epidermal Necrolysis with Severe Ocular Complications. Expert Rev. Clin. Immunol. 16, 285–291. doi:10.1080/1744666x.2020.1729128
Ueta, M., Tamiya, G., Tokunaga, K., Sotozono, C., Ueki, M., Sawai, H., et al. (2012). Epistatic Interaction between Toll-like Receptor 3 (TLR3) and Prostaglandin E Receptor 3 (PTGER3) Genes. J. Allergy Clin. Immunol. 129, 1413–1416. doi:10.1016/j.jaci.2012.01.069
Ueta, M., Tokunaga, K., Sotozono, C., Sawai, H., Tamiya, G., Inatomi, T., et al. (2012). HLA-A*0206 with TLR3 Polymorphisms Exerts More Than Additive Effects in Stevens-Johnson Syndrome with Severe Ocular Surface Complications. PLoS One 7, e43650. doi:10.1371/journal.pone.0043650
Ueta, M., Tokunaga, K., Sotozono, C., Sawai, H., Yoon, K.-C., Kum Kim, M., et al. (2015). HLA-A*02:06 and PTGER3 Polymorphism Exert Additive Effects in Cold Medicine-Related Stevens-Johnson Syndrome with Severe Ocular Complications. Hum. Genome Var. 2, 15023. doi:10.1038/hgv.2015.23
Ueta, M., Uematsu, S., Akira, S., and Kinoshita, S. (2009). Toll-like Receptor 3 Enhances Late-phase Reaction of Experimental Allergic Conjunctivitis. J. Allergy Clin. Immunol. 123, 1187–1189. doi:10.1016/j.jaci.2009.03.008
Wakamatsu, T. H., Ueta, M., Inoue, C., Costa, K. A., Sakano, L. Y., Sallum, J. M. F., et al. (2021). Human Leukocyte Antigen Class I and II Genes Associated with Dipyrone-Related Stevens-Johnson Syndrome and Severe Ocular Complications in a Brazilian Population. Ocular Surf. 20, 173–175. doi:10.1016/j.jtos.2021.02.008
Wakamatsu, T. H., Ueta, M., Tokunaga, K., Okada, Y., Loureiro, R. R., Costa, K. A., et al. (2017). Human Leukocyte Antigen Class I Genes Associated With Stevens-Johnson Syndrome and Severe Ocular Complications Following Use of Cold Medicine in a Brazilian Population. JAMA Ophthalmol. 135, 355–360. doi:10.1001/jamaophthalmol.2017.0074
Wei, C.-Y., Chung, W.-H., Huang, H.-W., Chen, Y.-T., and Hung, S.-I. (2012). Direct Interaction between HLA-B and Carbamazepine Activates T Cells in Patients with Stevens-Johnson Syndrome. J. Allergy Clin. Immunol. 129, 1562–1569. doi:10.1016/j.jaci.2011.12.990
Yamane, Y., Aihara, M., and Ikezawa, Z. (2007). Analysis of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Japan from 2000 to 2006. Allergol. Int. 56, 419–425. doi:10.2332/allergolint.o-07-483
Keywords: HLA, cold medicine, Stevens–Johnson syndrome, Toxic epidermal necrolysis, severe ocular complications, TLR3, PTGER3, IKZF1
Citation: Ueta M (2022) Susceptibility Genes and HLA for Cold Medicine-Related SJS/TEN with SOC. Front. Genet. 13:912478. doi: 10.3389/fgene.2022.912478
Received: 04 April 2022; Accepted: 14 June 2022;
Published: 11 July 2022.
Edited by:
Chonlaphat Sukasem, Mahidol University, ThailandReviewed by:
Chuang-Wei Wang, Linkou Chang Gung Memorial Hospital, TaiwanCopyright © 2022 Ueta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mayumi Ueta, bXVldGFAa290by5rcHUtbS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.