
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Genet. , 13 June 2022
Sec. Evolutionary and Population Genetics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.908367
This article is part of the Research Topic Population Genetics and Conservation of Aquatic Species View all 12 articles
To protect the germplasm resources of Schizothorax biddulphi, we developed and used 20 pairs of polymorphic microsatellite primers to analyze the genetic diversity and structure of populations. A total of 126 samples were collected from the Qarqan River (CEC), Kizil River (KZL), and Aksu River (AKS) in Xinjiang, China. The results showed that 380 alleles were detected in 20 pairs of primers and the average number of alleles was 19.0. The effective allele numbers and Nei’s gene diversity ranged from 1.1499 to 1.1630 and 0.0962 to 0.1136, respectively. The Shannon index range suggested low levels of genetic diversity in all populations. The genetic distance between the CEC and AKS populations was the largest, and the genetic similarity was the smallest. There was a significant genetic differentiation between CEC and the other two populations. The UPGMA clustering tree was constructed based on population genetic distance, and the clustering tree constructed by individuals showed that the AKS population and KZL population were clustered together, and the CEC population was clustered separately. Also, the group structure analysis also got the same result. It can be seen that although the three populations of S. biddulphi do not have high genetic diversity, the differentiation between the populations was high and the gene flow was limited, especially the differentiation between the CEC population and the other two populations. This study not only provided genetic markers for the research of S. biddulphi but the results of this study also suggested the need for enhanced management of S. biddulphi populations.
Schizothorax biddulphi, in the order Cypriniformes and family Cyprinidae, is a symbolic species distributed only in the Tarim River system of Xinjiang Uygur Autonomous Region, China (Le and Chen, 1998; Luo et al., 2012). Due to its slow growth, late sexual maturity, low fecundity, and higher requirements for living environment, coupled with the destruction of the ecological environment and human interference in recent years, the habitat of S. biddulphi has been destroyed and continuously reduced, and the population has been drastically reduced (Nie et al., 2014). Therefore, S. biddulphi was listed in the China Red Data Book of Endangered Animals–Fish Volume in 1998 (Le and Chen, 1998) and in the Xinjiang Provincial Second-Class Protected Animals in 2004. In 2020, S. biddulphi upgraded to the national secondary protection fish of China.
Microsatellite DNA, also known as simple sequence repeats (SSRs) and short tandem repeats (STRs), is a nucleotide sequence consisting of 1–6 nucleotides in the repeat motif (Tautz and Renz, 1984). SSR has the advantages of large number, wide distribution, high polymorphism, high conservation, easy detection, high repeatability, and co-dominant characteristics, so it is widely distributed in eukaryotic genomes as a molecular marker (Li et al., 2008; Mohanty et al., 2016). SSR plays an important role in biological genetics research and has been increasingly used in conservation genetics research of endangered animals that lack genetic information, such as Rhesus macaque (Macaca mulatta), Asian elephant (Elephas maximus), Arabian oryx (Oryx leucoryx), and land snail (Satsuma myomphala), to guide the management and conservation of endangered animals (Li et al., 2012; Kang et al., 2017; Elmeer et al., 2019; Marasinghe et al., 2021; Qiu et al., 2021).
Most genetic diversity assessment methods are only applicable to diploid organisms, but studies showed that schizothoracine was polyploidy, mainly tetraploid, hexaploid, and octaploid (Wang et al., 2016; Guo et al., 2019). However, in most of the existing studies on S. biddulphi, the complexity of polyploidy was ignored, and the analyses followed the diploid approaches (Gong et al., 2012; Luo et al., 2012). This neglect might not explain population genetic diversity and genetic differentiation among populations more accurately. To overcome it, the most common solution was to convert microsatellite's polyploid genotypes to pseudodiploid genotypes, which allowed the use of many popular methods (Wang and Scribner, 2014; Liu et al., 2017). A few studies attempted to develop microsatellite primers for S. biddulphi to analyze genetic differences in populations based on polyploidy information, but the available markers were still very limited (Yang et al., 2014), and the polymorphism of these markers was low, which is difficult to revealing genetic differentiation among populations. Therefore, in this study, we wanted to develop more high polymorphic microsatellites for the research and population management of S. biddulphi. Also, three populations from the Tarim River Basin were investigated to provide support for the germplasm resource conservation of S. biddulphi.
From July 2017 to July 2019, 126 tail fin samples were collected from 3 populations, including 44 from Cherchen River (CEC; 37°42′37″N, 85°41′45″E), 69 from Kizil River (KZL; 39°31′12″N, 75°14′20″E), and 13 from Akesu River (AKS; 40°14′4″N, 80°5′36″E), Xinjiang, China (Figure 1). All samples were stored in absolute alcohol and brought back to the laboratory and stored at −20°C. Total genomic DNA was extracted from the tail fin using the TIANamp Genomic DNA Kit (Tiangen, Beijing, China) following the manufacturer’s instructions. The concentration and quality of DNA were estimated using a DeNovix DS-11 spectrophotometer (DeNovix, Wilmington, United States).
The samples for the genome survey were randomly selected from 3 S. biddulphi from Cherchen River. Five DNA libraries of 270 bp(2), 350 bp(2), and 500 bp(1) insert size were constructed and sequenced by Illumina Hi-Seq 2000.
The Perl script MIcroSAtellite (MISA, http://pgrc.ipk-gatersleben.de/misa/misa.html) was used for genic-SSR markers with perfect repeat units of 2–6 nucleotides. The minimum SSR length criteria were defined as six reiterations for dinucleotide and five reiterations for other repeat units. About 288 microsatellite loci were selected, of which 288 with enough flanking sequence were used for primer design using Primer Premier 5.0 (Premier Biosoft International).
All SSR primer pairs were initially screened for a sample set comprising 42 randomly selected specimens, optimized for reproducible amplification using standard PCR conditions with annealing temperatures altered according to the primer sequences. By performing electrophoresis on an 8% non-denaturation polyacrylamide gel, 20 of 30 loci were successfully amplified and shown to be polymorphic, which were deposited in the GenBank (Accession Nos. MT211579–MT211598).
The forward primers for the 20 SSR loci were labeled with fluorescent dyes (6-FAM, HEX, TAMRA, and ROX). PCR amplification was carried out using genomic DNA extracted from 126 S. biddulphi. Each 50 μL PCR contained 2 μL genomic DNA, 25 µL RTaq DNA Premix, 1 µL forward and reverse primers, and 21 µL double-distilled water. The thermocycling conditions were as follows: initial denaturation for 5 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at the annealing temperature (Table 1), and 40 s at 72°C, and finally 10 min at 72°C. The PCR products were separated by capillary electrophoresis using an ABI3730xl DNA analyzer (Applied Biosystems) after confirmation of PCR amplification on a 1.5% agarose gel. The genotyping of polymorphism microsatellite locus analysis was performed using the software GeneMapper v4.0 (Applied Biosystems).
This study referred to the method of Zhu et al. (2002) to analyze the electrophoretic patterns. The polymorphic bands amplified by each primer were counted, a band at the same migration position was marked as “1,” and no band was marked as “0.” According to the capillary electrophoresis pattern analyzed by Gene mapper 4.1, the standard for adopting the data is that the signal value is above 400, there is no interference of other spurious peaks, and the peak shapes from the same site are all similar. Finally, the original data matrix is established. Data editing and format conversion were performed in GenAlEx v 6.501 software.
Population genetic parameters such as the polymorphic percentage, allele number (Na), effective number of alleles (Ne), Nei’s genetic distance (H), and Shannon’s Information index (I) were analyzed using GenePop v 4.14. Poptree V2 was used to establish an NJ clustering tree representing population genetic relationships based on allele frequency (Takezaki et al., 2010). Pcoa-genalex V6.5 analyzed genetic relationships among populations based on genetic distance (Smouse and Peakall, 2012). Structure 2.3.4 (http://pritch. bsd. uchicago. edu/) was used to analyze the genetic structure of the population, and the mixed model and frequency correlation model were selected. Online software Structure Harvest was used to estimate the theoretical population number according to the ΔK algorithm (Evanno et al., 2005).
The genomic DNA of S. biddulphi was used to construct two 270 bp libraries, two 350 bp libraries, and one 500 bp library, which were sequenced and filtered to obtain 165.34 Gb of high-quality data. The total sequencing depth was about 174 ×, the Q20 ratio of sequencing data was above 95.63%, and the Q30 ratio was above 90.48% (Supplementary Table SA1).
The corresponding Kmer depth of the main peak is 120. There are three peaks in Kmer, the main peak, the 1/2 peak, and the 1/4 peak, suggesting that the species may be tetraploid (Figure 2). The total number of Kmer obtained from the sequencing data was 11,754,486,585. After removing the Kmer with abnormal depth, a total of 114,172,843,993 Kmer were used for genome length estimation, and the calculated genome length was about 947.30 Mbp. According to the Kmer distribution, the content of repeated sequences was estimated to be about 39.45%, and the heterozygosity was estimated to be about 0.81%. Therefore, the genome of this species belongs to a complex genome with high heterozygosity. Scaffold N50 was about 3.84 Kb and Contig N50 was about 869 bp (Supplementary Table SA2).
A total of 558,993 unigenes with a total nucleotide number of 1,125,446,683 were obtained from the genomic data of S. biddulphi. A total of 743,118 SSR sequences were detected, and 155,018 sequences contained more than 1 SSR (Supplementary Table SA3). The microsatellite-type richness of the detected S. biddulphi genome was high, and di-nucleotide repeat unit content was the most (42.74%), followed by mono-nucleotide repeat units (39.07%), and the content of tri-nucleotide (9.09%) and tetra-nucleotide (7.94%) repeat units was similar, the content of penta-nucleotide (0.92%) and hexa-nucleotide (0.24%) repeat units was less. The distribution density of the six nucleotide repeat types was positively correlated with the corresponding SSR content (Table 2).
S. biddulphi microsatellites include 4 different mono-nucleotide repeat microsatellite types, 12 different di-nucleotide repeat microsatellite types, 59 different tri-nucleotide repeat microsatellite types, 215 different tetra-nucleotide repeat microsatellite types, 582 different penta-nucleotide repeat microsatellite types, and 418 different hexa-nucleotide repeat microsatellite types. Among the mono-nucleotide repeats, A/T was the main base composition (93.23%). AC/TG (30.02%) was more in di-nucleotide repeat SSRs. AAT/TTA (29.44%) and ATT/TTA (23.57%) had high frequency of tri-nucleotide repeats. TCTA/AGAT and GATA/CTAT appeared more frequently in tetra-nucleotide repeats. TATTA/ATAAT and AAAAT/TTTTA appeared more frequently in penta-nucleotide repeats. GTGTGA/CACACT and ATATAC/TATATG appeared more frequently in hexa-nucleotide repeat microsatellite.
Among the 288 pairs of primers, 30 pairs of primers with high polymorphism were screened by agarose gel electrophoresis and 8% non-denaturing polyacrylamide gel electrophoresis. According to the detection results of capillary electrophoresis, 20 pairs of SSR primers with high polymorphism were screened out from the above 30 pairs of primers (Table 1). The polymorphic parameters of the 20 primer pairs are shown in Supplementary Table SA4.
A total of 380 alleles (Na) were detected, and the number of alleles per SSR locus ranged from 5 (T166) to 56 (T269), with an average of 19 alleles. The number of effective alleles (Ne) ranged from 1.0595 (T269) to 1.5804 (T166), and the average number was 1.2517. Nei’s genetic distance (H) ranged from 0.0540 (T269) to 0.3449 (T166), with an average of 0.1635. Shannon’s Information index (I) for each SSR locus ranged from 0.1206 (T269) to 0.5188 (T166), with an average of 0.2773 (Table 3). In terms of population genetic diversity parameters, H and I were consistent, showing that the CEC was the highest and the AKS was the lowest (Table 4).
The range of genetic differentiation index and gene flow was 0.107–0.224 and 0.866–2.086, respectively. The genetic differentiation index among CEC, AKS, and KZL was great genetic differentiation (0.15 < Fst < 0.25), and there was moderate genetic differentiation between AKS and KZL (0.05 < Fst < 0.15). The gene flow between AKS and KZL was the highest, while the gene flow between CEC and the other two populations was low (Table 5).

TABLE 5. Gene flow Nm (above diagonal) and Fst values for pairwise comparison (blow diagonal) among the three populations of Schizothorax biddulphi.
The range of Nei’s genetic distance and genetic identity between every two populations was 0.021–0.044 and 0.957–0.980, respectively. The genetic distance of Nei’s between CEC and AKS was the longest (0.044), and the genetic identity was the least. The Nei’s genetic distance between AKS and KZL was the closest (0.021), and the genetic identity was the largest (Table 6).

TABLE 6. Nei’s genetic distance D (above diagonal) and genetic identity (blow diagonal) among the three populations of Schizothorax biddulphi.
Cluster analysis was performed on an individual (Figure 3) and population (Figure 4) of S. biddulphi. The results showed that 126 samples of S. biddulphi were roughly divided into two clusters, with AKS and KZL individuals clustered into one cluster and CEC individuals clustered into another cluster. At the same time, the UPGMA tree constructed according to population genetic distance was consistent with the clustering tree constructed by individuals, the AKS and KZL clustered into a cluster, and the CEC clustered into a single cluster.
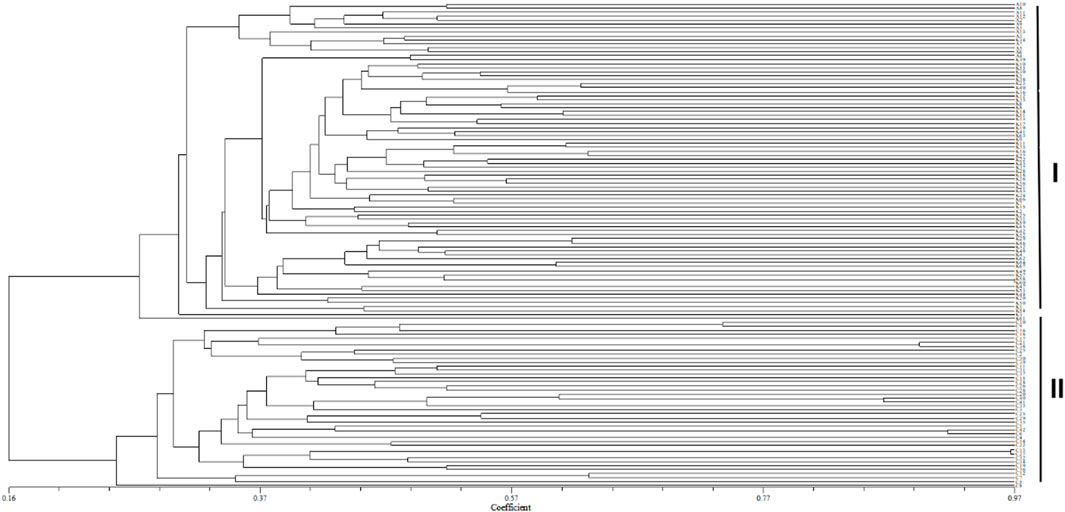
FIGURE 3. UPGMA clustering tree of 126 samples of Schizothorax biddulphi. I: all individuals of the Aksu and Kyzyl rivers; II: all individuals of the Qarqan River.
AMOVA results showed that 20% of the genetic variation of S. biddulphi came from inter-population and 80% of the genetic variation came from intra-population (Table 7). Therefore, the genetic variation within populations was larger than that between populations.
Division of the dataset into two subclusters (K = 2) produced the best assignment of individuals to subclusters, and subsequent analysis of each subset determined the appropriate number of populations within each subset (Figure 5A). There are two gene banks in the three wild populations (Figure 5B). When K = 2, the gene composition of AKS and KZL samples mainly came from gene bank 1. The gene composition of CEC was mainly derived from gene bank 2 (Table 8).
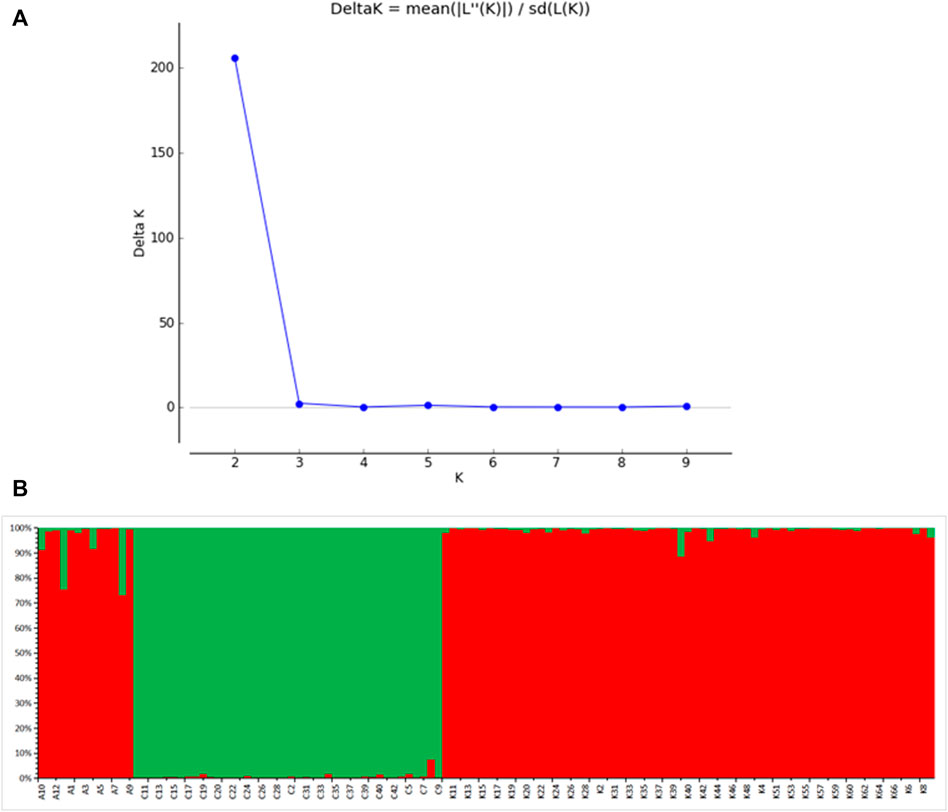
FIGURE 5. Model choice criterion lnP (D) of the structure analysis for each K value (A); K = 2, structure analysis in all three Schizothorax biddulphi populations (B), CEC (green), and other two populations (red).

TABLE 8. Proportion of ancestry of each population in three gene pools defined with the model-based clustering method.
S. biddulphi is an economic fish only distributed in the Tarim River system of Xinjiang, China, and is endangered at present. However, there are few nucleotide sequences related to S. biddulphi microsatellites found in the GeneBank database, and the number of markers that can be used for population genetic diversity research is limited. Therefore, this study obtains microsatellite sequences through genome sequencing of S. biddulphi, and develops and screens out microsatellite markers with high polymorphism. SSRs in the genome of S. biddulphi are not only abundant but also more numerous, with a predominance of dinucleotide repeats. This is a common phenomenon in the most aquatic animal genome, such as Takifugu rubripes (Cui et al., 2006), Scophthalmus maximus (Ruan et al., 2011), Ietalurus punetaus (Serapion et al., 2004), Fugu rubripes (Edwards et al., 1998), and Marsupenaeus japonicus (Lu et al., 2017).
Genetic diversity parameters, such as the effective number of alleles, Nei’s genetic distance, and Shannon’s Information index, can be used to reflect the size of the genetic diversity; in a fixed parameter range, the greater the value of these parameters, the higher the gene richness of the population (Lu et al., 2017). In this study, the average number of alleles of 20 pairs of polymorphic primers in 3 populations of S. biddulphi was 19, which was all higher than 9.5, 4.9, and 3.5 of the studies of Gong et al. (2012), Yang et al. (2014), and Luo et al. (2012). However, there was a large difference between the number of alleles at the SSR loci and the number of effective alleles, which indicated that alleles were unevenly distributed in the populations (Kimura and Ohta, 1969). Compared with the polymorphic locus frequency of other related species such as Schizopygopsis malacanthus (68.22%), Schizopygopsis malacanthus chengi (66.36%), Schizothorax wangchiachii (61.21%), Schizothorax griseus (61.24%), Schizothorax grahami (60.63%), Schizothorax dolichonema (57.01%), and Schizopygopsis stoliczkae (57.48%), the genetic diversity of S. biddulphi is relatively lower (Meng and Zhang, 2007; He et al., 2012). Molecular variance analysis of genetic differences between populations showed that genetic variation was mainly from within populations, with small differences between populations, which was consistent with the result of the study by Yang et al. on Tashikuergan, Duolang headworks, Muzhati River, Yulongkashi River, and Kalakashi River populations (Yang et al., 2014). At the same time, the results of Nei’s genetic distance and Shannon’s Information index in this study were consistent, indicating that the genetic diversity of CEC was the highest among the three sampling sites, while that of AKS was the lowest. In a finite population, the number of generations required for accidental new selectively neutral mutations depends on the effective population size, and low-density populations favor the accumulation of selectively neutral mutations (Kimura, 1979; Barros et al., 2020). Therefore, the uneven distribution of alleles in the population of S. biddulphi may be caused by the decrease in population size. The dams built on the Tarim River in recent years have blocked the migration of fish, leading to a significant increase in the frequency of their inbreeding, which in turn causes low heterozygosity and genetic diversity of the offspring. If this situation is not effectively improved, the genetic diversity of the population will continue to decline (or the inbreeding coefficient will continue to increase), eventually resulting in an irreversible decline of the population and risk of extinction. The genetic variation obtained by microsatellite markers was similar among different populations of S. biddulphi, and the genetic variation mainly occurred within populations rather than between populations. CEC may retain the original population resources, or the result may be caused by the small number of AKS samples.
Fst and Nm are two important indicators to measure population genetic structure (Pierson et al., 2015). In this study, the genetic differentiation between AKS and KZL was moderate, while that between other populations in pairs was high. However, the size of gene flow between populations was the opposite; that is, there was more gene exchange between AKS and KZL than between other populations. This may be due to the Kizil River being an upstream tributary of the Kashgar River, which originates from the eastern parts of the Pamir Mountains (Hayward, 1869). The Kashgar River eventually flows eastward into the Tarim River, while the Aksu River is the main tributary of the Tarim River, so there is a wide range of gene exchange between the two populations (Wang et al., 2021). Although the Kashgar River has been separated from the mainstream of the Tarim River at present, the separation time is relatively short, and the resulting genetic differentiation is relatively small. The reason for the large genetic differentiation between CEC and the other two populations is as follows: As early as 220 BC, the Tarim River and Cherchen River converged in Lop Nur (Xinjiang, China), and the gene exchange between aquatic organisms in the two rivers was directly influenced by the evolution of Lop Nur (Haysa et al., 2016). Studies have shown that Lop Nur appeared in the Upper Miocene about 5 mega annum ago and dried up at the end of the Pliocene due to climatic reasons. Subsequently, the climate gradually improved and the dryness decreased during 2.7–1.5 mega annum ago, and the surface of Lop Nur recovered before 1.5 mega annum (Yuan and Yuan, 1998; Luo et al., 2006; Chang and Chang, 2013). The genetic differentiation between CEC and other populations may have been caused during the period of Lop Nur drying, which caused geographical isolation between CEC and other populations and the fragmentation of its habitat. Although Cherchen River and Tarim River intersect at Taitema Lake, the genetic differentiation between CEC and other populations still exists significantly. For aquatic organisms, geographical isolation barriers between different water areas usually lead to obvious population genetic differentiation, so the pattern of fish distribution often determines its genetic differentiation pattern (Bergek and Björklund, 2007; Riginos et al., 2011). For example, the Arctic grayling (Thymallus arcticus) in the Itkillik, Kuparuk, and Sagavanirktok basins in the foothills of the Brooks Mountains undergo altered dispersal and gene flow due to movement restrictions caused by river drying (Golden et al., 2021).
Based on Nei’s genetic distance, the UPMGA method was used for cluster analysis of three S. biddulphi populations and individuals. The results showed that AKS and KZL clustered into a cluster, and CEC clustered into a cluster. Structure cluster analysis also shows that the CEC population had great genetic differences from the other two populations, which further verifies the Cherchen River due to early break with the Tarim River, cutoff of the genes within or between is a kind of communication, increase the genetic distance, and then the genetic similarity between CEC and the other two populations was small. After 1950s, although the Kizil River also gradually lost its connection with the Tarim River, the time of geographical isolation between KZL and AKS was relatively short, so the genetic distance between the two populations was relatively close and the genetic similarity coefficient was high.
In this study, 20 new SSR markers of S. biddulphi were developed by genome survey. These markers used were informative enough and could detect genetic diversity among S. biddulphi. The results of this study indicated that Cherchen River had preserved relatively primitive S. biddulphi population resources due to its early separation from the mainstream of Tarim River, and showed higher genetic diversity than the other two populations. However, the genetic diversity of S. biddulphi was still relatively low compared with other schizothoracin. If it is not its protection and intervention, S. biddulphi gene flow between populations appears greatly reduced or even cutoff and will lead to further genetic differentiation between populations, and make it into a vicious circle. Therefore, we must protect the genetic resources of S. biddulphi scientifically and rationally, strengthen the protection of its ecological water environment, and improve gene exchange between populations (Caballero and Toro, 2002, Jasim Aljumaili et al., 2018, Roques et al., 2016).
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA818042.
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Scientific Ethics Committee of Tarim University.
ZN and JW: conceptualization. YR and LZ: experimental operation. YR and RG: field sampling. ZN and YR: sample determination. ZN: writing—original draft preparation. JW: writing—reviewing, and editing. All authors read and approved the final manuscript.
This study was funded by the National Natural Science Foundation of China (31860729 and 31560721); The Young and Middle-aged Science and Technology Innovation Leading Talent Program Pro-ject of Xinjiang Production and Construction Corps (2018CB033); United Fund of the Ocean University of China and Tarim University (ZHYLH201902); President’s Fund of Tarim University-Germplasm resources and Genetic Breeding of Schizothorax in Southern XinjiangInnovative research team project (TDZKCX202204).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.908367/full#supplementary-material
Barros, J., Winkler, F. M., and Velasco, L. A. (2020). Assessing the Genetic Diversity in Argopecten Nucleus (Bivalvia: Pectinidae), a Functional Hermaphrodite Species with Extremely Low Population Density and Self‐fertilization: Effect of Null Alleles. Ecology Evol. 10, 3919–3931. doi:10.1002/ece3.6080
Bergek, S., and Björklund, M. (2007). Cryptic Barriers to Dispersal within a Lake Allow Genetic Differentiation of Eurasian Perch. Evolution 61, 2035–2041. doi:10.1111/j.1558-5646.2007.00163.x
Caballero*, A., and Toro, M. A. (2002). Analysis of Genetic Diversity for the Management of Conserved Subdivided Populations. Conserv. Genet. 3, 289–299. doi:10.1023/a:1019956205473
Chang, Q., and Chang, H. (2013). The Environmental Magnetism Study of Core Ls2 in Lop Nur, Tarim since 7.1Ma. Quat. Sci. 33, 876–888. doi:10.3969/j.issn.1001-7410.2013.05.06
Cui, J., Shen, X., Yang, G., Gong, Q., and Gu, Q. (2006). The Analysis of Simple Sequence Repeats in Takifugu rubripes Genome. Period. Ocean. Univ. China. 2, 249–254. doi:10.16441/j.cnki.hdxb.2006.02.015
Edwards, Y. J. K., Elgar, G., Clark, M. S., and Bishop, M. J. (1998). The Identification and Characterization of Microsatellites in the Compact Genome of the Japanese Pufferfish, Fugu Rubripes: Perspectives in Functional and Comparative Genomic Analyses. J. Mol. Biology 278, 843–854. doi:10.1006/jmbi.1998.1752
Elmeer, K., Matatt, I., Al-Malki, A., and Alam, M. S. (2019). Genetic Diversity and Relatedness in Arabian oryx (Oryx leucoryx) Revealed by SSR Markers. QScience Connect. 2019, 3. doi:10.5339/connect.2019.3
Evanno, G., Regnaut, S., and Goudet, J. (2005). Detecting the Number of Clusters of Individuals Using the Software Structure: a Simulation Study. Mol. Ecol. 14, 2611–2620. doi:10.1111/j.1365-294x.2005.02553.x
Golden, H. E., Holsinger, K. E., Deegan, L. A., Mackenzie, C. J. A., and Urban, M. C. (2021). River Drying Influences Genetic Variation and Population Structure in an Arctic Freshwater Fish. Conserv. Genet. 22, 369–382. doi:10.1007/s10592-021-01339-0
Gong, X., Cui, Z., and Wang, C. (2012). Isolation and Characterization of Polymorphic Microsatellite Loci from the Endangered Tarim Schizothoracin (Schizothorax Biddulphi Günther). Conserv. Genet. Resour. 4, 795–797. doi:10.1007/s12686-012-9646-1
Guo, X.-Z., Zhang, G.-R., Wei, K.-J., Ji, W., Yan, R.-J., Wei, Q.-W., et al. (2019). Phylogeography of the Threatened Tetraploid Fish, Schizothorax Waltoni, in the Yarlung Tsangpo River on the Southern Qinghai-Tibet Plateau: Implications for Conservation. Sci. Rep. 9, 2704. doi:10.1038/s41598-019-39128-y
Hayward, G. W. (1869). Journey from Leh to Yarkand and Kashgar, and Exploration of the Sources of the Yarkand River. Proc. R. Geogr. Soc. Lond. 14, 41–74. doi:10.2307/1799609
He, Y., An, M., and Li, X. (2012). “RAPD Analysis on Genetic Diversity of Three Kind of Schizothoraxs in Guizhou,”. Editor J. Guizhou, 28, 1
Jasim Aljumaili, S., Rafii, M. Y., Latif, M. A., Sakimin, S. Z., Arolu, I. W., Miah, G., et al. (2018). Genetic Diversity of Aromatic Rice Germplasm Revealed by SSR Markers. Biomed. Res. Int. 2018, 7658032. doi:10.1155/2018/7658032
Kang, S. W., Patnaik, B. B., Hwang, H.-J., Park, S. Y., Chung, J. M., Song, D. K., et al. (2017). Sequencing and De Novo Assembly of Visceral Mass Transcriptome of the Critically Endangered Land Snail Satsuma Myomphala : Annotation and SSR Discovery. Comp. Biochem. Physiology Part D Genomics Proteomics 21, 77–89. doi:10.1016/j.cbd.2016.10.004
Kimura, M. (1979). Model of Effectively Neutral Mutations in Which Selective Constraint Is Incorporated. Proc. Natl. Acad. Sci. U.S.A. 76, 3440–3444. doi:10.1073/pnas.76.7.3440
Kimura, M., and Ohta, T. (1969). The Average Number of Generations until Fixation of a Mutant Gene in a Finite Population. Genetics 61, 763–771. doi:10.1093/genetics/61.3.763
Le, P., and Chen, Y. (1998). China Red Data Book of Endangered Animals: Pisces. Beijing: Science Press.
Li, D., Yao, Y., Huang, X., Cheng, A., Xu, H., Ni, Q., et al. (2012). Simple Sequence Repeat (SSR) Polymorphisms and Population Genetics in Sichuan Wild Rhesus Macaques. Adv. Mat. Res. 343-344, 690–697. doi:10.4028/www.scientific.net/AMR.343-344.690
Li, Q., Zheng, X., and Yu, R. (2008). Inheritance Mode of Microsatellite Loci and Their Use for Kinship Analysis in the Pacific Oyster (Crassostrea gigas). Chin. J. Ocean. Limnol. 26, 256–262. doi:10.1007/s00343-008-0256-4
Liu, Y., Chen, Y., Gong, Q., Lai, J., Du, J., and Deng, X. (2017). Paternity Assignment in the Polyploid Acipenser Dabryanus Based on a Novel Microsatellite Marker System. PLoS One 12, e0185280. doi:10.1371/journal.pone.0185280
Lu, X., Luan, S., Kong, J., Hu, L., Mao, Y., and Zhong, S. (2017). Genome-wide Mining, Characterization, and Development of Microsatellite Markers in Marsupenaeus japonicus by Genome Survey Sequencing. Chin. J. Ocean. Limnol. 35, 203–214. doi:10.1007/s00343-016-5250-7
Luo, C., Peng, Z., Yang, D., Liu, W., and He, J. (2006). Research on the Environmental Evolution of Lop-Nur in Xinjiang, China. Chin. J. Nat. 28, 37
Luo, W., Nie, Z., Zhan, F., Wei, J., Wang, W., and Gao, Z. (2012). Rapid Development of Microsatellite Markers for the Endangered Fish Schizothorax Biddulphi (Günther) Using Next Generation Sequencing and Cross-Species Amplification. Int. J. Mol. Sci. 13, 14946–14955. doi:10.3390/ijms131114946
Marasinghe, M. S. L. R. P., Nilanthi, R. M. R., Hathurusinghe, H. A. B. M., Sooriyabandara, M. G. C., Chandrasekara, C. H. W. M. R. B., Jayawardana, K. A. N. C., et al. (2021). Revisiting Traditional SSR Based Methodologies Available for Elephant Genetic Studies. Sci. Rep. 11, 8718. doi:10.1038/s41598-021-88034-9
Meng, L., and Zhang, J. (2007). RAPD Analysis of Genetic Relationships Among 5 Species (Subspecies) of Schizothoracinae in the Yalong River. China Fish. 6, 73–76. doi:10.3969/j.issn.1002-6681.2007.06.046
Mohanty, P., Sahoo, L., Pillai, B. R., Jayasankar, P., and Das, P. (2016). Genetic Divergence in Indian Populations ofM. Rosenbergiiusing Microsatellite Markers. Aquac. Res. 47, 472–481. doi:10.1111/are.12508
Nie, Z.-L., Wei, J., Ma, Z.-H., Zhang, L., Song, W., Wang, W.-M., et al. (2014). Morphological Variations of Schizothoracinae Species in the Muzhati River. J. Appl. Ichthyol. 30, 359–365. doi:10.1111/jai.12376
Peakall, R., Smouse, P. E., Takezaki, N., Nei Takezaki, N., Nei, M., and Tamura, K. (20122010). GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research-Aan updatePOPTREE2: Software for Constructing Population Trees from Allele Frequency Data and Computing Other Population Statistics with Windows Interface. BioinformaticsMol. Biol. Evol. 2827, 2537747–2539752. doi:10.1093/bioinformatics/bts460
Pierson, J., Luikart, G., and Schwartz, M. (2015). “The Application of Genetic Indicators in Wild Populations: Potential and Pitfalls for Genetic Monitoring,” in Indicators and Surrogates of Biodiversity and Environmental Change. Editors D. B. Lindenmayer, P. Barton, and J. C. Pierson (Melbourne: CSIRO Publishing), 149
Qiu, J., Guo, R., Li, Y., Zhang, Y., Jia, K., Lei, Y., et al. (2021). De Novo transcriptome Assembly, Functional Annotation and SSR Marker Discovery of Qinling Takin (Budorcas Taxicolor Bedfordi). Animals 11, 2366. doi:10.3390/ani11082366
Riginos, C., Douglas, K. E., Jin, Y., Shanahan, D. F., and Treml, E. A. (2011). Effects of Geography and Life History Traits on Genetic Differentiation in Benthic Marine Fishes. Ecography 34, 566–575. doi:10.1111/j.1600-0587.2010.06511.x
Roques, S., Berrebi, P., Chèvre, P., Rochard, E., and Acolas, M. L. (2016). Parentage Assignment in the Critically Endangered European Sturgeon (Acipenser sturio) Based on a Novel Microsatellite Multiplex Assay: a Valuable Resource for Restocking, Monitoring and Conservation Programs. Conserv. Genet. Resour. 8, 313–322. doi:10.1007/s12686-016-0538-7
Ruan, X., Wang, W., Kong, J., and Hu, J. (2011). Isolation and Analysis of Microsatellites in the Genome of Turbot (Scophthalmus maximus L.). Afr. J. Biotechnol. 10, 507–518. doi:10.5897/AJB10.482
Serapion, J., Kucuktas, H., Feng, J., and Liu, Z. (2004). Bioinformatic Mining of Type I Microsatellites from Expressed Sequence Tags of Channel Catfish (Ictalurus punctatus). Mar. Biotechnol. 6, 364–377. doi:10.1007/s10126-003-0039-z
Tautz, D., and Renz, M. (1984). Simple Sequences Are Ubiquitous Repetitive Components of Eukaryotic Genomes. Nucl. Acids Res. 12, 4127–4138. doi:10.1093/nar/12.10.4127
Wang, J., and Scribner, K. T. (2014). Parentage and Sibship Inference from Markers in Polyploids. Mol. Ecol. Resour. 14, 541–553. doi:10.1111/1755-0998.12210
Wang, X., Gan, X., Li, J., Chen, Y., and He, S. (2016). Cyprininae Phylogeny Revealed Independent Origins of the Tibetan Plateau Endemic Polyploid Cyprinids and Their Diversifications Related to the Neogene Uplift of the Plateau. Sci. China Life Sci. 59, 1149–1165. doi:10.1007/s11427-016-0007-7
Wang, X., Luo, Y., Sun, L., and Shafeeque, M. (2021). Different Climate Factors Contributing for Runoff Increases in the High Glacierized Tributaries of Tarim River Basin, China. J. Hydrology Regional Stud. 36, 100845. doi:10.1016/j.ejrh.2021.100845
Yang, T., Meng, W., Gao, T., Guo, Y., Ma, Y., and Zhang, F. (2014). Genetic Polymorphism of Microsatellite DNA in Schizothorax Biddulphi Populations. Arid Zone Res. 31, 1109–1114. doi:10.13866/j.azr.2014.06.19
Yuan, G., and Yuan, L. (1998). An Approach to the Environmental Changes in Lop-Nur History. Acta Geogr. Sin. 53, 83–89. doi:10.11821/xb1998s1011
Keywords: Schizothorax biddulphi, microsatellite, genetic diversity, genetic structure, genome survey
Citation: Nie Z, Ren Y, Zhang L, Ge R and Wei J (2022) Analysis of Population Genetic Diversity and Genetic Structure of Schizothorax biddulphi Based on 20 Newly Developed SSR Markers. Front. Genet. 13:908367. doi: 10.3389/fgene.2022.908367
Received: 30 March 2022; Accepted: 02 May 2022;
Published: 13 June 2022.
Edited by:
Cong Zeng, Shanghai Jiao Tong University, ChinaReviewed by:
Qianhong Gu, Hunan Normal University, ChinaCopyright © 2022 Nie, Ren, Zhang, Ge and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Wei, d2VpamllZGt5QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.