- 1Department of Urology, Huashan Hospital & Institute of Urology, Fudan University, Shanghai, China
- 2Clinical Research Center of Urolithiasis, Shanghai Medical College, Fudan University, Shanghai, China
- 3Department of Urology, Shanghai Children’s Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China
A previous genome-wide association study (GWAS) reported several novel loci for nephrolithiasis in British and Japanese population, some of which were predicted to influence CaSR signaling. In this study, we aimed to evaluate the association of these loci with calcium nephrolithiasis in Chinese Han population. We performed a case-control association analysis involving 691 patients with calcium nephrolithiasis and 1008 control subjects. We were able to genotype a total of 17 single-nucleotide polymorphisms (SNPs), which were previously reported to be significantly associated with nephrolithiasis in GWAS. rs578595 at WDR72 was significantly associated with calcium nephrolithiasis in Chinese Han population (p < 0.001, OR = 0.617). Moreover, rs12654812 at SLC34A1 (p = 0.0427, OR = 1.170), rs12539707 at HIBADH (p = 0.0179, OR = 0.734), rs1037271 at DGKH (p = 0.0096, OR = 0.828) and rs12626330 at CLDN14 (p = 0.0080, OR = 1.213) indicated suggestive associations with calcium nephrolithiasis. Our results elucidated the significance of genetic variation at WDR72, DGKH, CLDN14, SLC34A1, and HIBADH in Chinese patients with nephrolithiasis. Since polymorphisms of WDR72, DGKH, and CLDN14 are predicted to influence in CaSR signaling, our results emphasized the role of abnormal calcium homeostasis in calcium nephrolithiasis.
Introduction
Nephrolithiasis is one of the most frequent disorders affecting almost all populations. Epidemiological studies have reported that the incidence of nephrolithiasis is about 5% among females and 12% among males (Lewandowski and Rodgers, 2004), and almost 70% of all kidney stones are composed of calcium oxalate and/or phosphate (Croppi et al., 2012; Liu et al., 2021). In recent years, the incidence and prevalence of nephrolithiasis is increasing globally. Nephrolithiasis is commonly recurrent, with up to 50% of individuals experiencing a second episode within 10 years of the initial presentation, and recurrent stone disease has been linked to renal function decline (Pearle et al., 2014; Yan et al., 2021). The cause of nephrolithiasis is considered multifactorial, including but not limited to diet, ethnic, climate, and genetic factors. Recent studies estimated that up to 65% of kidney stone formers have a family history of nephrolithiasis (Stechman et al., 2007). Moreover, twin studies have reported a heritability of >45% for nephrolithiasis, and a strong family history of nephrolithiasis, including a parent and two siblings, have a standard incidence ratio for stone formation of >50 (Hemminki et al., 2018).
Up till now, six genome-wide association studies (GWAS) in different ethnicity have identified about 25 loci associated with nephrolithiasis (Thorleifsson et al., 2009; Gudbjartsson et al., 2010; Urabe et al., 2012; Oddsson et al., 2015; Howles et al., 2019; Tanikawa et al., 2019). Loci identified by GWAS in patients with nephrolithiasis could provide possible insight into the pathogenesis of the disorder. Howles et al. (2019) reported a trans-ethnic GWAS meta-analysis of British and Japanese cohorts, which identified 7 novel loci associated with nephrolithiasis. Among them, five of the loci, WDR72, GPIC1, DGKD, DGKH, and BCR, were predicted to influence calcium-sensing receptor (CaSR) signaling. The CaSR is a G protein-coupled receptor, which is highly expressed in the kidneys and parathyroid (Litvinova et al., 2021). CaSR has a central role in calcium hemostasis, including increasing kidney calcium reabsorption while stimulating parathyroid hormone (PTH) release to enhance bone resorption, urinary calcium reabsorption and 1,25-dihydroxyvitamin D3 synthesis in the kidney (Hannan et al., 2016). In this study, we conducted the research regarding the association between polymorphisms of those CaSR-related genes and calcium nephrolithiasis in Chinese Han population.
Materials and Methods
Subjects
In total, 691 unrelated Chinese Han patients with nephrolithiasis (467 males and 224 females, mean aged 50.47 years) were recruited at Huashan Hospital of Fudan University. Patients with nephrolithiasis secondary to known causes, such as chronic kidney disease, renal failure, chronic diarrhea, gout, renal tubular acidosis, primary and secondary hyperparathyroidism, osteoporosis, or cancer were excluded. Patients with radioparent stones, including struvite, uric acid and cystine stones were excluded. We also excluded patients with history of medications that affected urinary calcium excretion or taking vitamin D and/or calcium supplements. The control group consisted of 1008 subjects were age/gender matched individuals without a history of nephrolithiasis or a family history of kidney stone disease. Nephrolithiasis was diagnosed clinically either with plane radiography of kidney-ureter-bladder (KUB) or non-contrast computed tomography (CT) scan. All the patients with nephrolithiasis and the control subjects were of the same racial, ethnic, geographical and environmental strata.
We assessed the effect of genetic variations on serum calcium, sodium, potassium, magnesium, phosphorus, chloride, carbon dioxide (CO2), creatinine, urea, uric acid, alkaline phosphatase (ALP), parathyroid hormone (PTH), serum 25-hydroxycholecalciferol, albumin, glucose, cholesterol, triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL) levels; urine calcium and phosphorus levels; estimated glomerular filtration rate (eGFR); and body mass index (BMI). Laboratory measurements were performed within 2 weeks preoperatively.
Blood Sample Collection and DNA Extraction
Peripheral blood samples were collected by venipuncture in a tube containing EDTA and was stored at −80°C. Genomic DNA was extracted using QiAamp DNA Blood Midi Kit (Qiagen, Germany). The concentration and quality of DNA was quantified by Qubit dsDNA HS Assay Kit (Promega, United States).
SNP Selection and Genotyping
Genotyping was performed using the Illumina Asian Screening Array (ASA) BeadChip platform covering ∼660k variants across the genome. Imputation was performed with the IMPUTE computer program using the 1000 Genomes Project Han Chinese in Beijing (CHB) population as the reference, with imputation information score >0.90. We intended to replicate 20 single-nucleotide polymorphisms (SNPs) which were identified in the transethnic GWAS by Howles et al. (2019). We also evaluated the association of other 5 SNPs (rs1256328 at ALPL, rs7627468 at CASR, rs12654812 at SLC34A1, rs199565725 and rs219780 at CLDN 14) and calcium nephrolithiasis, which were identified in GWAS but not replicated in Chinese Han population (Thorleifsson et al., 2009; Oddsson et al., 2015). A standard quality control procedure was applied to select SNPs for further analysis. SNPs were excluded if they had: 1) genotype call rate <90% or 2) p < 0.001 for the Hardy-Weinberg Equilibrium (HWE) test. Therefore, eight SNPs were excluded because of low call rate of genotyping (<90%) and we were able to genotype a total of 17 SNPs. Population stratification analysis was performed by using an ancestry informative marker panel (UT-AIM250) (Wang et al., 2019).
Statistical Analysis
Quantitative variables were presented as mean ± standard deviation (SD). An independent t test was used to compare the differences between the means of continuous variables. Categorical variables were analyzed using the Chi-square test. Genotype distributions for the SNP were tested for Hardy-Weinberg equilibrium (HWE). The association of SNPs with nephrolithiasis was tested by a Cochran-Armitage trend test. Results are expressed as odds ratio (OR) and 95% confidence intervals (CI). A p value lower than 2.94E-03 (0.05/17) was considered statistically significant. SNPs with p value less than 0.05 were also considered of interest. Multiple linear regression analyses were used to test association between genotype and clinical parameters, including serum calcium, phosphorus, creatinine, urea, uric acid, etc. with relevant covariates. We conducted association and QTL analyses using the PLINK-1.07 toolset. p-values were two tailed. An α of 0.05 was used to claim statistical significance.
Results
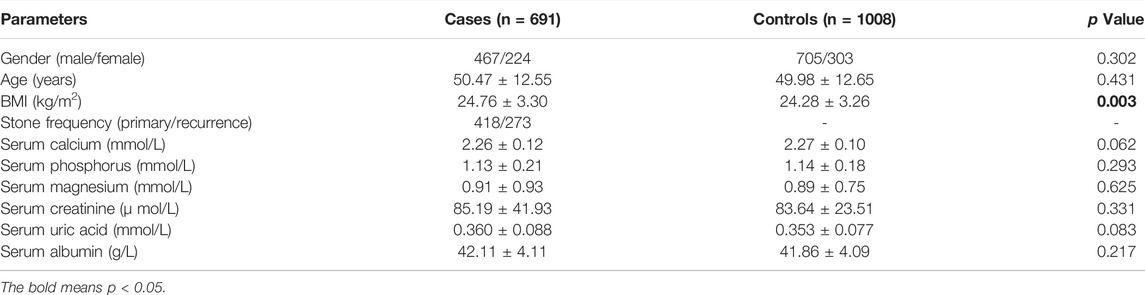
The clinical characteristics of case and control samples were shown in Table 1. Compared with the healthy controls, body mass index (BMI) was significantly higher in patients with calcium nephrolithiasis (p = 0.003). There showed no significant difference in the distribution of serum calcium, phosphorus, magnesium, creatinine, and uric acid among the patients and controls.
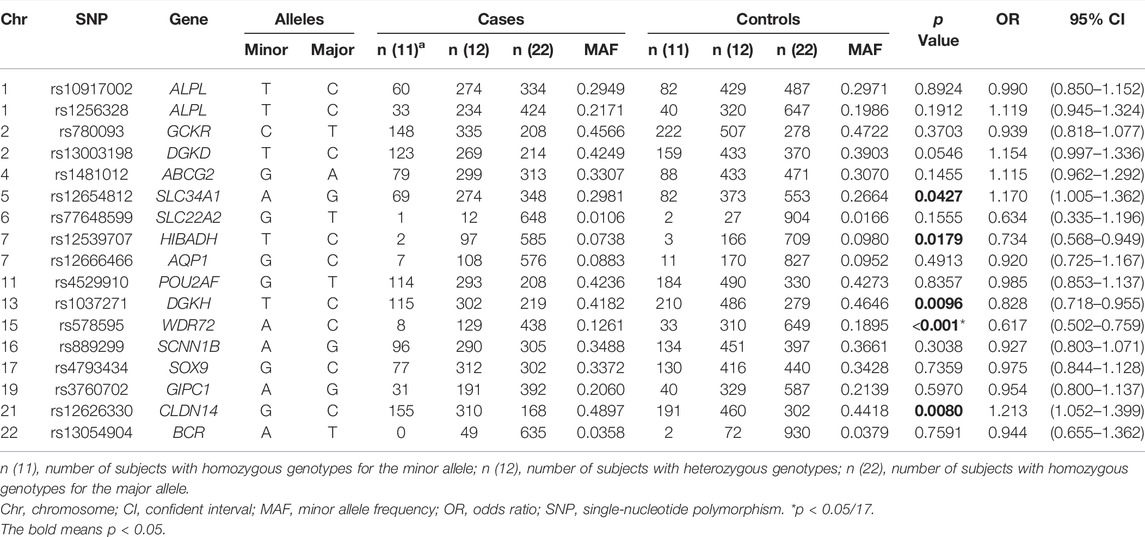
Population stratification analysis indicated that data of both cases and controls overlapped with Asian populations (Supplementary Figure S1). Therefore, no population stratification was detected between cases and controls. We were able to genotype a total of 17 SNPs (Supplementary Table S1), which were previously reported to be significantly associated with nephrolithiasis in GWAS. Table 2 shows the genotype frequencies of polymorphism among all subjects. The genotype frequencies of 17 SNPs among case and control subjects were distributed in accordance with the Hardy-Weinberg equilibrium (Supplementary Table S2). SNP rs578595 at WDR72 was significantly associated with calcium nephrolithiasis in Chinese Han population (p < 0.001, OR = 0.617). Four SNPs at 4 loci—— rs12654812 at SLC34A1 (p = 0.0427, OR = 1.170), rs12539707 at HIBADH (p = 0.0179, OR = 0.734), rs1037271 at DGKH (p = 0.0096, OR = 0.828) and rs12626330 at CLDN14 (p = 0.0080, OR = 1.213) indicated suggestive associations with calcium nephrolithiasis.
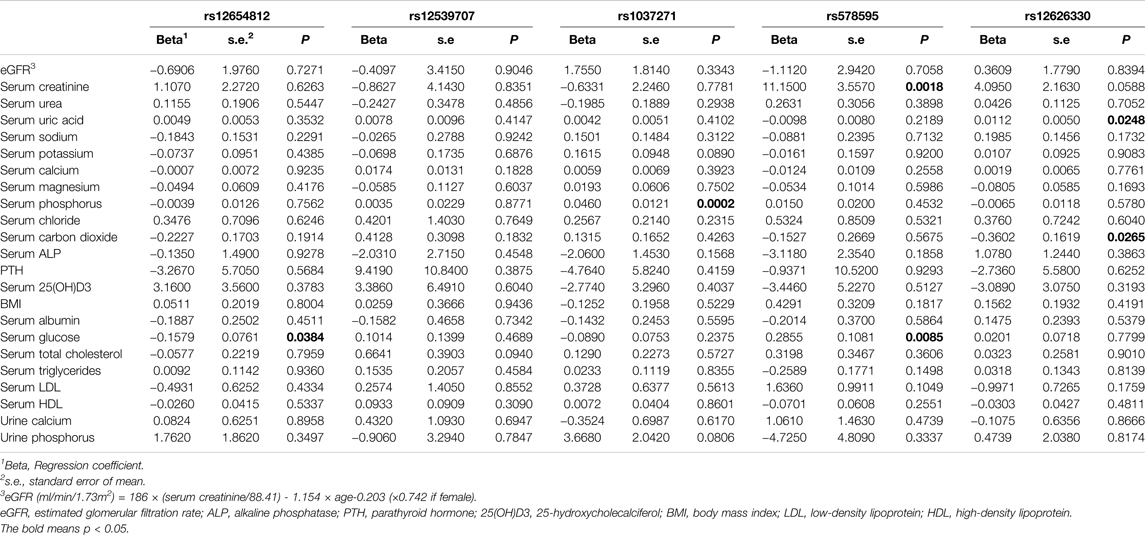
As shown in Table 3, the A allele of rs12654812 was significantly correlated with lower level of serum glucose (p = 0.0384). The C allele of rs1037271 was significantly correlated with higher level of serum phosphorus (p = 0.0002). The C allele of rs578595 was significantly correlated with higher level of serum creatinine (p = 0.0018) and serum glucose (p = 0.0085). The G allele of rs12626330 was significantly correlated with higher level of serum uric acid (p = 0.0248) and serum carbon dioxide (p = 0.0265).
Discussion
In Howles study, which is the largest nephrolithiasis GWAS to date and integrates data from 12,123 stone formers and 417,378 controls from British and Japanese populations, identified 20 loci associated with nephrolithiasis, 7 of which have not previously been reported to associate with nephrolithiasis (Howles et al., 2019). In this study, we evaluated the association of 17 SNPs of 16 loci identified in former GWAS with nephrolithiasis in Chinese Han population. Our results indicated that rs578595 at WDR72, rs1037271 at DGKH, rs12654812 at SLC34A1, rs12539707 at HIBADH, and rs12626330 at CLDN14 were associated with the risk of calcium nephrolithiasis in Chinese Han population. Among them, WDR72 and DGKH were predicted to influence calcium-sensing receptor (CaSR) signaling.
WDR72 encodes WD repeat containing protein 72 (WDR72), an intracellular protein of 1102 amino acids with no known functional domains except a β-propeller structure composed of WD40 repeat domains in its N-terminus. This domain organization is the characteristic of vesicle coat proteins that mediate membrane deformation complexes to regulate intracellular vesicle trafficking (Katsura et al., 2014). Mutations in WDR72 have been previously identified as the cause of amelogenesis imperfecta (AI), a hereditary disease that affect tooth enamel formation (El-Sayed et al., 2009). Okada et al. (2012) reported that loss-of-function mutations of WDR72 result in AI, whereas missense mutations of WDR72 cause distal renal tubular acidosis (dRTA), possibly without the presence of AI. dRTA is characterized by an impairment of urinary acidification resulting in metabolic acidosis, hypokalemia, and inappropriately elevated urine pH. If not treated, this chronic condition eventually leads to nephrocalcinosis, nephrolithiasis and impaired renal function (D'Ambrosio et al., 2021). Recently, a trans-ethnic GWAS in British and Japanese populations identified that rs578595, an intronic variant in WDR72, was significantly associated with nephrolithiasis (Howles et al., 2019). WDR72 are thought to play a role in Clathrin-mediated endocytosis, a process central to sustained intracellular CaSR signaling (Wang et al., 2015). Our results demonstrated that rs578595 was significantly associated with nephrolithiasis in Chinese Han ethnicity. The risk allele of rs578595 was significantly correlated with higher level of serum creatinine, but not correlated with estimated-GFR or level of serum urea. A GWAS in Caucasian population indicated that WDR72 was associated with renal function and chronic kidney diseases (Kottgen et al., 2010). SNPs on WDR72 were also found to be associated with the estimated-GFR variance in American Indians (Franceschini et al., 2014). Patients with chronic kidney disease are more likely to develop nephrolithiasis. Moreover, impaired renal function and nephrolithiasis may both probably caused by a dRTA-related mechanism. It is not surprising that rs578595 is correlated with higher level of serum glucose, since WDR72 has been reported to be associated with higher HbA1c level and poorer blood glucose control (Paterson et al., 2010). Patients with diabetes mellitus are at increased risk for nephrolithiasis, since diabetes might cause stone formation by affecting the composition of urine. It is reported that patients with diabetes excrete more oxalate and have lower urine pH than non-diabetic people (Eisner et al., 2010). Howles et al. (2019) identified rs3760702, ∼300 bp upstream of GIPC1, as a significant locus associated with nephrolithiasis. GIPC1 encodes Regulator of G-protein signaling 19 interacting protein 1 (GIPC1), which is also postulated to play a role in clathrin-mediated endocytosis in CaSR signaling (Shang et al., 2017). In the current study, however, rs3760702 showed insignificant association with nephrolithiasis in Chinese Han population.
DGKH encodes for diacylglycerol kinase eta (DGKH), and DGKD encodes for diacylglycerol kinase delta (DGKD). DGKD and DGKH phosphorylates diacylglycerol, a component of the intracellular CaSR-signaling pathway inducing CaSR-mediated membrane ruffling and activating protein kinase C (PKC) signaling cascades including mitogen-activated protein kinase (MAPK) and intracellular calcium release (Schlam and Canton, 2017; Gorvin et al., 2018). SNPs of DGKH was predicted to promote kidney stone formation by influencing CaSR signaling. In a genome-wide association meta-analysis, DGKH was identified to be associated with serum calcium concentrations (O'Seaghdha et al., 2013). In our study, rs1037271, an intronic variant in DGKH, showed suggestive association with nephrolithiasis in Chinese Han ethnicity. The risk allele of rs1037271 was significantly correlated with higher level of serum phosphorus. Previously, DGKD was identified as a new loci associated with serum calcium in a genome-wide association meta-analysis in Europeans. They characterized the expression of in kidney, and demonstrated that both DGKD and DGKH were significantly upregulated in response to low calcium diet, which suggested specific involved of these genes in calcium homeostasis (O'Seaghdha et al., 2013). In a GWAS in British and Japanese populations, rs13003198, ∼6 kb upstream of DGKD, was identified as a significant locus for calcium nephrolithiasis. Moreover, they verified that DGKD knockdown could impair CaSR-signal transduction pathway in vitro, and this effect can be rectified with the calcimimetic cinacalcet (Howles et al., 2019). In the current study, SNP rs13003198 was not successfully replicated in Chinese Han population.
BCR encodes Breakpoint Cluster Region (BCR) protein, which is a GTPase-activating protein for RAC1 (Rac Family Small GTPase 1). RAC1 activation was postulated to mediate CaSR-induced membrane ruffling (Schlam and Canton, 2017). SNP rs13054904 located ∼110 kb upstream of BCR, which was identified to be associated with nephrolithiasis in British population but not Japanese population through GWAS (Howles et al., 2019). We failed to replicate rs13054904 in Chinese Han population, which suggested that this locus predisposed to nephrolithiasis in European rather than East Asian populations.
The CLDN14 gene encodes claudin-14, which belongs to the claudin family of membrane proteins. Claudin-14, a 239-amino acid protein with 4 transmembrane domains and intracellular N and C termini, is an important component of epithelial tight junctions (Tsukita and Furuse, 2000). In the kidney, claudin-14 is predominantly expressed in the thick ascending limb of the Henle’s loop (TALH) where a quarter of filtered calcium is reabsorbed through a passive paracellular pathway involving claudin-14, claudin-16 and claudin-19 (Olinger et al., 2018). CaSR activation is thought to increase expression levels of claudin-14 in the TALH and thereby decrease paracellular calcium reabsorption (Dimke et al., 2013). Moreover, genetic variants may attenuate claudin-14 activity and lead to enhanced paracellular divalent cation reabsorption in the TALH. The first GWAS on nephrolithiasis was reported in 2009, which identified CLDN14 as a significant locus for nephrolithiasis. Howles et al. (2019) demonstrated that rs12626330, an intronic variant in CLDN14, was associated with nephrolithiasis. In this study, rs12626330 showed suggestive association with nephrolithiasis, and rs12626330 was correlated with higher level of serum uric acid and lower level of serum CO2. It has been reported that serum CO2 level was negatively correlated with the risk of uric acid stone formation (Moreira et al., 2015). Our results suggested that the risk allele of rs12626330 might increase the risk of calcium nephrolithiasis through abnormal metabolism associated with hyperuricemia.
SLC34A1 gene encodes NPT2a, which is a member of the type II a sodium-phosphate co-transporter family. The NPT2a expressed in the brush border membrane of proximal tubular cells where the bulk of phosphate reabsorption takes place. Mutations in SLC34A1 have been reported to cause hypophosphatemic nephrolithiasis and osteoporosis in human (Prie et al., 2002). In knockout mice, severe renal phosphate wasting, hypercalciuria and skeletal abnormalities were observed (Beck et al., 1998). In 2012, a GWAS in a Japanese population identified SLC34A1 as a novel locus associated with nephrolithiasis (Urabe et al., 2012). In 2015, a GWAS in Icelanders reported that common variants of rs12654812 was associated with nephrolithiasis, and rs12654812 associated significantly with decreased serum PTH levels and serum phosphate (Oddsson et al., 2015). In GWAS of Howles et al. (2019) identified rs56235845 as a significant SNP associated with nephrolithiasis in both British and Japanese populations. In our results, we discovered a suggestive correlation between rs12654812, whereas rs56235845 was not significantly associated with nephrolithiasis. The risk allele of rs12654812 was associated with lower level of serum glucose. Presumably, reduction in serum PTH levels associated with rs12654812 may result from a decrease in serum phosphate levels caused by diminished renal reabsorption. Since patients with hyperparathyroidism are usually associated with impair glucose tolerance (Aojula et al., 2021), that kidney stone variance may negatively correlated with serum glucose level through decreased serum PTH.
HIBADH encodes 3-hydroxyisobutyrate dehydrogenase (HIBADH). HIBADH is an NAD+ -dependent mitochondrial enzyme that catalyzes oxidation of 3-hydroxyisobutyrate, an intermediate of valine catabolism, to methylmalonate semialdehyde. HIBADH is considered as a key enzyme in the gluconeogenesis pathway (Tasi et al., 2013). HIBADH gene was identified as a candidate gene for type 2 diabetes mellitus (Chen et al., 2013). However, its involvement in nephrolithiasis has not been fully elucidated. Howles et al. (2019) first identified rs12539707, an intronic variant in HIBADH, as a significant SNP associated with nephrolithiasis in British and Japanese populations. Our results showed suggestive association between rs12539707 and nephrolithiasis in Chinese Han population, which suggests that genes related to glucose metabolism might be involved in the mechanism of nephrolithiasis formation.
To conclude, the results of the present study elucidate that rs578595 at WDR72 is significantly associated with calcium nephrolithiasis, whereas rs1037271 at DGKH, rs12626330 at CLDN14, rs12654812 at SLC34A1 and rs12539707 at HIBADH show suggestive associations with nephrolithiasis in Chinese Han population. As mentioned above, the expression of CLDN14 localized to the TALH of the kidney was demonstrated to be regulated via the calcium-sensing receptor (CaSR) signaling. Moreover, WDR72 and DGKH are predicted to influence CaSR signaling, but it remains to be confirmed in the kidney. Although further investigation is required, we assumed that the polymorphism of WDR72, DGKH, and CLDN14 could increase the risk of calcium nephrolithiasis by influencing the CaSR signaling. Our results emphasized the role of abnormal calcium homeostasis in Chinese patients with calcium nephrolithiasis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Huashan Institutional Review Board of Fudan University (HIRB). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Study design: WZ and WLJ. Data analysis and visualization: WLJ and LXL. Experiment validation and statistical analysis: ZZJ and YYY. Manuscript draft and revise: WLJ, WZ and GP.
Funding
This project was supported by grants from the National Natural Science Foundation of China (No. 81970603 and 82100807) and the Scientific Research Foundation of Huashan Hospital Northern Branch (grant numbers: HSBY2019003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.897051/full#supplementary-material
References
Aojula, N., Khan, S., Gittoes, N., and Hassan-Smith, Z. (2021). Normocalcaemic Primary Hyperparathyroidism: What is the Role of Parathyroid Surgery? Ther. Adv. Endocrinol. 12, 204201882199537. doi:10.1177/2042018821995370
Beck, L., Karaplis, A. C., Amizuka, N., Hewson, A. S., Ozawa, H., and Tenenhouse, H. S. (1998). Targeted Inactivation of Npt2 in Mice Leads to Severe Renal Phosphate Wasting, Hypercalciuria, and Skeletal Abnormalities. Proc. Natl. Acad. Sci. U.S.A. 95 (9), 5372–5377. doi:10.1073/pnas.95.9.5372
Chen, J., Meng, Y., Zhou, J., Zhuo, M., Ling, F., Zhang, Y., et al. (2013). Identifying Candidate Genes for Type 2 Diabetes Mellitus and Obesity through Gene Expression Profiling in Multiple Tissues or Cells. J. Diabetes Res. 2013, 1–9. doi:10.1155/2013/970435
Croppi, E., Ferraro, P. M., Ferraro, P. M., Taddei, L., and Gambaro, G. (2012). Prevalence of Renal Stones in an Italian Urban Population: A General Practice-Based Study. Urol. Res. 40 (5), 517–522. doi:10.1007/s00240-012-0477-z
D’Ambrosio, V., Azzarà, A., Sangiorgi, E., Gurrieri, F., Hess, B., Gambaro, G., et al. (2021). Results of a Gene Panel Approach in a Cohort of Patients with Incomplete Distal Renal Tubular Acidosis and Nephrolithiasis. Kidney Blood Press Res. 46 (4), 469–474. doi:10.1159/000516389
Dimke, H., Desai, P., Borovac, J., Lau, A., Pan, W., and Alexander, R. T. (2013). Activation of the Ca2+-Sensing Receptor Increases Renal Claudin-14 Expression and Urinary Ca2+ Excretion. Am. J. Physiol. Renal Physiol. 304 (6), F761–F769. doi:10.1152/ajprenal.00263.2012
Eisner, B. H., Porten, S. P., Bechis, S. K., and Stoller, M. L. (2010). Diabetic Kidney Stone Formers Excrete More Oxalate and Have Lower Urine pH Than Nondiabetic Stone Formers. J. Urol. 183 (6), 2244–2248. doi:10.1016/j.juro.2010.02.007
El-Sayed, W., Parry, D. A., Shore, R. C., Ahmed, M., Jafri, H., Rashid, Y., et al. (2009). Mutations in the Beta Propeller WDR72 Cause Autosomal-Recessive Hypomaturation Amelogenesis Imperfecta. Am. J. Hum. Genet. 85 (5), 699–705. doi:10.1016/j.ajhg.2009.09.014
Franceschini, N., Haack, K., Almasy, L., Laston, S., Lee, E. T., Best, L. G., et al. (2014). Generalization of Associations of Kidney-Related Genetic Loci to American Indians. Clin. J. Am. Soc. Nephrol. 9 (1), 150–158. doi:10.2215/CJN.02300213
Gorvin, C. M., Rogers, A., Hastoy, B., Tarasov, A. I., Frost, M., Sposini, S., et al. (2018). AP2σ Mutations Impair Calcium-Sensing Receptor Trafficking and Signaling, and Show an Endosomal Pathway to Spatially Direct G-Protein Selectivity. Cell Rep. 22 (4), 1054–1066. doi:10.1016/j.celrep.2017.12.089
Gudbjartsson, D. F., Holm, H., Indridason, O. S., Thorleifsson, G., Edvardsson, V., Sulem, P., et al. (2010). Association of Variants at UMOD with Chronic Kidney Disease and Kidney Stones-Role of Age and Comorbid Diseases. PLoS Genet. 6 (7), e1001039. doi:10.1371/journal.pgen.1001039
Hannan, F. M., Babinsky, V. N., and Thakker, R. V. (2016). Disorders of the Calcium-Sensing Receptor and Partner Proteins: Insights into the Molecular Basis of Calcium Homeostasis. J. Mol. Endocrinol. 57 (3), R127–R142. doi:10.1530/JME-16-0124
Hemminki, K., Hemminki, O., Försti, A., Sundquist, K., Sundquist, J., and Li, X. (2018). Familial Risks in Urolithiasis in the Population of Sweden. BJU Int. 121 (3), 479–485. doi:10.1111/bju.14096
Howles, S. A., Wiberg, A., Goldsworthy, M., Bayliss, A. L., Gluck, A. K., Ng, M., et al. (2019). Genetic Variants of Calcium and Vitamin D Metabolism in Kidney Stone Disease. Nat. Commun. 10 (1), 5175. doi:10.1038/s41467-019-13145-x
Katsura, K. A., Horst, J. A., Chandra, D., Le, T. Q., Nakano, Y., Zhang, Y., et al. (2014). WDR72 Models of Structure and Function: A Stage-specific Regulator of Enamel Mineralization. Matrix Biol. 38, 48–58. doi:10.1016/j.matbio.2014.06.005
Köttgen, A., Pattaro, C., Böger, C. A., Fuchsberger, C., Olden, M., Glazer, N. L., et al. (2010). New Loci Associated with Kidney Function and Chronic Kidney Disease. Nat. Genet. 42 (5), 376–384. doi:10.1038/ng.568
Lewandowski, S., and Rodgers, A. L. (2004). Idiopathic Calcium Oxalate Urolithiasis: Risk Factors and Conservative Treatment. Clin. Chim. Acta 345 (1-2), 17–34. doi:10.1016/j.cccn.2004.03.009
Litvinova, M. M., Khafizov, K., Korchagin, V. I., Speranskaya, A. S., Asanov, A. Y., Matsvay, A. D., et al. (2021). Association of CASR, CALCR, and ORAI1 Genes Polymorphisms with the Calcium Urolithiasis Development in Russian Population. Front. Genet. 12, 621049. doi:10.3389/fgene.2021.621049
Liu, C.-J., Cheng, C.-W., Tsai, Y.-S., and Huang, H.-S. (2021). Crosstalk between Renal and Vascular Calcium Signaling: The Link between Nephrolithiasis and Vascular Calcification. Int. J. Mol. Sci. 22 (7), 3590. doi:10.3390/ijms22073590
Moreira, D. M., Friedlander, J. I., Carons, A., Hartman, C., Leavitt, D. A., Smith, A. D., et al. (2015). Association of Serum Biochemical Metabolic Panel with Stone Composition. Int. J. Urol. 22 (2), 195–199. doi:10.1111/iju.12632
Oddsson, A., Sulem, P., Helgason, H., Edvardsson, V. O., Thorleifsson, G., Sveinbjörnsson, G., et al. (2015). Common and Rare Variants Associated with Kidney Stones and Biochemical Traits. Nat. Commun. 6, 7975. doi:10.1038/ncomms8975
Okada, Y., Sim, X., Sim, X., Go, M. J., Wu, J.-Y., Gu, D., et al. (2012). Meta-analysis Identifies Multiple Loci Associated with Kidney Function-Related Traits in East Asian Populations. Nat. Genet. 44 (8), 904–909. doi:10.1038/ng.2352
Olinger, E., Houillier, P., and Devuyst, O. (2018). Claudins: A Tale of Interactions in the Thick Ascending Limb. Kidney Int. 93 (3), 535–537. doi:10.1016/j.kint.2017.09.032
O'Seaghdha, C. M., Wu, H., Yang, Q., Kapur, K., Guessous, I., Zuber, A. M., et al. (2013). Meta-analysis of Genome-wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations. PLoS Genet. 9 (9), e1003796. doi:10.1371/journal.pgen.1003796
Paterson, A. D., Waggott, D., Boright, A. P., Hosseini, S. M., Shen, E., Sylvestre, M.-P., et al. (2010). A Genome-wide Association Study Identifies a Novel Major Locus for Glycemic Control in Type 1 Diabetes, as Measured by Both A1C and Glucose. Diabetes 59 (2), 539–549. doi:10.2337/db09-0653
Pearle, M. S., Goldfarb, D. S., Assimos, D. G., Curhan, G., Denu-Ciocca, C. J., Matlaga, B. R., et al. (2014). Medical Management of Kidney Stones: AUA Guideline. J. Urol. 192 (2), 316–324. doi:10.1016/j.juro.2014.05.006
Prié, D., Huart, V., Bakouh, N., Planelles, G., Dellis, O., Gérard, B., et al. (2002). Nephrolithiasis and Osteoporosis Associated with Hypophosphatemia Caused by Mutations in the Type 2a Sodium-Phosphate Cotransporter. N. Engl. J. Med. 347 (13), 983–991. doi:10.1056/NEJMoa020028
Schlam, D., and Canton, J. (2017). Every Day I'm Rufflin': Calcium Sensing and Actin Dynamics in the Growth Factor-independent Membrane Ruffling of Professional Phagocytes. Small GTPases 8 (2), 65–70. doi:10.1080/21541248.2016.1197873
Shang, G., Brautigam, C. A., Chen, R., Lu, D., Torres-Vázquez, J., and Zhang, X. (2017). Structure Analyses Reveal a Regulated Oligomerization Mechanism of the PlexinD1/GIPC/myosin VI Complex. Elife 6, e27322. doi:10.7554/eLife.27322
Stechman, M. J., Loh, N. Y., and Thakker, R. V. (2007). Genetics of Hypercalciuric Nephrolithiasis: Renal Stone Disease. Ann. N. Y. Acad. Sci. 1116, 461–484. doi:10.1196/annals.1402.030
Tanikawa, C., Kamatani, Y., Terao, C., Usami, M., Takahashi, A., Momozawa, Y., et al. (2019). Novel Risk Loci Identified in a Genome-wide Association Study of Urolithiasis in a Japanese Population. J. Am. Soc. Nephrol. 30 (5), 855–864. doi:10.1681/asn.2018090942
Tasi, Y.-C., Chao, H.-C. A., Chung, C.-L., Liu, X.-Y., Lin, Y.-M., Liao, P.-C., et al. (2013). Characterization of 3-hydroxyisobutyrate Dehydrogenase, HIBADH, as a Sperm-Motility Marker. J. Assist. Reprod. Genet. 30 (4), 505–512. doi:10.1007/s10815-013-9954-8
Thorleifsson, G., Holm, H., Edvardsson, V., Walters, G. B., Styrkarsdottir, U., Gudbjartsson, D. F., et al. (2009). Sequence Variants in the CLDN14 Gene Associate with Kidney Stones and Bone Mineral Density. Nat. Genet. 41 (8), 926–930. doi:10.1038/ng.404
Tsukita, S., and Furuse, M. (2000). Pores in the Wall: Claudins Constitute Tight Junction Strands Containing Aqueous Pores. J. Cell Biol. 149 (1), 13–16. doi:10.1083/jcb.149.1.13
Urabe, Y., Tanikawa, C., Takahashi, A., Okada, Y., Morizono, T., Tsunoda, T., et al. (2012). A Genome-wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1. PLoS Genet. 8 (3), e1002541. doi:10.1371/journal.pgen.1002541
Wang, S. K., Hu, Y., Yang, J., Smith, C. E., Nunez, S. M., Richardson, A. S., et al. (2015). Critical Roles for WDR 72 in Calcium Transport and Matrix Protein Removal during Enamel Maturation. Mol. Genet. Genomic Med. 3 (4), 302–319. doi:10.1002/mgg3.143
Wang, L.-J., Zhang, C. W., Su, S. C., Chen, H.-I. H., Chiu, Y.-C., Lai, Z., et al. (2019). An Ancestry Informative Marker Panel Design for Individual Ancestry Estimation of Hispanic Population Using Whole Exome Sequencing Data. BMC Genomics 20 (Suppl. 12), 1007. doi:10.1186/s12864-019-6333-6
Keywords: nephrolithiasis, WDR72, DGKH, CLDN14, SLC34A1, HIBADH, CaSR signaling, Chinese Han population
Citation: Wang L, Zhou Z, Yang Y, Gao P, Lin X and Wu Z (2022) A Genetic Polymorphism in the WDR72 Gene is Associated With Calcium Nephrolithiasis in the Chinese Han Population. Front. Genet. 13:897051. doi: 10.3389/fgene.2022.897051
Received: 12 April 2022; Accepted: 24 June 2022;
Published: 14 July 2022.
Edited by:
Mikhail Churnosov, Belgorod National Research University, RussiaReviewed by:
Zhe Han, University of Maryland, Baltimore, United StatesKuanjun He, Inner Mongolia University for Nationalities, China
Copyright © 2022 Wang, Zhou, Yang, Gao, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Lin, bHhsaW5nLjg1QDE2My5jb20=; Zhong Wu, ZHJ6aG9uZ3d1MTk2NEAxMjYuY29t
†These authors have contributed equally to this work
 Lujia Wang1,2†
Lujia Wang1,2† Xiaoling Lin
Xiaoling Lin Zhong Wu
Zhong Wu