- 1Department of Ophthalmology, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 2Glaucoma Research Chair in Ophthalmology, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 3King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
- 4Department of Ophthalmology and Visual Neurosciences, University of Minnesota, Minneapolis, MN, United States
Objective: It is plausible that common disease mechanisms exist in glaucoma pathophysiology. Accordingly, we investigated the genetic association of two previously reported primary open-angle glaucoma (POAG)-related gene polymorphisms, rs2472493 (A > G) in ABCA1 and rs7636836 (C > T) in FNDC3B, in primary angle-closure glaucoma (PACG) and pseudoexfoliation glaucoma (PXG).
Methods: TaqMan genotyping was performed in a total of 442 subjects consisting of 246 healthy controls, 102 PACG patients, and 94 PXG patients. Statistical evaluations were performed to detect allelic and genotype association of the variants with the disease and clinical variables such as intraocular pressure (IOP) and cup/disc ratio.
Results: Overall, there was no allelic or genotype association of these variants in PACG and PXG. However, rs7636836[T] allele significantly increased the risk of PXG among men (p = 0.029, odds ratio [OR] = 2.69, 95% confidence interval = 1.11–6.51). Similarly, rs2472493 and rs7636836 genotypes also showed significant association with PXG among men in over-dominant model (p = 0.031, OR = 1.98, 95% CI = 1.06–3.71) and co-dominant model (p = 0.029, OR = 2.69, 95% CI = 1.11–6.51), respectively. However, none survived Bonferroni’s correction. Besides, the synergic presence of rs2472493[G] and rs7636836[T] alleles (G-T) was found to significantly increase the risk of PACG (p = 0.026, OR = 2.85, 95% CI = 1.09–7.46). No significant genotype influence was observed on IOP and cup/disc ratio.
Conclusion: Our results suggest that the polymorphisms rs2472493 in ABCA1 and rs7636836 in FNDC3B genes may be associated with PXG among men, and a G-T allelic combination may confer an increased risk of PACG in the middle-eastern Saudi cohort. Further research in a larger population-based sample is needed to validate these findings.
Introduction
Glaucoma is a complex multifactorial disease with high heritability and ethnic-specific predisposition, suggesting the involvement of genetic components in its pathogenesis (Chan et al., 2016). Among the various forms of glaucoma, primary angle-closure glaucoma (PACG) is a far more common type than primary open-angle glaucoma (POAG) in Asian populations, including Saudi Arabia (Al Obeidan et al., 2011; Chan et al., 2016) and involves anatomical blockage of the aqueous flow pathway (Weinreb et al., 2014). Likewise, pseudoexfoliation glaucoma (PXG) is the most severe type of open-angle glaucoma highly prevalent among the elderly, characterized by the abnormal deposition of pseudoexfoliative material (fibrillar extracellular matrix) in the anterior segment of the eye causing the aqueous blockage and associated with worse prognosis (Vazquez and Lee, 2014). Several disease-causing mutations and multiple susceptibility loci have been associated with different types of glaucoma in various ethnicities (Zukerman et al., 2021). However, the precise role of these genetic components and their underlying molecular mechanisms in glaucoma pathogenesis remains unclear.
Overlapping clinical manifestations among glaucoma types, such as high intraocular pressure (IOP), optic nerve damage, and retinal ganglion cell (RGC) apoptosis, suggest the plausibility of common disease pathways and genetic mechanisms in glaucoma pathogenesis. The previous genetic association studies of MYOC (Dai et al., 2008), CYP1B1 (Chakrabarti et al., 2007), LOXL1 (Shiga et al., 2018; Eliseeva et al., 2021), ARHGEF12 (Aung et al., 2018), and ACVR1 (Kondkar et al., 2020) that have been common to different types of glaucoma and therefore lend further support to this hypothesis. Besides, most of the studies investigating glaucoma genetics were conducted in Asian or Caucasian populations. Depending on the population being assessed, the same variants may or may not show an association. Therefore, replication studies must be performed to confirm the effects of these single nucleotide variants in other regions or with other ethnicities. The genetic basis of PACG and PXG in middle-eastern patients of Saudi origin is still largely unknown (Abu-Amero et al., 2010; Abu-Amero et al., 2013, 2015; Kondkar et al., 2021) and therefore warrants further genetic investigation. Accordingly, we hypothesized that genetic polymorphisms rs2472493 located upstream of the ABCA1 gene and rs7636836 in FNDC3B were previously reported to be associated with POAG and elevated IOP (Hysi et al., 2014; Shiga et al., 2018), may also be associated with PACG and PXG in Saudi patients.
ABCA1 functions as a cholesterol efflux pump, and recent findings suggest its role in neuroinflammation, neurodegeneration, and RGC apoptosis (Howell et al., 2011; Awadalla et al., 2013). Whereas FNDC3B codes for an extracellular matrix protein involved in several signaling pathways including, transforming growth factor-beta (TGFβ) signaling (Prendes et al., 2013). Both genes are highly expressed in the human retina, optic nerve, trabecular meshwork, and RGC supporting its alleged role in glaucoma development and/or progression (Shiga et al., 2018). The genetic contribution of the variants at the ABCA1 and FNDC3B locus among Saudi PACG and PXG patients is unknown. To date, there has been no study to address the relationship between the polymorphisms in ABCA1 and FNDC3B in PACG and PXG in Saudi Arabs. Thus, in this exploratory study, we investigated whether single-nucleotide polymorphisms (SNPs) rs2472493 and rs7636836 upstream of ABCA1 and in FNDC3B genes, respectively, were associated with PACG and PXG in a Saudi cohort.
Materials and Methods
Study Design and Population
The retrospective case-control study was approved by the Institutional Review Board Ethics Committee at the College of Medicine of King Saud University (IRB protocol approval number # 08–657) and adhered to the Declaration of Helsinki principles with all the participants providing written informed consent. Patients of Saudi origin with a clinical diagnosis of PACG, PXG, and non-glaucoma controls were recruited at King Abdulaziz University Hospital, Riyadh, Saudi Arabia from April 2017 through December 2019.
PACG patients (n = 102) were diagnosed based on clinical evidence of anatomically closed-angle showing the occurrence of appositional or synechial closure of the anterior chamber angle (at least 270° of the angle is occluded); raised IOP (≥21 mmHg); the presence of optic disk damage with cup/disc ratio of at least 0.7 (at least in one eye); and loss of peripheral or advanced visual field (Abu-Amero et al., 2013). PXG patients (n = 94) showed the presence of flaky exfoliation material along the pupil edges or anterior lens capsule, glaucomatous optic neuropathy and associated visual field loss, and high IOP in either or both the eyes as described previously (Kondkar et al., 2018). Patients harboring secondary forms of glaucoma, history of optic neuropathies or visual impairment unrelated to glaucoma, steroid usage, ocular trauma, absence of sufficient fundus visualization for disk assessment, or refusal to enroll were excluded. A group of healthy Saudi Arab participants (n = 246) recruited from our ophthalmology screening clinics served as controls. These participants were: >40 years of age, with normal IOP (<21 mmHg), open angles on gonioscopy, healthy optic disc (cup/disc ratio <0.5), free from any form of glaucoma on examination, and no family history of glaucoma. Subjects refusing to participate in the study were excluded.
Genotyping of rs2472493 and rs7636836
Commercially available pre-designed TaqMan® assays, C__16235609_10 and C_189412462_10 purchased from Applied Biosystems (Catalog number: 4351379; Applied Biosystems Inc., Foster City, CA, United States) were used to genotype rs2472493 (A > G) and rs7636836 (C > T), respectively on ABI 7500 real-time PCR System (Applied Biosystems) according to the manufacturer instructions under recommended amplification conditions (Abu-Amero et al., 2013). Briefly, each assay utilizes two unlabeled PCR primers and two allele-specific probes. Each probe is labeled with a different color reporter dye (VIC® for allele 1 and 6-carboxy-fluorescein (FAM) for allele 2) at the 5′ end. The allele-specific fluorogenic probes when hybridized to the DNA template are cleaved by the 5′ nuclease activity of the Taq polymerase resulting in fluorescence emission from the reporter dye. Each PCR reaction was performed as recommended by the supplier in a total volume of 25 μl and consisted of 1X TaqMan® Genotyping Master Mix (Applied Biosystems), 1X SNP Genotyping Assay Mix and 20 ng DNA or molecular grade water in no template control well. The amplification conditions consisted of incubation at 95°C for 10 min, followed by 40 cycles, denaturation at 92°C for 15 s and annealing/ extension at 60°C for 1 min. The VIC® and FAM fluorescence levels of the PCR products were measured at 60°C for 1 min. Analysis of fluorescence using the automated 2-color allele discrimination software on ABI 7500 showed clear discrimination of all genotypes on a two-dimensional graph.
Statistical Analysis
Hardy-Weinberg Equilibrium (HWE), gender distribution, allele and genotype associations were tested using Pearson’s Chi-square analysis and Fisher’s test where applicable. Normality testing of continuous variables was done using the Kolmogorov–Smirnov test. Accordingly, age differences and genotypes effects on glaucoma indices such as IOP, cup/disc ratio, and number of antiglaucoma medications were estimated using Mann-Whitney U test (2-groups comparison) and Kruskal–Wallis test (3-groups comparison). Binary logistic regression analysis was performed to test the effects of multiple factors (age, sex, genotypes) on glaucoma outcome. The analyses were performed using SPSS version 22 (IBM Inc., Chicago, Illinois, United States), Stat View software version 5.0 (SAS Institute, Cary, NC, United States), and SNPStats online software (https://www.snpstats.net/start.htm). The combined allelic (haplotype) effect was estimated using SHEsis (http://analysis.bio-x.cn/myAnalysis.php). Power analysis was done using Power Genetic Association (PGA) software (https://dceg.cancer.gov/tools/design/pga). A p < 0.05 (2-tailed) was considered significant. Bonferroni’s correction p-value for multiple testing was considered where applicable.
Results
Demographic Data Distribution
The demographic data of subjects included in the study is shown in Supplementary Figure S1. In comparison to the mean age of controls (59.5 years, ± 7.2), the mean age in PACG (60.6 years, ± 8.5) was not significantly different (p = 0.225), but the PXG cases (66.4 years, ± 9.7) were significantly older (p < 0.001). Besides, the frequency of gender distribution did not differ significantly in the PACG (p = 0.105) and PXG (p = 0.078) groups than in the controls.
Allele Frequency of rs2472493 in ABCA1 and rs7636836 in FNDC3B Genes
Of the total number of 442 subjects genotyped for rs2472493 and rs7636836 polymorphisms in this study, there were seven samples with missing genotypes for rs2472493 that failed to amplify and none for rs7636836, giving an estimated call rate of 98% and 100%, respectively. The samples with missing genotypes were excluded from rs2472493 and combined genotype analysis. Table 1 shows the minor allele frequency (MAF) distribution of rs2472493 and rs7636836 according to glaucoma types and gender in cases and controls. No significant deviation from HWE was observed (p > 0.05). Overall, the MAFs of both the polymorphisms showed no significant association with PACG and PXG. However, rs7636836 [T] variant in FNDC3B was significantly associated with increased risk of PXG among men (OR = 2.69, 95% CI = 1.11–6.51, p = 0.029). No such gender-specific association was observed for rs2472493 in ABCA1.
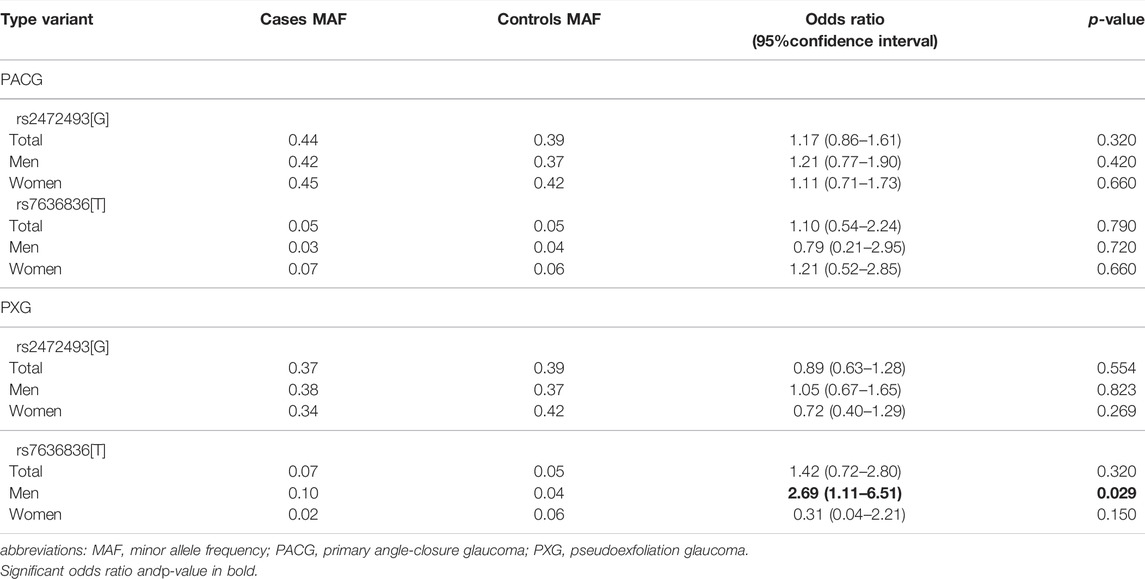
TABLE 1. Minor allele frequency of rs2472493 in ABCA1 and rs7636836 in FNDC3B genes according to glaucoma types and gender.
Genotype Analysis of rs2472493 (ABCA1) and rs7636836 (FNDC3B) in PACG
Genotype association analysis was performed in co-dominant, dominant, recessive, over-dominant, and log-additive genetic models using SNPStats software. Both rs2472493 and rs7636836 genotypes did not show any significant association with PACG (Table 2). Furthermore, genotype analysis of both the variants in PACG did not reveal any gender-specific association in any of the tested genetic models (Supplementary Tables S1, S2).
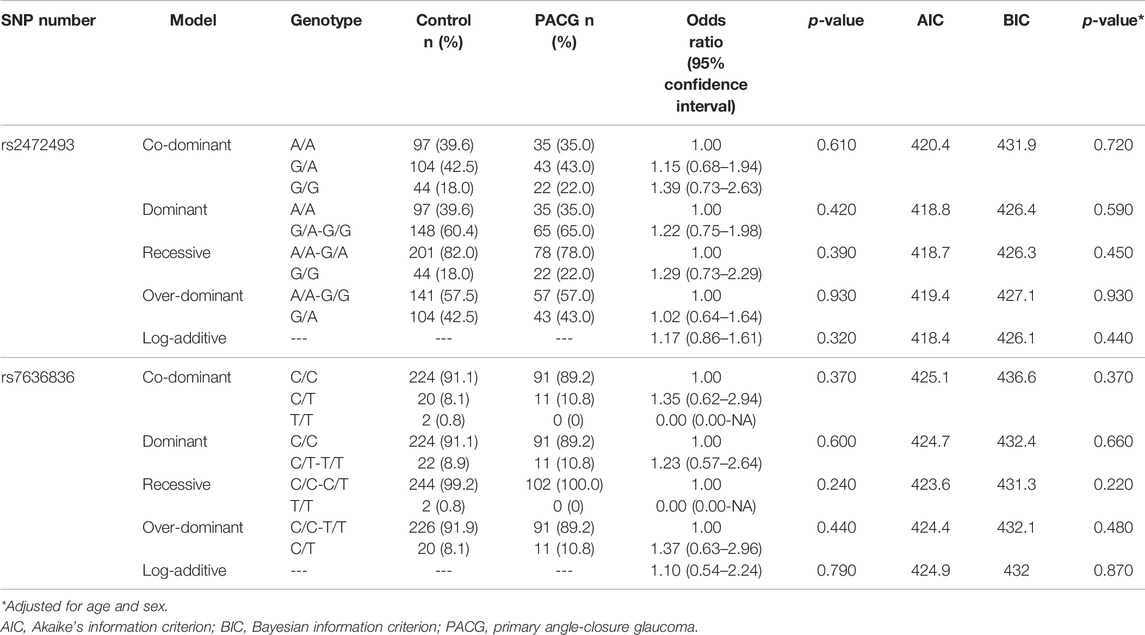
TABLE 2. Association of rs2472493 (ABCA1) and rs7636836 (FNDC3B) polymorphisms with the risk of primary angle-closure glaucoma compared to control under different genetic models.
Genotype Analysis of rs2472493 (ABCA1) and rs7636836 (FNDC3B) in PXG
Overall, the polymorphisms rs2472493 and rs7636836 showed no significant association with PXG. However, a further gender stratification showed a significant moderate association of these polymorphisms with PXG among men (Tables 3, 4). Rs2472493 in ABCA1 showed a significantly increased risk of PXG in men in the over-dominant model (OR = 1.98, 95% CI = 1.06–3.71, p = 0.031) that did not remain significant after adjustment for age, sex, and Bonferroni correction (Table 3). Similarly, although no homozygous rs7636836 [T/T] genotypes were observed in the PXG group, the heterozygous rs7636836 [C/T] genotype in FNDC3B showed significant association with PXG in men (OR = 2.69, 95% CI = 1.11–6.51, p = 0.029) in the co-dominant model that was significant after adjustment for age and sex (p = 0.026) but did not survive Bonferroni correction (Table 4).
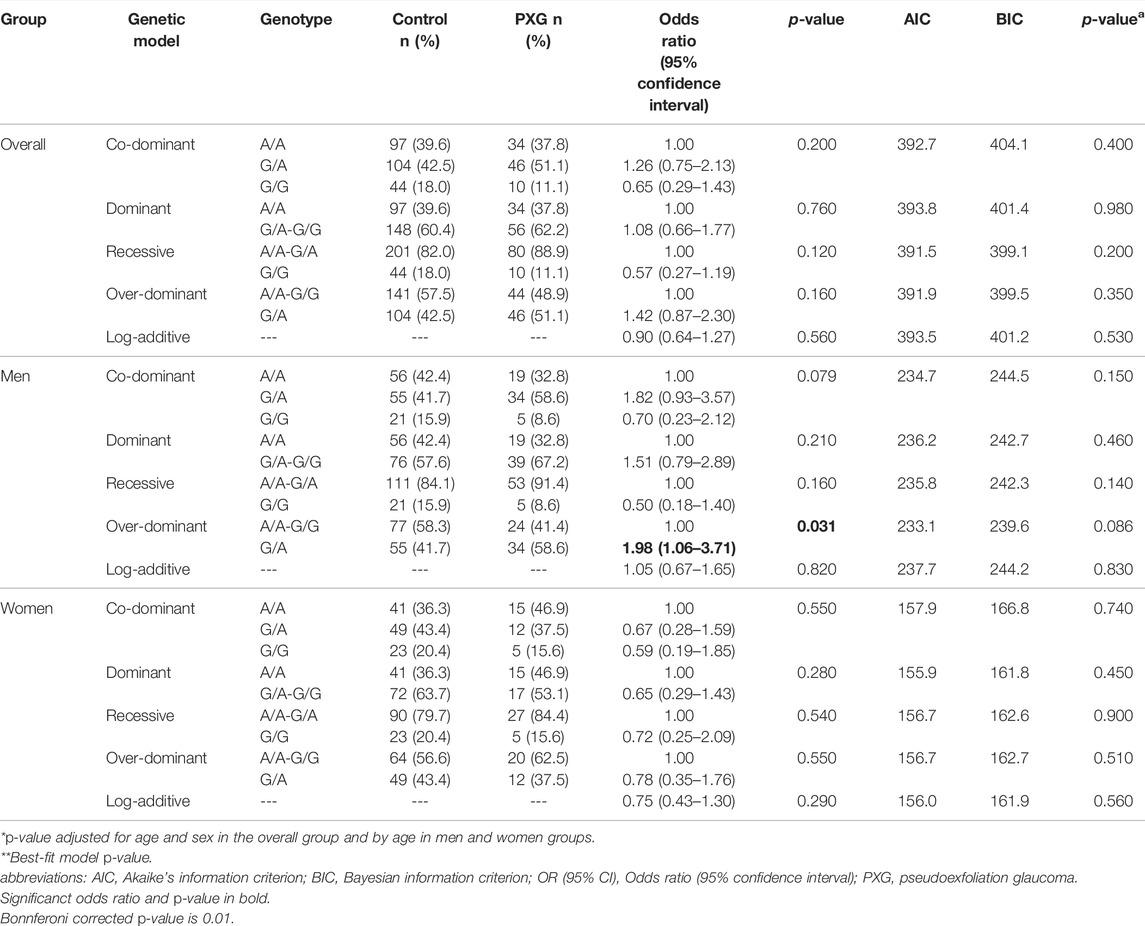
TABLE 3. Genotype association analysis of polymorphism rs2472493 in ABCA1 in pseudoexfoliation glaucoma.

TABLE 4. Genotype association analysis of rs7636836 variant in FNDC3B in pseudoexfoliation glaucoma.
Combined Genotype and Allele Frequency Analysis in PACG and PXG
The combined genotype analysis of the ABCA1 rs2472493 (A > G) and FNDC3B rs7636836 (C > T) polymorphisms did not show any significant effect on PACG susceptibility but did suggest that the presence of GG-CT genotype would increase the risk of PACG by 2.5-fold (OR = 2.55, 95% CI = 0.49–13.30), albeit non-significantly (p = 0.356) (Supplementary Table S3). In addition, the analysis of synergic effects of ABCA1 rs2472493 and FNDC3B rs7636836 alleles showed that the synergic presence of rs2472493[G] and rs7636836[T] alleles (G-T) could lead to a significantly (p = 0.026) increased risk of PACG (OR = 2.85, 95% CI = 1.09–7.46) (Supplementary Table S4). The analysis, however, did not account for multiple testing corrections. In contrast, combined genotype and allele frequency analysis in PXG yielded no significant result (Supplementary Tables S5, S6).
Regression Analysis and Effect of Genotypes on Clinical Indices of Glaucoma
A binary logistic regression analysis was performed to detect the effect of age, sex, and polymorphisms rs2472493 in ABCA1 and rs7636836 in FNDC3B on disease outcomes. None of these factors were found to have any significant effect on PACG and PXG outcomes. However, age remained a significant predictor of PXG (Supplementary Table S7). Furthermore, the genotype effect of rs2472493 (ABCA1) and rs7636836 (FNDC3B) polymorphisms on IOP, cup/disc ratio, and the number of antiglaucoma medications in the PACG and PXG patient groups were also examined. These phenotypes are clinical indicators related to disease severity. The analysis revealed no significant genotype effect on any of these clinical markers in both the PACG and PXG patient groups (Supplementary Figure S2).
Discussion
Glaucoma is a complex polygenic disorder affected by multiple genetic and environmental factors. Previously, several genome-wide association studies have identified numerous genetic polymorphisms and susceptibility loci in different populations worldwide, few of which have been unique to specific ethnic groups (Zukerman et al., 2021). However, the genetic etiology of glaucoma in middle-eastern population is still lacking and therefore warrants further studies. Given the overlapping clinical manifestations and common genetic variants reported among glaucoma types, we investigated the association of two POAG-related gene polymorphisms, rs2472493 in ABCA1 gene and rs7636836 near FNDC3B, in the PACG and PXG patient cohort of Saudi origin.
In our study, the MAF of rs2472493 in ABCA1 was 0.44 and 0.37 in PACG and PXG, respectively, and were comparable to those in controls (0.39) and thus non-significant. The allele frequencies were similar to those reported in the Han Chinese and Uygur Chinese population of PACG (0.45) and PXG (0.40) patients, respectively (Luo et al., 2015). Likewise, the MAFs were comparable to the Hispanics (0.37), non-Hispanics (0.42), and African American (0.35) POAG subjects (Choquet et al., 2018), but lower than the East-Asians (0.56) (Choquet et al., 2018) and the European (0.51) POAG patients (Gharahkhani et al., 2014) highlighting slight ethnic and glaucoma type variability.
Previously, SNP rs2472493 in ABCA1 was associated with POAG in the genome-wide meta-analysis of 18 population cohorts from the International Glaucoma Genetics Consortium (Hysi et al., 2014). Similar genome-wide findings were reported by Gharahkhani et al., in the Australian cohort (Gharahkhani et al., 2014) and by Chen et al., in POAG patients from Southern China (Chen et al., 2014). Thus, multiple population studies indicated that the ABCA1 gene might play a crucial role in POAG pathogenesis. In contrast, negative ABCA1 gene associations have been reported in the Han Chinese cohort of 1,311 healthy controls and 1122 PACG patients (Luo et al., 2015), and in the 52 Jordanian Arab glaucoma patients (POAG and congenital) and 96 healthy individuals investigated for the ABCA1 rs2472493 variant by Alkhatib et al. (2019). Although our study did not replicate the findings in the PXG and PACG patient cohort of Saudi origin, we observed a significant association of ABCA1 rs2472493 polymorphism among men in the PXG cohort. However, there are no published reports of this variant being examined in PXG.
ABCA1 belongs to a large superfamily of ABC transmembrane transporters and is best known for its role in cholesterol efflux to lipid-free apolipoprotein AI and apolipoprotein E (Wang and Smith, 2014). ABCA1 is expressed in all the major tissues of the eye, including the trabecular meshwork, Schlemm’s canal endothelial cells, RGCs, and optic nerve that are primarily involved in glaucoma (Chen et al., 2014). However, the exact mechanism(s) by which ABCA1 may be involved in glaucoma pathogenesis is still unclear. ABCA1 was demonstrated to inhibit ocular inflammation via activation of liver X-receptor in an experimental model of autoimmune uveitis (Yang et al., 2014). Using the glaucoma model, Li et al. demonstrated that ABCA1 was related to RGC death (Li et al., 2018). These studies suggest that ABCA1 may have a significant role in eye research. Likewise, using the Encyclopedia of DNA Elements (ENCODE) project data and Genevar database, Gharahkhani and colleagues, reported that the polymorphism rs2472493 located upstream of ABCA1 is an expression quantitative trait locus (eQTL) in lymphoblastoid cell lines that might alter the motif sequences for proteins such as FOXJ2 and SIX5 (Gharahkhani et al., 2014). Also, rs2472493 was found to be in high linkage disequilibrium with polymorphism rs2472494 near ABCA1 that alters the regulatory motif for binding of PAX6, a gene involved in eye development. The authors thereby predicted a possible regulatory role for this polymorphism in gene expression in a pathway similar to that of rs2472494 variant near ABCA1 gene (Gharahkhani et al., 2014). However, the exact mechanism(s) by which rs2472493 polymorphism in ABCA1 might increase PXG risk in men is unknown. The effect of gender, gene-gene, and gene-environment interactions on ABCA1 gene polymorphisms in lipid and lipoprotein metabolism have been well documented (Junyent et al., 2010; Coban et al., 2014; Shi et al., 2021). The ABCA1 polymorphism might plausibly modulate the risk of PXG among men in a similar manner.
FNDC3B (also known as FAD104) is a known regulator of adipogenesis (Tominaga et al., 2004). FNDC3B codes for an extracellular matrix protein that has a vital role in cell adhesion and growth signaling pathways, including TGFβ, and Wnt/β-catenin signaling (Nishizuka et al., 2009; Goto et al., 2017; Li et al., 2020) all of which have been strongly implicated in glaucoma pathogenesis (Prendes et al., 2013; Zhong et al., 2013; Webber et al., 2018). Furthermore, FNDC3B is expressed in all the primary eye tissues relevant to glaucoma (Li et al., 2015; Shiga et al., 2018).
The variant rs7636836 in FNDC3B was one of the novel loci associated with POAG in a two-stage genome-wide study consisting of 7,378 Japanese POAG cases and 36,385 controls conducted by Shiga et al. (2018). However, further validation in Singapore Chinese, European, and Africans did not show any significant association (Shiga et al., 2018). In our study cohort, the MAF of rs7636836 in FNDC3B was 0.05 and 0.07 in PACG and PXG, respectively. The frequency distribution was similar to European (0.06) but lower than the Japanese (0.40) and Singaporean Chinese (0.21) POAG subjects reported by Shiga et al. (2018). Our data showed no significant allelic and genotype association between rs7636836 in FNDC3B and PACG/PXG. However, in men, a modest allelic and genotype association of rs7636836 in FNDC3B was observed in PXG. The potential mechanism(s) by which rs7636836 in FNDC3B would modulate the risk of PXG in men is unclear but could possibly be hormonal (androgen) related via Wnt signaling which can be stimulated by FNDC3B (Li et al., 2020). There is evidence for crosstalk between androgen receptor and Wnt/ß-catenin signaling pathway (Mumford et al., 2018). The androgen receptors are expressed in ocular tissues (Tachibana et al., 2000) and Wnt signaling is strongly implicated in the maintenance of glaucomatous trabecular meshwork (Dhamodaran et al., 2020). To the best of our knowledge, there are no published reports of studies evaluating this polymorphism in PACG and PXG patients.
Gene-gene and gene-environmental interactions have been suggested to play an essential role in the pathogenesis of complex human diseases and highlight the possible contribution of genetic background and environmental triggers in disease development and progression (Marchini et al., 2005; Pan, 2008; Brossard et al., 2015). Glaucoma is also a complex polygenic disease with no clear inheritance pattern, and similar mechanisms may exist to influence the disease outcomes (Zakharov et al., 2013). Interestingly, a combined allelic and genotype analysis of rs2472493 in ABCA1 and rs7636836 in FNDC3B showed that G-T allele haplotype of ABCA1 and FNDC3B was associated with a significantly increased risk of PACG. Although it is difficult to ascertain whether the risk observed in our study is attributable to a real haplotype effect or probably reflects a linkage with other variant(s) not included in this study, the possible role of rs2472493 in ABCA1 and rs7636836 in FNDC3B in PACG cannot be completely ruled out and would need further research in a larger cohort.
Polymorphisms in ABCA1 and FNDC3B have also been shown to influence IOP (Hysi et al., 2014). A recent study showed that ABCA1 regulated IOP by modulating caveolin-1, nitric oxide/endothelial nitric oxide synthase signaling pathway (Hu et al., 2020). However, the analysis of polymorphisms effect on clinical endophenotypes of PACG and PXG, such as IOP and cup/disc ratio in our cohort, showed no significant association.
Thus, our results show that rs2472493 in ABCA1 and rs7636836 in FNDC3B may not have a major direct role in PACG and PXG in this ethnic group. However, the association of rs7636836 in FNDC3B and rs2472493 in ABCA1 observed among PXG men; and that of G-T haplotype in PACG suggests that they may have a significant indirect role (possibly via epistatic interaction(s)) in PXG and PACG. But since the study was performed in a relatively small sample size and does not provide any mechanistic evidence, the results would require a cautious interpretation. Based on the observed allele frequencies, the study had an estimated power of >0.9 per allele for the ABCA1 variant but was underpowered (0.6 per allele) for the FNDC3B variant to detect a relative risk of 2.0 with an alpha type I error of 0.05. Thus, the possibility of chance association cannot be ruled out and further emphasizes the need for replication in a large sample cohort potentially with age and gender-matched controls to confirm these findings.
In conclusion, our results suggest that the polymorphisms rs2472493 in ABCA1 and rs7636836 in FNDC3B genes may be associated with PXG among men, and a G-T allelic combination may confer an increased risk of PACG in the middle-eastern Saudi cohort. However, further investigations in larger population-based samples and different ethnicity are needed to draw definite conclusions and validate these findings. Moreover, considering the genetic heterogeneity of glaucoma per se, the plausible involvement of gene-gene and/or gene–environmental interactions must be important considerations for future research.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The study involving human subjects was approved by the Institutional review board (IRB) and the research ethics committee of the College of Medicine, King Saud University (IRB protocol# 08–657). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AK: Conceptualization, Analysis, Investigation, Project administration, Writing—original draft, review and editing; TS, TA: Investigation, Methodology, Writing—review and editing; EO, FA: Data curation, Investigation, Writing—review and editing; GL: Data interpretation, Investigation, Writing—review and editing; SAA-O: Conceptualization, Data curation, Investigation, Project administration, Writing—review and editing. All authors read and approved the final manuscript.
Funding
This work was supported by King Saud University through the Vice Deanship of Scientific Research Chair and Glaucoma Research Chair in Ophthalmology. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the Vice Deanship of Scientific Research Chair, Glaucoma Research Chair in Ophthalmology at King Saud University, for their support. The authors would also like to thank Mr. Abdulrahman Al-Mosa for his clinical assistance in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.877174/full#supplementary-material
References
Abu-Amero, K. K., Osman, E. A., Dewedar, A. S., Schmidt, S., Allingham, R. R., and Al-Obeidan, S. A. (2010). Analysis of LOXL1 Polymorphisms in a Saudi Arabian Population with Pseudoexfoliation Glaucoma. Mol. Vis. 16, 2805–2810. [pii].
Abu-Amero, K. K., Azad, T. A., Mousa, A., Osman, E. A., Sultan, T., and Al-Obeidan, S. A. (2013). A Catalase Promoter Variant Rs1001179 Is Associated with Visual Acuity but Not with Primary Angle Closure Glaucoma in Saudi Patients. BMC Med. Genet. 14, 84. doi:10.1186/1471-2350-14-84
Abu-Amero, K. K., Azad, T. A., Mousa, A., Osman, E. A., Sultan, T., and Al-Obeidan, S. A. (2015). Association of SOD2 Mutation (c.47T > C) with Various Primary Angle Closure Glaucoma Clinical Indices. Ophthalmic Genet. 36 (2), 180–183. doi:10.3109/13816810.2013.838276
Al Obeidan, S. A., Dewedar, A., Osman, E. A., and Mousa, A. (2011). The Profile of Glaucoma in a Tertiary Ophthalmic University Center in Riyadh, Saudi Arabia. Saudi J. Ophthalmol. 25 (4), 373–379. doi:10.1016/j.sjopt.2011.09.001
Alkhatib, R., Abudhaim, N., Al-Eitan, L., Abdo, N., Alqudah, A., and Aman, H. (2019). Genetic Analysis of ABCA1 Gene of Primary Glaucoma in Jordanian Arab Population. Tacg Vol 12, 181–189. doi:10.2147/TACG.S213818
Aung, T., Chan, A. S., and Khor, C.-C. (2018). Genetics of Exfoliation Syndrome. J. Glaucoma 27 (Suppl. 1), S12–S14. doi:10.1097/IJG.0000000000000928
Awadalla, M. S., Thapa, S. S., Hewitt, A. W., Burdon, K. P., and Craig, J. E. (2013). Association of Genetic Variants with Primary Angle Closure Glaucoma in Two Different Populations. PLoS One 8 (6), e67903. doi:10.1371/journal.pone.0067903
Brossard, M., Fang, S., Vaysse, A., Wei, Q., Chen, W. V., Mohamdi, H., et al. (2015). Integrated Pathway and Epistasis Analysis Reveals Interactive Effect of Genetic Variants atTERF1andAFAP1L2loci on Melanoma Risk. Int. J. Cancer 137 (8), 1901–1909. doi:10.1002/ijc.29570
Chakrabarti, S., Devi, K. R., Komatireddy, S., Kaur, K., Parikh, R. S., Mandal, A. K., et al. (2007). Glaucoma-AssociatedCYP1B1Mutations Share Similar Haplotype Backgrounds in POAG and PACG Phenotypes. Invest. Ophthalmol. Vis. Sci. 48 (12), 5439–5444. [pii]. doi:10.1167/iovs.07-0629
Chan, E. W. e., Li, X., Tham, Y.-C., Liao, J., Wong, T. Y., Aung, T., et al. (2016). Glaucoma in Asia: Regional Prevalence Variations and Future Projections. Br. J. Ophthalmol. 100 (1), 78–85. doi:10.1136/bjophthalmol-2014-306102
Chen, Y., Lin, Y., Vithana, E. N., Jia, L., Zuo, X., Wong, T. Y., et al. (2014). Common Variants Near ABCA1 and in PMM2 Are Associated with Primary Open-Angle Glaucoma. Nat. Genet. 46 (10), 1115–1119. doi:10.1038/ng.3078
Choquet, H., Paylakhi, S., Kneeland, S. C., Thai, K. K., Hoffmann, T. J., Yin, J., et al. (2018). A Multiethnic Genome-wide Association Study of Primary Open-Angle Glaucoma Identifies Novel Risk Loci. Nat. Commun. 9 (1), 2278. doi:10.1038/s41467-018-04555-4
Coban, N., Onat, A., Komurcu Bayrak, E., Gulec, C., Can, G., and Erginel Unaltuna, N. (2013). Gender Specific Association of ABCA1 Gene R219K Variant in Coronary Disease Risk through Interactions with Serum Triglyceride Elevation in Turkish Adults. Anadolu Kardiyol. Derg. 14 (1), 18–25. doi:10.5152/akd.2013.234
Dai, X., Nie, S., Ke, T., Liu, J., Wang, Q., and Liu, M. (2008). Two Variants in MYOC and CYP1B1 Genes in a Chinese Family with Primary Angle-Closure Glaucoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 25 (5), 493–496. 940625107 [pii].
Dhamodaran, K., Baidouri, H., Sandoval, L., and Raghunathan, V. (2020). Wnt Activation after Inhibition Restores Trabecular Meshwork Cells toward a Normal Phenotype. Invest. Ophthalmol. Vis. Sci. 61 (6), 30. doi:10.1167/iovs.61.6.30
Eliseeva, N., Ponomarenko, I., Reshetnikov, E., Dvornyk, V., and Churnosov, M. (2021). LOXL1 Gene Polymorphism Candidates for Exfoliation Glaucoma Are Also Associated with a Risk for Primary Open-Angle Glaucoma in a Caucasian Population from Central Russia. Mol. Vis. 27, 262–269. [pii].
Gharahkhani, P., Burdon, K. P., Burdon, K. P., Fogarty, R., Sharma, S., Hewitt, A. W., et al. (2014). Common Variants Near ABCA1, AFAP1 and GMDS Confer Risk of Primary Open-Angle Glaucoma. Nat. Genet. 46 (10), 1120–1125. doi:10.1038/ng.3079
Goto, M., Osada, S., Imagawa, M., and Nishizuka, M. (2017). FAD104, a Regulator of Adipogenesis, Is a Novel Suppressor of TGF-β-Mediated EMT in Cervical Cancer Cells. Sci. Rep. 7 (1), 16365. doi:10.1038/s41598-017-16555-3
Howell, G. R., Macalinao, D. G., Sousa, G. L., Walden, M., Soto, I., Kneeland, S. C., et al. (2011). Molecular Clustering Identifies Complement and Endothelin Induction as Early Events in a Mouse Model of Glaucoma. J. Clin. Invest. 121 (4), 1429–1444. doi:10.1172/jci44646
Hu, C., Niu, L., Li, L., Song, M., Zhang, Y., Lei, Y., et al. (2020). ABCA1 Regulates IOP by Modulating Cav1/eNOS/NO Signaling Pathway. Invest. Ophthalmol. Vis. Sci. 61 (5), 33. doi:10.1167/iovs.61.5.33
Hysi, P. G., Cheng, C. Y., Springelkamp, H., Macgregor, S., Bailey, J. N. C., Wojciechowski, R., et al. (2014). Genome-wide Analysis of Multi-Ancestry Cohorts Identifies New Loci Influencing Intraocular Pressure and Susceptibility to Glaucoma. Nat. Genet. 46 (10), 1126–1130. doi:10.1038/ng.3087
Junyent, M., Tucker, K. L., Smith, C. E., Lane, J. M., Mattei, J., Lai, C. Q., et al. (2010). The Effects of ABCG5/G8 Polymorphisms on HDL-Cholesterol Concentrations Depend on ABCA1 Genetic Variants in the Boston Puerto Rican Health Study. Nutr. Metabolism Cardiovasc. Dis. 20 (8), 558–566. doi:10.1016/j.numecd.2009.05.005
Kondkar, A. A., Sultan, T., Alobaidan, A. S., Azad, T. A., Osman, E. A., Almobarak, F. A., et al. (2021). Association Analysis of Variants Rs35934224 in TXNRD2 and Rs6478746 in LMX1B in Primary Angle-Closure and Pseudoexfoliation Glaucoma. Eur. J. Ophthalmol. 11206721211042547, 112067212110425. doi:10.1177/11206721211042547
Kondkar, A. A., Sultan, T., Azad, T. A., Osman, E. A., Almobarak, F. A., and Al-Obeidan, S. A. (2020). Association Analysis of Polymorphisms Rs12997 in ACVR1 and Rs1043784 in BMP6 Genes Involved in Bone Morphogenic Protein Signaling Pathway in Primary Angle-Closure and Pseudoexfoliation Glaucoma Patients of Saudi Origin. BMC Med. Genet. 21 (1), 145. doi:10.1186/s12881-020-01076-0
Kondkar, A., Azad, T. A., Almobarak, F., Kalantan, H., Al-Obeidan, S., and Abu-Amero, K. (2018). Elevated Levels of Plasma Tumor Necrosis Factor Alpha in Patients with Pseudoexfoliation Glaucoma. Opth Vol 12, 153–159. doi:10.2147/OPTH.S155168
Li, L., Xu, L., Chen, W., Li, X., Xia, Q., Zheng, L., et al. (2018). Reduced Annexin A1 Secretion by ABCA1 Causes Retinal Inflammation and Ganglion Cell Apoptosis in a Murine Glaucoma Model. Front. Cell. Neurosci. 12, 347. doi:10.3389/fncel.2018.00347
Li, Y. Q., Chen, Y., Xu, Y. F., He, Q. M., Yang, X. J., Li, Y. Q., et al. (2020). FNDC3B 3′‐UTR Shortening Escapes from microRNA‐mediated Gene Repression and Promotes Nasopharyngeal Carcinoma Progression. Cancer Sci. 111 (6), 1991–2003. doi:10.1111/cas.14394
Li, Z., Allingham, R. R., Nakano, M., Jia, L., Chen, Y., Ikeda, Y., et al. (2015). A Common Variant Near TGFBR3 Is Associated with Primary Open Angle Glaucoma. Hum. Mol. Genet. 24 (13), 3880–3892. doi:10.1093/hmg/ddv128
Luo, H., Chen, Y., Ye, Z., Sun, X., Shi, Y., Luo, Q., et al. (2015). Evaluation of the Association between Common Genetic Variants Near theABCA1Gene and Primary Angle Closure Glaucoma in a Han Chinese Population. Invest. Ophthalmol. Vis. Sci. 56 (11), 6248–6254. doi:10.1167/iovs.15-16741
Marchini, J., Donnelly, P., and Cardon, L. R. (2005). Genome-wide Strategies for Detecting Multiple Loci that Influence Complex Diseases. Nat. Genet. 37 (4), 413–417. ng1537 [pii]. doi:10.1038/ng1537
Mumford, P. W., Romero, M. A., Mao, X., Mobley, C. B., Kephart, W. C., Haun, C. T., et al. (20181985). Cross Talk between Androgen and Wnt Signaling Potentially Contributes to Age-Related Skeletal Muscle Atrophy in Rats. J. Appl. Physiology 125 (2), 486–494. doi:10.1152/japplphysiol.00768.2017
Nishizuka, M., Kishimoto, K., Kato, A., Ikawa, M., Okabe, M., Sato, R., et al. (2009). Disruption of the Novel Gene Fad104 Causes Rapid Postnatal Death and Attenuation of Cell Proliferation, Adhesion, Spreading and Migration. Exp. Cell Res. 315 (5), 809–819. doi:10.1016/j.yexcr.2008.12.013
Pan, W. (2008). Network-based Model Weighting to Detect Multiple Loci Influencing Complex Diseases. Hum. Genet. 124 (3), 225–234. doi:10.1007/s00439-008-0545-1
Prendes, M. A., Harris, A., Wirostko, B. M., Gerber, A. L., and Siesky, B. (2013). The Role of Transforming Growth Factor β in Glaucoma and the Therapeutic Implications. Br. J. Ophthalmol. 97 (6), 680–686. doi:10.1136/bjophthalmol-2011-301132
Shi, Z., Tian, Y., Zhao, Z., Wu, Y., Hu, X., Li, J., et al. (2021). Association between the ABCA1 (R219K) Polymorphism and Lipid Profiles: a Meta-Analysis. Sci. Rep. 11 (1), 21718. doi:10.1038/s41598-021-00961-9
Shiga, Y., Akiyama, M., Nishiguchi, K. M., Sato, K., Shimozawa, N., Takahashi, A., et al. (2018). Genome-wide Association Study Identifies Seven Novel Susceptibility Loci for Primary Open-Angle Glaucoma. Hum. Mol. Genet. 27 (8), 1486–1496. doi:10.1093/hmg/ddy053
Tachibana, M., Kobayashi, Y., Kasukabe, T., Kawajiri, K., and Matsushima, Y. (2000). Expression of Androgen Receptor in Mouse Eye Tissues. Invest. Ophthalmol. Vis. Sci. 41 (1), 64–66.
Tominaga, K., Kondo, C., Johmura, Y., Nishizuka, M., and Imagawa, M. (2004). The Novel Gene Fad104, Containing a Fibronectin Type III Domain, Has a Significant Role in Adipogenesis. FEBS Lett. 577 (1-2), 49–54. doi:10.1016/j.febslet.2004.09.062
Vazquez, L. E., and Lee, R. K. (2014). Genomic and Proteomic Pathophysiology of Pseudoexfoliation Glaucoma. Int. Ophthalmol. Clin. 54 (4), 1–13. doi:10.1097/IIO.0000000000000047
Wang, S., and Smith, J. D. (2014). ABCA1 and Nascent HDL Biogenesis. Biofactors 40 (6), 547–554. doi:10.1002/biof.1187
Webber, H. C., Bermudez, J. Y., Millar, J. C., Mao, W., and Clark, A. F. (2018). The Role of Wnt/β-Catenin Signaling and K-Cadherin in the Regulation of Intraocular Pressure. Invest. Ophthalmol. Vis. Sci. 59 (3), 1454–1466. doi:10.1167/iovs.17-21964
Weinreb, R. N., Aung, T., and Medeiros, F. A. (2014). The Pathophysiology and Treatment of Glaucoma. JAMA 311 (18), 1901–1911. doi:10.1001/jama.2014.3192
Yang, H., Zheng, S., Qiu, Y., Yang, Y., Wang, C., Yang, P., et al. (2014). Activation of Liver X Receptor Alleviates Ocular Inflammation in Experimental Autoimmune Uveitis. Invest. Ophthalmol. Vis. Sci. 55 (4), 2795–2804. doi:10.1167/iovs.13-13323
Zakharov, S., Wong, T., Aung, T., Vithana, E., Khor, C., Salim, A., et al. (2013). Combined Genotype and Haplotype Tests for Region-Based Association Studies. BMC Genomics 14, 569. doi:10.1186/1471-2164-14-569
Zhong, Y., Wang, J., and Luo, X. (2013). Integrins in Trabecular Meshwork and Optic Nerve Head: Possible Association with the Pathogenesis of Glaucoma. BioMed Res. Int. 2013, 1–8. doi:10.1155/2013/202905
Keywords: genetics, genotyping, glaucoma, intraocular pressure, ophthalmology, polymorphism, rs2472493, rs7636836
Citation: Kondkar AA, Sultan T, Azad TA, Osman EA, Almobarak FA, Lobo GP and Al-Obeidan SA (2022) Evaluation of ABCA1 and FNDC3B Gene Polymorphisms Associated With Pseudoexfoliation Glaucoma and Primary Angle-Closure Glaucoma in a Saudi Cohort. Front. Genet. 13:877174. doi: 10.3389/fgene.2022.877174
Received: 16 February 2022; Accepted: 18 May 2022;
Published: 01 June 2022.
Edited by:
Zi-Bing Jin, Capital Medical University, ChinaReviewed by:
Karen Curtin, The University of Utah, United StatesTeera Poyomtip, Ramkhamhaeng University, Thailand
Copyright © 2022 Kondkar, Sultan, Azad, Osman, Almobarak, Lobo and Al-Obeidan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Altaf A. Kondkar, YWtvbmRrYXJAZ21haWwuY29t
 Altaf A. Kondkar
Altaf A. Kondkar Tahira Sultan1
Tahira Sultan1 Taif A. Azad
Taif A. Azad Glenn P. Lobo
Glenn P. Lobo Saleh A. Al-Obeidan
Saleh A. Al-Obeidan