- 1Department of Cardiology, Institute of Cardiovascular Diseases, The First Affiliated Hospital, Guangxi Medical University, Nanning, China
- 2Department of Neurology, The First Affiliated Hospital, Guangxi Medical University, Nanning, China
- 3Department of Prevention and Health Care, The Fourth Affiliated Hospital, Guangxi Medical University, Liuzhou, China
The associations among the EH domain-binding protein 1 (EHBP1), tubulin beta class I (TUBB), and WW domain-containing oxidoreductase (WWOX) single nucleotide polymorphisms (SNPs) and coronary artery disease (CAD) and ischemic stroke (IS) are not yet understood. This study aimed to detect the associations of these SNPs, gene-gene and gene-environment interactions and CAD and IS in the Guangxi Han population. A total of 1853 unrelated subjects were recruited into normal control (n = 638), CAD (n = 622), and IS (n = 593) groups. Related genotypes were determined by high-throughput sequencing. The genotypic and minor allelic frequencies of rs2278075 were different between the CAD and control groups, and those of rs2710642, rs3130685, and rs2278075 were also different between the IS and control groups. The rs2278075T allele, rs3130685-rs2222896-rs2278075, rs3130685-rs2222896-diabetes, rs3130685-rs2222896-drinking, and haplotype rs2710642A-rs10496099C-diabetes interactions were associated with increased risk, while G-T-G-C-G-A and G-T-T-T-G-T-drinking were associated with reduced risk of CAD. The rs2278075T and rs2710642G alleles, rs2710642G-rs10496099C haplotype, rs3130685-rs2278075-rs2222896, and rs2710642-rs2278075-hypertension interactions aggravated the association with IS, whereas the rs3130685T allele, rs2710642A-rs10496099C haplotype and the interactions of H1 (s2710642A-rs10496099C)-H2 (rs2710642G-rs10496099C)-drinking and I1 (A-C-G-C-A-A)-I3 (A-C-G-T-A-A)-I4 (A-C-G-T-G-A)-I5 (G-T-G-C-G-A) diminished the association with IS. Carrying WWOX rs2278075T was strongly associated with CAD or IS, while EHBP1 rs2710642 and TUBB rs3130685 might alter the association of IS by modifying the serum lipid profile. This study demonstrates that the EHBP1, TUBB, and WWOX SNPs, gene-gene and gene-environment interactions are associated with the risk of CAD and IS in the Guangxi Han population.
Introduction
Cardiovascular disease (CVD) including cerebrovascular disease is the leading causes of death worldwide. In 2015, the global mortality of CVD was approximately 32.1%, of which coronary artery disease (CAD) accounted for 49.8% and ischemic stroke (IS) accounted for 68% (Arsava et al., 2017; Bede et al., 2020). Dyslipidemia is the key trigger of atherosclerosis, leading to odds ratios of CAD and IS of 3.25 and 1.84, respectively. However, dyslipidemia is also a controllable risk factor (Bede et al., 2020).
Dyslipidemia is genetically susceptible. The difference in lipid-related gene expression determines the diversity of serum lipid phenotypes. Several studies have reported associations between the SNPs in the EH domain-binding protein 1 gene (EHBP1) (Colafella and Denton, 2018), tubulin beta class I gene (TUBB), and WW domain-containing oxidoreductase gene (WWOX) (Denis et al., 2011; Deng et al., 2020) and dyslipidemia (GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, 2017; Diener and Hankey, 2020), atherosclerosis and CAD. The mechanisms involved in lipid metabolism for these genes have been described. EHBP1 regulates vesicle transport by its encoded protein, binds endocytic vesicles to the actin cytoskeleton, and plays a core role in modulating glucose transporter 4 in adipocyte transport (Fan et al., 2019). EHBP1 is an effector molecule for oncogene family member 8 (Rab8) and Rab10. Rab10-EHBP1-EHD2 forms a trimer complex that plays a critical role in hepatocyte lipid phagocytosis and the autophagic digestion of lipid droplets (Fuchs and Whelton, 2020). The proprotein convertase subtilisin/kexin type 9 gene (PCSK9) reduces the recycling of low-density lipoprotein receptors (LDLRs) and redirects LDLRs to late endosomes and lysosomes for degradation, facilitating an increase in serum lipids. A study found that the expression of the EHBP1 protein was downregulated in cells overexpressing PCSK9. This revealed the existence of the EHBP1 accompanying pathway linked to lipid metabolism (Gabb et al., 2016). Moreover, SNPs of the rs2710642 and rs10496099 in the EHBP1 were correlated with low-density lipoprotein cholesterol (LDL-C) (Giagtzoglou et al., 2013) and atherosclerosis (Golan et al., 2019), respectively. The TUBB encodes β-tubulin. Mutations in TUBB cause microtubule damage, resulting in complex diseases (Denis et al., 2011). A genome-wide association study (GWAS) analyzed the susceptibility to genetic variants of early-onset hyperlipidemia in 8,073 Japanese individuals (age ≤65 years) and found that the SNPs of the rs3132584 and rs3130685 in the TUBB were associated with serum LDL-C levels (Guilherme et al., 2004). WWOX encodes a 414 amino acid protein that is involved in a variety of important cellular processes, including steroid metabolism. Studies have demonstrated that WWOX plays an important physiological role in lipid and lipoprotein metabolism in mouse and human genetic models. It is mediated through the ABCA1/apolipoprotein (Apo) A1 pathway, and it binds to a series of potential ligands of proline-rich PPxY motifs and other kinds of cell proteins. It is also involved in a variety of other pathways, including cholesterol homeostasis, fatty acid biosynthesis and triglyceride (TG) metabolism (Iatan et al., 2014; Hasan et al., 2020), GWAS indicated that there was an association between the fasting lipid profile of 1,087 participants in an offspring cohort (mean age 47 years, 52% female) and WWOX. The correlation between the WWOX rs2222896AA genotype and LDL-C was two times that of the rs2222896AG or rs2222896 GG genotypes (Johannsen et al., 2018). Additionally, the WWOX rs2278075 SNP was associated with CVD (p = 5.0 × 10−9) (Joseph et al., 2017). In a previous study in Guangxi Maonan population, we revealed that the EHBP1 rs2710642 and rs10496099, TUBB rs3132584 and rs3130685, and WWOX rs2222896 SNPs interacted with several environmental factors to modify blood lipid profiles (Kathiresan et al., 2007; Liu et al., 2020). However, the association between these genes and the risk of CAD or IS in Chinese populations is unclear.
This study investigated the genotypes of SNPs (rs2710642, rs10496099, rs3132584, rs3130685, rs2222896, and rs2278075) in EHBP1, TUBB, and WWOX in the Guangxi Han population and analyzed the relationship between SNPs, gene-gene (G × G) and gene-environment (G × E) interactions, and the risk of CAD and IS, to explore the possible pathogenic mechanism of these genes and provides a theoretical basis for early CAD or IS prevention and treatment.
Materials and Methods
Study Population
The subjects of our cohort were inpatients and health physical examiners in the First Affiliated Hospital of Guangxi Medical University from September 2009 to December 2011. Data were listed in the database of our study team project, including their demographic characteristics, socioeconomic status, medical history, lifestyle factors, blood samples, and laboratory results. This study was conducted in accordance with the Declaration of Helsinki (2008 revised edition) and approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. Informed consent was obtained from all subjects.
A total of 622 patients in the CAD group were diagnosed according to the following criteria: ① typical clinical manifestations, myocardial enzymes, and electrocardiogram changes; ② coronary angiography examination revealing at least one major coronary artery (right coronary artery, left circumflex, or left anterior descending artery) stenosis ≥50%; and ③ no cerebrovascular disease, valvular heart disease, congenital heart disease, or aneurysm. A total of 593 patients in the IS group were diagnosed according to the following findings: ① large atherosclerotic stroke and/or small-artery occlusive stroke (Khawaled et al., 2020); and ② no CAD, hemorrhagic cerebral infarction, transient ischemic attack, and cerebral embolism. A total of 638 normal subjects without a history of CAD and IS were used as a control group.
All participants were Han population and unrelated individuals whose ancestors lived in Guangxi for at least three generations. The ages of the subjects ranged from 18 to 80 years. Mean age and sex ratio of the three groups were matched (p > 0.05). The mean age and percentage of women in the control, CAD and IS groups were 62.07 ± 12.19 years, 26.5%; 62.34 ± 10.50 years, 27.8%; and 62.85 ± 12.17 years, 27.0%, respectively. They had no evidence of liver or kidney disease, autoimmune diseases, hematological diseases, tumors, severe infections, metabolic diseases (except diabetes), or mental diseases.
Metabolic Parameters
A peripheral venous blood specimen (5 ml) was collected in the morning after fasting for more than 8 h. Serum lipid parameters were determined by an automatic analyzer (Model 7170A, Hitachi Co., Ltd., Tokyo, Japan). Serum LDL-C, total cholesterol (TC), TG and high-density lipoprotein cholesterol (HDL-C) were measured by commercially available enzyme assay kits, and serum ApoA1 and ApoB were measured by a turbidimetric immunoassay.
Dyslipidemia is defined as elevated levels of TC, TG or LDL-C or decreased HDL-C levels (Larsson et al., 2016). The reference ranges of serum TC, TG, HDL-C, LDL-C, ApoA1, and ApoB levels and the ApoA1/ApoB ratio in our hospital’s laboratory were 3.10–5.17, 0.56–1.70, 0.90–1.81, 2.70–3.10 mmol/L, 1.00–1.78, 0.63–1.14 g/L, and 1.00–2.50, respectively. Therefore, in this study, dyslipidemia was delimited by any of the following either alone or in combination: TC > 5.17, TG > 1.7, LDL-C > 3.10, and/or HDL-C < 0.9 mmol/L. Subjects with systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg were defined as having hypertension (Leitwein et al., 2020). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Overweight and obese categories in China are defined as BMI >24 kg/m2 and >28 kg/m2, respectively (Levy et al., 2007).
Genotyping
The basic principles of gene and SNP selection were as follows: ① EHBP1, TUBB, and WWOX were derived from GWAS associated with lipid metabolism. ② SNPs linked to lipid metabolism were selected by Haploview (Broad Institute of MIT and Harvard, Cambridge, MA, United States version 4.2). ③ Information on each SNP came from NCBI dbSNP Build 132 (http://www.ncbi.nlm.nih.gov/snp/). ④ SNPs with minor allele frequencies (MAFs) > 10% were included. ⑤ Associations between these SNPs (rs2710642, rs10496099, rs3132584, rs3130685, rs2222896, and rs2278075) and lipid metabolism or atherosclerotic cardiovascular disease in European and American populations were identified by previous studies.
DNA from peripheral blood samples was extracted using the phenol-chloroform method and stored at −80°C. Approximately 10 μL (10 ng/μL) of each sample was sent to the Department for Next-Generation Sequencing, Sangon Biotech Co., Ltd. (Shanghai, China) for genotyping. The absorbance of DNA samples met a ratio of A260 nm/A280 nm = 1.8 via a Shimadzu UV-1601 spectrophotometer, indicating that the DNA sample was pure. A HiSeqXTen sequencer (Illumina, San Diego, CA) was employed for SNP genotyping.
Statistical Analysis
Statistical analysis was performed using SPSS software version 25 (SPSS Inc., Chicago, Illinois, United States). Normally distributed data are presented as the means ± standard deviation, and differences among groups were analyzed by Student’s unpaired t-test (the control vs. CAD groups, the control vs. IS groups) or one-way analysis of variance (associations between three genotypes and lipid levels). Nonnormally distributed data are shown as interquartile ranges and medians, and differences in groups were determined by Mann-Whitney nonparametric tests. Differences in qualitative variables were analyzed by chi square tests. Associations between genotypes or haplotypes and continuous serum lipid levels were analyzed by multivariable linear regression. Differences in serum lipid levels associated with genotypes or haplotypes were considered statistically significant at p < 0.0004 or p < 0.0005, and G × G or G × E interactions were considered significant at p < 0.001 (corresponding to p < 0.05 after Bonferroni correction for six SNPs and seven environmental factors). The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by multiple logistic regression analysis and adjusted by stratified risk factors, including age, sex, cigarette smoking, alcohol consumption, BMI, diabetes, and hypertension. The Hardy-Weinberg equilibrium (HWE), genotypic and haplotypic frequencies, and linkage disequilibrium (LD) described by D′ and R2 values were calculated by SHEsis online software (http://analysis.bio-x.cn/myAnalysis.php). Generalized multifactor dimensionality reduction (GMDR) online software (http://sourceforge.net/projects/gmdr/) was used to screen optimal SNP-SNP, haplotype-haplotype, G × G, and G × E interaction models. The models meet 10/10 cross-validation consistency (CV) indicating 1,000 permutation tests; both training balance accuracy and test balance accuracy were ≥50%. Furthermore, all permutation tests had p < 0.001, suggesting their accuracy predictive value of diseases. An intuitive interactive tree diagram was used to depict the association of each factor in the model, here, the factors were no more than four. All other data visualizations were generated using GraphPad Prism (version 8.0.0).
Results
General Characteristics and Clinical Indicators
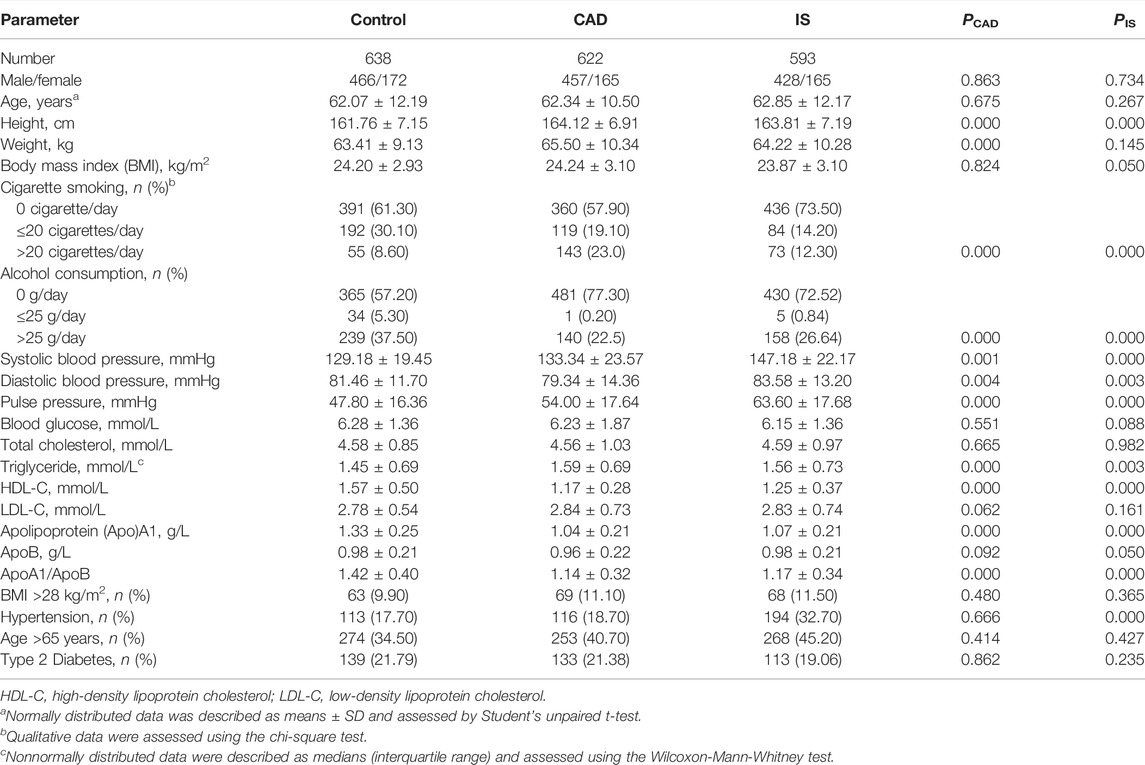
As shown in Table 1, mean height, weight, SBP, pulse pressure, TG, and the percentage of subjects who smoked cigarettes were higher (p ≤ 0.001), whereas mean DBP, HDL-C, ApoA1, the ratio of ApoA1 to ApoB, and the percentage of subjects who consumed alcohol were lower (p ≤ 0.004) in CAD than in control groups. Mean height, SBP, DBP, pulse pressure, TG, the percentages of subjects who consumed alcohol or smoked cigarettes, and the prevalence of hypertension were higher (p ≤ 0.003), whereas mean HDL-C, ApoA1, and the ratio of ApoA1 to ApoB were lower (p ≤ 0.001) in IS than in control groups.
EHBP1, TUBB, and WWOX Genotype and Allele Frequencies
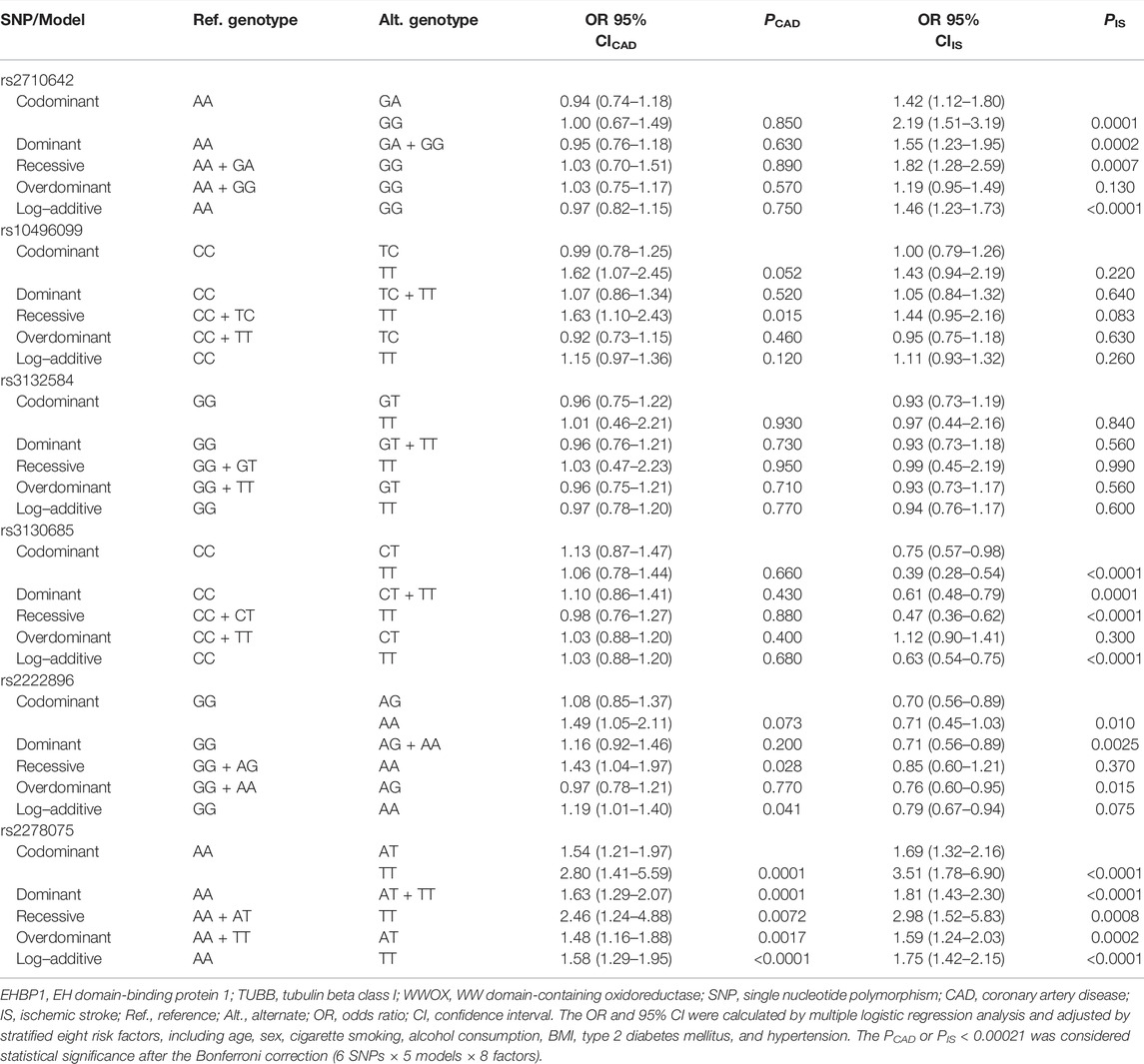
As listed in Table 2, the genotype distribution of the six SNPs in each group met HWE (p > 0.05 for all). The genotype and allele frequencies of the rs2278075 SNP were different between the CAD and control groups (p < 0.001 for each), and the genotype and allele frequencies of the rs2710642, rs3130685, and rs2278075 SNPs were different between the IS and control groups (p < 0.003).
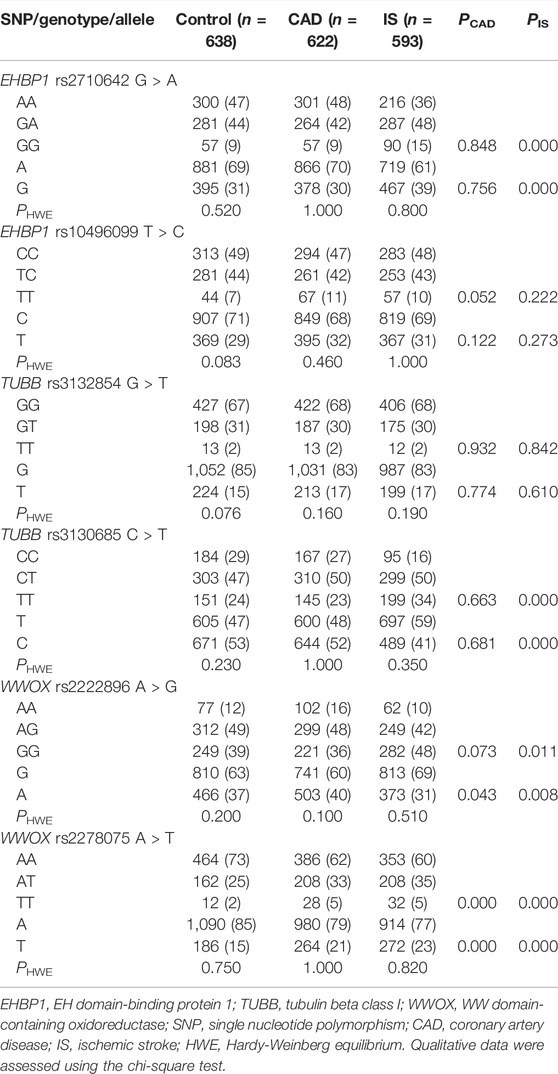
TABLE 2. Genotypic and allelic frequencies of the EHBP1, TUBB, and WWOX SNPs in the control and disease groups [n (%)].
Association Between the SNPs and CAD/IS
The genetic models of six EHBP1, TUBB, and WWOX SNPs are shown in Table 3. The rs2278075AT/TT genotypes were associated with increased risk of CAD (adjusted OR = 1.63, 95% CI = 1.29–2.07, p = 0.0001 for the dominant model). The rs2710642GA/GG and rs2278075AT/TT genotypes were associated with increased risk of IS (adjusted OR = 1.55, 95% CI = 1.23–1.95, p = 0.0002 and OR = 1.81, 95% CI = 1.43–2.30, p < 0.0001 for the dominant model; respectively), whereas the rs3130685CT/TT genotypes were associated with decreased risk of IS (adjusted OR = 0.61, 95% CI = 0.48–0.79, p = 0.0001 for the dominant model).
Association Between the SNPs and Serum Lipid Levels in Controls
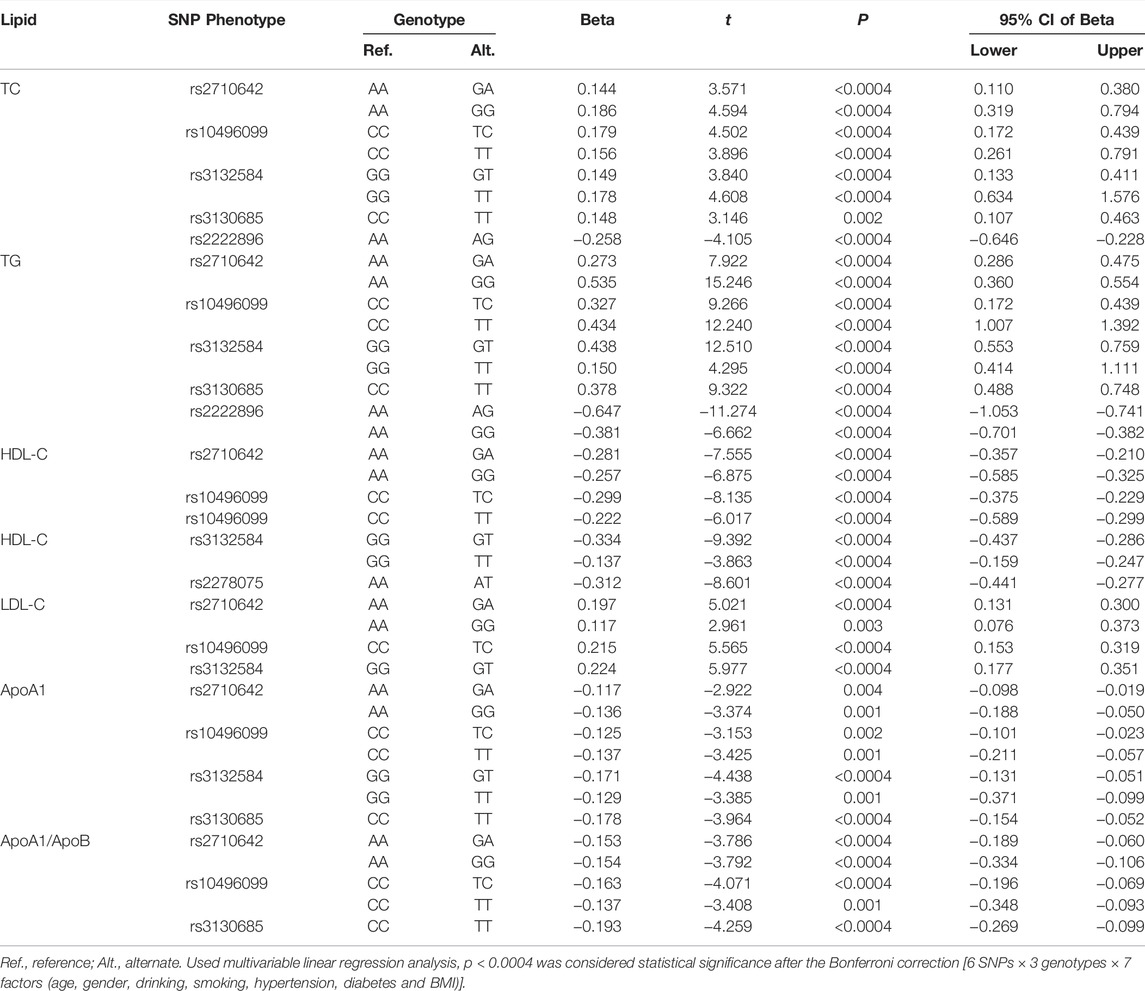
As shown in Figure 1, the levels of TC, TG, HDL-C, and LDL-C and the ratio of ApoA1/ApoB in the control group were significantly different among the three genotypes of the six SNPs, and the levels of ApoA1 were also significantly different among the three genotypes of five SNPs, except for rs3130685 SNP (p < 0.0004). High levels of TC and TG were associated with the rs2710642G, rs10496099T, rs3132584T, rs3130685T, and rs2222896A alleles; low levels of HDL-C were associated with the rs2710642G, rs10496099T, rs3132584T, and rs2278075T alleles. High levels of LDL-C were associated with the rs2710642G, rs10496099T, and rs3132584T alleles. Low levels of ApoA1 were associated with the rs2710642G, rs10496099T, rs3132584T, and rs3130685T alleles; low ratios of ApoA1/ApoB were associated with the rs2710642G, rs10496099T, and rs3130685T alleles (Table 4). The results were adjusted for confounds including hypertension, obesity, aging, drinking, female sex and diabetes (p < 0.0004 for all).
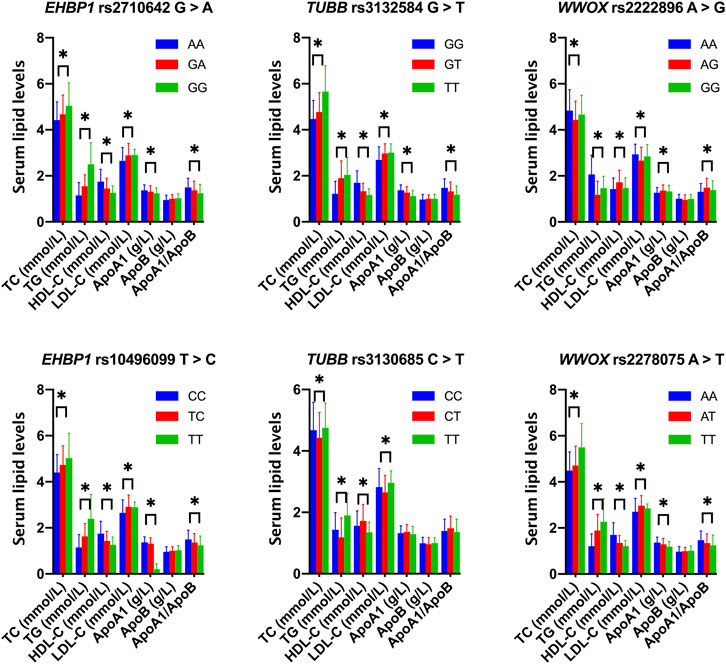
FIGURE 1. Associations between the 18 EHBP1, TUBB, and WWOX genotypes and serum lipid parameters in the control group. *p < 0.0004 was considered statistical significance after the Bonferroni correction (6 SNPs × 3 genotypes × 7 lipid phenotypes).
Associations Between Haplotypes and CAD/IS, and Serum Lipid Profiles in Controls
There was strong LD between the EHBP1 rs2710642 and rs10496099 SNPs in the three groups (control, D' = 0.951 or R2 = 0.821; CAD, D' = 1.000 or R2 = 0.938; and IS, D' = 1.000 or R2 = 0.690). Four common haplotypes were detected in the control and disease groups (Table 5). The rs2710642G-rs10496099C haplotype was associated with an increased risk of IS (OR = 2.94, 95% CI = 2.01–4.30, p = 7.76 × 10−9), whereas the rs2710642A-rs10496099C haplotype was associated with a reduced risk of IS (OR = 0.70, 95% CI = 0.59–0.83, p = 2.66 × 10−5). Moreover, the two haplotypes interacted with other risk factors for IS (Figure 2) exhibited that, the rs2710642A-rs10496099C haplotype interacted with sex (female), hypertension, and a small amount of smoking or alcohol to decrease the association of IS risk. On the other hand, the rs2710642G-rs10496099C haplotype interacted with sex (female), elderly (age >65 years), a small amount of smoking or alcohol, hypertension and dyslipidemia to increase the association of IS risk (p < 0.05 for all). However, there was no association between haplotypes and CAD (p > 0.05).
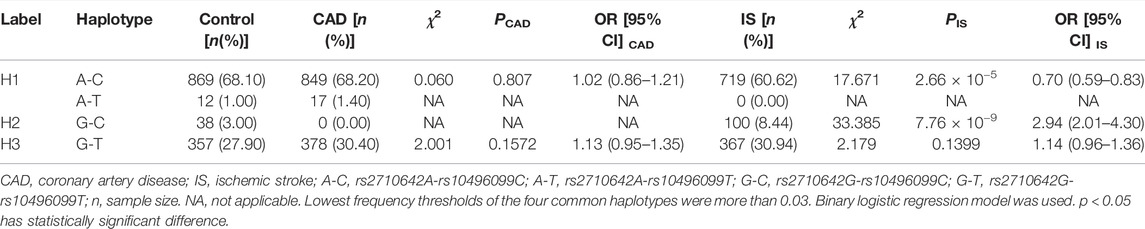
TABLE 5. Associations between haplotype frequencies of the two EHBP1 SNPs and CAD or IS [n (frequency)].
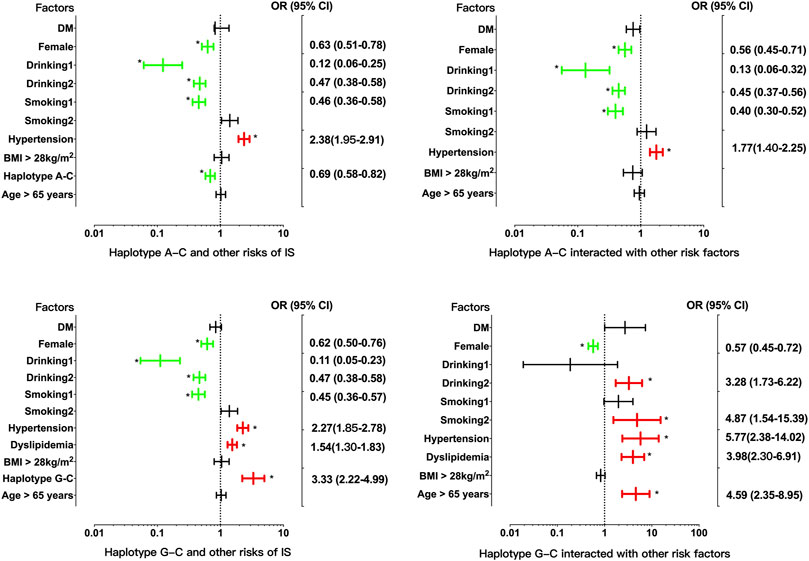
FIGURE 2. Associations between the EHBP1 haplotypes, environmental factors, and their interactions on ischemic stroke. p < 0.025 was considered statistical significance after the Bonferroni correction for two haplotypes. The red and green colors in the picture mean the increased risk and decreased risk factors, respectively.
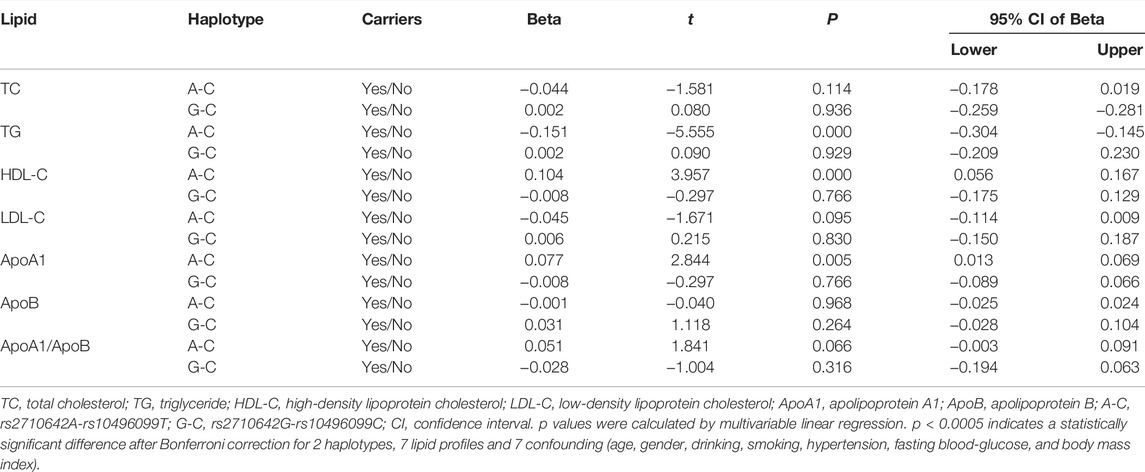
In the control group, the levels of TG, HDL-C and ApoA1 were different between the rs2710642A-rs10496099C haplotype carriers and the haplotype noncarriers (p < 0.025; Figure 3). After Bonferroni correction, we found that the rs2710642A-rs10496099C haplotype carriers had lower TG and higher HDL-C levels than the haplotype noncarriers (p < 0.0005; Table 6). There was no association between the rs2710642G-rs10496099C haplotype and any serum lipid parameters.
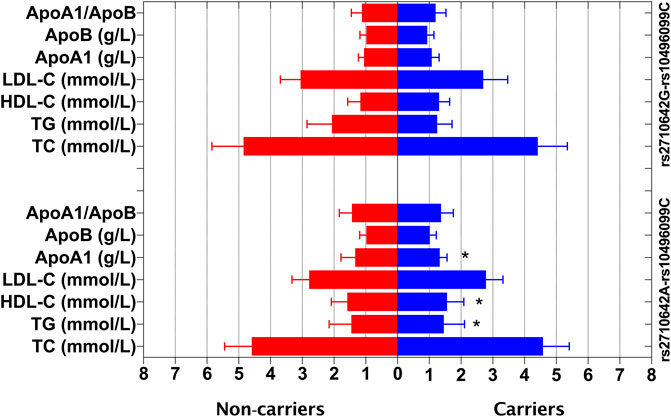
FIGURE 3. Associations between the EHBP1 haplotypes and serum lipid levels in the control group. *p < 0.025 was considered statistical significance after the Bonferroni correction for two haplotypes.
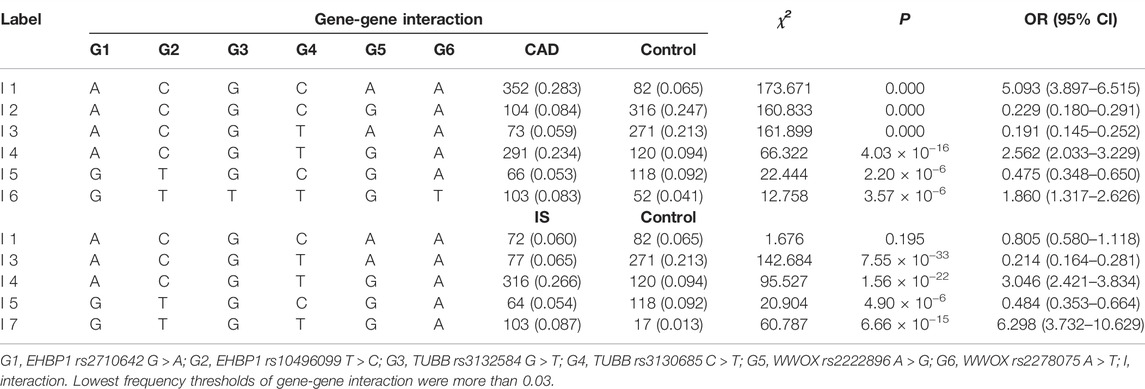
G × G Interactions on CAD or IS
As listed in Table 7, six interactions of rs2710642-rs10496099-rs3132584-rs3130685-rs2222896-rs2278075 were associated with CAD. Namely, A-C-G-C-A-A (OR = 5.093, 95% CI = 3.897–6.515, p = 0.000), A-C-G-T-G-A (OR = 2.562, 95% CI = 2.033–3.229, p = 4.03 × 10−16), and G-T-T-T-G-T (OR = 1.860, 95% CI = 1.317–2.626, p = 3.57 × 10−6) were correlated with an increased risk of CAD. On the other hand, A-C-G-C-G-A (OR = 0.229, 95% CI = 0.180–0.291, p = 0.000), A-C-G-T-A-A (OR = 0.191, 95% CI = 0.145–0.252, p = 0.000), and G-T-G-C-G-A (OR = 0.475, 95% CI = 0.348–0.650, p = 2.22 × 10−6) were correlated with a decreased risk of CAD. Additionally, four interactions of rs2710642-rs1,0496099-rs3132584-rs3130685-rs2222896-rs2278075 were associated with IS. Namely, A-C-G-T-G-A (OR = 3.046, 95% CI = 2.421–3.834, p = 1.56 × 10−22) and G-T-G-T-G-A (OR = 6.298, 95% CI = 3.732–10.629, p = 6.66 × 10−15) were correlated with an augmented risk of IS, while A-C-G-T-A-A (OR = 0.214, 95% CI = 0.164–0.281, p = 7.55 × 10−33) and G-T-G-C-G-A (OR = 0.484, 95% CI = 0.353–0.664, p = 4.90 × 10−6) were correlated with a reduced risk of IS.
Different Interaction Models on CAD or IS
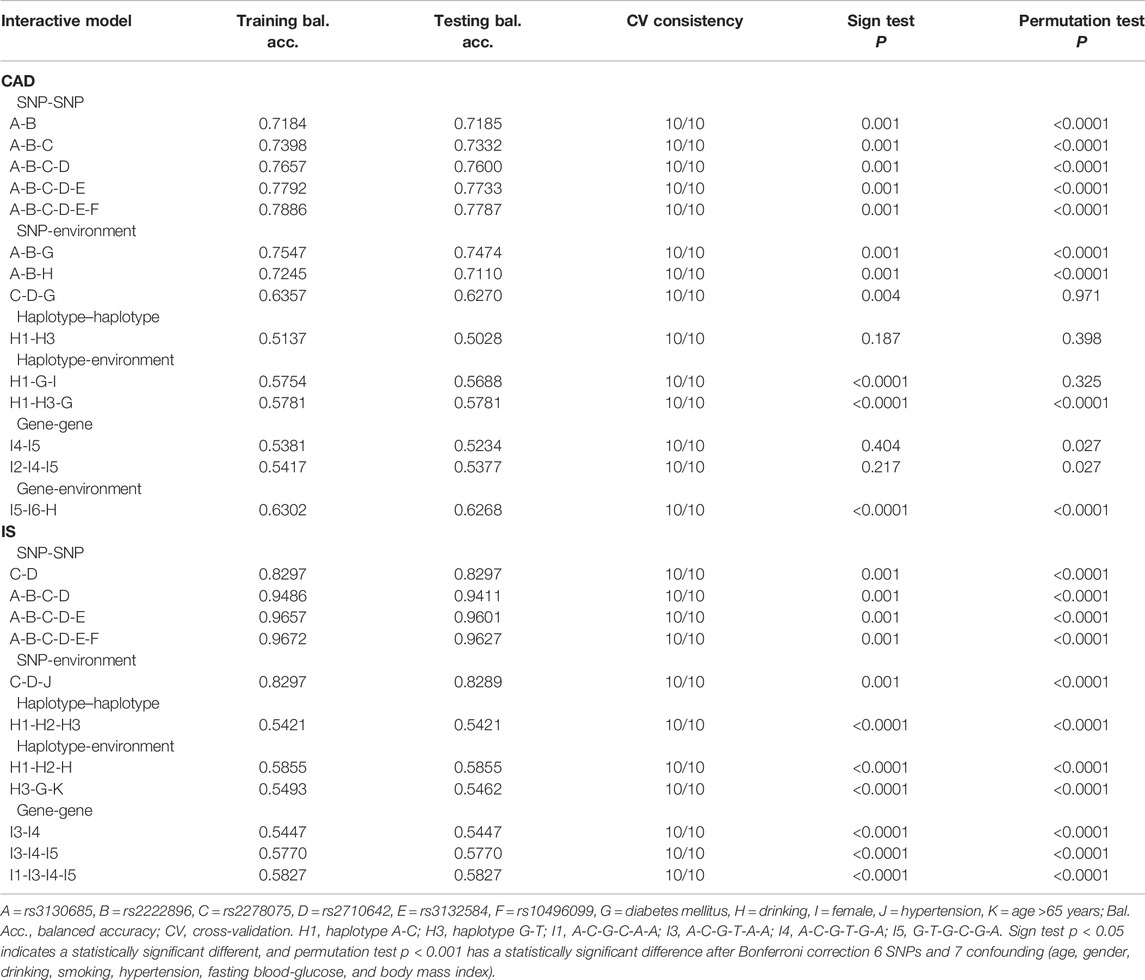
Using GMDR, we screened several models of SNP-SNP, haplotype-haplotype, G × G, and G × E interactions on the risk of CAD and IS, respectively. Nine optimal models (CV constancy of 10 of 10, balanced accuracy test ≥50.28%, and permutation test p < 0.001 for all) were significantly associated with CAD (Table 8). They were rs3130685-rs2222896, rs3130685-rs2222896-rs2278075, rs3130685-rs2222896-rs2278075-rs2710642, rs3130685-rs2222896-rs2278075-rs2710642-rs3132584, rs3130685-rs2222896-rs2278075-rs2710642-rs3132584-rs10496099, rs3130685-rs2222896-diabetes, rs3130685-rs2222896-drinking, H1 (rs2710642A-rs10496099C)-H3 (rs2710642G-rs10496099T)-diabetes, and I5 (G-T-G-C-G-A)-I6 (G-T-T-T-G-T)-drinking.
Eleven interaction models (CV constancy of 10 of 10, balanced accuracy test ≥54.21%, and permutation test p < 0.001 for all) were significantly related to IS (Table 8). They were rs2278075-rs2710642, rs3130685-rs2222896-rs2278075-rs2710642, rs3130685-rs2222896-rs2278075-rs2710642-rs3132584, rs3130685-rs2222896-rs2278075-rs2710642-rs3132584-rs10496099, rs2278075-rs2710642-hypertension, H1-H2 (rs2710642G-rs10496099C)-H3, H1-H2-drinking, H3-diabetes-aging, I1 (A-C-G-C-A-A)-I3 (A-C-G-T-A-A)-I4 (A-C-G-T-G-A)-I5, I3-I4-I5, and I3-I4.
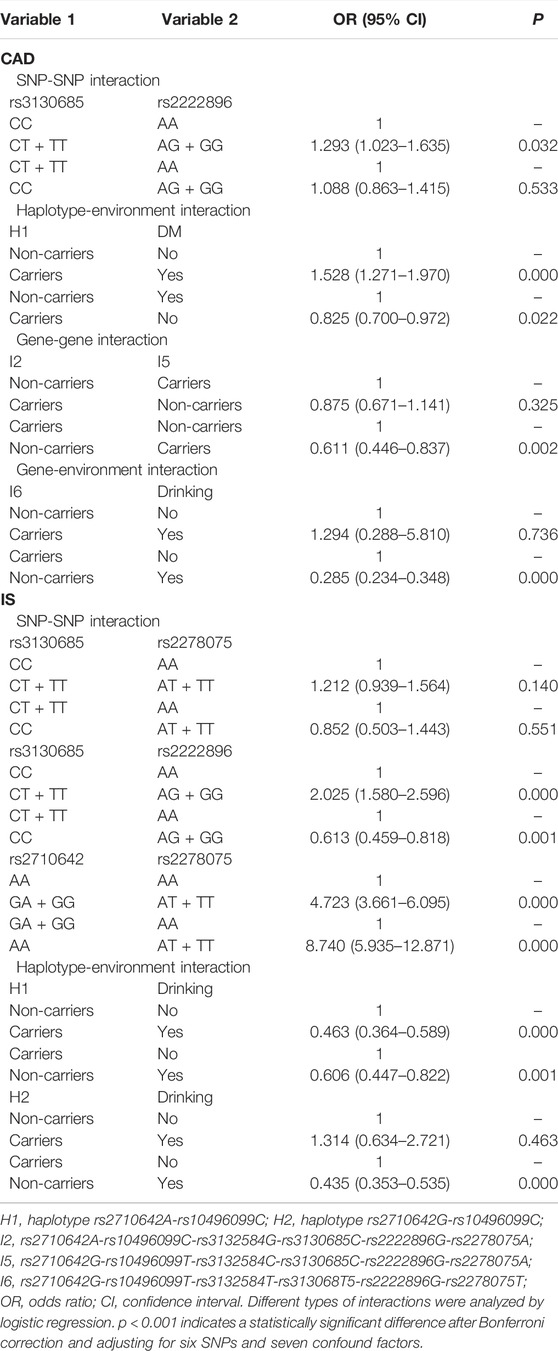
Logistic regression analysis was used to further verify the association between the interactions and CAD or IS (Table 9), and the interactive tree diagram (Figure 4) directly exhibited the interaction between risk factors. In the CAD group, subjects carrying H1 and diabetes had higher risk of CAD than those not carrying H1 and diabetes (OR = 1.528, 95% CI = 1.271–1.970, p < 0.001), and those drinking but not carrying G-T-T-T-G-T (I6) had lower risk of CAD than those carrying I6 but not drinking (OR = 0.285, 95% CI = 0.234–0.348, p < 0.001). In the IS group, compared to those carrying the rs3130685CC or rs2222896AA genotypes, subjects carrying the rs3130685CT + TT and rs2222896AG + GG genotypes were more likely to have a high risk of IS (OR = 2.025, 95% CI = 1.580–2.596, p < 0.001). Compared to those carrying the rs2710642GA + GG and rs2278075AA genotypes, subjects carrying the rs2710642AA and rs2278075AT + TT genotypes had an increased risk of IS (OR = 8.740, 95% CI = 5.935–12.871, p < 0.001). Compared to those carrying the rs2710642AA and rs2278075 TT genotypes, subjects carrying the rs2710642GA + GG and the rs2278075AT + TT genotypes had an increased risk of IS (OR = 4.723, 95% CI = 3.661–6.095, p < 0.001). In addition, compared to those not carrying H1 and not drinking, subjects carrying H1 and drinking had lower risk of IS (OR = 0.463, 95% CI = 0.364–0.589, p < 0.001), and compared to those not drinking but carrying H2, subjects drinking but not carrying H2 had lower risk of IS (OR = 0.435, 95% CI = 0.353–0.535, p < 0.001).
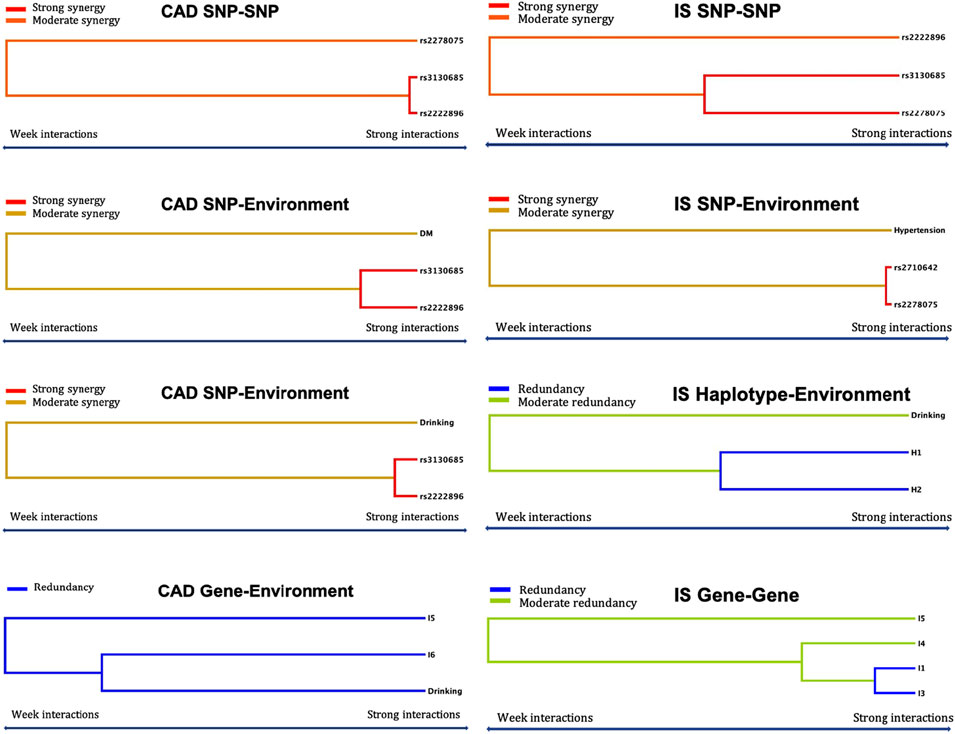
FIGURE 4. Optimal interactions affecting diseases shown in a dendrogram. Elements that interact strongly with each other appear close together in the leaves of the tree, while elements that interact weakly appear far from each other. The yellow, orange and red colors indicate synergy effect between two factors, and blue and green colors indicate redundancy effect between two factors. The darker the color, the stronger the effect.
Discussion
The principal findings of the present study are as follows: ① The rs2278075T allele was associated with an increased risk of CAD. The rs2278075T and rs2710642G alleles were associated with an increased risk, while the rs3130685T allele with a reduced risk of IS. ② The rs2710642G-rs10496099C haplotype was correlated with an increased risk of IS, but the rs2710642A-rs10496099C haplotype was correlated with a decreased risk of IS. ③ Serum lipid phenotypes of rs2278075T, rs2710642G and rs3130685T allele carriers were associated with dyslipidemia, and the haplotype of rs2710642A-rs10496099C was associated with beneficial lipid profiles in the control group. ④ The SNPs of rs2278075, rs2710642 and rs3130685 might be associated with CAD or IS by modifying the serum lipid profiles.
This study found that the MAF of rs2278075 was different between the CAD and control groups, and the MAFs of rs2710642, rs3130685, and rs2278075 were different between the IS and control groups. Moreover, subjects carrying rs2278075TT had an association of a 1.58-fold risk of CAD to those carrying rs2278075AA. This was consistent with a previous GWAS in Caucasian participants (Joseph et al., 2017), and another GWAS reported that smokers carrying WWOX were related to coronary artery calcification (Li et al., 2016). These can be explained by the function of WWOX in regulating lipoprotein metabolism and lipid homeostasis. In addition, carrying the rs2278075T and rs2710642G alleles were associated with increased risks of IS, while carrying the rs3130685T allele was associated with a reduced risk of IS. We speculate that the rs2278075, rs2710642, and rs3130685 SNPs played a role in regulating blood lipid profiles to change risk of IS. Furthermore, study on the synergistic effects of these SNPs showed that the rs2710642A-rs10496099C-rs3132584G-rs3130685C-rs2222896A-rs2278075A interaction was associated with an increased risk of CAD, and the rs2710642A-rs10496099C-rs3132584G-rs3130685T-rs2222896A-rs2278075A and rs2710642A-rs10496099C-rs3132584G-rs3130685T-rs2222896G-rs2278075A interactions reduced and increased the risk of CAD and IS, respectively. The rs2710642A-rs10496099C-rs3132584G-rs3130685C-rs2222896G-rs2278075A and rs2710642G-rs10496099T-rs3132584C-rs3130685C-rs2222896G-rs2278075A interactions were associated with a reduced risk of CAD, and the rs2710642G-rs10496099T-rs3132584T-rs3130685T-rs2222896G-rs2278075T interaction was associated with an increased risk of CAD. The rs2710642G-rs10496099T-rs3132584G-rs3130685C-rs2222896G-rs2278075A and rs2710642G-rs10496099T-rs3132584G-rs3130685T-rs2222896G-rs2278075A interactions were associated with reduced and increased IS risk, respectively. Many studies have identified that haplotypes have a more significant impact on phenotype than a single SNP (Liu et al., 2020), and the association between haplotypes and phenotypes is more helpful for understanding local ancestral genome information and population genetic structure (Locke et al., 2015). There was strong LD (D' > 0.8 or R2 > 0.5) between the EHBP1 rs2710642 and rs10496099 SNPs in the three groups. We found that the rs2710642A-rs10496099C haplotype reduced the risk of IS by 0.7 times, and rs2710642G-rs10496099C haplotype increased the risk of IS by 2.94 times. These results indicate that either coeffects or haplotypes of SNPs have a better predictive effect than any single SNP in the disease risk model of CAD or IS.
Previous studies reported that the rs2710642A and rs3130685T alleles were associated with LDL-C in European, Japanese and the Maonan population in China, and the rs2278075T allele was correlated with CAD in Caucasian (Kathiresan et al., 2007; Giagtzoglou et al., 2013; Joseph et al., 2017). This study observed that in the control group, high levels of TC and TG were associated with the rs2710642G and rs3130685T alleles, and high levels of LDL-C were associated with the rs2710642G allele. However, low levels of HDL-C were associated with rs2278075T allele. These findings suggest that the association of rs2710642, rs3130685 and rs2278075 SNPs with CAD and IS may be achieved by altering blood lipid levels. According to information from the International 1,000 Genomes database (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/), the genotypic frequencies and MAFs of the SNPs in Han Chinese individuals in Beijing, China (CHB) were as follows: rs2278075AA 68.3%, AT 19.5%, TT 12.2%, and T 22.0%; rs2710642AA 36.6%, AG 56.1%, GG 7.3% and G 35.4%; and rs3130685CC 20.0%, CT 51.1%, TT 28.9%, and C 45.6%. It exhibited that the mutations and minor alleles of these SNPs were similar between the CHB and Guangxi Han population. Therefore, these SNPs might be associated with an aggravate risk of CAD or IS in CHB population.
The interactions between EHBP1, TUBB, and WWOX and G × E were involved in the pathogenesis of CAD and IS. The interaction of rs2710642 and rs2278075 SNPs was associated with higher risk of IS. The results also showed that the haplotype of rs2710642A-rs10496099C or rs2710642G-rs10496099C interacted with some factors (female sex, few cigarettes, and drinking, especially a small amount of alcohol) to significantly reduce the risk of IS. Some studies have shown that the level of sex hormones and complement of sex chromosomes determined sex differences in CVD patients. Many lifestyles and environmental factors (such as smoking, drinking, and diet) were associated with CVD in gender-specific ways. Differences in sex hormone-dependent receptors that control endothelial function may lead to increased CVD and IS risks in elderly men and postmenopausal women and a slightly lower risk of CVD and IS in premenopausal women (O’Keefe et al., 2018; Owolabi et al., 2018; Polfus et al., 2013). Our study also found that female sex factor was independent or synergistic with lipid-related genes to modulate the risk of IS. The synergy between the haplotype H2 and hypertension and dyslipidemia significantly facilitated the risk of IS. In addition, this study screened the following optimal models to predict the risk of CAD or IS by the GMDR method and binary logistic regression. The interactions of rs3130685-rs2222896-rs2278075, rs3130685-rs2222896-diabetes, and rs3130685-rs2222896-drinking increased the risk of CAD, whereas those of I5-I6-drinking decreased the risk of CAD. The rs3130685-rs2278075-rs2222896 and rs2710642-rs2278075-hypertension interactions strengthened the risk of IS, while H1-H2-drinking and I1-I3-I4-I5 interactions weakened the risk of IS. Many studies have reported that minimal or moderate drinking (≤1 drink/day for women, ≤ 1-2 drink/day for men) is beneficial to protect arteries (Saez et al., 2010; Simino et al., 2013; Sferra et al., 2020). A small amount of red wine before or during dinner could improve the prognosis of CVD (Stanhewicz et al., 2018). The results might be attributed to the fact that grapes are rich in resveratrol, which reduces glutathione peroxidase and serum IL6 levels and has antioxidant and anti-inflammatory activities (Stewart et al., 2019). In this study, drinking and minimal drinking reduced the risk of IS and antagonized the effects of H1 and H2 to reduce IS risk. The explanation might be that the daily alcohol consumption of the subjects in the CAD and IS groups was generally low, or the alcohol content was relatively low. Moreover, this study observed that there was a redundancy effect of the drinking and I6 and I5 interaction in which the drinking factor played a dominant protective effect; thus, the coeffect of I5-I6-drinking reduced the risk of CAD. However, the coeffect of rs3130685-rs2222896-drinking increased CAD risk, here, the mutation of genes was the dominant factor for increasing risk of CAD. Smoking is an independent risk factor for accelerating atherosclerosis (Taskinen and Borén, 2015; Voigt et al., 2020). However, in this study, it showed that minimal smoking independently reduced the risk of IS, and the synergy of minimal smoking with haplotype H1 significantly reduced the risk of IS. Also, minimal smoking also antagonized the risk of IS caused by carrying the haplotype H2. This may be because minimal smoking reduced the psychological pressure of some patients, changing the risk of IS. Also, other protective factors overlapped with minimal smoking, which could mask its adverse effects. It may also be because there were too few subjects with minimal smoking in the IS group, leading to bias in this result. Therefore, a larger sample is needed to replicate the assessment to evaluate the accuracy of this result. Certainly, we have hardly found that minimal smoking has a similar effect on CAD. Hypertension, diabetes and dyslipidemia are independent and traditional risk factors for CVD and cerebrovascular disease (Willer et al., 2013; Wood et al., 2018). Clinical studies have found that with a lower level of normal blood pressure at baseline, hypertension will increase the risk of CAD and IS to a greater extent (Yamada et al., 2019). This study observed that diabetes enhanced the synergistic effect of the rs3130685-rs2222896 interaction, increasing the risk of CAD, and hypertension enhanced the synergistic effect of the rs2710642-rs2278075 interaction, increasing the risk of IS. These results suggest that the interaction of G × E was the risk of CAD and IS. To improve prognosis, intervention for these patients should be individualized, starting with environmental factors or treatments targeting lipid-related genes and their pathways.
Some deficiencies in this study cannot be ignored. First, it did not investigate the dietary habits of the subjects in the three groups and did not analyze the effect of diet as an environmental factor. Second, we did not record the types of antihypertensive or hypoglycemic drugs taken, which cannot be ruled out because of the effect of these drugs on blood lipid levels. Third, there was no resequencing of gene expression after treatment and no follow-up of the prognosis of susceptible gene carriers in the control group. Fourth, a larger cohort sample is needed to verify whether the study has selection bias. Finally, some CAD and IS patients were taking some drugs that may affect serum lipid profiles. Therefore, we could not determine the association between the SNPs and serum lipid levels in CAD and IS groups.
Conclusion
This study illustrated the associations among EHBP1, TUBB, and WWOX SNPs, G × G and G × E, and CAD and IS in the Guangxi Han population. We found that the WWOX rs2278075T allele was associated with the risk of CAD and IS, and EHBP1 rs2710642 and TUBB rs3130685 SNPs might be correlated with IS risk by regulating serum lipid profiles. The haplotypes of EHBP1 rs2710642 and rs10496099 were more predictive of IS risk than a single SNP. The interactions of G × G and G × E, such as female sex, drinking, smoking, hypertension and diabetes, may alter the association between single risk factor and CAD or IS.
Data Availability Statement
The data presented in the study have been included in the article/Supplementary Material (the genotypic data), further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital, Guangxi Medical University (No. Lunshen 2014-KY-Guoji-001; March 7, 2014). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
C-XL and R-XY had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: C-XL and R-XY. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: C-XL and R-XY. Statistical analysis: C-XL and Z-HS. Obtained funding: R-XY.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81460169) and the Youthful Science Foundation of Guangxi Province (No. 2017GXNSFBA198067).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.843661/full#supplementary-material
References
Arsava, E. M., Helenius, J., Avery, R., Sorgun, M. H., Kim, G.-M., Pontes-Neto, O. M., et al. (2017). Assessment of the Predictive Validity of Etiologic Stroke Classification. JAMA Neurol. 74, 419–426. doi:10.1001/jamaneurol.2016.5815
Bede, T. P., de Jesus, V., Rosse de Souza, V., Mattoso, V., Abreu, J. P., Dias, J. F., et al. (2020). Effect of Grape Juice, Red Wine and Resveratrol Solution on Antioxidant, Anti-inflammatory, Hepactic Function and Lipid Profile in Rats Feds with High-Fat Diet. Nat. Product. Res. 35, 5255–5260. doi:10.1080/14786419.2020.1747458
Colafella, K. M. M., and Denton, K. M. (2018). Sex-specific Differences in Hypertension and Associated Cardiovascular Disease. Nat. Rev. Nephrol. 14, 185–201. doi:10.1038/nrneph.2017.189
Deng, G.-X., Yin, R.-X., Guan, Y.-Z., Liu, C.-X., Zheng, P.-F., Wei, B.-L., et al. (2020). Association of the NCAN-TM6SF2-CILP2-PBX4-SUGP1-MAU2 SNPs and Gene-Gene and Gene-Environment Interactions with Serum Lipid Levels. Aging 12, 11893–11913. doi:10.18632/aging.103361
Denis, N., Palmer-Smith, H., Elisma, F., Busuttil, A., Wright, T. G., Khalil, M. B., et al. (2011). Quantitative Proteomic Analysis of PCSK9 Gain of Function in Human Hepatic HuH7 Cells. J. Proteome Res. 10, 2011–2026. doi:10.1021/pr2000072
Diener, H.-C., and Hankey, G. J. (2020). Primary and Secondary Prevention of Ischemic Stroke and Cerebral Hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 75, 1804–1818. doi:10.1016/j.jacc.2019.12.072
Fan, Y., Li, W., Liu, H., Wang, L., Zhang, S., Li, W., et al. (2019). Effects of Obesity and a History of Gestational Diabetes on the Risk of Postpartum Diabetes and Hyperglycemia in Chinese Women: Obesity, GDM and Diabetes Risk. Diabetes Res. Clin. Pract. 156, 107828. doi:10.1016/j.diabres.2019.107828
Fuchs, F. D., and Whelton, P. K. (2020). High Blood Pressure and Cardiovascular Disease. Hypertension 75, 285–292. doi:10.1161/HYPERTENSIONAHA.119.14240
Gabb, G. M., Mangoni, A. A., Anderson, C. S., Cowley, D., Dowden, J. S., Golledge, J., et al. (2016). Guideline for the Diagnosis and Management of Hypertension in Adults - 2016. Med. J. Aust. 205, 85–89. doi:10.5694/mja16.00526
GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017). Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990-2016: a Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. doi:10.1016/S0140-6736(17)32154-2
Giagtzoglou, N., Li, T., Yamamoto, S., and Bellen, H. J. (2013). dEHBP1 Regulates Scabrous Secretion during Notch Mediated Lateral Inhibition. J. Cel. Sci. 126, 3686–3696. doi:10.1242/jcs.126292
Golan, R., Gepner, Y., and Shai, I. (2019). Wine and Health-New Evidence. Eur. J. Clin. Nutr. 72, 55–59. doi:10.1038/s41430-018-0309-5
Guilherme, A., Soriano, N. A., Bose, S., Holik, J., Bose, A., Pomerleau, D. P., et al. (2004). EHD2 and the Novel EH Domain Binding Protein EHBP1 Couple Endocytosis to the Actin Cytoskeleton. J. Biol. Chem. 279, 10593–10605. doi:10.1074/jbc.M307702200
Hasan, K. M., Friedman, T. C., Parveen, M., Espinoza-Derout, J., Bautista, F., Razipour, M. M., et al. (2020). Electronic Cigarettes Cause Alteration in Cardiac Structure and Function in Diet-Induced Obese Mice. Plos. One 15, e0239671. doi:10.1371/journal.pone.0239671
Iatan, I., Choi, H. Y., Ruel, I., Reddy, M. V. P. L., Kil, H., Lee, J., et al. (2014). The WWOX Gene Modulates High-Density Lipoprotein and Lipid Metabolism. Circ. Cardiovasc. Genet. 7, 491–504. doi:10.1161/CIRCGENETICS.113.000248
Johannsen, J., Kortüm, F., Rosenberger, G., Bokelmann, K., Schirmer, M. A., Denecke, J., et al. (2018). A Novel Missense Variant in the SDR Domain of the WWOX Gene Leads to Complete Loss of WWOX Protein with Early-Onset Epileptic Encephalopathy and Severe Developmental Delay. Neurogenetics 19, 151–156. doi:10.1007/s10048-018-0549-5
Joseph, P., Leong, D., McKee, M., Anand, S. S., Schwalm, J.-D., Teo, K., et al. (2017). Reducing the Global Burden of Cardiovascular Disease, Part 1: The Epidemiology and Risk Factors. Circ. Res. 121, 677–694. doi:10.1161/CIRCRESAHA.117.308903
Kathiresan, S., Manning, A. K., Demissie, S., D'Agostino, R. B., Surti, A., Guiducci, C., et al. (2007). A Genome-wide Association Study for Blood Lipid Phenotypes in the Framingham Heart Study. BMC. Med. Genet. 8 (Suppl. 1), S17. doi:10.1186/1471-2350-8-S1-S17
Khawaled, S., Nigita, G., Distefano, R., Oster, S., Suh, S.-S., Smith, Y., et al. (2020). Pleiotropic Tumor Suppressor Functions of WWOX Antagonize Metastasis. Sig. Transduct. Target. Ther. 5, 43. doi:10.1038/s41392-020-0136-8
Larsson, S. C., Wallin, A., Wolk, A., and Markus, H. S. (2016). Differing Association of Alcohol Consumption with Different Stroke Types: a Systematic Review and Meta-Analysis. BMC. Med. 14, 178. doi:10.1186/s12916-016-0721-4
Leitwein, M., Duranton, M., Rougemont, Q., Gagnaire, P.-A., and Bernatchez, L. (2020). Using Haplotype Information for Conservation Genomics. Trends Ecol. Evol. 35, 245–258. doi:10.1016/j.tree.2019.10.012
Levy, D., Larson, M. G., Benjamin, E. J., Newton-Cheh, C., Wang, T. J., Hwang, S.-J., et al. (2007). Framingham Heart Study 100K Project: Genome-wide Associations for Blood Pressure and Arterial Stiffness. BMC. Med. Genet. 8, S3. doi:10.1186/1471-2350-8-S1-S3
Li, Z., Schulze, R. J., Weller, S. G., Krueger, E. W., Schott, M. B., Zhang, X., et al. (2016). A Novel Rab10-EHBP1-EHD2 Complex Essential for the Autophagic Engulfment of Lipid Droplets. Sci. Adv. 2, e1601470. doi:10.1126/sciadv.1601470
Liu, C.-X., Yin, R.-X., Shi, Z.-H., Deng, G.-X., Zheng, P.-F., Wei, B.-L., et al. (2020). EHBP1 SNPs, Their Haplotypes, and Gene-Environment Interactive Effects on Serum Lipid Levels. ACS. Omega. 5, 7158–7169. doi:10.1021/acsomega.9b03522
Locke, A. E., Kahali, B., Berndt, S. I., Justice, A. E., Pers, T. H., Day, F. R., et al. (2015). Genetic Studies of Body Mass index Yield New Insights for Obesity Biology. Nature 518, 197–206. doi:10.1038/nature14177
O'Keefe, E. L., DiNicolantonio, J. J., O'Keefe, J. H., and Lavie, C. J. (2018). Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog. Cardiovasc. Dis. 61, 68–75. doi:10.1016/j.pcad.2018.02.001
Owolabi, M. O., Sarfo, F., Akinyemi, R., Gebregziabher, M., Akpa, O., Akpalu, A., et al. (2018). Dominant Modifiable Risk Factors for Stroke in Ghana and Nigeria (SIREN): a Case-Control Study. Lancet Glob. Health 6, e436–e446. doi:10.1016/S2214-109X(18)30002-0
Polfus, L. M., Smith, J. A., Shimmin, L. C., Bielak, L. F., Morrison, A. C., Kardia, S. L. R., et al. (2013). Genome-wide Association Study of Gene by Smoking Interactions in Coronary Artery Calcification. Plos. One 8, e74642. doi:10.1371/journal.pone.0074642
Saez, M. E., González-Pérez, A., Martínez-Larrad, M. T., Gayán, J., Real, L. M., Serrano-Ríos, M., et al. (2010). WWOX Gene Is Associated with HDL Cholesterol and Triglyceride Levels. BMC. Med. Genet. 11, 148. doi:10.1186/1471-2350-11-148
Sferra, A., Petrini, S., Bellacchio, E., Nicita, F., Scibelli, F., Dentici, M. L., et al. (2020). TUBB Variants Underlying Different Phenotypes Result in Altered Vesicle Trafficking and Microtubule Dynamics. Int. J. Mol. Sci. 21, 1385. doi:10.3390/ijms21041385
Simino, J., Sung, Y. J., Kume, R., Schwander, K., and Rao, D. C. (2013). Gene-alcohol Interactions Identify Several Novel Blood Pressure Loci Including a Promising Locus Near SLC16A9. Front. Genet. 4, 277. doi:10.3389/fgene.2013.00277
Stanhewicz, A. E., Wenner, M. M., and Stachenfeld, N. S. (2018). Sex Differences in Endothelial Function Important to Vascular Health and Overall Cardiovascular Disease Risk across the Lifespan. Am. J. Physiol. Heart Circulatory Physiol. 315, H1569–H1588. doi:10.1152/ajpheart.00396.2018
Stewart, A. L., Barinas-Mitchell, E., Matthews, K. A., El Khoudary, S. R., Magnani, J. W., Jackson, E. A., et al. (2019). Social Role-Related Stress and Social Role-Related Reward as Related to Subsequent Subclinical Cardiovascular Disease in a Longitudinal Study of Midlife Women: The Study of Women's Health across the Nation. Psychosom. Med. 81, 821–832. doi:10.1097/PSY.0000000000000733
Taskinen, M.-R., and Borén, J. (2015). New Insights into the Pathophysiology of Dyslipidemia in Type 2 Diabetes. Atherosclerosis 239, 483–495. doi:10.1016/j.atherosclerosis.2015.01.039
Voigt, S., van Os, H., van Walderveen, M., van der Schaaf, I., Kappelle, L., Broersen, A., et al. (2020). Sex Differences in Intracranial and Extracranial Atherosclerosis in Patients with Acute Ischemic Stroke. Int. J. Stroke 16, 385–391. doi:10.1177/1747493020932806
Willer, C. J., Schmidt, E. M., Sengupta, S., Peloso, G. M., Gustafsson, S., Kanoni, S., et al. (2013). Discovery and Refinement of Loci Associated with Lipid Levels. Nat. Genet. 45, 1274–1283. doi:10.1038/ng.2797
Wood, A. M., Kaptoge, S., Butterworth, A. S., Willeit, P., Warnakula, S., Bolton, T., et al. (2018). Risk Thresholds for Alcohol Consumption: Combined Analysis of Individual-Participant Data for 599 912 Current Drinkers in 83 Prospective Studies. Lancet 391, 1513–1523. doi:10.1016/S0140-6736(18)30134-X
Keywords: EH domain-binding protein 1, tubulin beta class I, WW domaincontaining oxidoreductase, single nucleotide polymorphism, coronary artery disease, ischemic stroke
Citation: Liu C-X, Yin R-X, Cao X-L, Shi Z-H, Huang F, Wei B-L, Deng G-X, Zheng P-F and Guan Y-Z (2022) EHBP1, TUBB, and WWOX SNPs, Gene-Gene and Gene-Environment Interactions on Coronary Artery Disease and Ischemic Stroke. Front. Genet. 13:843661. doi: 10.3389/fgene.2022.843661
Received: 04 January 2022; Accepted: 17 March 2022;
Published: 26 April 2022.
Edited by:
Marcelo Rizzatti Luizon, Federal University of Minas Gerais, BrazilReviewed by:
Kei Hang Katie Chan, City University of Hong Kong, Hong Kong SAR, ChinaRuixue Hou, Icahn School of Medicine at Mount Sinai, United States
Lizbeth Gonzalez-Herrera, Universidad Autónoma de Yucatán, Mexico
Copyright © 2022 Liu, Yin, Cao, Shi, Huang, Wei, Deng, Zheng and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui-Xing Yin, eWlucnVpeGluZ0AxNjMuY29t
 Chun-Xiao Liu
Chun-Xiao Liu Rui-Xing Yin
Rui-Xing Yin Xiao-Li Cao2
Xiao-Li Cao2 Feng Huang
Feng Huang Bi-Liu Wei
Bi-Liu Wei Guo-Xiong Deng
Guo-Xiong Deng Peng-Fei Zheng
Peng-Fei Zheng Yao-Zong Guan
Yao-Zong Guan