- 1Haematology Department, Nnamdi Azikiwe University Teaching Hospital, Nnewi, Nigeria
- 2Department of Medical Laboratory Science, Faculty of Basic Medical Sciences, Nnamdi Azikiwe University, Nnewi, Nigeria
- 3Haematology Department, University of Port Harcourt, Port Harcourt, Nigeria
Background: Hemoglobin polymerization in sickle cell anemia (SCA) leads to abnormally rigid and adhesive erythrocytes that obstruct blood vessels, leading to poor tissue perfusion, hence provoking inflammation and damage of surrounding tissues. Adiponectin, a protein hormone, presumptively has anti-inflammatory characteristics, hence may be an important therapeutic target in SCA.
Aim: The aim of the study was to evaluate the status of adiponectin and its correlation with disease severity in SCA.
Patients and Methods: A total of 84 subjects were recruited for the study comprising 34 homozygous sickle cell (HbSS) subjects (25 in the steady state and nine in the resolving crisis state) and 50 controls (25 heterozygous sickle cell [HbAS] and 25 hemoglobin phenotype AA subjects). The hemoglobin phenotype, adiponectin levels, and full blood counts were evaluated. Anthropometric measurements were also conducted.
Results: A significant difference was observed in the mean body mass index between the different hemoglobin phenotype groups and also between the SCA in crisis resolution patients and the control group (p < 0.05). There was no significant difference in the median serum levels of adiponectin in the different hemoglobin phenotype groups and between SCA patients in the steady state compared with those in the crisis resolution state. Also, there was no correlation between disease severity and adiponectin in SCA patients in the steady state (p = 0.87).
Conclusion: Our study seems to suggest that in our data set of sickle cell anemia patients in the steady state, adiponectin does not constitute part of the endocrinopathy that affects these patients.
Introduction
Sickle cell disease (SCD) is a group of genetic conditions that result from the inheritance of abnormal genes, thereby resulting in the production of abnormal hemoglobin in red blood cells (Akinyanju, 2015). The most common and most severe form of SCD, sickle cell anemia (SCA), refers to homozygosity for the sickle hemoglobin (Hb), known as HbSS (Piel et al., 2017). Hemoglobin polymerization leads to abnormally rigid and adhesive red blood cells (RBCs) that obstruct blood vessels, leading to tissue damage from poor perfusion in HbSS, hence provoking inflammation, as they stimulate and damage surrounding tissues and cells (Serjeant, 1997) (Platt, 2000). Keikhaei et al. showed that pro-inflammatory cytokines, especially lL-8 and IL-17 were increased in SCD patients in the steady state when compared to those in controls (apparently normal individuals) and increased in the crisis state when compared to the steady state (Keikhaei et al., 2013). This suggests that there is a background inflammation in the steady state which escalates during crisis.
The body mass index (BMI) is broadly used to categorize a person as underweight, normal weight, overweight, or obese based on tissue mass (muscle, fat, and bone) and height (Malcolm, 2015). Historically, it is well documented that children with SCD were underweight, particularly those with HbSS due to a characteristic basal hypercatabolic metabolism (Thomas et al., 2000; Barden et al., 2000).
Adiponectin is a protein hormone that modulates a number of metabolic processes, including glucose regulation and fatty acid oxidation (Díez and Iglesias, 2003). Adiponectin is secreted from adipose tissue (and also from the placenta during pregnancy) into the blood. It is a specific protein with presumptive antiatherogenic, insulin-sensitizing, and anti-inflammatory characteristics (Chen et al., 2006; (Hotta et al., 2000; (Bełtowski et al., 2008). Adiponectin is considered important in the etiopathogenesis of many vascular and inflammatory disorders due to its anti-inflammatory actions (Zhu et al., 2008; (Cui et al., 2011). It also inhibits the synthesis of pro-inflammatory cytokines such as IL-6, IL-18, and TNF-α synthesis by blocking NF-κB activation (Yamaguchi et al., 2005; Chandrasekar et al., 2008; Ouchi and Walsh, 2017).
Exploring the role of adiponectin in sickle cell anemia hence becomes important, given that sickle cell anemia has a background chronic inflammatory state and adiponectin may be an important therapeutic target in its management.
Subjects and Methods
Patient Selection
A total of 84 subjects were recruited for the study from the hematology clinic of a tertiary health center and during community interactive sessions with patients. These included 25 homozygous sickle cell (HbSS) subjects in the steady state [the selection of the steady state group was dependent on subjects’ not experiencing crisis for at least 4 weeks, not receiving blood transfusion for at least 3 months, and not having fever for at least 2 weeks prior to the study (Okocha et al., 2014)]. A total of nine homozygous sickle cell (HbSS) patients, whose crisis was resolving, were defined as HbSS patients who did not meet the criteria for the steady state disease but were not in the acute phase of SCA crisis; while 25 heterozygous sickle cell (HbAS) and 25 hemoglobin phenotype AA subjects were used as controls. The ethical approval for this research was obtained from the Nnamdi Azikiwe University Teaching Hospital Ethics Committee, and written informed consent was sought and received from the subjects or their caregivers.
Disease Severity Score in Hemoglobin SS Patients
Disease severity was calculated using an objective severity scoring system, where points were assigned using the following characteristics: number of hospital admissions for crisis per year, rate of transfusions, white blood cell count, number of complications, and the degree of anemia. Scores of ≤3 were considered mild disease; scores of >3–≤ 7, moderate disease; while scores of >7 were considered severe disease (Okocha et al., 2017).
Specimen Collection, Preparation, and Storage
About 5 ml of venous blood was collected aseptically by venipuncture from each subject via the antecubital vein using a plastic syringe with minimum stasis, and 2 ml was then dispensed into ethylene diamine tetra acetic acid bottles for the determination of the hemoglobin phenotype and the full blood count. The remaining 3 ml was emptied into a plain bottle and centrifuged at 4000 rpm for 10 min. The serum (supernatant) was stored at −22°C and then used for the determination of adiponectin levels. The hemoglobin phenotype was determined using the Zip Zone electrophoresis chamber and EV 243 power supply (Helena Biosciences, United Kingdom). The adiponectin level was determined using commercially available adiponectin test-kits, and its assay was based on enzyme-linked immunosorbent assay. The full blood count was performed using a Sysmex automated hematology analyzer (KX2IN model, Sysmex Corporation, Kobe, Japan)
Statistical Analysis
Data obtained were analyzed using the Statistical Package for Social Sciences software package version 20 (SPSS Inc., IL, Chicago, United States). Descriptive statistics were used to summarize the variables and characterize the demographics. The statistical analysis was performed using the Kruskal–Wallis test to compare the differences in medians between two and three groups. The Spearman’s correlation coefficient was used for correlation of nonparametric variables. The values were deemed significant when p < 0.05.
Results
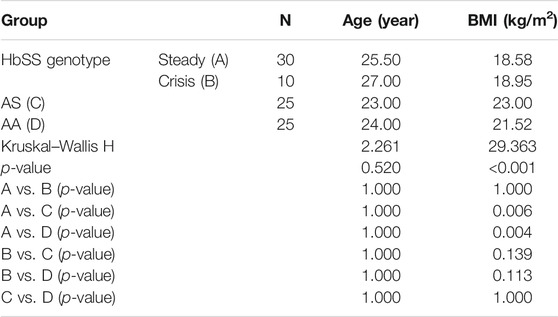
There was a total of 84 subjects recruited for this study which included 25 homozygous sickle cell (HbSS) subjects in the steady state, nine homozygous sickle cell (HbSS) patients whose crisis were resolving, 25 heterozygous sickle cell (HbAS) subjects, and 25 hemoglobin phenotype AA subjects. The HbSS subjects in the steady state were 14 nonpregnant females and 11 males; HbSS in crisis resolution included eight females and one male; HbAS included 16 males and 9 females, while HbAA included 14 males and 11 females. Their age range was between 10 and 48 years with the median age of the homozygous sickle cell subjects in the steady state being 25.50 years; the median age for the homozygous sickle cell subjects in crisis resolution was 27.00 years and that for the heterozygous sickle cell subjects was 23.00 years and the HbAA controls was 24.00 years (Table 1). Table 1 also shows the mean BMI (kg/m) in the various hemoglobin phenotype groups. A significant difference was observed in the mean BMI between the different hemoglobin phenotype groups. However, there was no significant difference in the mean BMI between the homozygous sickle cell anemia patients in the steady and crisis resolution states and between the sickle cell anemia in crisis resolution and the control group (p > 0.05).
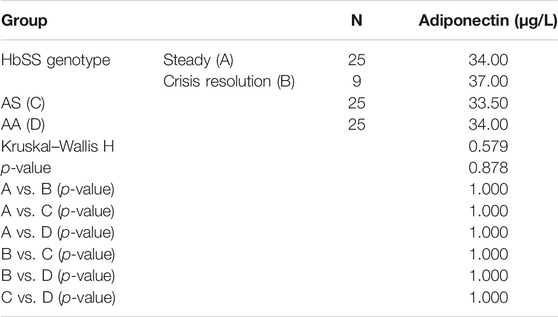
Table 2 shows the median levels of adiponectin in the different hemoglobin phenotype groups. There was no significant difference in the median serum levels of adiponectin in the different hemoglobin phenotype groups and between sickle cell anemia patients in the steady state and crisis resolution state.
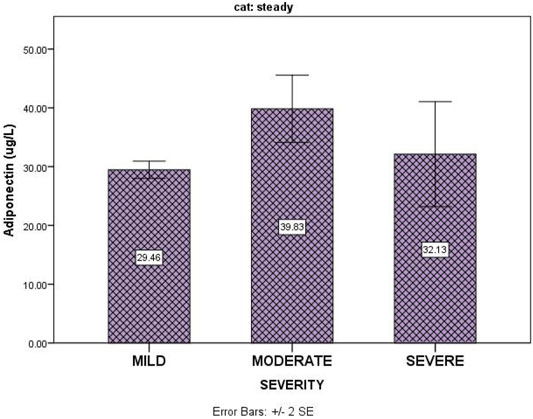
Table 3 shows that disease severity in SCA patients in the steady state had no correlation with serum adiponectin (p = 0.87). Figure 1 shows the relationship between serum levels of adiponectin and sickle cell disease severity for subjects in the steady state.
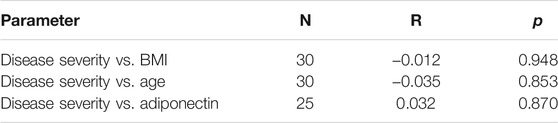
TABLE 3. Correlation studies of BMI, age, and adiponectin levels with disease severity in subjects with sickle cell anemia in the steady state.
Discussion
We have shown from this study that the mean serum adiponectin levels were not significantly different amongst the subjects from the different hemoglobin phenotypes, which is in contrast to the findings by Makis et al., who reported elevated adiponectin levels in steady state sickle cell anemia patients when compared with control subjects. This seems to suggest that in our data set of sickle cell anemia patients in the steady state, adiponectin does not constitute part of the endocrinopathy that affects these patients. Genetic variation could be the likely explanation of this difference between our data set and that of Makis et al. (2012).
The finding from this study reveals that the BMI is significantly decreased in sickle cell anemia in the steady state when compared with that of the control group (HbAA) is corroborated by Odetunde et al. (2016). Poor growth and nutrition are common in children with sickle cell anemia (SCA), which was demonstrated by Oredugba et al. in which the nutritional status in children with SCA negatively affected the anthropometric status, disease severity, and body composition of the patients (Oredugba, 2002). Some have hypothesized that underweight in SCA is caused by a hypermetabolic state, which increases energy demand that may lead to an undernourished state if not offset by increased nutrient consumption (Hibbert et al., 2006). Increased expenditure of energy at rest is a major metabolic change associated with HbSS. Barden et al. reported that energy expenditure at rest is approximately 15–20% higher in adolescents with HbSS than HbAA (Barden et al., 2000). This study showed no significant disparity in the BMI of subjects in the steady state when compared to those in crisis resolution, which is in agreement with the study by Iwalokun et al. (2011). This may be because the crisis duration may not be long enough to significantly alter the patients weight.
Although Makis et al. found a positive correlation between adiponectin and inflammatory markers, we found no correlation between adiponectin and disease severity. This may be because our disease severity scoring system is a composite of many parameters and therefore is more robust in predicting actual disease severity.
Our finding agrees with the report by Hall et al. (2018) which showed no association between BMI of sickle cell anemia subjects and disease severity. Also, no correlation was found between age and disease severity, which contrasts with the study by Adegoke and Kuti (2013), who found a positive correlation between age and disease severity.
Conclusion
Our data suggest that adiponectin does not seem to constitute part of sickle cell endocrinopathy in steady state sickle cell anemia patients at Nnewi, Southeast Nigeria. Also from the study, we found that the subjects with sickle cell anemia in crisis resolution and steady states had lower BMI values than HbAA and HbAS subjects. We therefore recommend that the nutritional needs of patients living with sickle cell anemia should be given more attention to improve the general well-being of the patients.
Limitation of the Study
Although this study has presented some insight into the dynamics of adiponectin in SCA clearly, it is limited because a larger longitudinal study starting from when the patients are in steady state through crisis state would have given further insight into the dynamics of adiponectin and BMI in these patients, thereby making for a better patient treatment, monitoring, and outcome.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics Statement
The ethical approval for this research was obtained from Nnamdi Azikiwe University Teaching Hospital ethics committee, and written informed consent was sought and received from the subjects or their caregivers.
Author Contributions
CEO designed the work, wrote the manuscript, and reviewed the manuscript. PO designed the work and wrote the manuscript. CI collected data, performed the experiments, and wrote the manuscript. UO wrote the manuscript. CO performed the statistical analysis. CE wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past collaboration with one of the authors CEO.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adegoke, S. A., and Kuti, B. P. (2013). Evaluation of Clinical Severity of Sickle Cell Anemia in Nigerian Children. J. Appl. Hematol. 4, 58–64.
Akinyanju, O. O. (2015). A Profile of Sickle Cell Disease in Nigeria. Ann. N. Y Acad. Sci. 565, 126–136. doi:10.1111/j.1749-6632.1989.tb24159.x
Barden, E. M., Zeme, B. S., and Kawchak, D. A. (2000). Total and Resting Energy Expenditure in Children with Sickle Cell Disease. J. Pediatr. 136, 73–79. doi:10.1016/s0022-3476(00)90053-2
Bełtowski, J., Jamroz-Wisniewska, A., and Widomska, S. (2008). Adiponectin and its Role in Cardiovascular Diseases. Cardiovasc. Hematological Disord. Drug Targets 8, 7–46. doi:10.2174/187152908783884920
Chandrasekar, B., Boylston, W. H., Venkatachalam, K., Webster, N. J., Prabhu, S. D., and Valente, A. J. (2008). Adiponectin Blocks Interleukin-18-Mediatedendothelial Cell Death via APPL1-dependent AMP-Activated Protein Kinase (AMPK) Activation and IKK/NF-kappaB/PTEN Suppression. J. Biol. Chem. 283, 24889–24898. doi:10.1074/jbc.m804236200
Chen, J., Tan, B., Karteris, E., Zervou, S., Digby, J., Hillhouse, E. W., et al. (2006). Secretion of Adiponectin by Human Placenta: Differential Modulation of Adiponectin and its Receptors by Cytokines. Diabetologia 49 (6), 1292–1302. doi:10.1007/s00125-006-0194-7
Cui, J., Panse, S., and Falkner, B. (2011). The Role of Adiponectin in Metabolic and Vascular Disease: a Review. Clin. Nephrol. 75, 6–33.
Díez, J. J., and Iglesias, P. (2003). "The Role of the Novel Adipocyte-Derived Hormone Adiponectin in Human Disease”. Eur. J. Endocrinol. 148(3), 293–300. doi:10.1530/eje.0.1480293
Hall, R., Gardner, K., Rees, D. C., and Chakravorty, S. (2018). High Body Mass Index in Children With Sickle Cell Disease: A Retrospective Single Centre Audit. Brit. Med. J. Paediat. 2 (1), e000302.
Hibbert, J. M., Hsu, L. L., Bhathena, S. J., Irune, I., Sarfo, B., Creary, M. S., et al. (2006). Proinflammatory Cytokines and the Hypermetabolism of Children with Sickle Cell Disease. Exp. Biol. Med. (Maywood) 230, 68–746. doi:10.1177/153537020523000109
Hotta, K., Funahashi, T., Arita, Y., Takahashi, M., Matsuda, M., and Okamoto, Y. (2000). Plasma Concentrations of a Novel, Adipose-specific Protein, Adiponectin, in Type 2 Diabetic Patients. Arteriosclerosis Thromb. Vasc. Biol. 20, 1595–1599. doi:10.1161/01.atv.20.6.1595
Iwalokun, A. B., Iwalokun, O. S., Hodonu, O. S., Aina, O. A., and Phillip, U. (2011). Agomo. Serum Levels of Leptin in Nigerian Patients with Sickle Cell Anemia. Blood Disord. 11, 2. doi:10.1186/1471-2326-11-2
Keikhaei, B., Mohseni, A. R., Norouzirad, R., Alinejadi, M., Ghanbari, S., Shiravi, F., et al. (2013). Altered Levels of Pro-inflammatory Cytokines in Sickle Cell Disease Patients during Vaso-Occlusive Crises and the Steady State Condition. Eur. Cytokine Netw. 24 (1), 45–52. doi:10.1684/ecn.2013.0328
Makis, A., Anna, C., Freideriki, K., Eleftheria, H., Xanthi, P., and Evangelos, B. (2012). Leptin and Adiponectin Blood Levels in Patients with Steady State Sickle Cell Disease Are Related to Chronic Hemolytic and Inflammatory Biomarkers. Blood 120.
Malcolm, K. (2015). Why Being 'overweight' Means You Live Longer: The Way Scientists Twist the Facts. Availableat: https://www.independent.co.uk (Accessedon August 8, 2019).
Odetunde, O. I., Chinawa, J. M., Achigbu, K. I., and Achigbu, E. O. (2016). Body Mass Index and Other Anthropometric Variables in Children with Sickle Cell Anemia. Pak J. Med. Sci. 32 (2), 341–346. doi:10.12669/pjms.322.9046
Okocha, C. E., Manafa, P. O., Ozomba, J. O., Ulasi, T. O., Chukwuma, G. O., and Aneke, J. C. (2014). C-reactive Protein and Disease Outcome in Nigerian Sickle Cell Disease Patients. Ann. Med. Health Sci. Res. 4, 701–705. doi:10.4103/2141-9248.141523
Okocha, E. C., Manafa, O. P., Aneke, C. J., Onwuzuruike, E. C., and Ibeh, C. N. (2017). Serum Superoxide Dismutase Activity: A Predictor of Disease Severity in Nigerian Sickle Cell Anemia Patients in Steady State. Med. J. DY Patil Univ. 10, 406–411. doi:10.4103/MJDRDYPU.MJDRDYPU_90_17
Oredugba, F. A. (2002). Anthropometric Findings in Nigerian Children with Sickle Cell Disease. Pediatr. Dentistry 24, 321–325.
Ouchi, N., and Walsh, K. (2017). Adiponectin as an Anti-inflammatory Factor. Clinica Chim. Acta 380, 24–30. doi:10.1016/j.cca.2007.01.026
Piel, F. B., Steinberg, M. H., and Rees, D. C. (2017). Sickle Cell Disease. N. Engl. J. Med. 376, 1561–1573. doi:10.1056/nejmra1510865
Platt, O. S. (2000). Sickle Cell Anemia as an Inflammatory Disease. J. Clin. Invest. 106, 337–338. doi:10.1172/jci10726
Serjeant, G. R. (1997). Sickle-cell Disease. The Lancet 350, 725–730. doi:10.1016/s0140-6736(97)07330-3
Thomas, P. W., Singhal, A., Hemmings, M., and Graham, S. (2000). Height and Weight Reference Curves for Homozygous Sickle Cell Disease. Arch. Dis. Child 82, 204–208. doi:10.1136/adc.82.3.204
Yamaguchi, N., Argueta, J. G., Masuhiro, Y., Kagishita, M., Nonaka, K., Saito, T., et al. (2005). Adiponectin Inhibits Toll-like Receptor Family-Induced Signaling. FEBS Lett. 579, 6821–6826. doi:10.1016/j.febslet.2005.11.019
Keywords: adiponectin, sickle cell anemia, body mass index, sickle cell disease severity, endocrinopathy
Citation: Okocha CE, Manafa PO, Igwe CN, Okite UP, Onah CE and Efobi C (2022) Adiponectin and Disease Severity in Sickle Cell Anemia Patients Attending a Tertiary Health Institution in Nnewi, Southeast Nigeria. Front. Genet. 13:799425. doi: 10.3389/fgene.2022.799425
Received: 21 October 2021; Accepted: 28 January 2022;
Published: 23 February 2022.
Edited by:
Vivian Paintsil, Kwame Nkrumah University of Science and Technology, GhanaReviewed by:
Prasenjit Mitra, Post Graduate Institute of Medical Education & Research (PGIMER), IndiaYvonne Dei-Adomakoh, University of Ghana, Ghana
Mathias Emokpae, University of Benin, Nigeria
Copyright © 2022 Okocha, Manafa, Igwe, Okite, Onah and Efobi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chide Emmanuel Okocha, b255aWNoaWRlb2tvY2hhQHlhaG9vLmNvbQ==
 Chide Emmanuel Okocha
Chide Emmanuel Okocha Patrick O. Manafa2
Patrick O. Manafa2 Chioma Nkechinyere Igwe
Chioma Nkechinyere Igwe