
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 05 April 2022
Sec. Human and Medical Genomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.796444
This article is part of the Research Topic Cancer systems biology View all 16 articles
 Rong Sun 1
Rong Sun 1 Ryosuke Tanino 1
Ryosuke Tanino 1 Xuexia Tong 2
Xuexia Tong 2 Minoru Isomura 3
Minoru Isomura 3 Li-Jun Chen 4
Li-Jun Chen 4 Takamasa Hotta 1
Takamasa Hotta 1 Tamio Okimoto 1
Tamio Okimoto 1 Megumi Hamaguchi 1
Megumi Hamaguchi 1 Shunichi Hamaguchi 1
Shunichi Hamaguchi 1 Yasuyuki Taooka 5
Yasuyuki Taooka 5 Takeshi Isobe 1
Takeshi Isobe 1 Yukari Tsubata1*
Yukari Tsubata1*The single nucleotide polymorphisms of COX-2 gene, also known as PTGS2, which encodes a pro-inflammatory factor cyclooxygenase-2, alter the risk of developing multiple tumors, but these findings are not consistent for lung cancer. We previously reported that the homozygous COX-2 –1195A genotype is associated with an increased risk for chronic obstructive pulmonary disease (COPD) in Japanese individuals. COPD is a significant risk factor for lung cancer due to genetic susceptibility to cigarette smoke. In this study, we investigated the association between COX-2 –1195G/A polymorphism and lung cancer susceptibility in the Japanese population. We evaluated the genotype distribution of COX-2 –1195G/A using a polymerase chain reaction-restriction fragment length polymorphism assay for 330 newly diagnosed patients with lung cancer and 162 healthy controls. Our results show that no relationship exists between the COX-2 –1195G/A polymorphism and the risk of developing lung cancer. However, compared to the control group, the homozygous COX-2 –1195A genotype increased the risk for lung squamous cell carcinoma (odds ratio = 2.902; 95% confidence interval, 1.171–7.195; p = 0.021), whereas no association is observed with the risk for adenocarcinoma. In addition, Kaplan-Meier analysis shows that the genotype distribution of homozygous COX-2 –1195A does not correlate with the overall survival of patients with lung squamous cell carcinoma. Thus, we conclude that the homozygous COX-2 –1195A genotype confers an increased risk for lung squamous cell carcinoma in Japanese individuals and could be used as a predictive factor for early detection of lung squamous cell carcinoma.
Lung cancer has the highest morbidity and mortality among cancers worldwide (Bray et al., 2018). There are many environmental risk factors for lung cancer and the most common is tobacco smoking (de Groot et al., 2018). However, exposure to environmental risk factors and genetic susceptibility are necessary for the development of lung carcinogenesis (Barta et al., 2019). Therefore, identifying genetic variants is vital for early prevention and screening of lung cancer.
Chronic inflammation plays an important role in carcinogenesis. Airway injuries related to inflammation caused by tobacco smoke or other environmental exposures increase the risk of developing lung cancer (Hecht, 2008). Moreover, inflammation-related genetic variants are highly associated with carcinogenesis for various cancers, including lung cancer (Rudnicka et al., 2019; Tan et al., 2019; Xiong et al., 2019).
The expression level of cyclooxygenase-2, a pro-inflammatory factor encoded by the COX-2 gene, is negligible in normal cells (Gurram et al., 2018). However, it is commonly overexpressed in various types of cancers and is implicated in the tumorigenesis, proliferation, metastasis, prognosis, and treatment of cancers (Tomozawa et al., 2000; Liu et al., 2001; Patti et al., 2002; Li et al., 2020). COX-2 is also involved in the molecular pathogenesis of chronic lung diseases (Park and Christman, 2006). Polymorphisms in the COX-2 gene alter the risk for chronic lung disease (Szczeklik et al., 2004; Xaubet et al., 2010). Three single nucleotide polymorphisms (SNPs) of the potential function, –1290G/A (rs689465), –1195G/A (rs689466), and –765G/C (rs20417), were identified in the COX-2 gene in esophageal cancer (Zhang et al., 2005). We previously reported that the homozygous COX-2 –1195A genotype is associated with an increased risk for chronic obstructive pulmonary disease (COPD) in the Japanese population (Chen et al., 2013). COPD is the single risk factor identified for the development of lung cancer after smoking exposure (Young et al., 2009). Chronic inflammation increases the risk for lung cancer by 2- to 3- fold in patients with COPD (Koshiol et al., 2009; Schwartz et al., 2016). Meta-analysis suggests that the emphysema detected visually on chest computed tomography (CT) and reduced forced expiratory volume in 1 s (FEV1) have strong effect on the increased odds of developing lung cancer (Wasswa-Kintu et al., 2005; Smith et al., 2012). Genetic analysis suggests that the genetic risk factors predisposing smokers to COPD and lung cancer may overlap (Young and Hopkins, 2011), and the key inflammatory-related genes and pathways impact the risk for lung cancer in a COPD-dependent manner (Watza et al., 2020). COX-2 is reported to be one of the candidate susceptibility genes related to inflammation involved in both COPD and lung cancer (Sekine et al., 2012).
The COX-2 –1195G/A gene polymorphism is functional and associated with an increased risk for various human cancers; however, the results are controversial in lung cancer (Zhang et al., 2005; Dong et al., 2010; Coskunpinar et al., 2011; Tang et al., 2011; Moraes et al., 2017). Therefore, this case-controlled study aimed to investigate the association between the COX-2 –1195G/A gene polymorphism and lung cancer susceptibility in the Japanese population.
This study included 492 participants from the Japanese population. The enrolled 330 patients with lung cancer were newly diagnosed at the Shimane University Hospital or Higashi Hiroshima Medical Center between 2009 and 2012. The lung cancer cases consisted of 221 patients with lung adenocarcinoma, 85 patients with lung squamous cell carcinoma, 9 patients with small cell lung cancer, and 15 patients with the other types. The 162 healthy controls were randomly selected from participants who received an annual health screening at the Shimane Institute of Health Science between 2009 and 2012. Those who were diagnosed with any cancer or any respiratory disease should be excluded from the controls. Ethical approval was obtained from the Institutional Review Board at the Shimane University Faculty of Medicine and the Higashi Hiroshima Medical Center (approved number 1022). Each enrolled participant signed an informed consent form.
DNA from enrolled participants was isolated from whole blood. A polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay was used for COX-2 polymorphism determination. The PCR reactions were performed in a reaction mixture system volume of 50 μl that contained 2.5 U Taq and 1 μl template DNA at a concentration of 50–150 ng/ml. The genotype of COX-2 –1195G/A was determined using the following specific primers: 5′- CCC TGA GCA CTA CCC ATG AT -3′ (forward) and 5′- GCC CTT CAT AGG AGA TAC TGG -3′ (reverse). The PCR cycling program was as follows: incubation at 96°C for 5 min followed by 32 cycles of 96°C for 20 s, 52°C for 30 s, and 72°C for 30 s with a final extension at 72°C for 6 min. The COX-2 273 bp PCR product was digested into 220 bp and 53 bp fragments with PvuII for the –1195G allele (restriction products: AA, 273 bp; GG, 220 bp + 53 bp; GA, 220 bp + 53 bp + 273 bp). The digested products were observed in a 2% agarose gel stained with ethidium bromide, and images were obtained under ultraviolet light.
Data are presented as the number (%) of participants. All statistical analyses were performed using SPSS Statistics version 27.0 (IBM, Armonk, NY, United States). Demographic characteristics were analyzed using a Mann-Whitney U-test or a Chi-squared test. The distribution of genotypes was assessed using the Hardy-Weinberg equilibrium. Differences in the genotype distribution of COX-2 gene were analyzed using a Chi-squared test. The association between the genotype distribution of COX-2 –1195G/A and lung cancer risk was estimated using odds ratios (ORs) and a 95% confidence interval (95% CI) that were computed using logistic regression analysis. Survival distributions were estimated using Kaplan-Meier analysis and compared using the log-rank test. P
The demographic characteristics of all study participants are summarized in Table 1, which includes age, sex, and smoking history. We observed the lung cancer group had a higher median age than that of the control group (p
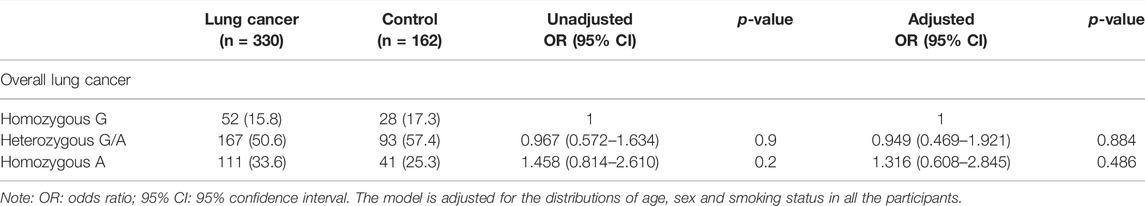
TABLE 3. The genotype distribution of COX-2 –1195G/A in patients with lung cancer and control participants.
No association on the risk of developing lung cancer with the genotype distribution of COX-2 –1195G/A was observed between the lung cancer and the control groups in both unadjusted analyses and adjusted analyses with age, sex, and smoking status (Table 3). Even though stratified by smoking status or sex in this study, we did not observe any association on the genotype distribution of COX-2 –1195G/A with the risk for lung cancer in both unadjusted and adjusted analyses with their respective factors (Table 4 and Table 5). In addition, no significant difference of the genotype distribution of COX-2 –1195G/A was found among the disease stages and the controls (Table 6). In the subgroup analyses, no increased risk was obsearved on the genotype distribution of the homozygous –1195A compared to the homozygous –1195G in the patients with stage IV (OR = 0.664; 95% CI, 0.346–1.272; p = 0.271). However, the multinomial logistic regression analysis represented that the genotype distribution of COX-2 –1195G/A was associated with the increasing risk for squamous cell carcinoma while not associated with adenocarcinoma (Table 7). The homozygous –1195A genotype increased the risk of 2.902 times for squamous cell carcinoma than the homozygous –1195G genotype (OR = 2.902; 95% CI, 1.171–7.195; p = 0.021). While no significant difference was observed from the heterozygous –1195G/A genotype for developing squamous cell carcinoma (OR = 1.618; 95% CI, 0.681–3.843; p = 0.275). To assess whether the prognosis was affected by the genotype of COX-2 –1195G/A, we compared the overall survival (OS). The median OS did not correlate with the genotype distribution of COX-2 –1195G/A among patients with squamous cell carcinoma (log-rank: p = 0.299) (Figure 1). In addition, the clinicopathology and sex were not related to the OS in patients with lung cancer (Supplementary Figure S1 and Supplementary Figure S2). Furthermore, there is no significant difference in the OS between the genotype of COX-2 –1195G/A stratified by sex (Supplementary Figure S3 and Supplementary Figure S4). Based on the above outcome, we summarized that the homozygous COX-2 –1195A genotype might increase the risk for lung squamous cell carcinoma in the Japanese population but no effect on the prognosis of squamous cell carcinoma.
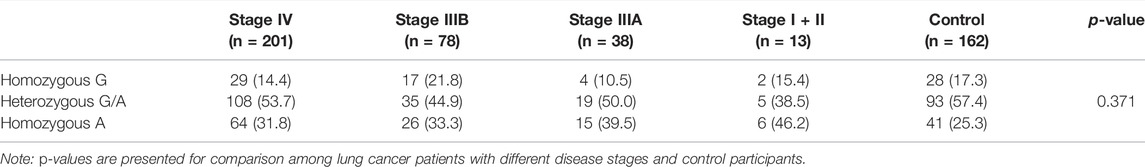
TABLE 6. The comparison on the genotype distribution of COX-2 –1195G/A gene among lung cancer patients with different disease stages and control participants.
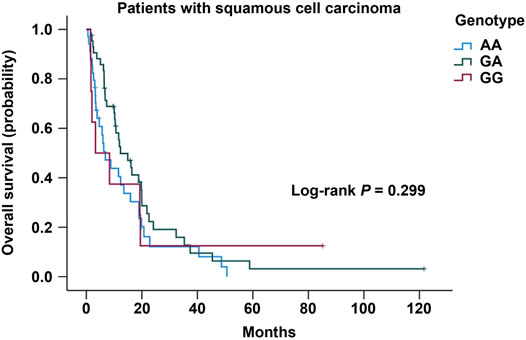
FIGURE 1. Kaplan-Meier analysis of overall survival in patients with lung squamous cell carcinoma stratified by the genotype of COX-2 –1195G/A.
This study analyzed the association between genotypes of the COX-2 –1195G/A polymorphism and different clinicopathology of lung cancer, and the results demonstrate that the homozygous COX-2 –1195A genotype was associated with the increased risk of developing lung squamous cell carcinoma. To date, the related studies have inconsistent results. The homozygous COX-2 –1195A genotype increased the risk of lung cancer development in the Turkish population, wherein patients with lung squamous cell carcinoma represented 53.2% (Coskunpinar et al., 2011). By contrast, a study from the Brazilian population concluded that the COX-2 –1195G/A polymorphism was not associated with the risk for lung cancer (Moraes et al., 2017), which is in agreement with the results from our present study. On the other hand, the number of patients diagnosed with lung squamous cell carcinoma observed in the Brazilian study is 39.4% (Moraes et al., 2017), and that of our study is 25.8%. Based on these results, we hypothesized that the discrepancy observed in the number of patients with homozygous COX-2 –1195A might be due to the different distribution of lung cancer clinicopathology. Indeed, our results reveal that the homozygous COX-2 –1195A genotype increases the risk for lung squamous cell carcinoma.
Increased levels of COX-2 expression were observed in bronchial precursors of squamous cell carcinoma using immunohistochemistry (Petkova et al., 2004; Mascaux et al., 2005). The substitution of –1195G
We previously demonstrated that the homozygous COX-2 –1195A genotype increased the risk for COPD in Japanese individuals (Chen et al., 2013). A Swedish study showed that the association with a lower FEV1 was higher for patients with lung squamous cell carcinoma than those with lung adenocarcinoma (Purdue et al., 2007). Further, the presence of emphysema, a typical manifestation of COPD on a chest CT scan, is associated with significantly increased odds of developing squamous cell carcinoma (Wang et al., 2018). Moreover, smoking is a major risk factor in the pathogenesis of lung squamous cell carcinoma and COPD, and it upregulates inflammation-related genes, including COX-2, in tracheal smooth muscle cells (Yang et al., 2009). Therefore, a potential link might exist between the functional COX-2 SNPs, COPD, and lung cancer, particularly for lung squamous cell carcinoma (Figure 2) (Lee et al., 2009). Considering the possible relationship between pulmonary function, emphysema CT scan parameters, smoking status, and COX-2 –1195A homozygosity, further studies are required.
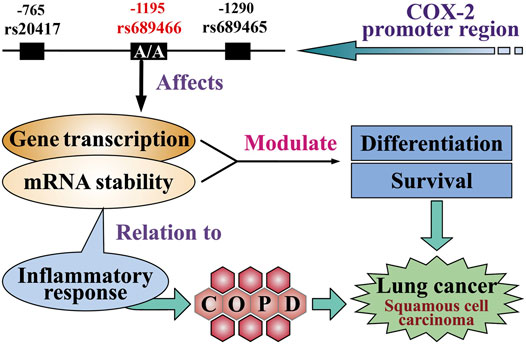
FIGURE 2. Potential function of the COX-2 single nucleotide polymorphisms in lung cancer. The genotype of homozygous COX-2 –1195A in the COX-2 promoter region affects gene transcription, thereby increasing the expression of COX-2 in lung cells. The inflammatory response is related to mRNA stability. Both factors enhance the level of differentiation of lung cells and promote the development of lung squamous cell carcinoma. The inflammatory response also leads to chronic obstructive pulmonary disease and lung cancer.
We did not observe any association between the risk for lung cancer and the COX-2 –1195G/A polymorphism stratified by smoking status. By contrast, a previous study from the Taiwanese population reported that the enrolled patients who smoked and carried the A allele of rs2066826 in the COX-2 intron 6 had an increased risk of 2.21 for lung cancer. (Liu et al., 2010). Further studies are needed to comprehensively analyze the functional COX-2 polymorphisms in addition to geographic populations.
The relationship between COX-2, COPD, and lung cancer is complicated. Epithelial-to-mesenchymal transition (EMT) is critical for lung carcinogenesis and observation of a malignant phenotype, and inhibition of COX-2 reverses EMT-induced changes in lung cancer patients (Dohadwala et al., 2006; Peebles et al., 2007). EMT in COPD and the resultant association with the risk for lung cancer have not been completely elucidated. Fundamental research is necessary to identify the molecular mechanisms linking these diseases.
Genotyping patients and identifying those with homozygous COX-2 –1195A could be combined with identifying emphysema using chest CT scans to serve as predictive markers for the early prevention and screening of lung squamous cell carcinoma. The increased odds of developing lung cancer in the presence of emphysema on CT may prove to be useful in targeting resources for the prevention and screening of lung squamous cell carcinoma. In addition, our findings suggest that either shared host susceptibility or an uncharacterized novel mechanism promotes the pathogenesis of both COPD and lung squamous cell carcinoma. It is necessary to further explore the benefit of clinical interventions to prevent or detect lung cancer after a patient is diagnosed with emphysema.
On the other hand, it has been reported that high levels of COX-2 mRNA transcription are associated with a more aggressive phenotype and poor prognosis for patients with non-small cell lung cancer (NSCLC) (Brabender et al., 2002). The homozygous COX-2 –1195A genotype is associated with poor overall survival in Chinese patients with NSCLC treated with chemoradiotherapy or radiotherapy alone (Bi et al., 2010). Although the homozygous COX-2 –1195A increased the risk for lung squamous cell carcinoma, this genotype did not correlate with poor prognosis in our study when evaluated using median overall survival. One reason for this discrepancy might be the fact that certain genetic markers are ethnicity-specific; another reason might be that different treatment regimens play a role in the prognosis of lung cancer and influence the effects of the COX-2 genotypes.
The three limitations of this study are listed as follows: the number of enrolled participants was low; only patients from the Japanese population were included; it was an imbalance of the baseline characteristics between the patients with lung cancer and the control participants.
In conclusion, the homozygous COX-2 –1195A increased the risk of developing lung squamous cell carcinoma and might be used as a predictive marker for early detection and screening of lung squamous cell carcinoma in Japanese individuals, but not as a predictive marker for the prognosis.
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available from Shimane university hospital but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Requests to access these datasets should be directed to Yukari Tsubata, ytsubata@med.shimane-u.ac.jp.
The studies involving human participants were reviewed and approved by the Institutional Review Board at the Shimane University Faculty of Medicine and the Higashi Hiroshima Medical Center (approved number 1022). The patients/participants provided their written informed consent to participate in this study.
RS, RT, TI, and YT: conception, design, supervision, resources, manuscript writing, revising, and editing. MI and LC: experiments conduction and data analysis. RS, MI, RT, XT, TH, TO, MH, SH, and YT: data visualization and statistical analysis. All authors contributed to the article and approved the submitted version.
TI reports personal fees from Boehringer Ingelheim, Pfizer, AstraZeneca and Daiichi-Sankyo outside the submitted work. YT reports personal fees from AstraZeneca, Daiichi-Sankyo, Chugai Pharmaceutical Co., Ltd. and Ono Pharmaceutical Co., Ltd. outside the submitted work. YT also reports scholarship grants from Pfizer Health Research Foundation outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
RS would like to thank the Otsuka Toshimi Scholarship Foundation for its support.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2022.796444/full#supplementary-material
Barta, J. A., Powell, C. A., and Wisnivesky, J. P. (2019). Global Epidemiology of Lung Cancer. Ann. Glob. Health 85. doi:10.5334/aogh.2419
Bi, N., Yang, M., Zhang, L., Chen, X., Ji, W., Ou, G., et al. (2010). Cyclooxygenase-2 Genetic Variants Are Associated with Survival in Unresectable Locally Advanced Non-small Cell Lung Cancer. Clin. Cancer Res. 16, 2383–2390. doi:10.1158/1078-0432.ccr-09-2793
Brabender, J., Park, J., Metzger, R., Schneider, P. M., Lord, R. V., Hölscher, A. H., et al. (2002). Prognostic Significance of Cyclooxygenase 2 mRNA Expression in Non-small Cell Lung Cancer. Ann. Surg. 235, 440–443. doi:10.1097/00000658-200203000-00017
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer J. clinicians 68, 394–424. doi:10.3322/caac.21492
Chen, L. J., Xu, W., Taooka, Y., Ohe, M., Takahashi, H., Sutani, A., et al. (2013). Cyclooxygenase 2 1195G > A Polymorphism Is Associated with Chronic Obstructive Pulmonary Disease in Japanese and Chinese Patients$\\gtrbin $. Chin. Med. J. (Engl) 126, 2215–2221.
Coskunpinar, E., Eraltan, I. Y., Turna, A., and Agachan, B. (2011). Cyclooxygenase-2 Gene and Lung Carcinoma Risk. Med. Oncol. 28, 1436–1440. doi:10.1007/s12032-010-9627-8
de Groot, P. M., Wu, C. C., Carter, B. W., and Munden, R. F. (2018). The Epidemiology of Lung Cancer. Transl. Lung Cancer Res. 7, 220–233. doi:10.21037/tlcr.2018.05.06
Dohadwala, M., Yang, S.-C., Luo, J., Sharma, S., Batra, R. K., Huang, M., et al. (2006). Cyclooxygenase-2-Dependent Regulation of E-Cadherin: Prostaglandin E2Induces Transcriptional Repressors ZEB1 and Snail in Non-small Cell Lung Cancer. Cancer Res. 66, 5338–5345. doi:10.1158/0008-5472.can-05-3635
Dong, J., Dai, J., Zhang, M., Hu, Z., and Shen, H. (2010). Potentially functionalCOX-2−1195G>A Polymorphism Increases the Risk of Digestive System Cancers: A Meta-Analysis$\\gtrbin $A Polymorphism Increases the Risk of Digestive System Cancers: A Meta-Analysis. J. Gastroenterol. Hepatol. 25, 1042–1050. doi:10.1111/j.1440-1746.2010.06293.x
Gurram, B., Zhang, S., Li, M., Li, H., Xie, Y., Cui, H., et al. (2018). Celecoxib Conjugated Fluorescent Probe for Identification and Discrimination of Cyclooxygenase-2 Enzyme in Cancer Cells. Anal. Chem. 90, 5187–5193. doi:10.1021/acs.analchem.7b05337
Hecht, S. S. (2008). Progress and Challenges in Selected Areas of Tobacco Carcinogenesis. Chem. Res. Toxicol. 21, 160–171. doi:10.1021/tx7002068
Koshiol, J., Rotunno, M., Consonni, D., Pesatori, A. C., De Matteis, S., Goldstein, A. M., et al. (2009). Chronic Obstructive Pulmonary Disease and Altered Risk of Lung Cancer in a Population-Based Case-Control Study. PloS one 4, e7380. doi:10.1371/journal.pone.0007380
Lee, G., Walser, T. C., and Dubinett, S. M. (2009). Chronic Inflammation, Chronic Obstructive Pulmonary Disease, and Lung Cancer. Curr. Opin. Pulm. Med. 15, 303–307. doi:10.1097/mcp.0b013e32832c975a
Li, M., Li, M., Wei, Y., and Xu, H. (2020). Prognostic and Clinical Significance of Cyclooxygenase-2 Overexpression in Endometrial Cancer: a Meta-Analysis. Front. Oncol. 10, 1202. doi:10.3389/fonc.2020.01202
Liu, C. H., Chang, S.-H., Narko, K., Trifan, O. C., Wu, M.-T., Smith, E., et al. (2001). Overexpression of Cyclooxygenase-2 Is Sufficient to Induce Tumorigenesis in Transgenic Mice. J. Biol. Chem. 276, 18563–18569. doi:10.1074/jbc.m010787200
Liu, C. J., Hsia, T. C., Wang, R. F., Tsai, C. W., Chu, C. C., Hang, L. W., et al. (2010). Interaction of Cyclooxygenase 2 Genotype and Smoking Habit in Taiwanese Lung Cancer Patients. Anticancer Res. 30, 1195–1199.
Mascaux, C., Martin, B., Verdebout, J.-M., Ninane, V., and Sculier, J.-P. (2005). COX-2 Expression during Early Lung Squamous Cell Carcinoma Oncogenesis. Eur. Respir. J. 26, 198–203. doi:10.1183/09031936.05.00001405
Moraes, J. L., Moraes, A. B., Aran, V., Alves, M. R., Schluckbier, L., Duarte, M., et al. (2017). Functional Analysis of Polymorphisms in the COX-2 Gene and Risk of Lung Cancer. Mol. Clin. Oncol. 6, 494–502. doi:10.3892/mco.2017.1167
Park, G. Y., and Christman, J. W. (2006). Involvement of Cyclooxygenase-2 and Prostaglandins in the Molecular Pathogenesis of Inflammatory Lung Diseases. Am. J. Physiology-Lung Cell Mol. Physiol. 290, L797–L805. doi:10.1152/ajplung.00513.2005
Patti, R., Gumired, K., Reddanna, P., Sutton, L. N., Phillips, P. C., and Reddy, C. D. (2002). Overexpression of Cyclooxygenase-2 (COX-2) in Human Primitive Neuroectodermal Tumors: Effect of Celecoxib and Rofecoxib. Cancer Lett. 180, 13–21. doi:10.1016/s0304-3835(02)00003-4
Peebles, K. A., Lee, J. M., Mao, J. T., Hazra, S., Reckamp, K. L., Krysan, K., et al. (2007). Inflammation and Lung Carcinogenesis: Applying Findings in Prevention and Treatment. Expert Rev. anticancer Ther. 7, 1405–1421. doi:10.1586/14737140.7.10.1405
Petkova, D. K., Clelland, C., Ronan, J., Pang, L., Coulson, J. M., Lewis, S., et al. (2004). Overexpression of Cyclooxygenase-2 in Non-small Cell Lung Cancer. Respir. Med. 98, 164–172. doi:10.1016/j.rmed.2003.09.006
Purdue, M. P., Gold, L., Jarvholm, B., Alavanja, M. C. R., Ward, M. H., and Vermeulen, R. (2007). Impaired Lung Function and Lung Cancer Incidence in a Cohort of Swedish Construction Workers. Thorax 62, 51–56. doi:10.1136/thx.2006.064196
Ramsay, R. G., Barton, A. L., and Gonda, T. J. (2003). Targeting C-Myb Expression in Human Disease. Expert Opin. Ther. Targets 7, 235–248. doi:10.1517/14728222.7.2.235
Rudnicka, K., Backert, S., and Chmiela, M. (2019). Genetic Polymorphisms in Inflammatory and Other Regulators in Gastric Cancer: Risks and Clinical Consequences. Mol. Mech. Inflamm. induction, resolution escape by Helicobacter pylori, 53–76. doi:10.1007/978-3-030-15138-6_3
Schwartz, A. G., Lusk, C. M., Wenzlaff, A. S., Watza, D., Pandolfi, S., Mantha, L., et al. (2016). Risk of Lung Cancer Associated with COPD Phenotype Based on Quantitative Image Analysis. Cancer Epidemiol. Biomarkers Prev. 25, 1341–1347. doi:10.1158/1055-9965.epi-16-0176
Sekine, Y., Katsura, H., Koh, E., Hiroshima, K., and Fujisawa, T. (2012). Early Detection of COPD Is Important for Lung Cancer Surveillance. Eur. Respir. J. 39, 1230–1240. doi:10.1183/09031936.00126011
Smith, B. M., Pinto, L., Ezer, N., Sverzellati, N., Muro, S., and Schwartzman, K. (2012). Emphysema Detected on Computed Tomography and Risk of Lung Cancer: a Systematic Review and Meta-Analysis. Lung cancer 77, 58–63. doi:10.1016/j.lungcan.2012.02.019
Szczeklik, W., Sanak, M., and Szczeklik, A. (2004). Functional Effects and Gender Association of COX-2 Gene Polymorphism G-765C in Bronchial Asthma. J. Allergy Clin. Immunol. 114, 248–253. doi:10.1016/j.jaci.2004.05.030
Tan, N., Song, J., Yan, M., Wu, J., Sun, Y., Xiong, Z., et al. (2019). Association between IL‐4 Tagging Single Nucleotide Polymorphisms and the Risk of Lung Cancer in China. Mol. Genet. Genomic Med. 7, e00585. doi:10.1002/mgg3.585
Tang, Z., Nie, Z.-L., Pan, Y., Zhang, L., Gao, L., Zhang, Q., et al. (2011). The Cox-2 -1195 G > A Polymorphism and Cancer Risk: a Meta-Analysis of 25 Case-Control Studies$\\gtrbin $A Polymorphism and Cancer Risk: a Meta-Analysis of 25 Case-Control Studies. Mutagenesis 26, 729–734. doi:10.1093/mutage/ger040
Tomozawa, S., Tsuno, N. H., Sunami, E., Hatano, K., Kitayama, J., Osada, T., et al. (2000). Cyclooxygenase-2 Overexpression Correlates with Tumour Recurrence, Especially Haematogenous Metastasis, of Colorectal Cancer. Br. J. Cancer 83, 324–328. doi:10.1054/bjoc.2000.1270
Wang, W., Xie, M., Dou, S., Cui, L., Zheng, C., and Xiao, W. (2018). The Link between Chronic Obstructive Pulmonary Disease Phenotypes and Histological Subtypes of Lung Cancer: a Case–Control Study. Copd Vol. 13, 1167–1175. doi:10.2147/copd.s158818
Wasswa-Kintu, S., Gan, W., Man, S., Pare, P., and Sin, D. (2005). Relationship between Reduced Forced Expiratory Volume in One Second and the Risk of Lung Cancer: a Systematic Review and Meta-Analysis. Thorax 60, 570–575. doi:10.1136/thx.2004.037135
Watza, D., Lusk, C. M., Dyson, G., Purrington, K. S., Wenzlaff, A. S., Neslund‐Dudas, C., et al. (2020). COPD‐dependent Effects of Genetic Variation in Key Inflammation Pathway Genes on Lung Cancer Risk. Int. J. Cancer 147, 747–756. doi:10.1002/ijc.32780
Xaubet, A., Fu, W. J., Li, M., Serrano-Mollar, A., Ancochea, J., Molina-Molina, M., et al. (2010). A Haplotype of Cyclooxygenase-2 Gene Is Associated with Idiopathic Pulmonary Fibrosis. Sarcoidosis Vasc. Diffuse Lung Dis. 27, 121–130.
Xiong, Z., Sun, Y., Wu, J., Niu, F., Jin, T., and Li, B. (2019). Genetic Polymorphisms in IL1R1 and IL1R2 Are Associated with Susceptibility to Thyroid Cancer in the Chinese Han Population. J. Gene Med. 21, e3093. doi:10.1002/jgm.3093
Yang, C.-M., Lee, I.-T., Lin, C.-C., Yang, Y.-L., Luo, S.-F., Kou, Y. R., et al. (2009). Cigarette Smoke Extract Induces COX-2 Expression via a PKCα/c-Src/EGFR, PDGFR/PI3K/Akt/NF-κB Pathway and P300 in Tracheal Smooth Muscle Cells. Am. J. Physiology-Lung Cell Mol. Physiol. 297, L892–L902. doi:10.1152/ajplung.00151.2009
Young, R. P., Hopkins, R. J., Christmas, T., Black, P. N., Metcalf, P., and Gamble, G. D. (2009). COPD Prevalence Is Increased in Lung Cancer, Independent of Age, Sex and Smoking History. Eur. Respir. J. 34, 380–386. doi:10.1183/09031936.00144208
Young, R. P., and Hopkins, R. J. (2011). How the Genetics of Lung Cancer May Overlap with COPD. Respirology 16, 1047–1055. doi:10.1111/j.1440-1843.2011.02019.x
Keywords: cyclooxygenase-2, single nucleotide polymorphism, promoter region, lung cancer risk, squamous cell carcinoma, Japanese
Citation: Sun R, Tanino R, Tong X, Isomura M, Chen L-J, Hotta T, Okimoto T, Hamaguchi M, Hamaguchi S, Taooka Y, Isobe T and Tsubata Y (2022) The Association Between Cyclooxygenase-2 –1195G/A (rs689466) Gene Polymorphism and the Clinicopathology of Lung Cancer in the Japanese Population: A Case-Controlled Study. Front. Genet. 13:796444. doi: 10.3389/fgene.2022.796444
Received: 16 October 2021; Accepted: 17 February 2022;
Published: 05 April 2022.
Edited by:
Jesús Espinal-Enríquez, Instituto Nacional de Medicina Genómica (INMEGEN), MexicoReviewed by:
Benjamin Rybicki, Henry Ford Health System, United StatesCopyright © 2022 Sun , Tanino , Tong , Isomura , Chen , Hotta , Okimoto , Hamaguchi , Hamaguchi , Taooka , Isobe and Tsubata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yukari Tsubata, eXRzdWJhdGFAbWVkLnNoaW1hbmUtdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.