
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Genet. , 06 January 2023
Sec. Human and Medical Genomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.1102953
This article is part of the Research Topic Genomic alteration landscapes of aging, metabolic disorders, and cancer: emerging overlaps and clinical importance View all 12 articles
Editorial on the Research Topic
Genomic alteration landscapes of aging, metabolic disorders, and cancer: Emerging overlaps and clinical importance
The biology of aging, cancer, and various metabolic disorders shows a clear association with genetic and epigenetic changes. These genomic alterations arise from diverse intrinsic and extrinsic/environmental factors. The efficiency of a cell to proofread its newly synthesized DNA strand gradually decreases with age hampering its genomic integrity. An increased burden of genomic changes, therefore, gives rise to multiple health issues like metabolic disorders (Abou Ziki and Mani, 2016; Varshavi et al., 2018). However, on the other hand, recent studies provide evidence for the role of metabolic perturbations in accelerated aging (Spinelli et al., 2020). These transformations, following either way, involve diverse interactions between molecular players of aging, metabolism, and redox biology (including mitochondria fitness, Ca2+ signaling, and bioenergetics); all encrypted in the genomic sequence. Accumulation of irreversible genomic changes over a long time then leads to the onset and progression of cancer. Cancer cells have been shown to operate with reengineered metabolic processes to satisfy their surplus needs during uncontrolled proliferation (Liu et al., 2022). Therefore, aging, metabolic changes, and cancer exist as a network of crossroads (Tidwell et al., 2017; Golubev and Anisimov, 2019; Poljsak et al., 2019). These broadly categorized pathologies share common genomic signatures that further strengthen the link between aging, metabolic disorders, and cancer (Aunan et al., 2017; Lacroix et al., 2020). Along with metabolic alterations, occurrence of aberrant mutations in the mitochondrial genome is also a common characteristic of aging and cancer (Smith et al., 2022). Therefore, it becomes important to uncover the contribution of genomic changes in the context of these cellular health states and the sequential order that defines these states, if any. In line with this, the original research articles and reviews published in the present Research Topic focus on genomic alteration landscapes of aging, metabolic disorders and cancer, the existing and emerging overlaps, and its clinical importance for therapeutic interventions (Figure 1).
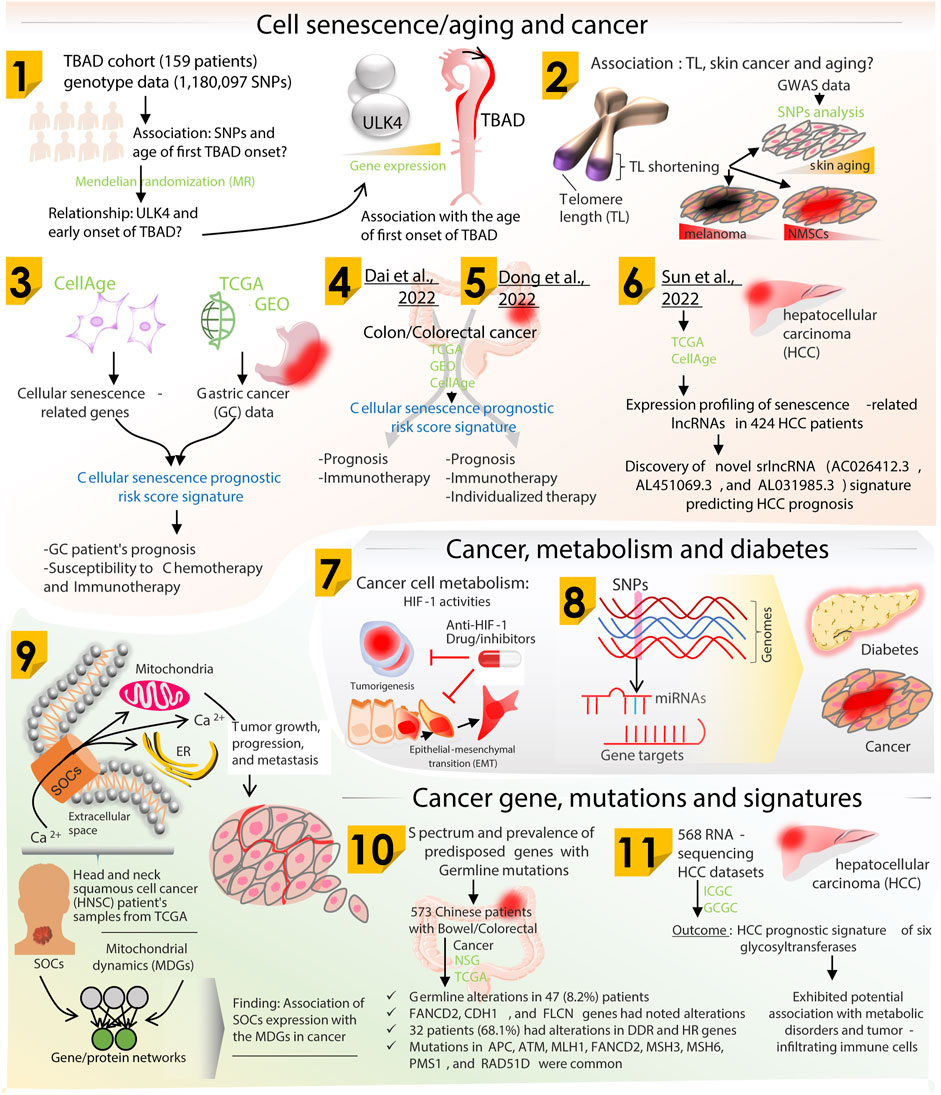
FIGURE 1. Schematic diagram showing research summarized majorly on three themes including cell senescence/aging and cancer, metabolism and cancer gene mutations/signatures. Research Topic articles are marked by serial number within the related schematic section.
The role of autophagy has been recently established in the pathogenesis of aortic dissection; however, the complete molecular mechanism has not been uncovered yet. In an original report, Huang et al. has studied the correlation between the family of ULK (UNC51-like enzymes) genes and the age of first onset of type B aortic dissection (TBAD). The authors analyzed the genome of 159 TBAD patients from Chinese population. A pool of 1,180,097 SNPs was included. Among the different ULK genes, only ULK4 was found to be significantly associated with the first onset age. They concluded that high level of ULK4 gene expression was related to delayed onset of TBAD among these patients. Further experimental validation of these findings can suggest ULK4 to be a diagnostic target for TBAD.
Telomere shortening is one of the important hallmarks of cellular senescence, however, telomere length related cellular senescence has been shown to have varying effects in different cancers. To delineate this paradoxical relationship, Son et al. made use of 42 telomere length associated SNPs, and performed Mendelian randomization analysis to explore the causal relationship between telomere length, skin aging and the susceptibility risk of different skin cancer types. The authors found that telomere shortening can promote aging of the skin and reduce the risk of cutaneous melanoma and non-melanoma skin cancer.
As mentioned before, cellular senescence has often been correlated to oncogenic activation and tumor suppression leading to cancer development. In line with this, Dai et al. has constructed a prognostic risk score signature using the senescence related genes differentially expressed in gastric cancer samples. A total of 135 such genes were identified with significant dys-regulation. Integration of survival data associated 24 of these genes with gastric cancer prognosis. Patients with high expression of SMARCA4 (gene with highest mutational frequency) were associated with higher overall survival and progression-free survival. A total of 11 genes were then identified using LASSO Cox regression analysis to develop the prognostic risk score signature. Testing using an independent data showed that this signature could accurately distinguish low-risk and high-risk samples. The authors further showed that the low-risk score group was also more susceptible to chemotherapy and immunotherapy, and hence can be used for better decision making for treatment to be given. Another study by Dai et al. on similar grounds looked into the cellular senescence related genes that can be used for prediction of prognosis and immunotherapy response in colon cancer patients. Dong et al. and Sun et al. also reported a senescence-related prognostic model that has been shown to predict the prognosis, immunotherapeutic response, and identify potential drug targets for colorectal and hepatocellular carcinoma patients, respectively. These studies show the potential of using huge amount of publicly available clinical data for learning and developing predictive models to design personalized treatment regimen for cancer patients.
Intracellular calcium levels play an important role in homeostasis and various cell signalling processes. Dysregulated levels of calcium have been shown to be remarkably associated with cancer growth, angiogenesis, and metastasis. Elevated serum calcium level is a proposed diagnostic marker for head and neck malignancy. In association with this, Hegde et al. carried out in silico analysis to demonstrate the role of store-operated calcium channels in regular mitochondrial function, and further suggest that alteration in these calcium channels might be a predictive and prognostic marker for head and neck squamous cell cancer patients.
Though cancer in general is thought to arise from accumulation of somatic mutations, they do have a substantial hereditary component. To look at the contribution of the pathogenic germline variants in the development of bowel cancer in Chinese population, Xie et al. analyzed the mutation profile of 573 patients accounting for various stages of bowel/colorectal cancer. The profiled germline mutations were categorized as pathogenic, likely-pathogenic and non-pathogenic. Some rare germline alterations in genes like ANCD2, CDH1, and FLCN were also observed. The other germline mutations were enriched in genes involved in DNA-damage repair and homologous recombination. Patients carrying germline mutations also showed a distinctive somatic mutation profile and tumor mutation burden, which also affected the overall survival of these patients. This study provides an assessment of a wider range of susceptibility genes in Chinese bowel/colorectal patients.
Along with accumulating genetic mutations, cancer cells also reprogram the other biochemical processes to generate conditions favorable for sustenance and continuous proliferation. Metabolic reprogramming to switch from oxidative phosphorylation to aerobic glycolysis is one of the major hallmarks of cancer. In this Research Topic of articles, Sharma et al. has presented a comprehensive review highlighting the role of hypoxia-inducible factor-1 (HIF-1) in imparting aggressive behavior in cancer cells through hypoxic glycolysis, and novel therapeutic strategies currently available for targeting HIF-1 in cancer.
Not only gene expression, but its regulation by non-coding RNAs like miRNA also plays a crucial role in onset and progression of various diseases. A review article from Chhichholiya et al. gives information about the reported single nucleotide polymorphisms in miRNA(s) and their target sequences known to be involved in cancer and diabetic pathologies.
In summary, the present Research Topic gathers original research and comprehensive reviews highlighting the genomic factors behind aging, metabolic perturbations and cancer. These studies confirm the interconnecting links between these pathologies, and the need to understand these to identify the cross-points that can be further explored for diagnosis, prognosis and other therapeutic interventions.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
DT is supported in part by National Institutes of Health (NIH) grant R00DK120876. AKA is supported by a Career Development Grant from the American Heart Association award number 19CDA34780005.
We thank all the contributing authors and reviewers for their valuable contributions to this Research Topic.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abou Ziki, M. D., and Mani, A. (2016). Metabolic syndrome: Genetic insights into disease pathogenesis. Curr. Opin. Lipidol. 27, 162–171. doi:10.1097/MOL.0000000000000276
Aunan, J. R., Cho, W. C., and Soreide, K. (2017). The biology of aging and cancer: A brief overview of shared and divergent molecular hallmarks. Aging Dis. 8, 628–642. doi:10.14336/AD.2017.0103
Golubev, A. G., and Anisimov, V. N. (2019). Aging and cancer: Is glucose a mediator between them? Oncotarget 10, 6758–6767. doi:10.18632/oncotarget.27344
Lacroix, M., Riscal, R., Arena, G., Linares, L. K., and Le Cam, L. (2020). Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 33, 2–22. doi:10.1016/j.molmet.2019.10.002
Liu, Y. H., Hu, C. M., Hsu, Y. S., and Lee, W. H. (2022). Interplays of glucose metabolism and KRAS mutation in pancreatic ductal adenocarcinoma. Cell Death Dis. 13, 817. doi:10.1038/s41419-022-05259-w
Poljsak, B., Kovac, V., Dahmane, R., Levec, T., and Starc, A. (2019). Cancer etiology: A metabolic disease originating from life's major evolutionary transition? Oxid. Med. Cell. Longev. 2019, 7831952. doi:10.1155/2019/7831952
Smith, A. L. M., Whitehall, J. C., and Greaves, L. C. (2022). Mitochondrial DNA mutations in ageing and cancer. Mol. Oncol. 16, 3276–3294. doi:10.1002/1878-0261.13291
Spinelli, R., Parrillo, L., Longo, M., Florese, P., Desiderio, A., Zatterale, F., et al. (2020). Molecular basis of ageing in chronic metabolic diseases. J. Endocrinol. Invest.. 43, 1373–1389. doi:10.1007/s40618-020-01255-z
Tidwell, T. R., Soreide, K., and Hagland, H. R. (2017). Aging, metabolism, and cancer development: From peto's paradox to the warburg effect. Aging Dis. 8, 662–676. doi:10.14336/AD.2017.0713
Keywords: genetic alterations, cancer, aging, metabolic disorder, mutations, senescence
Citation: Dhanjal JK, Kalra RS, Tomar D and Ajay AK (2023) Editorial: Genomic alteration landscapes of aging, metabolic disorders, and cancer: Emerging overlaps and clinical importance. Front. Genet. 13:1102953. doi: 10.3389/fgene.2022.1102953
Received: 19 November 2022; Accepted: 23 November 2022;
Published: 06 January 2023.
Edited and reviewed by:
Maxim B. Freidin, Queen Mary University of London, United KingdomCopyright © 2023 Dhanjal, Kalra, Tomar and Ajay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaspreet Kaur Dhanjal, amFzcHJlZXRAaWlpdGQuYWMuaW4=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.