- 1Key Laboratory of Freshwater Fisheries and Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi, China
- 2Wuxi Fisheries College, Nanjing Agricultural University, Wuxi, China
- 3National Demonstration Center for Experimental Fisheries Science Education, Shanghai Ocean University, Shanghai, China
Cyclin A (CycA) plays essential roles in regulating multiple steps of the cell cycle, and it affects gonad development in mammals and invertebrates. Previous RNA interference (RNAi) analysis revealed that knocking-down the expression of CycA in female oriental river prawns (Macrobrachium nipponense) inhibited ovarian development. CycA was also predicted to have regulatory roles in reproductive development of male M. nipponense based on significant changes of Mn-CycA expression after eyestalk ablation. The goal of this study was to investigate the potential functions of CycA in the reproductive development of male M. nipponense using RNAi and histological observations. Quantitative real-time PCR analysis revealed that both single-side and double-side eyestalk ablation stimulated the expressions of Mn-CycA, and the expression was higher in prawns with double-side eyestalk ablation (p < 0.05). Mn-CycA expression was significantly higher in the testis and androgenic gland during the reproductive season than during the non-reproductive season (p < 0.05). In the RNAi analysis, Mn-CycA expression significantly decreased after prawns were injected with dsCycA, and the expression of insulin-like androgenic gland hormone (Mn-IAG) also decreased as Mn-CycA expression decreased. This result indicated that CycA positively regulated the expression of IAG in M. nipponense. Histological observations revealed that the number of sperm decreased dramatically to <5% of the total cells in the testis of the dsCycA-treated group compared to that of control group on day 14, indicating that knockdown of Mn-CycA expression inhibited testis development by affecting the expression of Mn-IAG in M. nipponense. These results highlighted the functions of CycA in male reproductive development of M. nipponense, which can be applied to future studies of male reproduction in other crustacean species.
Introduction
The oriental river prawn (Macrobrachium nipponense) (Crustacea; Decapoda; Palaemonidae) is widely distributed in China and other Asian countries, where it inhabits freshwater and low salinity estuarine regions (Jin S. B. et al., 2021). It is an economically important freshwater prawn in China, with annual aquaculture production of 225,321 metric tons in 2019 (Zhang et al., 2020). Previous histological observations revealed that newly hatched male and female M. nipponense can reach sexual maturity within 40 days after hatching during the reproductive season (Jin et al., 2016). The rapid gonad development of hatchlings leads to inbreeding between young prawns, resulting in mating and propagation of multiple generations in the same ponds. This leads to prawns with smaller market size, thereby restricting the sustainable development of the M. nipponense industry (Jin et al., 2022). Thus, the long-term goal of genetic improvements in M. nipponense is to regulate the process of gonad development.
Jin S. B. et al. (2021) previously reported that the expression of Mn-Cyclin A (Mn-CycA) increased after the ablation of eyestalks from male M. nipponense. They also found that eyestalk ablation promoted testis development by stimulating the expression of insulin-like androgenic gland hormone (IAG) (Jin S. B. et al., 2021; Jin et al., 2021b). The negative regulatory relationship between CycA and eyestalks suggested that CycA may be involved in regulating male reproductive development in M. nipponense.
Cyclins are synthesized and degraded in a cell cycle-dependent fashion. They are involved in cell cycle regulation through the formation of integral regulatory subunits of protein kinase complexes with cyclin-dependent kinases (CDKs). CycA is involved in the regulation of multiple steps in the cell cycle, including the S and G2/M phases of Saccharomyces cerevisiae and animal cells (Tarn and Lai, 2011). CycA binds with CDK2 during G1/S conversion to promote replication of meiotic chromosomes and to enhance transcriptional activity of genes encoding estrogen and progesterone receptors (Rogatsky et al., 1986; Narayanan et al., 2005). CycA also forms a complex with CDK1 during the late S phase, and the compound activates and stabilizes cyclin B and CDK1 (Minshull et al., 1990; Lees and Harlow, 1993; Li et al., 2004).
The full-length cDNA sequence of Mn-CycA has been submitted to NCBI with the accession number of MT802360.1. In a previous study of M. nipponense, CycA expression was highest in the ovary and then testis, and the values were significantly higher than those of the other tested tissues, indicating the CycA may play regulatory roles in gonad development in M. nipponense (Zhou Z. Y. et al., 2021). RNA interference (RNAi) analysis in female M. nipponense revealed that knockdown of CycA expression inhibited ovarian development (Zhou Z. Y. et al., 2021). However, the regulatory roles of CycA on the reproductive development of male M. nipponense still needs to be investigated.
The goal of this study was to analyze the potential functions of CycA in male reproductive development of M. nipponense using quantitative real-time PCR (qPCR), in situ hybridization, RNAi, and histological observations. The results of this study highlighted the functions of CycA in M. nipponense, providing a basis for further studies of the mechanism of male reproduction in other crustacean species.
Materials and methods
Ethics statement
We obtained permission from the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Wuxi, China) to conduct all experiments involving M. nipponense.
The animal study was reviewed and approved by We obtained the permission from the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Wuxi, China) for all experiments involving M. nipponense. Written informed consent was obtained from the owners for the participation of their animals in this study.
The qPCR analysis
The qPCR was used to measure the relative mRNA expression of Mn-CycA in the testis after the ablation of eyestalks from male M. nipponense and the expression in the testis and androgenic gland of male prawns collected during the reproductive and non-reproductive seasons. Three hundred healthy male M. nipponense (body weight, 3.47–3.89 g) were collected from the Dapu M. nipponense Breeding Base in Wuxi, China (120°13′44″E, 31°28′ 22″N). The prawns were randomly divided into three groups: control (CG), single-side eyestalk ablation (SS), and double-side eyestalk ablation (DS). They were transferred into three 500 L tanks and maintained in aerated freshwater for 3 days before eyestalk ablation. The testes were collected from prawns in each group on days 1, 4, and 7 and immediately preserved in liquid nitrogen until used for qPCR analysis. Testes from five different prawns were pooled to form a biological replicate, and three biological replicates were analyzed for each time point.
For the temporal comparison, testis and androgenic gland were collected from male M. nipponense during the reproductive season in July, when the water temperature was 30 ± 2 °C and the illumination time was ≥16 h. Tissues were also collected during the non-reproductive season in January, when the water temperature was 13 ± 2 °C and illumination time was ≤12 h. Five prawns were pooled to form one biological replicate, and three biological replicates were analyzed.
Details about the procedures used for RNA isolation and cDNA synthesis were described in previous studies (Jin et al., 2014; Jin et al., 2018). Briefly, total RNA from each tissue was extracted using the UNlQ-10 Column TRIzol Total RNA Isolation Kit (Sangon, Shanghai, China) following the manufacturer’s protocol. The PrimeScript™ RT Reagent Kit (Takara, Shiga, Japan) was used to synthesize the cDNA template according to the manufacturer’s instructions. The UltraSYBR Mixture (CWBIO, Beijing, China) was used to determine the expression level of Mn-CycA in each tissue. All of the SYBR Green RT-qPCR assays were performed using the Bio-Rad iCycler iQ5 Real-Time PCR System (Bio-Rad, Hercules, CA, United States ), and the qPCR reaction was run for three technical replicates for each tissue. Table 1 lists the primers used for qPCR analysis. Eukaryotic translation initiation factor 5A was used as the reference gene (Hu et al., 2018). The relative mRNA expressions of Mn-CycA were calculated based on the 2−ΔΔCT comparative CT method.
In situ hybridization
In situ hybridization was used to determine the mRNA locations of Mn-CycA in the testis and androgenic gland in shrimp collected during the reproductive season in July. The tissues were fixed in paraformaldehyde (4%) (Sangon, Shanghai, China), and then they were embedded in paraffin and sliced into 4 µm thick sections for the analysis. Details about the primer design and in situ hybridization can be found in previous reports (Jin et al., 2018; Li et al., 2018; Jin et al., 2022). Briefly, the specific anti-sense (experimental group) and sense (control group) probes were designed using Primer5 software based on the cDNA sequence of Mn-CycA. The specific primers with a digoxigenin (DIG) tag were synthesized by Shanghai Sangon Biotech Company (Table 1). The Zytofast PLUS CISH Implementation Kit (Zyto Vision GmBH, Bremerhaven, Germany) was used to perform the in situ hybridization analysis of the sectioned tissues following the manufacturer’s protocol. Slides were examined under a light microscope (Olympus Corporation, Tokyo, Japan).
RNAi analysis
The potential function of CycA in reproductive development of male M. nipponense was investigated by RNAi. Snap Dragon tools (http://www.flyrnai.org/cgibin/RNAifind_primers.pl) was used to design the specific RNAi primer with a T7 promoter site based on the open reading frame of Mn-CycA (Table 1). The Mn-CycA dsRNA (dsCycA) was synthesized using the Transcript Aid™ T7 High Yield Transcription kit (Fermentas, Waltham, MA, United States ) following the manufacturer’s protocol. The green fluorescent protein dsRNA (dsGFP) was also synthesized and used as the negative control (Zhang et al., 2016).
Six hundred male M. nipponense were collected from the Dapu M. nipponense Breeding Base approximately 5 months after hatching (body weight, 3.15–4.37 g) and randomly divided into the dsCycA group (RNAi) and dsGFP group (control). The injected dose of dsCycA and dsGFP was 4 μg/g (Jiang et al., 2014; Li et al., 2018). Seven days after the first injection, 4 μg/g of dsCycA or dsGFP were injected again. Androgenic gland samples were collected from both the control group and the RNAi group on days 1, 7, and 14 after dsGFP or dsCycA injection. Androgenic gland samples from five different prawns at each time point were collected and pooled to form a biological replicate, and three biological replicates were tested. The Mn-CycA mRNA expression in the samples was measured by qPCR in order to confirm the silencing efficiency. The mRNA expression of Mn-IAG was also measured using the same cDNA templates to evaluate the regulatory relationship between CycA and IAG in M. nipponense.
Haematoxylin and eosin (HE) staining
Morphological differences in the testis between prawns in the control and RNAi groups were assessed by HE staining following previously reported procedures (ShangGuan et al., 1991; Ma et al., 2006). Briefly, the tissues were dehydrated in different concentrations of ethanol and then embedded in xylene and wax. The embedded tissues were sectioned to a thickness of 5 µm using a microtome (Leica, Wetzlar, Germany). The sectioned tissues were placed on a slide and stained with HE for 3–8 min. The slides were observed under an Olympus SZX16 microscope (Olympus Corporation, Tokyo, Japan).
Statistical analysis
All statistical analyses were conducted using SPSS Statistics 23.0 (IBM, Armonk, NY, United States ). The independent samples t-test was used to evaluate differences between the control and RNAi groups on the same day. Statistical differences were also identified by analysis of variance followed by least significant difference and Duncan’s multiple range tests. Quantitative data were expressed as mean ± standard deviation. A p-value < 0.05 was considered to be statistically significant.
Results
The qPCR analysis
The physiological functions of a gene can be preliminary reflected by tissues distribution. The mRNA expression of Mn-CycA did not differ significantly in the testes between different days in control prawns (p > 0.05) (Figure 1). However, both single-side and double-side eyestalk ablation stimulated the mRNA expression of Mn-CycA. Expression continuously increased from day 1 to day 7, when the expression levels after single-side and double-side eyestalk ablation were 5.12-fold and 8.17-fold higher than that of day 1 in control prawns, respectively (p < 0.05). Furthermore, Mn-CycA expression in the prawns with double-side ablation was significantly higher than those of the control and single-side ablation groups on the same day after eyestalk ablation (p < 0.05). The qPCR analysis also showed that Mn-CycA expression in the testis and androgenic gland were 4.12-fold and 2.98-fold higher, respectively, during the reproductive season than during the non-reproductive season (p < 0.05) (Figure 2).
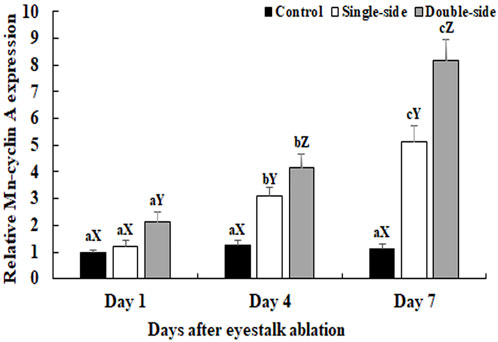
FIGURE 1. Measurement of the Mn-CycA expressions after the ablations of single-side and double-side eyestalk from male M. nipponense by qPCR. The amount of Mn-CycA mRNA was normalized to the EIF transcript level. Data are shown as mean ± SD (standard deviation) of tissues from three separate individuals. Lowercases indicate expression difference between different days in the same group (p < 0.05). Capital letters indicated the expression difference between different groups at the same day (p < 0.05).
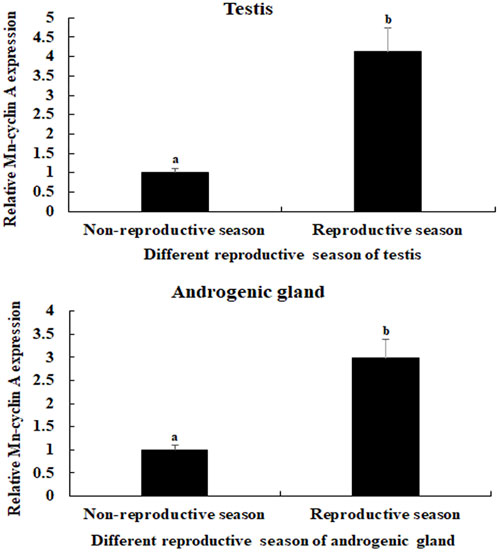
FIGURE 2. Measurement of the Mn-CycA expression in the testis and androgenic gland taken from different reproductive season. The amount of Mn-CycA mRNA was normalized to the EIF transcript level. Data are shown as mean ± SD (standard deviation) of tissues from three separate individuals. Lowercases indicate expression difference between different samples. (A) Mn-CycA expression in the testes taken from different reproductive season. (B) Mn-CycA expression in the androgenic gland taken from different reproductive season.
In situ hybridization
In the in situ hybridization assay, HE staining revealed that the testis was composed of spermatogonia, spermatocytes, and sperm, and the androgenic gland includes the ejaculatory bulb and androgenic gland cells (Figure 3). No signal was directly observed in the androgenic gland cells, whereas DIG signals were observed in the ejaculatory bulb surrounding the androgenic gland cells. In addition, strong DIG signals were observed in the spermatogonia of the testis, whereas no signal was observed in the spermatocytes and sperm (Figure 3).
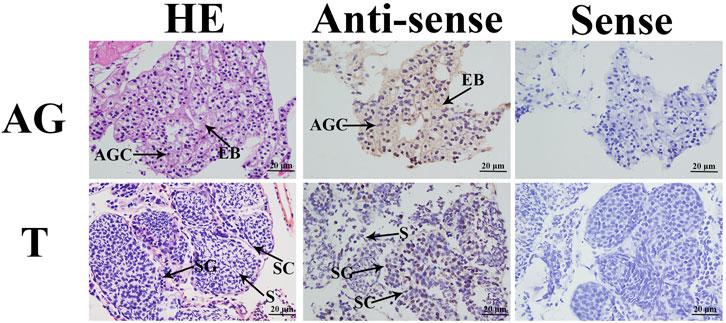
FIGURE 3. In situ hybridization analysis of Mn-CycA in the testis and androgenic gland taken from the reproductive season. SG: Spermatogonia; SC: spermatocyte; S: sperm; AGC: androgenic gland cells; EB: ejaculatory bulb. Scale bars = 20 μm.
RNAi analysis
In the RNAi experiment, the mRNA expression of Mn-CycA in the androgenic gland did not differ significantly between different days after the dsGFP treatment, and this result was verified by qPCR analysis (p > 0.05). However, a decrease of >90% was detected on days 7 and 14 after dsCycA treatment compared to that of the dsGFP-injected group on the same day (p < 0.01) (Figure 4A). The expression of Mn-IAG did not differ significantly on day 1 after dsCycA injection compared to that of the dsGFP group (p > 0.05). However, it decreased by > 80% on days 7 and 14 in the dsCycA-injected group compared to the dsGFP group on the same day (p < 0.01) (Figure 4B).
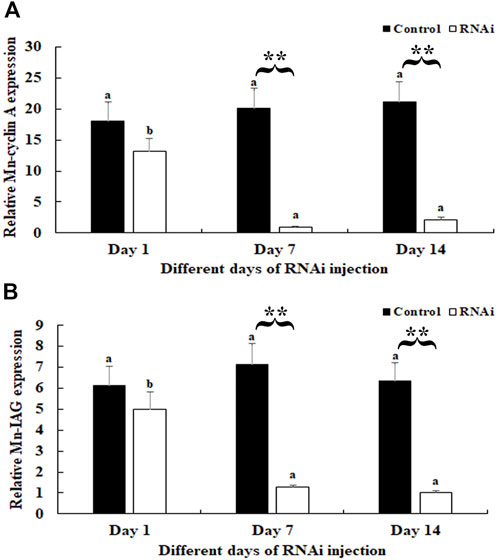
FIGURE 4. Measurement of Mn-CycA and Mn-IAG expression at different days after dsCycA and dsGFP injection. The amount of Mn-CycA and Mn-IAG mRNA was normalized to the EIF transcript level. Data are shown as mean ± SD (standard deviation) of tissues from three separate individuals. Lowercases indicated expression difference between different days after dsGFP and dsCycA injection. ** (p < 0.01) indicates significant expression difference between the dsCycA and dsGFP treated prawns at the sample day. (A) Measurement of Mn-CycA expression at different days after dsGFP and dsCycA injection. (B) Measurement of Mn-IAG expression at different days after dsGFP and dsCycA injection.
Histological observations
Histological observations revealed that the cell types did not differ on different days in the control group, and over 50% of the cells were sperm in the testis of dsGFP-treated prawns. In contrast, knockdown of the expression of Mn-CycA significantly decreased the number of sperm in the testis, and <5% cells were sperm on day 14 in the dsCycA-treated prawns (Figure 5).
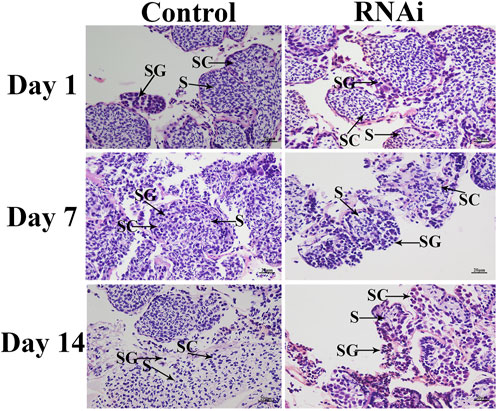
FIGURE 5. The histological observations of testis between dsCycA and dsGFP treated prawns. SG: Spermatogonia; SC: spermatocyte; S: sperm. Scale bars = 20 μm.
Discussion
Tarn and Lai (2011) reported that CycA regulates multiple steps of the cell cycle and plays a critical role in the S and G2/M phases in animal cells. A previous study of CycA in M. nipponense revealed that knockdown of CycA expression resulted in delayed ovarian development in M. nipponense (Zhou Z. Y. et al., 2021). In addition, Zhou Z. Y. et al. (2021) found that CycA expression was highest in the ovary and then the testis, and Jin S. B. et al. (2021) reported that its expression significantly increased in the androgenic gland at 7 days after eyestalk ablation from male M. nipponense. These results suggested that CycA was involved in the regulation of reproductive development in male M. nipponense, and in this study we further investigated the potential roles of CycA in this process.
To the best of our knowledge, we are the first to analyse the regulatory relationship between the eyestalk and CycA expression in a crustacean species. The qPCR analysis revealed that the expression of Mn-CycA significantly increased over time after both single-side and double-side eyestalk ablation, and expression was higher in the double-side eyestalk ablation group. This result indicated that double-side eyestalk ablation had a greater effect on promoting male reproductive development than single-side eyestalk ablation, which is consistent with results of previous studies (Jin S. B. et al., 2021; Jin et al., 2021b). The X-organ–SG complex is located in the eyestalk and is a principal neuroendocrine gland that stores and releases many neurosecretory hormones (Hopkins, 2012), including ion transport peptides, gonad-inhibiting hormone (GIH), crustacean hyperglycemic hormone (CHH), molt inhibiting hormone (MIH), and mandibular organ-inhibiting hormone. These hormones play essential roles in many biological functions of crustacean species (Santos et al., 1997; Almeida et al., 2004; Tiu and Chan, 2007; Sainz-Hernandez et al., 2008; Treerattrakool et al., 2011; Diarte-Plata et al., 2012; Pamuru et al., 2012; Salma et al., 2012; Revathi et al., 2013; Shen et al., 2013; Treerattrakool et al., 2013). In M. nipponense, CHH and MIH were reported to be involved in regulation of testis development and molting, respectively (Jin et al., 2013; Qiao et al., 2018). Additionally, the neurosecretory hormones secreted by eyestalks were found to have negative regulatory effects on both testis and ovarian development in M. nipponense. Qiao et al. (2015) showed that RNAi of GIH significantly promoted ovarian development in M. nipponense. Ablation of eyestalks from male M. nipponense stimulated the expression of Mn-IAG (Jin S. B. et al., 2021), with promoted testis development (Jin et al., 2021b). In the current study, the continuous increase of Mn-CycA expression after eyestalk ablation from male M. nipponense indicated that CycA may positively regulate reproductive development of male prawns. qPCR analysis also revealed higher Mn-CycA expression in the testis and androgenic gland during the reproductive season than during the non-reproductive season, which further supported the role of CycA in regulating reproductive development of male M. nipponense.
Prior to this study, in situ hybridization analysis of CycA had only been reported for female M. nipponense. Zhou Z. Y. et al. (2021) detected DIG signals of Mn-CycA throughout ovarian development, and they were mainly located in oogonia and oocytes. In male crustaceans, the androgenic gland and testis are the main reproductive organs (Sagi et al., 1986; Sagi and Cohen, 1990; Qiu et al., 1995). In the current study, DIG signals were observed in the ejaculatory bulb that surrounds the androgenic gland but not in androgenic gland cells, indicating that CycA did not directly involved in process of sex determination and differentiation in M. nipponense, while it played essential roles in the formation and maintenance of the normal structure of the androgenic gland (Jin et al., 2018; JinC et al., 2021; Jin et al., 2022). In the testis, DIG signals were only observed in the spermatogonia, indicating that CycA was involved in the process of spermatogenesis in M. nipponense, especially that of the spermatogonia proliferation (JinC et al., 2021; Jin et al., 2022).
The expression of CycA is known to affect the process of testis and ovarian development in many mammals and invertebrates. Liu et al. (1998) reported that targeted deletion of CycA1 from male mice blocked spermatozoa development before the first meiosis, resulting in male infertility. Female fruit flies (Drosophila) can lay eggs after knockdown of the expressions of CycA, but the eggs cannot hatch. In addition, low expression of CycA can result in delayed rupture of the meiotic nuclear membrane and mislocalization of the middle of the spindle at the end of mitosis (Bergman et al., 2015; Bourouh et al., 2016). Sandra et al. (2012) found that the development of germinal vesicle rupture and G2/M was inhibited during meiotic phase I after injection of CycA2 antibody into mouse oocytes during meiosis. Knockdown of CycA2 expression in oocytes at meiotic stage II inhibited the separation of sister chromatids. In the silk moth Bombyx mori, ovarian development was blocked in the G1 phase after knockdown of the expression of CycA in ovarian cells, which effectively inhibited cell proliferation (Liu et al., 2015). Knockdown of the expression of CycA in female M. nipponense by RNAi resulted in delayed ovarian development (Zhou Z. Y. et al., 2021). In the present study, RNAi was also employed to investigate the potential functions of CycA in the reproduction of male M. nipponense. The injection of dsCycA resulted in a significant decrease of Mn-CycA and Mn-IAG expressions on days 7 and 14, indicating that the synthesized dsCycA efficiently knocked-down the expression of these genes and that CycA has a positive regulatory role in the expression of IAG in M. nipponense. IAG is the main gene expressed in the androgenic gland, and its regulatory effects on male differentiation and reproduction have been well studied in Macrobrachium rosenbergii, especially those related to spermatogenesis (Sagi et al., 1986; Sagi and Cohen, 1990; Ventura et al., 2012). Similar functions of IAG have been reported in many other crustacean species (Li et al., 2012; Huang et al., 2014; Ma et al., 2016; Zhou T. T. et al., 2021; Liu, 2021). Histological observations revealed that sperm were the main cell type in the testis of dsGFP-injected prawns but that they accounted for <5% of cells on day 14 after dsCycA injection. This result indicated that RNAi of Mn-CycA had a significant inhibitory role on testis development of M. nipponense, which confirmed the positive regulatory roles of CycA in the regulation of testis development in this prawn.
In conclusion, eyestalk ablation from male M. nipponense continuously stimulated the expression of Mn-CycA, and Mn-CycA expression was higher in the testis and androgenic gland during the reproductive season than during the non-reproductive season. Knockdown of the expression of Mn-CycA by RNAi in male M. nipponense revealed that CycA had a positive regulatory effect on testis development of this prawn, which was verified by histological observations of testis samples from dsGFP-injected and dsCycA-injected prawns. These results showed that CycA had an important role in the reproductive development of male M. nipponense, which can be applied to the development of techniques to regulate testis development in M. nipponense.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Wuxi, China) for all experiments involving M. nipponense. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
WZ wrote the manuscript. YX provided the experimental prawns. PW performed the qPCR analysis. TC synthesized the dsRNA. SJ performed the in situ hybridization analysis. HQ performed the histological observations. YG ablated the eyestalk from male M. nipponense. YW extracted RNA for this study. SJ revised the manuscript. HF supervised the experiment.
Funding
This research was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20221207), Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2021JBFM02 and 2020TD36), National Key R&D Program of China (2018YFD0900201), Jiangsu Agricultural Industry Technology System (JATS[2020]461), China Agriculture Research System-48 (CARS-48), and New Cultivar Breeding Major Project of Jiangsu Province (PZCZ201745).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almeida, E. A., Petersen, R. L., Andreatta, E. R., and Bainy, A. C. (2004). Effects of captivity and eyestalk ablation on antioxidant status of shrimps (Farfantepenaeus paulensis). Aquaculture 238, 523–528. doi:10.1016/j.aquaculture.2004.04.010
Bergman, Z. J., McLaurin, J. D., Eritano, A. S., Johnson, B. M., Sims, A. Q., and Riggs, B. (2015). Spatial reorganization of the endoplasmic reticulum during mitosis relies on mitotic kinase cyclin A in the early Drosophila embryo. PLoS ONE 10, e0117859. doi:10.1371/journal.pone.0117859
Bourouh, M., Dhaliwal, R., Rana, K., Sinha, S., Guo, Z., and Swan, A. (2016). Distinct and overlapping requirements for cyclins A, B, and B3 in drosophila female meiosis. G3 6, 3711–3724. doi:10.1534/g3.116.033050
Diarte-Plata, G., Sainz-Hernández, J. C., Aguiñaga-Cruz, J. A., Fierro-Coronado, J. A., Polanco-Torres, A., and Puente-Palazuelos, C. (2012). Eyestalk ablation procedures tominimize pain in the freshwater prawn Macrobrachium Americanum. Appl. Anim. Behav. Sci. 140, 172–178. doi:10.1016/j.applanim.2012.06.002
Hopkins, P. M. (2012). The eyes have it: A brief history of crustacean neuroendocrinology. Gen. Comp. Endocrinol. 175, 357–366. doi:10.1016/j.ygcen.2011.12.002
Hu, Y. N., Fu, H. T., Qiao, H., Sun, S. M., Zhang, W. Y., Jin, S. B., et al. (2018). Validation and evaluation of reference genes for Quantitative real-time PCR in Macrobrachium nipponense. Int. J. Mol. Sci. 19, 2258. doi:10.3390/ijms19082258
Huang, X. S., Ye, H. H., Huang, H. Y., Yang, Y. N., and Gong, J. (2014). An insulin-like androgenic gland hormone gene in the mud crab, Scylla paramamosain, extensively expressed and involved in the processes of growth and female reproduction. Gen. Comp. Endocrinol. 204, 229–238. doi:10.1016/j.ygcen.2014.06.002
Jiang, F. W., Fu, H. T., Qiao, H., Zhang, W. Y., Jiang, S. F., Xiong, Y. W., et al. (2014). The RNA interference regularity of transformer-2 gene of oriental river prawn Macrobrachium nipponense. Chin. Agricul. Sci. Bul. 30 (32), 32–37.
Jin, S. B., Fu, H. T., Jiang, S. F., Xiong, Y. W., Sun, S. M., Qiao, H., et al. (2018). Molecular cloning, expression, and in situ hybridization analysis of forkhead box protein L2 during development in Macrobrachium nipponense. J. World Aquac. Soc. 49 (10), 429–440. doi:10.1111/jwas.12510
Jin, S. B., Fu, Y., Hu, Y. N., Fu, H. T., Jiang, S. F., Xiong, Y. W., et al. (2021a). Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation. Sci. Rep. 11, 19855. doi:10.1038/s41598-021-99022-4
Jin, S. B., Jiang, S. F., Xiong, Y. W., Qiao, H., Sun, S. M., Zhang, W. Y., et al. (2014). Molecular cloning of two tropomyosin family genes and expression analysis during development in oriental river prawn, Macrobrachium nipponense. Gene 546 (2), 390–397. doi:10.1016/j.gene.2014.05.014
Jin, S. B., Wang, N., Qiao, H., Fu, H. T., Wu, Y., Gong, Y. S., et al. (2013). Molecular cloning and expression of a full-length cDNA encoding crustacean hyperglycemic hormone (CHH) in oriental river pawn (Macrobrachium nipponense). J. Fish. Sci. China 20, 82–92. doi:10.3724/sp.j.1118.2013.00082
Jin, S. B., Zhang, Y., Guan, H. H., Fu, H. T., Jiang, S. F., Xiong, Y. W., et al. (2016). Histological observation of gonadal development during post-larva in oriental river prawn, Macrobrachium nipponense. Chin. J. Fish. 29, 11–16.
Jin, S., Fu, H., Jiang, S., Xiong, Y., Qiao, H., Zhang, W., et al. (2022). RNA interference analysis reveals the positive regulatory role of ferritin in testis development in the oriental river prawn, Macrobrachium nipponense. Front. Physiol. 13, 805861. doi:10.3389/fphys.2022.805861
Jin, S., Fu, Y., Hu, Y., Fu, H., Jiang, S., Xiong, Y., et al. (2021b). Transcriptome profiling analysis of the testis after eyestalk ablation for selection of the candidate genes involved in the male sexual development in Macrobrachium nipponense. Front. Genet. 12, 675928. doi:10.3389/fgene.2021.675928
Jin, S., Hu, Y., Fu, H., Jiang, S., Xiong, Y., Qiao, H., et al. (2021). Identification and characterization of the pyruvate dehydrogenase E1 gene in the oriental river prawn, Macrobrachium nipponense. Front. Endocrinol. 12, 752501. doi:10.3389/fendo.2021.752501
Lees, E. M., and Harlow, E. (1993). Sequences within the conserved cyclin box of human cyclin A are sufficient for binding to and activation of cdc2 kinase. Mol. Cell. Biol. 13, 1194–1201. doi:10.1128/mcb.13.2.1194
Li, C. J., Vassilev, A., and DePamphilis, L. D. (2004). Role for Cdk1 (Cdc2)/Cyclin A in preventing the mammalian origin recognition complex’s largest subunit (Orc1) from binding to chromatin during mitosis. Mol. Cell. Biol. 24, 5875–5886. doi:10.1128/MCB.24.13.5875-5886.2004
Li, F., Qiao, H., Fu, H. T., Sun, S. M., Zhang, W. Y., Jin, S. B., et al. (2018). Identification and characterization of opsin gene and its role in ovarian maturation in the oriental river prawn Macrobrachium nipponense. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 218, 1–12. doi:10.1016/j.cbpb.2017.12.016
Li, S. H., Li, F. H., Sun, Z., and Xiang, J. H. (2012). Two spliced variants of insulin-like androgenic gland hormone gene in the Chinese shrimp, Fenneropenaeus chinensis. Gen. Comp. Endocrinol. 177 (2), 246–255. doi:10.1016/j.ygcen.2012.04.010
Liu, D., Matzuk, M. M., Sung, W. K., Guo, Q., Wang, P., and Wolgemuth, D. J. (1998). Cyclin A1 is required for meiosis in the male mouse. Nat. Genet. 20, 377–380. doi:10.1038/3855
Liu, F., Shi, W., Ye, H., Liu, A., and Zhu, Z. (2021). RNAi reveals role of insulin-like androgenic gland hormone 2 (IAG2) in sexual differentiation and growth in hermaphrodite shrimp. Front. Mar. Sci. 8, 666763. doi:10.3389/fmars.2021.666763
Liu, L., Shen, W., Bing, L. I., Qin, J., Youlan, Y. U., Pengyu, M. A., et al. (2015). RNA interference impact on silkworm cyclin A gene and cell proliferation. J. Nanjing Agric. Univ. 38 (1), 168–171.
Ma, K. Y., Li, J. L., and Qiu, G. F. (2016). Identification of putative regulatory region of insulin-like androgenic gland hormone gene (IAG) in the prawn Macrobrachium nipponense and proteins that interact with IAG by using yeast two-hybrid system. Gen. Comp. Endocrinol. 229, 112–118. doi:10.1016/j.ygcen.2016.03.019
Ma, X. K., Liu, X. Z., Wen, H. S., Xu, Y. J., and Zhang, L. J. (2006). Histological observation on gonadal sex differentiation in Cynoglossus semilaevis Günther. Mar. Fish. Res. 27 (2), 55–61.
Minshull, J., Golsteyn, R., Hill, C. S., and Hunt, T. (1990). The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 9, 2865–2875. doi:10.1002/j.1460-2075.1990.tb07476.x
Narayanan, R., Adigun, A. A., Edwards, D. P., and Weigel, N. L. (2005). Cyclin-dependent kinase activity is required for progesterone receptor function: Novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol. Cell. Biol. 25, 264–277. doi:10.1128/MCB.25.1.264-277.2005
Pamuru, R. R., Rosen, O., Manor, R., Chung, J. S., Zmora, N., Glazer, L., et al. (2012). Stimulation of molt by RNA interference of the molt inhibiting hormone in the crayfish Cherax quadricarinatus. Gen. Comp. Endocrinol. 178, 227–236. doi:10.1016/j.ygcen.2012.05.007
Qiao, H., Jiang, F. W., Xiong, Y. W., Jiang, S. F., Fu, H. T., Li, F., et al. (2018). Characterization, expression patterns of molt-inhibiting hormone gene of Macrobrachium nipponense and its roles in molting and growth. PloS one 13, e0198861. doi:10.1371/journal.pone.0198861
Qiao, H., Xiong, Y. W., Zhang, W. Y., Fu, H. T., Jiang, S. F., Sun, S. M., et al. (2015). Characterization, expression, and function analysis of gonad-inhibiting hormone in Oriental River prawn, Macrobrachium nipponense and its induced expression by temperature. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 185, 1–8. doi:10.1016/j.cbpa.2015.03.005
Qiu, G. F., Du, N. S., and Lai, W. (1995). Studies on the male reproductive system of the freshwater prawn. Macrobrachium Nippon. J. Shanghai Fish. Univ. 4, 107–111.
Revathi, P., Iyapparaj, P., Vasanthi, L. A., Jeyanthi, S., and Krishnan, M. (2013). Impact of eyestalk ablation on the androgenic gland activity in the freshwater prawn Macrobrachium rosenbergii (De Man). World 5, 373–381.
Rogatsky, I., Trowbridge, J. M., and Garabedian, M. J. (1986). Potentiation of human estrogen receptor α transcriptional activation through phosphorylation of serines 104 and 106 by the Cyclin A-CDK2 complex. J. Biol. Chem. 274, 22296–22302. doi:10.1074/jbc.274.32.22296
Sagi, A., and Cohen, D. (1990). Growth, maturation and progeny of sex-reversed Macrobrachium rosenbetgii males. World Aquacult 21 (4), 87–90.
Sagi, A., Ra’anan, Z., Cohen, D., and Wax, Y. (1986). Production of Macrobrachium rosenbetgii in momosex population: Yield characteristes under intensive monoculture conditions in cages. Aquaculture 51, 265–275. doi:10.1016/0044-8486(86)90318-2
Sainz-Hernandez, J. C., Racotta, I. S., Dumas, S., and Hernandez-Lopez, J. (2008). Effect of unilateral and bilateral eyestalk ablation in Litopenaeus vannamei male and female on several metabolic and immunologic variables. Aquaculture 283, 188–193. doi:10.1016/j.aquaculture.2008.07.002
Salma, U., Uddowla, M. H., Kim, M., Kim, J. M., Bo, K. K., Baek, H. J., et al. (2012). Five hepatopancreatic and one epidermal chitinases from a pandalid shrimp (Pandalopsis japonica): Cloning and effects of eyestalk ablation on gene expression. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 161, 197–207. doi:10.1016/j.cbpb.2011.11.005
Sandra, A. T., Damien, C., Lisa, M. L., Ioanna, L., Jean-Philippe, C., Ahmed, R., et al. (2012). Cyclin A2 is required for sister chromatid segregation, but not separase control, in mouse oocyte meiosis. Cell Rep. 2, 1077–1087. doi:10.1016/j.celrep.2012.10.002
Santos, E. A., Eduardo, L., Nery, M., Goncalves, A. A., and Keller, R. (1997). Evidence for the involvement of the crustacean hyperglycemic hormone in the regulation of lipid metabolism. Physiol. Zool. 70, 415–420. doi:10.1086/515846
ShangGuan, B. M., Liu, Z. Z., and Li, S. Q. (1991). Histological studies on ovarian development in Scylla serrata. J. Fish. Sci. China. 15 (2), 96–103.
Shen, H., Zhou, X., Bai, A., Ren, X., and Zhang, Y. (2013). Ecdysone receptor gene from the freshwater prawn Macrobrachium nipponense: Identification of different splice variants and sexually dimorphic expression, fluctuation of expression in the molt cycle and effect of eyestalk ablation. Gen. Comp. Endocrinol. 193, 86–94. doi:10.1016/j.ygcen.2013.07.014
Tarn, W. Y., and Lai, M. C. (2011). Translational control of cyclins. Cell Div. 6, 5. doi:10.1186/1747-1028-6-5
Tiu, S. H. K., and Chan, S. M. (2007). The use of recombinant protein and RNA interference approaches to study the reproductive functions of a gonad-stimulating hormone from the shrimp Metapenaeus ensis. FEBS J. 274, 4385–4395. doi:10.1111/j.1742-4658.2007.05968.x
Treerattrakool, S., Chartthai, C., Phromma-in, N., Panyim, S., and Udomkit, A. (2013). Silencing of gonad-inhibiting hormone gene expression in Penaeus monodon by feeding with GIH dsRNA-enriched Artemia. Aquaculture 404, 116–121. doi:10.1016/j.aquaculture.2013.04.024
Treerattrakool, S., Panyim, S., and Udomkit, A. (2011). Induction of ovarian maturation and spawning in Penaeus monodon broodstock by double-stranded RNA. Mar. Biotechnol. 13, 163–169. doi:10.1007/s10126-010-9276-0
Ventura, T., Manor, R., Aflalo, E. D., Weil, S., Rosen, O., and Sagi, A. (2012). Timing sexual differentiation: Full functional sex reversal achieved through silencing of a single insulin-like gene in the prawn, Macrobrachium rosenbergii. Biol. Reprod. 86 (3), 90. doi:10.1095/biolreprod.111.097261
Zhang, S. B., Jiang, P., Wang, Z. Q., Long, S. R., Liu, R. D., Zhang, X., et al. (2016). Dsrna-mediated silencing of nudix hydrolase in Trichinella spiralis inhibits the larval invasion and survival in mice. Exp. Parasitol. 162, 35–42. doi:10.1016/j.exppara.2016.01.005
Zhang, X. L., Cui, L. F., Li, S. M., Liu, X. Z., Han, X., Jiang, K. Y., et al. (2020). Bureau of Fisheries, ministry of agriculture, P.R.C. Fisheries economic statistics. China Fishery Yearbook. Beijing China Agricultural Press, 24.
Zhou, T. T., Wang, W., Wang, C. G., Sun, C. B., Shi, L. L., and Chan, S. F. (2021a). Insulin-like androgenic gland hormone from the shrimp fenneropenaeus merguiensis: Expression, gene organization and transcript variants. Gene 782, 145529. doi:10.1016/j.gene.2021.145529
Keywords: Macrobrachium nipponense, cyclin A, RNA interference, insulin-like androgenic gland hormone, male reproduction
Citation: Zhang W, Xiong Y, Wang P, Chen T, Jiang S, Qiao H, Gong Y, Wu Y, Jin S and Fu H (2022) RNA interference analysis of potential functions of cyclin A in the reproductive development of male oriental river prawns (Macrobrachium nipponense). Front. Genet. 13:1053826. doi: 10.3389/fgene.2022.1053826
Received: 26 September 2022; Accepted: 31 October 2022;
Published: 17 November 2022.
Edited by:
You-Yi Kuang, Heilongjiang River Fisheries Research Institute (CAFS), ChinaReviewed by:
Yingying Zhao, Shenyang Agricultural University, ChinaWenming Ma, Zhejiang Wanli University, China
Copyright © 2022 Zhang, Xiong, Wang, Chen, Jiang, Qiao, Gong, Wu, Jin and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shubo Jin, amluc2JAZmZyYy5jbg==; Hongtuo Fu, ZnVodEBmZnJjLmNu
 Wenyi Zhang1
Wenyi Zhang1 Sufei Jiang
Sufei Jiang Hui Qiao
Hui Qiao Shubo Jin
Shubo Jin Hongtuo Fu
Hongtuo Fu