
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 08 November 2022
Sec. Cancer Genetics and Oncogenomics
Volume 13 - 2022 | https://doi.org/10.3389/fgene.2022.1047326
This article is part of the Research Topic Translational Medicine in the Diagnosis and Treatment of Cancer based on Oncogenetics: From Bench to Bedside View all 16 articles
 Jie Cheng1†
Jie Cheng1† Qianyuan Li1†
Qianyuan Li1† Sheng Xiao1†
Sheng Xiao1† Lu Nie1
Lu Nie1 Jianping Liao1
Jianping Liao1 Qingjie Jiang1
Qingjie Jiang1 Biyu Xiang1
Biyu Xiang1 Hongfei Zhang1
Hongfei Zhang1 Yanhong Jiang1
Yanhong Jiang1 Chenjiao Yao1,2*
Chenjiao Yao1,2*Objective: This study aims to determine the clinical significance of the advanced lung cancer inflammation index (ALI) in predicting prognosis, chemotherapy response, and infection risk in newly diagnosed multiple myeloma (MM) patients receiving induction therapy.
Methods: A retrospective analysis of the clinical characteristics and laboratory data of 111 newly diagnosed MM patients from the Haematology Department of the Third Xiangya Hospital of Central South University from January 2014 to March 2020 was performed. We first determined the relationship between ALI and overall survival (OS), as well as clinical and laboratory parameters. Second, predictive factors for chemotherapy response were analysed by univariate and multivariate regression analyses. Third, univariate regression analysis of risk factors was performed using infection as the evaluable outcome.
Results: Of the 111 evaluable patients, the low ALI group (<32.7) exhibited significantly poorer survival than the high ALI group (51 months versus 77 months). Multivariable analysis showed that advanced age, chemotherapy response and serum calcium level were independent prognostic factors for OS. Better chemotherapy efficacy in the high ALI group (89.3%) than in the low ALI group (42.2%) (p < 0.001) was noted. Multivariate analysis suggested that only ALI [HR: 0.110, 95% CI (0.035–0.350), p = 0.000] is an independent predictive factor in evaluating the efficiency of induction chemotherapy. Forty patients (36.04%) presented with infection after induction chemotherapy. Univariate analysis suggested that low ALI and abnormal renal function increase risk of infection in newly diagnosed MM patients.
Conclusion: Our study confirmed that ALI is not only a prognostic biomarker for newly diagnosed patients, but also predicts chemotherapy efficacy in newly diagnosed MM patients receiving induction therapy.
Multiple myeloma (MM) is the second most common malignant tumour of the haematological system, accounting for 1% of haematological tumours (Raab et al., 2009; Palumbo and Anderson, 2011). Revised International Staging System (R-ISS) and the Durie-Salmon (D&S) staging system have been established to predict survival based on prognostic classification of MM patients (Boyd et al., 2012; Palumbo et al., 2015). Induction therapy is the most crucial treatment in newly diagnosed multiple myeloma (NDMM) patients, and bortezomib (PI) and dexamethasone (VD) remain the current standard of care. The recently updated 2021 EHA-ESMO clinical practice guidelines recommend the use of either lenalidomide-VD (VRD) or daratumumab-thalidomide-VD (Dara-VTD) as first-line options for transplant-eligible NDMM patients; when not available, thalidomide-VD (VTD) or cyclophosphamide-VD (VCD) are acceptable alternatives (Bazarbachi et al., 2022). The majority of MM patients are in a refractory or relapsed state (Röllig et al., 2015). Clinical evidence confirms that infections represent a major cause of morbidity and mortality in patients with MM, especially for those with severe infection, pneumonia, and neutropenia in relapsed/refractory settings (Blimark et al., 2015; Balmaceda et al., 2021). In NDMM patients admitted for the first time, risk of infection correlates with poor prognosis, particularly in those with ISS stage III or low haemoglobin levels (Lin et al., 2020). MM patients with a risk of infection and complications are recommended to receive optimal preventive strategies (Bladé and Rosiñol, 2005; Raje et al., 2022). Additionally, biological mechanism research has demonstrated that the proinflammatory cytokine interleukin (IL)-18 is involved in tumour-promoting inflammation, and its high expression suggessts poor overall survival in MM (Nakamura et al., 2018). All of these results indicate that inflammatory markers have potential as prognostic markers (Hanahan and Weinberg, 2011). The inflammation-based neutrophil-to-lymphocyte ratio (NLR) has been demonstrated to be a prognostic biomarker, with higher NLR indicating poorer prognosis in MM patients, as demonstrated by meta-analysis (Mu et al., 2018). Similarly, the advanced lung cancer inflammation index (ALI), which is a modification of NLR that includes albumin (ALB) and body mass index (BMI), has been developed and demonstrated to be a prognostic marker of poor survival in several cancers, including non-small cell lung cancer (NLSCL) (Jafri et al., 2013). ALI also represents a simple tool to predict immunotherapy efficacy in patients with advanced NSCLC treated with PD-L1 inhibitors alone, and the ALI score has a stronger predictive effect than NLR, the PD-L1 tumour proportion score and other biomarkers (Shiroyama et al., 2018; Mountzios et al., 2021). Therefore, ALI, which is calculated based on body composition, nutrition, and systemic inflammation, may represent a comprehensive indicator for predicting prognosis of NDMM patients. Although ALI reflects the systemic inflammation and cachexia provoked by cancer, its prognostic and predictive value in NDMM patients is unknown. We hypothesized that low ALI is associated with poor survival and can predict chemotherapy efficacy in NDMM patients. We also sought to evaluate the predictive capacity of ALI on the overall incidence of infection in patients receiving induction chemotherapy.
This was a single-centre, observational, retrospective study. Our study collected data for 111 MM patients at the Department of Haematology, Third Xiangya Hospital, Central South University from January 2014 to March 2020 under approval of the Institutional Review Board of Third Xiangya Hospital, Central South University (No. 22081). We then collected clinical data and performed classification according to ISS staging standards and the DS staging system. Abnormal secretion of clonal immunoglobulins was classified into the following categories: IgG, IgD, IgM, IgE, double clonal, kappa, and lambda light chain or nonsecreting types. NDMM patients were treated consecutively with bortezomib-based triplet regimens, consisting of proteasome inhibitor (PI) bortezomib plus dexamethasone (VD) backbone, with the addition of a third agent, such as thalidomide (VTD), cyclophosphamide (VCD), lenalidomide (VRD) or doxorubicin (PAD). The number of induction cycles varied from 4 to 6. The curative effect was evaluated according to the traditional efficacy standard of International Myeloma Working Group in 2016 (Kumar et al., 2016). Complete remission in a strict sense (SCR), complete remission (CR), very good partial remission (VGPR), and partial remission (PR) are regarded as effectively curative effects; stable disease (SD) and progressive disease (PD) are regarded as ineffective curative effects. In addition, patients who meet any of the following conditions after initial diagnosis of chemotherapy should be judged as having complicated infection: 1) continuous fever for more than 2 days (axillary temperature greater than 38°C), and fever caused by blood transfusion or related drugs should be excluded; 2) exact clinical symptoms and/or signs of infection, such as bladder irritation, frequent micturition and urgent micturition or signs, such as tenderness of the ureter point when urinary system infection occurs; and 3) exact imaging-related examinations and/or aetiological evidence suggesting the existence of infected foci. The exclusion criteria were as follows: 1) acute or chronic inflammation at the time of initial diagnosis; 2) a history of blood system diseases and malignant tumours; 3) immune disease, such as inflammatory bowel disease, Sjogren’s syndrome, or rheumatoid arthritis; 4) complication of acute and chronic hepatitis or liver disease; 5) complication of severe damage to the functions of other important organs except the kidneys; 6) indicators, such as height and weight, not recorded at the time of admission; 7) relevant biochemical tests not completed within 48 h after admission; and 8) treatment discontinuation or incomplete clinical data.
Data were collected retrospectively from the electronic medical records. Weight, height, neutrophil, lymphocyte, and ALB (g/dl) data were collected at baseline before chemotherapy administration. BMI was calculated by dividing weight (kg) by height (m) squared. The neutrophil-to-lymphocyte ratio (NLR) was computed as the absolute neutrophil count divided by the absolute lymphocyte count. We calculated the advanced lung cancer inflammation index (ALI) as follows: ALI = BMI ×ALB/NLR.
The χ2 test or Fisher’s exact test was used to determine the significance of differences between discrete variables. Significant variables in univariate analysis were included in binary multivariate logistic analysis. A receiver operating characteristic curve (ROC) was used to determine specificity and sensitivity. Univariate and multivariate analyses were performed using the Cox proportional risk regression model to evaluate prognostic factors and their impact on OS. Associations between ALI and OS were analysed using Kaplan‒Meier survival curve estimates and compared using the log-rank test. The statistical analysis was performed using SPSS version 26.0, and p < 0.05 was considered statistically significant.
Overall, we analysed 111 patients with NDMM at first hospitalization (66 males and 45 females). The average age was 58 years (44–75), and 39.6% of the patients were ≥60 years. A total of 49 patients (44.1%) had MM of the IgG type, 27% had the IgA type, and 18% had the λ light chain type. In total, 10.8% of the patients were classified with other types (κ light chain type and IgD type or others). Based on the Durie-Salmon scale, four patients were at stage I, 12 at stage II, and 95 at stage III. In total, 90 patients had normal renal function, whereas 21 had abnormal renal function. Based on the International Staging System (ISS) scale, 12 patients were at stage I, 28 at stage II, and 71 at stage III. All patients received two cycles of induction chemotherapy, and curative effects were evaluated. In total, the response in 78 patients was considered effective, whereas the response was classified as ineffective in 33 patients.
We first analysed the association between ALI and patient prognosis. Using the X-tile program (Camp et al., 2004), an optimal cut-off value for ALI of 32.7 was determined. All cases were categorized into the following two groups: a high ALI group (n = 66 cases) and a low ALI group (n = 45). Patients in the low ALI group had significantly poorer survival than those in the high ALI group (51 months versus 77 months, p = 0.001, Figure 1). Univariate analyses showed that OS correlated with NLR, abnormal renal function, advanced age (>60 years), ß 2-MG level, ISS stage, chemotherapy efficacy, and serum calcium level. Multivariable analyses showed that advanced age, chemotherapy response, and serum calcium were independent prognostic factors for OS (Table 1).
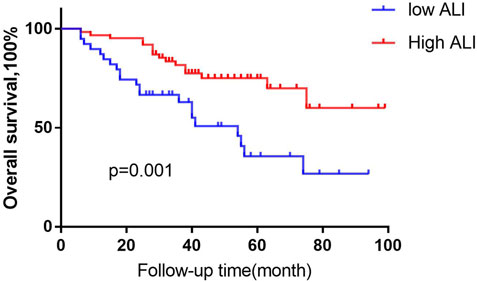
FIGURE 1. Kaplan-Meier analysis of OS based on ALI status. Using the X-tile program, an optimal cut-off value for ALI of 32.7 was determined. We defined MM patients the high- ALI group, (n = 66 cases) and the low -ALI group, (n = 45). Low-ALI patients with MM were significantly correlated with poor prognosis compared with high-ALI patients with respect to overall survival (OS; p = 0.001, log-rank test).
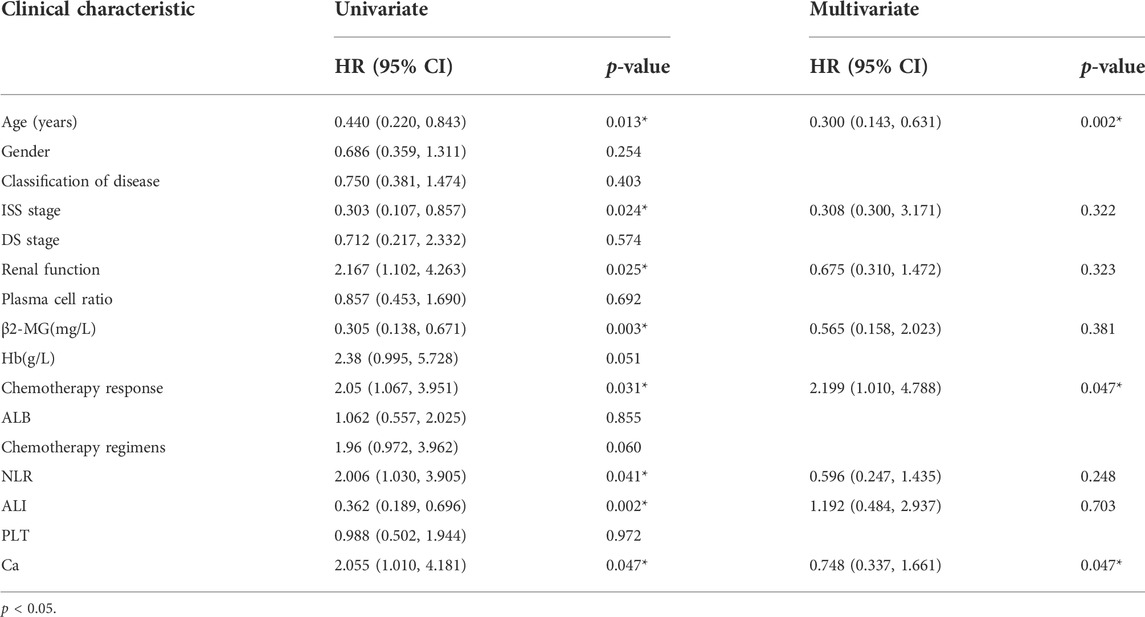
TABLE 1. Cox regression analysis of predictive factors for overall survival in patients with MM (n = 111).
Next, we analysed the relationship between ALI and patient characteristics as well as laboratory parameters. Table 2 shows that no differences in age, sex, disease classification, plasma cell ratio, ISS stage, DS stage, haemoglobin level, or ß 2-MG level were noted between the two groups. However, abnormal renal function and increased serum calcium were significantly associated with the low ALI group. Better chemotherapy efficacy was observed in the high ALI group (89.3%) than in the low ALI group (42.2%) (p < 0.001), but there was no significant difference between the high ALI group and the low ALI group in whether bortezomib was used (p > 0.05). In univariate analyses, abnormal renal function, lower ALI, and high NLR level was related to poor chemotherapy efficiency, but multivariate analysis suggested that only ALI [HR: 0.110, 95% CI (0.035–0.350), p = 0.000] was an independent predictive factor in evaluating the efficiency of induction chemotherapy (Table 3). We compared the predictive value of NLR, ALB, and ALI in patients with MM treated with chemotherapy. The areas under the ROC curves of NLR, ALB, and ALI were calculated (Figure 2), and ALI and NLR were significantly associated with OS. Notably, based on ROC curve analyses, ALI was identified as a more powerful prognostic marker than NLR and ALB.
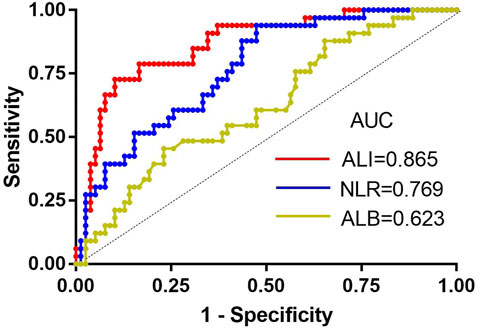
FIGURE 2. The receiver-operating characteristic (ROC) curves for ALI, ALB, and NLR. AUC, area under the curve; ALI, advanced lung cancer inflammation index; NLR, neutrophil-to-lymphocyte ratio; ALB, albumin.
Among the MM patients, 40 (36.04%) presented with infection after induction chemotherapy. The most common site of infection was the respiratory system (32 cases, 80%), followed by the digestive system (3, 7.5%) and the urinary system (2, 5%). In addition, three patients (7.5%) had more than one site of infection. Univariate analysis suggested that ALI and abnormal renal function were significant in predicting infection in NDMM patients (Table 4).
The ALI score, comprising BMI, ALB, and NLR, reflects inflammatory immunity and nutritional status. In this study, advanced age, chemotherapy response and serum calcium were independent prognostic factors for OS. Regarding the prognostic potential of ALI in MM patients, a lower ALI score reduced OS, indicating that ALI can serve as an outcome biomarker (Jafri et al., 2013; Park et al., 2017; Mandaliya et al., 2019; Kusunoki et al., 2020; Yin et al., 2021). Similar research has reported that ALI is a predictive marker for PD-L1 inhibitor monotherapy and has stronger predictive ability compared with other analysed parameters, such as NLR and PD-L1 TPS (Mountzios et al., 2021), which have also been confirmed as predictors of OS in NDMM patients treated with bortezomib-based regimens (Romano et al., 2017; Zhou et al., 2018). A retrospective single-centre study in China showed that age, haemoglobin, ALB, serum calcium, β2-MG, LDH, CRP, the ratio of plasma cells and the percentage of abnormal plasma cells in bone marrow were all independent prognostic factors for OS, which is different from our results (Qian et al., 2017). However, the strict inclusion criteria for NDMM patients potentially led to sample selection bias. In addition, the sample size of our study was relatively small, which might also lead to bias in our results. Renal dysfunction is an important characteristic of MM, and the development of renal dysfunction is a negative prognostic factor for MM patients undergoing induction chemotherapy (Li et al., 2021). Our study suggests that low ALI is positively associated with renal dysfunction. Kidney damage often manifests as proteinuria and hypoproteinaemia (Pei et al., 1992), which reduce ALB and result in low ALI. Therefore, ALI also represents a potential negative prognostic factor for patients with MM.
Chemotherapy response is an important prognostic factor in NDMM patients receiving bortezomib-based therapy. Based on univariate analyses, we found that abnormal renal function, high NLR, and lower ALI level are related to poor chemotherapy efficiency. Chen’s research demonstrated that renal function is associated with a high incidence of chemotherapy-related toxicities in metastatic colorectal cancer and even decreases the efficacy of chemotherapy (Chen et al., 2015), which is consistent with our research. In addition, a signature consisting of 11 cytokine genes in the lung environment is able to predict lymph node metastasis and prognosis of lung adenocarcinoma based on IL-8 and TNFα as the top two genes for predicting prognosis (Seike et al., 2007). IL-8 was originally described as a monocyte-derived neutrophil chemotactic factor (MNDCF) that specifically attracts neutrophils (Mukaida et al., 1998), which explains why the NLR value is increased in tumour cells. Hirahara et al. (2019) evaluated the relationship between tumour response and NLR/PLR and found that the score can serve as a new blood predictor of tumour response and prognosis. As discussed above, NLR is related to renal function, and the ALI index is calculated from the NLR value. Therefore, ALI is extremely closely related to the efficacy of chemotherapy. This finding is consistent with our multivariate result that ALI is an independent predictor of chemotherapy effect. Moreover, based on ROC analysis, ALI outperformed other biomarkers of chemotherapy response, including NLR and ALB (Szudy-Szczyrek et al., 2020).
Given that NDMM patients have a high risk of infections at first admission, especially infections with Epstein‒Barr virus (EBV), hepatitis B virus (HBV) and Escherichia coli (Lin et al., 2020), we excluded MM patients with concurrent infection from this study. Interestingly, our results highlight that ALI is not only an independent predictive marker of chemotherapy efficacy but also a predictor of susceptibility to infection in NDMM patients during induction chemotherapy. Our study also showed that patients with lower ALI may be more susceptible to infectious complications after receiving induction chemotherapy. Some studies have shown that renal impairment is a risk factor for infections in the early phase of MM or in NDMM patients receiving bortezomib-based induction chemotherapy (Sørrig et al., 2019; Soekojo et al., 2020). We also found that renal failure is a significant risk factor for NDMM patients. Therefore, the presence of low ALI and renal dysfunction may be of greater practical value in predicting infection for MM patients without comorbidities at admission. On the other hand, patients with lower ALI may have a higher NLR value, which may indicate lymphocytopaenia and worse inflammatory immunity and nutritional status (Hirahara et al., 2019), which are significant risk factors for NDMM patients. The incidence of infection after chemotherapy in MM patients is 36.04%, and pulmonary infection is most common, which is consistent with existing research results (O'Brien et al., 2003). Based on data on antimyeloma therapy in previous studies, chemotherapy can increase the risk of bacterial infection, and bortezomib can increase that of influenza infection (Nucci and Anaissie, 2009; Teh et al., 2015). However, we found that chemotherapeutic regimens, including bortezomib, did not increase the risk of infection, which is not consistent with existing research (Nucci and Anaissie, 2009). This finding may be due to our inclusion criteria of MM patients without the presence of comorbidities or multiple organ dysfunctions.
In conclusion, our study confirms that ALI is not only a prognostic biomarker for patients newly diagnosed with MM but also predicts the efficacy of chemotherapy in NDMM patients receiving bortezomib-based therapy. Importantly, those with abnormal renal function have poorer prognosis and are more susceptible to serious infection complications. Therefore, ALI can be adapted in clinical practice to stratify MM patients for future trials. Furthermore, large-scale multicentre prospective studies are required to completely validate our findings.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
This study involving human participants was approved by the Ethics Committee of the Third Xiangya Hospital, Central South University (No. 22081).
CY contributed to the conception and design of the study. JC and QL performed the statistical analysis and SX wrote the article. LN, JL, BX contributed to data collection. YJ, HZ, QJ contributed to manuscript revision. All authors contributed to the study conception and design.
This research was supported by a grant from the Key Research and Development program of Hainan Province (ZDYF2020135) and Changsha Natural Science Foundation (kq2202427).
The authors would like to thank the Third Xiangya Hospital of Central South University for its support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Balmaceda, N., Aziz, M., Chandrasekar, V. T., McClune, B., Kambhampati, S., Shune, L., et al. (2021). Infection risks in multiple myeloma: A systematic review and meta-analysis of randomized trials from 2015 to 2019. BMC Cancer 21 (1), 730. doi:10.1186/s12885-021-08451-x
Bazarbachi, A. H., Al Hamed, R., Malard, F., Bazarbachi, A., Harousseau, J. L., and Mohty, M. (2022). Induction therapy prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma: An update. Blood Cancer J. 12 (3), 44. doi:10.1038/s41408-019-0205-9
Bladé, J., and Rosiñol, L. (2005). Renal, hematologic and infectious complications in multiple myeloma. Best. Pract. Res. Clin. Haematol. 18 (4), 635–652. doi:10.1016/j.beha.2005.01.013
Blimark, C., Holmberg, E., Mellqvist, U. H., Landgren, O., Björkholm, M., Hultcrantz, M., et al. (2015). Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica 100 (1), 107–113. doi:10.3324/haematol.2014.107714
Boyd, K. D., Ross, F. M., Chiecchio, L., Dagrada, G. P., Konn, Z. J., Tapper, W. J., et al. (2012). A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: Analysis of patients treated in the MRC myeloma IX trial. Leukemia 26 (2), 349–355. doi:10.1038/leu.2011.204
Camp, R. L., Dolled-Filhart, M., and Rimm, D. L. (2004). X-Tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res. 10 (21), 7252–7259. doi:10.1158/1078-0432.CCR-04-0713
Chen, J., Wang, X. T., Luo, P. H., and He, Q. J. (2015). Effects of unidentified renal insufficiency on the safety and efficacy of chemotherapy for metastatic colorectal cancer patients: A prospective, observational study. Support. Care Cancer 23 (4), 1043–1048. doi:10.1007/s00520-014-2461-3
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell. 144 (5), 646–674. doi:10.1016/j.cell.2011.02.013
Hirahara, T., Arigami, T., Yanagita, S., Matsushita, D., Uchikado, Y., Kita, Y., et al. (2019). Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer 19 (1), 672. doi:10.1186/s12885-019-5903-y
Jafri, S. H., Shi, R., and Mills, G. (2013). Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 13, 158. doi:10.1186/1471-2407-13-158
Kumar, S., Paiva, B., Anderson, K. C., Durie, B., Landgren, O., Moreau, P., et al. (2016). International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet. Oncol. 17 (8), e328–e346. doi:10.1016/S1470-2045(16)30206-6
Kusunoki, K., Toiyama, Y., Okugawa, Y., Yamamoto, A., Omura, Y., Ohi, M., et al. (2020). Advanced lung cancer inflammation index predicts outcomes of patients with colorectal cancer after surgical resection. Dis. Colon Rectum 63 (9), 1242–1250. doi:10.1097/DCR.0000000000001658
Li, S., Gong, T., Kou, C., Fu, A., Bolanos, R., and Liu, J. (2021). Clinical outcomes associated with chronic kidney disease in elderly medicare patients with multiple myeloma. Clin. Lymphoma Myeloma Leuk. 21 (6), 401–412.e24. doi:10.1016/j.clml.2021.01.015
Lin, C., Shen, H., Zhou, S., Liu, M., Xu, A., Huang, S., et al. (2020). Assessment of infection in newly diagnosed multiple myeloma patients: Risk factors and main characteristics. BMC Infect. Dis. 20 (1), 699. doi:10.1186/s12879-020-05412-w
Mandaliya, H., Jones, M., Oldmeadow, C., and Nordman, II (2019). Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl. Lung Cancer Res. 8 (6), 886–894. doi:10.21037/tlcr.2019.11.16
Mountzios, G., Samantas, E., Senghas, K., Zervas, E., Krisam, J., Samitas, K., et al. (2021). Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open 6 (5), 100254. doi:10.1016/j.esmoop.2021.100254
Mu, S., Ai, L., Fan, F., Sun, C., and Hu, Y. (2018). Prognostic role of neutrophil-lymphocyte ratio in multiple myeloma: A dose-response meta-analysis. Onco. Targets. Ther. 11, 499–507. doi:10.2147/OTT.S153146
Mukaida, N., Harada, A., and Matsushima, K. (1998). Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 9 (1), 9–23. doi:10.1016/s1359-6101(97)00022-1
Nakamura, K., Kassem, S., Cleynen, A., Chrétien, M. L., Guillerey, C., Putz, E. M., et al. (2018). Dysregulated IL-18 is a Key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer Cell. 33 (4), 634–648. e5. doi:10.1016/j.ccell.2018.02.007
Nucci, M., and Anaissie, E. (2009). Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin. Infect. Dis. 49 (8), 1211–1225. doi:10.1086/605664
O'Brien, S. N., Blijlevens, N. M., Mahfouz, T. H., and Anaissie, E. J. (2003). Infections in patients with hematological cancer: Recent developments. Hematol. Am. Soc. Hematol. Educ. Program 2003, 438–472. doi:10.1182/asheducation-2003.1.438
Palumbo, A., and Anderson, K. (2011). Multiple myeloma. N. Engl. J. Med. 364 (11), 1046–1060. doi:10.1056/NEJMra1011442
Palumbo, A., Avet-Loiseau, H., Oliva, S., Lokhorst, H. M., Goldschmidt, H., Rosinol, L., et al. (2015). Revised international staging system for multiple myeloma: A report from international myeloma working group. J. Clin. Oncol. 33 (26), 2863–2869. doi:10.1200/JCO.2015.61.2267
Park, Y. H., Yi, H. G., Lee, M. H., Kim, C. S., and Lim, J. H. (2017). Prognostic value of the pretreatment advanced lung cancer inflammation index (ALI) in diffuse large B cell lymphoma patients treated with R-CHOP chemotherapy. Acta Haematol. 137 (2), 76–85. doi:10.1159/000452991
Pei, Y., Cattran, D., and Greenwood, C. (1992). Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int. 42 (4), 960–966. doi:10.1038/ki.1992.374
Qian, J., Jin, J., Luo, H., Jin, C., Wang, L., Qian, W., et al. (2017). Analysis of clinical characteristics and prognostic factors of multiple myeloma: A retrospective single-center study of 787 cases. Hematology 22 (8), 472–476. doi:10.1080/10245332.2017.1309493
Raab, M. S., Podar, K., Breitkreutz, I., Richardson, P. G., and Anderson, K. C. (2009). Multiple myeloma. Lancet 374 (9686), 324–339. doi:10.1016/S0140-6736(09)60221-X
Raje, N. S., Anaissie, E., Kumar, S. K., Lonial, S., Martin, T., Gertz, M. A., et al. (2022). Consensus guidelines and recommendations for infection prevention in multiple myeloma: A report from the international myeloma working group. Lancet. Haematol. 9 (2), e143–e161. doi:10.1016/S2352-3026(21)00283-0
Röllig, C., Knop, S., and Bornhäuser, M. (2015). Multiple myeloma. Lancet 385 (9983), 2197–2208. doi:10.1016/S0140-6736(14)60493-1
Romano, A., Laura Parrinello, N., Cerchione, C., Letizia Consoli, M., Parisi, M., Calafiore, V., et al. (2017). The NLR and LMR ratio in newly diagnosed MM patients treated upfront with novel agents. Blood Cancer J. 7 (12), 649. doi:10.1038/s41408-017-0019-6
Seike, M., Yanaihara, N., Bowman, E. D., Zanetti, K. A., Budhu, A., Kumamoto, K., et al. (2007). Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J. Natl. Cancer Inst. 99 (16), 1257–1269. doi:10.1093/jnci/djm083
Shiroyama, T., Suzuki, H., Tamiya, M., Tamiya, A., Tanaka, A., Okamoto, N., et al. (2018). Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 7 (1), 13–20. doi:10.1002/cam4.1234
Soekojo, C. Y., Low, J. Z., Oh, J., Ooi, M., De Mel, S., and Chng, W. J. (2020). Bacterial infection among patients with multiple myeloma treated with bortezomib-based induction therapy: Real-world experience in an asian cancer center. Clin. Lymphoma Myeloma Leuk. 20 (4), e165–e170. doi:10.1016/j.clml.2019.12.024
Sørrig, R., Klausen, T. W., Salomo, M., Vangsted, A., and Gimsing, P. (2019). Risk factors for infections in newly diagnosed multiple myeloma patients: A Danish retrospective nationwide cohort study. Eur. J. Haematol. 102 (2), 182–190. doi:10.1111/ejh.13190
Szudy-Szczyrek, A., Mlak, R., Mielnik, M., Szczyrek, M., Nowaczyńska, A., Homa-Mlak, I., et al. (2020). Prognostic value of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in multiple myeloma patients treated with thalidomide-based regimen. Ann. Hematol. 99 (12), 2881–2891. doi:10.1007/s00277-020-04092-5
Teh, B. W., Worth, L. J., Harrison, S. J., Thursky, K. A., and Slavin, M. A. (2015). Risks and burden of viral respiratory tract infections in patients with multiple myeloma in the era of immunomodulatory drugs and bortezomib: Experience at an Australian cancer hospital. Support. Care Cancer 23 (7), 1901–1906. doi:10.1007/s00520-014-2550-3
Yin, C., Toiyama, Y., Okugawa, Y., Omura, Y., Kusunoki, Y., Kusunoki, K., et al. (2021). Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: A propensity score matching analysis. Clin. Nutr. 40 (3), 1130–1136. doi:10.1016/j.clnu.2020.07.018
Keywords: advanced lung cancer inflammation index, multiple myeloma, chemotherapy response, infection, overall survival (OS)
Citation: Cheng J, Li Q, Xiao S, Nie L, Liao J, Jiang Q, Xiang B, Zhang H, Jiang Y and Yao C (2022) The advanced lung cancer inflammation index predicts chemotherapy response and infection risk in multiple myeloma patients receiving induction chemotherapy. Front. Genet. 13:1047326. doi: 10.3389/fgene.2022.1047326
Received: 18 September 2022; Accepted: 24 October 2022;
Published: 08 November 2022.
Edited by:
Rui Cao, Affiliated Beijing Friendship Hospital, Capital Medical University, ChinaReviewed by:
Yi Zhang, Xuzhou Medical University, ChinaCopyright © 2022 Cheng, Li, Xiao, Nie, Liao, Jiang, Xiang, Zhang, Jiang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenjiao Yao, eWFvY2hlbmppYW9AY3N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.