- 1Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2Fuyang People’s Hospital Pediatrics, Fuyang, China
This study aims to evaluate the incidence of low birth weight (LBW) and related maternal risk factors (during pregnancy or childbirth) and neonatal outcomes. A retrospective cross-sectional study design was used to select 7,421 pregnant women who gave birth in our hospital from January 2018 to June 2021. The data were analyzed using STATA 14.1, and the dependent variable (LBW) and risk were analyzed by the chi-square test of independence. The association between factors is used to determine the factors related to LBW through bivariate and multivariate logistic regression. The incidence of LBW in this study was 4.77%. Compared with single pregnant women, the probability of newborn LBW in married pregnant women is 40% lower (AOR = 0.60 95%CI: 0.40–0.90, p = 0.013). Compared with gestational age less than 37 weeks, the LBW probability of gestational age 37–42 and 42 weeks or older is 85 and 81% lower respectively (AOR = 0.15 95% CI: 0.10–0.24, p = 0.001; AOR = 0.19 95 %CI: 0.09–38, p = 0.001), compared with normal pregnant women, the probability of neonatal LBW among pregnant women with hypertension is 94% higher [AOR = 1.94 (95% CI: 1.39–2.74, p = 0.001). Compared with neonates with normal birth weight, neonates with LBW are at Apgar 1 min And Apgar 5 min score is lower than 7 (AOR = 0.52 95%CI: 0.37–0.73, p = 0.001, AOR = 0.54 95%CI: 0.38–0.75, p = 0.001) higher risk. In conclusion, women’s marital status (single), gestational age (<37 weeks), and combined hypertension are independently associated with LBW, and the higher risk of Apgar 1 min and Apgar 5 min scores <7 is an independent result of LBW.
Introduction
Birth weight is critical for neonatal survival, physical health, and development (Sharma et al., 2015). Low birth weight (LBW), defined as birth weight below 2,500 g, is considered a major health problem for newborns (World Health Organization, 2014). It is the leading cause of 40%–60% of neonatal deaths worldwide (UNICEF and United Nations Childr en’s Fund (UNICEF), 2008). Currently, the incidence of LBW worldwide is approximately 20 percent, of which 95 percent occur in low- and middle-income countries (World Health Organization, 2014). The incidence of LBW has not declined in any way over the past decade, which is of concern to medical staff, policymakers, and researchers (World Health Organization, 2014). Babies born with LBW are at risk for many health problems, including: hypothermia, hypoglycemia, cognitive impairment, malnutrition, etc (Qian and Gu, 2020; Shandong Province Multi-center Prognosis Evaluation Cooperative Group for Very Low Birth Weight Infants, 2020). In addition, LBW infants are at risk of Complication mortality is 20 times higher (Sharma et al., 2015).
The incidence of LBW continues to increase year by year despite efforts and a number of strategies specified (World Health Organization, 2014). The UNICEF-WHO 2019 report shows that global progress in reducing the incidence of LBW was slower in the period 2010–2015 compared to the period 2000–2009, thereby impacting the prevention of neonatal mortality and reducing stunting and the number of wasted children (World Health Organization, 2019). Understanding the incidence and influencing factors of LBW births is important for medical staff, hospital administrators, and policy makers because the feedback gathered can be used to implement the right strategies related to LBW prevention/reduction. Therefore, this study aimed to assess the incidence of LBW and associated maternal risk factors (during pregnancy or delivery) and neonatal outcomes.
Methods
Study population
This study adopted a retrospective cross-sectional study design, and selected 7,421 pregnant women who delivered in our hospital from September 2018 to June 2021 for retrospective analysis. Inclusion criteria: singleton pregnancy; neonates without congenital diseases. Exclusion criteria: incomplete maternal and infant information, unknown neonatal status (i.e., dead or alive), stillbirth, missing birth weight information, non-singleton pregnancy. The study was approved by the hospital ethics approval committee (Ethical approval K20180412), but no informed consent was obtained because it was a retrospective study.
Data collection
Based on a review of relevant literature (Agbozo et al., 2016; Mohammed et al., 2019), a structured data extraction questionnaire was designed and data was collected through the hospital electronic information recording system. The data was divided into two parts: sociodemographic characteristics of pregnant women and newborns, and obstetric characteristics. Sociodemographic characteristics include: age, marital status, education level, occupation of pregnant women. Obstetric characteristics were as follows: number of pregnancies, fetal presentation, gestational age, mode of delivery, hypertensive disorders, bleeding disorders, UTI/STI, premature rupture of membranes (PROM), and preeclampsia, and neonatal factors included: Neonatal gender, Apgar 1 min score, Apgar 5 min score, neonatal resuscitation, crying at birth.
Data analysis
Data were analyzed by STATA 14.1, descriptive statistics were used for categorical data, chi-square test was used to test the association between the dependent variable (LBW) and LBW risk factors, and bivariate and multivariate logistic regression was used to identify factors associated with LBW, significant variables in bivariate logistic regression were used for multivariate logistic regression and p < 0.05 values were considered statistically significant.
Result
Sociodemographic characteristics
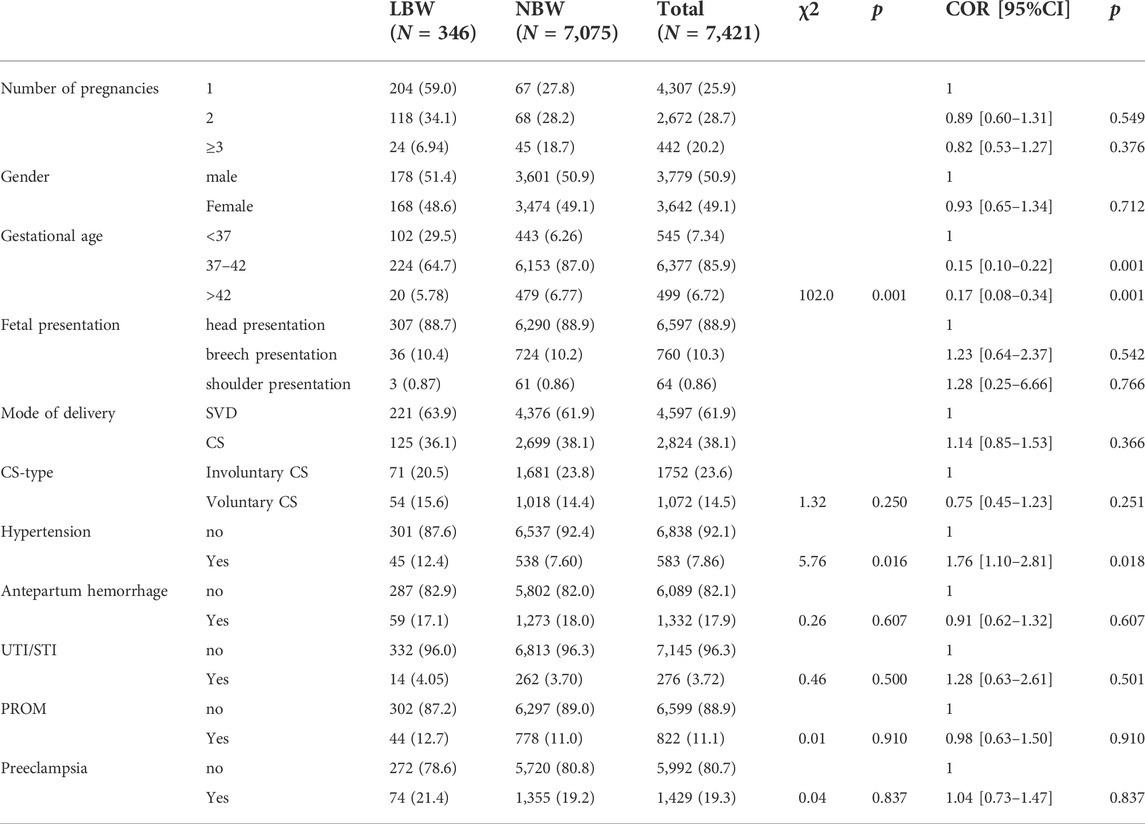
346 (4.78%) newborns weighed less than 2,500 g, most (58.2%) pregnant women were between 21–30 years old, 83.6% were married, the majority (63.0%) of pregnant women had secondary or higher education, and male newborns (50.9%) were slightly higher than females (50.9%, Table 1).
Maternal sociodemographic factors associated with low birth weight
Bivariate logistic regression analysis showed that compared with single pregnant women, married pregnant women were 31% less likely to have low birth weight neonates [COR = 0.69, 95%CI: 0.47–1.00, p = 0.043, Table 1; Figure 1).
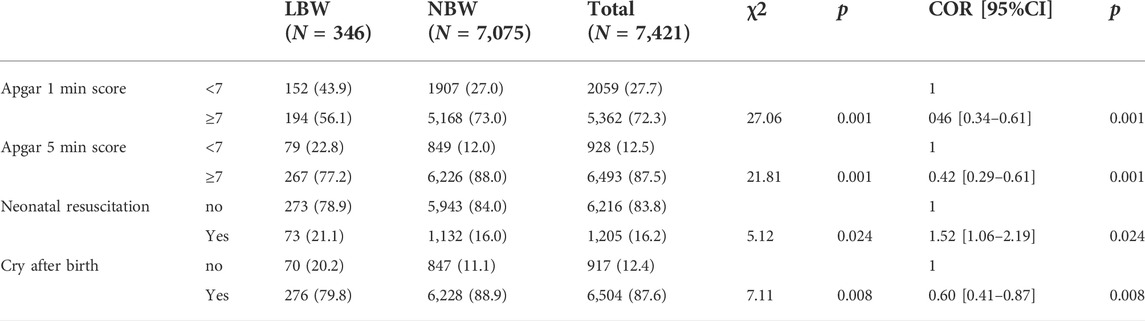
Antenatal and obstetric factors associated with low birth weight
Chi-square test of independence showed that gestational age (χ2 = 102.0, p = 0.001, Figure 2) and hypertension (χ2 = 5.76, p = 0.016, Figure 3) were significantly associated with LBW. Bivariate logistic regression analysis further showed that compared with gestational age less than 37 weeks, the incidence of LBW was reduced by 85% (COR = 0.15 95%CI: 0.10–0.22) at gestational age of 37–42 weeks, and the incidence of LBW was reduced by 83% at gestational age of more than 42 weeks (COR = 0.17 95%CI: 0.08–0.34, p < 0.001), the prevalence of LBW was 76% higher among pregnant women with hypertensive disorders (COR = 1.76 95%CI: 1.10–2.81, p = 0.018, Table 2).
Estimating risk of neonatal outcomes based on birth weight
Chi-square test of independence showed that LBW and Apgar 1 min score <7 (χ2 = 27.06, p = 0.001), Apgar 5 min score <7 (χ2 = 21.81, p = 0.001), and neonatal resuscitation (χ2 = 5.12, p = 0.024) was associated with crying at birth (χ2 = 7.11, p = 0.008). Bivariate logistic regression analysis also showed that LBW neonates had a higher risk of Apgar 1 min and Apgar 5 min scores less than 7 and a 52% higher neonatal resuscitation rate compared with normal-weight neonates (COR = 1.52 95%CI:1.06–2.19, p = 0.024) and a 40% lower rate of postnatal crying (COR = 0.60 95%CI: 0.60–0.87, p = 0.008, Table 3).
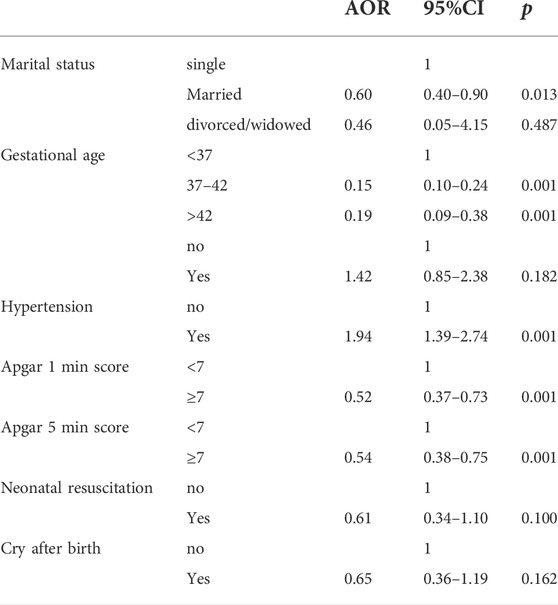
Multivariate logistic regression showing factors associated with low birth weight
Married pregnant women had a 40% lower chance of neonatal LBW compared with single pregnant women (AOR = 0.60 95%CI: 0.40–0.90, p = 0.013), compared with less than 37 weeks gestational age, the odds of LBW were 85 and 81% lower for gestational age 37–42 weeks and over 42 weeks (AOR = 0.15 95%CI: 0.10–0.24, p = 0.001; AOR = 0.19 95%CI: 0.09–0.38, p = 0.001), compared with normal pregnant women, pregnant women with hypertensive disorders had a 94% higher chance of neonatal LBW (AOR = 1.94 95%CI: 1.39–2.74, p = 0.001), compared with neonates with normal birth weight, LBW neonates had Apgar 1min and Apgar 5 min scores of less than 7 (AOR = 0.52 95%CI: 0.37–0.73, p = 0.001, AOR = 0.54 95%CI: 0.38–0.75, p = 0.001, Table 4) risk was higher.
Discussion
Birth weight is critical for neonatal survival, physical health, and development, so regional assessments of the incidence of LBW and its specific causes are recommended to alert healthcare workers and policy makers to appropriate interventions to improve women’s health before, during and after pregnancy. A total of 7,421 mother-infant pairs were included in this study, and the incidence of neonatal LBW was 4.77%. Our findings are lower than the 5.3% reported in Hu Meina’s article “Low Birth Weight Incidence and Influencing Factors”, and higher than the 4.3% reported in Wan Lixin’s “2018 Neonatal Low Birth Weight Incidence and Influencing Factors Analysis in Jilin Province” (Hu et al., 2019; Wan et al., 2020), and the difference in prevalence in different regions may be due to different living habits, living environment and economic factors.
The present study found a positive association between marital status and LBW in a logistic regression model, with married women having a 40% lower chance of having a newborn with LBW. This finding is consistent with previous studies reporting that pregnant women are married as a preventive factor for LBW (Agorinya et al., 2018). This is because most men (husbands) are the head of the household and have an important role in meeting the needs of their wives, including the decision to seek skilled nursing services during pregnancy. The lower odds of LBW newborns among married women may be due to the financial, mental, and physical support of the husband to keep the woman in a good state of mind during pregnancy and childbirth. This study observed a significant association between gestational age and LBW. Neonates born at term (37 weeks gestation and older) were 85% less likely to develop LBW compared with preterm infants (AOR = 0.15 95%CI: 0.10–0.24, p = 0.001). The results of the current study are similar to those reported in the previous literature (Liu et al., 2021). It was further shown that neonates born before 37 weeks of gestational age were more likely to develop LBW than neonates born at term. Furthermore, the present findings are consistent with another previously conducted study that indicated that gestational age was independently associated with the incidence of LBW: neonates born at 37 weeks of gestation or older were protective against LBW (Gebremedhin et al., 2015; Mengesha et al., 2017). It is clear that if the gestational age of the fetus is below the normal time frame (37 weeks gestation), there will be a dramatic loss of fetal weight due to preterm birth (World Health Organization, 2017). Therefore, in order to prevent preterm birth, any gynecological, medical or other conditions that may lead to preterm birth during pregnancy must be addressed promptly.
The current study found a clear association between hypertensive disorders of pregnancy (HDP) and LBW. Multiple studies worldwide have reported that women with HDP have a higher incidence of LBW than women with normotensive blood pressure (Wang et al., 2016; Zhang et al., 2016; Shen et al., 2019). Their findings are consistent with a secondary analytical survey conducted by WHO in low- and middle-income countries, confirming that women with HDP have twice the risk of having LBW babies (Adu-Bonsaffoh et al., 2017). In vitro experiments have confirmed that in gestational hypertension, trophoblast invasion of the spiral arteries supplying the placenta is reduced, in addition, due to reduced uteroplacental blood flow, resulting in small gestational age, preterm birth and intrauterine growth restriction, thus predisposing newborns to suffering from LBW (de Souza Rugolo et al., 2011; Amaral et al., 2017). Therefore, prompt and effective care for women with HDP is essential to reduce their complications and LBW. LBW infants were at higher risk for lower Apgar 1 min and Apgar 5 min scores than infants with normal birth weight. Likewise, previous studies have reported that infants born with LBW have an increased risk of low Apgar scores at the first and fifth minutes (Mitao et al., 2016). A study by Coutinho et al. (2018) found a significant association between neonates with LBW at birth and low Apgar scores at the first and fifth minutes. The similarity of the findings confirms that the incidence of low Apgar scores is inversely related to birth weight.
In conclusion, this study revealed a low incidence of LBW (4.77%), and that women’s marital status (single pregnant women), gestational age (<37 weeks), and comorbid hypertensive disorders were independent risk factors associated with LBW, while in Apgar Higher risk of 1 min and Apgar 5 min scores less than seven was an independent outcome of low birth weight. The current findings contribute to an understanding of the incidence of LBW and its influencing factors, which can guide healthcare providers, hospital administrators, and planners in the development and implementation of appropriate clinical and public health strategies aimed at reducing LBW. However, this study still has certain limitations. For example, although the sample size investigated is rich, most of them are from a single region, and the reference significance for the whole country is still limited. Secondly, the clinical factors included in this study are relatively few, and further research is needed supplement.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Fuyang People’s Hospital Pediatrics. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
YS and ZB collected data and wrote articles, LM, ZJ, QP, HY, and ZL collected data and analyzed statistics, ZN and MG provided guidance and funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adu-Bonsaffoh, K., Ntumy, M. Y., Obed, S. A., and Seffah, J. D. (2017). Perinatal outcomes of hypertensive disorders in pregnancy at a tertiary hospital in Ghana. BMC Pregnancy Childbirth 17 (1), 388–394. doi:10.1186/s12884-017-1575-2
Agbozo, F., Abubakari, A., Der, J., and Jahn, A. (2016). Prevalence of low birth weight, macrosomia and stillbirth and their relationship to associated maternal risk factors in Hohoe Municipality, Ghana. Midwifery 40 (18), 200–206. doi:10.1016/j.midw.2016.06.016
Agorinya, I. A., Kanmiki, E. W., Nonterah, E. A., Tediosi, F., Akazili, J., Welaga, P., et al. (2018). Socio-demographic determinants of low birth weight: Evidence from the kassena-nankana districts of the upper east region of Ghana. PloS One 13 (11), 02062077–e207135. doi:10.1371/journal.pone.0206207
Amaral, L. M., Wallace, K., Owens, M., and LaMarca, B. (2017). Pathophysiology and current clinical management of preeclampsia. Curr. Hypertens. Rep. 19 (8), 61–65. doi:10.1007/s11906-017-0757-7
Coutinho, P. R., Cecatti, J. G., Surita, F. G., Costa, M. L., and Morais, S. S. (2018). Perinatal outcomes associated with low birth weight in a historical cohort. Reprod. Health 8 (1), 18–22. doi:10.1186/1742-4755-8-18
De Souza Rugolo, L. M. S., Bentlin, M. R., and Trindade, C. E. P. (2011). Preeclampsia: Effect on the fetus and newborn. NeoReviews 12 (4), 198–206. doi:10.1542/neo.12-4-e198
Gebremedhin, M., Ambaw, F., Admassu, E., and Berhane, H. (2015). Maternal associated factors of low birth weight: A hospital based cross-sectional mixed study in tigray, northern Ethiopia. BMC Pregnancy Childbirth 15 (1), 222–224. doi:10.1186/s12884-015-0658-1
Hu, Me, Zhao, Y, and Zou, X (2019). The incidence and influencing factors of low birth weight[J]. Chin. J. Reproductive Health 30 (02), 127–130.
Liu, M, Fu, C, and Ma, G (2021). Analysis of risk factors for low birth weight infants in Guangxi [J]. Mod. Prev. Med. 48 (01), 63–66.
Mengesha, H. G., Wuneh, A. D., Weldearegawi, B., and Selvakumar, D. L. (2017). Low birth weight and macrosomia in tigray, northern Ethiopia: Who are the mothers at risk? BMC Pediatr. 17 (1), 144–149. doi:10.1186/s12887-017-0901-1
Mitao, M., Philemon, R., Obure, J., Mmbaga, B. T., Msuya, S., and Mahande, M. J. (2016). Risk factors and adverse perinatal outcome associated with low birth weight in northern Tanzania: A registry-based retrospective cohort study. Asian Pac. J. Reproduction 5 (1), 75–79. doi:10.1016/j.apjr.2015.12.014
Mohammed, S., Bonsing, I., Yakubu, I., and Wondong, W. P. (2019). Maternal obstetric and sociodemographic determinants of low birth weight: A retrospective cross-sectional study in Ghana. Reprod. Health 16 (1), 70–74. doi:10.1186/s12978-019-0742-5
Qian, Ye, and Gu, X (2020). Analysis of risk factors for low birth weight in Gusu District, Suzhou City from 2017 to 2018 [J]. China Public Health Manag. 36 (03), 385–387. doi:10.19568/j.cnki.23-1318.2020.03.025
Shandong Province Multi-center Prognosis Evaluation Cooperative Group for Very Low Birth Weight Infants (2020). A multi-center study of risk factors for extrauterine growth retardation in very low birth weight infants[J]. Chin. J. Pediatr. 58 (08), 653–660. doi:10.3760/cma.j.cn112140-20200326-00308
Sharma, S. R., Giri, S., Timalsina, U., Bhandari, S. S., Basyal, B., Wagle, K., et al. (2015). Low birth weight at term and its determinants in a tertiary hospital of Nepal: A case control study. PloS One 10 (4), 01239622–e124142. doi:10.1371/journal.pone.0123962
Shen, Z, Wang, Y, Ma, S, Zhan, Y. L., Wu, S. S., Feng, Y. H., et al. (2019). Risk factors for preterm birth, low birth weight and small for gestational age: A prospective cohort study. Chin. J. Epidemiol. 40 (09), 1125–1129. doi:10.3760/cma.j.issn.0254-6450.2019.09.020
UNICEF, United Nations Children's Fund (UNICEF) (2008). The state of the world's children 2009: Maternal and newborn health. New York, United States: Unicef.
Wan, L, Yi, L, and He, H (2020). Analysis on the incidence and influencing factors of low birth weight of newborns in Jilin Province in 2018 [J]. Mod. Prev. Med. 47 (21), 3903–3907.
Wang, S, Deng, Y, and Wu, Y (2016). Analysis of related factors of low birth weight infants [J]. China Maternal Child Health 31 (13), 2646–2648. doi:10.3969/j.issn.1001-4411.2003.05.023
World Health Organization (2017). Care of the preterm and low-birth-weight newborn: World prematurity day “let them thrive”. Available at: https:www.int/entity/maternal_child_adolescent/topics/newborn/care_of_preterm.
World Health Organization (2014). Global nutrition targets 2025: Low birth weight policy brief. Geneva, Switzerland: World Health Organization.
World Health Organization (2019). UNICEF-WHO low birthweight estimates: Levels and trends 2000-2015. Geneva, Switzerland: World Health Organization.
Keywords: low birth weight, risk factors, marital status, gestational age, incidence
Citation: Shaohua Y, Bin Z, Mei L, Jingfei Z, Pingping Q, Yanping H, Liping Z, Jiexin Y and Guoshun M (2022) Maternal risk factors and neonatal outcomes associated with low birth weight. Front. Genet. 13:1019321. doi: 10.3389/fgene.2022.1019321
Received: 15 August 2022; Accepted: 09 September 2022;
Published: 28 September 2022.
Edited by:
Qiu-Ning Liu, Yancheng Teachers University, ChinaReviewed by:
Kunimoto Dennis, University of Alberta, CanadaSteinhart Brian David, Samuel S Fels Fund, United States
Copyright © 2022 Shaohua, Bin, Mei, Jingfei, Pingping, Yanping, Liping, Jiexin and Guoshun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Shaohua, eWFuZ3NoYW9odWEyMDIyMDhAMTYzLmNvbQ==; Mao Guoshun, MTM3MDU1ODU4NjBAMTYzLmNvbQ==
 Yang Shaohua
Yang Shaohua Zheng Bin
Zheng Bin Liu Mei2
Liu Mei2 Yan Jiexin
Yan Jiexin