- 1Department of Medical Genetics, Capital Institute of Pediatrics, Beijing, China
- 2Department of Child Health Care, Children’s Hospital, Capital Institute of Pediatrics, Beijing, China
- 3Department of General Surgery, Children’s Hospital, Capital Institute of Pediatrics, Beijing, China
- 4Institute of Basic Medicine, Chinese Academy of Medical Sciences & School of Basic Medicine, Peking Union Medical College, Beijing, China
Background: To report detailed knowledge about the clinical manifestations, genetic spectrum as well as physical, language, neurodevelopment features and genotype-phenotype correlations of Chinese patients with Mowat-Wilson syndrome (MWS).
Methods: We retrospectively collected and analyzed clinical data for twenty-two patients with molecularly confirmed diagnoses. We used Gesell Developmental Schedules (GDS) to assess their neurodevelopment and the Diagnostic Receptive and Expressive Assessment of Mandarin-Infant & Toddler (DREAM-IT) to evaluate their language ability and compared the data with the two types of underlying pathogenic variations.
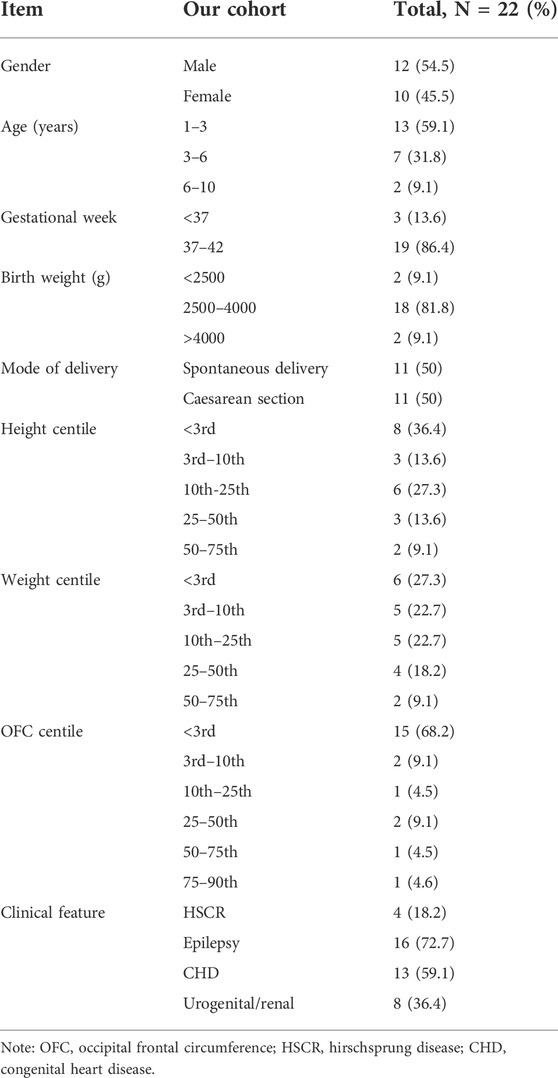
Results: The height and weight of all patients were below the 75th percentile, and microcephaly was observed in 16 of 22 patients (72.7%). Four patients carrying chromosome deletions encompassing the ZEB2 gene were more severely affected. All MWS patients exhibited better performance in cognitive play and social communication than in receptive and expressive language. In the receptive language area, the types of words that children with MWS understood most were nouns, followed by adjectives and verbs.
Conclusion: This study delineated the phenotypic spectrum of the largest MWS cohort in China and provided comprehensive profiling of their physical, language, neurodevelopment features and genotype-phenotype correlations.
Introduction
Mowat-Wilson syndrome (MWS, OMIM 235730) is a rare autosomal dominant disorder caused by a heterozygous deletion or loss-of-function variant of the ZEB2 gene (zinc finger E-box binding homeobox 2). Clinical manifestations of MWS are characterized by typical facial dysmorphism, moderate to severe intellectual disability (ID), global developmental delay, and multiple congenital anomalies, which may involve short stature, microcephaly, Hirschsprung disease (HSCR), corpus callosum agenesis, malformations of the brain, genitourinary anomalies (most commonly hypospadias), and congenital heart defects (Mowat et al., 2003). Since the first description in 1998, more than 350 MWS individuals have been reported (Garavelli et al., 2017; Ivanovski et al., 2018; Ivanovski et al., 2020), and the incidence of the syndrome is estimated to be 1:50,000–70,000 live births (Ghoumid et al., 2013).
The ZEB2 gene is a member of the ZEB family of zinc finger transcription factors, located on chromosome 2q22-q23, originally identified as a transcriptional corepressor for the transforming growth factor β signaling pathway through binding to SMAD proteins. To date, more than 220 pathogenic variants of ZEB2 have been associated with MWS according to the Human Gene Mutation Database (HGMD, http://www.hgmd.cf.ac.uk). Among them, point mutations have been identified in 70%–80% of MWS patients; full or partial deletion of the gene accounts for another 15%–50%, resulting in either truncated or absent protein products, and missense mutations are present in a small percentage (less than 2%) (Ivanovski et al., 2018). Although no obvious genotype-phenotype correlation has been established, MWS patients with large deletions of ZEB2 are suspected to have more severe phenotypes (Cerruti Mainardi et al., 2004; Ishihara et al., 2004).
In addition to a distinctive facial appearance, most patients with typical MWS have moderate to severe intellectual disabilities, accompanied by severe language impairment (Cordelli et al., 2021). As reported (Ivanovski et al., 2018; Ho et al., 2020), speech in MWS patients rarely extends to more than a few words, and many even have an absence of speech. Meanwhile, receptive language skills are believed to be less affected, and patients with absent speech can sometimes communicate successfully using alternative methods, such as gestures. Systematic language assessments of MWS patients to date have been sparse, and the correlation between its severity and the type of ZEB2 variation remains unclear. Herein, a comprehensive profile of the physical, language and neurodevelopment features in twenty-two MWS patients of Chinese ancestry was attempted. We used the GDS to assess their neurodevelopment and DREAM-IT questionnaires to evaluate their language ability. To our knowledge, this is the largest case series in China for detailed assessment of phenotypic, genetic spectrum and phenotype-genotype correlations, which will provide important guidance for appropriate intervention and patient care in the future.
Materials and methods
Study cohort
Altogether twenty-two MWS patients of Chinese ancestry were recruited, whose molecular confirmation of ZEB2 variations was identified. Basic clinical information was obtained through a web-based highly detailed questionnaire.
The number of involved systems was calculated for each patient according to their neurological, cardiac, gastrointestinal tract, urogenital/renal, eye, teeth, musculoskeletal and behavioral phenotypes. This study was approved by the Ethics Committee of Capital Institute of Pediatrics (SHERLL 2013039). Written informed consent was obtained from the guardians of all of the patients.
Assessment of neurodevelopment
The Gesell Developmental Schedules (GDS) (Gesell and Amatruda, 1941) is a classic international child development scale that examines five domains: adaptability, gross motor, fine motor, language, and social personality. The Chinese version of the GDS has been modified by the Chinese Pediatric Association with good validation against the Chinese reference population (Song and Zhu, 1987; Yang, 2016). GDS is widely used not only for assessing the neuropsychological development of children aged 0–6 years but also serves as one of the standardized methods for assessing the intellectual impairment of children aged 0–6 years (Chen et al., 2020). GDS is of diagnostic and rehabilitative significance for children who may have neuropsychological developmental delays (Liu et al., 2016). The results are expressed in terms of the developmental quotient (DQ), which is calculated as DQ = developmental age (DA)/chronological age (CA) × 100. Children with a DQ ≥ 85 were deemed normal in terms of developmental status, whereas those with a DQ < 40 were diagnosed as having severe neurodevelopmental disabilities. Based on the degree of neurodevelopmental disability, the DQ scores were divided into mild (55 ≤ DQ < 75), moderate (40 ≤ DQ < 55), severe (25 ≤ DQ < 40), and suspected (75 ≤ DQ < 84) neurodevelopmental disability.
Assessment of language development
The Diagnostic Receptive and Expressive Assessment of Mandarin-Infant & Toddler (DREAM-IT) is a standardized language test used to evaluate children’s language development levels and has good reliability and validity (De Villiers et al., 2018; Liu, 2019). DREAM-IT has 4 dimensions, including receptive language, expressive language, cognitive play and social interaction. The test is standardized on normal children 0–3 years old. The developmental age refers to the norm of children aged 0–3, and the results are expressed by the developmental quotient (DQ), which is calculated as DQ = developmental age (DA)//chronological age (CA) × 100. A quotient of greater than or equal to 80 points indicates no delay. In addition, DREAM-IT also calculates word number and types and evaluates the consonant development of the tested children.
Quality control
Three experienced psychologists who have obtained evaluation qualification certificates participated in this study. Before the formal investigation, conformance tests of the GDS and DREAM-IT were conducted, and the intragroup correlation coefficients were both above 0.9. For the same MWS child, the two tests were completed by one evaluator within 24 h, and the original score was recorded.
Statistical analysis
Statistical analysis was performed with SPSS Version 12 (SPSS China, Beijing, China). Differences in the listed categorical characteristics between patients were assessed using Student’s t test. p < 0.05 was considered statistically significant.
Results
Cohort analysis
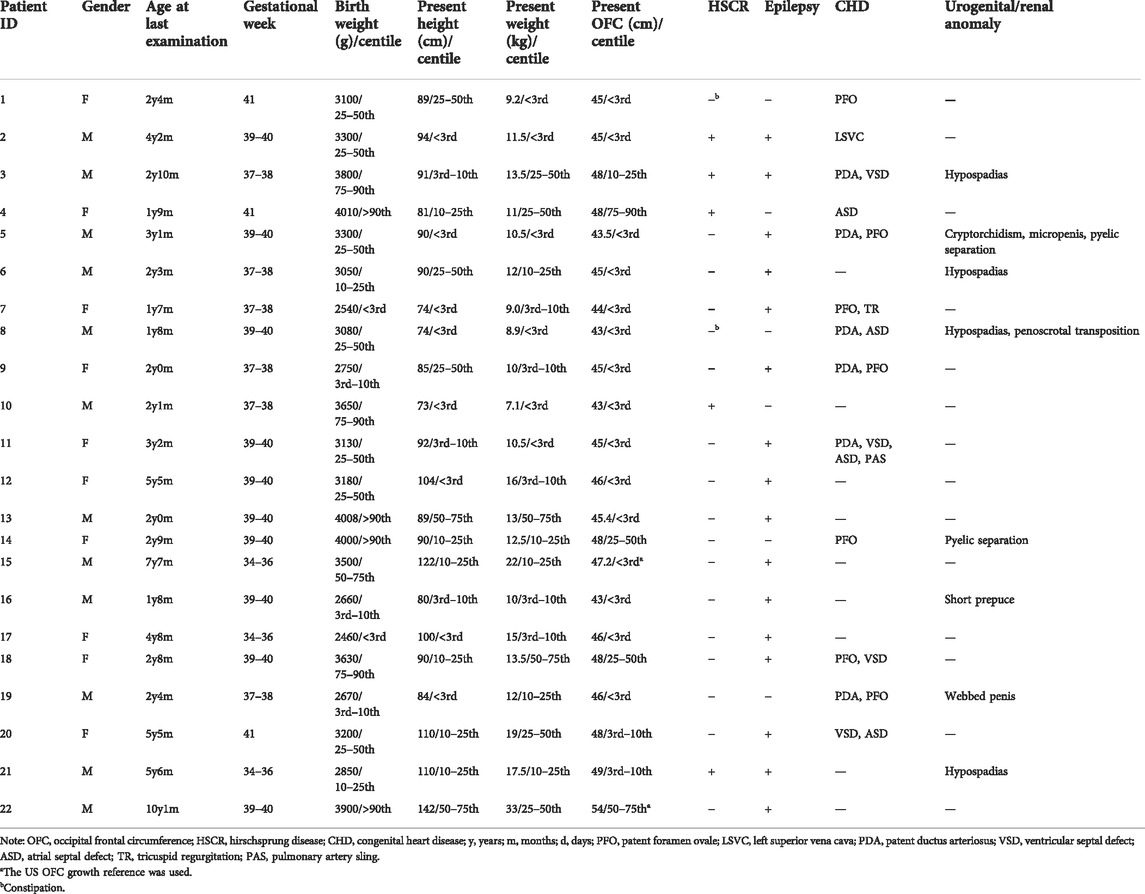
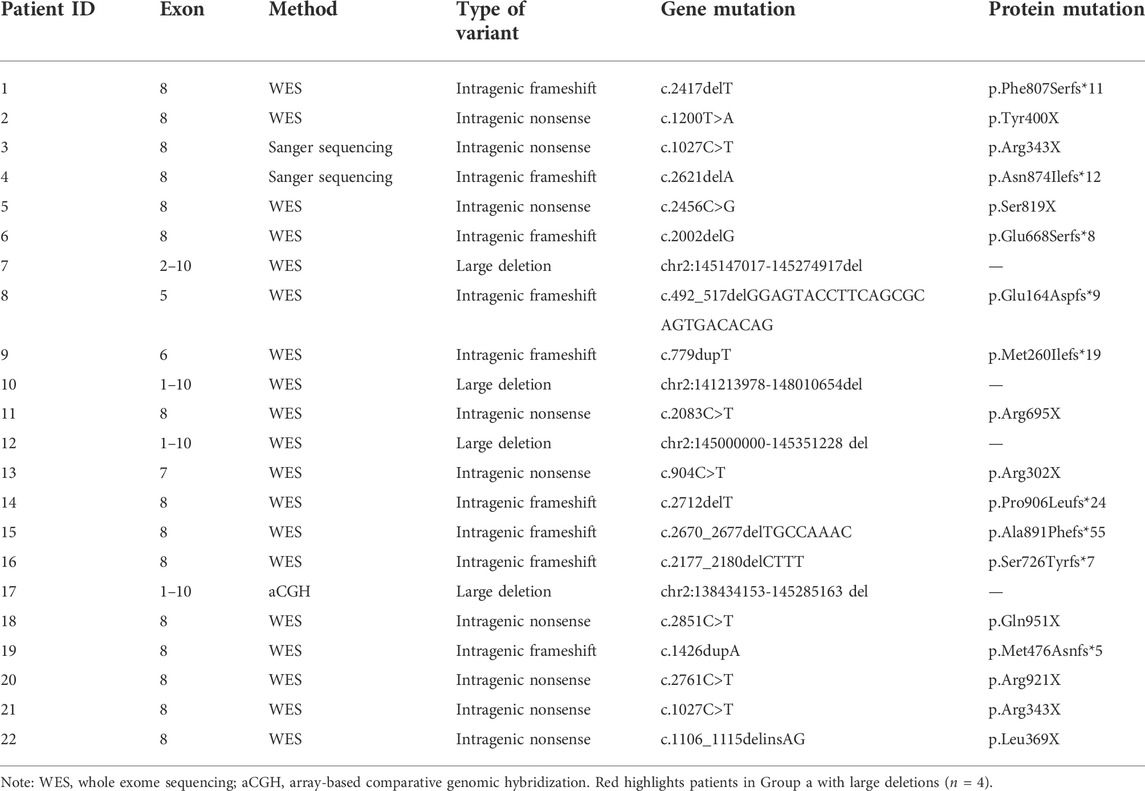
Overall, twenty-two patients were reported, ranging in age from 1 to 10 years (Tables 1, 2). The mean age at the last clinical evaluation was 3 years and 6 months. The male-to-female ratio was 12:10. All patients were sporadic, and all variants were de novo, heterozygous and classified as pathogenic according to the ACMG criteria. No consanguinity was reported. Nine patients were discovered to carry frameshift variants (40.9%), including small deletions or insertions. Nine patients had nonsense variants (40.9%), with two of them carrying the same point mutation (c.1027C>T, p.Arg343X). In addition, chromosome deletions encompassing the ZEB2 gene were found in four patients (18.2%), among which three cases were affected by whole gene deletion (0.35–6.85 Mb), and the latter was affected by intragenic deletion comprising exons 2–10 (0.13 Mb). The genetic and molecular study results are summarized in Figures 1A,B and Table 3.
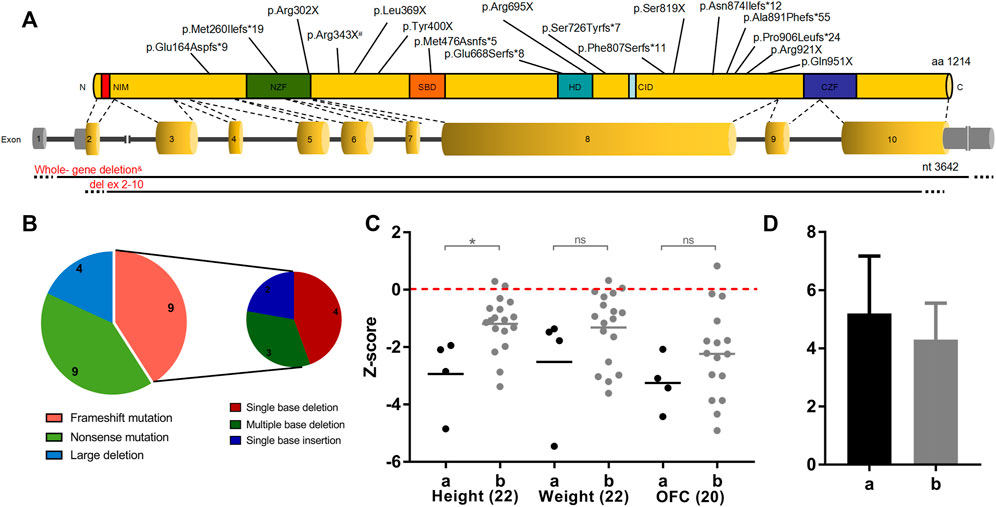
FIGURE 1. ZEB2 variations and the genotype-phenotype correlation with clinical features. (A) Position of genetic defects identified in our cohort on a schematic representation of the ZEB2 transcript and protein. Red represents Group a with large deletion (n = 4), black represents Group b with intragenic nonsense/frameshift variant (n = 18). #Two patients had the same nonsense mutation (c.1027C>T) of ZEB2, and &three patients had the whole ZEB2 gene deletion (0.35–6.85 Mb). (B) Distribution of the different ZEB2 defects found in our cohort. (C) Correlation between physical development (height, weight and OFC) and broad categories of the underlying heterozygous ZEB2 variants. Student’s t test was performed to compare between groups. *p < 0.05, ns, not significant. OFC, occipital frontal circumference. (D) Number of involved systems in relation to broad categories of the underlying ZEB2 variants.
Variants in the current study were stratified into two groups: a, large deletion, including all coding exons of a ZEB2 allele (n = 4); b, intragenic nonsense/frameshift variant, including nonsense and frameshift mutations within ZEB2 (n = 18). Based on these categories, we further delineated the clinical features in our patients.
Genotype-phenotype correlations with physical development
As shown in Table 1, both the height and weight of all twenty-two patients at the last examination were lower than the 75th percentile, with only 9% higher than the 50th percentile (2 in 22 patients) (Li et al., 2009; Zong and Li, 2013). Because the Chinese OFC (occipital frontal circumference) standard is set for children younger than 6 years old, the approximate percentile of two older MWS patients aged 7 years (<3rd) and 10 years (50–75th) were estimated with the US OFC growth reference chart (Rollins et al., 2010) and not included in the following statistical analysis of the Z score value for consistency. Only two MWS OFCs were beyond the 50th percentile, and 72.7% of cases were microcephalic (16 of 22 patients, at least 2 SDs below the mean). After adjustment for age, Group b showed better status (Figure 1C). The mean Z score values of all 3 indices in Group a were lower than those in Group b, with statistical significance detected in height. In addition, the average number of involved systems in Group a was 5.2, which was higher than that in Group b (4.5) (Figure 1D).
Genotype-phenotype correlations with neurodevelopment
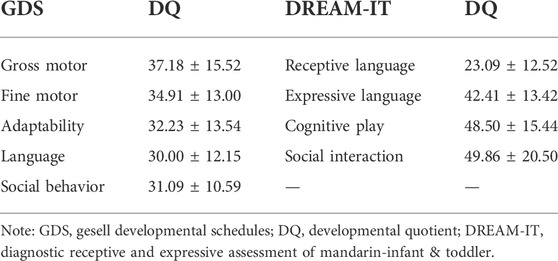
The GDS was applied to evaluate the mental development of each child with MWS. The average quotients of twenty-two MWS patients are listed in Table 4. The developmental quotients of all five domains were less than 40 points, which were comprehensively delayed, especially language. According to the variant categories, the developmental quotients in Group a were lower than those of Group b, except for the fine motor and gross motor subscale (Figure 2).
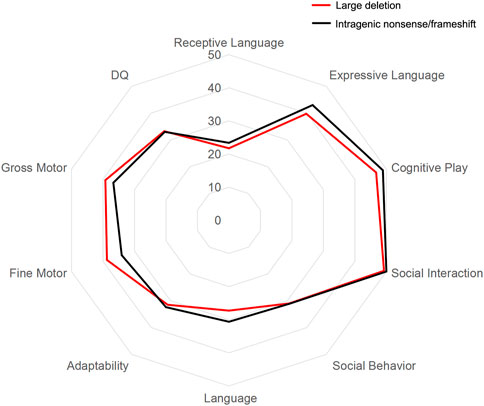
FIGURE 2. Results of Gesell Developmental Schedules (GDS) and Diagnostic Receptive and Expressive Assessment of Mandarin-Infant & Toddler (DREAM-IT) assessment in MWS patients. The mean scores of the general and subscale quotients of GDS and DREAM-IT assessment in two groups are shown. Red represents Group a with large deletion (n = 4), black represents Group b with intragenic nonsense/frameshift variant (n = 18).
Genotype-phenotype correlations with language development
DREAM-IT tests were conducted to assess the language development of all MWS children. As shown in Table 4, the scores of the four dimensions were all less than 80, indicating developmental delay, especially in receptive language function. In the receptive language area, the types of words that MWS children understood most were nouns, followed by adjectives and verbs. The word cloud analysis showed the most frequently occurring words in each category, including person (mommy, grandmother, brother), food (milk, steamed bun, water), common objects (bed, bowl, lamp), verbs (go, wait, wipe) and adjectives (no more, big, full) (Figure 3). According to the variant categories, the developmental scores of all four dimensions in Group a were lower than those in Group b (Figure 2).

FIGURE 3. Representative words most understood by MWS patients in our study. Word cloud analysis of all (A) and summary categories for nouns (B,C), adjectives (D) and verbs (E) using R package (wordcloud2, version 0.2.1) shows the most frequently occurring words. Font size denotes the frequency in each panel.
Discussion
MWS is caused by deleterious de novo heterozygous variations in the ZEB2 gene (Garavelli et al., 2017). The majority of variants lead to haploinsufficiency through premature stop codons or large deletions and thus can be conceived as in vivo models of the consequence of protein dysfunction on human neurodevelopment (Birkhoff et al., 2021). Moderate to severe intellectual disabilities are always present in MWS patients, accompanied by varying extents of language impairment (Garavelli et al., 2017; Ivanovski et al., 2018). A clear genotype-phenotype correlation has not yet been established. Our study attempted to comprehensively investigate physical, language and neurodevelopment in the largest MWS cohort of Chinese ancestry to date.
The average developmental quotients of twenty-two MWS patients evaluated by the GDS of all five domains were less than 40 points, suggesting a severe comprehensive delay, especially in language with the lowest quotient score. The ZEB2 gene might be particularly important in language/vocal learning, in addition to other roles in neurodevelopment. We hope the present study will not remain just another endpoint of research and instead act as a starting point to further explore its role in language and the underlying molecular mechanism. The DREAM-IT test can provide four-dimensional analysis of language ability. Of note, all of our patients were demonstrated to have developmental delays, with a score lower than 80. In the receptive language area, the words that children with MWS understood consist of various types, including nouns, verbs, and adjectives. However, in expressive language, they can only speak a few words or emit only some vowels or consonants. According to previous studies (Ivanovski et al., 2018; Ho et al., 2020), receptive language skills were comparatively preserved in MWS patients, especially in those with speech absence. Surprisingly, the score of receptive language was the lowest among the others, at 23.09 ± 12.52. This is related to the rapid development of receptive language compared with the expressive development of normal children at an early age (Luinge et al., 2006). Although the receptive language of children with MWS was comparatively preserved, their actual ability fell behind the normal range when the developmental age of receptive language was compared with the chronological age, resulting in a relatively low developmental quotient score. In addition, there are several objective factors that can limit the accurate assessment of language development in MWS patients: 1) neurodevelopmental profiles are not necessarily reported from standardized clinical protocols for each affected child; 2) measurement of the expressive ability can be very difficult in those with minimal verbal skills, such as MWS patients; and 3) it is possible that the sociability of many individuals with MWS leads to the impression of relative receptive strength, but this may not be the case in actuality.
In comparison with expressive language, receptive language was a more effective manner of communication between children with MWS and their caregivers. In addition, receptive language training, such as simple instructions or gestures, was more easily performed for parents compared to the former. It should be noted that the scores of cognitive play and social communication in our cohort were higher than those of receptive and expressive language (48.50 ± 15.44 and 49.86 ± 20.50 versus 23.09 ± 12.52 and 42.41 ± 13.42, respectively). Playing and social communication can effectively encourage children to receive and study language. The relatively higher cognitive play and social communication abilities revealed here should thus be stressed to help caregivers conduct language studies of MWS patients. In humans, accelerated genomic regions (ARs, the fastest-evolving sequences in the genome) are postulated as having neurological functions, potentially related to the evolution of larger brains and language (Whalen and Pollard, 2022). ARs have been identified as enriched in noncoding regions near genes with known speech functions in humans and vocal learning birds, including ZEB2, and this convergent evolution might be an indication of the particular importance of ZEB2 in vocal acquisition (Kamm et al., 2013; Oksenberg et al., 2013; Boyd et al., 2015; Cahill et al., 2021). However, the underlying mechanism of ZEB2 involvement in language development is still unknown.
ZEB2, a zinc finger transcription factor, plays an essential role during embryonic development. It is expressed throughout the central nervous system, from the mesencephalon to the spinal cord, indicating an important participation in the process of neurogenic and gliogenic specialization. To date, no obvious genotype-phenotype association has been established in MWS patients, but cases with large deletions tend to be correlated with more severe phenotypes (Zweier et al., 2006). In the current study, whole or partial gene deletion of ZEB2 was identified in 4 patients and assigned to Group a. Compared with the other 18 children in Group b, these patients exhibited more serious delays in physical, language and neurodevelopment, together with an increased severity with multiple systems affected, confirming the previous conclusion. The language ability of all patients within Group a exhibited a worse status in all four dimensions, and the three body indices were also lower than those of Group b, although at a limited sample size. Ivanovski’s study indicated that milder clinical presentations could be identified with variant ZEB2 proteins that were predicted to preserve some functionality (Ivanovski et al., 2018). Some researchers suspected that the defective protein may influence the expression level of the wild-type allele in a dominant-negative fashion. Others have debated that the dominant-negative fashion may be partial and not devastating for various reasons, such as less stability of the defective protein or the potential partial compensation of the loss of protein from the mutant allele (Rossi et al., 2015). Anyhow, more studies are needed to better understand the genotype-phenotype association, particularly correlating formal assessments of ID, speech or other phenotypes with functional genomic studies of these atypical variants in ZEB2.
In conclusion, our study delineated the phenotypic spectrum of the largest MWS cohort in China and provided comprehensive profiling of their physical, language and neurodevelopment features, which will provide important guidance for appropriate intervention and patient care in the future. The scarcity of functional data makes it difficult to establish genotype–phenotype correlations and assess their statistical significance, and further elucidation of this would require a deeper molecular analysis of the mutant ZEB2 function.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Capital Institute of Pediatrics (SHERLL 2013039). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
LHW and JW conceptualized and designed the study, drafted the initial manuscript. LHW and JW contributed equally to this paper. LeW, QX, and BZ assisted in the patient’s language and neurodevelopment assessment. ZZ, QL, and HW collected the data and carried out the initial analyses. LH conducted the word cloud analysis. QJ and LiW took overall responsibility for the design, implementation and analysis of the study. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This work was made possible by a grant from the Beijing Municipal Administration of Hospitals Incubating Program (PX2022053) to JW. QJ was supported by the National Natural Science Foundation of China (82070532, 81771620), the Public Service Development and Reform Pilot Project of Beijing Medical Research Institute (BMR 2019-11), the CAMS Innovation Fund for Medical Sciences (CIFMS) 2016-I2M-1-008 and the Research Foundation of Capital Institute of Pediatrics (CXYJ-2021-05). LiW was supported by the Capital’s Funds for Health Improvement and Research (2020-2-2104), the Public Service Development and Reform Pilot Project of Beijing Medical Research Institute (BMR 2019-11, BMR 2021-03), the Beijing Hospitals Authority’s Ascent Plan (DFL20221103) and the Research Foundation of Capital Institute of Pediatrics (FX-2019-06, QN-2020-08, CXYJ-2021-08, LCPY-2021-11, LCPY-2021-27).
Acknowledgments
We would like to express our sincere gratitude to all our patients and their family for participating in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Birkhoff, J. C., Huylebroeck, D., and Conidi, A. (2021). ZEB2, the mowat-wilson syndrome transcription factor: Confirmations, novel functions, and continuing surprises. Genes (Basel) 12 (7), 1037. doi:10.3390/genes12071037
Boyd, J. L., Skove, S. L., Rouanet, J. P., Pilaz, L. J., Bepler, T., Gordân, R., et al. (2015). Human-chimpanzee differences in a FZD8 enhancer alter cell-cycle dynamics in the developing neocortex. Curr. Biol. 25 (6), 772–779. doi:10.1016/j.cub.2015.01.041
Cahill, J. A., Armstrong, J., Deran, A., Khoury, C. J., Paten, B., Haussler, D., et al. (2021). Positive selection in noncoding genomic regions of vocal learning birds is associated with genes implicated in vocal learning and speech functions in humans. Genome Res. 31 (11), 2035–2049. doi:10.1101/gr.275989.121
Cerruti Mainardi, P., Pastore, G., Zweier, C., and Rauch, A. (2004). Mowat-wilson syndrome and mutation in the zinc finger homeo box 1B gene: A well defined clinical entity. J. Med. Genet. 41 (2), e16. doi:10.1136/jmg.2003.009548
Chen, Z., Li, R., Liu, H., Duan, J., Yao, C., Yang, R., et al. (2020). Impact of maternal hypertensive disorders on offspring's neurodevelopment: A longitudinal prospective cohort study in China. Pediatr. Res. 88 (4), 668–675. doi:10.1038/s41390-020-0794-9
Cordelli, D. M., Di Pisa, V., Fetta, A., Garavelli, L., Maltoni, L., Soliani, L., et al. (2021). Neurological phenotype of mowat-wilson syndrome. Genes (Basel) 12 (7), 982. doi:10.3390/genes12070982
De Villiers, J., Liu, X., Lee, w., Hutchings, T., Rolfhus, E., and Yao, L. (2018). Development of an early language and developmental screener for Chinese infants and toddlers: The DREAM-IT. Boston: ASHA Convention. ( United States).
Garavelli, L., Ivanovski, I., Caraffi, S. G., Santodirocco, D., Pollazzon, M., Cordelli, D. M., et al. (2017). Neuroimaging findings in mowat-wilson syndrome: A study of 54 patients. Genet. Med. 19 (6), 691–700. doi:10.1038/gim.2016.176
Gesell, A., and Amatruda, C. S. (1941). Clinical methods and practical applications. New York: Hoeber.Developmental diagnosis: Normal and abnormal child development
Ghoumid, J., Drevillon, L., Alavi-Naini, S. M., Bondurand, N., Rio, M., Briand-Suleau, A., et al. (2013). ZEB2 zinc-finger missense mutations lead to hypomorphic alleles and a mild Mowat-Wilson syndrome. Hum. Mol. Genet. 22 (13), 2652–2661. doi:10.1093/hmg/ddt114
Ho, S., Luk, H. M., Chung, B. H., Fung, J. L., Mak, H. H., and Lo, I. F. M. (2020). Mowat-wilson syndrome in a Chinese population: A case series. Am. J. Med. Genet. A 182 (6), 1336–1341. doi:10.1002/ajmg.a.61557
Ishihara, N., Yamada, K., Yamada, Y., Miura, K., Kato, J., Kuwabara, N., et al. (2004). Clinical and molecular analysis of Mowat-Wilson syndrome associated with ZFHX1B mutations and deletions at 2q22-q24.1. J. Med. Genet. 41 (5), 387–393. doi:10.1136/jmg.2003.016154
Ivanovski, I., Djuric, O., Broccoli, S., Caraffi, S. G., Accorsi, P., Adam, M. P., et al. (2020). Mowat-wilson syndrome: Growth charts. Orphanet J. Rare Dis. 15 (1), 151. doi:10.1186/s13023-020-01418-4
Ivanovski, I., Djuric, O., Caraffi, S. G., Santodirocco, D., Pollazzon, M., Rosato, S., et al. (2018). Phenotype and genotype of 87 patients with Mowat-Wilson syndrome and recommendations for care. Genet. Med. 20 (9), 965–975. doi:10.1038/gim.2017.221
Kamm, G. B., Pisciottano, F., Kliger, R., and Franchini, L. F. (2013). The developmental brain gene NPAS3 contains the largest number of accelerated regulatory sequences in the human genome. Mol. Biol. Evol. 30 (5), 1088–1102. doi:10.1093/molbev/mst023
Li, H., Ji, C. Y., Zong, X. N., and Zhang, Y. Q. (2009). Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi 47 (7), 487–492.
Liu, X. M. (2019). An overview of child language disorders and assessment. Chin. Sci. J. Lang. Speech Res. 17 (3), 161–165. doi:10.3969/j.issn.1672-4933.2019.03.001
Liu, Z. H., Li, Y. R., Lu, Y. L., and Chen, J. K. (2016). Clinical research on intelligence seven needle therapy treated infants with brain damage syndrome. Chin. J. Integr. Med. 22 (6), 451–456. doi:10.1007/s11655-015-1977-9
Luinge, M. R., Post, W. J., Wit, H. P., and Goorhuis-Brouwer, S. M. (2006). The ordering of milestones in language development for children from 1 to 6 years of age. J. Speech Lang. Hear. Res. 49 (5), 923–940. doi:10.1044/1092-4388(2006/067
Mowat, D. R., Wilson, M. J., and Goossens, M. (2003). Mowat-Wilson syndrome. J. Med. Genet. 40 (5), 305–310. doi:10.1136/jmg.40.5.305
Oksenberg, N., Stevison, L., Wall, J. D., and Ahituv, N. (2013). Function and regulation of AUTS2, a gene implicated in autism and human evolution. PLoS Genet. 9 (1), e1003221. doi:10.1371/journal.pgen.1003221
Rollins, J. D., Collins, J. S., and Holden, K. R. (2010). United States head circumference growth reference charts: Birth to 21 years. J. Pediatr. 156 (6), 907e902–913. doi:10.1016/j.jpeds.2010.01.009
Rossi, A., Kontarakis, Z., Gerri, C., Nolte, H., Hölper, S., Krüger, M., et al. (2015). Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524 (7564), 230–233. doi:10.1038/nature14580
Song, J., and Zhu, Y. M. (1987). Children’s neuropsychological tests. 2nd edn. Shanghai: Shanghai Scientific and Technological Publishing Company.
Whalen, S., and Pollard, K. S. (2022). Enhancer function and evolutionary roles of human accelerated regions. Annu. Rev. Genet. 56. doi:10.1146/annurev-genet-071819-103933
Yang, Y. F. (2016). Rating scales for children’s developmental behavior and mental Health. Beijing: People 's medical Publishing House.
Zong, X. N., and Li, H. (2013). Construction of a new growth references for China based on urban Chinese children: Comparison with the WHO growth standards. PLoS One 8 (3), e59569. doi:10.1371/journal.pone.0059569
Keywords: physical development, language assessment, Mowat-Wilson syndrome, phenotype-genotype correlation, ZEB2
Citation: Wu L, Wang J, Wang L, Xu Q, Zhou B, Zhang Z, Li Q, Wang H, Han L, Jiang Q and Wang L (2022) Physical, language, neurodevelopment and phenotype-genotype correlation of Chinese patients with Mowat-Wilson syndrome. Front. Genet. 13:1016677. doi: 10.3389/fgene.2022.1016677
Received: 11 August 2022; Accepted: 24 October 2022;
Published: 03 November 2022.
Edited by:
Paolo Bonanni, Eugenio Medea (IRCCS), ItalyReviewed by:
Meredith Wilson, Children’s Hospital at Westmead, AustraliaAglaia Vignoli, University of Milan, Italy
Livia Garavelli, AUSL IRCCS Reggio Emilia, Italy
Copyright © 2022 Wu, Wang, Wang, Xu, Zhou, Zhang, Li, Wang, Han, Jiang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Jiang, dGVhY29AMTI2LmNvbQ==; Lin Wang, Y2Fyb2xpbl93YW5nQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work
 Lihua Wu1†
Lihua Wu1† Bo Zhou
Bo Zhou Zhen Zhang
Zhen Zhang Hui Wang
Hui Wang Lu Han
Lu Han Qian Jiang
Qian Jiang Lin Wang
Lin Wang