- 1Department of Anesthesiology, Affiliated Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China
- 2Department of Pain Medicine, Harbin Medical University Cancer Hospital, Harbin, China
- 3Department of Anesthesiology, Ningbo First Hospital, Zhejiang, China
- 4Department of Anesthesiology, The First Affiliated Hospital of Dalian Medical University, Dalian, China
This study aims to investigate the prognostic impact of peripheral blood markers in patients with advanced non-small cell lung cancer (NSCLC) undergoing immunotherapy. In the current multicenter study, 157 advanced NSCLC cases treated by immunotherapy at three institutions were included. Biochemical parameters in baseline peripheral blood were collected. The associations between biochemical parameters and prognosis were investigated by the Kaplan–Meier survival analyses and Cox regression, and the predictive performances of biomarkers were evaluated via receiver operating characteristic analysis. The neutrophil-to-lymphocyte ratio (NLR) (progression-free survival [PFS]: hazard ratio [HR], 1.766; 95% confidence interval [CI], 1.311–2.380; p < 0.001; overall survival [OS]: HR, 1.283; 95% CI, 1.120–1.469; p < 0.001) and red blood cell distribution width (RDW) (PFS: HR, 1.052; 95% CI, 1.005–1.102; p = 0.031; OS: HR, 1.044; 95% CI, 1.001–1.091; p = 0.042) were revealed as independent predictors for both PFS and OS. In addition, NLR ≥3.79 (1-year PFS, 24.2% [95% CI, 15.2%–38.4%] versus 27.3% [95% CI, 18.2%–41.1%], p = 0.041; 1-year OS, 44.2% [95% CI, 32.5%–60.1%] versus 71.8% [95% CI, 60.6%–85.2%], p < 0.001) or RDW ≥44.8 g/L (1-year PFS, 19.2% [95% CI, 11.4%–32.3%] versus 31.7% [95% CI, 21.9%–46.0%], p = 0.049; 1-year OS, 54.0% [95% CI, 42.7%–68.3%] versus 63.1% [95% CI, 50.6%–78.6%], p = 0.014) was significantly correlated to poorer PFS and OS than NLR < 3.79 or RDW <44.8 g/L. Moreover, NLR and RDW achieved areas under the curve with 0.651 (95% CI, 0.559–0.743) and 0.626 (95% CI, 0.520–0.732) for predicting PFS, and 0.660 (95% CI, 0.567–0.754) and 0.645 (95% CI, 0.552–0.739), for OS. Therefore, PLR and RDW could help predict the immunotherapeutic efficacy of advanced NSCLC.
Introduction
Immune checkpoint inhibitors (ICIs), which target programmed cell death 1 (PD-1) and its ligand (PD-L1), are capable of inducing sustained antitumor effects, ushering in the therapeutic era for multiple malignant neoplasms (Okazaki et al., 2013; Ribas and Wolchok, 2018). In spite of this significant breakthrough, potent immunotherapeutic responses were only observed in approximately 20% advanced non-small cell lung cancer (NSCLC) population (Borghaei et al., 2015; Brahmer et al., 2015; Reck et al., 2016). Hence, precise recognition of patients who have the potential to derive additional benefits from ICIs is essential for the personalized treatment of advanced NSCLC.
Several biomarkers for immunotherapeutic efficacy of advanced NSCLC, such as tumor mutation burden (TMB), PD-L1, and tumor-infiltrating lymphocytes, have been revealed in previous publications (Kerr et al., 2015; Meng et al., 2015; High TMB Predicts Immunotherapy Benefit, 2018). However, in the current clinical practice, recognizing these signatures primarily resorts to core biopsy, which is unable to quantify the whole heterogeneity of tumors attributable to the limited specimens and simultaneously brings about a significant morbidity risk considering its invasive manipulation (Kerr et al., 2015; McLaughlin et al., 2016). As a result, a reliable and noninvasive instrument to predict the immunotherapeutic efficacy of advanced NSCLC is urgently needed.
Previous studies indicated that tumor-related inflammation played an important role in regulating tumor progression and immune infiltration (Jomrich et al., 2021). Moreover, biochemical parameters in peripheral blood provide a convenient and cost-effective path for reflecting the inflammatory status and their predictive potentials for immunotherapeutic efficacy have been investigated in various types of cancers receiving ICIs (Fukui et al., 2019; Nenclares et al., 2021; Valero et al., 2021). However, pieces of evidence for the value of biochemical parameters in peripheral blood in advanced NSCLC are insufficient. Therefore, this study, based on a multicenter population, proposes to explore the associations between pretreatment peripheral blood markers and prognosis in advanced NSCLC populations treated with ICIs.
Materials and methods
Study population
Approval of the institutional review boards and ethics committees of Harbin Medical University Cancer Hospital, Affiliated Drum Tower Hospital, and The First Affiliated Hospital of Dalian Medical University and a waiver for informed consent were obtained. Consecutive advanced NSCLC patients who underwent ICIs treatment in the abovementioned institutions between January 2016 to December 2020 were reviewed (Figure 1). Patients were included in this study when meeting the following criteria: 1) pathologically confirmed NSCLC; 2) stage III–IV; 3) administration of treatment ICIs, regardless of pretreatment line. The exclusion criteria included incomplete baseline data and lost to follow-up. All patients completed the follow-up survey before August 2022.
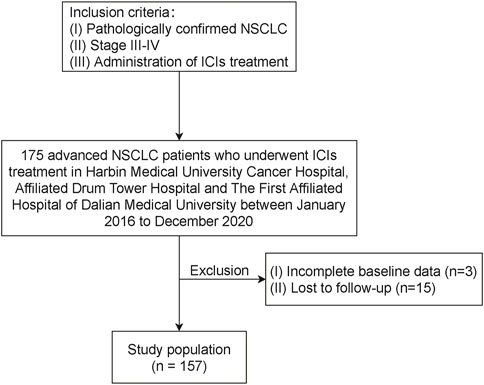
FIGURE 1. Flow chart illustrating patient inclusion. NSCLC, non-small cell lung cancer; ICI, immune checkpoint inhibitor.
Data collection
Clinicopathologic information was collected from electronic medical systems. Follow-up data were obtained through outpatient visits and telephone surveys. Overall survival (OS) was determined as the interval from initial ICI treatment to death or last follow-up. Progression-free survival (PFS) was calculated as the duration between initial ICI treatment and disease progress, death, or last follow-up.
Baseline peripheral blood samples were acquired within 7 days before immunotherapy, and routine blood biochemical parameters were collected. The inflammatory indexes were obtained based on the following formula: platelet-to-lymphocyte ratio (PLR) and absolute platelet count/absolute lymphocyte count; neutrophil-to-lymphocyte ratio (NLR) and absolute neutrophil count/absolute lymphocyte count; derived NLR (dNLR) and absolute neutrophil count/(white blood cell count-absolute neutrophil count); monocyte-to-lymphocyte ratio (MLR) and absolute monocyte count/absolute lymphocyte count; and systemic immune-inflammation index (SII), absolute neutrophil count×absolute platelet count/absolute lymphocyte count.
Statistical analysis
Pearson’s chi-squared test and Student’s t-test were implemented to compare the categorical and continuous parameters, respectively. Cox regressions and Kaplan–Meier survival analyses were conducted to recognize predictors for OS and PFS via the backward stepwise selection. The abovementioned statistical analyses were done using SPSS (version 23.0, IBM, Armonk, NY, United States) R software (version 4.1.1, http://www.R-project.org). A p value less than 0.05 was considered statistically significant.
Results
Clinicopathologic characteristics
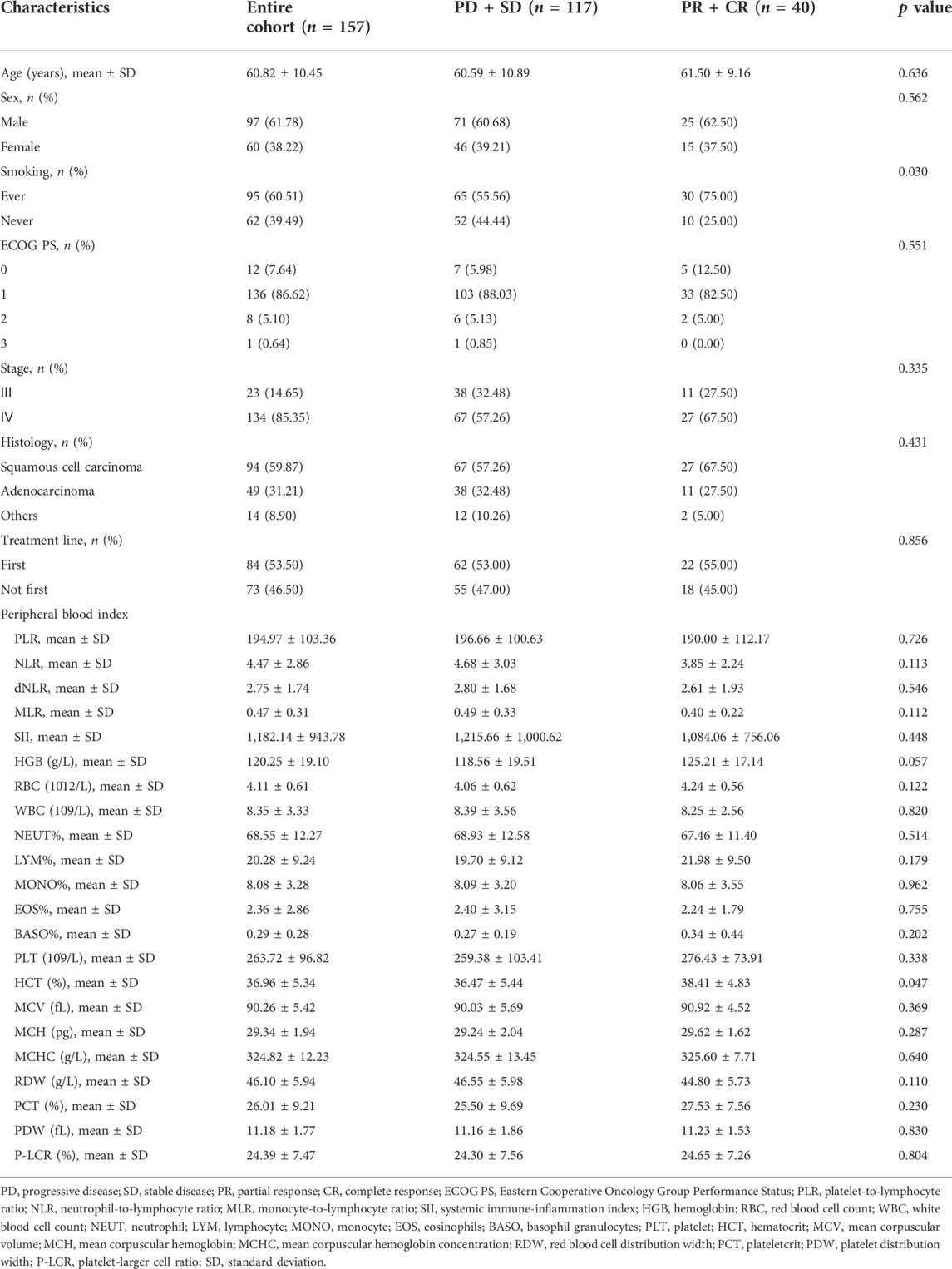
The clinicopathologic characteristics were displayed in Table 1. The entire cohort included 97 (61.78%) men and 60 (38.22%) women, and the mean age for the whole population was 60.82 years. Smoking history was identified in 95 (60.51%) patients. ECOG PS 1 (n = 136, 86.62%) accounted for the largest proportion. Most patients were diagnosed as stage Ⅳ (n = 134, 85.35%) and squamous cell carcinoma (n = 94, 59.87%). Regarding the peripheral blood indexes, the mean level of PLR, NLR, dNLR, MLR, SII, hemoglobin (HGB), red blood cell count (RBC), white blood cell count (WBC), percentage of neutrophil (NEUT%), percentage of lymphocyte (LYM%), percentage of monocyte (MONO), percentage of eosinophils (EOS), percentage of basophil granulocytes (BASO), platelet (PLT), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cell distribution width (RDW), plateletcrit (PCT), platelet distribution width (PDW), and platelet-larger cell ratio (P-LCR) were 194.97, 4.47, 2.75, 0.47, 1,182.14, 120.25 g/L, 4.11 × 10^12/L, 8.35 × 10^9/L, 68.55%, 20.28%, 8.08%, 2.36%, 0.29%, 263.72 × 10^9/L, 36.96%, 90.26 fL, 29.34 ng, 324.82 g/L, 46.10 g/L, 26.01%, 11.18 fL and 24.39%. In addition, in subgroup analyses between 117 patients evaluated as progressive disease (PD) or stable disease (SD) and 40 patients with partial response (PR) or complete response (CR), patients classified as PR or CR were associated with a significantly higher proportion of smoking history (75% versus 55.56%, p = 0.030) and a higher level of HCT (38.41% versus 36.47%, p = 0.047).
Prognostic impact of peripheral blood markers
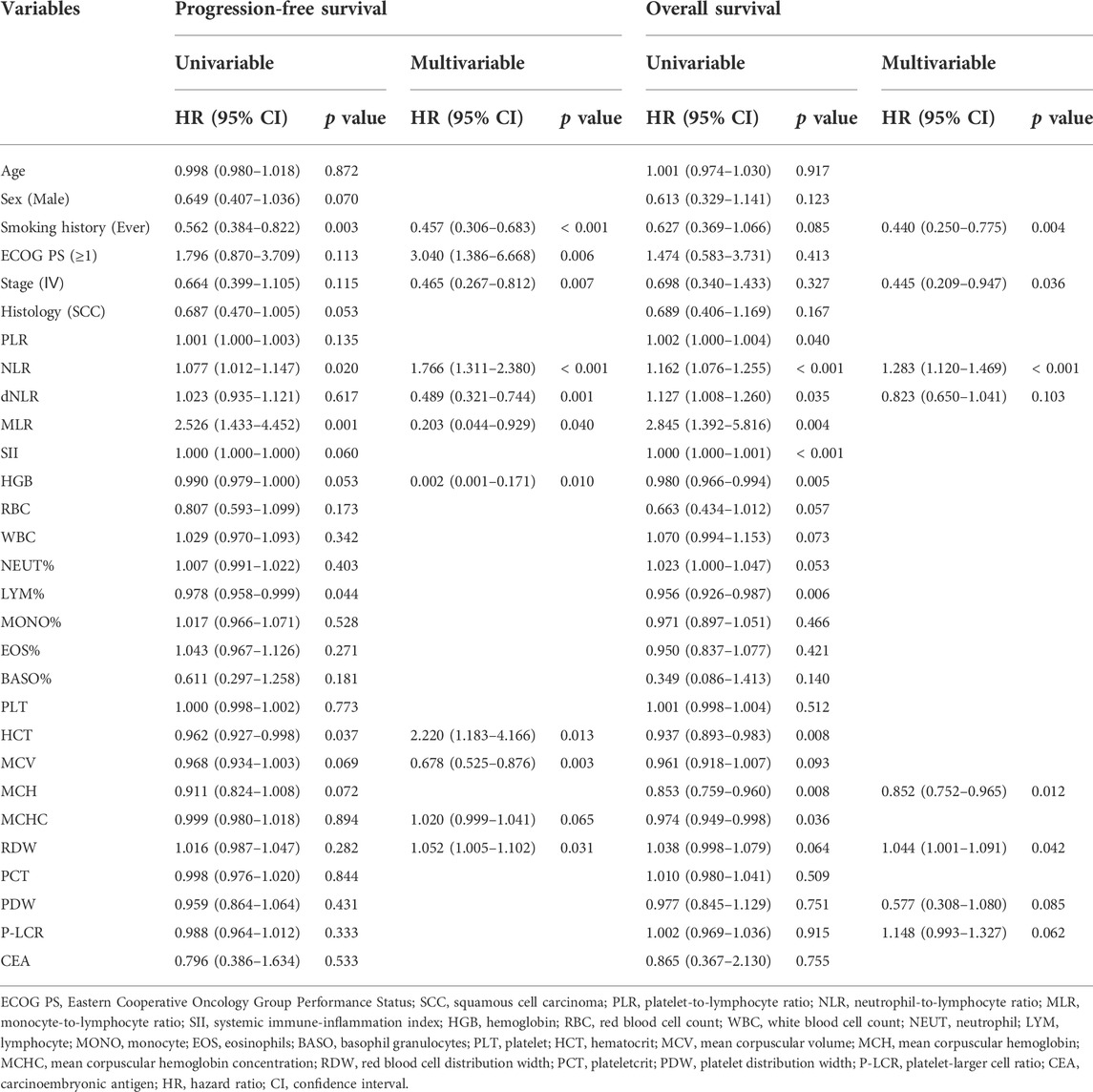
In the Cox survival analyses (Table 2), smoking history (hazard ratio [HR], 0.457; 95% confidence interval [CI], 0.306–0.683; p < 0.001), ECOG PS ≥ 1 (HR, 3.040; 95% CI, 1.386–6.668; p = 0.006), stage Ⅳ (HR, 0.465; 95% CI, 0.267–0.812; p = 0.007), NLR (HR, 1.766; 95% CI, 1.311–2.380; p < 0.001), dNLR (HR, 0.489; 95% CI, 0.321–0.744; p = 0.001), MLR (HR, 0.203; 95% CI, 0.044–0.929; p = 0.040), HGB (HR, 0.002; 95% CI, 0.001–0.171; p = 0.010), HCT (HR, 2.220; 95% CI, 1.183–4.166; p = 0.013), MCV (HR, 0.678; 95% CI, 0.525–0.876; p = 0.003), and RDW (HR, 1.052; 95% CI, 1.005–1.102; p = 0.031) were independent predictors for PFS. Similarly, smoking history (HR, 0.440; 95% CI, 0.250–0.775; p = 0.004), stage Ⅳ (HR, 0.445; 95% CI, 0.209–0.947; p = 0.036), NLR (HR, 1.283; 95% CI, 1.120–1.469; p < 0.001), MCH (HR, 0.852; 95% CI, 0.752–0.965; p = 0.012), and RDW (HR, 1.044; 95% CI, 1.001–1.091; p = 0.042) independently predicted OS.
As illustrated in Figure 2, ever-smoking patients achieved significantly better PFS (1-year PFS, 31.2% [95% CI, 22.2%–43.9%] versus 16.3% [95% CI, 8.3%–31.7%], p = 0.003) and OS (1-year OS, 64.0% [95% CI, 53.8%–76.3%] versus 49.1% [95% CI, 34.8%–69.1%], p = 0.042) compared with never-smoking patients. However, ECOG PS and stage failed to stratify the prognosis after immunotherapy. Moreover, as shown in Figure 3, by utilizing the median value as the cut-off, NLR ≥3.79 (1-year PFS, 24.2% [95% CI, 15.2%–38.4%] versus 27.3% [95% CI, 18.2%–41.1%], p = 0.041; 1-year OS, 44.2% [95% CI, 32.5%–60.1%] versus 71.8% [95% CI, 60.6%–85.2%], p < 0.001) or RDW ≥44.8 g/L (1-year PFS, 19.2% [95% CI, 11.4%–32.3%] versus 31.7% [95% CI, 21.9%–46.0%], p = 0.049; 1-year OS, 54.0% [95% CI, 42.7%–68.3%] versus 63.1% [95% CI, 50.6%–78.6%], p = 0.014) was significantly correlated to poorer PFS and OS than NLR< 3.79 or RDW< 44.8 g/L. In addition, patients with dNLR ≥2.41 (1-year OS, 48.5% [95% CI, 36.6%–64.4%] versus 67.8% [95% CI, 56.3%–81.5%], p = 0.013) or HGB <120 g/L (1-year OS, 51.1% [95% CI, 39.6%–65.9%] versus 67.6% [95% CI, 56.0%–81.7%], p = 0.046) showed inferiority only in OS than those with dNLR< 2.41 or HGB ≥120 g/L. However, other blood biochemical parameters did not stratify the prognosis of NSCLC receiving immunotherapy.
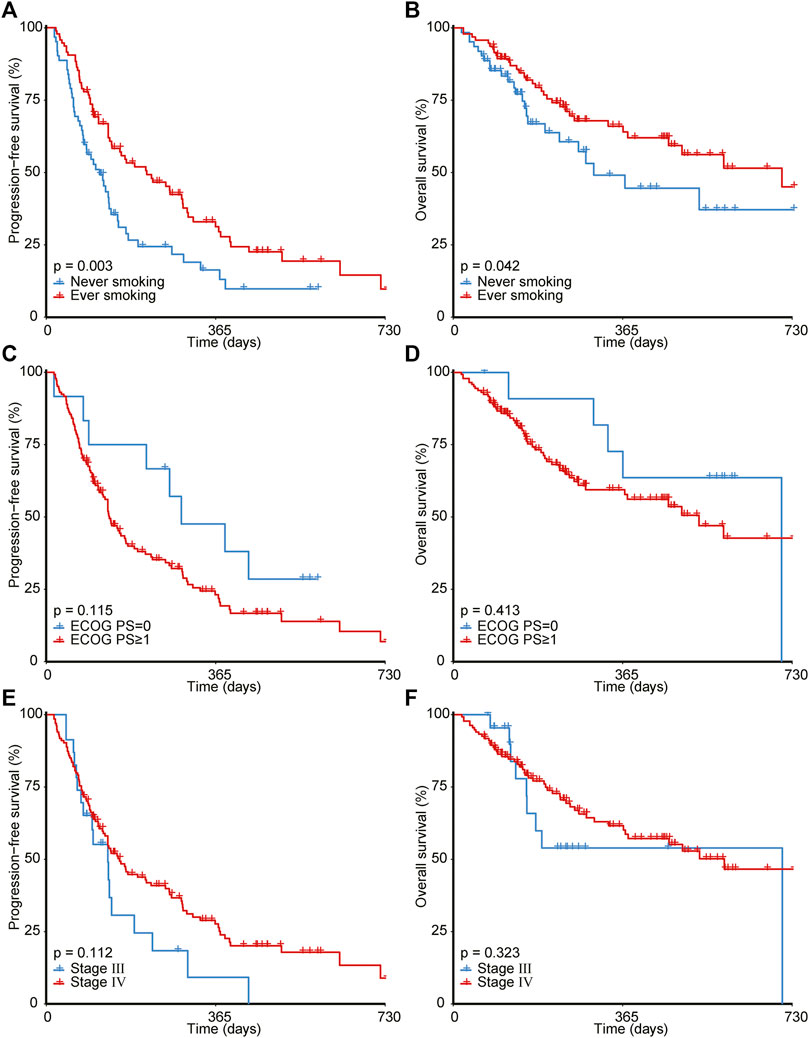
FIGURE 2. Survival analyses for patients with different smoking history (A,B), ECOG PS (C,D), and tumor stage (E,F). ECOG PS, Eastern Cooperative Oncology Group Performance Status.
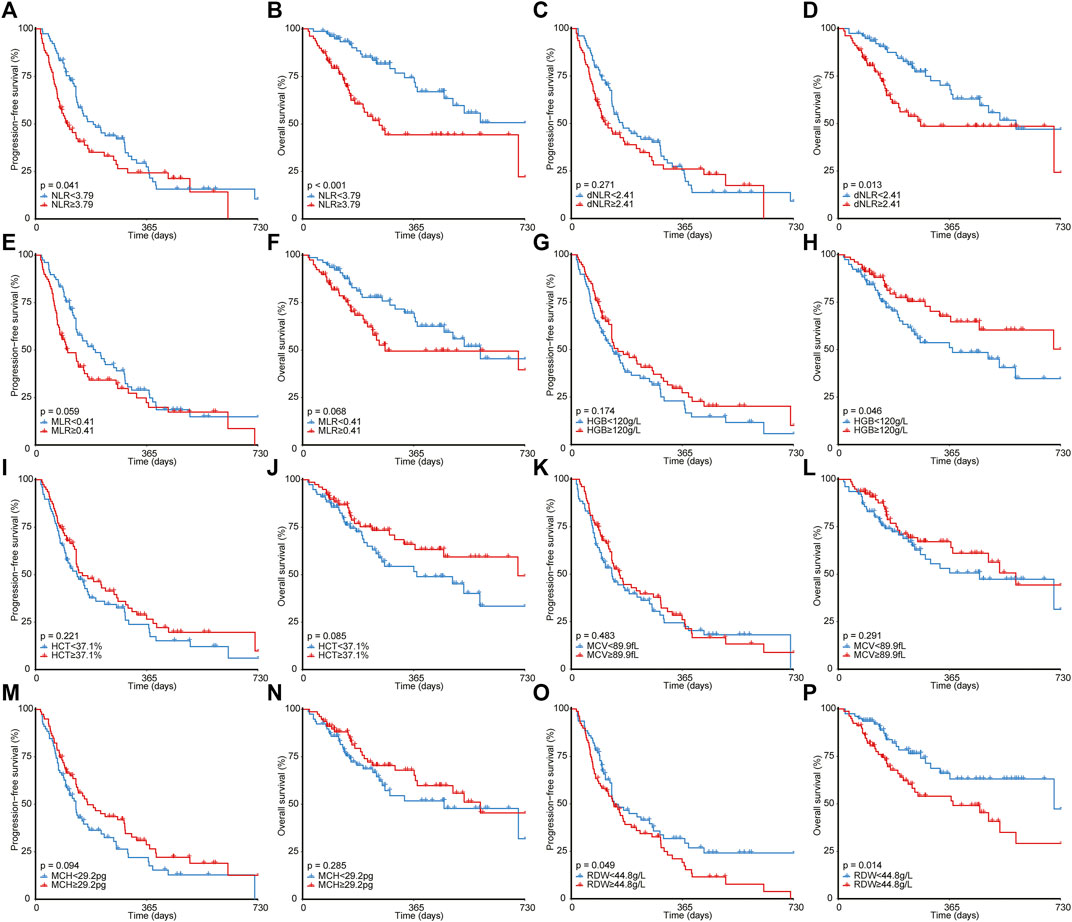
FIGURE 3. Survival analyses for patients with different NLR (A,B), dNLR (C,D), MLR (E,F), HGB (G,H), HCT (I,J), MCV (K,L), MCH (M,N), and RDW (O,P). NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; RDW, red blood cell distribution width.
Predictive performance of peripheral blood markers
Considering PLR and RDW were two independent inflammatory biomarkers for both PFS and OS, the receiver operating characteristic analysis was implemented to quantify the predictive performance of PLR and RDW (Figure 4). For predicting PFS, NLR and RDW achieved areas under the curves (AUCs) with 0.651 (95% CI, 0.559–0.743) and 0.626 (95% CI, 0.520–0.732). Similarly, in the prediction for OS, the performances of NLR and RDW were shown to have AUCs of 0.660 (95% CI, 0.567–0.754) and 0.645 (95% CI, 0.552–0.739).
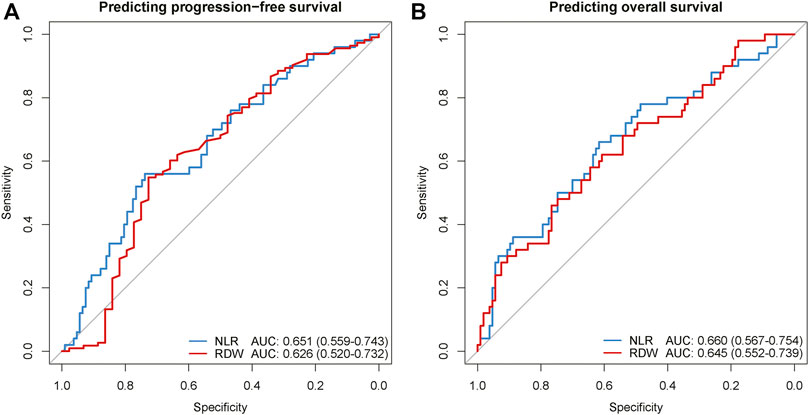
FIGURE 4. The ROC curves of NLR and RDW for predicting regression-free survival (A) and overall survival (B). ROC, receiver operating characteristic; AUC, area under the curve of the receiver operating characteristic; NLR, neutrophil-to-lymphocyte ratio; RDW, red blood cell distribution width.
Discussion
Despite immunotherapy having revolutionized the treatment paradigms of NSCLC (Okazaki et al., 2013; Ribas and Wolchok, 2018), the low response rate, therapy-related adverse effects, and high medical expense emphasize the significance of biomarkers for immunotherapeutic efficacy (Borghaei et al., 2015; Brahmer et al., 2015; Reck et al., 2016). In this study based on a multicenter population, we demonstrated that higher NLR and RDW in baseline peripheral blood were significantly correlated with poor PFS and OS in NSCLC patients undergoing ICIs treatment.
Previously, a number of studies have made investigations on this topic and revealed that TMB, PD-L1, and tumor-infiltrating lymphocytes derived from core biopsy specimens were correlated with immunotherapy prognosis of NSCLC (Kerr et al., 2015; Meng et al., 2015; High TMB Predicts Immunotherapy Benefit, 2018). However, these biomarkers suffered from biopsy-related morbidities due to their invasive nature. To overcome this limitation, further studies found that these markers in the peripheral blood also hold the potential to predict immunotherapy efficacy (Gandara et al., 2018; Wang et al., 2019; Bratman et al., 2020). Despite this breakthrough, these blood biomarkers were quantified based on peripheral blood mononuclear cells, which are too costly and time-consuming to acquire. In contrast, peripheral blood markers derived from routine complete blood count (CBC) are easily accessible and cost-effective, and thereby could be utilized as a convenient instrument in routine clinical practice.
Findings in our study were in line with previous publications that higher NLR was an adverse factor for the prognosis of NSCLC receiving immunotherapy (Fukui et al., 2019; Valero et al., 2021). In addition, Diem et al. (2017) concluded that PLR also played an important role in predicting immunotherapy response and prognosis and NSCLC patients with higher PLR tended to have an inferior prognosis. However, our study failed to validate the predictive efficiency of PLR: we speculated it might be attributable to that our study also included other biochemical parameters in the routine peripheral blood examination. Interestingly, we proved that increment of RDW significantly predicted poorer PFS and OS in NSCLC treated by immunotherapy, which was also observed in diffuse large B-cell lymphoma receiving immunotherapy (Beltran et al., 2019), but limited previous studies demonstrated its value in the NSCLC population. As such, we first indicated the capability of RDW as the biomarker for immunotherapeutic efficacy, and this finding might imply further insight into the prediction of immunotherapy response.
In addition to clinical implications, it is important to understand the biological basis underlying the prediction of NLR and RDW. The predictive mechanism of NLR might be rooted in its contributions to an immunosuppressive tumor microenvironment. On the one hand, as neutrophils were capable of releasing components mediating immunosuppression and tumor angiogenesis, neutrophil infiltration, thereby, established a microenvironment promoting cancer initiation, proliferation, and metastasis (Gonzalez et al., 2018; Shaul and Fridlender, 2019). On the other hand, reduced densities of lymphocyte infiltration contributed to the decreased response of antitumor T-cell, and the high level of neutrophils might further restrain T-cell response (Restifo et al., 2012; Zito Marino et al., 2017).
RDW, as an indicator representing the variations in the shape and size of red blood cells, is easily accessible in a routine CBC examination. The increased level of RDW implies a sign of impairments in erythropoiesis and red blood cell metabolism. The mechanism underlying the correlation of RDW with immunotherapeutic efficacy has not been clarified. However, several publications revealed that increasing RDW might result from oxidative stress, inflammation, and poor nutritional status via variation of erythropoiesis (Salvagno et al., 2015), and emerging pieces of evidence indicate that RDW was an adverse predictor for the prognosis of multiple malignancies (Koma et al., 2013; Albayrak et al., 2014; Ay et al., 2015).
Still, several limitations existed in the current study. First, despite the inclusion of a multicenter population, this study was limited by its retrospective nature, which suffered from selection bias and potential confounders. We utilized the multivariable regression to adjust prognostic predictors, but the impact of some known biomarkers, such as TMB, could not be evaluated. Thus, future prospective studies are required to validate our conclusions. Second, the small sample size reduces the power of the current study, and to be confirmed, further follow‐up studies enrolling a larger sample size need to be performed. Finally, the underlying mechanism of the biomarkers has not been elucidated, and future studies focusing on the biological basis of NLR and RDW are warranted.
Conclusion
Our study demonstrated that NLR and RDW in baseline peripheral blood could help stratify the prognosis of advanced NSCLC patients receiving immunotherapy. Thus, NLR and RDW harbor the potential to serve as effective biomarkers for immunotherapeutic efficacy in NSCLC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review boards of Harbin Medical University Cancer Hospital, Affiliated Drum Tower Hospital, and The First Affiliated Hospital of Dalian Medical University. The ethics committee waived the requirement of written informed consent for participation.
Author contributions
GZ conceived the original idea. LZ collected the clinical data. SL analyzed the clinical data and wrote the manuscript. LZ helped modify the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albayrak, S., Zengin, K., Tanik, S., Bakirtas, H., Imamoglu, A., and Gurdal, M. (2014). Red cell distribution width as a predictor of prostate cancer progression. Asian pac. J. Cancer Prev. 15 (18), 7781–7784. doi:10.7314/apjcp.2014.15.18.7781
Ay, S., Eryilmaz, M. A., Aksoy, N., Okus, A., Unlu, Y., and Sevinc, B. (2015). Is early detection of colon cancer possible with red blood cell distribution width? Asian pac. J. Cancer Prev. 16 (2), 753–756. doi:10.7314/apjcp.2015.16.2.753
Beltran, B. E., Paredes, S., Castro, D., Cotrina, E., Sotomayor, E. M., and Castillo, J. J. (2019). High red cell distribution width is an adverse predictive and prognostic factor in patients with diffuse large B-cell lymphoma treated with chemoimmunotherapy. Clin. Lymphoma Myeloma Leuk. 19 (9), e551–e557. doi:10.1016/j.clml.2019.06.005
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crino, L., Eberhardt, W. E. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Bratman, S. V., Yang, S. Y. C., Iafolla, M. A. J., Liu, Z., Hansen, A. R., Bedard, P. L., et al. (2020). Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat. Cancer 1 (9), 873–881. doi:10.1038/s43018-020-0096-5
Diem, S., Schmid, S., Krapf, M., Flatz, L., Born, D., Jochum, W., et al. (2017). Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 111, 176–181. doi:10.1016/j.lungcan.2017.07.024
Fukui, T., Okuma, Y., Nakahara, Y., Otani, S., Igawa, S., Katagiri, M., et al. (2019). Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: A prospective observational study. Clin. Lung Cancer 20 (3), 208–214. doi:10.1016/j.cllc.2018.04.021
Gandara, D. R., Paul, S. M., Kowanetz, M., Schleifman, E., Zou, W., Li, Y., et al. (2018). Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 24 (9), 1441–1448. doi:10.1038/s41591-018-0134-3
Gonzalez, H., Hagerling, C., and Werb, Z. (2018). Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 32 (19-20), 1267–1284. doi:10.1101/gad.314617.118
High TMB predicts immunotherapy benefit. Cancer Discov., 2018. 8(6): p. 668, doi:10.1158/2159-8290.CD-NB2018-048
Jomrich, G., Paireder, M., Kristo, I., Baierl, A., Ilhan-Mutlu, A., Preusser, M., et al. (2021). High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann. Surg. 273 (3), 532–541. doi:10.1097/SLA.0000000000003370
Kerr, K. M., Tsao, M. S., Nicholson, A. G., Yatabe, Y., Wistuba, I. I., Hirsch, F. R., et al. (2015). Programmed death-ligand 1 immunohistochemistry in lung cancer: In what state is this art? J. Thorac. Oncol. 10 (7), 985–989. doi:10.1097/JTO.0000000000000526
Koma, Y., Onishi, A., Matsuoka, H., Oda, N., Yokota, N., Matsumoto, Y., et al. (2013). Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One 8 (11), e80240. doi:10.1371/journal.pone.0080240
McLaughlin, J., Han, G., Schalper, K. A., Carvajal-Hausdorf, D., Pelekanou, V., Rehman, J., et al. (2016). Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2 (1), 46–54. doi:10.1001/jamaoncol.2015.3638
Meng, X., Huang, Z., Teng, F., Xing, L., and Yu, J. (2015). Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat. Rev. 41 (10), 868–876. doi:10.1016/j.ctrv.2015.11.001
Nenclares, P., Gunn, L., Soliman, H., Bover, M., Trinh, A., Leslie, I., et al. (2021). On-treatment immune prognostic score for patients with relapsed and/or metastatic head and neck squamous cell carcinoma treated with immunotherapy. J. Immunother. Cancer 9 (6), e002718. doi:10.1136/jitc-2021-002718
Okazaki, T., Chikuma, S., Iwai, Y., Fagarasan, S., and Honjo, T. (2013). A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 14 (12), 1212–1218. doi:10.1038/ni.2762
Reck, M., Rodriguez-Abreu, D., Robinson, A. G., Hui, R., Csoszi, T., Fulop, A., et al. (2016). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375 (19), 1823–1833. doi:10.1056/NEJMoa1606774
Restifo, N. P., Dudley, M. E., and Rosenberg, S. A. (2012). Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12 (4), 269–281. doi:10.1038/nri3191
Ribas, A., and Wolchok, J. D. (2018). Cancer immunotherapy using checkpoint blockade. Science 359 (6382), 1350–1355. doi:10.1126/science.aar4060
Salvagno, G. L., Sanchis-Gomar, F., Picanza, A., and Lippi, G. (2015). Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 52 (2), 86–105. doi:10.3109/10408363.2014.992064
Shaul, M. E., and Fridlender, Z. G. (2019). Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 16 (10), 601–620. doi:10.1038/s41571-019-0222-4
Valero, C., Lee, M., Hoen, D., Weiss, K., Kelly, D. W., Adusumilli, P. S., et al. (2021). Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 12 (1), 729. doi:10.1038/s41467-021-20935-9
Wang, Z., Duan, J., Cai, S., Han, M., Dong, H., Zhao, J., et al. (2019). Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 5 (5), 696–702. doi:10.1001/jamaoncol.2018.7098
Keywords: non-small cell lung cancer, biomarker, peripheral blood, neutrophil-to-lymphocyte ratio, red blood cell distribution width
Citation: Liu S, Zhao L and Zhou G (2022) Peripheral blood markers predict immunotherapeutic efficacy in patients with advanced non-small cell lung cancer: A multicenter study. Front. Genet. 13:1016085. doi: 10.3389/fgene.2022.1016085
Received: 10 August 2022; Accepted: 15 September 2022;
Published: 20 October 2022.
Edited by:
Chang Gu, Tongji University, ChinaCopyright © 2022 Liu, Zhao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohua Zhou, ZmVpc2hpcWkxQDE2My5jb20=
†These authors have contributed equally to this work
 Shuai Liu1†
Shuai Liu1† Guohua Zhou
Guohua Zhou