- 1Department of Endocrinology and Metabolism, Institute of Endocrine, NHC Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, The First Hospital of China Medical University, Shenyang, China
- 2Department of Thyroid Surgery, The First Hospital of China Medical University, Shenyang, China
Resistance to thyroid hormone beta (RTHβ) is an autosomal dominant hereditary disorder that is difficult to diagnose because of its rarity and variable clinical features, which are caused by mutations in the thyroid hormone receptor beta (THRB) gene. Recent studies have indicated a close association between THRB mutations and human cancers, but the mechanistic role of THRB mutations in carcinogenesis is unknown. Herein, we report two cases of RTHβ coexisting with papillary thyroid carcinoma (PTC) and their follow-up results. Two female patients presented with elevated serum thyroid hormone levels and nonsuppressed thyrotropin (TSH). Genetic analysis showed that each patient had a THRB gene mutation (p.P453T and p. R320H). Based on the results of ultrasound-guided fine-needle aspiration biopsy, the thyroid nodules were suspected to be PTC. Intraoperative pathology confirmed that the two patients had PTC with multifocal carcinoma of both lobes. One patient underwent total thyroidectomy and central lymph node dissection, and the other underwent total thyroidectomy alone. Following surgery, large doses of levothyroxine were administered to suppress TSH levels and prevent recurrent or persistent disease. However, it is difficult to continually suppress TSH levels below the upper limit of the normal range. To date, the two patients have experienced no recurrence of PTC on ultrasound.
Introduction
Resistance to thyroid hormone (RTH) is a clinical syndrome defined by impaired sensitivity to thyroid hormone (TH), and its more common form, termed RTHβ, is caused by mutations in the thyroid hormone receptor beta (THRB) gene (Pappa and Refetoff, 2021). Surveys of 80,884 and 74,992 newborns using thyroid stimulating hormone (TSH) and thyroxine (T4) measurements identified 2 and 4 infants with THRB gene mutations, indicating a prevalence of 1 in 40,000 and 1 in 18,750 live births, respectively (Lafranchi et al., 2003; Vela et al., 2019).
RTHβ is typically characterized by elevated thyroid hormone levels and concentrations of TSH either within the normal range or mildly elevated. The clinical phenotypes of RTHβ can be highly variable and include thyroid goiter, tachycardia, and abnormal neuronal development (Ortiga-Carvalho et al., 2014).
Several studies have demonstrated that thyroid hormone receptors (TRs) are involved in human cancer (González-Sancho et al., 2003). The reduced expression of TRs, caused by hypermethylation or deletion of TR genes, found in human cancers suggests that TRs could function as tumor suppressors (Kim and Cheng, 2013). A close association between somatic mutations of TRs and human cancers further supports the notion that the loss of normal TR functioning could lead to uncontrolled growth and loss of cell differentiation (Kim and Cheng, 2013).
In the present case report, two female Chinese patients with RTHβ and papillary thyroid carcinoma (PTC) are described. Furthermore, we conduct a literature review of patients with PTC coexisting with RTHβ, and we especially discuss the follow-up of TSH suppression therapy after surgery in this rare manifestation of PTC.
Case one
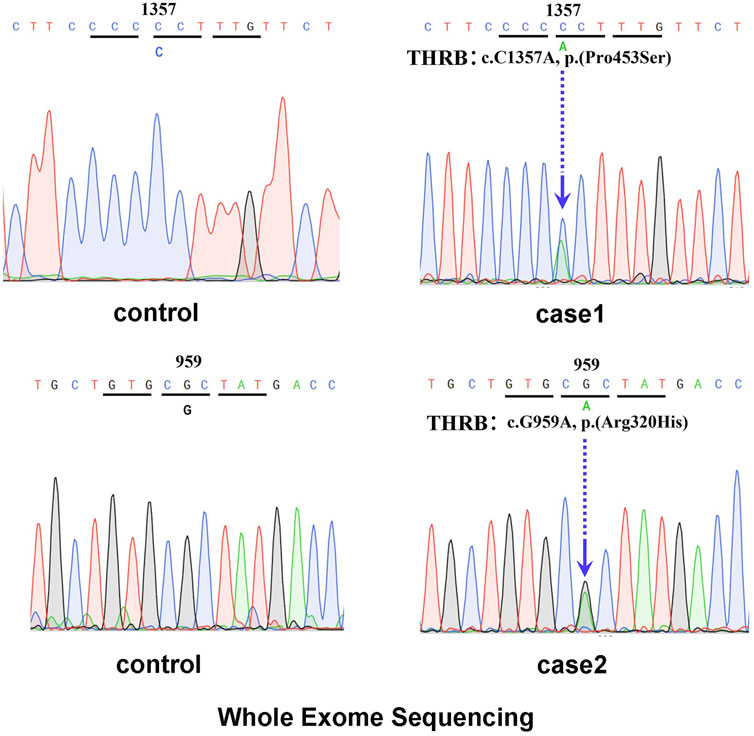
In February 2021, a 48-year-old Chinese female was referred to the Endocrinology Department due to thyroid disease. Two years prior, she noticed hyperthyroidism symptoms, such as palpitations and fatigue. She had a 3-month history of treatment with methimazole (MMI), allegedly for thyrotoxicosis. On physical examination, she presented with goiter, a heart rate of 100 beats per minute, no Graves’ ophthalmopathy and no hand tremor, although she complained of memory loss. Clinical measurements revealed out-of-range levels of circulating free thyroxine (FT4) and free triiodothyronine (FT3) in the presence of nonsuppressed TSH. Anti-thyroglobulin antibodies (TgAb) were slightly elevated, but anti-TSH receptor antibodies (TRAb) and anti-thyroid peroxidase antibodies (TPOAb) were negative (Table 1). Thyroid ultrasonography (USG) revealed multiple micronodules in the bilateral lobes that showed some suspicious features of malignancy (Thyroid Imaging Reporting and Data System (TI-RADS) grade 4c). Magnetic resonance imaging (MRI) did not show pituitary adenoma. Genetic analysis revealed a heterozygous missense mutation of THRB in exon 11 at codon 453 (P453T; c.1357C>A, Figure 1A). The same mutation was also detected in her mother and younger sister. Her mother also had multiple thyroid nodules (TI-RADS grade 4a), but her younger sister’s morphological features on thyroid USG were normal.
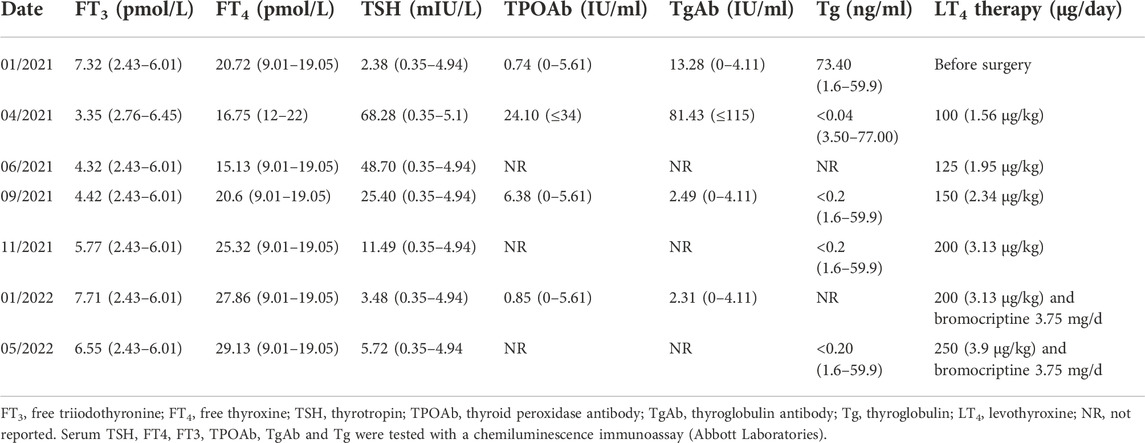
TABLE 1. Thyroid Indicators Measurement During Follow-Up of patient one as Related to Levothyroxine Doses.
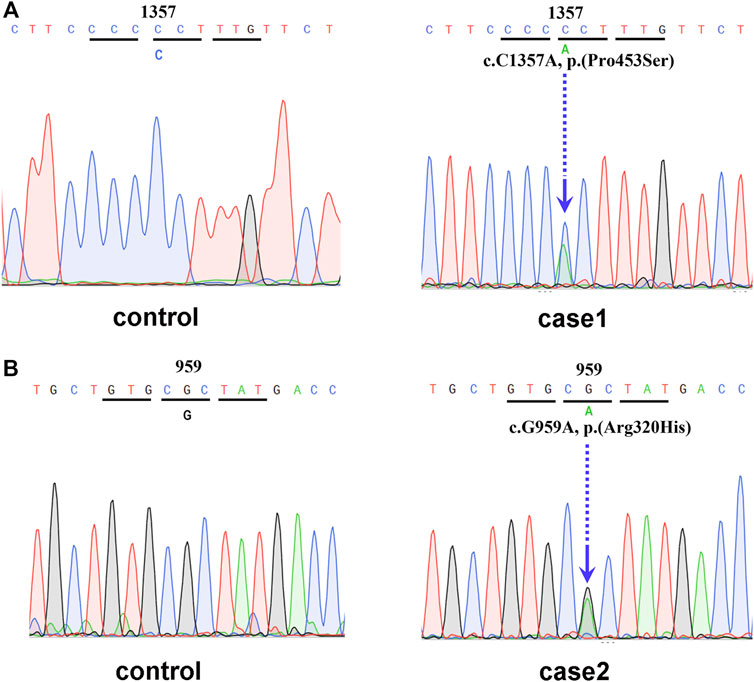
FIGURE 1. (A) Genetic analysis revealed a heterozygous mutation of THRB in exon 11 at codon 453 (P453T; c.1357C>A). (B) Genetic analysis revealed a heterozygous mutation of THRB in exon 10 at codon 320 (Arg320His, c.959G>A).
In March 2021, the patient underwent total thyroidectomy and central neck lymph node dissection. Pathology revealed multifocal micropapillary thyroid carcinomas in both lobes (5 mm diameter in the right lobe, 6 mm and 7 mm diameter in the left lobe), as well as metastases of PTC in 1 out of 14 central lymph nodes. Immunohistopathological staining was positive for a BRAF V600E mutation. The patient was diagnosed with RTHβ and PTC (T1aN1aM0 according to version 8 of the UICC/AJCC TNM system) and was rated as low risk in accordance with the 2015 American Thyroid Association (ATA) risk of recurrence stratification system. Since there were no signs of extrathyroidal extension or distant metastases, radioactive iodine remnant ablation was not performed.
After surgery, the patient received levothyroxine (LT4) at 75–100 μg/day (Table 1). Three months later, her TSH level was still elevated (TSH 48.7 mIU/L), and she complained of symptoms of hypothyroidism; therefore, we increased the dose of LT4 to 125 μg/day. After 3 months, her TSH level decreased to 25.4 mIU/L, and most of her hypothyroid symptoms disappeared. Due to the elevated TSH level, the dose of LT4 was increased to 150 μg/day. After 2 months, her serum TSH level was 11.49 mIU/L. To achieve a TSH level less than 0.5 mIU/L within the first year after surgery, the dose of LT4 was increased to 200 μg/day. Two months after this increase, the TSH level was 3.48 mIU/L. Subsequently, LT4 suppression therapy was continued together with 3.75 mg/day bromocriptine. The latest thyroid function tests 15 months postsurgery revealed that the patient’s TSH, FT4 and FT3 levels were slightly increased, but she exhibited no signs of hyperthyroidism. Thyroid USG demonstrated no signs of disease persistence or recurrence, and serum Tg levels were <0.20 ng/ml.
Case two
In November 2017, a 31-year-old Chinese female came to the clinic complaining of palpitations and dyspnea for 1.5 years that had worsened in the last 2 months. She also presented with tremor, fatigue, and poor memory. She reported a history of dyslipidemia and learning difficulties.
The laboratory examination revealed a normal TSH level despite elevated levels of thyroid hormone (Table 2). Thyroid USG showed several hypoechoic nodules with irregular borders and microcalcifications in both lobes, which indicated a grade of TI-RADS 4a. Furthermore, US-guided FNA was performed for the nodules that showed suspicious features, and the cytologic diagnosis was papillary carcinoma. BRAF V600E mutation analysis was positive. Pituitary MRI did not reveal any significant changes. Genetic analysis revealed a heterozygous missense mutation of THRB in exon 10 at codon 320 (Arg320His, c.959G>A; Figure 1B), which was also previously reported as a known mutation site in RTHβ.
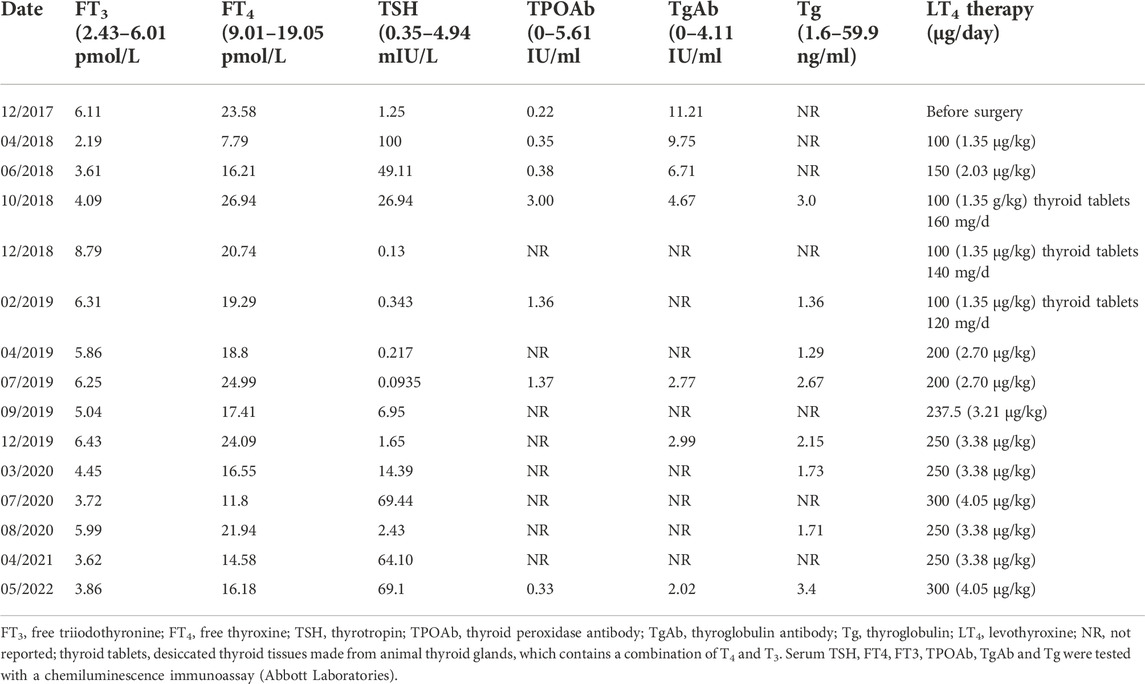
TABLE 2. Thyroid Indicators Measurement During Follow-Up of patient Two as Related to Levothyroxine Doses.
The patient underwent total thyroidectomy in March 2018. Histological examination showed micropapillary thyroid carcinomas (7 mm diameter in the right lobe and 2 mm diameter in the left lobe) without extrathyroidal invasion or lymph node metastasis (T1N0M0). Since the patient was evaluated as low risk for the recurrence of PTC, radioactive iodine (RAI) remnant ablation therapy was not administered. TSH suppression treatment was not initiated immediately after surgery since the patient refused to take levothyroxine. After the first month postsurgery, a thyroid function test showed a dramatic elevation in TSH concentration (>100 mIU/L). LT4 treatment was initiated to decrease the TSH concentration with an initial dose of 100 μg/day (Table 2). Two months later, the TSH concentration was reduced to 49.11 mU/L, and the LT4 dose was increased to 150 μg/day. Four months later, the thyroid function test showed a TSH concentration of 26.94 mIU/L and an elevated FT4 concentration of 26.936 mmol/L. Since LT3 was unavailable, 100 μg/day LT4 combined with 160 mg/day thyroid tablets derived from pig thyroid glands (containing both T3 and T4) was administered. The patient complained of obvious tachycardia. A thyroid function test showed a suppressed TSH concentration of 0.13 mIU/L, with elevated FT4 and FT3 concentrations. The dose of thyroid tablets was reduced to 120–140 mg/day. During the following 4 months, the results showed a suppressed TSH concentration of 0.22 mU/L, with normal FT4 and FT3 concentrations. However, even with β-blocker treatment, the tachycardia persisted, and the patient occasionally felt short of breath. Due to the cardiac side effects of the thyroid tablets, they were discontinued, and the dose of LT4 was increased to 200 μg/day. During the next 3 years, TSH was not persistently well controlled below the upper limit of the normal reference range, even though the dose of LT4 continued to increase (Table 2). She had no symptoms of hyperthyroidism. Her daily heart rate was approximately 60 bpm, and she was diagnosed with Grade 2 Type 1 atrioventricular block. In May 2022, considering her high TSH and Tg levels, she underwent a diagnostic 131I whole-body scan, which showed only normal thyroid remnants in the neck, without recurrence of thyroid carcinoma or metastasis.
Discussion
Since Taniyama et al. reported the first case of RTHβ coexisting with PTC in 2001, 17 cases of this rare disease have been reported, listed in Table 3. As a special type of PTC, the population characteristics of patients with this disease are similar to those of patients with classical PTC. For example, it occurs in all ages from 9 to 63, and 88% of them are adults, which is consistent with the much lower incidence of PTC in children than in adults. The ratio of males to females among patients with RTHβ coexisting with PTC is 1:3.25, which is similar to the ratio of males to females (approximately 1:4.39) with PTC (LeClair et al., 2021).
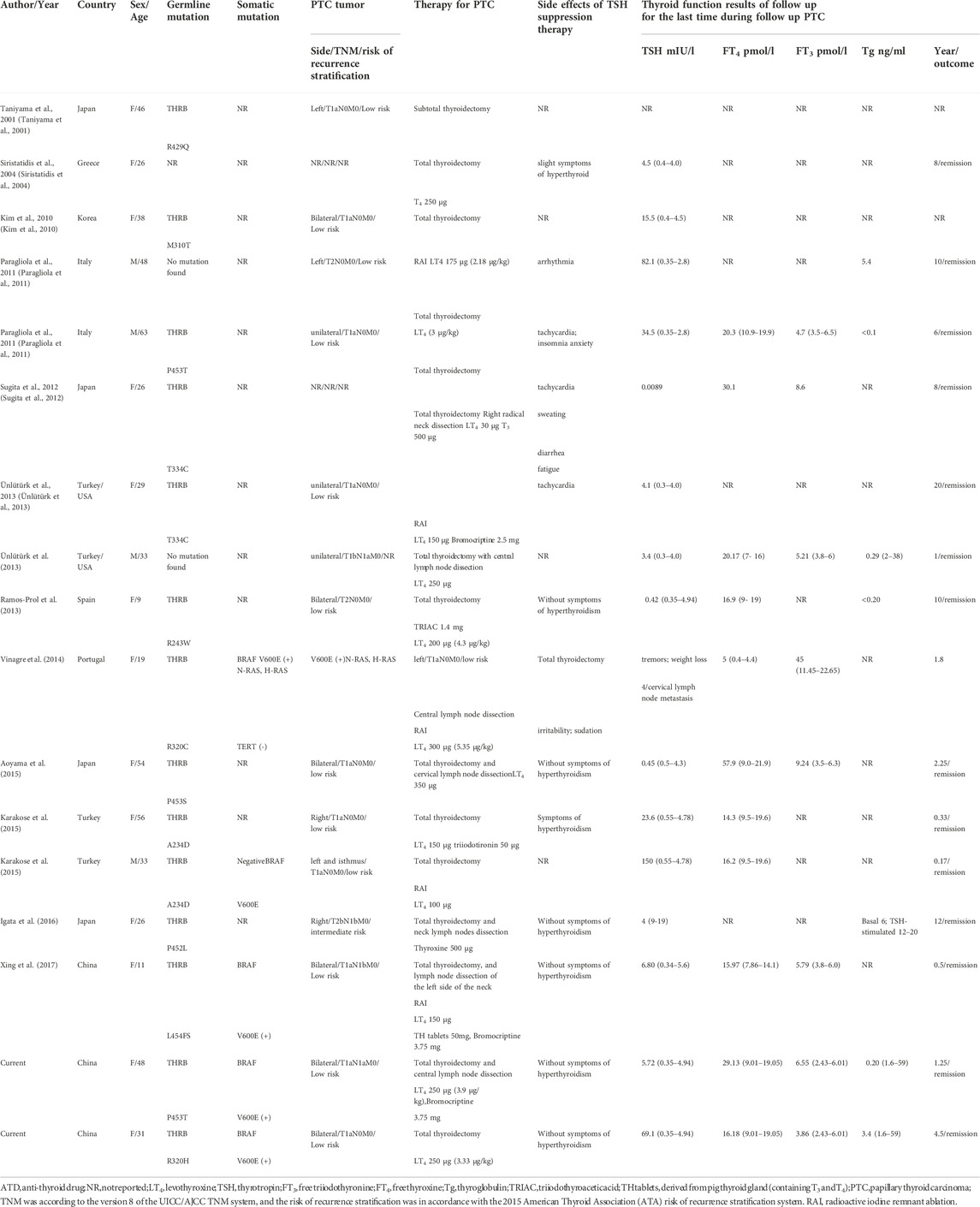
TABLE 3. Review of cases of resistance to thyroid hormone coexisting with papillary thyroid carcinoma.
Usually, PTC in children and adolescents strongly tends to be multifocal and aggressive and easily invades outside the thyroid capsule, directly involving the recurrent laryngeal nerve, trachea, blood vessels and esophagus. Pediatric papillary thyroid carcinoma has a higher probability of lymph node metastasis and distant metastasis at the time of diagnosis, reaching up to 40%–80% (Piciu et al., 2012; Almosallam et al., 2020). In our literature review of 17 cases, two were children. Consistent with the findings of pediatric PTC, both had bilateral and multifocal thyroid cancer, and one had central lymph node metastases at the time of diagnosis (Ramos-Prol et al., 2013; Xing et al., 2017).
Among the 17 patients with RTHβ and PTC, two patients were diagnosed with RTHβ due to a refractory increase in TSH after total thyroidectomy, but the sizes, number, and lymph node metastasis of the thyroid tumors were not described. After excluding these two cases, 11 of the remaining 15 patients had papillary thyroid microcarcinoma (PTMC), 4 patients had lymph node metastases at diagnosis, and 1 patient had lymph node metastases 4 years after surgery; thus, the proportion of lymph node metastases was 33.3%. Postoperative risk of recurrence assessment showed that 13 cases were at low risk, 1 case was at intermediate risk, and the risk of the last case was not available. However, no distant metastases were reported.
Of the 15 cases, histopathologic variants of thyroid carcinoma associated with more unfavorable outcomes (e.g., tall cell, columnar cell, and hobnail variants of PTC) were not reported, and more favorable outcomes, such as a follicular variant of PTC, were found in 2 cases. BRAF testing was performed in only 5 of the 15 patients, and the BRAFV600E mutation was found in 4 of those 5 patients (80%). Other molecular markers (including THRB, N-RAS, H-RAS, and TERT) were detected in only 1 patient, but no mutations were found.
A challenging issue in patients with RTHβ and PTC is the determination of the optimal surgical treatment strategies for the tumors. Table 3 shows that 7 of the 15 patients (47%) had bilateral and multifocal PTC. The review of the literature revealed that 16 of the 17 patients underwent total thyroidectomy; among them, only 1 patient developed central lymph node metastasis. The ATA guidelines state that total thyroidectomy and central lymph node dissection can help prevent tumor persistence and recurrence in patients with differentiated thyroid cancer (DTC) (Haugen et al., 2015). Table 3 shows that 10 patients who had no lymph node metastases at the time of diagnosis did not undergo prophylactic central lymph node dissection. Among them, 9 adult patients had no recurrence or metastasis, whereas one 19-year-old adolescent girl developed central lymph node metastasis after total thyroidectomy. Therefore, prophylactic central lymph node dissection would be recommended in adolescents with this rare disease. However, for adults, it is not yet clear if it is necessary to perform prophylactic central lymph node dissection.
Another issue for patients with RTHβ and PTC is determining whether to implement RAI therapy after total thyroidectomy. The ATA guidelines indicate that 131I adjuvant therapy can effectively improve overall survival (OS) and disease-free survival (DFS) in DTC patients with a high risk of recurrence. For intermediate-risk patients, the overall benefit of 131I adjuvant therapy is still controversial, and low-risk patients do not exhibit significantly improved OS or DFS (Haugen et al., 2015). Among the 17 patients, one child with intermediate risk received RAI, and one adult with intermediate risk did not. No recurrence or lymph node metastasis was found in the 2 patients during the follow-up period. However, due to the small number of intermediate-risk cases, whether intermediate-risk patients need RAI therapy is unclear. All 13 patients at low risk did not have tumor recurrence or lymph node metastasis during follow-up, regardless of whether they received radioiodine therapy.
Another challenging issue in patients with RTHβ and PTC is TSH suppression therapy. Usually, the dose of LT4 in TSH suppression therapy for classical PTC is between 1.5 and 2.5 μg/kg/day, but it is difficult to suppress TSH below the upper limit of the normal reference range in patients with RTHβ and PTC, even with very large doses of LT4 (Table 3). Four patients were treated with triiodothyronine or thyroid tablets (a mixture of T4 and T3) to assist TSH suppression, but their cardiac side effects were difficult to overcome. It was demonstrated that 3,5,3’ triiodothyroacetic acid (TRIAC) has a higher affinity for TRβ1 than T3, which may suppress TSH without causing as severe a peripheral tissue effect. LT4 combined with TRIAC was used in 1 of the 17 cases (Ramos-Prol et al., 2013), and TSH was well inhibited without cardiac side effects (Beck-Peccoz et al., 1983; Salmela et al., 1988). Although bromocriptine also had a suppressive effect on serum TSH, the inhibition did not appear to be significant in our patient. The safe range at which serum TSH levels should be controlled to not only prevent the recurrence of PTC but also avoid the probable occurrence of TSH tumors caused by long-term high TSH levels requires further study.
Confusingly, although a very large dose of LT4 was administered, the serum T3 and T4 concentrations in the patients with RTHβ and PTC were disproportionately elevated or within the normal reference range (Table 3). LT4 is the same as the thyroxine naturally secreted by the thyroid gland. Usually, the intake of large doses of LT4 can cause an increase in serum thyroxine levels. This prompted us to think about potential alterations in thyroxine metabolism in patients with RTHβ and PTC after total thyroidectomy, such as a shortened half-life of thyroxine or abnormal transformation from thyroxine to the other thyroid metabolites, but these hypotheses require further study.
The concomitant presence of RTHβ and PTC raises the question of whether patients with RTHβ are at an increased risk for thyroid cancer. The precise contribution of RTHβ to thyroid tumorigenesis is not fully understood, but there is evidence to suggest that it may play a contributory role. First, it has been postulated that TSH is a growth factor, and the continuous stimulation of TSH can promote the development of nodules and thyroid cancer. Second, the TRβ mutation itself can also be somewhat pro-oncogenic (Kim et al., 2010). The levels of TRβ mRNA were significantly higher in normal and hyperplastic thyroid tissues than in neoplastic thyroid tissues (Wallin et al., 1992). Sequencing of TRβ1 and TRα1 cDNAs cloned from 16 papillary thyroid cancers revealed that mutations affected receptor amino acid sequences in 93.75% and 62.5% of cases, respectively. In contrast, no mutations were found in healthy thyroid controls (Puzianowska-Kuznicka et al., 2002). In addition, mice that harbored a knock-in mutant TRβ gene (TRβ PV mutant) spontaneously developed thyroid cancer and distant metastasis similar to human follicular thyroid cancer (Furuya et al., 2006). Further study indicated that the more aggressive thyroid tumor progression in Thrb PV/PV mice was due not only to the loss of tumor suppressor functions of TR via mutation but also, importantly, to gain-of-function in the oncogenic activities of PV to drive thyroid carcinogenesis. This identifies a novel mechanism by which a mutated TRβ evolves with an oncogenic advantage to promote thyroid carcinogenesis (Lu and Cheng, 2011). Third, the BRAFV600E mutation, identified in between 29 and 83% of PTC cases (Trovisco et al., 2007; Xing et al., 2017) and considered an early or initiating event in PTC, is highly expressed in patients with RTH coexisting with PTC who have received BRAF testing (Table 3). Whether the BRAFV600E mutation and RTHβ are jointly involved in the occurrence of PTC deserves further study.
In conclusion, our literature review of the 17 cases of RTHβ coexisting with PTC revealed that patients with this rare disease seem to have a good prognosis. However, due to the limited number of patients and the short-term follow-up for many of them, the recurrence rate of this rare disease may be higher than that reported here. Unsuppressed or increased serum TSH levels in the background of RTHβ are associated with an increased risk of PTC recurrence and metastasis. Therefore, total thyroidectomy is recommended for adult patients, and total thyroidectomy and prophylactic central lymph node dissection are recommended for children and adolescents. Close follow-up of the current cases is needed, and benign or malignant thyroid nodules should be evaluated during the follow-up of patients with RTHβ.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Science Research Ethics Committee of First Affiliated Hospital of China Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YF and TL prepared the original draft. YF, TL, and HH performed the data collection and analysis. ZW, ZS, YC, and XT oversaw patient care. ZS, YC, and XT assisted in the critical revision of the manuscript. All coauthors read and approved the manuscript.
Funding
This study was supported by grants from the Chinese National Natural Science Foundation (Grant Nos. 81570711 and 81970681).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ATA, American Thyroid Association; DTC, differentiated thyroid cancer; DFS, disease-free survival; FT4, free thyroxine; FT3, free triiodothyronine; FNA, fine needle aspiration; LT4, levothyroxine; LT3, triiodothyronine; MMI, methimazole; MRI, magnetic resonance imaging; OS, overall survival; PTMC, papillary thyroid microcarcinoma; PTC, papillary thyroid carcinoma; RTH, resistance to thyroid hormone; RAI, radioactive iodine; THRβ, thyroid hormone receptor beta; TSH, thyrotropin; TH, thyroid hormone; TRs, thyroid hormone receptors; TgAb, anti-thyroglobulin antibodies; TRAb, anti-TSH receptor antibodies; TPOAb, anti-thyroid peroxidase antibodies; TRIAC, 3,5,3′ triiodothyroacetic acid; USG, ultrasonography.
References
Almosallam, O. I., Aseeri, A., Alhumaid, A., AlZahrani, A. S., Alsobhi, S., and AlShanafey, S. (2020). Thyroid surgery in 103 children in a single institution from 2000-2014. Ann. Saudi Med. 40 (4), 316–320. doi:10.5144/0256-4947.2020.316
Aoyama, M., Yamasaki, S., and Tsuyuguchi, M. (2015). A case of resistance to thyroid hormone diagnosed after total thyroidectomy for thyroid cancer. J. Med. Invest. 62 (3-4), 268–271. doi:10.2152/jmi.62.268
Beck-Peccoz, P., Piscitelli, G., Cattaneo, M. G., and Faglia, G. (1983). Successful treatment of hyperthyroidism due to nonneoplastic pituitary TSH hypersecretion with 3, 5, 3'-triiodothyroacetic acid (TRIAC). J. Endocrinol. Invest. 6 (3), 217–223. doi:10.1007/BF03350611
Furuya, F., Hanover, J. A., and Cheng, S. Y. (2006). Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone beta receptor. Proc. Natl. Acad. Sci. U. S. A. 103 (6), 1780–1785. doi:10.1073/pnas.0510849103
Haugen, B. R., Alexander, E. K., Bible, K. C., Doherty, G. M., Mandel, S. J., Nikiforov, Y. E., et al. (2015). 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26 (1), 1–133. doi:10.1089/thy.2015.0020
Igata, M., Tsuruzoe, K., Kawashima, J., Kukidome, D., Kondo, T., Motoshima, H., et al. (2016). Coexistence of resistance to thyroid hormone and papillary thyroid carcinoma. Endocrinol. Diabetes Metab. Case Rep. 2016, 160003. doi:10.1530/EDM-16-0003
Karakose, M., Caliskan, M., Arslan, M. S., Cakal, E., Yesilyurt, A., and Delibasi, T. (2015). Thyroid hormone resistance in two patients with papillary thyroid microcarcinoma and their BRAFV600E mutation status. Arch. Endocrinol. Metab. 59 (4), 364–366. doi:10.1590/2359-3997000000091
Kim, H. K., Kim, D., Yoo, E. H., Lee, J. I., Jang, H. W., Tan, A. H., et al. (2010). A case of resistance to thyroid hormone with thyroid cancer. J. Korean Med. Sci. 25 (9), 1368–1371. doi:10.3346/jkms.2010.25.9.1368
Kim, W. G., and Cheng, S. Y. (2013). Thyroid hormone receptors and cancer. Biochim. Biophys. Acta 1830 (7), 3928–3936. doi:10.1016/j.bbagen.2012.04.002
Lafranchi, S. H., Snyder, D. B., Sesser, D. E., Skeels, M. R., Singh, N., Brent, G. A., et al. (2003). Follow-up of newborns with elevated screening T4 concentrations. J. Pediatr. 143 (3), 296–301. doi:10.1067/S0022-3476(03)00184-7
LeClair, K., Bell, K. J. L., Furuya-Kanamori, L., Doi, S. A., Francis, D. O., and Davies, L. (2021). Evaluation of gender inequity in thyroid cancer diagnosis: Differences by sex in US thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern. Med. 181 (10), 1351–1358. doi:10.1001/jamainternmed.2021.4804
Lu, C., and Cheng, S. Y. (2011). Extranuclear signaling of mutated thyroid hormone receptors in promoting metastatic spread in thyroid carcinogenesis. Steroids 76 (9), 885–891. doi:10.1016/j.steroids.2011.03.016
Ortiga-Carvalho, T. M., Sidhaye, A. R., and Wondisford, F. E. (2014). Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 10 (10), 582–591. doi:10.1038/nrendo.2014.143
Pappa, T., and Refetoff, S. (2021). Resistance to thyroid hormone beta: A focused review. Front. Endocrinol. 12, 656551. doi:10.3389/fendo.2021.656551
Paragliola, R. M., Lovicu, R. M., Locantore, P., Senes, P., Concolino, P., Capoluongo, E., et al. (2011). Differentiated thyroid cancer in two patients with resistance to thyroid hormone. Thyroid 21 (7), 793–797. doi:10.1089/thy.2010.0233
Piciu, D., Piciu, A., and Irimie, A. (2012). Thyroid cancer in children: A 20-year study at a Romanian oncology institute. Endocr. J. 59 (6), 489–496. doi:10.1507/endocrj.ej11-0397
Puzianowska-Kuznicka, M., Krystyniak, A., Madej, A., Cheng, S. Y., and Nauman, J. (2002). Functionally impaired TR mutants are present in thyroid papillary cancer. J. Clin. Endocrinol. Metab. 87 (3), 1120–1128. doi:10.1210/jcem.87.3.8296
Ramos-Prol, A., Antonia Pérez-Lázaro, M., Isabel del Olmo-García, M., León-de Zayas, B., Moreno-Macián, F., Navas-de Solis, S., et al. (2013). Differentiated thyroid carcinoma in a girl with resistance to thyroid hormone management with triiodothyroacetic acid. J. Pediatr. Endocrinol. Metab. 26 (1-2), 133–136. doi:10.1515/jpem-2012-0230
Salmela, P. I., Wide, L., Juustila, H., and Ruokonen, A. (1988). Effects of thyroid hormones (T4, T3), bromocriptine and Triac on inappropriate TSH hypersecretion. Clin. Endocrinol. 28 (5), 497–507. doi:10.1111/j.1365-2265.1988.tb03684.x
Siristatidis, C., Mastorakos, G., Vitoratos, N., Gregoriou, O., Iakovidou, H., Salamalekis, E., et al. (2004). Thyroid hormone resistance and enlargement of the sella turcica during pregnancy. Arch. Gynecol. Obstet. 269 (2), 152–155. doi:10.1007/s00404-002-0455-8
Sugita, M., Harada, H., and Yamamoto, T. (2012). Perioperative management of a patient with thyroid hormone resistance who underwent total thyroidectomy for thyroid cancer. J. Anesth. 26 (4), 595–597. doi:10.1007/s00540-012-1365-y
Taniyama, M., Ishikawa, N., Momotani, N., Ito, K., and Ban, Y. (2001). Toxic multinodular goitre in a patient with generalized resistance to thyroid hormone who harbours the R429Q mutation in the thyroid hormone receptor beta gene. Clin. Endocrinol. 54 (1), 121–124. doi:10.1046/j.1365-2265.2001.01033.x
Trovisco, V., Soares, P., Preto, A., Castro, P., Máximo, V., and Sobrinho-Simões, M. (2007). Molecular genetics of papillary thyroid carcinoma: Great expectations. Arq. Bras. Endocrinol. Metabol. 51 (5), 643–653. doi:10.1590/s0004-27302007000500002
Ünlütürk, U., Sriphrapradang, C., Erdoğan, M. F., Emral, R., Güldiken, S., Refetoff, S., et al. (2013). Management of differentiated thyroid cancer in the presence of resistance to thyroid hormone and TSH-secreting adenomas: A report of four cases and review of the literature. J. Clin. Endocrinol. Metab. 98 (6), 2210–2217. doi:10.1210/jc.2012-4142
Vela, A., Pérez-Nanclares, G., Ríos, I., Rica, I., Portillo, N., Castaño, L., et al. (2019). Thyroid hormone resistance from newborns to adults: A Spanish experience. J. Endocrinol. Invest. 42 (8), 941–949. doi:10.1007/s40618-019-1007-4
Vinagre, J., Borges, F., Costa, A., Alvelos, M. I., Mazeto, G., Sobrinho-Simões, M., et al. (2014). Differentiated thyroid cancer in patients with resistance to thyroid hormone syndrome. A novel case and a review of the literature. Front. Mol. Biosci. 1, 10. doi:10.3389/fmolb.2014.00010
Wallin, G., Brönnegård, M., Grimelius, L., McGuire, J., and Tørring, O. (1992). Expression of the thyroid hormone receptor, the oncogenes c-myc and H-ras, and the 90 kD heat shock protein in normal, hyperplastic, and neoplastic human thyroid tissue. Thyroid 2 (4), 307–313. doi:10.1089/thy.1992.2.307
Keywords: resistance to thyroid hormone, papillary thyroid carcinoma, THRB gene mutation, TSH suppression therapy, thyroid hormone receptor
Citation: Fang Y, Liu T, Hou H, Wang Z, Shan Z, Cao Y and Teng X (2022) Resistance to thyroid hormone beta coexisting with papillary thyroid carcinoma—two case reports of a thyroid hormone receptor beta gene mutation and a literature review. Front. Genet. 13:1014323. doi: 10.3389/fgene.2022.1014323
Received: 08 August 2022; Accepted: 10 November 2022;
Published: 01 December 2022.
Edited by:
Mirjana Babić Leko, University of Split, CroatiaReviewed by:
Pietro Princi, Ospedale Cristo Re, ItalyHaixia Liu, Second Affiliated Hospital of Dalian Medical University, China
Copyright © 2022 Fang, Liu, Hou, Wang, Shan, Cao and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanli Cao, dmFuaWxsYTQyMUAxNjMuY29t; Xiaochun Teng, dGVuZ3hpYW9jaHVuQDEyNi5jb20=
†These authors have contributed equally to this work
 Yingxin Fang1†
Yingxin Fang1† Tingting Liu
Tingting Liu Huimin Hou
Huimin Hou Zhongyan Shan
Zhongyan Shan Xiaochun Teng
Xiaochun Teng