- 1Surgical Oncology Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
- 2Genetics Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
Pathogenic and likely pathogenic (P/LP) germline variants in the tumor suppressor gene CDH1 (E-cadherin) result in increased lifetime risk of diffuse-type gastric cancer and lobular breast cancer. CDH1 variants are also associated with hereditary cleft lip and palate (CLP), the mechanism of which is not well understood. We sought to determine the prevalence of CLP in families who carry P/LP CDH1 variants. Patients with P/LP CDH1 variants who were enrolled in a prospective clinical trial were reviewed (NCT03030404). The cohort included 299 individuals from 153 families that had 80 unique P/LP variants in CDH1. The rate of CLP was 19% (29/153) in families reporting CLP in at least one family member, and 2.7% (8/299) among individuals with confirmed germline CDH1 P/LP variants. There were 22 unique variants in CDH1 among the 29 families that reported CLP, or a CLP rate of 27.5% per variant (22/80). 10 of the variants were not previously reported to be associated with CLP. We observed that 24% (7/29) of CLP-associated gene variants involved large-scale (≥1 exon) deletions. Among families with CLP, 69% (20/29) had a member diagnosed with gastric cancer, and 79% (23/29) had a member with breast cancer, which were similar to rates observed in non-CLP families (p >0.3 for both). Our analysis suggests that the prevalence of CLP in families with germline CDH1 P/LP variants was high in this large cohort, and there was no genotype-phenotype pattern. Genetic testing for CDH1 variants should be considered in families with CLP and history of either diffuse-type gastric or lobular breast cancer.
Report
E-cadherin is a glycoprotein involved in maintaining the integrity of mucosal epithelium via trans-homophilic binding at cell-cell junctions (Takeichi, 2014; Mendonsa et al., 2018). Germline pathogenic or likely pathogenic (P/LP) variants in the CDH1 gene, which encodes E-cadherin, lead to the Diffuse Gastric and Lobular Breast Cancer (DGLBC, formerly hereditary diffuse gastric cancer, HDGC [MIM: 137215]) syndrome with an autosomal dominant pattern of inheritance. Lifetime disease penetrance estimates for gastric cancer and breast cancer in patients bearing a P/LP variant in CDH1 are approximately 25–42% and 42–55%, respectively (Roberts et al., 2019; Xicola et al., 2019).
In addition to cancer phenotypes, CDH1 variants are associated with Blepharocheilodontic syndrome (MIM: 119580) and cleft lip and palate (CLP). CLP, the most common congenital craniofacial abnormality, is a uni- or bilateral non-union of pharyngeal arch 1 structures and occurs in approximately 1 in 700 live births (Dixon et al., 2011). Most cases of CLP are idiopathic, but CLP may also present in the context of certain congenital syndromes (Venkatesh, 2009; Ghoumid et al., 2017). E-cadherin protein expression in the developing frontonasal prominence reportedly increases during weeks four–six of embryonic development (Frebourg et al., 2006), and epithelial cell adhesion is an important contributor to proper development of this structure (Cox et al., 2018). A prior study reported an association between variants in the linker regions of the E-cadherin protein and CLP, however, no mechanistic evidence has been provided to explain this phenomenon (Selvanathan et al., 2020).
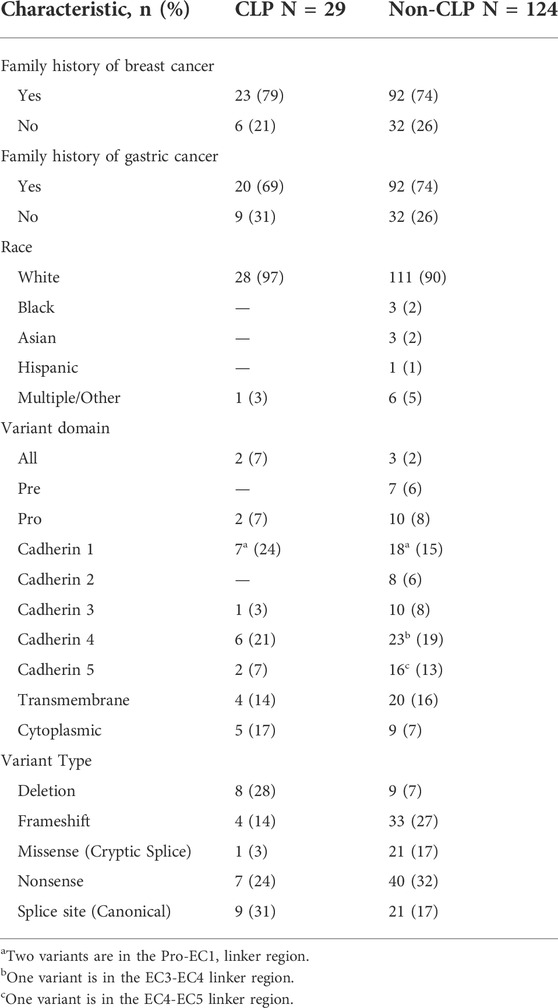
To evaluate the association between CDH1 variants and CLP, we analyzed a large single-institution cohort of 299 patients with confirmed CDH1 P/LP variants enrolled in a prospective natural history study from 2017 through 2021. A total of 299 individual study participants were enrolled (211 female, 88 male) from 153 different families, the majority of whom identified as White (Table 1). Although the individual rate of CLP among patients with germline CDH1 P/LP variants was 2.7% (8/299), 19% (29/153) of families identified at least one relative with CLP (Median: 1, range 1–5). Of the study participants and their relatives identified with CLP (n = 47), 15 were positive for a P/LP variant in CDH1, 1 was an obligate carrier, and 31 were untested but at-risk to carry the familial CDH1 variant. Individuals with CLP were 45% (21/47) female, 19% (4/21) of whom had a personal history of breast cancer. Advanced gastric cancer was identified in 13% (6/47) of individuals with CLP. For families with CLP, 69% (20/29) reported at least one member with advanced gastric cancer, and 79% (23/29) reported breast cancer, which were similar to rates observed in non-CLP families (breast cancer Χ2 = 0.33, p = 0.566; gastric cancer Χ2 = 0.33, p = 0.567).
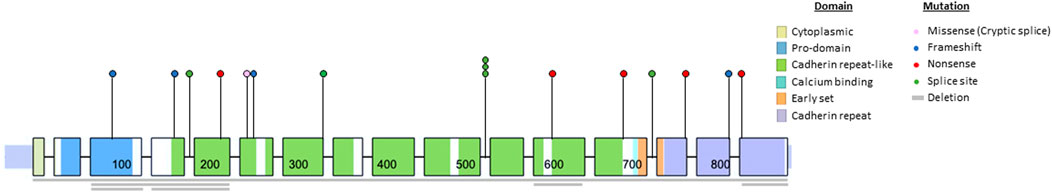
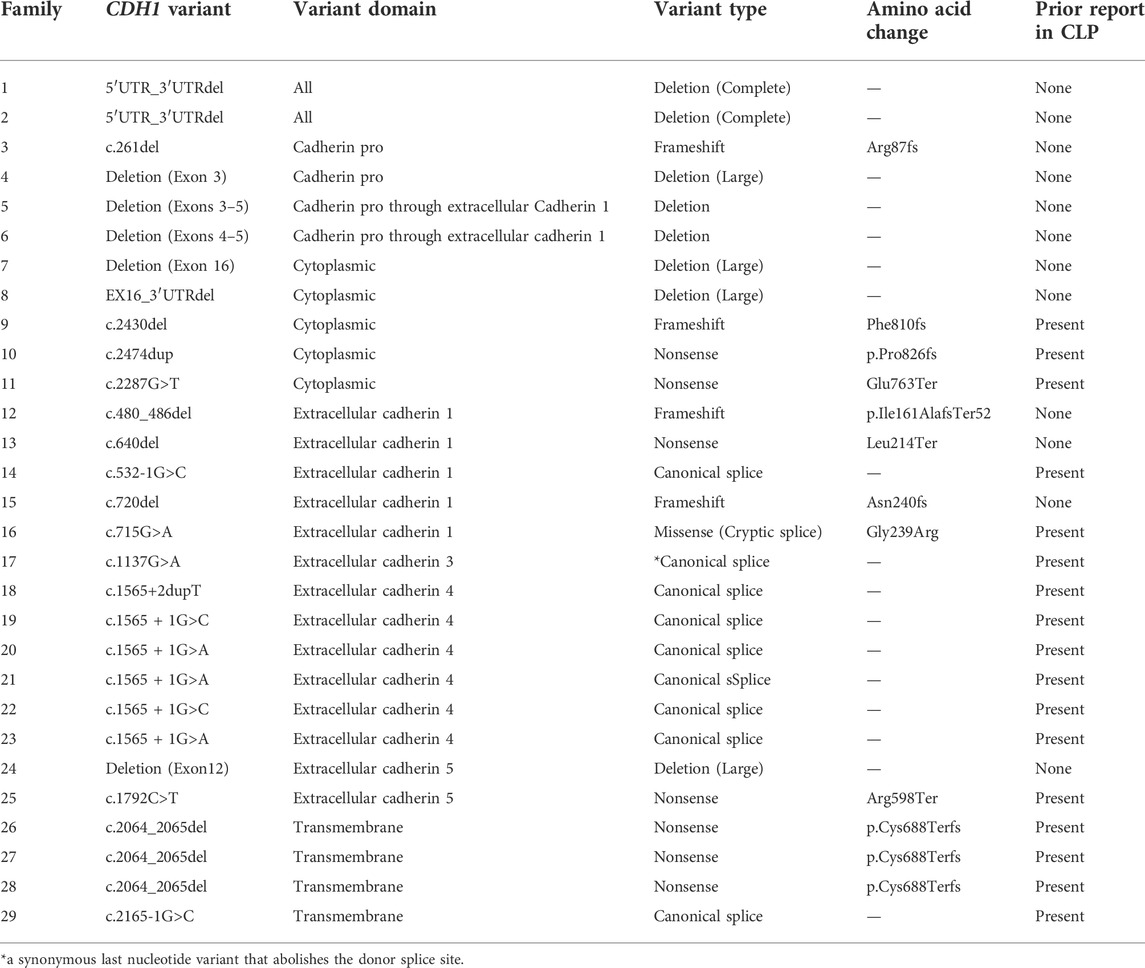
Next, we analyzed the CDH1 genotype of the cohort (Figure 1). There were 80 unique CDH1 P/LP variants among 153 different families. Of the 29 families that reported CLP, there were 22 unique variants in CDH1, 10 of which had not been associated previously with CLP (Table 2). The rate of CLP per unique CDH1 P/LP variant was 27.5% (22/80). Truncation of E-cadherin was predicted in 55% (16/29) of families reporting CLP based on either nonsense or frameshift variants in CDH1 (Table 2). An additional 24% (7/29) of CLP families had large deletions of ≥1 exon, including two families that were heterozygous for complete CDH1 gene deletion. Interestingly, there were two other families in the CLP-negative cohort heterozygous for the same complete CDH1 gene deletions that denied a known history of CLP. In contrast, there was only 1 missense cryptic splice variant in CLP-positive families (3%) compared with 21 missense mutations in the CLP-negative families (17%). The missense cryptic splice variant in the CLP-positive subgroup was not located within a cadherin-repeat linker region. Surprisingly, variants located at EC-EC linker regions were found in families without a history of CLP. The most common location for a CDH1 variant in both subgroups was in EC4. The frequency of variants of intracytoplasmic or transmembrane domains were similar in both CLP-positive and CLP-negative groups.
Here, we have reported the largest known single-institution analysis of CLP prevalence in subjects with germline CDH1 P/LP variants. The rarity of DGLBC syndrome and CDH1 P/LP variants presents challenges for any analysis. A prior study of CDH1 variant data pooled from the literature and public genetic variation databases found that 13% of CDH1 variants were associated with syndromic CLP (only DGLBC and Blepharocheilodontic syndrome) and non-syndromic CLP (Selvanathan et al., 2020). Our dataset, in contrast, allowed for CLP status to be systematically collected. We were able to determine that 27.5% of unique CDH1 P/LP variants were associated with CLP. Additionally, 19% of families with germline CDH1 P/LP variants reported at least one relative with CLP. These data demonstrate that CLP may be more prevalent in families with CDH1 P/LP variants than previously described.
Identification of individuals with a CDH1 P/LP variant provides opportunities for cancer risk reduction and early detection. Due to the high incidence of CLP in the general population, a diagnosis of isolated CLP at birth would be insufficient to recommend germline CDH1 genetic testing. Detailed individual and family criteria for CDH1 germline genetic testing have been developed by the International Gastric Cancer Linkage Consortium (Blair et al., 2020). Of the nine specific testing criteria, only one addresses CLP which recommends CDH1 testing for individuals with diffuse gastric cancer at any age and a personal or family history of CLP. Based on this report, it appears quite reasonable to expand the criteria to include a recommendation for CDH1 genetic testing in individuals with lobular breast cancer at any age with a personal or family history of CLP. Another consideration is that in families with features of hereditary cancer, there will be relatives with syndrome associated cancers who are deceased or uninterested/unable to undergo genetic testing. Therefore, we suggest that CDH1 genetic testing criteria also include testing for unaffected individuals with a family history of CLP and diffuse gastric cancer or lobular breast cancer.
Genotype-phenotype correlations have been elusive for CDH1. We found no difference in the rates of CLP in families reporting a history of gastric or breast cancer. Functionally, E-cadherin can form hetero- and homodimers on the cell surface and initiates intracellular signal transduction via β-catenin signaling and cytoskeletal modulation (Mendonsa et al., 2018). A previous study suggested mechanistic associations that might explain phenotypic differences between CLP and cancer development, specifically implicating linker regions of E-cadherin enriched for CLP-associated variants (Selvanathan et al., 2020). However, we found no evidence of region-specific variants that correlated with the presence of CLP. The CLP-positive subgroup demonstrated variants throughout the entire gene, including two patients with full CDH1 gene deletions which had not been reported previously. Interestingly, there were two additional families with full CDH1 gene deletions that reported no CLP. In addition, the only missense mutation in the CLP + group was a known cryptic splice site, generating premature termination codon that potentially resulted in reduced abundance of CDH1 mRNA via the nonsense-mediated decay (NMD) pathway (Kaurah et al., 2007; Karam et al., 2008). Together, these findings suggest that quantity, not quality, of functional E-cadherin may be a driver of CLP phenotype in CDH1 P/LP carriers, and that CLP is likely a multifactorial phenotype.
Materials and methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Patients were enrolled in National Institutes of Health (NIH) protocol number 17-C-0043 (NCT ID: NCT03030404) from 2017 to 2021. The study was approved by the institutional review board of the National Institutes of Health (reference number 385481) and informed consent was taken from all patients. Patients were enrolled if they had positive genotyping for a P/LP variant in CDH1. Patients had genetic testing at a CLIA certified lab. Results were reviewed by a certified genetic counselor. All data were analyzed by SPSS version 25® (IBM, IL, United States). Chi-squared statistical test was used where appropriate.
Summary
Approximately 1 in 5 families with germline CDH1 pathogenic variants identified a family member with cleft lip/palate. This rate of cleft lip/palate associated with germline CDH1 variants should be incorporated into considerations for genetic testing in patients with a personal or family history of diffuse gastric cancer or lobular breast cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the National Institutes. Informed consent was taken from the patients involved.
Author contributions
Conceptualization: BG and JD data curation: All authors formal analysis: All authors funding acquisition: JD investigation: BG and JD methodology: All authors project administration: BG and JD resources: JD software: n/a Supervision: JD validation: All authors visualization: BG, LG, SS writing—original draft: BG and JD writing—review and editing: All authors.
Funding
This research was supported in part by the Intramural Research Program, National Cancer Institute, National Institutes of Health.
Acknowledgments
The authors would like to acknowledge Dr. Chimene Kesserwan for providing variant classification and interpretation guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Blair, V. R., McLeod, M., Carneiro, F., Coit, D. G., D'Addario, J. L., van Dieren, J. M., et al. (2020). Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet. Oncol. 21 (8), e386–e397. doi:10.1016/S1470-2045(20)30219-9
Cox, L. L., Cox, T. C., Moreno Uribe, L. M., Zhu, Y., Richter, C. T., Nidey, N., et al. (2018). Mutations in the epithelial cadherin-p120-catenin complex cause mendelian non-syndromic cleft lip with or without cleft palate. Am. J. Hum. Genet. 102 (6), 1143–1157. doi:10.1016/j.ajhg.2018.04.009
Dixon, M. J., Marazita, M. L., Beaty, T. H., and Murray, J. C. (2011). Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 12 (3), 167–178. doi:10.1038/nrg2933
Frebourg, T., Oliveira, C., Hochain, P., Karam, R., Manouvrier, S., Graziadio, C., et al. (2006). Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J. Med. Genet. 43 (2), 138–142. doi:10.1136/jmg.2005.031385
Ghoumid, J., Stichelbout, M., Jourdain, A. S., Frenois, F., Lejeune-Dumoulin, S., Alex-Cordier, M. P., et al. (2017). Blepharocheilodontic syndrome is a CDH1 pathway-related disorder due to mutations in CDH1 and CTNND1. Genet. Med. 19 (9), 1013–1021. doi:10.1038/gim.2017.11
Karam, R., Carvalho, J., Bruno, I., Graziadio, C., Senz, J., Huntsman, D., et al. (2008). The NMD mRNA surveillance pathway downregulates aberrant E-cadherin transcripts in gastric cancer cells and in CDH1 mutation carriers. Oncogene 27 (30), 4255–4260. doi:10.1038/onc.2008.62
Kaurah, P., MacMillan, A., Boyd, N., Senz, J., De Luca, A., Chun, N., et al. (2007). Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 297 (21), 2360–2372. doi:10.1001/jama.297.21.2360
Mendonsa, A. M., Na, T. Y., and Gumbiner, B. M. (2018). E-cadherin in contact inhibition and cancer. Oncogene 37 (35), 4769–4780. doi:10.1038/s41388-018-0304-2
Roberts, M. E., Ranola, J. M. O., Marshall, M. L., Susswein, L. R., Graceffo, S., Bohnert, K., et al. (2019). Comparison of CDH1 penetrance estimates in clinically ascertained families vs families ascertained for multiple gastric cancers. JAMA Oncol. 5, 1325–1331. doi:10.1001/jamaoncol.2019.1208
Selvanathan, A., Nixon, C. Y., Zhu, Y., Scietti, L., Forneris, F., Uribe, L. M. M., et al. (2020). CDH1 mutation distribution and type suggests genetic differences between the etiology of orofacial clefting and gastric cancer. Genes 11 (4), E391. doi:10.3390/genes11040391
Takeichi, M. (2014). Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 15 (6), 397–410. doi:10.1038/nrm3802
Venkatesh, R. (2009). Syndromes and anomalies associated with cleft. Indian J. Plast. Surg. 42, S51–S55. doi:10.4103/0970-0358.57187
Keywords: cleft lip, cleft palate, CDH1, E-cadherin, cleft lip/palate, hereditary diffuse gastric cancer syndrome
Citation: Green BL, Fasaye G-A, Samaranayake SG, Duemler A, Gamble LA and Davis JL (2022) Frequent cleft lip and palate in families with pathogenic germline CDH1 variants. Front. Genet. 13:1012025. doi: 10.3389/fgene.2022.1012025
Received: 05 August 2022; Accepted: 09 September 2022;
Published: 28 September 2022.
Edited by:
Augusto Rojas-Martinez, Escuela de Medicina y Ciencias de la Salud Tec Salud, Tecnológico de Monterrey, MexicoReviewed by:
Alexandre Rezende Vieira, University of Pittsburgh, United StatesRachid Karam, Ambry Genetics, United States
Copyright © 2022 Green, Fasaye, Samaranayake, Duemler, Gamble and Davis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy L. Davis, jeremy.davis@nih.gov
 Benjamin L. Green
Benjamin L. Green