
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Genet. , 18 January 2022
Sec. Computational Genomics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.799807
This article is part of the Research Topic Genetic and proteomic biomarkers in solid tumor detection and treatment View all 64 articles
Lynch syndrome (LS) is a cancer-predisposing genetic disease mediated by pathogenic mutations in DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PMS2. Accumulating evidence demonstrates that there is significant biological heterogeneity across MMR genes. Compared to MLH1 and MSH2, PMS2 variant carriers have a much lower risk for LS-related cancers. Tumors in MLH1 and MSH2 variant carriers often display MMR deficiency (dMMR) and/or high microsatellite instability (MSI-H), two predictive biomarkers for immunotherapy efficacy. However, tumors in PMS2 variant carriers are largely microsatellite stable (MSS) instead of MSI. Therefore, the optimal management of cancer patients with LS requires the integration of disease stage, MMR gene penetrance, dMMR/MSI status, and tumor mutational burden (TMB). In this work, we presented a locally advanced lung cancer patient with dMMR/MSI-H/TMB-H tumor and selective loss of PMS2 by immunohistochemistry. Germline testing revealed a rare PMS2 splicing variant (c.1144+1G>A) in the proband and his healthy daughter. The diagnosis of LS was made based on genetic analysis of this variant and literature review. Given the incomplete penetrance of PMS2, the proband and the carrier received tailored genetic counseling. To reduce cancer risk, the proband received four cycles of nivolumab plus chemotherapy and achieved a disease-free survival of sixteen months.
In the past decade, cancer immunotherapy has shifted the landscape of cancer treatment (Tang et al., 2018). Predictive biomarkers such as PD-L1 expression level have greatly facilitated the selection of patients for immunotherapy in some cancer types (Doroshow et al., 2021). In 2017, FDA approved PD-1 antibody pembrolizumab to treat patients with unresectable or metastatic mismatch repair deficiency (dMMR) and/or microsatellite instability-high (MSI-H) solid tumors, making it the first tissue/site-agnostic predictive biomarker (Marcus et al., 2019).
MMR deficiency is mediated by somatic or germline mutations in MMR genes (MLH1, MSH2, MSH6, and PMS2) and rarely, EPCAM (Le et al., 2015). Pathogenic germline variants in MMR genes cause Lynch Syndrome (LS), a genetic disease predisposing patients to multiple types of cancers (Lynch et al., 2015). Therefore, the diagnosis of LS is essential for the treatment and cancer-risk reduction for LS patients and family members harboring pathogenic variants (Yurgelun and Hampel, 2018).
The optimal testing and treatment of LS patients require a good knowledge of the pan-cancer prevalence and heterogeneity of pathogenic MMR gene variants. To explore the prevalence of LS across solid tumors according to MSI status, researchers at the Memorial Sloan Kettering Cancer Center (MSKCC) screened 15,045 patients of more than 50 cancer types (Latham et al., 2019). Among LS patients with MSI tumors, half had tumors other than colorectal and endometrial cancer, including gastric, pancreas, small bowel, and germ cell tumors. However, none of the 1,952 lung cancer patients had LS. While most tumors from MLH1 and MSH2 carriers are MSI, more than two-thirds of tumors from PMS2 carriers are microsatellite stable (MSS) (Latham et al., 2019).
Recent studies including the Prospective Lynch Syndrome Database (PLSD) and the International Mismatch Repair Consortium revealed that MMR gene variants had distinct penetrance (Dominguez-Valentin et al., 2020; Dominguez-Valentin et al., 2021; International Mismatch Repair Consortium, 2021). Pathogenic MLH1 and MSH2 variants caused high penetrance in a broad spectrum of LS cancers, while pathogenic PMS2 variants were associated with low penetrance in few LS-related cancers. Therefore, the cancer screening, chemoprevention, and risk-reduction surgery protocol for carriers of MLH1 and MSH2 variants should be tailored to carriers of PMS2 variants (Balmaña et al., 2013; Stoffel et al., 2015; Gupta et al., 2019; Seppälä et al., 2021a). Here, we presented the diagnosis, tailored genetic counseling, and cancer prevention of a locally advanced lung cancer patient with dMMR/MSI-H/TMB-H tumor and PMS2-LS.
Our proband was a 62-year-old Chinese man with 40-pack-year smoking history, presented with a consolidation shadow in the lower lobe of the right lung discovered by chest computed tomography (CT) (Figure 1A). The CT scan also detected local occlusion of internal small bronchus, subpleural nodular ground-glass opacity on the posterior segment of the right superior lobe, as well as a nodular contour in the right adrenal gland. He underwent thoracoscopic right lower lobectomy and mediastinal lymphadenectomy. Postoperative pathology showed a stage IIIA lung adenocarcinoma (T1aN2M0) with regional lymph node metastases, which had a size of 0.9 × 0.6 × 0.6 cm. Genomic profiling with a multi-gene next-generation sequencing (NGS) panel (Onco Panscan™, Genetron Health) showed a KRAS G13D mutation, a TP53 R267W mutation, and a high tumor mutational burden (TMB-H, 13.62 mutations/Mb) (Table 1). PD-L1 immunochemistry (IHC), MMR IHC, and microsatellite instability (MSI) testing revealed that his tumor was PD-L1-positive (TPS 8%), MSI-H, and dMMR (Figures 1B,C).
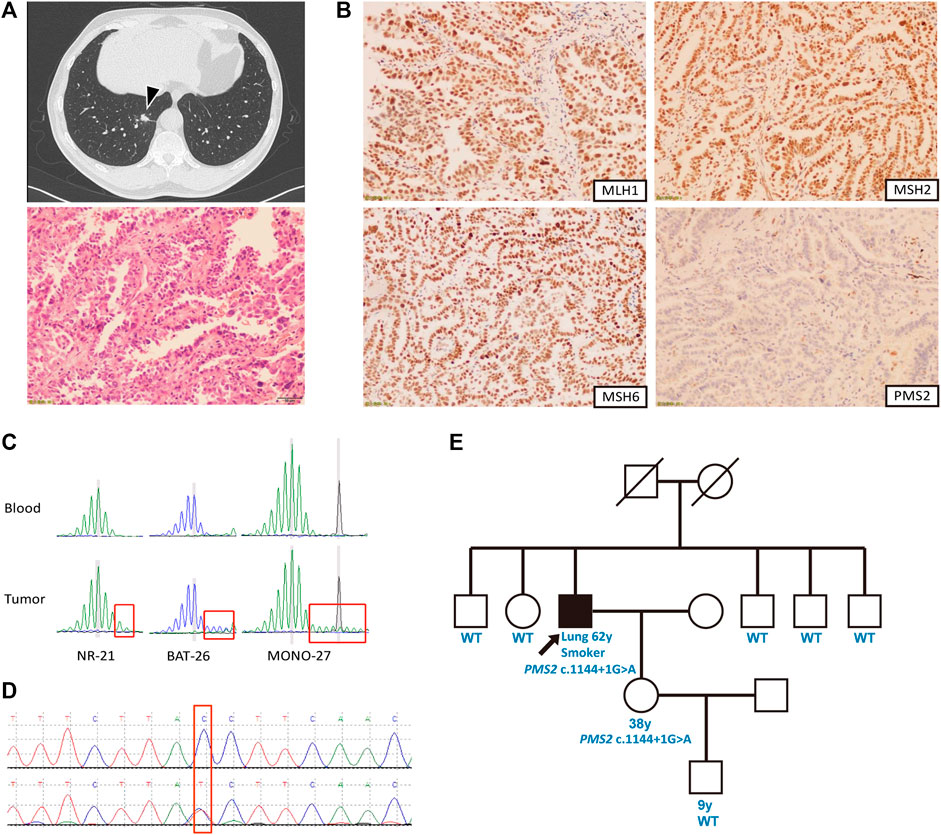
FIGURE 1. Case summary. (A) Clinical findings of the patient. Above: Chest computed tomography revealing a mass in the right lower lobe (arrow); below: Hematoxylin and eosin-stained tumor tissue sections presenting adenocarcinoma (magnification, ×400). (B) Immunohistochemical staining showing absence of PMS2 as well as presence of MLH1, MSH2 and MSH6 in the tumor cell nuclei. (C) Capillary electrophoresis results showed loss of stability of three microsatellite biomarkers in tumor compared to the blood control, indicating high microsatellite instability. (D) Sequence chromatogram of the patient’s daughter containing the same PMS2 c.1144+1G>A mutation. (E) Pedigree of the patient’s family. The proband is indicated with an arrow and black denotes the cancer-affected individual.
Based on the dMMR/MSI-H/TMB-H phenotype of the patient, we suspected Lynch syndrome (LS) even though his personal and family history did not fulfill the revised Amsterdam criteria or Bethesda guideline (Cohen et al., 2019). Germline testing revealed a c.1144+1G>A mutation located at the splice donor site of intron 10 of the PMS2 gene. This variant was not included in the InSiGHT database (http://www.insight-group.org/) (Thompson et al., 2014). In 2019, the two interpretations of this variant in the ClinVar database did not follow the 2015 ACMG–AMP guidelines (Richards et al., 2015) or the refined 2017 version (Sherloc) (Nykamp et al., 2017). To accurately determine its pathogenicity, we conducted a comprehensive genetic analysis. In silico analysis with three splice prediction programs including Alternative Splice Site Predictor (ASSP) (Wang and Marín, 2006), MaxEntScan (Yeo and Burge, 2004), and NetGene2 (Hebsgaard et al., 1996) suggested that the PMS2 c.1144+1G>A variant will generate aberrant splicing transcripts (Table 2). Data in the gnomAD database revealed that its population allele frequency is 2/282496 without homozygotes, which falls into the pathogenic range defined by the Sherloc guideline (Nykamp et al., 2017). The proband had an oncogenic KRAS G13D somatic mutation and his 38-years-old daughter with the same PMS2 variant had no cancer yet (Figure 1D). Moreover, Sanger sequencing results showed that this variant was segregated with his daughter but none of his five siblings (Figure 1E). We conducted an extensive literature review and found two probands harboring the same PMS2 variant, a breast cancer patient from a cohort of 480 patients during a genetic screen of germline hereditary breast cancer susceptibility genes (Wang et al., 2018) and a colorectal cancer patient in a cohort of 6,503 patients from the NHLBI Exome Sequencing Project (ESP) (Amendola et al., 2015). According to the ACMG/Sherloc guidelines, we classified the PMS2 c.1144+1G>A variant as class 4 (likely pathogenic). Interestingly, in a family with germline PMS2 c.1144+2T>A variant, which disrupts the same splice donor site as c.1144+1G>A, the cosegregation between this variant and LS-related cancers was seen (Hendriks et al., 2006). These reports and our findings indicated that disruption of this specific PMS2 splicing site can be pathogenic/likely pathogenic and result in LS.
Although the ESMO (2013), ASCO (2015), NCCN (2017) LS management guidelines gave the same recommendations for different MMR genes (Balmaña et al., 2013; Stoffel et al., 2015; Gupta et al., 2017), accumulating evidence demonstrated that the penetrance of PMS2 was much lower than other MMR genes (Møller et al., 2017; Ten Broeke et al., 2018a; Ten Broeke et al., 2018b). Therefore, we provided PMS2-specific genetic counseling to the proband and the carrier. 5-yearly colonoscopic surveillance was recommended to the patient and the carrier. For the female carrier, endometrial/ovarian cancer screen was recommended after she reaches 50 years old. Furthermore, given the dMMR/MSI-H/TMB-H phenotype of the tumor and the recurrence risk of locally advanced NSCLC with concurrent KRAS and TP53 mutations (Yagishita et al., 2015; Hames et al., 2016; Skoulidis and Heymach, 2019), the patient was then treated with cisplatin (75 mg/m2), pemetrexed (500 mg/m2), and nivolumab (200 mg) every 3 weeks for a total of 4 cycles after surgery for risk reduction. Sixteen months after the discontinuation of treatment, he developed progressive disease and was enrolled in a study with another investigational agent.
After the approval of pembrolizumab for patients with advanced dMMR/MSI-H solid tumor in 2017, the testing of dMMR/MSI-H has become a common diagnosis approach for cancer patients (Evans et al., 2021). If dMMR/MSI testing results are positive, germline testing will be recommended for the diagnosis of Lynch syndrome (LS), which is essential for the optimal care for cancer patients and their family members at risk (Yurgelun and Hampel, 2018). Currently, the standard of dMMR and MSI testing is the immunochemistry (IHC) assay of four MMR proteins and PCR-based assays of five microsatellite loci, respectively (André et al., 2020). One interesting observation in MMR IHC testing is that patients with pathogenic MSH2 (path_MSH2) and MLH1 (path_MLH1) mutations display simultaneous loss-of-expression for MSH2/MSH6 and MLH1/PMS2, respectively (Luchini et al., 2019). In contrast, patients with pathogenic MSH6 (path_MSH6) and PMS2 (path_PMS2) mutations retain expression of MSH2 and MLH1, respectively. This is because MSH6 forms a heterodimer complex with MSH2 and PMS2 forms a heterodimer complex with MLH1, which are required to maintain the protein stability of MSH6 and PMS2 but not MSH2 and MLH1, respectively. Therefore, the phenotypes of path_MSH2 and path_MLH1 single mutants mimic the phenotypes of path_MSH2/path_MSH6 and path_MLH1/path_PMS2 double mutants, respectively. According to these results, the cancer risk of path_MSH2 and path_MLH1 carriers should be significantly higher than path_MSH6 and path_PMS2 carriers.
This prediction was supported by multiple studies including the Prospective Lynch Syndrome Database (PLSD), an international, multicenter prospective observational study involving 6,350 path_MMR variants carriers and 1,808 observed cancers (Dominguez-Valentin et al., 2020). Path_MSH2 and path_MLH1 variants were associated with high penetrance dominant syndrome in colorectal, endometrial, and ovarian cancers while path_MSH6 variants were associated with high risk in endometrial cancer but modestly increased risk for colorectal cancer. In contrast, the risk of path_PMS2 variants for these three cancers was not increased before 50 years of age and only nonsignificantly increased after that.
The PLSD study series is a game changer for current cancer surveillance and risk-reduction practice for LS patients (Seppälä et al., 2021a; Dominguez-Valentin et al., 2021). According to the 2019 NCCN guideline for LS management, both the proband and carriers of path_MMR variants should take colonoscopy every 1–2 years for colorectal cancer surveillance and aspirin for risk reduction (Gupta et al., 2019). Additionally, the female carriers may consider an endometrial biopsy screen every 1–2 years for endometrial cancer surveillance and hysterectomy for risk-reduction. Given the incomplete penetrance of PMS2 (Møller et al., 2017; Ten Broeke et al., 2018a; Ten Broeke et al., 2018b), we provided PMS2-specific genetic counseling to the proband and carrier, which was different from the general recommendation of the 2013 ESMO/2015 ASCO LS managenment guidelines (Balmaña et al., 2013; Stoffel et al., 2015). For instance, we did not recommend chemoprevention with aspirin or colonoscopy every one or two years for the proband and carrier. Our practice was largely consistent with the 2021 NCCN LS guideline which provided gene-specific cancer surveillance and risk reduction recommendations (NCCN Guideline, 2021). For instance, chemoprevention with 600 mg/daily aspirin for 2 years is recommended for all path_MMR carriers except for path_PMS2 carriers. For path_PMS2 and path_MSH6 carriers, the colonoscopy screen age has been changed to 30–35 from 20–25 years old. Ovarian cancer screen or risk-reduction surgery are not recommended for path_PMS2 carriers, as they do not have increased risk. In addition, the risk of endometrial cancer for path_PMS2 carriers is only moderately increased compared to path_MLH1, path_MSH2, and path_MSH6 carriers. Similarly, the 2021 LS guideline developed by the European Hereditary Tumour Group (EHTG) and the European Society of Coloproctology (ESCP) also revised the colorectal cancer surveillance and risk-reduction procedures for path_MMR carriers (Seppälä et al., 2021b). The colonoscopy screen interval time for path_PMS2 and other path_MMR carriers are 5 years and 2–3 years, respectively. Moreover, the colonoscopy screen starting ages for path_PMS2 and path_MSH6 carriers are 35, but 25 for path_MLH1 and path_MSH2 carriers. Additionally, extended surgery is only recommended for path_MLH1 and path_MSH2 carriers but not for path_PMS2 and path_MSH6 carriers at the first diagnosis of colorectal cancer.
In addition to cancer surveillance and prevention, dMMR/MSI is also an important biomarker for cancer immunotherapy (Luchini et al., 2019). Due to the functional heterogeneity of MMR gene variants, sometimes we can see discordance between path_MMR variants and MSI status. This is well illustrated in the MSKCC pan-cancer MSI study of 15,045 patients including 103 LS cases (Latham et al., 2019). The microsatellite stable (MSS) cases in path_PMS2, path_MSH6, path_MSH2, and path_MLH1 carriers were 68.2% (15/22), 53.8% (14/26), 13.9% (5/36), and 16.7% (3/18), respectively. This result indicates that the diagnosis of LS alone does not justify the treatment decision of immunotherapy, especially for path_PMS2 and path_MSH6 carriers.
Besides the discordance between path_MMR variants and MSI status, the discordance between dMMR and TMB is another issue oncologists should consider during the treatment decision-making process for LS patients. Recently, Bielska et al. reported that low TMB level in LS patients with dMMR tumors was a mechanism of immunotherapy resistance (Bielska et al., 2021). Three LS patients developed two primary dMMR tumors, one TMB-H and the other TMB-L. While the dMMR tumors with high TMB responded to immunotherapy, those with low TMB did not.
Next, we briefly discuss the limitations of TMB-H as the pan-cancer biomarkers for immunotherapy. In 2020, FDA approved PD-1 antibody pembrolizumab for the treatment of TMB-H (TMB >10 mutations/megabase) solid tumors. This approval was based on the results of the KEYNOTE-158 trial (Marcus et al., 2021). Pembrolizumab achieved an overall response rate (ORR) of 29% in TMB-H patients (13%, n = 102). However, this approval should not be applied to colorectal cancer (CRC) as subgroup analysis of 137 advanced CRC patients treated with immunotherapy showed that there is no survival benefit in TMB-H patients after the removal of patients with dMMR or POLD/POLE1 mutations (Rousseau et al., 2021). Further pan-cancer analysis of 1,661 cancer patients treated with immunotherapy revealed that TMB-H was associated with improved overall survival in a limited subgroup of pMMR cancers including NSCLC (Rousseau et al., 2021). There are three important lessons that we can learn from these studies: first, TMB is not a pan-cancer immunotherapy biomarker; second, the combination of dMMR/MSI-H and TMB-H can provide better immunotherapy efficacy prediction than either alone; third, dMMR tumors with low TMB may not respond to immunotherapy.
LS is very rare in primary lung cancer. For instance, the MSKCC pan-cancer study did not find LS in 1,952 lung cancer patients including 94 MSI cases (Latham et al., 2019). We reviewed the literature and identified a few primary lung cancer cases associated with LS driven by path_MSH2 (n = 4), path_MLH1 (n = 1), path_MSH6 (n = 1), and path_PMS2 (n = 1) variants (Table 3). The path_PMS2 lung cancer case was a 74-year old female non-smoker with MSS, pMMR, and TMB-L tumor (Sun et al., 2019). Without actionable mutations, she was treated with gefitinib for two months and then switched to platinum-based chemotherapy. The path_MSH6 lung cancer case was a 76-year old male smoker with PD-L1-positive, pMMR, and TMB-L tumor (Long et al., 2021). Without actionable mutations, pembrolizumab was administered with SD at 4 cycles and PD at 8 cycles, likely due to an acquired STK11 mutation. The path_MLH1 lung cancer case was a 36-year old male non-smoker with dMMR/MSI-H tumor (Masuzawa et al., 2020). He received nivolumab as the fifth-line therapy for 15 cycles with a partial response lasting more than 20 months. Among the four path_MSH2 lung cancer cases (Canney et al., 2009; Nolan et al., 2009; Kawashima et al., 2019; Sun et al., 2019), only one patient had dMMR/MSI tumor (Kawashima et al., 2019). He received nivolumab as the third-line therapy with a partial response lasting more than 10 months (Kawashima et al., 2019). Our patient was positive for three immune biomarkers (dMMR/MSI-H/TMB-H). These results suggested that he could benefit from immunotherapy.
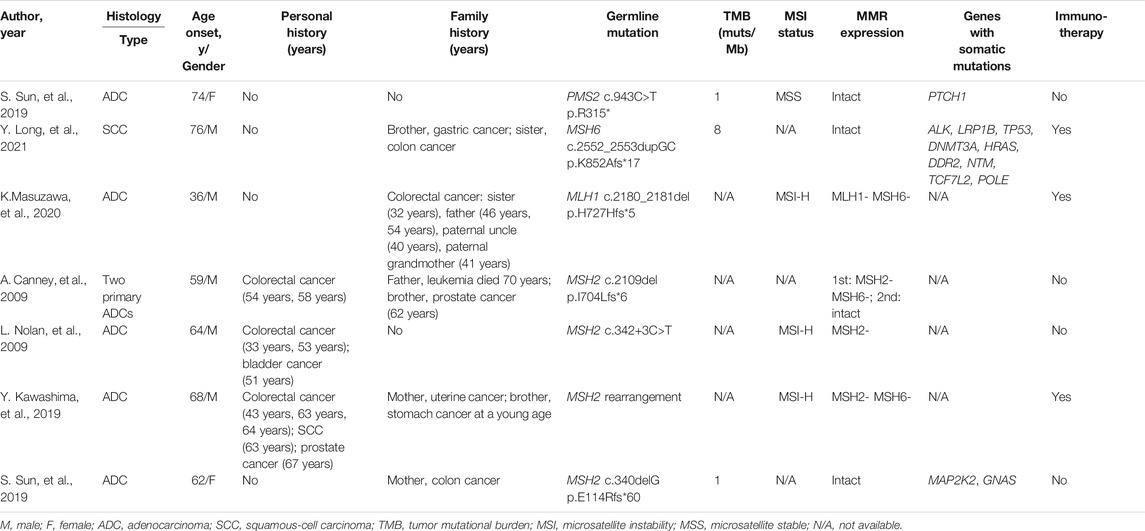
TABLE 3. Clinical information and genetic testing results for seven lung cancer patients associated with LS.
Despite complete surgical resection, stage III NSCLC patients have high rates of relapse (Evison, 2020). Given the success of PD(L)-1 checkpoint inhibitors in metastatic NSCLC treatment, multiple trials are testing their efficacy in earlier stages of disease. Recently, results of the phase 3 IMpower010 trial showed a disease-free survival benefit with atezolizumab versus best supportive care after adjuvant chemotherapy in patients with PD-L1-positive resected stage II–IIIA NSCLC (Felip et al., 2021). This led to the FDA approval of atezolizumab as the adjuvant therapy for this patient population. Similarly, in the phase 2 KEYNOTE-799 trial, pembrolizumab plus concurrent chemoradiation therapy demonstrated objective response rates of 71% in locally advanced, stage III NSCLC (Jabbour et al., 2021). Because our case was a locally advanced dMMR/MSI-H tumor, we were also interested in the efficacy of adjuvant immunotherapy in this setting. For dMMR/MSI-H solid tumors, results of the phase 3 KEYNOTE-177 trial established that first-line pembrolizumab therapy resulted in significantly longer PFS than chemotherapy for dMMR/MSI-H metastatic CRC (André et al., 2020). Currently, two ongoing randomized phase 3 trials are testing the efficacy of adjuvant immunochemotherapy in patients with resected dMMR/MSI stage III CRC. The ATOMIC trial (NCT02912559) and the POLEM trial (NCT03827044) are evaluating the combination of chemotherapy with atezolizumab or avelumab, respectively (Sinicrope et al., 2019; Lau et al., 2020). Results of these two trials will provide proof-of-concept for the use of immune checkpoint inhibitors in the curative setting of dMMR/MSI-H stage III CRC which could be extended to other LS-associated solid tumors.
In summary, we encountered a locally advanced lung cancer patient with untargetable driver mutations, dMMR/MSI-H/TMB-H tumor, and a germline PMS2 splicing variant which led to the diagnosis of LS. We provided PMS2-specific genetic counseling to the proband and the carrier in his family. The proband received 4 cycles of nivolumab plus chemotherapy for cancer-risk reduction, which led to a disease-free survival time of 16 months. Further efforts are required to investigate the efficacy of adjuvant immunotherapy in LS patients with locally advanced dMMR/MSI-H/TMB-H tumors.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Concept and design: GD. Acquisition, analysis, and interpretation of data: QH, SL, XL. Drafting of the manuscript: QH, SL, XL. Critical revision of the manuscript for important intellectual content: TM, XL, GD. Technical and material support: SL, ZC, QW, LJ. Study supervision: GD. All the authors gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
SL, XL, TM and LJ are employees of Genetron Health (Beijing) Technology, Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Yi Cai for his valuable and constructive suggestions during the preparation of this manuscript. The authors are grateful to the patient and family members for their kind cooperation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.799807/full#supplementary-material
Amendola, L. M., Dorschner, M. O., Robertson, P. D., Salama, J. S., Hart, R., Shirts, B. H., et al. (2015). Actionable Exomic Incidental Findings in 6503 Participants: Challenges of Variant Classification. Genome Res. 25, 305–315. doi:10.1101/gr.183483.114
André, T., Shiu, K.-K., Kim, T. W., Jensen, B. V., Jensen, L. H., Punt, C., et al. (2020). Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 383, 2207–2218. doi:10.1056/NEJMoa2017699
Balmaña, J., Balaguer, F., Cervantes, A., and Arnold, D. (2013). Familial Risk-Colorectal Cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 24 (Suppl. 6), vi73–vi80. doi:10.1093/annonc/mdt209
Bielska, A. A., Chatila, W. K., Walch, H., Schultz, N., Stadler, Z. K., Shia, J., et al. (2021). Tumor Mutational Burden and Mismatch Repair Deficiency Discordance as a Mechanism of Immunotherapy Resistance. J. Natl. Compr. Cancer Netw. 19, 130–133. doi:10.6004/jnccn.2020.7680
Canney, A., Sheahan, K., Keegan, D., Tolan, M., Hyland, J., and Green, A. (2009). Synchronous Lung Tumours in a Patient with Metachronous Colorectal Carcinoma and a Germline MSH2 Mutation. J. Clin. Pathol. 62, 471–473. doi:10.1136/jcp.2008.063008
Cohen, S. A., Pritchard, C. C., and Jarvik, G. P. (2019). Lynch Syndrome: From Screening to Diagnosis to Treatment in the Era of Modern Molecular Oncology. Annu. Rev. Genom. Hum. Genet. 20, 293–307. doi:10.1146/annurev-genom-083118-015406
Dominguez-Valentin, M., Sampson, J. R., Seppälä, T. T., Ten Broeke, S. W., Plazzer, J. P., Nakken, S., et al. (2020). Cancer Risks by Gene, Age, and Gender in 6350 Carriers of Pathogenic Mismatch Repair Variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 22, 15–25. doi:10.1038/s41436-019-0596-9
Dominguez-Valentin, M., Crosbie, E. J., Engel, C., Aretz, S., Macrae, F., Winship, I., et al. (2021). Risk-Reducing Hysterectomy and Bilateral Salpingo-Oophorectomy in Female Heterozygotes of Pathogenic Mismatch Repair Variants: A Prospective Lynch Syndrome Database Report. Genet. Med. 23, 705–712. doi:10.1038/s41436-020-01029-1
Doroshow, D. B., Bhalla, S., Beasley, M. B., Sholl, L. M., Kerr, K. M., Gnjatic, S., et al. (2021). PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 18, 345–362. doi:10.1038/s41571-021-00473-5
Evans, D. G., Lalloo, F., Ryan, N. A., Bowers, N., Green, K., Woodward, E. R., et al. (2021). Advances in Genetic Technologies Result in Improved Diagnosis of Mismatch Repair Deficiency in Colorectal and Endometrial Cancers. J. Med. Genet. doi:10.1136/jmedgenet-2020-107542
Evison, M. (2020). The Current Treatment Landscape in the UK for Stage III NSCLC. Br. J. Cancer 123, 3–9. doi:10.1038/s41416-020-01069-z
Felip, E., Altorki, N., Zhou, C., Csőszi, T., Vynnychenko, I., Goloborodko, O., et al. (2021). Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB-IIIA Non-small-cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. The Lancet 398, 1344–1357. doi:10.1016/s0140-6736(21)02098-5
Gupta, S., Provenzale, D., Llor, X., Halverson, A. L., Grady, W., Chung, D. C., et al. (2019). NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 2.2019. J. Natl. Compr. Cancer Netw. 17, 1032–1041. doi:10.6004/jnccn.2019.0044
Gupta, S., Provenzale, D., Regenbogen, S. E., Hampel, H., Slavin, T. P., Hall, M. J., et al. (2017). NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 3.2017. J. Natl. Compr. Canc Netw. 15, 1465–1475. doi:10.6004/jnccn.2017.0176
Hames, M. L., Chen, H., Iams, W., Aston, J., Lovly, C. M., and Horn, L. (2016). Correlation between KRAS Mutation Status and Response to Chemotherapy in Patients with Advanced Non-small Cell Lung Cancer☆. Lung Cancer 92, 29–34. doi:10.1016/j.lungcan.2015.11.004
Hebsgaard, S., Korning, P., Tolstrup, N., Engelbrecht, J., Rouzé, P., and Brunak, S. (1996). Splice Site Prediction in Arabidopsis Thaliana Pre-mRNA by Combining Local and Global Sequence Information. Nucleic Acids Res. 24, 3439–3452. doi:10.1093/nar/24.17.3439
Hendriks, Y. M. C., Jagmohan–Changur, S., van der Klift, H. M., Morreau, H., van Puijenbroek, M., Tops, C., et al. (2006). Heterozygous Mutations in PMS2 Cause Hereditary Nonpolyposis Colorectal Carcinoma (Lynch Syndrome). Gastroenterology 130, 312–322. doi:10.1053/j.gastro.2005.10.052
International Mismatch Repair Consortium (2021). Variation in the Risk of Colorectal Cancer in Families with Lynch Syndrome: A Retrospective Cohort Study. Lancet Oncol. 22, 1014–1022. doi:10.1016/s1470-2045(21)00189-3
Jabbour, S. K., Lee, K. H., Frost, N., Breder, V., Kowalski, D. M., Pollock, T., et al. (2021). Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients with Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer. JAMA Oncol. 7, 1351–1359. doi:10.1001/jamaoncol.2021.2301
Kawashima, Y., Nishikawa, S., Ninomiya, H., Yoshida, R., Takano, N., Oguri, T., et al. (2019). Lung Adenocarcinoma with Lynch Syndrome and the Response to Nivolumab. Intern. Med. 58, 1479–1484. doi:10.2169/internalmedicine.1673-18
Latham, A., Srinivasan, P., Kemel, Y., Shia, J., Bandlamudi, C., Mandelker, D., et al. (2019). Microsatellite Instability Is Associated with the Presence of Lynch Syndrome Pan-Cancer. J. Clin. Oncol. 37, 286–295. doi:10.1200/jco.18.00283
Lau, D., Kalaitzaki, E., Church, D. N., Pandha, H., Tomlinson, I., Annels, N., et al. (2020). Rationale and Design of the POLEM Trial: Avelumab Plus Fluoropyrimidine-Based Chemotherapy as Adjuvant Treatment for Stage III Mismatch Repair Deficient or POLE Exonuclease Domain Mutant colon Cancer: a Phase III Randomised Study. ESMO open 5, e000638. doi:10.1136/esmoopen-2019-000638
Le, D. T., Uram, J. N., Wang, H., Bartlett, B. R., Kemberling, H., Eyring, A. D., et al. (2015). PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 372, 2509–2520. doi:10.1056/NEJMoa1500596
Long, Y., Tang, Y., Cai, C., Yu, M., Zhang, M., Chen, R., et al. (2021). The Influence of STK11 Mutation on Acquired Resistance to Immunotherapy in Advanced Non-small Cell Lung Cancer with Lynch Syndrome: a Case Report and Literature Review. Ann. Palliat. Med. 10, 7088–7094. doi:10.21037/apm-20-1639
Luchini, C., Bibeau, F., Ligtenberg, M. J. L., Singh, N., Nottegar, A., Bosse, T., et al. (2019). ESMO Recommendations on Microsatellite Instability Testing for Immunotherapy in Cancer, and its Relationship with PD-1/pd-L1 Expression and Tumour Mutational burden: a Systematic Review-Based Approach. Ann. Oncol. 30, 1232–1243. doi:10.1093/annonc/mdz116
Lynch, H. T., Snyder, C. L., Shaw, T. G., Heinen, C. D., and Hitchins, M. P. (2015). Milestones of Lynch Syndrome: 1895-2015. Nat. Rev. Cancer 15, 181–194. doi:10.1038/nrc3878
Marcus, L., Fashoyin-Aje, L. A., Donoghue, M., Yuan, M., Rodriguez, L., Gallagher, P. S., et al. (2021). FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 27, 4685–4689. doi:10.1158/1078-0432.Ccr-21-0327
Marcus, L., Lemery, S. J., Keegan, P., and Pazdur, R. (2019). FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 25, 3753–3758. doi:10.1158/1078-0432.Ccr-18-4070
Masuzawa, K., Asakura, T., Ikemura, S., Yasuda, H., Kawada, I., Takaoka, S., et al. (2020). Long-Lasting Response to Nivolumab for a Patient with Lynch Syndrome-Associated Lung Adenocarcinoma. JCO Precision Oncol. 4, 74–78. doi:10.1200/po.19.00156
Møller, P., Seppälä, T., Bernstein, I., Holinski-Feder, E., Sala, P., Evans, D. G., et al. (2017). Cancer Incidence and Survival in Lynch Syndrome Patients Receiving Colonoscopic and Gynaecological Surveillance: First Report from the Prospective Lynch Syndrome Database. Gut 66, 464–472. doi:10.1136/gutjnl-2015-309675
Nolan, L., Eccles, D., Cross, E., Crawford, G., Beck, N., Bateman, A., et al. (2009). First Case Report of Muir-Torre Syndrome Associated with Non-Small Cell Lung Cancer. Fam. Cancer 8, 359–362. doi:10.1007/s10689-009-9247-7
Nykamp, K., Anderson, M., Powers, M., Garcia, J., Herrera, B., Ho, Y.-Y., et al. (2017). Sherloc: A Comprehensive Refinement of the ACMG-AMP Variant Classification Criteria. Genet. Med. 19, 1105–1117. doi:10.1038/gim.2017.37
National Comprehensive Cancer Network (2021). NCCN Clinical Practice Guidelines in Oncology: Genetic/Familinal High-Risk Assessment: Colorectal. Available at https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf [Accessed on: May 11, 2021].
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and Guidelines for the Interpretation of Sequence Variants: a Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. doi:10.1038/gim.2015.30
Rousseau, B., Foote, M. B., Maron, S. B., Diplas, B. H., Lu, S., Argilés, G., et al. (2021). The Spectrum of Benefit from Checkpoint Blockade in Hypermutated Tumors. N. Engl. J. Med. 384, 1168–1170. doi:10.1056/NEJMc2031965
Seppälä, T. T., Dominguez-Valentin, M., Sampson, J. R., and Møller, P. (2021). Prospective Observational Data Informs Understanding and Future Management of Lynch Syndrome: Insights from the Prospective Lynch Syndrome Database (PLSD). Fam. Cancer 20, 35–39. doi:10.1007/s10689-020-00193-2
Seppälä, T. T., Latchford, A., Negoi, I., Sampaio Soares, A., Jimenez-Rodriguez, R., Sánchez-Guillén, L., et al. (2021). European Guidelines from the EHTG and ESCP for Lynch Syndrome: An Updated Third Edition of the Mallorca Guidelines Based on Gene and Gender. Br. J. Surg. 108, 484–498. doi:10.1002/bjs.11902
Sinicrope, F. A., Ou, F.-S., Zemla, T., Nixon, A. B., Mody, K., Levasseur, A., et al. (2019). Randomized Trial of Standard Chemotherapy Alone or Combined with Atezolizumab as Adjuvant Therapy for Patients with Stage III colon Cancer and Deficient Mismatch Repair (ATOMIC, Alliance A021502). J. Clin. Oncol. 37, e15169. doi:10.1200/JCO.2019.37.15_suppl.e15169
Skoulidis, F., and Heymach, J. V. (2019). Co-Occurring Genomic Alterations in Non-Small-Cell Lung Cancer Biology and Therapy. Nat. Rev. Cancer 19, 495–509. doi:10.1038/s41568-019-0179-8
Stoffel, E. M., Mangu, P. B., Gruber, S. B., Hamilton, S. R., Kalady, M. F., Lau, M. W. Y., et al. (2015). Hereditary Colorectal Cancer Syndromes: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the Familial Risk-Colorectal Cancer: European Society for Medical Oncology Clinical Practice Guidelines. J. Clin. Oncol. 33, 209–217. doi:10.1200/jco.2014.58.1322
Sun, S., Liu, Y., Eisfeld, A.-K., Zhen, F., Jin, S., Gao, W., et al. (2019). Identification of Germline Mismatch Repair Gene Mutations in Lung Cancer Patients with Paired Tumor-Normal Next Generation Sequencing: A Retrospective Study. Front. Oncol. 9, 550. doi:10.3389/fonc.2019.00550
Tang, J., Shalabi, A., and Hubbard-Lucey, V. M. (2018). Comprehensive Analysis of the Clinical Immuno-Oncology Landscape. Ann. Oncol. 29, 84–91. doi:10.1093/annonc/mdx755
Ten Broeke, S. W., van Bavel, T. C., Jansen, A. M. L., Gómez-García, E., Hes, F. J., van Hest, L. P., et al. (2018). Molecular Background of Colorectal Tumors from Patients with Lynch Syndrome Associated with Germline Variants in PMS2. Gastroenterology 155, 844–851. doi:10.1053/j.gastro.2018.05.020
Ten Broeke, S. W., van der Klift, H. M., Tops, C. M. J., Aretz, S., Bernstein, I., Buchanan, D. D., et al. (2018). Cancer Risks for PMS2-Associated Lynch Syndrome. J. Clin. Oncol. 36, 2961–2968. doi:10.1200/jco.2018.78.4777
Thompson, B. A., Spurdle, A. B., Spurdle, A. B., Plazzer, J.-P., Greenblatt, M. S., Akagi, K., et al. (2014). Application of a 5-Tiered Scheme for Standardized Classification of 2,360 Unique Mismatch Repair Gene Variants in the InSiGHT Locus-specific Database. Nat. Genet. 46, 107–115. doi:10.1038/ng.2854
Wang, M., and Marín, A. (2006). Characterization and Prediction of Alternative Splice Sites. Gene 366, 219–227. doi:10.1016/j.gene.2005.07.015
Wang, Y. A., Jian, J.-W., Hung, C.-F., Peng, H.-P., Yang, C.-F., Cheng, H.-C. S., et al. (2018). Germline Breast Cancer Susceptibility Gene Mutations and Breast Cancer Outcomes. BMC cancer 18, 315. doi:10.1186/s12885-018-4229-5
Yagishita, S., Horinouchi, H., Sunami, K. S., Kanda, S., Fujiwara, Y., Nokihara, H., et al. (2015). Impact of KRAS Mutation on Response and Outcome of Patients with Stage III Non‐squamous Non‐Small Cell Lung Cancer. Cancer Sci. 106, 1402–1407. doi:10.1111/cas.12740
Yeo, G., and Burge, C. B. (2004). Maximum Entropy Modeling of Short Sequence Motifs with Applications to RNA Splicing Signals. J. Comput. Biol. 11, 377–394. doi:10.1089/1066527041410418
Keywords: Lynch syndrome, lung cancer, PMS2, splicing variant, incomplete penetrance
Citation: Han Q, Liu S, Cui Z, Wang Q, Ma T, Jiang L, Li X and Dai G (2022) Case Report and Literature Review: Diagnosis, Tailored Genetic Counseling and Cancer Prevention for a Locally Advanced dMMR/MSI-H/TMB-H Lung Cancer Patient With Concurrent Lynch Syndrome Mediated by a Rare PMS2 Splicing Variant (c.1144+1G>A). Front. Genet. 12:799807. doi: 10.3389/fgene.2021.799807
Received: 22 October 2021; Accepted: 20 December 2021;
Published: 18 January 2022.
Edited by:
Ruowen Zhang, Jiahehongsheng (Shenzhen) Health Industry Group, ChinaReviewed by:
Luke Hesson, Garvan Institute of Medical Research, AustraliaCopyright © 2022 Han, Liu, Cui, Wang, Ma, Jiang, Li and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghai Dai, ZGFpZ2gzMDFAdmlwLnNpbmEuY29t; Xiaomo Li, eGlhb21vLmxpQGdlbmV0cm9uaGVhbHRoLmNvbQ==
†These authors have contributed equally to this work and share first authorship.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.