- Department of Human Genetics and Molecular Medicine, Central University of Punjab, Bathinda, India
miRNAs are fascinating molecular players for gene regulation as individual miRNA can control multiple targets and a single target can be regulated by multiple miRNAs. Loss of miRNA regulated gene expression is often reported to be implicated in various human diseases like diabetes and cancer. Recently, geneticists across the world started reporting single nucleotide polymorphism (SNPs) in seed sequences of miRNAs. Similarly, SNPs are also reported in various target sequences of these miRNAs. Both the scenarios lead to dysregulated gene expression which may result in the progression of diseases. In the present paper, we explore SNPs in various miRNAs and their target sequences reported in various human cancers as well as diabetes. Similarly, we also present evidence of these mutations in various other human diseases.
Introduction
MicroRNAs (miRNAs) are endogenous single stranded, non-coding, 20–22 nucleotides long molecules that are processed from pre-miRNA. miRNAs have been demonstrated to be tremendously versatile in their function. miRNAs have significant roles in the nucleus as well as cytoplasm in terms of controlling gene expression. They play a significant role in post-transcriptional regulation of gene expression either via translational repression or mRNA degradation (Iorio and Croce, 2012; Peng and Croce, 2016). miRNAs recognize targets by specific base-pairing complementarity between their seed sequence of miRNA (5′ end) and untranslated region (3′UTR) of target gene/mRNA (Ling et al., 2011; Si et al., 2019).
However, in some exceptional cases, base pairing is also reported between 5′ UTR region of the specific mRNA and coding regions (O'Brien et al., 2018; Valinezhad Orang et al., 2014). The standard size of 3′UTR in the human gene is about 950 nucleotides whereas the seed sequence of miRNA is around 6 to 8 nucleotides. The 3′UTR region of a particular mRNA may be recognized by a specific miRNA or by multiple miRNAs. Sequence complementarity is shared by miRNAs with respect to their mRNA targets, resulting in the interaction of a single miRNA with many genes whereas a single gene can probably be regulated by multiple miRNAs (Hashimoto et al., 2013; Mariella et al., 2019).
Around 10 million SNPs are known to be present in both coding as well as non-coding regions of the human genome at a frequency of one in every 300 bp (Moszyńska et al., 2017). Since SNPs have also been reported to be present in seed sequence, it is most likely that the presence of these alterations might disrupt or create new interaction of miRNA with its target site (Palmero et al., 2011; Bhattacharya and Cui, 2017). Furthermore, the SNPs in the 3′UTRs of gene/mRNA can also modulate miRNA-mRNA interactions, protein-mRNA interactions, polyadenylation, all of which might have a serious impact on translation efficiency and mRNA stability (Malhotra et al., 2019a). This in turn might result in the development of various diseases including neurodevelopment disorders, cardiovascular diseases, cancer, autoimmune diseases, and many more (Bruno et al., 2012; Moszyńska et al., 2017).
Cancer and diabetes are multifactorial life threatening human diseases in which various miRNAs have been reported in the pathogenesis as well as the severity of these diseases (Ayaz Durrani et al., 2021). Tens of millions of people are diagnosed with cancer each year around the world, with more than half of those diagnosed dying from it. miRNA profiling and high throughput sequencing in the recent past revealed that miRNA expression is dysregulated in cancer and that its fingerprints might be utilized to classify, diagnose, and prognosis of tumors. miRNAs have been reported to act as oncogenes or tumor suppressors under certain biological conditions. Cancer hallmarks such as sustaining proliferative signals, evading growth suppressors, resisting cell death, activating invasion and metastasis, and initiating angiogenesis have been linked to dysregulated miRNAs (Peng and Croce, 2016).
Diabetes mellitus (DM) affects 347 million people worldwide. Diabetes-related fatalities are expected to double between 2005 and 2030, according to the World Health Organization (Chen et al., 2014a). High blood glucose levels are a defining feature of DM. Diabetes is classified into two types. A deficiency of insulin synthesis in pancreatic cells causes type 1 diabetes (T1D), whereas type 2 diabetes (T2D) is caused by insulin resistance, which causes the body to utilize insulin inefficiently. In both T1D and T2D, long-term hyperglycemia can cause macrovascular (coronary artery disease, peripheral arterial disease, and stroke) as well as microvascular complications (diabetic nephropathy, neuropathy, and retinopathy) (Fowler, 2008). miRNAs are implicated in the etiology and pathogenesis of diabetes and associated complications (Chen et al., 2014a). However, the role of miRNAs in diabetes and its complications are comparatively explored less.
Very few reports are available on SNPs reported in the seed sequence of miRNAs and the 3′UTR region of their target genes. The current review has been compiled with an aim to evaluate the role of genetic variation in the seed sequence of the miRNA and the 3′UTR of their specific target genes in association with the development of the two most common prevalent diseases—cancer and diabetes.
SNPs in the Seed Sequence of miRNA and the 3′UTR of Specific Target Gene in Cancer
Many miRNAs have been discovered to play a role in the genesis of cancer, either as tumor suppressor genes or as oncogenes. The study of tumor-specific miRNA expression profiles in a variety of malignancies revealed extensive dysregulation of these molecules, some of the overexpression and underexpression of various miRNA (Song and Chen, 2011). As evidenced by multiple findings demonstrating the importance of miRNAs in carcinogenesis, miRNA dysregulation leads to modulation of tumor cell signaling, changes in DNA repair or stress response, and function of the effector protein (Moszyńska et al., 2017; Malhotra et al., 2019a; Galka-Marciniak et al., 2019).
SNPs in the Seed Sequence of miRNA
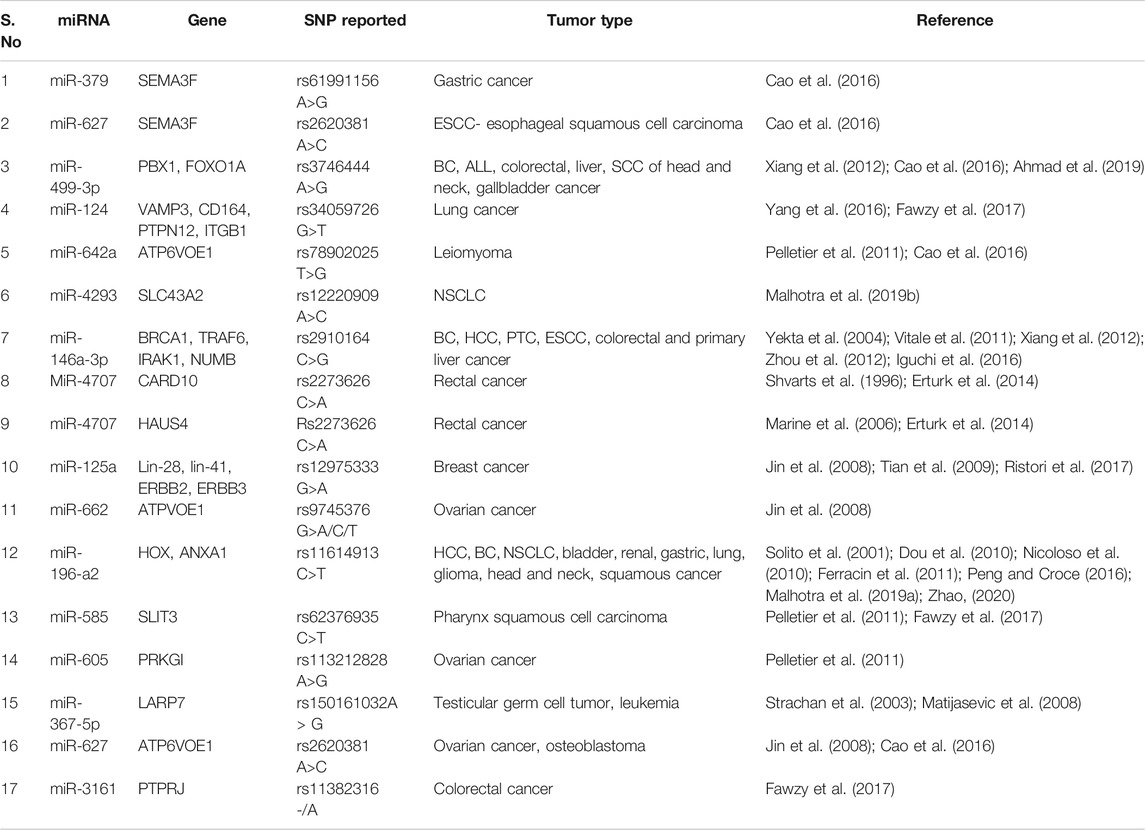
SNPs in the mature or primary miRNA or seed sequence might affect miRNA processing or binding. Several SNPs present in miRNA main sequences or upstream regulatory regions have been linked to increased cancer risk as well as its prognosis (Duan et al., 2007). In cancer, SNPs reported in seed sequence of various miRNA include rs2910164 in miR-146a; rs3746444 in miR-499; rs12975333 in miR-125a; rs34059726 in miR-124; and rs11614913 in miR-196-a2. Information about SNPs in the seed sequence of miRNAs is summed up in Table 1 and elaborated functional role of these miRNAs in the development of cancer has been depicted in Figure 1.
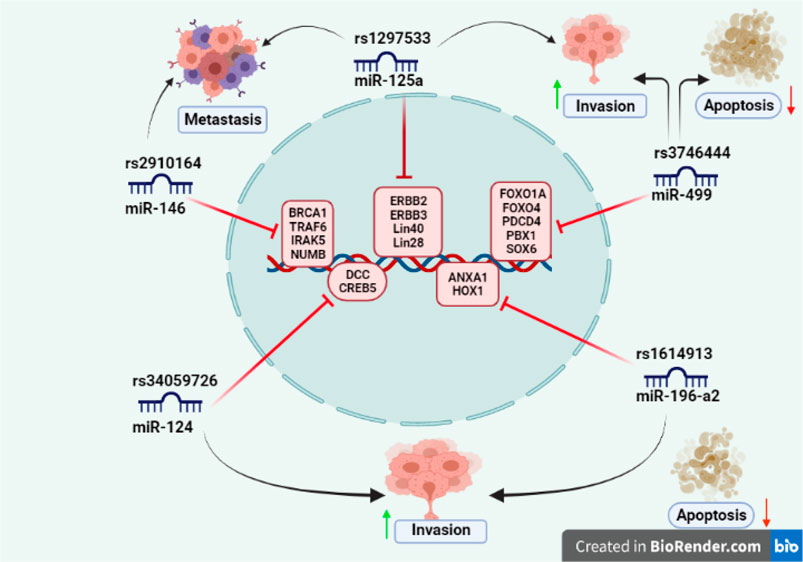
FIGURE 1. SNPs reported in seed sequence of miRNA involved in cancer: miR-146 (rs2910164) targets include BRCA1, TRAF6, IRAKS, and NUMB gene and associated with cancer metastasis; miR-125a (rs1297533) found to be involved in invasion and metastasis and known targets include ERBB2, ERBB3, lin-40, and lin-28 gene; miR-499 (rs3746444) promotes invasion and inhibits cell apoptosis by targeting FOXO1A, FOXO4, PDCD4, PBX1, and SOX6 gene; miR-124 (rs34059726) has target genes DCC, CREB gene, and enhances invasion; miR-196-a2 (rs1614913) inhibits apoptosis and promotes invasion having target genes ANXA1 and HOX gene.
miRNA-146a
miR-146a is a widely expressed miRNA in mammalian cells. Multiple studies have shown that miR-146a is involved in inflammation, differentiation, and function of adaptive and innate immune cells. miR-146a has been found to be a regulator of cell function and differentiation in innate and adaptive immunity. A subset of human T cells exhibits different expression level of niR-146a. Memory T cells and naive T lymphocytes have different levels of miR-146a expression (Nahand et al., 2020). This miRNA is produced by T-cell receptor activation, and the binding sites of c-ETS and transcription factor nuclear factor-κB (NF-kB) are required for miR-146a transcription in T lymphocyte cells (Curtale et al., 2010; Lu et al., 2010). Some studies observed an association between the NF-kB signaling pathway and miR-146a expression (Rusca and Monticelli, 2011). Taganov et al. (2006) found that LPS stimulation enhanced miR-146a expression in an NF-kB-dependent manner and that miR-146a targeted the IRAK1 and TRAF6 genes (Taganov et al., 2006; Rusca and Monticelli, 2011). After a cell surface receptor (such as TLR4) is activated, a biochemical cascade involving IRAK1 and TRAF6 causes IkBa to be phosphorylated and degraded, resulting in the activation of NF-kB and its nuclear translocation. Furthermore, NF-kB activation causes some genes, such as pri-miR-146a, to be expressed. miR-146a matures on the RISC and contributes to the attenuation of receptor signaling by downregulating TRAF6 and IRAK1. As a result, miR-146a inhibits the signaling pathway leading to NF-B activation (Taganov et al., 2006; Taganov et al., 2007; Labbaye and Testa, 2012).
miR-146a polymorphism, rs2910164, involves a G>C nucleotide alteration on the seed region of miR146a-3p, resulting in G:U pair to a C:U mismatch pairing in the stem of the miR-146a affecting the specificity of mature miR-146a binding to its targets and results in elevated expression of miR-146a (Brincas et al., 2020). Previous studies have established the association of rs2910164 in pre-miR-146a with strong association with breast cancer (BC), hepatocellular carcinoma (HCC), papillary thyroid carcinoma (PTC), esophageal squamous cell carcinoma (ESCC), primary liver cancer, and colorectal cancer (Hu et al., 2009; Vitale et al., 2011; Xiang et al., 2012; Zhou et al., 2012).
A microarray-based expression study carried out in a Chinese population found that miR-146a was significantly upregulated in breast carcinoma tissues compared to normal tissues (Omrani et al., 2014). Its expression level was three times higher in triple negative tumors in comparison with other tumor subgroups. However, this association has been reported to vary in different ethnic groups. The allele C is associated with BC risk in the European population but did not show any association with BC in the Asian population. This discrepancy might be on account of ethnicity, different exposure to carcinogens, or linkage disequilibrium with different causal variants (Brincas et al., 2020).
Molecular targets of miR-146a include BRCA1, TRAF6, IRAK1, and NUMB genes (Brincas et al., 2020). The variant allele of rs2910164 leads to increased levels of mature miR-146a that binds with greater affinity to the BRCA1 gene. Alternatively, rs2910146 might disrupt the well-documented role of miR-146a as a mediator of the pro-apoptotic transcriptional factor NF-κB. Also, the expression levels of miR146a-5p were observed three times higher in triple negative tumors compared to other subgroups of mammary tumors (Brincas et al., 2020). Two other significant targets of miR-146a, TRAF6, and IRAK1 are important adapter molecules downstream of the toll-like and cytokine receptors that have a vital role in signaling cell growth and immune recognition (Omrani et al., 2014). Both the genes have been associated with progression and metastasis. The reduced TRAF6 and IRAK1 levels reduce the activity of NF-kB, a potential inducer of proliferation, survival, angiogenesis, and metastasis (Brincas et al., 2020).
rs2910164 of miR-146a has been reported to induce liver metastasis in colorectal cancer (CRC) via Notch and JAK/STAT signaling pathways. Migratory response of NUMB has been observed in CRC cell lines (RKO, HT29, LoVo). NUMB protein is a negative regulator of Notch signaling, miR-146a activates Notch and JAK/STAT3 signaling through suppression of NUMB protein thereby enhancing the metastatic risk. Further, patients of gastric cancer bearing the altered genotype showed a higher expression of miR-146a than the ones bearing the normal genotype (Iguchi et al., 2016). In addition, this polymorphism has also been reported to increase the risk of PTC in a heterozygous condition (Vitale et al., 2011). The reduction in expression level of miR-146a led to less efficient inhibition of target genes TRAF6 and IRAF1 involved in the Toll-like receptor and cytokine signaling pathways and thereby increase risk of PTC (Slaby et al., 2012).
miRNA-499
miR-499 is a microRNA that regulates multiple genes and signaling pathways post-transcriptionally, especially in hypoxic-ischemic situations like cancer and myocardial infarction (Wilson et al., 2010; Ando et al., 2014). Wang et al. (2015) observed reduced expression of miR-499-5p disrupted the PI3K/AKT/GSK signaling pathway (Liu et al., 2011). miR-499 functions as a tumor suppressor by decreasing cell proliferation causing apoptosis, which inhibits cancer progression. In addition, it also prevents metastases. FOXO4 and programmed cell death 4 (PDCD4) genes have been reported to be the targets of miR-499 (Liu et al., 2011). PDCD4 is an RNA-binding protein that stops particular mRNAs from being translated (Ohnheiser et al., 2015). PDCD4 modulates several signal transduction pathways and impacts the translation and transcription of many genes as a tumor suppressor (Wang et al., 2013a). It may play a key role in halting cell cycle progression and preventing tumor metastasis by inhibiting cell proliferation (Wei et al., 2012). Wei et al. (2012) demonstrate that PDCD4 may be important in stopping cell cycle progression at a critical checkpoint, limiting cell proliferation and suppressing tumor spread. In ovarian cancer cells, the PI3K-Akt pathway was thought to be involved in the regulation of PDCD4 degradation (Wei et al., 2012).
An SNP rs3746444 (T>C) has been reported in the seed region of mature miR-499 (Chen et al., 2014b). This SNP has been associated with increased susceptibility to various cancers like BC, cervical squamous cell carcinoma, acute lymphoblastic leukemia (ALL), colorectal cancer, liver cancer, gallbladder cancer, lung cancer, gastric cancer, squamous cell carcinoma of the head and neck, and prostate cancer. rs3746444 has been reported to be associated with an elevated risk of BC in the Chinese, German, and Italian populations; gastric cancer in the Japanese population; prostate cancer in the Indian population; cervical squamous cell carcinoma and lung cancer in the Chinese population, and ALL in the Iranian population (Tian et al., 2009; Catucci et al., 2010; Liu et al., 2010; Srivastava et al., 2010; Zhou et al., 2010; George et al., 2011; Okubo et al., 2011; Hasani et al., 2014). In contrast, Asian populations with the T allele of the miR-SNP are thought to have a lower risk of BC whereas Caucasians bearing the same variant allele have been reported at a higher risk of BC (Chen et al., 2014c).
rs3746444 leads to overexpression of miR-499 resulting in its enhanced binding to its target genes including FOXO4, PDCD4, and SOX6 gene (Dai et al., 2016). FOXO transcription factors regulate a variety of physiological activities, including fuel metabolism, oxidative stress response, and redox signaling, cell cycle progression, and apoptosis (Urbánek and Klotz, 2017). FOXO4 is a tumor suppressor protein that has associated with metastasis (Yang et al., 2006; Lee et al., 2009; Zhang et al., 2009). PDCD4 is a well-known tumor suppressor regulating the growing, invading, or metastasis of the tumors. The study reported that prometastatatic action of miR-499 is on account of the suppression of FOXO4 and PDCD4 expression (Liu et al., 2011). PDCD4 inhibits the expression of mitogen-activated protein kinase (MAP4K1) via Jun N-terminal kinase (JNK) pathway. This was established by cDNA microarray analysis of PDCD4-overexpressing in RKO human colon cancer cells (Yang et al., 2006).
rs3746444 has also been reported to regulate the expression level of SOX gene. The anti-apoptosis action of miR-499 (rs3746444 T>C) can be reversed by up-regulating the SOX6 gene (Li et al., 2013a). Deregulation of the SOX gene activates the Wnt/-catenin signaling pathway, which has been linked to cancer development (Yan et al., 2017).
miRNA-125a
miR-125 plays a role in disease prevention and promotion, especially in cancer and host immunological responses. miR-125 inhibits a variety of genes, including transcription factors, matrix metalloproteinases, Bcl-2 family members, and others, causing aberrant cell proliferation, metastasis, and invasion, as well as carcinomas (Sun et al., 2013). BC, stomach cancer, and medulloblastoma all have lower levels of miR-125a, which promotes disease development. In human medulloblastoma cells, overexpression of miR-125a resulted in cell growth arrest and apoptosis. Furthermore, in stomach cancer cells, identical ectopic expressions inhibited growth. In BC cells, overexpression of miR-125a resulted in decreased anchorage-dependent proliferation. It was discovered that miR-125a modulates these cellular processes through Erbb2 in the context of gastric cancer and BC investigations (Scott et al., 2007; Ferretti et al., 2009).
The polymorphism rs12975333 (G>T) in miR-125a is in the seed sequence at the 8th nucleotide of mature miRNA. The T allele has been shown to inhibit the conversion of pri-miRNA to pre-miRNA precursor and is extremely rare, having been found only once in a panel of 1200 people from various ethnic origins and correlated with an elevated risk of BC in the Belgium population (Peterlongo et al., 2011). The reduced expression of mature miR-125a leads to the overexpression of the target genes.
The known targets of miR-125a like ERBB2 and ERBB3 have previously been reported to be associated with BC tumorigenesis (Morales et al., 2018). ERBB2 encodes the BC marker HER2 and alterations of ERBB2 and ERBB3 have been reported to promote malignancy. For example, ERBB2 overexpression is associated with approximately 25% of all human BC which drives the key aggressive features including cell proliferation, motility, and invasion (Lehmann et al., 2013). Malignant transformation can be induced by deregulation of ERBB2 and ERBB3 alone or in combination. Amplification and overexpression of ERBB2 have been associated with 25% of all human breast tumors. Overexpression of ERBB2 in particular promotes cell survival, proliferation, motility, and invasion, all of which are hallmarks of this aggressive form of human BC (Scott et al., 2007).
miRNA- 124
miR-124 is one of the most abundant miRNAs in the adult brain and is expressed primarily in the CNS. Mature miR-124 family includes three members, namely miR-124-1, miR-124-2, and miR-124-3. miR-124 has been demonstrated to induce cell differentiation while inhibiting cell proliferation in general (Ristori et al., 2017). Several cancers, including colon, breast, and lung cancers, as well as leukemia and lymphoma, are linked to miR-124 (Pal et al., 2015).
A G>T (rs3405972) has been reported in the seed sequence of miR-124-3 (Gong et al., 2012; Beretta et al., 2017). The major miR-124-3 targets include vesicle-associated membrane protein 3 (VAMP3), sialomucin core protein 24 (CD164), tyrosine-protein phosphatase non-receptor type 12 (PTPN12), neuronal growth regulator 1 (NEGR1), cyclin-dependent kinase 6 (CDK6), integrin Beta 1 (ITGB1), and insulin-like growth factor-binding protein 7 (IGFBP7) (Leong, 2013).
The 3′UTR of oncogene CDK6 is the target of mature miR-124. miR-124 epigenetic masking causes CDK6 activation and subsequent phosphorylation of retinoblastoma (Rb), resulting in cell growth acceleration which is directly involved in brain cancer (Pal et al., 2015). miR-124 expression leads to the downregulation of PTPN12 protein which regulates tyrosine phosphorylation and is implicated in cancer and cellular physiology. As PTPN12 reduces mammary cell proliferation and transformation, the targeting of PTPN12 by miR-124 suppresses its tumor suppressor behavior which promotes the oncogenic shift in breast and lung cancer (Sun et al., 2011). Leong Pei predicted that miR-124-3 with a variant allele targets novel genes DCC (deleted in colorectal cancer) and CREB5 (cyclic AMP-responsive element-binding protein 5) rather than PTPN12 (as predicted by public databases). The variant miR-124-3 is unable to suppress PTPN12 tumor suppressor and may alternatively behave as a tumor suppressor instead of an oncogene in breast or lung cancer (Leong, 2013). Hunt et al. (2011) reported miR-124 reduces oral squamous cell carcinoma (OSCC) invasion by targeting ITGB1, which is responsible for regulating intracellular signaling cascades and tissue homeostasis. Thus, miR-124 has a strong potential to be used as a prognostic marker in OSCC (Sun et al., 2011).
miRNA-196-a2
miR-196 family of molecules can operate as tumor suppressors. miR-196a, for example, inhibits metastasis in melanoma and BC cells, while miR-196b is downregulated in many types of leukemia cells. Bioinformatics research revealed that miR-196a2 could target multiple genes involved in cell cycle regulation, survival, and apoptosis, all of which could be relevant in GI malignancies. Cell proliferation, migration, invasion, and radio resistance are all functions of miR-196 family molecules’ carcinogenic impacts (Fawzy et al., 2017).
The polymorphic C>T (rs11614913) is in the mature sequence of miR-196a-3p that negatively affects the processing of precursor miRNA to mature and subsequently its capability to regulate its target genes. Variant T allele influences the stability of the secondary structure of miR-196a2 (Wojcicka et al., 2014). This variant has been associated with an increased risk of bladder cancer; renal cancer; gastric cancer; lung cancer, HCC; glioma; head and neck squamous carcinoma; NSCLC and familial BC (Hu et al., 2008; Tian et al., 2009; Dou et al., 2010; Stenholm et al., 2013; Dai et al., 2015; Liu et al., 2018).
C allele impairs mature miRNA expression, resulting in lower levels of mature miR-196a2 (Yekta et al., 2004; Hoffman et al., 2009). In the Chinese population thus miR-SNP-induced decrease in miRNA expression could be used as a predictive biomarker for assessing BC risk (Qi et al., 2015). Other studies, on the other hand, have suggested that in some groups, the rs11614913 polymorphism predicts a lower risk of BC (Dai et al., 2016). A meta-analysis of 16 studies was carried out and observed that Caucasian patients had a lower risk of BC, with no significant influence on total risk (Zhang et al., 2017). It has been found that people with the CC genotype of this SNP were more likely to develop BC (Ferracin et al., 2011; Wang et al., 2013b).
rs11614913 associated with decreased risk of glioma and BC in Chinese populations and it has been found to be associated with reduced risk of cervical cancer in the Indian population (Hu et al., 2009; Dou et al., 2010; Thakur et al., 2019). According to recent studies, miR-196a2 TT genotype was associated with decreased risk for cervical cancer whereas miR-196a2 and CC/CT genotype was associated with higher risk. In another study, it was shown that C allele exhibited association with HCC in the Asian population but not in Caucasians whereas it increased the risk of colorectal, glioma, and prostate cancer in the non-African population compared to the African population (Zhao, 2020).
The potential molecular targets of miR-196a2 include HOX and annexin-A1(ANXA1) genes. ANXA1 was shown to be a key modulator of apoptosis and has since been linked to glucocorticoid activities such as cell proliferation suppression and cell migration control. ANXA1 plays a significant role in membrane trafficking, exocytosis, signal transduction, cell differentiation, and apoptosis, among other biological roles (Luthra et al., 2008). Overexpression of miR-196a2 due to variant rs11614913 leads to the suppression of ANXA1 thereby promoting cell proliferation and suppressing apoptosis (Solito et al., 2001; Luthra et al., 2008; Rahim et al., 2019). ANXA1 exhibits varied expression in different cancers. It is upregulated in glioma and oropharyngeal cancer and downregulated in prostate cancer, esophageal squamous cell, and head and neck squamous carcinoma (Rahim et al., 2019). As an upstream regulator, miR-196a has been found to partially direct the cleavage of the mRNA of the HOX gene clusters. HOX genes were shown to be abnormally expressed in BC, and HOXD10 was found to initiate tumor invasion and metastasis (Yekta et al., 2004).
SNPs in 3′UTR of miRNA Target Sequence
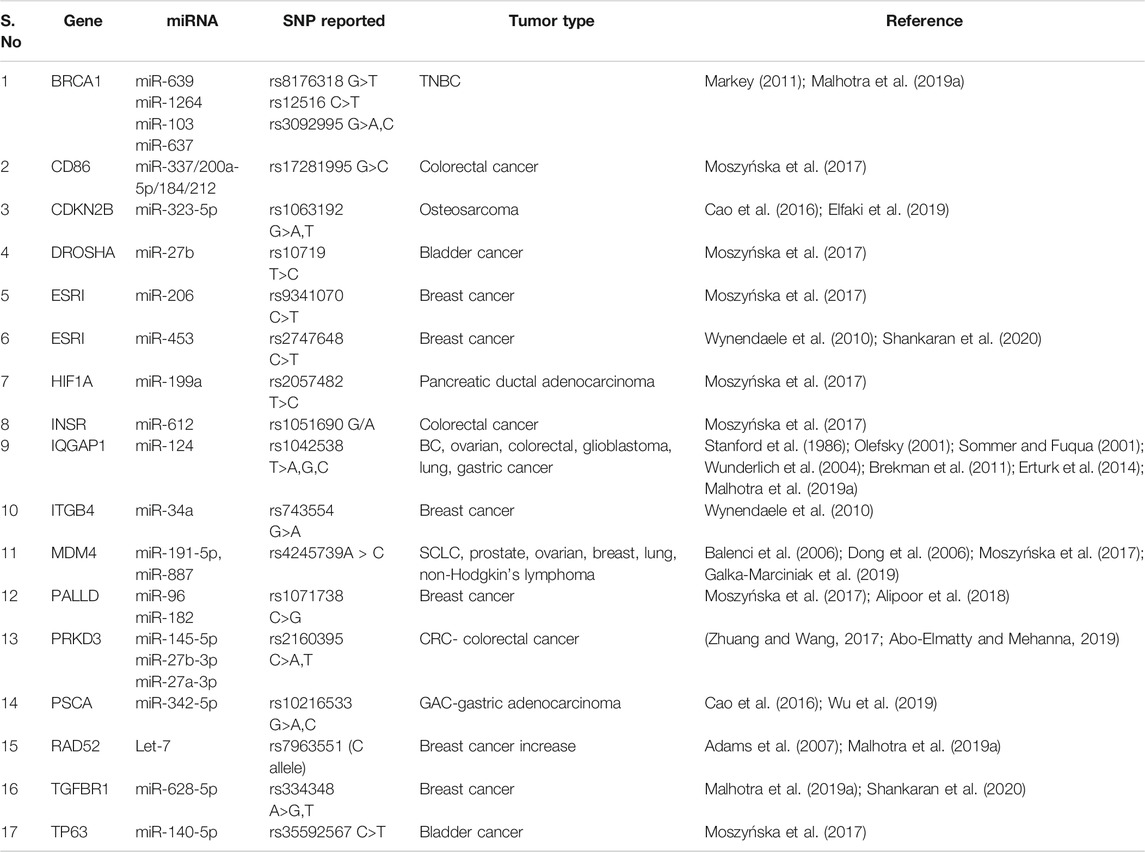
Several SNPs in miRNA binding sites or miRNA target gene (3′UTR) disrupt the capability to recognize the specific target. This results in dysregulation of target genes due to changes in miRNA and mRNA interactions (Nicoloso et al., 2010; Zheng et al., 2011). The presence of SNPs in the 3′UTR in an oncogene or a tumor regulatory gene might cause changes in gene regulation can shift the balance of cellular homeostasis toward cancer (Zheng et al., 2011). Variations in the 3′UTR of target genes involved in the stress response or DNA repair modify the activity of effector proteins, resulting in changes in the ability to repair damaged DNA and raising the risk of cancer (Cao et al., 2016). SNPs reported in 3′UTR of target genes of specific miRNA includes rs3092995, rs12516, rs8176318 in BRCA1 gene; rs4245739 in MDM4; rs1042538 in IQGAP1; rs7963551 in RAD52; rs9341070 in ESRI; and rs1071738 in PALLD gene. Information about genetic variation (SNPs) reported in 3′UTR of miRNA target gene along with functional outcome implicated in the pathogenesis of cancer have been summed up in Table 2 and Figure 2.
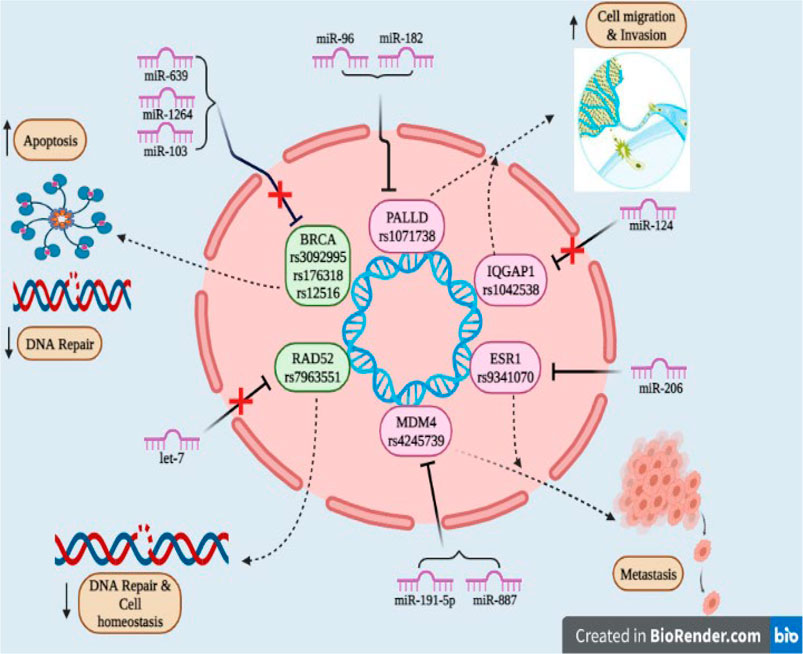
FIGURE 2. SNPs reported in 3′UTR region of the target gene involved in cancer: Due to SNPs in the BRCA gene (rs3092995, rs176318, rs12516) and RAD52 gene (rs7963551) binding site for miR-639, miR-1264, and miR-103 within BRCA and for let-7 within the RAD52 gene found to be disrupted and as tumor suppressor genes they promote cell apoptosis, decrease the DNA repair mechanism and maintain cell homeostasis; PALLD (rs1071738), IQGAP1 (rs1042538), ESR1 (rs9341070), and MDM4 (rs4245739) genes act as oncogenes and they are responsible for metastasis, cell migration, and invasion.
BRCA 1
BRCA is one of the well-studied genes associated with BC. This gene has a highly conserved 3′UTR of 1381 nucleotides encodes a 1863 amino acid protein and functions as a tumor suppressor gene that regulates various cellular processes including cell cycle checkpoint control, chromatin remodeling, DNA repair, regulation of transcription, protein ubiquitination, and apoptosis (Yang et al., 2016; Ahmad et al., 2019). The 3′UTR region of the BRCA1 gene plays a pivotal role in the localization, stability, and mRNA transport. SNPs in 3′UTR of this gene might alter genes expression and therefore increase the risk of BC. The prevalence of SNPs in 3′UTR of BRCA1 modulating the miRNA binding site that can emerge as a significant biomarker of the disease (Ahmad et al., 2019).
SNPs including rs3092995, rs12516, and rs8176318 are in the 3′UTR of this gene. rs3092995 (G>A, C) is in the sequence of BRCA1 gene that interacts with the seed sequence of miR-103. rs3092995 is strongly associated with increased risk of BC in African American women. The variant alleles of rs8176318 (G>T) and rs12516 (G>A,T) are associated with ovarian and familial BC in Thai women (Pelletier et al., 2011). rs8176318 is located at the region where miR-639 is assumed to bind. Enhanced risk of BC was reported in individuals with GT or GG compared to TT genotype in the Pakistani population (Ahmad et al., 2019). This SNP is also associated with the risk of TNBC and ovarian cancer in an Irish population. The bearer of the rs8176318 variant allele has been linked to an increased risk of BC in menopausal women, as estrogen levels in these women drop after menopause (Malhotra et al., 2019b).
SNP rs12516 in the 3′UTR of BRCA1 gene alters the binding site of miR-1264, affecting its binding affinity and at the same time creating a binding site for other miRNAs including miR- 4278, miR-4704-5p, and miR-637. Binding of these miRNAs to the 3′UTR of BRCA1 has been associated with higher NC risk. This rs12516 present in the 3′UTR of BRCA1 has been reported to be a genetic marker in the Turkish population associated with an increased risk of BC development (Erturk et al., 2014; Malhotra et al., 2019a).
MDM4
The murine double minute 4 (MDM4) protein was found as a p53-binding protein and has a fundamental amino acid sequence that is very similar to MDM2 (Shvarts et al., 1996). Over half of all human malignancies have a mutation in p53, the most frequently inactivated gene in cancer. p53 functions as a transcriptional factor that transactivates a set of genes involved in multiple cellular processes such as cell cycle arrest, cellular senescence, energy metabolism, and apoptosis in response to various extra- and intracellular stresses such as oncogene activation, DNA damage, and hypoxia. High concentrations of p53 inhibitors can also inactivate p53 signaling. MDM2 and MDM4, two main negative regulators of p53, are substantially responsible for p53 activity suppression (Marine et al., 2006). MDM4 has also been reported to bind with p21 and direct it to proteasomal destruction without ubiquitination. Mdm4-mediated p21 degradation is independent of MDM2, yet it works together with MDM2 to break the G1 cell cycle arrest (Jin et al., 2008). Deletion of MDM4 causes multipolar spindle formation, increased chromosomal loss, higher proliferative potentials, and increased spontaneous tumor transformation in p53-null cells (Matijasevic et al., 2008). Because MDM4 and MDM2 form heterodimers, Mdm4 depletion may stimulate MDM2 interaction with other proteins such as Rb and p21, enhancing carcinogenesis. MDM4 also inhibits E2F1 transactivation by disrupting E2F1-DNA binding or changing the location of the E2F1 transcription complex (Strachan et al., 2003). E2F1 overexpression enhances the G1/S transition in cells and has been linked to cancer development. E2F1 overexpression, on the other hand, causes both p53-dependent and p53-independent apoptosis (Crosby and Almasan, 2004; Wunderlich et al., 2004; Stanelle and Pützer, 2006). These findings point to MDM4 upregulation, which is found in many malignant malignancies (Markey, 2011).
An SNP rs4245739 (A>C) is located within the 3′UTR region of MDM4. The C allele creates a new binding site for three miRNAs, miRNA-191-5p, miR-887, and miR-3669 (Wynendaele et al., 2010; Stegeman et al., 2015; Anwar et al., 2017). The variant allele of this polymorphism has been reported as a risk factor for many cancers including ovarian cancer, lung cancer, prostate cancer, BC, non-Hodgkin’s lymphoma, esophageal cancer, and retinoblastoma (Wynendaele et al., 2010; Zhou et al., 2013; Gao et al., 2015a; Stegeman et al., 2015; Xu et al., 2016; Anwar et al., 2017).
miR-191-5p showed a greater binding affinity with C allele of rs4245739. In a genotype-based mRNA expression analysis, it was found that C allele was associated with decreased risk of ovarian cancer and retinoblastoma in an Asian population (Xu et al., 2016). MDM4 is overexpressed in A allele genotype and enhances the risk of BC and ESCC in a Chinese population (Zhou et al., 2013; Stegeman et al., 2015). The patients bearing homozygous AA allele were found to have 5.5-fold increased risk of tumor-associated mortality and 4.2-fold increased risk of recurrence (Guo et al., 2016). In an in vitro study carried out in PC3 cells in the case of C allele bearers, since it binds with miR-191-5p and miR-887. On other hand, A allele is un-targeted, that is, it directly enhances the risk of prostate cancer (Stegeman et al., 2015). Individuals carrying the rs4245739 C allele express low levels of MDM4 resulting in high DNA repair ability mediated by p53 and thus decreased cancer risk (Zhou et al., 2013).
IQGAP1
IQ-domain GTPase-activating proteins (IQGAPs) are a multi-domain protein family that regulate a variety of cellular processes such as cell adhesion, migration, extracellular signaling, and cytokinesis (Brown and Sacks, 2006). The first of three human IQGAP homologues, IQGAP1 is expressed throughout the body, whereas IQGAP2 and IQGAP3 are mostly found in the liver and intestine, the brain, and the testis (Weissbach et al., 1994; Nojima et al., 2008; Schmidt et al., 2008). IQGAP1 is hypothesized to contribute to the changed cancer cell phenotype by modulating signaling pathways involved in cell proliferation and transformation, cell-cell adhesion weakening cell motility and invasion stimulation (Johnson et al., 2009). Calmodulin, a ubiquitous calcium-binding protein, regulates IQGAP1 function via the IQ motifs, which are common calmodulin-interacting domains present in many proteins. Calmodulin is thought to affect IQGAP1 function by producing a conformational shift that affects IQGAP1-protein interactions and/or IQGAP1 subcellular localization (Briggs and Sacks, 2003). Both ERK and b-catenin-dependent signaling are aided by IQGAP1. IQGAP1 binds to B-Raf, MEK, and ERK leading to the activation of MAPK cascade. Constitutive MAPK pathway activation is a common oncogenic trigger in a variety of cancers, particularly those caused by Ras and B-Raf activating mutations. H-Ras and R-Ras were shown to have no detectable binding whereas active M-Ras had a favorable association (Roy et al., 2005; Nussinov et al., 2018). IQGAP1 is overexpressed in several cancers including ovarian cancer, colorectal cancer, glioblastoma, lung cancer, and gastric cancer (Nabeshima et al., 2002; Nakamura et al., 2005; Balenci et al., 2006; Dong et al., 2006).
miR-124 regulates IQGAP1 through a binding site in its 3′UTR. This target site sequence is disrupted by rs1042538 (T>A,C,G) in the core binding region. The presence of this variation at the miR-124 binding region has been suggested as a possible predictor of BC risk and prognosis. Based on a case-control study carried out in the Chinese population, the TT genotype was associated with a lower BC in comparison AA genotype, depicting that the T allele protects against BC (Zheng et al., 2011).
RAD52
Radiation sensitive 52 (RAD52) is a DNA strand exchange protein that mediates the DNA-DNA interaction required for complementary DNA strands to anneal during homologous recombination in DNA damage repair in order to maintain cell viability and homeostasis. Recent research has found that RAD52 plays a key function in mammalian cell genomic stability and cancer suppression (Feng et al., 2011; Lok et al., 2013). RAD52 stimulates the creation of nuclear foci, which appears to correspond to DNA repair sites, in response to DNA damage. RAD52 activity increases progressively as cells enter phase S, peaking in the S phase, and then disappearing at the start of G2. Phosphorylation and sumoylation are two post-translational changes that RAD52 can undergo. All these processes appear to work together to control the timing of RAD52 recruitment, as well as its stability and function (Liu et al., 1999; Barlow and Rothstein, 2010; Nogueira et al., 2019). RAD52 has also been shown to have a role in the response to oncogene-induced DNA replication stress (Sotiriou et al., 2016). High levels of RAD52 expression have been reported in tumor cells, particularly in lung squamous cell carcinomas and nasopharyngeal carcinoma tissues (Lieberman et al., 2016).
An SNP (C>A) rs7963551 is in the 3′UTR of RAD52 that is the binding site of let-7 miRNA (Jiang et al., 2013). In Chinese women, this SNP has been linked to an increased risk of BC. The presence of this variation reduces the binding capacity of let-7 to its target regions in the RAD52 3′UTR that has been suggested to boost its expression (Jiang et al., 2013). rs7963551 polymorphism with A allele was found to be strongly related with a lower incidence of SCLC in the Chinese population, according to a study. The functional genetic variant was only substantially associated with SCLC susceptibility among smokers but not with nonsmokers (Han et al., 2015).
ESR1
The nuclear hormone receptor and oncoprotein estrogen receptor alpha/estrogen receptor 1 (ER/ESR1) is overexpressed in around 70% of breast tumors (Stanford et al., 1986; Sommer and Fuqua, 2001). The ESR1 gene encodes estrogen receptor (ER), which is primarily a nuclear protein that operates as a ligand-dependent transcription factor (ER’s genomic activity) (Olefsky, 2001). In primary human BC and human BC cell lines, MDM2 expression has been reported to be correlated with ER expression. The ER has been postulated to upregulate MDM2 expression (Baunoch et al., 1996; Hori et al., 2002; Brekman et al., 2011). MDM2 also forms a protein complex with ER, making it easier for ER to be ubiquitinated and degraded resulting in a negative feedback loop. However, the ability of ER and MDM4 (another member of the MDM family) to interact and regulate each other’s expression in a comparable way has yet to be established (Liu et al., 2000).
The SNP rs9341070 (C>T) is one of the known polymorphisms located at 3′UTR of ESR1 gene at the binding site of miR-206. This variant influences the binding between miRNA and 3′UTR of ESR1 results in lower expression of ESR1 gene (Brucker et al., 2017). The T allele at 3′UTR allows binding of miR-206 to ESR1 and it is significantly downregulated (Adams et al., 2007). This SNP has been associated with an increased risk of BC (Anwar et al., 2017; Brucker et al., 2017).
PALLD
Palladin (PALLD), an actin-associated protein whose expression is intimately linked to the pathogenic cell motility properties of aggressive cancer cells, is encoded by the PALLD gene. Palladin expression is higher in invasive and malignant BC cell types than in noninvasive and normal cell lines. Palladin stimulates podosome formation, modulates the actin cytoskeleton via numerous routes, participates in matrix breakdown, and hence it aids in BC spread (Goicoechea et al., 2009; von Nandelstadh et al., 2014).
miR-96 and miR-182 inhibit BC cell migration and invasion by downregulating Palladin protein levels. This mechanism is disturbed by an SNP rs1071738 in the 3′UTR of the PALLD gene. The variation rs1071738 (C>G) is a very normal functional variant of the PALLD gene. The alternate G allele is substantially more prevalent than the ancestral minor C allele. The mRNA target sequence at the 3′UTR of PALLD is entirely complementary to the miR-96 and miR-182 seed areas. The presence of C allele favors the interaction of these miRNAs with the 3′UTR of PALLD. However, the presence of the variant G allele results in a mismatch between the two. miR-96 and miR-182 regulate the expression of PALLD reducing its expression by about 30 and 70%, respectively, in the presence of the normal CC genotype. However, the presence of the G allele leads to abolition of interaction between the two regulatory miRNAs and PALLD, on account of the mismatch between these two miRNAs and seed sequence of PALLD. In normal conditions miR-96 and miR-182 are involved in the prevention of BC metastasis. However, the G allele counteracts this impact (Gilam et al., 2016). The functional significance of rs1071738 has been proved by in vitro study carried out by MCF-7 (non-invasive BC cell lines) and Hs578 (highly invasive BC cell line) (Gilam et al., 2016; Moszyńska et al., 2017).
SNPs in Seed Sequence of miRNA and the 3′UTR of Specific Target Genes in Diabetes
SNPs in Seed Sequence of miRNA
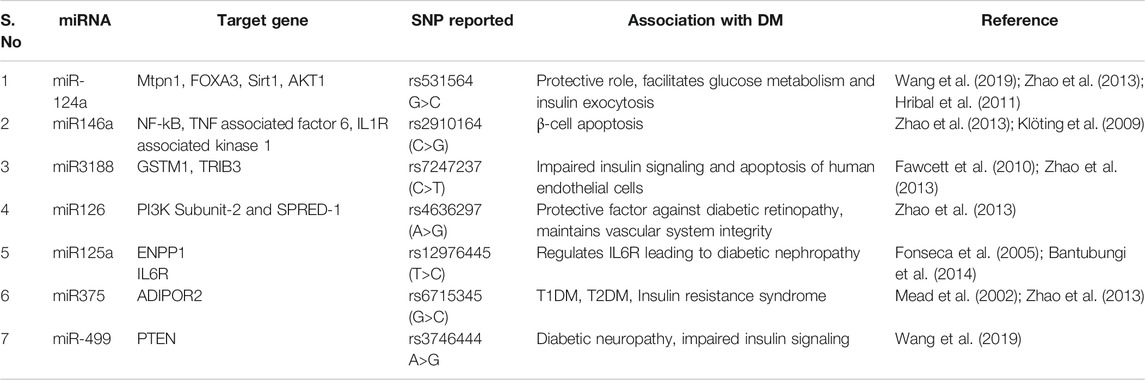
SNPs reported in the seed sequence of miRNA associated with diabetes or its complications including rs3746444 in miR-499a; rs2910164 in miR-146a; rs7247237 in miR-3188; and rs34059726 in miR-124-3p. A detailed information involving SNPs in seed sequence of miRNA associated with diabetes and their functional implications has been given in Table 3 and Figure 3.

FIGURE 3. SNPs reported in seed sequence of miRNA involved in diabetes: (A) miR146a with SNP rs2910164 (C>G) raises vascular complications caused by upregulation of inflammatory factors (TNF associated factor 6 and IL1 associated kinase 1) in endothelial cells and induces apoptosis in pancreatic β-cells via NF-kB mediated pathway. (B) Reduced expression of miR499a due to SNP rs3746444 (A>G) provokes mitochondrial stress, impairs insulin signaling via PTEN mediated pathway, and promotes hepatic insulin resistance. (C) The miR124 rs34059726 (G>T) creates complimentary sequence for INSR; causing failure to transport GLUT4 transporter vesicle to outer membrane. This SNP also inhibits glycogenesis process via GSK-3β activation. (D) miR-3188 with rs7247237 (C>T) inhibits PI3K/AKT pathway dysregulating protein synthesis. Its target genes overexpression (GSTM1 and TRIB3) curtails nitric oxide pathway which increases vascular complications and induces β-cell apoptosis.
miRNA-499a
The diabetic neuropathy including cardiovascular autonomic neuropathy (CAN) and diabetic neuropathy (DPN) have been reported to impact the quality of life in diabetics since these complications have been reported in a large percentage of diabetics. The miR-499a is an antiapoptotic and cardioprotective miRNA (Wang et al., 2011; de Carvalho et al., 2019). It has been reported that polymorphisms in miR499 are involved in perturbed insulin secretion, CAN, and peripheral neuropathy (Ciccacci et al., 2018).
The genetic variation in miR-499a has been associated with the development of diabetic neuropathies. Especially the patients carrying the GG genotype of rs3746444 (A>G) present in the seed region of this miRNA are at higher risk of developing the CAN (Ciccacci et al., 2018). A study carried out in an Italian population investigate the association between mitochondrial DNA (mtDNA) copy number and rs3746444 in DPN patients. A decline in the mtDNA copy number in T2DM patients affecting DPN was observed in comparison with healthy controls (Latini et al., 2020). The increase in the copy number of mtDNA in association with the variant allele has been hypothesized to be on account of mitochondrial fission due to oxidative stress (Ghaedi et al., 2016). Increased ROS and mitochondrial injury might be contributing to nervous system dysfunction (Wang et al., 2011).
Apart from mitochondrial dysfunction, dyslipidemia was also observed in patients. Dyslipidemia plays a significant role in the pathogenesis of DN, synergistically with hyperglycemia (Vincent et al., 2009). An excess of long-chain fatty acids in T2D can lead to an accumulation of acetyl-CoA, as a product of mitochondrial beta-oxidation (Fan et al., 2020). miR499a-5p over-expression can enhance the glycogen level and improve insulin signaling by PTEN inhibition. Reduced miR-499-5p level is observed in hepatic insulin resistance (Wang et al., 2015a). miR-499-5p is involved in the signaling pathway of IRS1/PI3K/AKT and in particular miR-499-5p targets PTEN, which is an important regulator of the insulin signaling pathway (Peyrou et al., 2015). Therefore, unstable secondary structure with GG genotype reduces miR-499-5p levels, as a consequence an increase in PTEN impairs the insulin signaling.
miRNA-146a
The key biological role of miR146a is as immunosuppressive modulator which regulates inflammatory response. It downregulates innate immune response by suppressing expression of IRAK1 and TRAF2, decreasing NFkB activity (Gholami et al., 2020). Therefore, it functions as a negative regulator of NFkB and its inflammatory cascade and promotes apoptosis and inhibits migratory capacity by negative regulation of EGFR signaling pathway (Chen et al., 2013; Park et al., 2015).
SNP rs2910164 C>G is present within the seed sequence of miR146a which reduces its expression (Alipoor et al., 2018). This SNP plays a significant role in the pathogenesis of diabetes by participating in β-cell metabolism, proliferation, and death. The suppressed expression of miR146a enhances the activity of NFkB inflammatory events and induction of β-cell apoptosis responsible for diabetes and related complications (Elfaki et al., 2019). The potential targets of miR146a include TNF associated factor 6 and IL-1 receptor associated kinase 1 which regulate endothelial inflammation. The C>G transition causes overexpression of these target mRNAs resulting in T2DM (Shankaran et al., 2020). This polymorphism also increases the incidence of preeclampsia in gestational diabetes mellitus (GDM) (Abo-Elmatty and Mehanna, 2019).
In a study involving the Chinese population, rs2910164 was associated with an increased risk of T2DM. In some other studies, this SNP is also responsible for risk like diabetic nephropathy in T1DM patients and diabetic macular edema in T2DM patients of Caucasian population. Further it is also associated with diabetic polyneuropathy and GDM in the Italian and Egyptian populations (Zhuang and Wang, 2017).
miRNA-3188
miR-3188 is involved in regulation of the mTOR-P-PI3k/AkT pathway and has been reported to affect the pathogenesis of diabetic complications. It is one of the miRNAs discovered quite early.
rs7247237 (C>T) is considered to be located in the seed sequence of miR-3188 and has been associated with T2DM in the Chinese population. In vitro studies on HUVEC cell lines showed that the C allele expression was five times higher than T allele suggesting that C>T transition reduces its level which results in the overexpression of its targets; GSTM1 (glutathione S-transferase M1) and Trib3 (Tribbles pseudokinase3). This in turn reduces nitric oxide (NO) production in the endothelial cells through inhibition of endothelial NO synthase. There is also evidence that the overexpression of TRIB3 is associated with apoptosis in human endothelial cells, which could probably have an important role in the progression and pathogenesis of vascular complications in diabetes. As according to a study, miR3188 regulating mTOR and PI3K/AKT pathway involved in insulin signaling in endothelial cell; its reduced expression on account of the presence of rs7247237 resulting in T2DM (Wang et al., 2017; Wu et al., 2019).
RhoA/ROCK is another downregulated pathway by miR-3188 is RhoA/ROCK pathway via targeting ETS transcription factor ELK4. Elk4 is involved in various cancers and atherosclerosis. Its potential role via RhoA/ROCK pathway needs to be further elucidated (Li et al., 2017). The potential therapeutic value of miR-3188 could be further explored to mitigate the effect of pathogenic SNP (Wang et al., 2019)
miR-124-3p
The miR-124 is highly expressed in the brain and involved in epigenetic regulation of neurogenesis (Coolen et al., 2015). However, the in vitro studies miR124 overexpression of miR-124 in MIN6 pseudoislet cells caused impaired glucose induced secretion of insulin. Its silencing in MIN6 pseudoislet cells resulted in upregulation of its target genes FOXA2, Mtpn, Flot2, AKT3, Sirt1, and NeuroD1. All these targets are involved in normal Beta-cell functioning (Sebastiani et al., 2015). A study carried out in a mouse model demonstrated that miR-124 mediates triglyceride accumulation in the liver induced by high fat diet by directly targeting tribbles pseudokinase 3 (TRB3) and enhancing AKT signaling (Liu et al., 2016). An SNP rs34059726 located in the seed region of miR-124-3p, curated in PolymiRTS database is predicted to target insulin receptor transcript (INSR) (Gong et al., 2012). INSR belongs to tyrosine kinase receptor family which mediates insulin signaling via PI3K/AKT pathway. This pathway is responsible for maintaining glucose homeostasis, proliferation, and differentiation and inhibition of apoptosis (Chen et al., 2019). Down regulation of INSR via miR-124-3p leads to dysregulation of glucose uptake due to inhibition of GLUT4 vesicle transport to membrane and glucose transfer into cells (Jaakson et al., 2018). Lower PI3K/AKT signaling stimulates GSK-3β and inhibits glycogenesis. Failure of AKT to inhibit proapoptotic protein expression leads to apoptosis of the cells.
SNPs in 3′UTR of miRNA Target Sequence
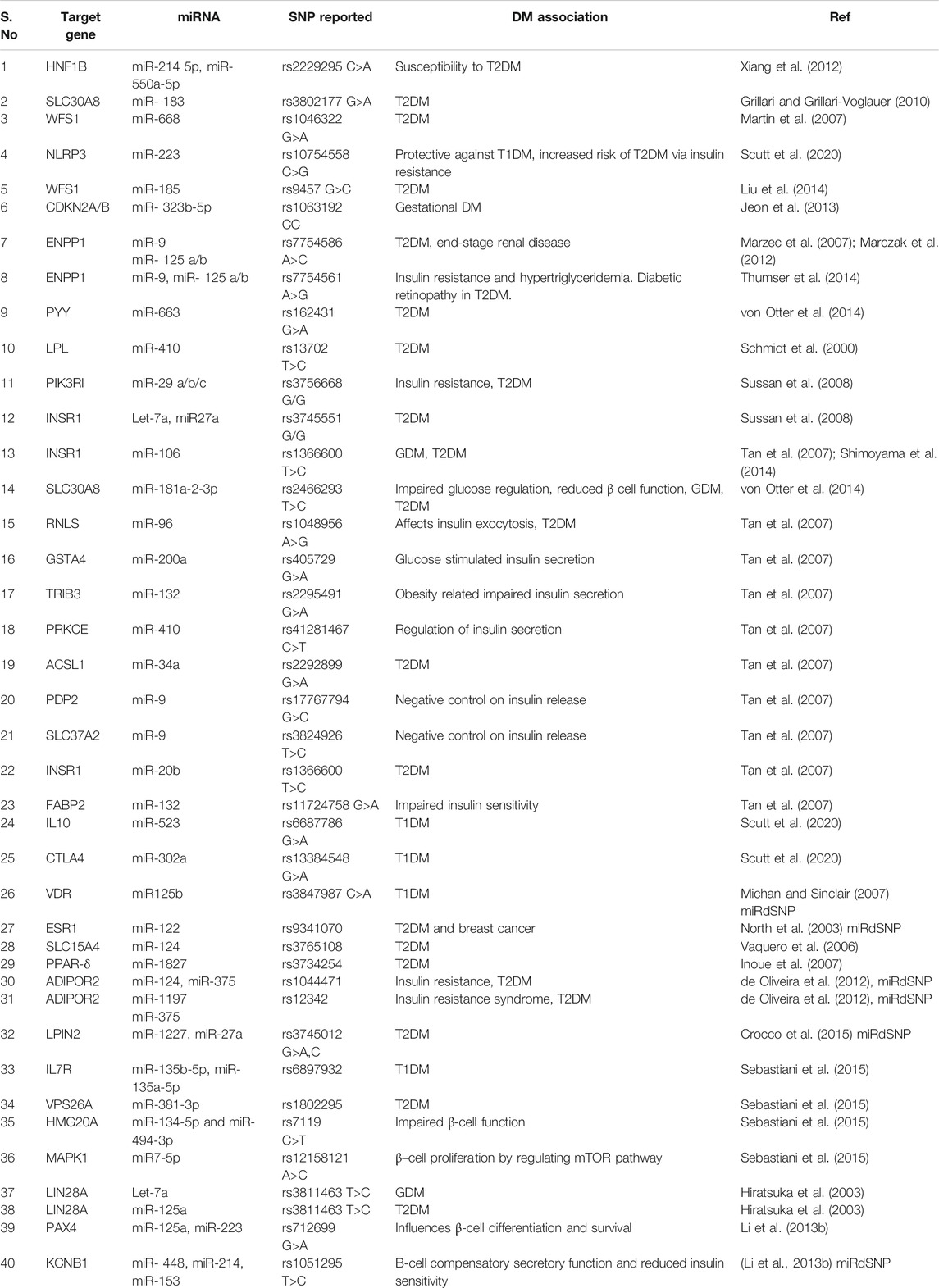
The SNPs in 3′UTR of miRNA target genes reported in diabetes include rs11724758 in FABP-2; rs1046322 in WFS-1; rs2229295 in HNF1B; rs1063192 in CDKN2B; and rs13702 in LPL genes. Further details of other SNPs in 3′UTR of miRNA target genes implicated in pathogenesis of diabetes have been summed up in Table 4 and the functional role in Figure 4.
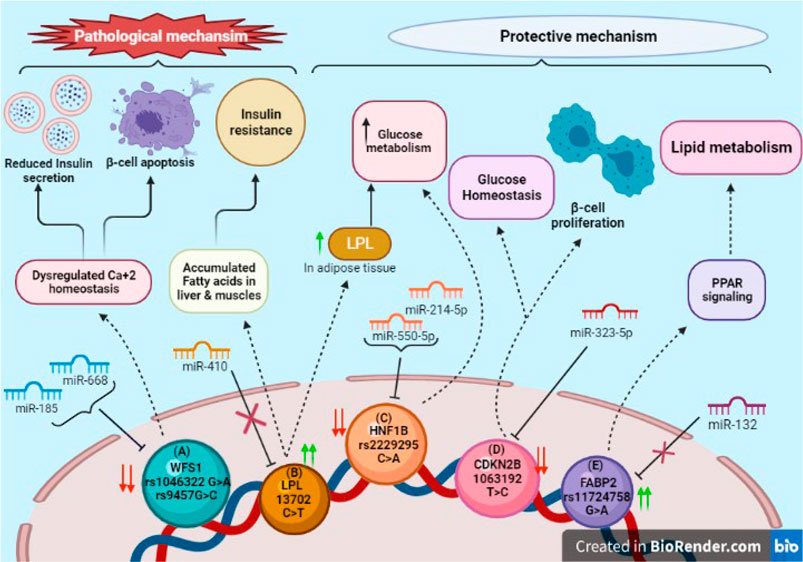
FIGURE 4. SNPs in 3′UTR of target genes associated with diabetes: (A) Downregulation of WFS1 due to SNPs rs1046322 (G>A) and rs9457 (G>C) via miRNAs miR185 and miR668 induces β-cell apoptosis and declines insulin secretion due to ER Ca+2 stress. (B) SNP rs13702 (C>T) in LPL gene disrupts binding site for miR410. Its overexpression has pathological impact on liver and muscles leading to insulin resistance; whereas in adipose tissue it increases glucose metabolism. (C) SNP rs2229295 (C>A) of HNF1B creates new binding site for miR214-5p and miR550-5p, increasing glucose metabolism. (D) CDKN2B’s downregulation due to SNP rs1063192 (T>C) by miR323-5p maintains glucose homeostasis and promotes proliferation of β-cells. (E) Over-expression of FABP2 due to SNP rs11724758 (G>A) activates PPAR signaling pathway which in-turn leads to lipid metabolism.
FABP-2
Fatty acid binding protein-2 belongs to ubiquitous lipid chaperones family is expressed in intestines which regulate fat absorption by intracellular trafficking of long chain fatty acids, eicosanoids, and other lipids (Haunerland and Spener, 2004). Its dysregulation has been associated with non-alcoholic hepatic liver disease and obesity (Thumser et al., 2014). Around 20–30% T2DM patients have renal impairment and FABP 2 is a novel biomarker for diabetic nephropathy (Tsai et al., 2020). FABP2 has been associated with insulin resistance mechanisms, indicating its essential role in protection against T2DM (Baier et al., 1995).
The 3′UTR polymorphism of rs11724758 (G>A) in FABP2 gene causes loss of binding site for miR-132. The miR-132 plays a significant role in adipose tissue dysfunction and obesity associated diabetes (Klöting et al., 2009). The AA genotype of FABP2 has been associated with decreased risk of T2DM compared to GG genotype (Zhao et al., 2013). Therefore, G>A transition functions as a protective factor against T2DM. FABP2 is involved in intracellular fatty acid transportation and fat absorption via PPAR signaling (Zhao et al., 2013).
WFS-1
Wolframin or WFS1 gene encodes for endoplasmic reticulum trans-membrane protein highly expressed in brain, pancreas, and heart (Hofmann et al., 2003). Mutation in this gene leads to a metabolic condition known as Wolfram Syndrome inherited in an autosomal recessive manner (Harel et al., 2015). Two SNPs within 3′UTR of WFS1—rs1046322 and rs9457—have been reported to be as risk factors for T1DM and T2DM, respectively (Fawcett et al., 2010; Kovacs-Nagy et al., 2013; Elfaki et al., 2019). WFS1 is an ER transmembrane protein highly expressed in pancreas and insulinoma β-cell lines. It plays a significant role in maintaining Ca+2 ER homeostasis (Hofmann et al., 2003). In T2DM, it indicated that glucose induced insulin secretion has been found to increase WFS1 expression. The increased insulin production caused by insulin resistance in T2DM leads to chronic ER stress contributing to the death of β-cells by apoptosis. It was demonstrated that glucose induced insulin secretion leads to increased WFS1 expression in wild-type mice, whereas ER stress and β-cell dysfunction can be observed in WFS1 knock-out animals (Fonseca et al., 2005). In vitro interaction between WFS1 3′ UTR and miR-668 signified the rs1046322 influenced the affinity of miR-668 to WFS1 mRNA. In an in vitro luciferase assay it was observed that variation in 3′utr of WFS1 gene rs1046322 “A” and rs9457 “C” is sensitive to both miR-185 and miR-668, although the effect of miR-185 seemed to be stronger (Elek et al., 2015). miR-185 was reported to be strongly associated with diabetes mellitus via its targets SOCS3 and WFS1 (Bao et al., 2015; Elek et al., 2015). They showed that these different pathways can be in the background of the same phenotype, as miR-185 is suggested to be related to diabetes mellitus via WFS1 target (Bao et al., 2015).
HNF1B
HNF1B encodes for HNF1β (hepatocyte nuclear factor 1-β) homeodomain containing transcription factor expressed in pancreas, liver, and kidney (Coffinier et al., 1999). It regulates the critical function of pancreatic development, glucose metabolism, and hepatic insulin activity (Goda et al., 2015). It is the most common transcription factor associated with monogenic diabetes leading to young adult onset of T1DM with dominant inheritance patterns in familial cases. An SNP rs2229295 (C>A) within 3′-UTR of HNF1β acts as a protective factor against T2DM (Moszynska et al., 2017). In silico analysis revealed that rs2229295 in 3′UTR of HNF1β creates the binding site for miR-214-5p and miR-550-5p. The A allele of HNF1β is responsible for post-transcriptional regulation by miR214-5p and miR-550-5p. Therefore, due to this variation, expression of HNF1β is downregulated and thereby it acts as a protective factor against T2DM (Goda et al., 2015).
CDKN2B
CDKN2A/B highly expressed in pancreas is considered as a strong determinant of diabetes mellitus. The SNP rs1063192 T>C located within 3′UTR region of CDKN2A/B is associated with increased risk of GDM in pregnant Chinese Han women population (Wang et al., 2015b). The tumor-suppressor products of CDKN2A/B, p15INK4b, and p16INK4a inhibit important CDKs, i.e., CDK4 and CDK6, essential for β-cell proliferation and regeneration (Krishnamurthy et al., 2006). The T>C transition creates complimentary sequence of miR-323-5p which reduces the expression of CDKN2A/B (Hribal et al., 2011). The decreased expression of CDKN2A/B due to rs1063192 T>C results in reduced inhibition of CDK6 by p15INK4b and facilitates β-cell proliferation, lowering DM risk. On the other hand, increased expression of p15INK4b regulates glucose homeostasis. It can be speculated that miR-323-5p may also regulate other crucial genes responsible for β-cell hyperplasia and insulin signaling. Moreover, duality of p15INK4b in glucose homeostasis and deficiency of p16INK4a inducing in vivo hepatic glucose production via PKA-CREBPGC1a pathway possibly explains its role in GDM (Bantubungi et al., 2014).
LPL
Lipoprotein lipase enzyme is involved in hydrolysis of low-density lipoproteins and circulating chylomicrons into non-esterified fats which can be absorbed by the tissues. Disturbance in this conversion could lead to various abnormalities such as Alzheimer’s, dyslipidemia, and diabetes (Mead et al., 2002). In liver and muscle tissues, the free fatty acid generated by LPL activity gets accumulated, leading to insulin resistance (Kim et al., 2001). The SNP rs13702 C>T in 3′UTR of LPL mRNA is located within the seed recognizing region of miR-410 (Hatefi et al., 2018). This C>T transition disrupts the binding site of miR-410 which leads to increased expression of LPL (Richardson et al., 2013). However, in adipose tissue, LPL increases glucose metabolism and insulin tolerance (Walton et al., 2015). Knockdown studies in MIN6 cells indicated decreased ability of glucose induced insulin secretion. In the Iranian population, the T allele of rs13702 showed protective association whereas C allele was found to be a risk factor against T2DM (Hatefi et al., 2018).
SNPs in the Seed Sequence of miRNA and Target Genes Associated with Various Diseases
SNPs within seed sequence of miRNA and their target genes have been also implicated in the development of various human diseases like Parkinson disease, asthma, periodontal disease, neurodegenerative disease, cardiovascular disease, and kidney and liver diseases (Martin et al., 2007; Tan et al., 2007; Rademakers et al., 2008; Wang et al., 2008; Schaefer et al., 2010; Bruno et al., 2012). The presence of SNPs in miRNA seed regions has a major impact on miRNA target loss and gain (generates a new repertoire of target genes), resulting in a considerable change in miRNA biological function (Xu et al., 2013; Zhang et al., 2019).
Among human diseases, ischemic stroke is one of the complicated diseases that consist of a variety of conditions with different hereditary and environmental risk factors. miRNAs played a role in a variety of physiopathological processes, and frequent SNPs in pre-miRNAs have been linked to disease vulnerability in humans (Liu et al., 2014). According to a case-control study, SNP (A>G) rs3746444 located within seed sequence of miR-499 may be significantly linked with higher risk of ischemic stroke in the Chinese community (Liu et al., 2014). miR-499 has been associated with ischemia condition, apoptosis, and cell death in anoxia via knockdown or calcineurin over-expression, inhibiting Drp1 dephosphorylation and mitochondrial fragmentation caused by anoxia (Wang et al., 2011). The rs3746444 polymorphism changed the stem structure of the miR-499 precursor from an A:U pair to a G:U mismatch, which changed the function or expression of mature miR-499, as well as the regulation of target mRNAs, influencing the risk of ischemic stroke (Hu et al., 2009; Xiang et al., 2012). Its targets includes peptyl arginine deiminase type 4, regulatory factor X4, IL-2, IL-2 receptor B (IL-2R), IL-6, IL-17 receptor B (IL-17RB), IL-18 receptor (IL-18R), IL-21, IL-23a, and B and T lymphocyte attenuator (Yang et al., 2012). miR-499/rs3746444 bound to its mentioned targets and can influence inflammation, fibrinogen, and CRP formation. Higher plasma CRP levels can raise blood pressure, BMI, insulin resistance, and lipids making CRP one of the common causes of cerebral ischemia (Yang et al., 2012; Jeon et al., 2013). Increased CRP, inflammation, and fibrinogen in the allele G and carried G genotypes of rs3746444 A/G may play a predisposing role in the development of ischemic stroke (Liu et al., 2014).
SNP in the 3′UTR of mRNA/target gene might disrupt or create the binding sites for miRNA. The renin-angiotensin system (RAS) is a key player in blood pressure regulation and is thought to be a contributing element in the development of hypertension (Laragh et al., 1991). Angiotensin II is a key player in the RAS pathway, inducing vasoconstriction, salt retention, and water retention, and is closely linked to the inflammatory, thrombotic, and fibrotic factors. Angiotensin II receptor type 1 (AGTR1) and type 2 (AGTR2) mediate these effects both directly and indirectly (AGTR2). AGTR1 is mostly found in vascular smooth muscle cells, as well as the heart, adrenal gland, and kidney (Oparil and Weber, 2000). A study on miR-155 and SNPs in the angiotensin II receptor, type 1 gene has been conducted by Sethupathy et al. (2007). They discovered that miRNA miR-155 could bind to the A allele of the SNP rs5186 (A>C) in the 3′UTR of the AGTR1 mRNA more efficiently than the C allele (which is more common in essential hypertension) (Sethupathy et al., 2007). In persons with the A allele, the binding of miR-155 has the capacity to reduce the level of AGTR1 mRNA and hence cause the pressor effect of Angiotensin II. Protein levels of AGTR1 in untreated essential hypertension patients homozygous for the C allele of rs5186 were also favorably linked with systolic and diastolic blood pressure. The expression levels of miR-155 were also negatively linked with AGTR1 protein levels, and miRNA levels were lower in those with the CC genotype that is directly associated with hypertensions (Ceolotto et al., 2011). Tables 5 and 6 give a brief glimpse of SNPs reported in miRNAs or target gene sequences in various diseases.
SNPs Role in Aging
Human aging is a complicated process that has been related to dysregulation of a variety of cellular and molecular processes, including telomere shortening, altered DNA damage response, protein homeostasis loss, cellular senescence, and mitochondrial failure. These cellular and molecular processes can result in a wide range of illnesses, including cancer, cardiovascular disease, and neurological disease, as well as an increased chance of death (Huan et al., 2018). The study of the mechanics of the aging process could also benefit from the determination of an individual’s SNPs. A comparison of the DNA sequences of healthy young individuals with the DNA sequences of healthy, extremely elderly people could reveal genes that play a big role in determining how long people live. Animal models had already been researched, and specific genes, such as DNA repair genes, had also been studied because of the role of repair processes in aging (Schmidt et al., 2000; Ruttan and Glickman, 2002). In recent years, it has also been suggested that post-transcriptional control by miRNAs may play a role in the phenotypic changes seen throughout aging by epigenetically modifying the expression of important regulatory proteins (Grillari and Grillari-Voglauer, 2010; Liu et al., 2012).
Human aging is linked to increased susceptibility to adverse drug reactions (ADRs), multimorbidity, and frailty, however, the intensity and age at which people become ill varies greatly. Identifying genetic indicators for this phenotype’s higher risk might aid in the stratification of individuals who would benefit from specialized intervention. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) controls the expression of enzymes involved in drug metabolism, as well as the cell’s response to stresses. In animal aging models, its expression has been demonstrated to diminish (Scutt et al., 2020). In the promoter region of the human Nrf2 gene (NFE2L2), there are many single-nucleotide polymorphisms (SNPs) that influence Nrf2 expression in vivo. Specific age-related disorders, such as acute lung damage, reduced forearm vasodilator response, and Parkinson’s disease, have been linked to these SNPs. Individuals with a variant allele may be more susceptible to the negative effects of medications, have a higher number of comorbidities, and be frailer in the setting of an age-related reduction in Nrf2 (Marzec et al., 2007; Marczak et al., 2012; von Otter et al., 2014). According to a recent study, polymorphism rs35652124 (T>A,C,G) in NFE2L2/Nrf2 gene was found to be associated with aging. Because the G allele is linked to lower NFE2L2/Nrf2 expression, another possible reason for the AA genotype’s higher risk of multimorbidity and frailty is that high NFE2L2 levels are harmful in some disorders. When compared to control mice, Nrf2 knockout animals had a smaller atherosclerotic plaque (Sussan et al., 2008). Furthermore, the rs35652124 AA genotype is linked to a higher risk of high blood pressure and cardiovascular death in adults (Shimoyama et al., 2014). As a result, it is possible that cardiovascular pathology is to blame for the increased levels of multimorbidity and frailty (Scutt et al., 2020).
SIRT2 is one of seven mammalian sirtuins (Sir2-like proteins) that play critical roles in cellular activities such as metabolism and differentiation (Michan and Sinclair, 2007). It is mostly found in the cytoplasm, where it deacetylates -tubulin, but it also migrates to the nucleus during the G2/M phase, where it deacetylates histones, influencing cell cycle progression (North et al., 2003; Vaquero et al., 2006; Inoue et al., 2007). SIRT2 also deacetylates numerous additional substrates (PEPCK1, FOXO1, FOXO3a, p65, and p53) that are involved in key cellular processes linked to organism health, such as homeostasis, oxidative stress management, inflammation, and cell growth and death regulation (de Oliveira et al., 2012). SIRT2 variation rs45592833 (G>T) is located inside a binding region identified by three distinct miRNAs (miR-3170, miR-92a-1-5p, and miR-615-5p), all of which were expected to bind more firmly to the T allele, causing SIRT2 production to be reduced (Crocco et al., 2015). SIRT2 levels have been discovered to be low in various human malignancies, and SIRT2-deficient animals have been reported to develop tumors as they age (Hiratsuka et al., 2003; Kim et al., 2011; Li et al., 2013b). miR-615-5p, which has the highest binding energy change caused by rs45592833, has been found to be deregulated in cancer cell lines, patients with aging-related conditions such as Huntington’s and cardiovascular diseases, and in the muscles of old mice, implying that miR-615-5p downstream targets may be involved in signaling pathways that are important in the aging process (Hulf et al., 2011; Sondermeijer et al., 2011; Hoss et al., 2014; Gao et al., 2015b).
An SNP in the DRD2 gene, rs6276 (A>G), which encodes a G protein-coupled receptor found on postsynaptic dopaminergic neurons, was also found to have a substantial connection with the longevity phenotype. DRD2 signaling is required for the appropriate control of a variety of physiological activities, including locomotion, behavior, and hormone synthesis (Usiello et al., 2000). Six distinct miRNAs were projected to bind to the region containing the polymorphism rs6276 using in silico analysis, with miR-485-5p having the greatest energy binding level to the 3′UTR with the minor G allele (Crocco et al., 2015). As a result, G allele binding is likely to be linked to enhanced miRNA–mRNA binding, resulting in more severe DRD2 expression regulation. DRD2 expression has been found to be downregulated in both striatal and extrastriatal areas of the brain in elderly adults, and that changes in DRD2 receptor density or activity have been linked to age-related declines in motor and cognitive abilities (Noble, 2003; Bäckman et al., 2006; Crocco et al., 2015).
Conclusion
Dysregulation of miRNAs and their targets is often reported to be involved in cancer progression. Multiple mechanisms for regulation of target gene expression by miRNAs have been proposed. However, recent evidence suggested another layer of complexity in terms of SNPs in either the miRNA seed or their target sequences. These mutations may cause dysregulated gene expression leading to cancer progression. Similar evidence is emerging in diabetes mellitus as well. There is limited scientific literature reporting SNPs in miRNA seed sequences highlighting scope of further exploration.
Overall, the current evidence suggests the need for the in-depth sequence analysis of miRNAs and target genes as well as to correlate the genetic evidence with functional studies. Since single miRNA can target multiple genes and similarly single genes can be targeted by multiple miRNAs, understanding the functional implications of these SNPs can provide new information regarding mechanisms of disease progression.
Author Contributions
S.S. and A.M. conceptualized the idea. Y.C., A.S., and P.S. wrote the manuscript and prepared the figures and tables. S.S. and A.M. edited the manuscript. The manuscript has been read and approved by all the authors.
Funding
Y.C. is thankful to CSIR for providing SRF, P.S. is thankful to ICMR for providing RA fellowship. A.S. was Master’s student in the department. The authors are thankful to Central University of Punjab for support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abo-Elmatty, D. M., and Mehanna, E. T. (2019). MIR146A Rs2910164 (G/C) Polymorphism Is Associated with Incidence of Preeclampsia in Gestational Diabetes Patients. Biochem. Genet. 57 (2), 222–233. doi:10.1007/s10528-018-9886-1
Adams, B. D., Furneaux, H., and White, B. A. (2007). The Micro-ribonucleic Acid (miRNA) miR-206 Targets the Human Estrogen Receptor-α (ERα) and Represses ERα Messenger RNA and Protein Expression in Breast Cancer Cell Lines. Mol. Endocrinol. 21 (5), 1132–1147. doi:10.1210/me.2007-0022
Ahmad, M., Jalil, F., Haq, M., and Shah, A. A. (2019). Effect of Variation in miRNA-Binding Site (Rs8176318) of the BRCA1 Gene in Breast Cancer Patients. Turk J. Med. Sci. 49 (5), 1433–1438. doi:10.3906/sag-1905-17
Alipoor, B., Ghaedi, H., Meshkani, R., Omrani, M. D., Sharifi, Z., and Golmohammadi, T. (2018). The Rs2910164 Variant Is Associated with Reduced miR-146a Expression but Not Cytokine Levels in Patients with Type 2 Diabetes. J. Endocrinol. Invest. 41 (5), 557–566. doi:10.1007/s40618-017-0766-z
Ando, H., Asai, T., Koide, H., Okamoto, A., Maeda, N., Tomita, K., et al. (2014). Advanced Cancer Therapy by Integrative Antitumor Actions via Systemic Administration of miR-499. J. Controlled Release 181, 32–39. doi:10.1016/j.jconrel.2014.02.019
Anwar, S. L., Wulaningsih, W., and Watkins, J. (2017). Profile of the Breast Cancer Susceptibility Marker Rs4245739 Identifies a Role for miRNAs. Cancer Biol. Med. 14 (4), 387–395. doi:10.20892/j.issn.2095-3941.2017.0050
Ayaz Durrani, I., Bhatti, A., and John, P. (2021). Regulatory MicroRNAs in T2DM and Breast Cancer. Processes 9 (5), 819. doi:10.3390/pr9050819
Bäckman, L., Nyberg, L., Lindenberger, U., Li, S.-C., and Farde, L. (2006). The Correlative Triad Among Aging, Dopamine, and Cognition: Current Status and Future Prospects. Neurosci. Biobehavioral Rev. 30 (6), 791–807. doi:10.1016/j.neubiorev.2006.06.005
Baier, L. J., Sacchettini, J. C., Knowler, W. C., Eads, J., Paolisso, G., Tataranni, P. A., et al. (1995). An Amino Acid Substitution in the Human Intestinal Fatty Acid Binding Protein Is Associated with Increased Fatty Acid Binding, Increased Fat Oxidation, and Insulin Resistance. J. Clin. Invest. 95 (3), 1281–1287. doi:10.1172/jci117778
Balenci, L., Clarke, I. D., Dirks, P. B., Assard, N., Ducray, F., Jouvet, A., et al. (2006). IQGAP1 Protein Specifies Amplifying Cancer Cells in Glioblastoma Multiforme. Cancer Res. 66 (18), 9074–9082. doi:10.1158/0008-5472.can-06-0761
Bantubungi, K., Hannou, S.-A., Caron-Houde, S., Vallez, E., Baron, M., Lucas, A., et al. (2014). Cdkn2a/p16Ink4a Regulates Fasting-Induced Hepatic Gluconeogenesis Through the PKA-CREB-PGC1 Pathway. Diabetes 63 (10), 3199–3209. doi:10.2337/db13-1921
Bao, L., Fu, X., Si, M., Wang, Y., Ma, R., Ren, X., et al. (2015). MicroRNA-185 Targets SOCS3 to Inhibit Beta-Cell Dysfunction in Diabetes. PLoS One 10 (2), e0116067. doi:10.1371/journal.pone.0116067
Barlow, J. H., and Rothstein, R. (2010). Timing Is Everything: Cell Cycle Control of Rad52. Cell Div 5, 7. doi:10.1186/1747-1028-5-7
Baunoch, D., Watkins, L., Tewari, A., Reece, M., Adams, L., Stack, R., et al. (1996). MDM2 Overexpression in Benign and Malignant Lesions of the Human Breast. Int. J. Oncol. 8 (5), 895–899. doi:10.3892/ijo.8.5.895
Beretta, S., Maj, C., and Merelli, I. (2017). Rank miRNA: A Web Tool for Identifying Polymorphisms Altering miRNA Target Sites. Proced. Comp. Sci. 108, 1125–1134. doi:10.1016/j.procs.2017.05.189
Bhattacharya, A., and Cui, Y. (2017). Systematic Prediction of the Impacts of Mutations in MicroRNA Seed Sequences. J. Integr. Bioinform 14 (1), 20170001. doi:10.1515/jib-2017-0001
Brekman, A., Singh, K. E., Polotskaia, A., Kundu, N., and Bargonetti, J. (2011). A P53-independent Role of Mdm2 in Estrogen-Mediated Activation of Breast Cancer Cell Proliferation. Breast Cancer Res. 13 (1), R3. doi:10.1186/bcr2804
Briggs, M. W., and Sacks, D. B. (2003). IQGAP1 as Signal Integrator: Ca2+, Calmodulin, Cdc42 and the Cytoskeleton. FEBS Lett. 542 (1-3), 7–11. doi:10.1016/s0014-5793(03)00333-8
Brincas, H. M., Augusto, D. G., Mathias, C., Cavalli, I. J., Lima, R. S., Kuroda, F., et al. (2020). A Genetic Variant in microRNA-146a Is Associated with Sporadic Breast Cancer in a Southern Brazilian Population. Genet. Mol. Biol. 42 (4), e20190278. doi:10.1590/1678-4685-GMB-2019-0278
Brown, M. D., and Sacks, D. B. (2006). IQGAP1 in Cellular Signaling: Bridging the GAP. Trends Cel Biol. 16 (5), 242–249. doi:10.1016/j.tcb.2006.03.002
Brucker, S. Y., Frank, L., Eisenbeis, S., Henes, M., Wallwiener, D., Riess, O., et al. (2017). Sequence Variants inESR1andOXTRare Associated with Mayer-Rokitansky-Küster-Hauser Syndrome. Acta Obstet. Gynecol. Scand. 96 (11), 1338–1346. doi:10.1111/aogs.13202
Bruno, A. E., Li, L., Kalabus, J. L., Pan, Y., Yu, A., and Hu, Z. (2012). miRdSNP: A Database of Disease-Associated SNPs and microRNA Target Sites on 3'UTRs of Human Genes. BMC Genomics 13 (1), 44. doi:10.1186/1471-2164-13-44
Cao, J., Luo, C., Peng, R., Guo, Q., Wang, K., Ye, H., et al. (2016). MiRNA-binding Site Functional Polymorphisms in DNA Repair Genes RAD51, RAD52, and XRCC2 and Breast Cancer Risk in Chinese Population. Tumour Biol. [Epub ahead of print]. doi:10.1007/s13277-016-5459-2
Catucci, I., Yang, R., Verderio, P., Pizzamiglio, S., Heesen, L., Hemminki, K., et al. (2010). Evaluation of SNPs inmiR-146a,miR196a2andmiR-499as Low-Penetrance Alleles in German and Italian Familial Breast Cancer Cases. Hum. Mutat. 31 (1), E1052–E1057. doi:10.1002/humu.21141
Ceolotto, G., Papparella, I., Bortoluzzi, A., Strapazzon, G., Ragazzo, F., Bratti, P., et al. (2011). Interplay Between miR-155, AT1R A1166C Polymorphism, and AT1R Expression in Young Untreated Hypertensives. Am. J. Hypertens. 24 (2), 241–246. doi:10.1038/ajh.2010.211
Chen, C., Yang, S., Chaugai, S., Wang, Y., and Wang, D. W. (2014). Meta-analysis of Hsa-Mir-499 Polymorphism (Rs3746444) for Cancer Risk: Evidence from 31 Case-Control Studies. BMC Med. Genet. 15 (1), 126. doi:10.1186/s12881-014-0126-1
Chen, G., Umelo, I. A., Lv, S., Teugels, E., Fostier, K., Kronenberger, P., et al. (2013). miR-146a Inhibits Cell Growth, Cell Migration and Induces Apoptosis in Non-small Cell Lung Cancer Cells. PLoS One 8 (3), e60317. doi:10.1371/journal.pone.0060317
Chen, H., Lan, H.-Y., Roukos, D. H., and Cho, W. C. (2014). Application of microRNAs in Diabetes Mellitus. J. Endocrinol. 222 (1), R1–R10. doi:10.1530/joe-13-0544
Chen, Q.-H., Wang, Q.-B., and Zhang, B. (2014). Ethnicity Modifies the Association Between Functional microRNA Polymorphisms and Breast Cancer Risk: A HuGE Meta-Analysis. Tumor Biol. 35 (1), 529–543. doi:10.1007/s13277-013-1074-7
Chen, Y., Huang, L., Qi, X., and Chen, C. (2019). Insulin Receptor Trafficking: Consequences for Insulin Sensitivity and Diabetes. Int. J. Mol. Sci. 20 (20), 5007. doi:10.3390/ijms20205007
Ciccacci, C., Latini, A., Greco, C., Politi, C., D'Amato, C., Lauro, D., et al. (2018). Association Between a MIR499A Polymorphism and Diabetic Neuropathy in Type 2 Diabetes. J. Diabetes its Complications 32 (1), 11–17. doi:10.1016/j.jdiacomp.2017.10.011
Coffinier, C., Thepot, D., Babinet, C., Yaniv, M., and Barra, J. (1999). Essential Role for the Homeoprotein vHNF1/HNF1beta in Visceral Endoderm Differentiation. Development 126 (21), 4785–4794. doi:10.1242/dev.126.21.4785
Coolen, M., and Bally-Cuif, L. (2015). “MicroRNAs in Brain Development,” in MicroRNA in Regenerative Medicine. Editor C. K. Sen (Oxford, UK: Academic Press), 447–488. doi:10.1016/b978-0-12-405544-5.00018-6
Crocco, P., Montesanto, A., Passarino, G., and Rose, G. (2015). Polymorphisms Falling within Putative miRNA Target Sites in the 3′UTR Region ofSIRT2andDRD2Genes Are Correlated with Human Longevity. Gerona 71 (5), 586–592. doi:10.1093/gerona/glv058
Crosby, M. E., and Almasan, A. (2004). Opposing Roles of E2Fs in Cell Proliferation and Death. Cancer Biol. Ther. 3 (12), 1208–1211. doi:10.4161/cbt.3.12.1494
Curtale, G., Citarella, F., Carissimi, C., Goldoni, M., Carucci, N., Fulci, V., et al. (2010). An Emerging Player in the Adaptive Immune Response: microRNA-146a Is a Modulator of IL-2 Expression and Activation-Induced Cell Death in T Lymphocytes. Blood 115 (2), 265–273. doi:10.1182/blood-2009-06-225987
Dai, Z.-J., Shao, Y.-P., Wang, X.-J., Xu, D., Kang, H.-F., Ren, H.-T., et al. (2015). Five Common Functional Polymorphisms in microRNAs (Rs2910164, Rs2292832, Rs11614913, Rs3746444, Rs895819) and the Susceptibility to Breast Cancer: Evidence from 8361 Cancer Cases and 8504 Controls. Cpd 21 (11), 1455–1463. doi:10.2174/1381612821666141208143533
Dai, Z.-M., Kang, H.-F., Zhang, W.-G., Li, H.-B., Zhang, S.-Q., Ma, X.-B., et al. (2016). The Associations of Single Nucleotide Polymorphisms in miR196a2, miR-499, and miR-608 with Breast Cancer Susceptibility. Medicine (Baltimore) 95 (7), e2826. doi:10.1097/md.0000000000002826
de Carvalho, J. B., de Morais, G. L., Vieira, T. C. D. S., Rabelo, N. C., Llerena, J. C., Gonzalez, S. M. D. C., et al. (2019). miRNA Genetic Variants Alter Their Secondary Structure and Expression in Patients with RASopathies Syndromes. Front. Genet. 10, 1144. doi:10.3389/fgene.2019.01144
de Oliveira, R. M., Sarkander, J., Kazantsev, A. G., and Outeiro, T. F. (2012). SIRT2 as a Therapeutic Target for Age-Related Disorders. Front. Pharmacol. 3, 82. doi:10.3389/fphar.2012.00082
Dong, P., Nabeshima, K., Nishimura, N., Kawakami, T., Hachisuga, T., Kawarabayashi, T., et al. (2006). Overexpression and Diffuse Expression Pattern of IQGAP1 at Invasion Fronts Are Independent Prognostic Parameters in Ovarian Carcinomas. Cancer Lett. 243 (1), 120–127. doi:10.1016/j.canlet.2005.11.024
Dou, T., Wu, Q., Chen, X., Ribas, J., Ni, X., Tang, C., et al. (2010). A Polymorphism of microRNA196a Genome Region Was Associated with Decreased Risk of Glioma in Chinese Population. J. Cancer Res. Clin. Oncol. 136 (12), 1853–1859. doi:10.1007/s00432-010-0844-5
Duan, R., Pak, C., and Jin, P. (2007). Single Nucleotide Polymorphism Associated with Mature miR-125a Alters the Processing of Pri-miRNA. Hum. Mol. Genet. 16 (9), 1124–1131. doi:10.1093/hmg/ddm062
Elek, Z., Németh, N., Nagy, G., Németh, H., Somogyi, A., Hosszufalusi, N., et al. (2015). Micro-RNA Binding Site Polymorphisms in the WFS1 Gene Are Risk Factors of Diabetes Mellitus. PLoS One 10 (10), e0139519. doi:10.1371/journal.pone.0139519
Elfaki, I., Mir, R., Mir, M. M., AbuDuhier, F. M., Babakr, A. T., and Barnawi, J. (2019). Potential Impact of MicroRNA Gene Polymorphisms in the Pathogenesis of Diabetes and Atherosclerotic Cardiovascular Disease. J. Pers Med. 9 (4), 51. doi:10.3390/jpm9040051
Erturk, E., Cecener, G., Polatkan, V., Gokgoz, S., Egeli, U., Tunca, B., et al. (2014). Evaluation of Genetic Variations in miRNA-Binding Sites of BRCA1 and BRCA2 Genes as Risk Factors for the Development of Early-Onset And/or Familial Breast Cancer. Asian Pac. J. Cancer Prev. 15 (19), 8319–8324. doi:10.7314/apjcp.2014.15.19.8319
Fan, B., Chopp, M., Zhang, Z. G., and Liu, X. S. (2020). Emerging Roles of microRNAs as Biomarkers and Therapeutic Targets for Diabetic Neuropathy. Front. Neurol. 11, 558758. doi:10.3389/fneur.2020.558758
Fawcett, K. A., Wheeler, E., Morris, A. P., Ricketts, S. L., Hallmans, G., Rolandsson, O., et al. (2010). Detailed Investigation of the Role of Common and Low-Frequency WFS1 Variants in Type 2 Diabetes Risk. Diabetes 59 (3), 741–746. doi:10.2337/db09-0920
Fawzy, M. S., Toraih, E. A., Ibrahiem, A., Abdeldayem, H., Mohamed, A. O., and Abdel-Daim, M. M. (2017). Evaluation of miRNA-196a2 and Apoptosis-Related Target Genes: ANXA1, DFFA and PDCD4 Expression in Gastrointestinal Cancer Patients: A Pilot Study. PloS one 12 (11), e0187310. doi:10.1371/journal.pone.0187310
Feng, Z., Scott, S. P., Bussen, W., Sharma, G. G., Guo, G., Pandita, T. K., et al. (2011). Rad52 Inactivation Is Synthetically Lethal with BRCA2 Deficiency. Proc. Natl. Acad. Sci. 108 (2), 686–691. doi:10.1073/pnas.1010959107
Ferracin, M., Querzoli, P., Calin, G. A., and Negrini, M. (2011). MicroRNAs: Toward the Clinic for Breast Cancer Patients. Semin. Oncol. 38 (6), 764–775. doi:10.1053/j.seminoncol.2011.08.005
Ferretti, E., De Smaele, E., Po, A., Di Marcotullio, L., Tosi, E., Espinola, M. S. B., et al. (2009). MicroRNA Profiling in Human Medulloblastoma. Int. J. Cancer 124 (3), 568–577. doi:10.1002/ijc.23948
Fonseca, S. G., Fukuma, M., Lipson, K. L., Nguyen, L. X., Allen, J. R., Oka, Y., et al. (2005). WFS1 Is a Novel Component of the Unfolded Protein Response and Maintains Homeostasis of the Endoplasmic Reticulum in Pancreatic β-Cells. J. Biol. Chem. 280 (47), 39609–39615. doi:10.1074/jbc.m507426200
Fowler, M. J. (2008). Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 26, 77–82. doi:10.2337/diaclin.26.2.77
Galka-Marciniak, P., Urbanek-Trzeciak, M. O., Nawrocka, P. M., Dutkiewicz, A., Giefing, M., Lewandowska, M. A., et al. (2019). Somatic Mutations in miRNA Genes in Lung Cancer-Potential Functional Consequences of Non-coding Sequence Variants. Cancers 11 (6), 793. doi:10.3390/cancers11060793
Gao, F., Xiong, X., Pan, W., Yang, X., Zhou, C., Yuan, Q., et al. (2015). A Regulatory MDM4 Genetic Variant Locating in the Binding Sequence of Multiple MicroRNAs Contributes to Susceptibility of Small Cell Lung Cancer. PLOS ONE 10 (8), e0135647. doi:10.1371/journal.pone.0135647
Gao, W., Gu, Y., Li, Z., Cai, H., Peng, Q., Tu, M., et al. (2015). miR-615-5p Is Epigenetically Inactivated and Functions as a Tumor Suppressor in Pancreatic Ductal Adenocarcinoma. Oncogene 34 (13), 1629–1640. doi:10.1038/onc.2014.101
George, G. P., Gangwar, R., Mandal, R. K., Sankhwar, S. N., and Mittal, R. D. (2011). Genetic Variation in microRNA Genes and Prostate Cancer Risk in North Indian Population. Mol. Biol. Rep. 38 (3), 1609–1615. doi:10.1007/s11033-010-0270-4
Ghaedi, H., Bastami, M., Jahani, M. M., Alipoor, B., Tabasinezhad, M., Ghaderi, O., et al. (2016). A Bioinformatics Approach to the Identification of Variants Associated with Type 1 and Type 2 Diabetes Mellitus that Reside in Functionally Validated miRNAs Binding Sites. Biochem. Genet. 54 (3), 211–221. doi:10.1007/s10528-016-9713-5
Gholami, M., Asgarbeik, S., Razi, F., Esfahani, E. N., Zoughi, M., Vahidi, A., et al. (2020). Association of microRNA Gene Polymorphisms with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Res. Med. Sci. 25, 56. doi:10.4103/jrms.JRMS_751_19
Gilam, A., Conde, J., Weissglas-Volkov, D., Oliva, N., Friedman, E., Artzi, N., et al. (2016). Local microRNA Delivery Targets Palladin and Prevents Metastatic Breast Cancer. Nat. Commun. 7 (1), 12868–12914. doi:10.1038/ncomms12868
Goda, N., Murase, H., Kasezawa, N., Goda, T., and Yamakawa-Kobayashi, K. (2015). Polymorphism in microRNA-Binding Site in HNF1B Influences the Susceptibility of Type 2 Diabetes Mellitus: A Population Based Case-Control Study. BMC Med. Genet. 16, 75. doi:10.1186/s12881-015-0219-5
Goicoechea, S. M., Bednarski, B., García-Mata, R., Prentice-Dunn, H., Kim, H. J., and Otey, C. A. (2009). Palladin Contributes to Invasive Motility in Human Breast Cancer Cells. Oncogene 28 (4), 587–598. doi:10.1038/onc.2008.408
Gong, J., Tong, Y., Zhang, H.-M., Wang, K., Hu, T., Shan, G., et al. (2012). Genome-wide Identification of SNPs in microRNA Genes and the SNP Effects on microRNA Target Binding and Biogenesis. Hum. Mutat. 33 (1), 254–263. doi:10.1002/humu.21641
Grillari, J., and Grillari-Voglauer, R. (2010). Novel Modulators of Senescence, Aging, and Longevity: Small Non-coding RNAs Enter the Stage. Exp. Gerontol. 45 (4), 302–311. doi:10.1016/j.exger.2010.01.007
Guo, Z., Shu, Y., Zhou, H., and Zhang, W. (2016). Identification of Diagnostic and Prognostic Biomarkers for Cancer: Focusing on Genetic Variations in microRNA Regulatory Pathways (Review). Mol. Med. Rep. 13 (3), 1943–1952. doi:10.3892/mmr.2016.4782
Han, S., Gao, F., Yang, W., Ren, Y., Liang, X., Xiong, X., et al. (2015). Identification of an SCLC Susceptibility Rs7963551 Genetic Polymorphism in a Previously GWAS-Identified 12p13.33 RAD52 Lung Cancer Risk Locus in the Chinese Population. Int. J. Clin. Exp. Med. 8 (9), 16528–16535.
Harel, T., Pehlivan, D., Caskey, C. T., and Lupski, J. R. (2015). “Mendelian, Non-mendelian, Multigenic Inheritance, and Epigenetics,” in Rosenberg's Molecular and Genetic Basis of Neurological and Psychiatric Disease. Editors R. N. Rosenberg, and J. M. Pascual. 5th Edn (Boston. MA: Academic Press), 3–27. doi:10.1016/b978-0-12-410529-4.00001-2
Hasani, S.-S., Hashemi, M., Eskandari-Nasab, E., Naderi, M., Omrani, M., and Sheybani-Nasab, M. (2014). A Functional Polymorphism in the miR-146a Gene Is Associated with the Risk of Childhood Acute Lymphoblastic Leukemia: A Preliminary Report. Tumor Biol. 35 (1), 219–225. doi:10.1007/s13277-013-1027-1
Hashimoto, Y., Akiyama, Y., and Yuasa, Y. (2013). Multiple-to-multiple Relationships Between microRNAs and Target Genes in Gastric Cancer. PLoS One 8 (5), e62589. doi:10.1371/journal.pone.0062589
Hatefi, Z., Soltani, G., Khosravi, S., Kazemi, M., Salehi, A. R., and Salehi, R. (2018). Micro R-410 Binding Site Single Nucleotide Polymorphism Rs13702 in Lipoprotein Lipase Gene Is Effective to Increase Susceptibility to Type 2 Diabetes in Iranian Population. Adv. Biomed. Res. 7, 79. doi:10.4103/abr.abr_286_16
Haunerland, N. H., and Spener, F. (2004). Fatty Acid-Binding Proteins - Insights from Genetic Manipulations. Prog. Lipid Res. 43 (4), 328–349. doi:10.1016/j.plipres.2004.05.001
Hiratsuka, M., Inoue, T., Toda, T., Kimura, N., Shirayoshi, Y., Kamitani, H., et al. (2003). Proteomics-based Identification of Differentially Expressed Genes in Human Gliomas: Down-Regulation of SIRT2 Gene. Biochem. biophysical Res. Commun. 309 (3), 558–566. doi:10.1016/j.bbrc.2003.08.029
Hoffman, A. E., Zheng, T., Yi, C., Leaderer, D., Weidhaas, J., Slack, F., et al. (2009). microRNA miR-196a-2 and Breast Cancer: A Genetic and Epigenetic Association Study and Functional Analysis. Cancer Res. 69 (14), 5970–5977. doi:10.1158/0008-5472.can-09-0236
Hofmann, S., Philbrook, C., Gerbitz, K.-D., and Bauer, M. F. (2003). Wolfram Syndrome: Structural and Functional Analyses of Mutant and Wild-type Wolframin, the WFS1 Gene Product. Hum. Mol. Genet. 12 (16), 2003–2012. doi:10.1093/hmg/ddg214
Hori, M., Shimazaki, J., Inagawa, S., Itabashi, M., and Hori, M. (2002). Overexpression of MDM2 Oncoprotein Correlates with Possession of Estrogen Receptor Alpha and Lack of MDM2 mRNA Splice Variants in Human Breast Cancer. Breast Cancer Res. Treat. 71 (1), 77–84. doi:10.1023/a:1013350419426
Hoss, A. G., Kartha, V. K., Dong, X., Latourelle, J. C., Dumitriu, A., Hadzi, T. C., et al. (2014). MicroRNAs Located in the Hox Gene Clusters Are Implicated in huntington's Disease Pathogenesis. Plos Genet. 10 (2), e1004188. doi:10.1371/journal.pgen.1004188
Hribal, M. L., au, fnm., Presta, I., Procopio, T., Marini, M. A., Stančáková, A., et al. (2011). Glucose Tolerance, Insulin Sensitivity and Insulin Release in European Non-diabetic Carriers of a Polymorphism Upstream of CDKN2A and CDKN2B. Diabetologia 54 (4), 795–802. doi:10.1007/s00125-010-2038-8
Hu, Z., Chen, J., Tian, T., Zhou, X., Gu, H., Xu, L., et al. (2008). Genetic Variants of miRNA Sequences and Non-small Cell Lung Cancer Survival. J. Clin. Invest. 118 (7), 2600–2608. doi:10.1172/JCI34934
Hu, Z., Liang, J., Wang, Z., Tian, T., Zhou, X., Chen, J., et al. (2009). Common Genetic Variants in Pre-microRNAs Were Associated with Increased Risk of Breast Cancer in Chinese Women. Hum. Mutat. 30 (1), 79–84. doi:10.1002/humu.20837
Huan, T., Chen, G., Liu, C., Bhattacharya, A., Rong, J., Chen, B. H., et al. (2018). Age-associated microRNA Expression in Human Peripheral Blood Is Associated with All-Cause Mortality and Age-Related Traits. Aging Cell 17 (1), e12687. doi:10.1111/acel.12687
Hulf, T., Sibbritt, T., Wiklund, E. D., Bert, S., Strbenac, D., Statham, A. L., et al. (2011). Discovery Pipeline for Epigenetically Deregulated miRNAs in Cancer: Integration of Primary miRNA Transcription. BMC genomics 12 (1), 54–59. doi:10.1186/1471-2164-12-54
Iguchi, T., Nambara, S., Masuda, T., Komatsu, H., Ueda, M., Kidogami, S., et al. (2016). miR-146a Polymorphism (Rs2910164) Predicts Colorectal Cancer Patients' Susceptibility to Liver Metastasis. PLOS ONE 11 (11), e0165912. doi:10.1371/journal.pone.0165912
Inoue, T., Hiratsuka, M., Osaki, M., and Oshimura, M. (2007). The Molecular Biology of Mammalian SIRT Proteins: SIRT2 Functions on Cell Cycle Regulation. Cell Cycle 6 (9), 1011–1018. doi:10.4161/cc.6.9.4219
Iorio, M. V., and Croce, C. M. (2012). microRNA Involvement in Human Cancer. Carcinogenesis 33 (6), 1126–1133. doi:10.1093/carcin/bgs140
Jaakson, H., Karis, P., Ling, K., Ilves-Luht, A., Samarütel, J., Henno, M., et al. (2018). Adipose Tissue Insulin Receptor and Glucose Transporter 4 Expression, and Blood Glucose and Insulin Responses during Glucose Tolerance Tests in Transition Holstein Cows with Different Body Condition. J. Dairy Sci. 101 (1), 752–766. doi:10.3168/jds.2017-12877
Jeon, Y. J., Kim, O. J., Kim, S. Y., Oh, S. H., Oh, D., Kim, O. J., et al. (2013). Association of the miR-146a , miR-149 , miR-196a2 , and miR-499 Polymorphisms with Ischemic Stroke and Silent Brain Infarction Risk. Arterioscler Thromb. Vasc. Biol. 33 (2), 420–430. doi:10.1161/atvbaha.112.300251
Jiang, Y., Qin, Z., Hu, Z., Guan, X., Wang, Y., He, Y., et al. (2013). Genetic Variation in a Hsa-Let-7 Binding Site in RAD52 Is Associated with Breast Cancer Susceptibility. Carcinogenesis 34 (3), 689–693. doi:10.1093/carcin/bgs373
Jin, Y., Zeng, S. X., Sun, X.-X., Lee, H., Blattner, C., Xiao, Z., et al. (2008). MDMX Promotes Proteasomal Turnover of P21 at G 1 and Early S Phases Independently of, but in Cooperation with, MDM2. Mol. Cel Biol 28 (4), 1218–1229. doi:10.1128/mcb.01198-07
Johnson, M., Sharma, M., and Henderson, B. R. (2009). IQGAP1 Regulation and Roles in Cancer. Cell Signal. 21 (10), 1471–1478. doi:10.1016/j.cellsig.2009.02.023
Kim, H.-S., Vassilopoulos, A., Wang, R.-H., Lahusen, T., Xiao, Z., Xu, X., et al. (2011). SIRT2 Maintains Genome Integrity and Suppresses Tumorigenesis Through Regulating APC/C Activity. Cancer cell 20 (4), 487–499. doi:10.1016/j.ccr.2011.09.004
Kim, J. K., Fillmore, J. J., Chen, Y., Yu, C., Moore, I. K., Pypaert, M., et al. (2001). Tissue-specific Overexpression of Lipoprotein Lipase Causes Tissue-specific Insulin Resistance. Proc. Natl. Acad. Sci. 98 (13), 7522–7527. doi:10.1073/pnas.121164498
Klöting, N., Berthold, S., Kovacs, P., Schön, M. R., Fasshauer, M., Ruschke, K., et al. (2009). MicroRNA Expression in Human Omental and Subcutaneous Adipose Tissue. PLoS One 4 (3), e4699. doi:10.1371/journal.pone.0004699
Kovacs-Nagy, R., Elek, Z., Szekely, A., Nanasi, T., Sasvari-Szekely, M., and Ronai, Z. (2013). Association of Aggression with a Novel microRNA Binding Site Polymorphism in the Wolframin Gene. Am. J. Med. Genet. 162 (4), 404–412. doi:10.1002/ajmg.b.32157
Krishnamurthy, J., Ramsey, M. R., Ligon, K. L., Torrice, C., Koh, A., Bonner-Weir, S., et al. (2006). p16INK4a Induces an Age-dependent Decline in Islet Regenerative Potential. Nature 443 (7110), 453–457. doi:10.1038/nature05092
Labbaye, C., and Testa, U. (2012). The Emerging Role of MIR-146A in the Control of Hematopoiesis, Immune Function and Cancer. J. Hematol. Oncol. 5 (1), 13. doi:10.1186/1756-8722-5-13
Laragh, J., and Pickering, T. (1991). “Essential Hypertension,” in Brenner & Rector’s the Kidney. Editors B. M. Brenner, and F. C. Rector (Philadelphia, PA: W.B. Saunders Company), 1913–1967.
Latini, A., Borgiani, P., De Benedittis, G., D'Amato, C., Greco, C., Lauro, D., et al. (2020). Mitochondrial DNA Copy Number in Peripheral Blood Is Reduced in Type 2 Diabetes Patients with Polyneuropathy and Associated with a MIR499A Gene Polymorphism. DNA Cel Biol. 39 (8), 1467–1472. doi:10.1089/dna.2019.5326
Lee, M. J., Yu, G. R., Yoo, H. J., Kim, J. H., Yoon, B. I., Choi, Y. K., et al. (2009). ANXA8 Down-Regulation by EGF-FOXO4 Signaling Is Involved in Cell Scattering and Tumor Metastasis of Cholangiocarcinoma. Gastroenterology 137 (3), 1138–1150.e1–e9. doi:10.1053/j.gastro.2009.04.015
Lehmann, T. P., Korski, K., Ibbs, M., Zawierucha, P., Grodecka-gazdecka, S., and Jagodziński, P. P. (2013). rs12976445 Variant in the Pri-miR-125a Correlates with a Lower Level of Hsa-miR-125a and ERBB2 Overexpression in Breast Cancer Patients. Oncol. Lett. 5 (2), 569–573. doi:10.3892/ol.2012.1040
Leong, P. L. (2013). Single Nucleotide Polymorphism in the Seed Region of microRNA Alters the Expression of its Mature microRNA/Leong Pei Li. Kuala Lumpur, Malaysia: University of Malaya.
Li, N., Chen, J., Zhao, J., and Wang, T. (2017). MicroRNA-3188 Targets ETS-Domain Protein 4 and Participates in RhoA/ROCK Pathway to Regulate the Development of Atherosclerosis. Pharmazie 72 (11), 687–693. doi:10.1691/ph.2017.7686
Li, X., Wang, J., Jia, Z., Cui, Q., Zhang, C., Wang, W., et al. (2013). MiR-499 Regulates Cell Proliferation and Apoptosis during Late-Stage Cardiac Differentiation via Sox6 and Cyclin D1. PLOS ONE 8 (9), e74504. doi:10.1371/journal.pone.0074504
Li, Z., Xie, Q. R., Chen, Z., Lu, S., and Xia, W. (2013). Regulation of SIRT2 Levels for Human Non-small Cell Lung Cancer Therapy. Lung Cancer 82 (1), 9–15. doi:10.1016/j.lungcan.2013.05.013
Lieberman, R., Xiong, D., James, M., Han, Y., Amos, C. I., Wang, L., et al. (2016). Functional Characterization of RAD52 as a Lung Cancer Susceptibility Gene in the 12p13.33 Locus. Mol. Carcinog. 55 (5), 953–963. doi:10.1002/mc.22334
Ling, H., Zhang, W., and Calin, G. A. (2011). Principles of microRNA Involvement in Human Cancers. Chin. J. Cancer 30 (11), 739–748. doi:10.5732/cjc.011.10243
Liu, F.-J., Wen, T., and Liu, L. (2012). MicroRNAs as a Novel Cellular Senescence Regulator. Ageing Res. Rev. 11 (1), 41–50. doi:10.1016/j.arr.2011.06.001
Liu, G., Schwartz, J. A., and Brooks, S. C. (2000). Estrogen Receptor Protects P53 from Deactivation by Human Double Minute-2. Cancer Res. 60 (7), 1810–1814.
Liu, X., Zhang, Z., Sun, L., Chai, N., Tang, S., Jin, J., et al. (2011). MicroRNA-499-5p Promotes Cellular Invasion and Tumor Metastasis in Colorectal Cancer by Targeting FOXO4 and PDCD4. Carcinogenesis 32 (12), 1798–1805. doi:10.1093/carcin/bgr213
Liu, X., Zhao, J., Liu, Q., Xiong, X., Zhang, Z., Jiao, Y., et al. (2016). MicroRNA-124 Promotes Hepatic Triglyceride Accumulation Through Targeting Tribbles Homolog 3. Sci. Rep. 6, 37170. doi:10.1038/srep37170
Liu, Y., He, A., Liu, B., Zhong, Y., Liao, X., Yang, J., et al. (2018). rs11614913 Polymorphism in miRNA-196a2 and Cancer Risk: an Updated Meta-Analysis. Ott 11, 1121–1139. doi:10.2147/ott.s154211
Liu, Y., Li, M.-j., Lee, E. Y.-H. P., and Maizels, N. (1999). Localization and Dynamic Relocalization of Mammalian Rad52 During the Cell Cycle and in Response to DNA Damage. Curr. Biol. 9 (17), 975–978. doi:10.1016/s0960-9822(99)80427-8
Liu, Y., Ma, Y., Zhang, B., Wang, S.-X., Wang, X.-M., and Yu, J.-M. (2014). Genetic Polymorphisms in Pre-microRNAs and Risk of Ischemic Stroke in a Chinese Population. J. Mol. Neurosci. 52 (4), 473–480. doi:10.1007/s12031-013-0152-z
Liu, Z., Li, G., Wei, S., Niu, J., El-Naggar, A. K., Sturgis, E. M., et al. (2010). Genetic Variants in Selected Pre-microRNA Genes and the Risk of Squamous Cell Carcinoma of the Head and Neck. Cancer 116 (20), 4753–4760. doi:10.1002/cncr.25323
Lok, B. H., Carley, A. C., Tchang, B., and Powell, S. N. (2013). RAD52 Inactivation Is Synthetically Lethal with Deficiencies in BRCA1 and PALB2 in Addition to BRCA2 through RAD51-Mediated Homologous Recombination. Oncogene 32 (30), 3552–3558. doi:10.1038/onc.2012.391
Lu, L.-F., Boldin, M. P., Chaudhry, A., Lin, L.-L., Taganov, K. D., Hanada, T., et al. (2010). Function of miR-146a in Controlling Treg Cell-Mediated Regulation of Th1 Responses. Cell 142 (6), 914–929. doi:10.1016/j.cell.2010.08.012
Luthra, R., Singh, R. R., Luthra, M. G., Li, Y. X., Hannah, C., Romans, A. M., et al. (2008). MicroRNA-196a Targets Annexin A1: A microRNA-Mediated Mechanism of Annexin A1 Downregulation in Cancers. Oncogene 27 (52), 6667–6678. doi:10.1038/onc.2008.256
Malhotra, P., Read, G. H., and Weidhaas, J. B. (2019). Breast Cancer and miR-SNPs: The Importance of miR Germ-Line Genetics. Noncoding RNA 5 (1), 27. doi:10.3390/ncrna5010027
Malhotra, P., Read, G., and Weidhaas, J. (2019). Breast Cancer and miR-SNPs: The Importance of miR Germ-Line Genetics. ncRNA 5 (1), 27. doi:10.3390/ncrna5010027
Marczak, E. D., Marzec, J., Zeldin, D. C., Kleeberger, S. R., Brown, N. J., Pretorius, M., et al. (2012). Polymorphisms in the Transcription Factor NRF2 and Forearm Vasodilator Responses in Humans. Pharmacogenetics and genomics 22 (8), 620–628. doi:10.1097/fpc.0b013e32835516e5
Mariella, E., Marotta, F., Grassi, E., Gilotto, S., and Provero, P. (2019). The Length of the Expressed 3' UTR Is an Intermediate Molecular Phenotype Linking Genetic Variants to Complex Diseases. Front. Genet. 10 (714), 714. doi:10.3389/fgene.2019.00714
Marine, J.-C., Francoz, S., Maetens, M., Wahl, G., Toledo, F., and Lozano, G. (2006). Keeping P53 in Check: Essential and Synergistic Functions of Mdm2 and Mdm4. Cell Death Differ 13 (6), 927–934. doi:10.1038/sj.cdd.4401912
Martin, M. M., Buckenberger, J. A., Jiang, J., Malana, G. E., Nuovo, G. J., Chotani, M., et al. (2007). The Human Angiotensin II Type 1 Receptor +1166 A/C Polymorphism Attenuates microRNA-155 Binding. J. Biol. Chem. 282 (33), 24262–24269. doi:10.1074/jbc.m701050200
Marzec, J. M., Christie, J. D., Reddy, S. P., Jedlicka, A. E., Vuong, H., Lanken, P. N., et al. (2007). Functional Polymorphisms in the Transcription Factor NRF2 in Humans Increase the Risk of Acute Lung Injury. FASEB j. 21 (9), 2237–2246. doi:10.1096/fj.06-7759com
Matijasevic, Z., Steinman, H. A., Hoover, K., and Jones, S. N. (2008). MdmX Promotes Bipolar Mitosis to Suppress Transformation and Tumorigenesis in P53-Deficient Cells and Mice. Mol. Cel Biol 28 (4), 1265–1273. doi:10.1128/mcb.01108-07
Mead, J., Irvine, S., and Ramji, D. (2002). Lipoprotein Lipase: Structure, Function, Regulation, and Role in Disease. J. Mol. Med. 80 (12), 753–769. doi:10.1007/s00109-002-0384-9
Michan, S., and Sinclair, D. (2007). Sirtuins in Mammals: Insights into Their Biological Function. Biochem. J. 404 (1), 1–13. doi:10.1042/bj20070140
Morales, S., De Mayo, T., Gulppi, F., Gonzalez-Hormazabal, P., Carrasco, V., Reyes, J., et al. (2018). Genetic Variants in Pre-miR-146a, Pre-miR-499, Pre-miR-125a, Pre-miR-605, and Pri-miR-182 Are Associated with Breast Cancer Susceptibility in a South American Population. Genes 9 (9), 427. doi:10.3390/genes9090427
Moszyńska, A., Gebert, M., Collawn, J. F., and Bartoszewski, R. (2017). SNPs in microRNA Target Sites and Their Potential Role in Human Disease. Open Biol. 7 (4), 170019. doi:10.1098/rsob.170019
Moszynska, A., Gebert, M., Collawn, J. F., and Bartoszewski, R. (2017). SNPs in microRNA Target Sites and Their Potential Role in Human Disease. Open Biol. 7 (4), 170019. doi:10.1098/rsob.170019
Nabeshima, K., Shimao, Y., Inoue, T., and Koono, M. (2002). Immunohistochemical Analysis of IQGAP1 Expression in Human Colorectal Carcinomas: Its Overexpression in Carcinomas and Association with Invasion Fronts. Cancer Lett. 176 (1), 101–109. doi:10.1016/s0304-3835(01)00742-x
Nahand, J. S., Karimzadeh, M. R., Nezamnia, M., Fatemipour, M., Khatami, A., Jamshidi, S., et al. (2020). The Role of miR‐146a in Viral Infection. IUBMB Life 72 (3), 343–360. doi:10.1002/iub.2222
Nakamura, H., Fujita, K., Nakagawa, H., Kishi, F., Takeuchi, A., Aute, I., et al. (2005). Expression Pattern of the Scaffold Protein IQGAP1 in Lung Cancer. Oncol. Rep. 13 (3), 427–431. doi:10.3892/or.13.3.427
Nicoloso, M. S., Sun, H., Spizzo, R., Kim, H., Wickramasinghe, P., Shimizu, M., et al. (2010). Single-nucleotide Polymorphisms Inside microRNA Target Sites Influence Tumor Susceptibility. Cancer Res. 70 (7), 2789–2798. doi:10.1158/0008-5472.can-09-3541
Noble, E. P. (2003). D2 Dopamine Receptor Gene in Psychiatric and Neurologic Disorders and its Phenotypes. Am. J. Med. Genet. 116B (1), 103–125. doi:10.1002/ajmg.b.10005
Nogueira, A., Fernandes, M., Catarino, R., and Medeiros, R. (2019). RAD52 Functions in Homologous Recombination and its Importance on Genomic Integrity Maintenance and Cancer Therapy. Cancers 11 (11), 1622. doi:10.3390/cancers11111622
Nojima, H., Adachi, M., Matsui, T., Okawa, K., Tsukita, S., and Tsukita, S. (2008). IQGAP3 Regulates Cell Proliferation Through the Ras/ERK Signalling cascade. Nat. Cel Biol 10 (8), 971–978. doi:10.1038/ncb1757
North, B. J., Marshall, B. L., Borra, M. T., Denu, J. M., and Verdin, E. (2003). The Human Sir2 Ortholog, SIRT2, Is an NAD+-dependent Tubulin Deacetylase. Mol. Cel. 11 (2), 437–444. doi:10.1016/s1097-2765(03)00038-8
Nussinov, R., Zhang, M., Tsai, C.-J., and Jang, H. (2018). Calmodulin and IQGAP1 Activation of PI3Kα and Akt in KRAS, HRAS and NRAS-Driven Cancers. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1864 (6, Part B), 2304–2314. doi:10.1016/j.bbadis.2017.10.032
O'Brien, J., Hayder, H., Zayed, Y., and Peng, C. (2018). Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. (Lausanne) 9 (402), 402. doi:10.3389/fendo.2018.00402
Ohnheiser, J., Ferlemann, E., Haas, A., Müller, J. P., Werwein, E., Fehler, O., et al. (2015). Programmed Cell Death 4 Protein (Pdcd4) and Homeodomain-Interacting Protein Kinase 2 (Hipk2) Antagonistically Control Translation of Hipk2 mRNA. Biochim. Biophys. Acta (Bba) - Mol. Cel Res. 1853 (7), 1564–1573. doi:10.1016/j.bbamcr.2015.03.008
Okubo, M., Tahara, T., Shibata, T., Yamashita, H., Nakamura, M., Yoshioka, D., et al. (2011). Association Study of Common Genetic Variants in Pre-microRNAs in Patients with Ulcerative Colitis. J. Clin. Immunol. 31 (1), 69–73. doi:10.1007/s10875-010-9461-y
Olefsky, J. M. (2001). Nuclear Receptor Minireview Series. J. Biol. Chem. 276 (40), 36863–36864. doi:10.1074/jbc.r100047200
Omrani, M., Hashemi, M., Eskandari-Nasab, E., Hasani, S.-S., Mashhadi, M. A., Arbabi, F., et al. (2014). hsa-mir-499 Rs3746444 Gene Polymorphism Is Associated with Susceptibility to Breast Cancer in an Iranian Population. Biomarkers Med. 8 (2), 259–267. doi:10.2217/bmm.13.118
Oparil, S., and Weber, M. A. (2000). Hypertension: A Companion to Brenner & Rector’s the Kidney. Philadelphia, PA: W. B. Saunders Company.
Pal, D., Ghatak, S., and Sen, C. K. (2015). “Epigenetic Modification of MicroRNAs,” in MicroRNA in Regenerative Medicine. Editor C. K. Sen (Amsterdam, Netherlands: Elsevier), 77–109. doi:10.1016/b978-0-12-405544-5.00003-4
Palmero, E. I., Campos, S. G. P. d., Campos, M., Souza, N. C. N. d., Guerreiro, I. D. C., Carvalho, A. L., et al. (2011). Mechanisms and Role of microRNA Deregulation in Cancer Onset and Progression. Genet. Mol. Biol. 34 (3), 363–370. doi:10.1590/s1415-47572011000300001
Park, D. H., Jeon, H. S., Lee, S. Y., Choi, Y. Y., Lee, H. W., Yoon, S., et al. (2015). MicroRNA-146a Inhibits Epithelial Mesenchymal Transition in Non-small Cell Lung Cancer by Targeting Insulin Receptor Substrate 2. Int. J. Oncol. 47 (4), 1545–1553. doi:10.3892/ijo.2015.3111
Pelletier, C., Speed, W. C., Paranjape, T., Keane, K., Blitzblau, R., Hollestelle, A., et al. (2011). RareBRCA1haplotypes Including 3'UTR SNPs Associated with Breast Cancer Risk. Cell Cycle 10 (1), 90–99. doi:10.4161/cc.10.1.14359
Peng, Y., and Croce, C. M. (2016). The Role of MicroRNAs in Human Cancer. Sig Transduct Target. Ther. 1 (1), 15004. doi:10.1038/sigtrans.2015.4
Peterlongo, P., Caleca, L., Cattaneo, E., Ravagnani, F., Bianchi, T., Galastri, L., et al. (2011). The Rs12975333 Variant in the miR-125a and Breast Cancer Risk in Germany, Italy, Australia and Spain. J. Med. Genet. 48 (10), 703–704. doi:10.1136/jmedgenet-2011-100103
Peyrou, M., Bourgoin, L., Poher, A.-L., Altirriba, J., Maeder, C., Caillon, A., et al. (2015). Hepatic PTEN Deficiency Improves Muscle Insulin Sensitivity and Decreases Adiposity in Mice. J. Hepatol. 62 (2), 421–429. doi:10.1016/j.jhep.2014.09.012
Qi, P., Wang, L., Zhou, B., Yao, W. J., Xu, S., Zhou, Y., et al. (2015). Associations of miRNA Polymorphisms and Expression Levels with Breast Cancer Risk in the Chinese Population. Genet. Mol. Res. 14 (2), 6289–6296. doi:10.4238/2015.june.11.2
Rademakers, R., Eriksen, J. L., Baker, M., Robinson, T., Ahmed, Z., Lincoln, S. J., et al. (2008). Common Variation in the miR-659 Binding-Site of GRN Is a Major Risk Factor for TDP43-Positive Frontotemporal Dementia. Hum. Mol. Genet. 17 (23), 3631–3642. doi:10.1093/hmg/ddn257
Rahim, A., Afzal, M., and Naveed, A. K. (2019). Genetic Polymorphism of miRNA-196a and its Target Gene Annexin-A1 Expression Based on Ethnicity in Pakistani Female Breast Cancer Patients. Pak J. Med. Sci. 35 (6), 1598–1604. doi:10.12669/pjms.35.6.1322
Richardson, K., Nettleton, J. A., Rotllan, N., Tanaka, T., Smith, C. E., Lai, C.-Q., et al. (2013). Gain-of-function Lipoprotein Lipase Variant Rs13702 Modulates Lipid Traits Through Disruption of a microRNA-410 Seed Site. Am. J. Hum. Genet. 92 (1), 5–14. doi:10.1016/j.ajhg.2012.10.020
Ristori, E., and Nicoli, S. (2017). “Comparative Functions of miRNAs in Embryonic Neurogenesis and Neuronal Network Formation,” in Essentials of Noncoding RNA in Neuroscience. Editor D. D. P. Tonelli (Amsterdam, Netherlands: Elsevier), 265–282. doi:10.1016/b978-0-12-804402-5.00015-7
Roy, M., Li, Z., and Sacks, D. B. (2005). IQGAP1 Is a Scaffold for Mitogen-Activated Protein Kinase Signaling. Mol. Cel Biol 25 (18), 7940–7952. doi:10.1128/mcb.25.18.7940-7952.2005
Rusca, N., and Monticelli, S. (2011). MiR-146a in Immunity and Disease. Mol. Biol. Int. 2011, 437301. doi:10.4061/2011/437301
Ruttan, C. C., and Glickman, B. W. (2002). Coding Variants in Human Double-Strand Break DNA Repair Genes. Mutat. Res. 509 (1-2), 175–200. doi:10.1016/s0027-5107(02)00218-x
Schaefer, A. S., Richter, G. M., Nothnagel, M., Laine, M. L., Rühling, A., Schäfer, C., et al. (2010). A 3′ UTR Transition within DEFB1 Is Associated with Chronic and Aggressive Periodontitis. Genes Immun. 11 (1), 45–54. doi:10.1038/gene.2009.75
Schmidt, P. S., Duvernell, D. D., and Eanes, W. F. (2000). Adaptive Evolution of a Candidate Gene for Aging in Drosophila. Proc. Natl. Acad. Sci. 97 (20), 10861–10865. doi:10.1073/pnas.190338897
Schmidt, V. A., Chiariello, C. S., Capilla, E., Miller, F., and Bahou, W. F. (2008). Development of Hepatocellular Carcinoma in Iqgap2 -Deficient Mice Is IQGAP1 Dependent. Mol. Cel Biol 28 (5), 1489–1502. doi:10.1128/mcb.01090-07
Scott, G. K., Goga, A., Bhaumik, D., Berger, C. E., Sullivan, C. S., and Benz, C. C. (2007). Coordinate Suppression of ERBB2 and ERBB3 by Enforced Expression of Micro-RNA miR-125a or miR-125b. J. Biol. Chem. 282 (2), 1479–1486. doi:10.1074/jbc.m609383200
Scutt, G., Overall, A., Bakrania, P., Krasteva, E., Parekh, N., Ali, K., et al. (2020). The Association of a Single-Nucleotide Polymorphism in the Nuclear Factor (Erythroid-Derived 2)-Like 2 Gene with Adverse Drug Reactions, Multimorbidity, and Frailty in Older People. The Journals Gerontol. Ser. A 75 (6), 1050–1057. doi:10.1093/gerona/glz131
Sebastiani, G., Po, A., Miele, E., Ventriglia, G., Ceccarelli, E., Bugliani, M., et al. (2015). MicroRNA-124a Is Hyperexpressed in Type 2 Diabetic Human Pancreatic Islets and Negatively Regulates Insulin Secretion. Acta Diabetol. 52 (3), 523–530. doi:10.1007/s00592-014-0675-y
Sethupathy, P., Borel, C., Gagnebin, M., Grant, G. R., Deutsch, S., Elton, T. S., et al. (2007). Human microRNA-155 on Chromosome 21 Differentially Interacts with its Polymorphic Target in the AGTR1 3′ Untranslated Region: A Mechanism for Functional Single-Nucleotide Polymorphisms Related to Phenotypes. Am. J. Hum. Genet. 81 (2), 405–413. doi:10.1086/519979
Shankaran, Z. S., Walter, C. E. J., Ramachandiran, K., Gurramkonda, V. B., and Johnson, T. (2020). Association of microRNA-146a Rs2910164 Polymorphism with Type II Diabetes Mellitus in a South Indian Population and a Meta-Analysis. Gene Rep. 18, 100567. doi:10.1016/j.genrep.2019.100567
Shimoyama, Y., Mitsuda, Y., Tsuruta, Y., Hamajima, N., and Niwa, T. (2014). Polymorphism of Nrf2, an Antioxidative Gene, Is Associated with Blood Pressure and Cardiovascular Mortality in Hemodialysis Patients. Int. J. Med. Sci. 11 (7), 726–731. doi:10.7150/ijms.8590
Shvarts, A., Steegenga, W. T., Riteco, N., van Laar, T., Dekker, P., Bazuine, M., et al. (1996). MDMX: A Novel P53-Binding Protein with Some Functional Properties of MDM2. EMBO J. 15 (19), 5349–5357. doi:10.1002/j.1460-2075.1996.tb00919.x
Si, W., Shen, J., Zheng, H., and Fan, W. (2019). The Role and Mechanisms of Action of microRNAs in Cancer Drug Resistance. Clin. Epigenet 11 (1), 25. doi:10.1186/s13148-018-0587-8
Slaby, O., Bienertova-Vasku, J., Svoboda, M., and Vyzula, R. (2012). Genetic Polymorphisms and microRNAs: New Direction in Molecular Epidemiology of Solid Cancer. J. Cel Mol Med 16 (1), 8–21. doi:10.1111/j.1582-4934.2011.01359.x
Solito, E., De Coupade, C., Canaider, S., Goulding, N. J., and Perretti, M. (2001). Transfection of Annexin 1 in Monocytic Cells Produces a High Degree of Spontaneous and Stimulated Apoptosis Associated with Caspase-3 Activation. Br. J. Pharmacol. 133 (2), 217–228. doi:10.1038/sj.bjp.0704054
Sommer, S., and Fuqua, S. A. W. (2001). Estrogen Receptor and Breast Cancer. Semin. Cancer Biol. 11 (5), 339–352. doi:10.1006/scbi.2001.0389
Sondermeijer, B. M., Bakker, A., Halliani, A., de Ronde, M. W. J., Marquart, A. A., Tijsen, A. J., et al. (2011). Platelets in Patients with Premature Coronary Artery Disease Exhibit Upregulation of miRNA340* and miRNA624*. PloS one 6 (10), e25946. doi:10.1371/journal.pone.0025946
Song, F.-J., and Chen, K.-X. (2011). Single-nucleotide Polymorphisms Among microRNA: Big Effects on Cancer. Chin. J. Cancer 30 (6), 381–391. doi:10.5732/cjc.011.10142
Sotiriou, S. K., Kamileri, I., Lugli, N., Evangelou, K., Da-Ré, C., Huber, F., et al. (2016). Mammalian RAD52 Functions in Break-Induced Replication Repair of Collapsed DNA Replication forks. Mol. Cel. 64 (6), 1127–1134. doi:10.1016/j.molcel.2016.10.038
Srivastava, K., Srivastava, A., and Mittal, B. (2010). Common Genetic Variants in Pre-microRNAs and Risk of Gallbladder Cancer in North Indian Population. J. Hum. Genet. 55 (8), 495–499. doi:10.1038/jhg.2010.54
Stanelle, J., and Pützer, B. M. (2006). E2F1-induced Apoptosis: Turning Killers into Therapeutics. Trends Mol. Med. 12 (4), 177–185. doi:10.1016/j.molmed.2006.02.002
Stanford, J. L., Szklo, M., and Brinton, L. A. (1986). Estrogen Receptors and Breast Cancer. Epidemiol. Rev. 8, 42–59. doi:10.1093/oxfordjournals.epirev.a036295
Stegeman, S., Moya, L., Selth, L. A., Spurdle, A. B., Clements, J. A., and Batra, J. (2015). A Genetic Variant of MDM4 Influences Regulation by Multiple microRNAs in Prostate Cancer. Endocr. Relat. Cancer 22 (2), 265–276. doi:10.1530/erc-15-0013
Stenholm, L., Stoehlmacher-Williams, J., Al-Batran, S. E., Heussen, N., Akin, S., Pauligk, C., et al. (2013). Prognostic Role of microRNA Polymorphisms in Advanced Gastric Cancer: A Translational Study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Ann. Oncol. 24 (10), 2581–2588. doi:10.1093/annonc/mdt330
Strachan, G. D., Jordan-Sciutto, K. L., Rallapalli, R., Tuan, R. S., and Hall, D. J. (2003). The E2F-1 Transcription Factor Is Negatively Regulated by its Interaction with the MDMX Protein. J. Cel. Biochem. 88 (3), 557–568. doi:10.1002/jcb.10318
Sun, T., Aceto, N., Meerbrey, K. L., Kessler, J. D., Zhou, C., Migliaccio, I., et al. (2011). Activation of Multiple Proto-Oncogenic Tyrosine Kinases in Breast Cancer via Loss of the PTPN12 Phosphatase. Cell 144 (5), 703–718. doi:10.1016/j.cell.2011.02.003
Sun, Y.-M., Lin, K.-Y., and Chen, Y.-Q. (2013). Diverse Functions of miR-125 Family in Different Cell Contexts. J. Hematol. Oncol. 6, 6. doi:10.1186/1756-8722-6-6
Sussan, T. E., Jun, J., Thimmulappa, R., Bedja, D., Antero, M., Gabrielson, K. L., et al. (2008). Disruption of Nrf2, A Key Inducer of Antioxidant Defenses, Attenuates ApoE-Mediated Atherosclerosis in Mice. PloS one 3 (11), e3791. doi:10.1371/journal.pone.0003791
Taganov, K. D., Boldin, M. P., and Baltimore, D. (2007). MicroRNAs and Immunity: Tiny Players in a Big Field. Immunity 26 (2), 133–137. doi:10.1016/j.immuni.2007.02.005
Taganov, K. D., Boldin, M. P., Chang, K.-J., and Baltimore, D. (2006). NF- B-dependent Induction of microRNA miR-146, An Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. 103 (33), 12481–12486. doi:10.1073/pnas.0605298103
Tan, Z., Randall, G., Fan, J., Camoretti-Mercado, B., Brockman-Schneider, R., Pan, L., et al. (2007). Allele-specific Targeting of microRNAs to HLA-G and Risk of Asthma. Am. J. Hum. Genet. 81 (4), 829–834. doi:10.1086/521200
Thakur, N., Singhal, P., Mehrotra, R., and Bharadwaj, M. (2019). Impacts of Single Nucleotide Polymorphisms in Three microRNAs (miR-146a, miR-196a2 and miR-499) on the Susceptibility to Cervical Cancer Among Indian Women. Biosci. Rep. 39 (4), BSR20180723. doi:10.1042/BSR20180723
Thumser, A. E., Moore, J. B., and Plant, N. J. (2014). Fatty Acid Binding Proteins. Curr. Opin. Clin. Nutr. Metab. Care 17 (2), 124–129. doi:10.1097/mco.0000000000000031
Tian, T., Shu, Y., Chen, J., Hu, Z., Xu, L., Jin, G., et al. (2009). A Functional Genetic Variant in microRNA-196a2 Is Associated with Increased Susceptibility of Lung Cancer in Chinese. Cancer Epidemiol. Biomarkers Prev. 18 (4), 1183–1187. doi:10.1158/1055-9965.epi-08-0814
Tsai, I.-T., Wu, C.-C., Hung, W.-C., Lee, T.-L., Hsuan, C.-F., Wei, C.-T., et al. (2020). FABP1 and FABP2 as Markers of Diabetic Nephropathy. Int. J. Med. Sci. 17 (15), 2338–2345. doi:10.7150/ijms.49078
Urbánek, P., and Klotz, L.-O. (2017). Posttranscriptional Regulation ofFOXOexpression: microRNAs and Beyond. Br. J. Pharmacol. 174 (12), 1514–1532. doi:10.1111/bph.13471
Usiello, A., Baik, J.-H., Rougé-Pont, F., Picetti, R., Dierich, A., LeMeur, M., et al. (2000). Distinct Functions of the Two Isoforms of Dopamine D2 Receptors. Nature 408 (6809), 199–203. doi:10.1038/35041572
Valinezhad Orang, A., Safaralizadeh, R., and Kazemzadeh-Bavili, M. (2014). Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-specific Upregulation. Int. J. Genomics 2014, 970607. doi:10.1155/2014/970607
Vaquero, A., Scher, M. B., Lee, D. H., Sutton, A., Chen, H.-L., Alt, F. W., et al. (2006). SirT2 Is a Histone Deacetylase with Preference for Histone H4 Lys 16 During Mitosis. Genes Develop. 20 (10), 1256–1261. doi:10.1101/gad.1412706
Vincent, A. M., Hayes, J. M., McLean, L. L., Vivekanandan-Giri, A., Pennathur, S., and Feldman, E. L. (2009). Dyslipidemia-induced Neuropathy in Mice: The Role of oxLDL/LOX-1. Diabetes 58 (10), 2376–2385. doi:10.2337/db09-0047
Vitale, A. V., Tan, H., and Jin, P. (2011). MicroRNAs, SNPs and Cancer. J. Nucleic Acids Invest. 2 (1), e6. doi:10.4081/jnai.2011.2236
von Nandelstadh, P., Gucciardo, E., Lohi, J., Li, R., Sugiyama, N., Carpen, O., et al. (2014). Actin-associated Protein Palladin Promotes Tumor Cell Invasion by Linking Extracellular Matrix Degradation to Cell Cytoskeleton. MBoC 25 (17), 2556–2570. doi:10.1091/mbc.e13-11-0667
von Otter, M., Bergström, P., Quattrone, A., De Marco, E. V., Annesi, G., Söderkvist, P., et al. (2014). Genetic Associations of Nrf2-Encoding NFE2L2 Variants with Parkinson's Disease - A Multicenter Study. BMC Med. Genet. 15, 131. doi:10.1186/s12881-014-0131-4
Walton, R. G., Zhu, B., Unal, R., Spencer, M., Sunkara, M., Morris, A. J., et al. (2015). Increasing Adipocyte Lipoprotein Lipase Improves Glucose Metabolism in High Fat Diet-Induced Obesity. J. Biol. Chem. 290 (18), 11547–11556. doi:10.1074/jbc.m114.628487
Wang, G., van der Walt, J. M., Mayhew, G., Li, Y.-J., Züchner, S., Scott, W. K., et al. (2008). Variation in the miRNA-433 Binding Site of FGF20 Confers Risk for Parkinson Disease by Overexpression of α-Synuclein. Am. J. Hum. Genet. 82 (2), 283–289. doi:10.1016/j.ajhg.2007.09.021
Wang, J.-X., Jiao, J.-Q., Li, Q., Long, B., Wang, K., Liu, J.-P., et al. (2011). miR-499 Regulates Mitochondrial Dynamics by Targeting Calcineurin and Dynamin-Related Protein-1. Nat. Med. 17 (1), 71–78. doi:10.1038/nm.2282
Wang, L., Zhang, N., Pan, H.-p., Wang, Z., and Cao, Z.-y. (2015). MiR-499-5p Contributes to Hepatic Insulin Resistance by Suppressing PTEN. Cell Physiol Biochem 36 (6), 2357–2365. doi:10.1159/000430198
Wang, P.-Y., Gao, Z.-H., Jiang, Z.-H., Li, X.-X., Jiang, B.-F., and Xie, S.-Y. (2013). The Associations of Single Nucleotide Polymorphisms in miR-146a, miR-196a and miR-499 with Breast Cancer Susceptibility. PLoS One 8 (9), e70656. doi:10.1371/journal.pone.0070656
Wang, X., Li, W., Ma, L., Gao, J., Liu, J., Ping, F., et al. (2015). Association Study of the miRNA-Binding Site Polymorphisms of CDKN2A/B Genes with Gestational Diabetes Mellitus Susceptibility. Acta Diabetol. 52 (5), 951–958. doi:10.1007/s00592-015-0768-2
Wang, X., Li, W., Ma, L., Ping, F., Liu, J., Wu, X., et al. (2017). Investigation of miRNA-Binding Site Variants and Risk of Gestational Diabetes Mellitus in Chinese Pregnant Women. Acta Diabetol. 54 (3), 309–316. doi:10.1007/s00592-017-0969-y
Wang, X., Qin, X., Yan, M., Shi, J., Xu, Q., Li, Z., et al. (2019). Loss of Exosomal miR-3188 in Cancer-Associated Fibroblasts Contributes to HNC Progression. J. Exp. Clin. Cancer Res. 38 (1), 151. doi:10.1186/s13046-019-1144-9
Wang, Y.-Q., Guo, R.-D., Guo, R.-M., Sheng, W., and Yin, L.-R. (2013). MicroRNA-182 Promotes Cell Growth, Invasion, and Chemoresistance by Targeting Programmed Cell Death 4 (PDCD4) in Human Ovarian Carcinomas. J. Cel. Biochem. 114 (7), 1464–1473. doi:10.1002/jcb.24488
Wei, N., Liu, S. S., Chan, K. K. L., and Ngan, H. Y. S. (2012). Tumour Suppressive Function and Modulation of Programmed Cell Death 4 (PDCD4) in Ovarian Cancer. PLoS One 7 (1), e30311. doi:10.1371/journal.pone.0030311
Weissbach, L., Settleman, J., Kalady, M. F., Snijders, A. J., Murthy, A. E., Yan, Y. X., et al. (1994). Identification of a Human rasGAP-Related Protein Containing Calmodulin-Binding Motifs. J. Biol. Chem. 269 (32), 20517–20521. doi:10.1016/s0021-9258(17)32023-9
Wilson, K. D., Hu, S., Venkatasubrahmanyam, S., Fu, J.-D., Sun, N., Abilez, O. J., et al. (2010). Dynamic MicroRNA Expression Programs during Cardiac Differentiation of Human Embryonic Stem Cells. Circ. Cardiovasc. Genet. 3 (5), 426–435. doi:10.1161/circgenetics.109.934281
Wojcicka, A., de la Chapelle, A., and Jazdzewski, K. (2014). MicroRNA-related Sequence Variations in Human Cancers. Hum. Genet. 133 (4), 463–469. doi:10.1007/s00439-013-1397-x
Wu, B., Liu, G., He, F., Liu, R., Wang, Z., Wang, Y., et al. (2019). miR-3188 (rs7247237-C>T) Single-Nucleotide Polymorphism Is Associated with the Incidence of Vascular Complications in Chinese Patients with Type 2 Diabetes. J. Cardiovasc. Pharmacol. 74 (1), 62–70. doi:10.1097/fjc.0000000000000681
Wunderlich, M., Ghosh, M., Weghorst, K., and Berberich, S. J. (2004). MdmX Represses E2F1 Transactivation. Cell Cycle 3 (4), 472–478. doi:10.4161/cc.3.4.746
Wynendaele, J., Böhnke, A., Leucci, E., Nielsen, S. J., Lambertz, I., Hammer, S., et al. (2010). An Illegitimate microRNA Target Site Within the 3′ UTR of MDM4 Affects Ovarian Cancer Progression and Chemosensitivity. Cancer Res. 70 (23), 9641–9649. doi:10.1158/0008-5472.can-10-0527
Xiang, Y., Fan, S., Cao, J., Huang, S., and Zhang, L.-p. (2012). Association of the microRNA-499 Variants with Susceptibility to Hepatocellular Carcinoma in a Chinese Population. Mol. Biol. Rep. 39 (6), 7019–7023. doi:10.1007/s11033-012-1532-0
Xu, C., Zhu, J., Fu, W., Liang, Z., Song, S., Zhao, Y., et al. (2016). MDM4 Rs4245739 A > C Polymorphism Correlates with Reduced Overall Cancer Risk in a Meta-Analysis of 69477 Subjects. Oncotarget 7 (44), 71718–71726. doi:10.18632/oncotarget.12326
Xu, Y., Li, L., Xiang, X., Wang, H., Cai, W., Xie, J., et al. (2013). Three Common Functional Polymorphisms in microRNA Encoding Genes in the Susceptibility to Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Gene 527 (2), 584–593. doi:10.1016/j.gene.2013.05.085
Yan, W., Gao, X., and Zhang, S. (2017). Association of miR-196a2 Rs11614913 and miR-499 Rs3746444 Polymorphisms with Cancer Risk: A Meta-Analysis. Oncotarget 8 (69), 114344–114359. doi:10.18632/oncotarget.22547
Yang, B., Chen, J., Li, Y., Zhang, J., Li, D., Huang, Z., et al. (2012). Association of Polymorphisms in Pre-miRNA with Inflammatory Biomarkers in Rheumatoid Arthritis in the Chinese Han Population. Hum. Immunol. 73 (1), 101–106. doi:10.1016/j.humimm.2011.10.005
Yang, F., Chen, F., Xu, J., and Guan, X. (2016). Identification and Frequency of the Rs12516 and Rs8176318 BRCA1 Gene Polymorphisms Among Different Populations. Oncol. Lett. 11 (4), 2481–2486. doi:10.3892/ol.2016.4252
Yang, H.-S., Matthews, C. P., Clair, T., Wang, Q., Baker, A. R., Li, C.-C. H., et al. (2006). Tumorigenesis Suppressor Pdcd4 Down-Regulates Mitogen-Activated Protein Kinase Kinase Kinase Kinase 1 Expression to Suppress Colon Carcinoma Cell Invasion. Mol. Cel Biol 26 (4), 1297–1306. doi:10.1128/mcb.26.4.1297-1306.2006
Yekta, S., Shih, I.-h., and Bartel, D. P. (2004). MicroRNA-directed Cleavage of HOXB8 mRNA. Science 304 (5670), 594–596. doi:10.1126/science.1097434
Zhang, H., Zhang, Y., Yan, W., Wang, W., Zhao, X., Ma, X., et al. (2017). Association Between Three Functional microRNA Polymorphisms (miR-499 Rs3746444, miR-196a Rs11614913 and miR-146a Rs2910164) and Breast Cancer Risk: A Meta-Analysis. Oncotarget 8 (1), 393–407. doi:10.18632/oncotarget.13426
Zhang, S., Li, J., Jiang, Y., Xu, Y., and Qin, C. (2009). Programmed Cell Death 4 (PDCD4) Suppresses Metastastic Potential of Human Hepatocellular Carcinoma Cells. J. Exp. Clin. Cancer Res. 28 (1), 71–11. doi:10.1186/1756-9966-28-71
Zhang, Y., Bai, R., Liu, C., Ma, C., Chen, X., Yang, J., et al. (2019). MicroRNA Single-Nucleotide Polymorphisms and Diabetes Mellitus: A Comprehensive Review. Clin. Genet. 95 (4), 451–461. doi:10.1111/cge.13491
Zhao, D. (2020). Single Nucleotide Alterations in MicroRNAs and Human Cancer-A Not Fully Explored Field. Non-coding RNA Res. 5 (1), 27–31. doi:10.1016/j.ncrna.2020.02.003
Zhao, X., Ye, Q., Xu, K., Cheng, J., Gao, Y., Li, Q., et al. (2013). Single-nucleotide Polymorphisms Inside microRNA Target Sites Influence the Susceptibility to Type 2 Diabetes. J. Hum. Genet. 58 (3), 135–141. doi:10.1038/jhg.2012.146
Zheng, H., Song, F., Zhang, L., Yang, D., Ji, P., Wang, Y., et al. (2011). Genetic Variants at the miR-124 Binding Site on the Cytoskeleton-Organizing IQGAP1 Gene Confer Differential Predisposition to Breast Cancer. Int. J. Oncol. 38 (4), 1153–1161. doi:10.3892/ijo.2011.940
Zhou, B., Rao, L., Peng, Y., Wang, Y., Chen, Y., Song, Y., et al. (2010). Common Genetic Polymorphisms in Pre-microRNAs Were Associated with Increased Risk of Dilated Cardiomyopathy. Clin. Chim. Acta 411 (17-18), 1287–1290. doi:10.1016/j.cca.2010.05.010
Zhou, J., Lv, R., Song, X., Li, D., Hu, X., Ying, B., et al. (2012). Association Between Two Genetic Variants in miRNA and Primary Liver Cancer Risk in the Chinese Population. DNA Cel. Biol. 31 (4), 524–530. doi:10.1089/dna.2011.1340
Zhou, L., Zhang, X., Li, Z., Zhou, C., Li, M., Tang, X., et al. (2013). Association of a Genetic Variation in a miR-191 Binding Site in MDM4 with Risk of Esophageal Squamous Cell Carcinoma. PloS one 8 (5), e64331. doi:10.1371/journal.pone.0064331
Keywords: miRNA, microRNA, target genes, seed sequences, SNPs, cancer, diabetes mellitus
Citation: Chhichholiya Y, Suryan AK, Suman P, Munshi A and Singh S (2021) SNPs in miRNAs and Target Sequences: Role in Cancer and Diabetes. Front. Genet. 12:793523. doi: 10.3389/fgene.2021.793523
Received: 12 October 2021; Accepted: 28 October 2021;
Published: 01 December 2021.
Edited by:
Rajkumar S Kalra, Okinawa Institute of Science and Technology Graduate University, JapanReviewed by:
Choudhary Harsha, Indian Institute of Technology Guwahati, IndiaAlok Kumar, Kyoto University, Japan
Copyright © 2021 Chhichholiya, Suryan, Suman, Munshi and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anjana Munshi, YW5qYW5hLm11bnNoaUBjdXAuZWR1Lmlu; Sandeep Singh, c2FuZGVlcHNpbmdoODJAY3VwLmVkdS5pbg==
 Yogita Chhichholiya
Yogita Chhichholiya Aman Kumar Suryan
Aman Kumar Suryan Prabhat Suman
Prabhat Suman Sandeep Singh
Sandeep Singh