
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Genet. , 21 January 2022
Sec. Human and Medical Genomics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.772032
This article is part of the Research Topic 10 Years of Frontiers in Genetics: past discoveries, current challenges and future perspectives View all 31 articles
The tumor necrosis factor alpha (TNF-α) polymorphism may play an important role in chronic obstructive pulmonary disease (COPD) susceptibility. However, the results are still inconclusive. Eligible studies were searched in Cochrane Library database, EMBASE, Pudmed, Web of science, China National Knowledge Infrastructure, and Wanfang database. Finally, a total of 27 case-control studies with 3473 COPD cases and 4935 controls were included in the present analysis. We also performed trial sequential analysis (TSA) to confirm our results. Overall, association between TNF-α-308G/A polymorphism and COPD susceptibility was identified in allelic model (A vs. G, OR = 1.21, 95%CI: 1.01–1.45, p = 0.04) when smoking status was not adjusted. In ethnicity subgroup analysis, we found that the TNF-α -308G/A polymorphism was associated to COPD among Asians (GA vs. GG, OR = 1.35, 95%CI: 1.04–1.77, p = 0.02) when smoking status was not adjusted. However, no significant association was found in Asian smokers or Caucasian smokers. In conclusion, our study suggest that TNF-α-308 GA genotype is related to COPD in the Asian population. In addition, the TNF-α+489G/A, - 238G/A variants do not increase the risk of COPD.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42021273980.
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction and bronchial hyperresponsiveness, with increasing morbidity, mortality, and resource utilization worldwide (Celli et al., 2015). According to the latest figures, COPD has been estimated to become the fourth leading cause of death in the world by 2040 (Foreman et al., 2018).
Tumor necrosis factor alpha (TNF-α) gene is found on chromosome 6p21.33 (Nedwin et al., 1985). The TNF-α-308 G/A polymorphism appears to affect the multifunctional proinflammatory cytokine (Zhou et al., 2016). The signals are mediated through two transmembrane receptors, TNFR1 and TNFR2, to regulate the inflammatory cell functions such as cell proliferation, survival, differentiation, and apoptosis (Parameswaran and Patial, 2010). Some epidemiologic research on the association between TNF-α+489 G/A and -308 G/A polymorphism and COPD susceptibility have been performed (Cui et al., 2015; Zhang et al., 2016; Reséndiz-Hernández et al., 2018; Yu et al., 2021), but the results are still inconsistent. Additionally, the scarcity of meta-analysis about the relationship between TNF-α-238G/A and COPD risk impels us to study the issue in this article. In order to do this, we pooled 27 original studies and followed a stricter criterion to obtain an explicit understanding. Subgroup analysis based on ethnicity was also performed to gain a comprehensive view.
We followed the PRISMA guidelines and registered the review protocol on PROSPERO (CRD42021273980, September 21, 2021).
A comprehensive search of PubMed, EMBASE, Web of science, Cochrane Library database, Wanfang databases, and China National Knowledge Infrastructure were performed to find eligible studies. We retrieved articles using search strategies: “TNF-α or Tumor Necrosis Factor-α” and “COPD or COAD or chronic obstructive lung disease or chronic obstructive pulmonary disease or chronic obstructive airway disease” and “polymorphism or SNP or genotype or variant” published before July 25, 2021. In addition, we reviewed all references cited in the identified articles.
The included studies had to meet all four criteria: (Celli et al., 2015): Case-control or cohort studies, (Foreman et al., 2018), Availability of sufficient information to the odds rations (ORs) with 95% confidence intervals (CIs), (Nedwin et al., 1985), Alleles or genotypes frequencies in control groups and case groups could be received from the studies, and (Zhou et al., 2016) Literature in English. Accordingly, the exclusion criteria were as follows: (Celli et al., 2015): Results not focused on TNF-α-308, +489, or −238; (Foreman et al., 2018); Meta-analyses, letters, reviews, editorial articles, and studies that duplicated previous publications; (Nedwin et al., 1985); Detailed genotype data were not provided; or (Zhou et al., 2016) the genotype distribution of control was not in accordance with the Hardy-Weinberg equilibrium (HWE).
Two investigators retrieved data from the included studies separately, and all discordances were discussed to reach an agreement. The extraction information mainly includes the first author’s name, year of publication, country of origin, ethnicity, source of control, genotype method, the overall number of cases and controls, and genotype distribution of three TNF-α gene variants. The Newcastle-Ottawa Scale (NOS) was applied to estimate the quality of included articles, and the studies were classified as high quality (scores ≥7 stars) or low quality (scores<7 stars).
The odds ratio (OR) and its 95% confidence interval (95%CI) were used as an effect size to assess the risk of COPD caused by TNF-α polymorphism, as well as the strength of association between them. Z-test was used to estimate the statistical significance of pooled ORs. There were five genetic comparison models: allelic model (A vs. G), heterozygote model (GA vs. GG), homozygote model (AA vs. GG), dominant model (GA + AA vs. GG), and recessive model (AA vs. GG + GA). We checked the heterogeneity assumption by the Q-test. The outcome (p > 0.1 and I2<50%) indicated no heterogeneity among studies, and fixed-effects model (the Mantel-Haenszel method) was applied. Otherwise, when I2 ≥ 50% or p ≤ 0.10, we performed random-effects model (the DerSimonian and Laird method). Publication bias of the literature was evaluated by funnel plots and Egger’s test, and one-way sensitivity analyses were performed to assess the stability of the results.
The pooled OR and its 95%CI were calculated by Review Manager 5.4.1 software. All the p-values are two-sided.
We used the TSA v0.9.5.10 Beta software to perform the trial sequential analysis. Our study set the relative risk reduction to 20%, the first type of error α = 0.05, and power = 80% to evaluate required information size (RIS) and the trial sequential monitoring boundary (TSMB). The results are considered reliable and stable when the Z-value crosses TSMB. At the same time, the sample size can be deemed adequate. However, if the cumulative Z value does not cross the TSMB or RIS threshold, it means the sample size is not sufficient. And it still needs more studies to confirm the result.
The search strategy yielded 261 potentially relevant articles, and 27 publications with 3473 COPD cases and 4935 controls were ultimately included in the present analysis (Figure 1). All the cases were confirmed by the diagnostic criteria of COPD. There were 25 articles that studied the TNF-α-308 variant, five articles on the TNF-α+489 variant, and four articles on the TNF-α-238 variant. These studies fell into two groups: 16 Asian articles and 20 Caucasian articles. The genotype distributions of TNF-α were found to be in the Hardy-Weinberg equilibrium. Smoking status was adjusted for in 10 studies, and these were included for the second meta-analysis (Table 1).
The effect of the TNF-α-308G/A polymorphism on COPD was first investigated by pooling 25 studies where smoking status was not adjusted, comprising 3283 COPD cases and 4539 non-COPD controls. It indicated that the A allele was associated with an increased COPD risk in the overall population (A vs. G, OR = 1.21,95% CI: 1.01–1.45, p = 0.04), using an allelic model. According to the comparison between heterozygote model (GA vs. GG, OR = 1.22, 95% CI :1.02–1.45, p = 0.03) and dominant model (GA + AA vs. GG, OR = 1.22, 95%CI: 1.01–1.48, p = 0.04), GA genotype carriers of TNF-α-308 have a higher risk of developing COPD compared to GG carriers.
In the stratified analysis by ethnicity, we found that the TNF-α-308 G/A polymorphism was associated with COPD risk under the allelic model (A vs. G, OR = 1.40, 95% CI: 1.03–1.89, p = 0.03). The pooled OR of heterozygote model was 1.35 (GA vs. GG, OR = 1.35, 95% CI: 1.04–1.77, p = 0.02), indicating that the risk of COPD with TNF-α-308 GA genotype was 1.35 times higher than that with the GG genotype in an Asian population. A similar result was also observed in the dominant model (GA + AA vs. GG, OR = 1.40, 95% CI: 1.04–1.88, p = 0.03), which further supported our finding. In contrast, no significant association between the TNF-α-308 G/A SNP and COPD was found in Caucasians (Figure 2).
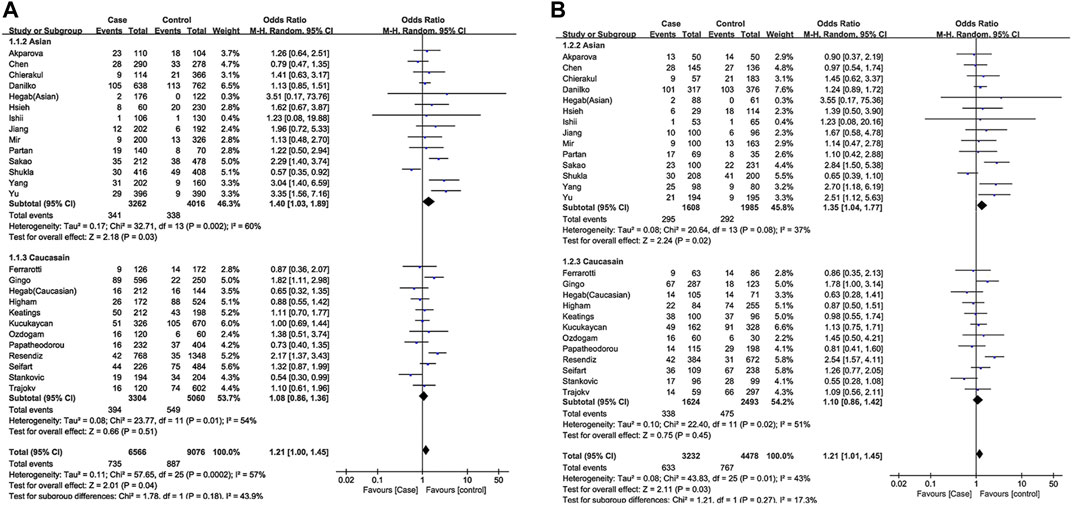
FIGURE 2. Forest plot of TNF-α-308 polymorphism and COPD risk [(A): overall for A vs. G, (B): overall for GA vs. GG].
Specific environmental factors, such as smoking, may contribute to the difference in the distribution of genetic polymorphism. Therefore, we conducted a second meta-analysis with 10 studies including 2749 smokers. In this meta-analysis, TNF-α-308 polymorphism was not associated with COPD either in Asian smokers or Caucasian smokers (Table 2).
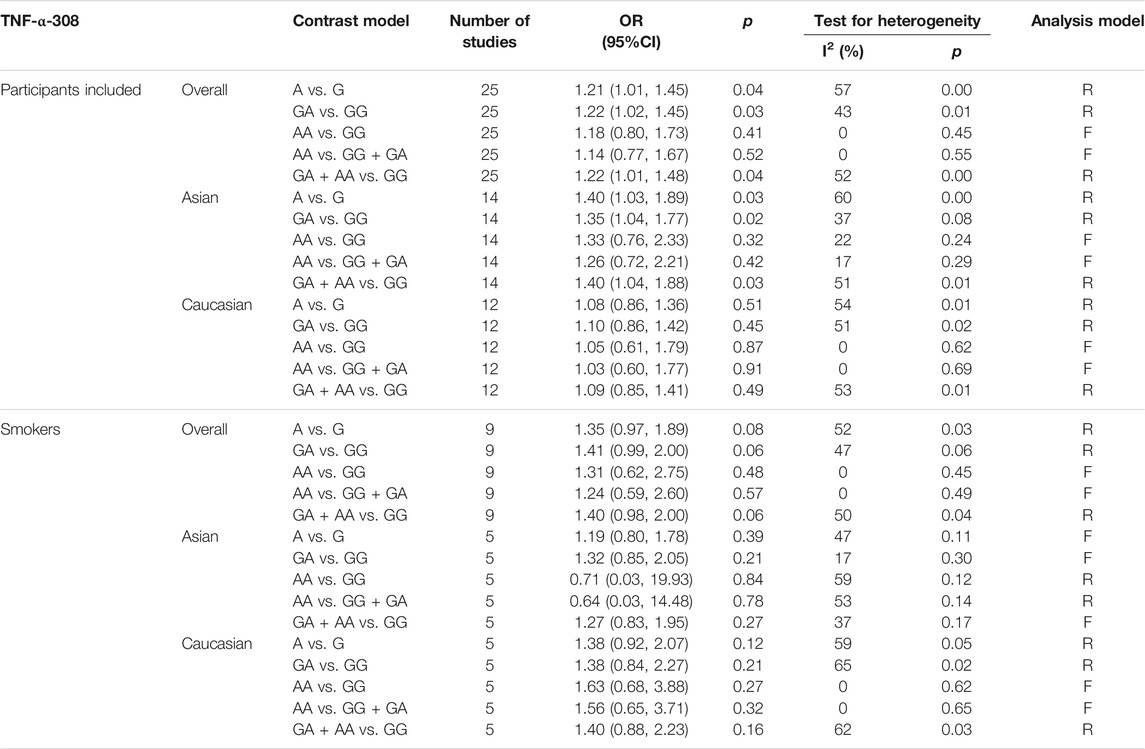
TABLE 2. Meta-analysis results for relationship between the TNF-α-308 polymorphism and COPD risk.; Abbreviation: CI = confidence interval, F = fixed effect model, OR = odds ratio, R = random-effect model.
For TNF-α+489G/A variant, five research papers, including 709 cases and 853 normal controls, were selected. The pooled results suggested that TNF-α+489 polymorphism was not associated with COPD risk (p > 0.05). Subgroups analysis by ethnicity also showed no association between TNF-α+489 and COPD among Asians (p > 0.05) and Caucasians (p > 0.05) (Table 3).
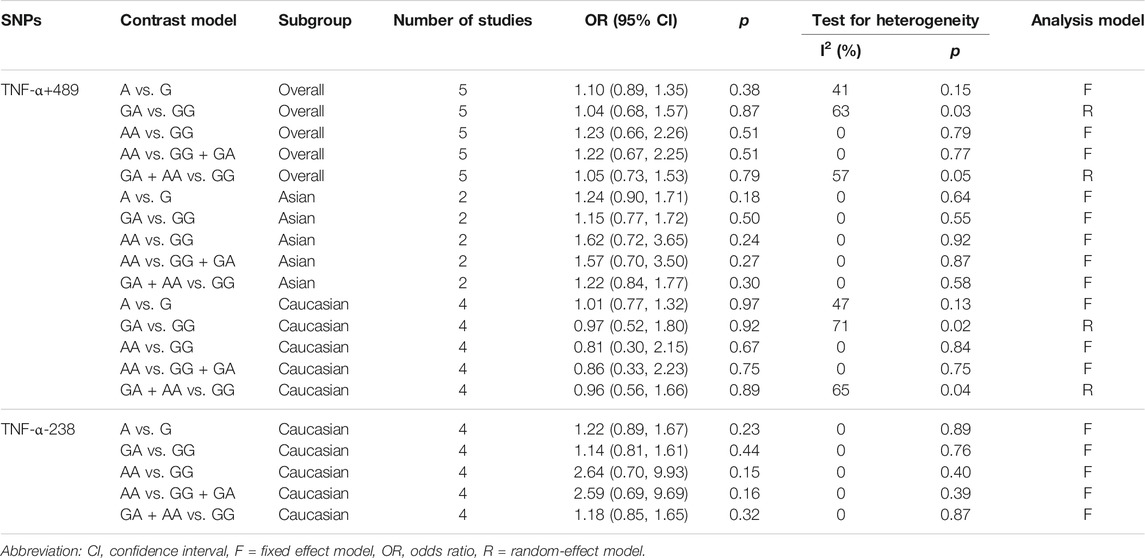
TABLE 3. Meta-analysis results for relationship between the TNF-α+489, -238 polymorphism and COPD risk.
624 cases and 1514 controls originated from four studies were included to probe the relevance between susceptibility to COPD and TNF-α-238G/A variant. We found that TNF-α-238 polymorphism was not associated with COPD risk in the overall population (p > 0.05). (Table 3).
Sensitivity analysis was performed by sequentially excluding each study to assess the stability of the results in this meta-analysis. As shown in Table 4, the results based on the overall meta-analysis were not stable (Table 4). However, in the Asian subgroup, the pooled OR did not vary significantly, implying that the findings were reliable (Figure 3). We speculated that the overall stability was affected by those studies restricted to Caucasians.
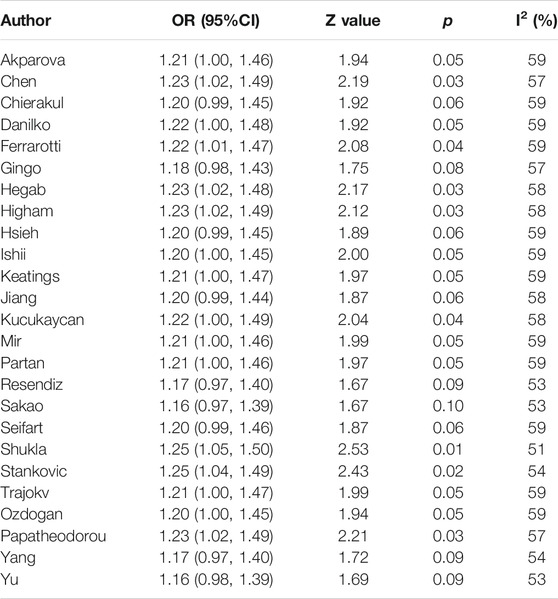
TABLE 4. Sensitivity analysis of the association between TNF-α-308 and COPD risk, under the allelic model in overall population.; Abbreviation: CI = confidence interval, OR = odds ratio.
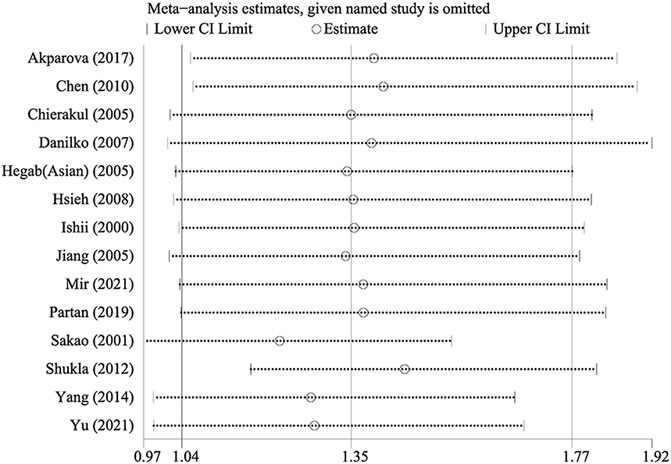
FIGURE 3. Sensitivity analysis of TNF-α-308 polymorphism and COPD risk (Asian population for GA vs. GG).
Begg’s funnel plots and Egger’s test were performed to assess the publication bias (all contrast models: p > 0.05), and the results suggested that there was no publication bias for the association between TNF-α-308 polymorphism and COPD in included studies (Figure 4).
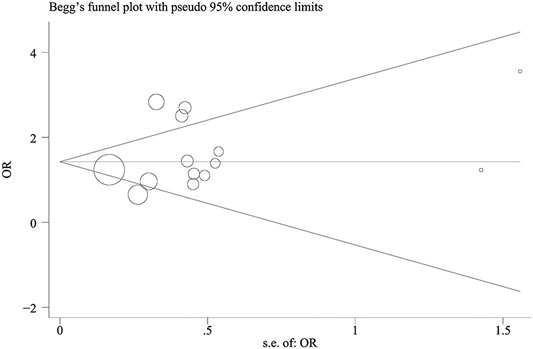
FIGURE 4. Begg’s funnel plot of TNF-α-308 polymorphism and COPD risk (Asian population for GA vs. GG).
We implemented TSA to reduce the risk of type I error and to evaluate the RIS by keeping the overall 5% risk of the type I error and the relative risk reduction of 20% (power of 80%). The results on TNF-α-308 showed that the Z-curve crossed the TSMB (Figure 5A). Therefore, it can be assumed that mutation from G to A on TNF-α-308 can increase the risk of COPD. The sample size did not reach the RIS among Asians in the allele model, heterozygote model, or dominant model (Figure 5B). Hence, more qualified studies are expected to confirm the relationship between TNF-α-308 variants and COPD susceptibility among Asians.
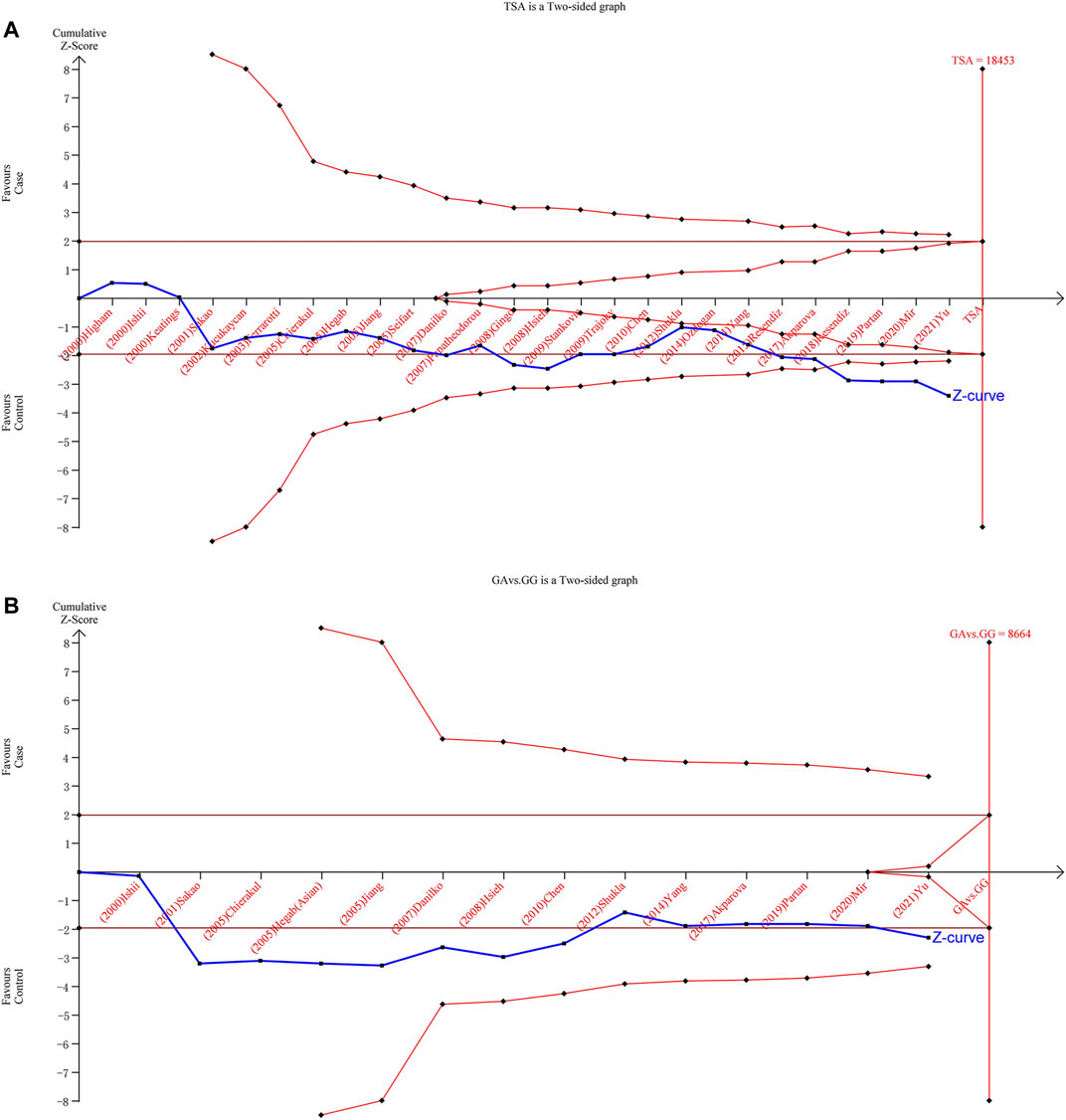
FIGURE 5. Results of TSA with TNF-α-308 variant. The required information size was calculated based on a two-sided a = 5%, ß = 15% (power 80%), and a relative risk reduction of 20%. [(A): overall for A vs. G, (B): Asian population for GA vs. GG].
For the TNF-α-308 G/A, the GA genotype was significantly associated with increased COPD risk, especially among Asians. Presumably, the GA variant was a stronger activator of TNF-α transcription than the GG genotype (Wilson et al., 1997). Some studies had shown that TNF-α-308 GA expression may cause damage to muscle (Baumert et al., 2016) and increase bronchial responsiveness (Moffatt et al., 1999). Moreover, Zhang (Zhang et al., 2016) and other previous studies (Hu et al., 2007; Zhan et al., 2011) found an increased risk of COPD associated with TNF-α-308 AA genotype. Nevertheless, we did not find any significant relationship between COPD susceptibility and TNF-α-308 AA genotype. Our meta-analysis updated five case-control studies (Akparova et al., 2017; Reséndiz-Hernández et al., 2018; Umi Partan and Hidayat, 2019; Mir et al., 2020; Yu et al., 2021) compared with Zhang’s research, and the cumulative Z-value crossed the TSA boundary. The TSA result could be due to the fact that our study had enough COPD cases and controls. This may be the reason for the difference between our analysis and Zhang’s study. Furthermore, we conducted the ethnicity subgroup analysis to gain a more comprehensive view (Salimi Asl et al., 2019; Shi and Zhao, 2019). In our study, a second meta-analysis restricted to smokers was also performed, and it indicated that TNF-α-308G/A polymorphism was not associated with COPD susceptibility among smokers. The result implied that cigarette smoking may conceal the influence of TNF-α polymorphism on COPD. The effect of smoking on the gene factor may be attributed to the following: 1) smoking might exert an interaction with TNF-α-308G/A polymorphism, and have a potential influence on TNF-α gene expression; and 2) smoking is the major risk factor for COPD. We speculated that compared with the effect of TNF-α-308 GA variant on COPD, there was a stronger association between smoking and COPD risk.
As for the TNF-α+489G/A, no significant association was found between TNF-α+489G/A polymorphism and COPD susceptibility. Due to the new case-control study (Yu et al., 2021) included, we proposed a converse conclusion compared with previous meta-analysis (Cui et al., 2015). The result remained controversial because of the limited sample size. Therefore, more case-control studies and cohort studies are in urgent need to determine a stable conclusion.
To the best of our knowledge, this is the first meta-analysis to analyze TNF-α -238G/A variant and its contribution to risk of COPD. Similarly, the pooled results suggested that TNF-α -238G/A polymorphism had no significant association with COPD risk. All the case-control studies about TNF-α-238G/A we retrieved were restricted to Caucasians. Hence, we are looking forward to more original studies on other ethnicities to confirm our conclusions.
Our study features some advantages. Firstly, we performed TSA to reduce the risk of type I error and evaluate required information size. The TSA result showed that the conclusion about TNF-α-308 G/A was based on a sufficient number of cases and controls. Secondly, we carried out a second meta-analysis restricted to smokers. As smoking is the major risk factor for the development of COPD, this sub-group analysis illustrates the additional information genetic polymorphism provides when studying risk factors of COPD. Thirdly, NOS quality test was performed to estimate the quality of the case-control studies included, making the results more reliable. In addition, sensitivity analysis and Egger’s test were performed to assess the publication bias. And the test showed our results were stable in the Asian subgroup.
Nevertheless, there are still some limitations that cannot be avoided in our meta-analysis. First, due to the lack of case-control studies related to nonsmokers, we failed to ascertain the association between the TNF-α-308 G/A variant and COPD risk in nonsmoking populations. Second, some unpublished studies might not be retrieved. Third, there were limited data to conduct subgroup analyses in Asians and Caucasians. The conclusions for TNF-α+489G/A and TNF-α -238G/A required more original studies to make them convincing.
Together, the present meta-analysis indicated that TNF-α-308G/A polymorphism was associated with an increased risk of COPD among Asians but not in Caucasians, and GA genotype carriers of TNF-α-308 had a higher risk of developing COPD compared to GG carriers when smoking status was not adjusted. However, in the meta-analysis with restrictions to smokers, no association was found between TNF-α-308 polymorphism and COPD susceptibility either in Asian smokers or Caucasian smokers. Moreover, the TNF-α+489G/A, -238G/A variants did not have an increased risk of COPD susceptibility.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Study design: ZX and YW; Data collection: FL and HS; Data analysis: ZX, YW, and PH; Writing: ZX and YW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akparova, A., Abdrakhmanova, B., Banerjee, N., and Bersimbaev, R. (2017). EPHX1 Y113H Polymorphism Is Associated with Increased Risk of Chronic Obstructive Pulmonary Disease in Kazakhstan Population. Mutat. Research/Genetic Toxicol. Environ. Mutagenesis 816-817, 1–6. doi:10.1016/j.mrgentox.2017.02.004
Baumert, P., Lake, M. J., Stewart, C. E., Drust, B., and Erskine, R. M. (2016). Genetic Variation and Exercise-Induced Muscle Damage: Implications for Athletic Performance, Injury and Ageing. Eur. J. Appl. Physiol. 116 (9), 1595–1625. doi:10.1007/s00421-016-3411-1
Celli, B. R., Decramer, M., Wedzicha, J. A., Wilson, K. C., Agustí, A., Criner, G. J., et al. (2015). An Official American Thoracic Society/European Respiratory Society Statement: Research Questions in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 191 (7), e4–e27. doi:10.1164/rccm.201501-0044ST
Chen, Y.-C., Liu, S.-F., Chin, C.-H., Wu, C.-C., Chen, C.-J., Chang, H.-W., et al. (2010). Association of Tumor Necrosis Factor-α-863c/A Gene Polymorphism with Chronic Obstructive Pulmonary Disease. Lung 188 (4), 339–347. doi:10.1007/s00408-010-9236-5
Chierakul, N., Wongwisutikul, P., Vejbaesya, S., and Chotvilaiwan, K. (2005). Tumor Necrosis Factor-Alpha Gene Promoter Polymorphism Is Not Associated with Smoking-Related COPD in Thailand. Respirology 10 (1), 36–39. doi:10.1111/j.1440-1843.2005.00626.x
Cui, K., Ge, X. Y., and Ma, H. L. (2015). Association of the TNF-Α+489 G/A Polymorphism with Chronic Obstructive Pulmonary Disease Risk in Asians: Meta-Analysis. Genet. Mol. Res. 14 (2), 5210–5220. doi:10.4238/2015.may.18.12
Danilko, K. V., Korytyna, G. F., Akhmadishina, L. Z., Yanbaeva, D. G., Zagidullin, S. Z., and Victorova, T. V. (2007). Association of Polymorphisms of Cytokine Genes (IL1B, IL1RN, TNFA, LTA, IL6, IL8, and IL10) with Chronic Obstructive Pulmonary Disease. Mol. Biol. 41 (1), 22–31. doi:10.1134/s0026893307010049
Ferrarotti, I., Zorzetto, M., Beccaria, M., Gilè, L. S., Porta, R., Ambrosino, N., et al. (2003). Tumour Necrosis Factor Family Genes in a Phenotype of COPD Associated with Emphysema. Eur. Respir. J. 21 (3), 444–449. doi:10.1183/09031936.03.00051303
Foreman, K. J., Marquez, N., Dolgert, A., Fukutaki, K., Fullman, N., McGaughey, M., et al. (2018). Forecasting Life Expectancy, Years of Life Lost, and All-Cause and Cause-specific Mortality for 250 Causes of Death: Reference and Alternative Scenarios for 2016-40 for 195 Countries and Territories. The Lancet 392 (10159), 2052–2090. doi:10.1016/s0140-6736(18)31694-5
Gingo, M. R., Silveira, L. J., Miller, Y. E., Friedlander, A. L., Cosgrove, G. P., Chan, E. D., et al. (2008). Tumour Necrosis Factor Gene Polymorphisms Are Associated with COPD. Eur. Respir. J. 31 (5), 1005–1012. doi:10.1183/09031936.00100307
Hegab, A. E., Sakamoto, T., Saitoh, W., Nomura, A., Ishii, Y., Morishima, Y., et al. (2005). Polymorphisms of TNFα, IL1β, and IL1RN Genes in Chronic Obstructive Pulmonary Disease. Biochem. Biophysical Res. Commun. 329 (4), 1246–1252. doi:10.1016/j.bbrc.2005.02.099
Higham, M. A., Pride, N. B., Alikhan, A., and Morrell, N. W. (2000). Tumour Necrosis Factor-α Gene Promoter Polymorphism in Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 15 (2), 281–284. doi:10.1034/j.1399-3003.2000.15b10.x
Hsieh, M.-H., Chong, I.-W., Hwang, J.-J., Lee, C.-H., Ho, C.-K., Yu, M.-L., et al. (2008). Lack of Associations between Several Polymorphisms in Cytokine Genes and the Risk of Chronic Obstructive Pulmonary Diseases in Taiwan. Kaohsiung J. Med. Sci. 24 (3), 126–137. doi:10.1016/s1607-551x(08)70140-2
Hu, G. P., Peng, G. Y., Hu, J. X., and Ran, P. X. (2007). Association of Tumor Necrosis Factor Alpha 308 G/A Gene Promoter Polymorphism with the Presence of Chronic Obstructive Pulmonary Disease: a Meta-Analysis. Zhonghua Jie He He Hu Xi Za Zhi 30 (8), 588–594.
Ishii, T., Matsuse, T., Teramoto, S., Matsui, H., Miyao, M., Hosoi, T., et al. (2000). Neither IL-1 β, IL-1 Receptor Antagonist, Nor TNF- α Polymorphisms Are Associated with Susceptibility to COPD. Respir. Med. 94 (9), 847–851. doi:10.1053/rmed.2000.0808
Jiang, L., He, B., Zhao, M. W., Ning, L. D., Li, X. Y., and Yao, W. Z. (2005). Association of Gene Polymorphisms of Tumour Necrosis Factor-Alpha and Interleukin-13 with Chronic Obstructive Pulmonary Disease in Han Nationality in Beijing. Chin. Med. J. (Engl) 118 (7), 541–547.
Keatings, V. M., Cave, S. J., Henry, M. J., Morgan, K., O'Connor, C. M., FitzGerald, M. X., et al. (2000). A Polymorphism in the Tumor Necrosis Factor-α Gene Promoter Region May Predispose to a Poor Prognosis in COPD. Chest 118 (4), 971–975. doi:10.1378/chest.118.4.971
Küçükaycan, M., Van Krugten, M., Pennings, H. J., Huizinga, T. W., Buurman, W. A., Dentener, M. A., et al. (2002). Tumor Necrosis Factor-Alpha +489G/A Gene Polymorphism Is Associated with Chronic Obstructive Pulmonary Disease. Respir. Res. 3, 29. doi:10.1186/rr194
Matokanovic, M., Rumora, L., Popovic-Grle, S., Cepelak, I., Culic, O., and Barisic, K. (2012). Association of Hsp70-2 (+1267A/G), Hsp70-Hom (+2437T/C), HMOX-1 (Number of GT Repeats) and TNF-Alpha (+489G/A) Polymorphisms with COPD in Croatian Population. Clin. Biochem. 45 (10-11), 770–774.
Mir, H., Koul, P. A., Bhat, D., and Shah, Z. A. (2020). A Case-Control Study of Tumor Necrosis Factor-Alpha Promoter Polymorphism and its Serum Levels in Patients with Chronic Obstructive Pulmonary Disease in Kashmir, North India. Lung India 37 (3), 204–209. doi:10.4103/lungindia.lungindia_477_19
Moffatt, M. F., James, A., Ryan, G., Musk, A. W., and Cookson, W. O. C. M. (1999). Extended Tumour Necrosis Factor/HLA-DR Haplotypes and Asthma in an Australian Population Sample. Thorax 54 (9), 757–761. doi:10.1136/thx.54.9.757
Nedwin, G. E., Naylor, S. L., Sakaguchi, A. Y., Smith, D., Jarrett-Nedwin, J., Pennica, D., et al. (1985). Human Lymphotoxin and Tumor Necrosis Factor Genes: Structure, Homology and Chromosomal Localization. Nucl. Acids Res. 13 (17), 6361–6373. doi:10.1093/nar/13.17.6361
Özdoğan, N., Tutar, N., Demir, R., Saatçi, Ç., Kanbay, A., and Büyükoğlan, H. (2014). Is TNF-α Gene Polymorphism Related to Pulmonary Functions and Prognosis as Determined by FEV1, BMI, COPD Exacerbation and Hospitalization in Patients with Smoking-Related COPD in a Turkish Population? Revista Portuguesa de Pneumologia 20 (6), 305–310. doi:10.1016/j.rppneu.2014.03.003
Papatheodorou, A., Latsi, P., Vrettou, C., Dimakou, A., Chroneou, A., Makrythanasis, P., et al. (2007). Development of a Novel Microarray Methodology for the Study of SNPs in the Promoter Region of the TNF-α Gene-Their Association with Obstructive Pulmonary Disease in Greek Patients. Clin. Biochem. 40 (12), 843–850. doi:10.1016/j.clinbiochem.2007.03.024
Parameswaran, N., and Patial, S. (2010). Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukar Gene Expr. 20 (2), 87–103. doi:10.1615/critreveukargeneexpr.v20.i2.10
Reséndiz-Hernández, J. M., Ambrocio-Ortiz, E., Pérez-Rubio, G., Lopez-Flores, L. A., Abarca-Rojano, E., Pavón Romero, G. F., et al. (2018). TNF Promoter Polymorphisms Are Associated with Genetic Susceptibility in COPD Secondary to Tobacco Smoking and Biomass Burning. Copd Vol. 13, 627–637. doi:10.2147/copd.s147688
Sakao, S., Tatsumi, K., Igari, H., Shino, Y., Shirasawa, H., and Kuriyama, T. (2001). Association of Tumor Necrosis Factor α Gene Promoter Polymorphism with the Presence of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 163 (2), 420–422. doi:10.1164/ajrccm.163.2.2006031
Salimi Asl, M., Ahmadi, A., Salimian, J., Shohani, S., Azimzadeh Jamalkandi, S., and Ghanei, M. (2019). TNF-α −308 G/A Variant and Susceptibility to Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. Cytokine 123, 154763. doi:10.1016/j.cyto.2019.154763
Seifart, C., Dempfle, A., Plagens, A., Seifart, U., Clostermann, U., Müller, B., et al. (2005). TNF-α-, TNF-β-, IL-6-, and IL-10-promoter Polymorphisms in Patients with Chronic Obstructive Pulmonary Disease. Tissue Antigens 65 (1), 93–100. doi:10.1111/j.1399-0039.2005.00343.x
Shi, C., and Zhao, H. (2019). Association between Tumor Necrosis Factor-308 G/A Polymorphism and Chronic Obstructive Pulmonary Disease Risk in Chinese Population: Evidence from a Meta-Analysis. Clin. Lab. 65 (10). doi:10.7754/Clin.Lab.2019.190313
Shukla, R. K., Kant, S., Bhattacharya, S., and Mittal, B. (2012). Association of Cytokine Gene Polymorphisms in Patients with Chronic Obstructive Pulmonary Disease. Oman Med. J. 27 (4), 285–290. doi:10.5001/omj.2012.71
Stankovic, M., Nestorovic, A., Tomovic, A., Petrovic-Stanojevic, N., Andjelic-Jelic, M., Dopudja-Pantic, V., et al. (2009). TNF-α-308 Promotor Polymorphism in Patients with Chronic Obstructive Pulmonary Disease and Lung Cancer. neo 56 (4), 348–352. doi:10.4149/neo_2009_04_348
Tarigan, A. P., Syafiuddin, T., Yunus, F., and Suradi, (2015). Association of Tumor Necrosis Factor Alpha and Lymphotoxin Alpha Gene Polymorphisms with the Presence of Chronic Obstructive Pulmonary Disease. Acta Med. Indones 47 (4), 283–290.
Trajkov, D., Mirkovska-Stojkovikj, J., Petlichkovski, A., Strezova, A., Efinska-Mladenovska, O., Sandevska, E., et al. (2009). Association of Cytokine Gene Polymorphisms with Chronic Obstructive Pulmonary Disease in Macedonians. Iran J. Allergy Asthma Immunol. 8 (1), 31–42. doi:10.08.01/ijaai.3142
Umi Partan, R., and Hidayat, R. (2019). The Relationship between TNF-α Gene Polymorphism, Pro-inflammatory Cytokines and Bone Turnover Markers in COPD Patients with Osteoporosis. J. Phys. Conf. Ser., 1246. doi:10.1088/1742-6596/1246/1/012035
Wilson, A. G., Symons, J. A., McDowell, T. L., McDevitt, H. O., and Duff, G. W. (1997). Effects of a Polymorphism in the Human Tumor Necrosis Factor Promoter on Transcriptional Activation. Proc. Natl. Acad. Sci. 94 (7), 3195–3199. doi:10.1073/pnas.94.7.3195
Yang, L., Li, F., Yan, M., and Su, X. (2014). Association of the CYP1A1 MspI and TNFα-308 Polymorphisms with Chronic Obstructive Pulmonary Disease in Inner Mongolia. Genet. Mol. Res. 13 (2), 3209–3217. doi:10.4238/2014.april.25.6
Yu, S., Xue, M., Yan, Z., Song, B., Hong, H., and Gao, X. (2021). Correlation between TNF-α -308 and +489 Gene Polymorphism and Acute Exacerbation of Chronic Obstructive Pulmonary Diseases. Biomed. Res. Int. 2021, 6661281. doi:10.1155/2021/6661281
Zhan, P., Wang, J., Wei, S.-Z., Qian, Q., Qiu, L.-X., Yu, L.-K., et al. (2011). TNF-308 Gene Polymorphism Is Associated with COPD Risk Among Asians: Meta-Analysis of Data for 6,118 Subjects. Mol. Biol. Rep. 38 (1), 219–227. doi:10.1007/s11033-010-0098-y
Zhang, L., Gu, H., Gu, Y., and Zeng, X. (2016). Association between TNF-α -308 G/A Polymorphism and COPD Susceptibility: a Meta-Analysis Update. Int. J. Chron. Obstruct Pulmon Dis. 11, 1367–1379. doi:10.2147/COPD.S105394
Keywords: COPD, single nucleotide polymorphism, meta-analysis, trial sequential analysis, TNF-α
Citation: Xia Z, Wang Y, Liu F, Shu H and Huang P (2022) Association Between TNF-α-308, +489, −238 Polymorphism, and COPD Susceptibility: An Updated Meta-Analysis and Trial Sequential Analysis. Front. Genet. 12:772032. doi: 10.3389/fgene.2021.772032
Received: 07 September 2021; Accepted: 01 December 2021;
Published: 21 January 2022.
Edited by:
William C Cho, QEH, Hong Kong SAR, ChinaReviewed by:
Pentti Nieminen, University of Oulu, FinlandCopyright © 2022 Xia, Wang, Liu, Shu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Huang, aHVhbmdwZW5nbmN1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.