
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Genet., 23 September 2021
Sec. Genetics of Common and Rare Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.741323
Woodhouse-Sakati syndrome (WSS, MIM 241080) is a rare neuroendocrine disease characterized by hair loss, hypogonadism, diabetes, hearing loss, and extrapyramidal syndrome, and is usually caused by mutations in the DCAF17 gene as an inherited disease. DCAF17 plays an important role in mammalian gonadal development and infertility. So far, there have been no WSS reports in China. The patient introduced in this case is from a consanguineous family. The main symptoms of the patient were alopecia and gonadal agenesis. Other symptoms such as hearing loss, intellectual disability, and hyperglycemia were remarkable, and these symptoms are often observed in WSS patients. We found a nonsense mutation in the 11th exon of the gene DCAF17 (Refseq: NM_025000) in the patient and her younger brother, which confirmed the diagnosis of WSS. The genetic results also showed that the mutation was inherited from their healthy first-cousin parents.
Woodhouse-Sakati syndrome (WSS) is a rare neuroendocrine and ectodermal disorder, which is caused by mutations in the gene encoding DCAF17. The phenotype of WSS is variable, which is also the reason why this disease is often undiagnosed or misdiagnosed. However, the main symptoms of WSS are alopecia, hypogonadotropic hypogonadism, sensorineural hearing loss, diabetes mellitus, and extrapyramidal movements. This syndrome was first reported by Woodhouse and Sakati (Woodhouse and Sakati, 1983). Currently, fewer than 100 WSS patients have been reported, and most of these patients originated from the Middle East. Some cases have also been recognized in other areas, such as in Europe, Turkey, India, Pakistan, Portugal, France, and Japan. In 2008, DCAF17, which is located on chromosome 2q31.1, was discovered as the causal gene for WSS by Woodhouse and Sakati (Sheridan et al., 2015; Hdiji et al., 2016; Louro et al., 2019; Sendur et al., 2019; Shah et al., 2020). According to the HGMD database, so far 24 mutations in DCAF17 have been reported in the literature, and 18 are responsible for WSS, as listed in Supplementary Table S1. These mutations include three nonsense mutations: c.906G>A, p.(Ser114Term), c387G>A, p.(Trp302Term), and c341C>A, p.(Trp129Term); five intronic mutations that are thought to result in mis-localization of the DCAF17 gene: c.321+1G>A, c.1422+5G>T, c.1091+2T>C, c.1091+1G>A, and c.1091+6T>G; nine frameshift mutations: c.1238delA, c.270delA, c.270dupA, c.289dupA, c.127-3_127-1delTAGinsAA, c.995delT, c.436delC, c.459-7_499del, and c.50delC; and one start loss mutation (c.1G>A) (Sheridan et al., 2015; Hdiji et al., 2016; Louro et al., 2019; Sendur et al., 2019; Shah et al., 2020). We report the first Chinese case and her younger brother, whose symptoms were suggestive of WSS on clinical presentation. The diagnosis was made by molecular genetic analyses. The proband had a nonsense mutation, and her younger brother also had the same mutation.
The proband was a 25-yr-old woman, the first child of healthy first cousin parents (Figure 1), with a height of 156 cm and a body weight of 56 kg. She complained of sparse hair and no menstruation. Her mother’s pregnancy, delivery, and feeding history were normal. Her younger brother had similar symptoms, such as alopecia and gonadal agenesis. She had another younger brother died at 7 mo, so his genetic analysis could not be investigated. It is unknown if the cause of death was related to the mutation of this gene. In addition, generations I and II above the patient’s parents died, and genetic analysis could not be performed (Figure 1). During her childhood, her parents noticed that her hair was sparse compared to other children’s. In adolescence, she did not show secondary sexual characteristics. Menarche did not occur. Her previous consultations in other hospitals showed that she had abnormal blood glucose, intellectual disability, dysaudia, an immature uterus and ovaries with few follicles, and her bone age was delayed by 9 yr. Over a year of progynova treatment, she had two menstruations. Then, the patient stopped taking the medicine and no longer menstruated. Physical examination on admission showed that her hair was sparse, especially on both temporal sides and forehead. Her face was progeric, with a large forehead and prominent ears. Secondary sexual characteristics were incompletely developed. Axillary or pubic hair was not present, and minimal breast budding was present with marked vulvar hypoplasia (Figure 2). Unlike some patients in the early literature (Agopiantz et al., 2014), the patient and her younger brother did not have extrapyramidal movement such as dysaudia, dyskinesia with oropharyngeal swallowing disorder, or trouble walking. The patient’s Mini-mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) scale scores were 22 and 19 respectively, which confirmed that she had mental retardation. Interestingly, we did not find abnormal extravertebral movement in this patient or her younger brother, which is common in other patients (Agopiantz et al., 2014). The neurological examination showed that the patient had no dysarthria, dysphagia, tremor and abnormal muscle tension; her coordination movements, posture and gait were also normal; At the same time, her muscle strength was normal and Babinski sign was negative.
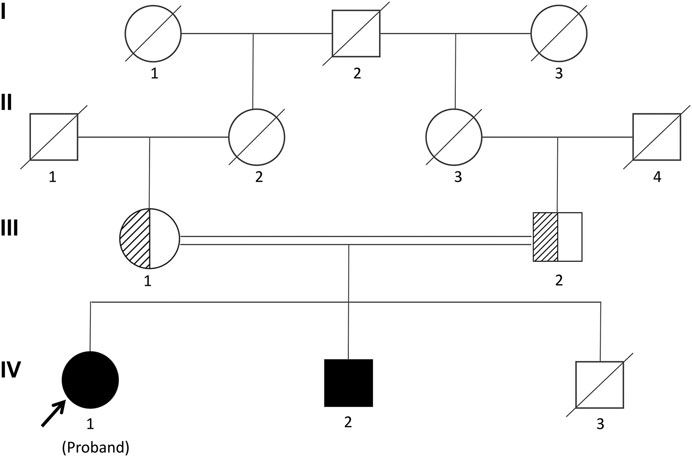
FIGURE 1. Pedigree of the Chinese family segregating Woodhouse-Sakati syndrome (WSS) in an autosomal recessive manner. Male individuals are represented by squares, and females are represented by circles. Half-filled symbols represent a DCAF17 heterozygous mutation, while filled symbols represent a DCAF17 homozygous mutation. The third child of the patient’s parents died at 7 mo and his genetic analyses could not be investigated.
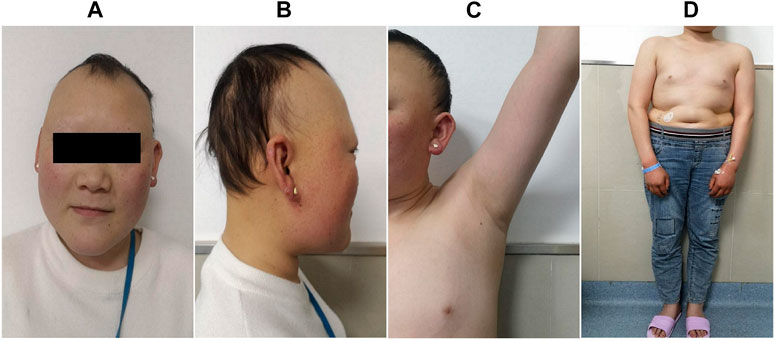
FIGURE 2. Clinical manifestations of the proband. She presented with sparse hair, especially on both temporal sides and on the forehead, with a progeric face and a large forehead with prominent ears (A,B). Secondary sexual characteristics were incompletely developed (C,D).
Sex hormone examinations disclosed low estradiol level (<5 pg/ml, reference range: 12.4–233 pg/ml) and low testosterone level (<0.025 pg/ml, reference range: 0.07–0.78 pg/ml). Her follicle-stimulating hormone level, prolactin level, and luteinizing hormone level were in the normal range. Other hormone investigations revealed high thyroid-stimulating hormone (6.36 uIU/ml, reference range: 0.27–4.2 uIU/ml), low free thyroxine (7.39 pmol/ml, reference range: 12–22 pmol/ml), and a normal free triiodothyronine level. These data were suggestive of subclinical hypothyroidism. She had a low insulin growth factor 1 (IGF-1) level (45.7 ng/ml, reference range: 69–200 ng/ml) and a high level of HbA1c (8.8%, reference range: 3.6–6%) (Table 1). Her C-peptide release test results were as follows: 0 min: 2.25 ng/ml, 30 min: 2.43 ng/ml, 60 min: 2.99 ng/ml, and 120 min: 5.58 ng/ml. A transabdominal ultrasound showed an immature uterus and small ovaries. Her breast ultrasound found tiny gland tissue in the bilateral breasts. A hearing assessment disclosed bilateral mild sensorineural deafness. A left wrist X-ray showed epiphyseal closure. An electrocardiograph (ECG) was unremarkable. Magnetic resonance imaging (MRI) revealed a small pituitary, partially empty sella, no iron deposits in the globus pallidus, and multiple intracranial white matter hyperintensities that may be of vascular origin (Figure 3). As mentioned above, the patient’s clinical features, metabolic disease, neurological findings, ectodermal appendages, and laboratory tests are summarized in Table 1.
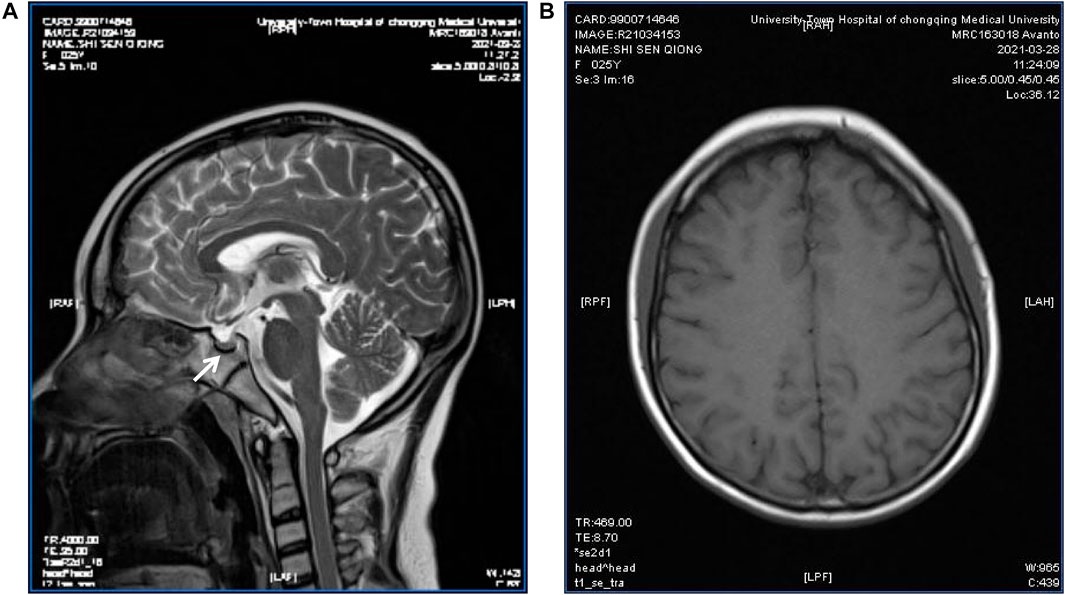
FIGURE 3. Pituitary MRI of the patient. T2-weighted MR imaging for the patient showed a partially empty sella and a small pituitary gland (arrow) (A); Magnetic sensitivity weighted imaging showed no abnormal iron deposition accumulation (B).
After obtaining informed consent from the patient and her family, peripheral blood was sampled from the patient, her younger brother, and their parents. In order to better describe identification of the variant, we have added more detailed steps for genetic testing, data screening and pathogenicity analysis based on previous articles published after the company’s WES technical services (Ma et al., 2019; Zhang et al., 2019). We isolated nucleic acid to capture DNA fragments, then constructed a library, using biotinylated probes to target and capture exons, and amplify the captured targets, qPCR was used for quality control and sequencing, and to analyze the captured information. We analyzed DCAF17 mutatifons and their pathogenicity. We used the MGI-T7 platform (BGI) for sequencing at a depth of 200× and a target coverage of 99.57%. Forward primer: 3′-CAGAATCTCCGAATTTGAAGGAG-5′, reverse primer: 3′-TCTTTAAATCTGAAATGTACATGGG-5’. Her parent’s and younger brother’s samples were tested via Sanger sequencing for verification (Supplementary Figure S1). With pathogenicity analysis (Zhang et al., 2019), we detected the pathogenic locus (Supplementary Figure S2). The patient’s DCAF17 harbored a homozygous mutation c.1111delA, p.(Ile371Term), her younger brother had the same mutation, and her parents were heterozygous for the mutation at this site. The c.1111delA mutation is a nonsense mutation leading to a premature stop codon p.(Ile371Term) (Figure 4).
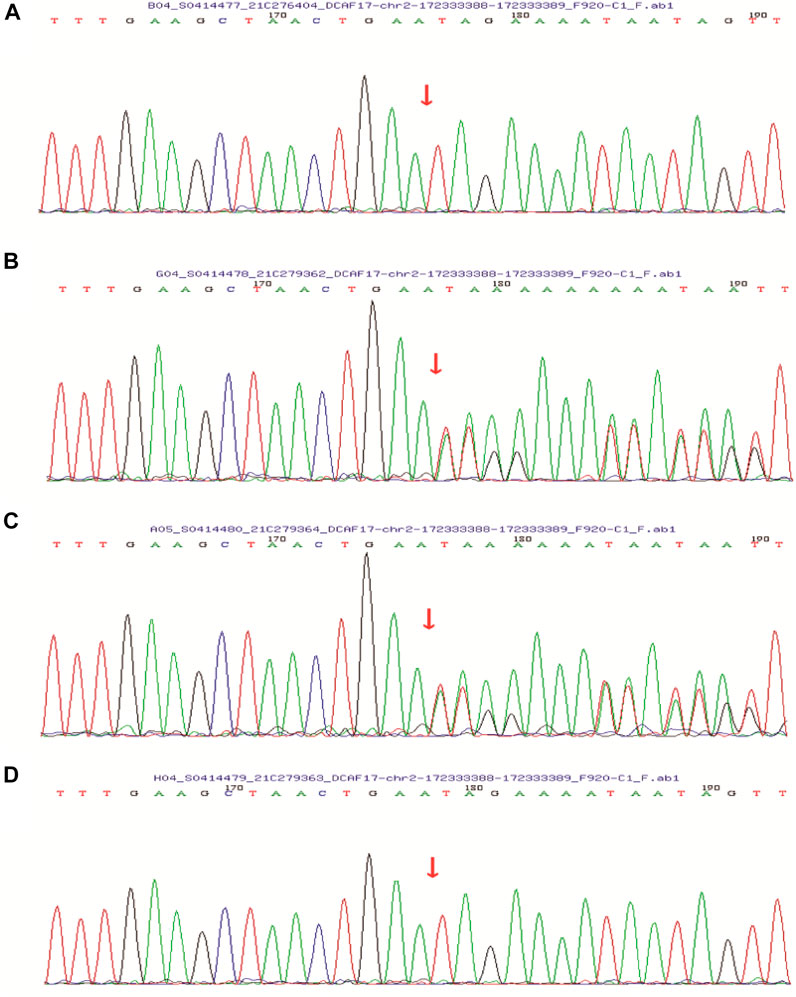
FIGURE 4. Sanger sequencing showed that the patient’s (A) and her younger brother’s (D) DCAF17 harbored a homozygous mutation c.1111delA, p.(Ile371Term), while their father (B) and mother (C) were heterozygous for this mutation at this site.
The most prominent manifestation of WSS is disorder of the neuroendocrine system (Agopiantz et al., 2014). Previously, reports revealed the presence of hypogonadism (100%), intellectual disability (87%), sensorineural hearing loss (76%), and extrapyramidal movements (42%) (Bohlega et al., 1993; Agopiantz et al., 2014). The first cases reported in 1983 were six Saudi Arabian patients from two highly inbred families (Woodhouse and Sakati, 1983). Until now, fewer than 100 WSS patients have been reported. The patient in this report sought medical treatment for 10 yr and was not diagnosed until she came to our hospital and we noticed that she had symptoms of WSS. This is consistent with previous reports (Schweitzer et al., 2003; Kurnaz et al., 2019; Louro et al., 2019; Shah et al., 2020), The patient presented with alopecia, hypogonadism, hearing loss, intellectual disability, and high blood sugar, but neither the patient nor her younger brother had other symptoms such as dysarthria, dyskinesia with oropharyngeal dysphagia, or difficulty walking, and no abnormal manifestations such as iron deposition on the MRI image. In this case, the mutation site was different from previous reports. Meanwhile, this is the first reported Chinese WSS family.
The DCAF17 gene, located on chromosome 2q31.1, encodes a nucleolar protein with two isoforms including alpha (453 amino acid residues; NP_001158293.1) and beta (520 amino acids residues; NP_079276.2) (Bohlega et al., 1993; Andersen et al., 2005). The protein is expressed in the brain, liver, and skin, as well as in seminiferous tubules in male mice, a pattern that is compatible with the neurosensory, endocrine, and cutaneous phenotypic manifestations (Agopiantz et al., 2014). Previous literature regarding this nucleolar protein showed that it functions as a substrate co-receptor for the ubiquitin ligase complex Cul4-DDB1 (Angers et al., 2006; Ali et al., 2016), which emphasizes the lesser-known functions of the nucleolus as a caretaker of normal development and post-development maintenance, including the cellular activities of growth, proliferation, and response to stress as well as in the cell cycle and apoptosis (Lee and Zhou, 2007). Mutation of DCAF17 leads to disruption of the nucleolus, and this damage results in dysregulated ribosome biogenesis, regulation of the cell cycle, cellular aging, signal recognition, small-RNA processing, mRNA transport, and apoptosis (Andersen et al., 2005). These disruptions may underlie the pathogenesis of WSS. Nevertheless, the pathogenic mechanism underlying the clinical variability associated with different mutations has not been explored extensively. Further experimental studies are necessary to reveal the exact pathogenic mechanisms of how these genes affect different tissues.
The mutation c.1111delA, p.(Ile371Term) in our case resulted in an adenine deletion at basepair 1111, and this deletion leads to translation termination as stated in the literature (Ali et al., 2016), We speculate that an incomplete protein with missing domains and motifs necessary to interact with the DDB1-CUL4 ubiquitin ligase complex and recruit substrates was formed. Another possible result of the mutation would be degeneration of gene transcript through mRNA decay (Kurosaki et al., 2021; Yang et al., 2021). This nonsense mutation was different from other nonsense mutations in previous reports (Bohlega et al., 1993; Boisvert et al., 2007; Alazami et al., 2010; Habib et al., 2011; Sheridan et al., 2015).
In conclusion, WSS is a genetic disorder of the neuroendocrine system that leads to neuroendocrine cell aging function loss, which is usually caused by mutations in the DCAF17 gene. Significantly, we have identified a novel mutation in the DCAF17 gene. Molecular genetic analyses should be performed as a crucial tool to inform early diagnosis. Additional work to explore the clinical and underlying biological mechanisms will be helpful to further understand the disease.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Ethics Committee of Xinqiao Hospital, Army Medical University (Third Military Medical University). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
GC and LZ performed a literature search and drafted the manuscript. LZ, QC, JW, and PJ acquired data; RS and ML revised the manuscript. GC and RS obtained study funding. HZ and ML contributed to the diagnosis and treatment. All authors read and approved the final manuscript.
This study was supported by the science and technology research youth project of Chongqing Municipal Education Commission(KJQN202000447), the Hospital Party Building and Medical Federation Project(2021DJ01), the Clinical Research Project of Xinqiao Hospital of Army Medical University(2018XLC3049).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Hengguang Zhao (dermatologist), Jing Tian (gynecologist), Ning Yan (neurologist), Deping Sun (otolaryngologist) and Tao Xie (Ultrasound physician) of University-Town Hospital of Chongqing Medical University for their contribution for the patient’s diagnosis and treatment.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.741323/full#supplementary-material
Agopiantz, M., Corbonnois, P., Sorlin, A., Bonnet, C., Klein, M., Hubert, N., et al. (2014). Endocrine Disorders in Woodhouse-Sakati Syndrome: A Systematic Review of the Literature. J. Endocrinol. Invest. 37, 1–7. doi:10.1007/s40618-013-0001-5
Alazami, A., Schneider, S., Bonneau, D., Pasquier, L., Carecchio, M., Kojovic, M., et al. (2010). C2orf37 Mutational Spectrum in Woodhouse-Sakati Syndrome Patients. Clin. Genet. 78, 585–590. doi:10.1111/j.1399-0004.2010.01441.x
Ali, R. H., Shah, K., Nasir, A., Steyaert, W., Coucke, P. J., and Ahmad, W. (2016). Exome Sequencing Revealed A Novel Biallelic Deletion in theDCAF17gene Underlying Woodhouse Sakati Syndrome. Clin. Genet. 90, 263–269. doi:10.1111/cge.12700
Andersen, J. S., Lam, Y. W., Leung, A. K. L., Ong, S.-E., Lyon, C. E., Lamond, A. I., et al. (2005). Nucleolar Proteome Dynamics. Nature 433, 77–83. doi:10.1038/nature03207
Angers, S., Li, T., Yi, X., MacCoss, M. J., Moon, R. T., and Zheng, N. (2006). Molecular Architecture and Assembly of the DDB1-CUL4A Ubiquitin Ligase Machinery. Nature 443, 590–593. doi:10.1038/nature05175
Bohlega, S. A., and Alkuraya, F. S. (1993). “Woodhouse-Sakati Syndrome,” in GeneReviews((R)). Editors M. P. Adam, H. H. Ardinger, R. A. Pagon, S. E. Wallace, L. J. H. Bean, G. Mirzaaet al. (Seattle, WA: University of Washington).
Boisvert, F.-M., van Koningsbruggen, S., Navascués, J., and Lamond, A. I. (2007). The Multifunctional Nucleolus. Nat. Rev. Mol. Cel Biol. 8, 574–585. doi:10.1038/nrm2184
Habib, R., Basit, S., Khan, S., Khan, M. N., and Ahmad, W. (2011). A Novel Splice Site Mutation in Gene C2orf37 Underlying Woodhouse-Sakati Syndrome (WSS) in a Consanguineous Family of Pakistani Origin. Gene 490, 26–31. doi:10.1016/j.gene.2011.09.002
Hdiji, O., Turki, E., Bouzidi, N., Bouchhima, I., Damak, M., Bohlega, S., et al. (2016). Woodhouse-Sakati Syndrome: Report of the First Tunisian Family with the C2orf37 Gene Mutation. J. Mov. Disord. 9, 120–123. doi:10.14802/jmd.16003
Kurnaz, E., Türkyılmaz, A., Yaralı, O., Demir, B., and Çayır, A. (2019). A Novel DCAF17 Homozygous Mutation in a Girl with Woodhouse-Sakati Syndrome and Review of the Current Literature. J. Pediatr. Endocrinol. Metab. 32, 1287–1293. doi:10.1515/jpem-2019-0173
Kurosaki, T., Imamachi, N., Pröschel, C., Mitsutomi, S., Nagao, R., Akimitsu, N., et al. (2021). Loss of the Fragile X Syndrome Protein FMRP Results in Misregulation of Nonsense-Mediated mRNA Decay. Nat. Cel Biol. 23, 40–48. doi:10.1038/s41556-020-00618-1
Lee, J., and Zhou, P. (2007). DCAFs, the Missing Link of the CUL4-DDB1 Ubiquitin Ligase. Mol. Cel. 26, 775–780. doi:10.1016/j.molcel.2007.06.001
Louro, P., Durães, J., Oliveira, D., Paiva, S., Ramos, L., and Macário, M. C. (2019). Woodhouse-Sakati Syndrome: First Report of a Portuguese Case. Am. J. Med. Genet. 179, 2237–2240. doi:10.1002/ajmg.a.61303
Ma, J., Zhang, Z., Jiang, H. C., Sun, H., Ming, C., Zhao, L. P., et al. (2019). A Novel Dominant Mutation in the SOX10 Gene in a Chinese Family with Waardenburg Syndrome Type II. Mol. Med. Rep. 19, 1775–1780. doi:10.3892/mmr.2019.9815
Schweitzer, D. N., Yano, S., Earl, D. L., and Graham, J. M. (2003). Johnson-McMillin Syndrome, a Neuroectodermal Syndrome with Conductive Hearing Loss and Microtia: Report of a New Case. Am. J. Med. Genet. 120A, 400–405. doi:10.1002/ajmg.a.20085
Sendur, S. N., Oguz, S., Utine, G. E., Dagdelen, S., Oguz, K. K., Erbas, T., et al. (2019). A Case of Woodhouse-Sakati Syndrome with Pituitary Iron Deposition, Cardiac and Intestinal Anomalies, with a Novel Mutation in DCAF17. Eur. J. Med. Genet. 62, 103687. doi:10.1016/j.ejmg.2019.103687
Shah, K., Jan, A., Ahmad, F., Basit, S., Ramzan, K., and Ahmad, W. (2020). Woodhouse-Sakati Syndrome in a Family Is Associated with a Homozygous Start Loss Mutation in the DCAF 17 Gene. Clin. Exp. Dermatol. 45, 159–164. doi:10.1111/ced.14046
Sheridan, M. B., Wohler, E., Batista, D. A., Applegate, C., and Hoover-Fong, J. (2015). The Use of High-Density SNP Array to Map Homozygosity in Consanguineous Families to Efficiently Identify Candidate Genes: Application to Woodhouse-Sakati Syndrome. Case Rep. Genet. 2015, 169482. doi:10.1155/2015/169482
Woodhouse, N. J., and Sakati, N. A. (1983). A Syndrome of Hypogonadism, Alopecia, Diabetes Mellitus, Mental Retardation, Deafness, and ECG Abnormalities. J. Med. Genet. 20, 216–219. doi:10.1136/jmg.20.3.216
Yang, Q., Lyu, X., Zhao, F., and Liu, Y. (2021). Effects of Codon Usage on Gene Expression Are Promoter Context Dependent. Nucleic Acids Res. 49, 818–831. doi:10.1093/nar/gkaa1253
Keywords: diabetes mellitus, hypogonadism, alopecia, woodhouse-Sakati syndrome, DCAF17, deletion mutation
Citation: Chen G, Zhou L, Chen Q, Wang J, Jiang P, Shen R, Long M and Zhou H (2021) Case Report: A Deletion Variant in the DCAF17 Gene Underlying Woodhouse-Sakati Syndrome in a Chinese Consanguineous Family. Front. Genet. 12:741323. doi: 10.3389/fgene.2021.741323
Received: 22 July 2021; Accepted: 13 September 2021;
Published: 23 September 2021.
Edited by:
Sulman Basit, Taibah University, Saudi ArabiaReviewed by:
Wasim Ahmad, Quaid-i-Azam University, PakistanCopyright © 2021 Chen, Zhou, Chen, Wang, Jiang, Shen, Long and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Long, bG9uZ21pbl9jYXNwZXJAMTYzLmNvbQ==; Houdi Zhou, Y3FtZW1qaUAxMjYuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.