- 1Department of Radiation Oncology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Radiotherapy Center, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 3Department of Oncology-Pathology, Karolinska Institutet, Solna, Sweden
- 4Clinical Pathology and Cancer Diagnostics, Karolinska University Hospital, Solna, Sweden
Background: Immunotherapy has recently shown remarkable efficacy for advanced bladder cancer patients. Accordingly, identifying a biomarker associated with the programmed cell death protein 1 (PD-1)/its ligand (PD-L1) genomic signature to predict patient prognosis is necessary.
Methods: In this study, we used mutation data and RNA-seq data of bladder cancer samples acquired from The Cancer Genome Atlas (TCGA) database to combine PD-1/PD-L1-associated mutational signatures with PD-1/PD-L1-associated differentially expressed genes (DEGs). Then, we performed a Kaplan-Meier analysis on the corresponding clinical data of the TCGA bladder urothelial carcinoma (BLCA) cohort to identify prognostic genes, and the results were validated using the GSE48075 cohort. The online platform UCSC Xena was used to analyze the relationship between the candidate genes and clinical parameters. We utilized the Human Protein Atlas (HPA) database to validate the protein expression levels. Then, correlation analysis, cell-type identification by estimating relative subsets of RNA transcripts (CIBERSORT) analysis, and gene set enrichment analysis (GSEA) were used to clarify the mechanism.
Results: We identified one prognostic gene, sortilin related receptor 1 (SORL1), whose downregulation was associated with a comparatively advanced BLCA stage. While further exploring this finding, we found that SORL1 expression was negatively correlated with PD-1/PD-L1 expression and M2 macrophage levels. Furthermore, we found that the downregulation of SORL1 expression was significantly associated with a higher epithelial-mesenchymal transition (EMT) score.
Conclusion: We described a novel PD-1/PD-L1-associated signature, SORL1, that predicts favorable outcomes in bladder cancer. SORL1 might reduce immune suppression and inhibit the M2 macrophage-induced EMT phenotype of tumor cells.
Introduction
Bladder urothelial carcinoma (BLCA) remains the 10th most commonly diagnosed malignancy globally (Sung et al., 2021). Approximately 75% of patients present with nonmuscle-invasive bladder cancer, which has a recurrence rate between 50 and 70%, and the progression rate is approximately 10–30%; the remaining patients have muscle-invasive disease or a metastatic disease and have worse prognoses (Soloway et al., 2002). However, the mortality rate of bladder cancer has been decreasing in recent years due to improvements in treatments, immunotherapy as a representative.
Immunotherapy has gained substantial public attention because it is a promising treatment approach for multiple tumors. In addition, an immune checkpoint inhibitor (ICI) is a powerful weapon for activating therapeutic antitumor immunity (Pardoll, 2012). For example, antibodies against cytotoxic T-lymphocyte antigen 4 (CTLA4), programmed cell death protein 1 (PD-1) and its ligand (PD-L1) target immune checkpoints and activate immune cells to better fight cancers, such as melanoma (Robert et al., 2015; Marzagalli et al., 2019) and non-small-cell lung cancer (NSCLC) (Fehrenbacher et al., 2016; Sun et al., 2020). However, not all tumors are sensitive to immunotherapy, such as pancreatic cancer (Leinwand and Miller, 2020) or glioblastoma (Jackson et al., 2019), and the therapeutic effects of immunotherapy are related to specific characteristics such as tissue specificity (Salmon et al., 2019) and tumor mutational load (Samstein et al., 2019).
For non-muscle-invasive bladder cancer, intravesical immunotherapy with live attenuated bacillus Calmette-Guérin (BCG) is a routine treatment (Lamm, 1992). Evidence has revealed that the resistance of bladder cancer patients to BCG therapy is related to PD-L1 expression (Inman et al., 2007). Although systemic cisplatin-based chemotherapy remains the first-line therapy for advanced bladder cancer, many ICIs have been approved by the Food and Drug Administration (FDA) as a second-line therapy for those with metastatic bladder carcinoma. Some of these drugs have even been used as first-line therapy for cisplatin-ineligible patients due to their remarkable efficacy (Kamat et al., 2016; Lopez-Beltran et al., 2021).
Given the growing importance of immunotherapy for bladder cancer, it is critical to identify an immune-associated biomarker to predict patient prognoses (Zhao et al., 2021; Zhou et al., 2021). Some studies also introduced effective prediction methods to enhanced the understanding of gene expression regulation and therapeutic biomarkers (Zhang L. et al., 2021). The first example we considered was PD-L1 expression levels, as quantified by immunohistochemistry (IHC). However, there are some limitations to measuring PD-L1 expression levels. For example, there is currently a lack of standardization among available PD-L1 assays and cut-offs, and the role of PD-L1 for predicting ICI agent outcomes has yielded mixed results (Xylinas et al., 2014; Bellmunt et al., 2015; Krabbe et al., 2017).
Herein, we integrated PD-1/PD-L1-relevant transcriptome profiles with the mutational signature following the acquisition of accessible RNA-seq data. Then we screened out an immune microenvironment-related gene—SORL1—whose expression is related to the prognosis of bladder cancer validated by various methods. We further determined the correlation of SORL1 with PD-1/PD-L1 expression and M2 macrophage levels, as well as epithelial-mesenchymal transition (EMT) score. Additional exploration on the prognostic signature—SORL1 which would be a predictive biomarker for bladder cancer need to be performed to see whether it is benefit for patients who were treated with ICI agents.
All in all, we identified a novel genomic signature—SORL1 that was closely related to immune microenvironment and predicted favorable outcomes in bladder cancer by exploiting public database.
Materials and Methods
Data Collection
We obtained publicly available RNA-seq data for bladder cancer patients and the patient clinical information and mutation data from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov). We transformed the RNA-seq data (fragments per kilobase million, FPKM) values into transcripts per kilobase million (TPM) values before performing log normalization. We performed gene annotation using the Ensembl database (http://asia.ensembl.org/biomart/martview). The GSE48075 cohort, which contains 142 bladder cancer samples, was retrieved from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi). By removing the samples with a vital state listed as “NA” using the “filter” function in R version 4.0.5 (https://www.r-project.org), we obtained 73 BLCA samples. We obtained a panel of gene signature matrices that distinguished 22 immune cell phenotypes, referred to as LM22, from the available literature (Newman et al., 2015). We also collected a set of 50 hallmark signatures (Liberzon et al., 2015) and a panel of biological function signatures deposited in the Molecular Signatures Database (MSigDB) (https://www.gsea-msigdb.org/gsea/msigdb) to conduct a GSVA analysis.
Identification of PD-1/PD-L1 Mutational Signatures
We analyzed the PD-1 and PD-L1 mutational signatures in the transcriptome and mutation profiles. For each gene out of a panel containing 16,542 genes, we regrouped 408 patients into mutant and wild-type cohorts. We carried out the Wilcoxon test to observe the differences in PD-1/PD-L1 expression between the mutant and wild-type groups and computed the expression fold change values between these two groups. The genes were only considered PD-1/PD-L1 mutational signatures under the following conditions: 1. mutational frequency >2%; 2. p value of Wilcoxon test <0.05; and 3. FC >1.5.
Identification of PD-1/PD-L1-Associated DEGs
We classified the 408 bladder cancer patients into two groups (high and low expression) using RNA-seq count data. The top 25% PD-1/PD-L1 expression values were treated as a cut-off to differentiate the “high” and “low” expression cohorts. We installed a DESeq2 R Bioconductor package to analyse differentially expressed genes (DEGs) between the two cohorts. The significant DEGs met the criteria of |log2FC|>1.5 and an adjusted p value < 0.05. We used the “Enhanced Volcano” R package to draw volcano plots.
Identification of PD-1/PD-L1 Genomic Signatures
We combined PD-1/PD-L1 mutational signatures with PD-1/PD-L1-associated DEGs to analyze PD-1/PD-L1 genomic signatures using the “draw_venn” function in R.
Prognosis Analysis for PD-1/PD-L1 Genomic Signatures
Based on the 401 TCGA BLCA samples with available RNA-seq data and clinical information, we performed a Kaplan-Meier analysis of the overall survival (OS) rates to screen genes related to prognosis from the PD-1/PD-L1 genomic signatures. Patients were stratified by gene expression values greater than or below the median score. We applied the log-rank test to observe the differences between the two groups using the “survdiff” function in the “survival” R package, with p < 0.05 as the significance threshold. Subsequently, the GSE48075 population was used to test these selected genes for prognostic significance. We adopted the “survminer” R package to plot survival curves.
Analysis of the Relationship Between SORL1 Expression and Clinical Parameters
We analyzed the relationship between the SORL1 expression level and BLCA phenotypes (pathologic T, pathologic N, pathologic M, pathologic stage, primary therapy outcome success, sample type) using the online platform UCSC Xena (http://xena.ucsc.edu/).
Validation of the Protein Expression of SORL1 in the Human Protein Atlas Database
We determined the protein expression of SORL1, both in BLCA and normal tissues, using IHC retrieved from the Human Protein Atlas (HPA, https://www.proteinatlas.org/) (Uhlen et al., 2010).
Correlation Analysis Between SORL1 and PD-1/PD-L1 Expression
We analysed the gene expression correlation using the online platform cBioPortal (http://www.cbioportal.org/), which comprises various cancer genomic data. We analysed 408 mRNA expression datasets derived from bladder cancer samples (TCGA, Cell 2017). Additionally, a scatter plot was used to visualize the correlation between the log2-normalized gene expression value of SORL1 and PD-1/PD-L1, and the p value was used to determine statistical significance.
Determination of Tumor-Infiltrating Immune Cells Associated With SORL1 Expression
We used the Cell-type Identification by Estimating Relative Subsets of RNA Transcripts (CIBERSORT) method (Newman et al., 2015; Zhang Z. et al., 2021) to quantify the infiltration level of 22 different immune cells. Contrasting the BLCA RNA-seq FPKM matrix and LM22, we estimated the abundances of immune cell types in 401 BLCA patients using the CIBERSORT R script obtained from the CIBERSORT platform (https://cibersort.stanford.edu/) after 1,000 permutations. We excluded the samples with a p value for the deconvolution algorithm greater than or equal to 0.05 and retained the remaining 204 samples for further analysis. We conducted a Wilcoxon test to evaluate the differences in immune infiltration levels between the SORL1 high and low expression groups.
Identification of Prognosis-Related Significantly Differentially Enriched Signatures
We applied the Gene Set Variation Analysis (GSVA) R Bioconductor package to the reference gene sets to estimate the pathway enrichment scores in different samples (Hänzelmann et al., 2013). We used the limma R package to perform a differential analysis between high and low expression groups divided by the median SORL1 expression level. We defined the significantly differentially enriched signature based on adjusted p-values < 0.001 and |log FC|>0.3.
Then, we performed a Kaplan-Meier analysis and a log-rank test to evaluate the association between the selected differentially enriched signatures and BLCA survival outcomes. p < 0.01 was considered statistically significant.
EMT Scores are Significantly Associated With the Abundance of M2 Macrophages
We obtained an epithelial-mesenchymal transition (EMT) signature enrichment score (defined as the EMT score) from the previous step and computed the M2 macrophage immune infiltration abundance using the CIBERSORT method. We generated the scatter plot of the correlation between the EMT scores and M2 infiltration abundance through the “ggplot2” function in R. We estimated the linear regression line using the “lm” function and estimated the Spearman correlation using the “stat_cor” function in R.
Results
Identification of the PD-1/PD-L1 Genomic Signature
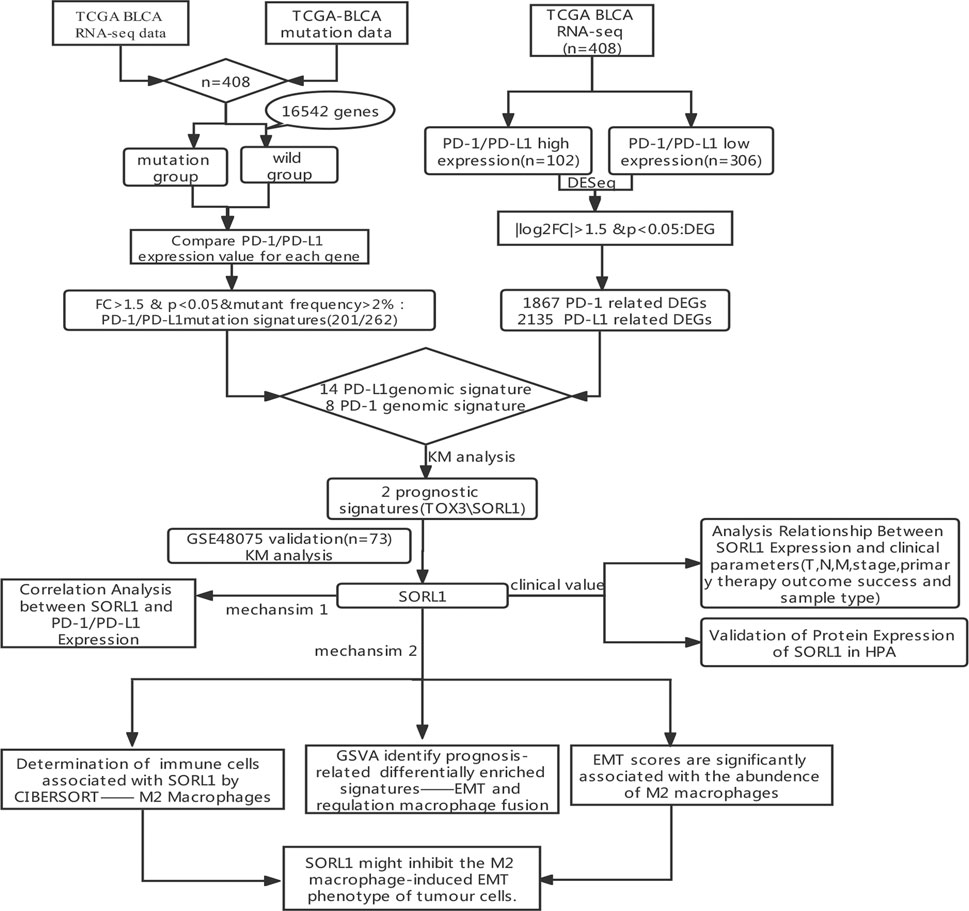
To identify immune-relevant biomarkers that can predict the prognosis of bladder carcinoma, we explored PD-1/PD-L1-relevant genomic profiles in bladder cancer. Figure 1 shows the flowchart of this study.
We selected 408 bladder tumour patients out of 433 TCGA BLCA samples. These 408 samples were accompanied by both transcriptomic and mutation profile data. We then evaluated the mutation state of 16,542 genes in the selected samples. Their mutation state was studied with a focus on PD-1- and PD-L1-related genomic profiles. For each gene, we regrouped samples into mutant and wild-type cohorts. With the criteria that the mutant group to wild-type ratio had a mean PD-1/PD-L1 expression value >1.5 and a mutational frequency >2%, we obtained the PD-1/PD-L1 mutational signatures listed in Figures 2A,B.
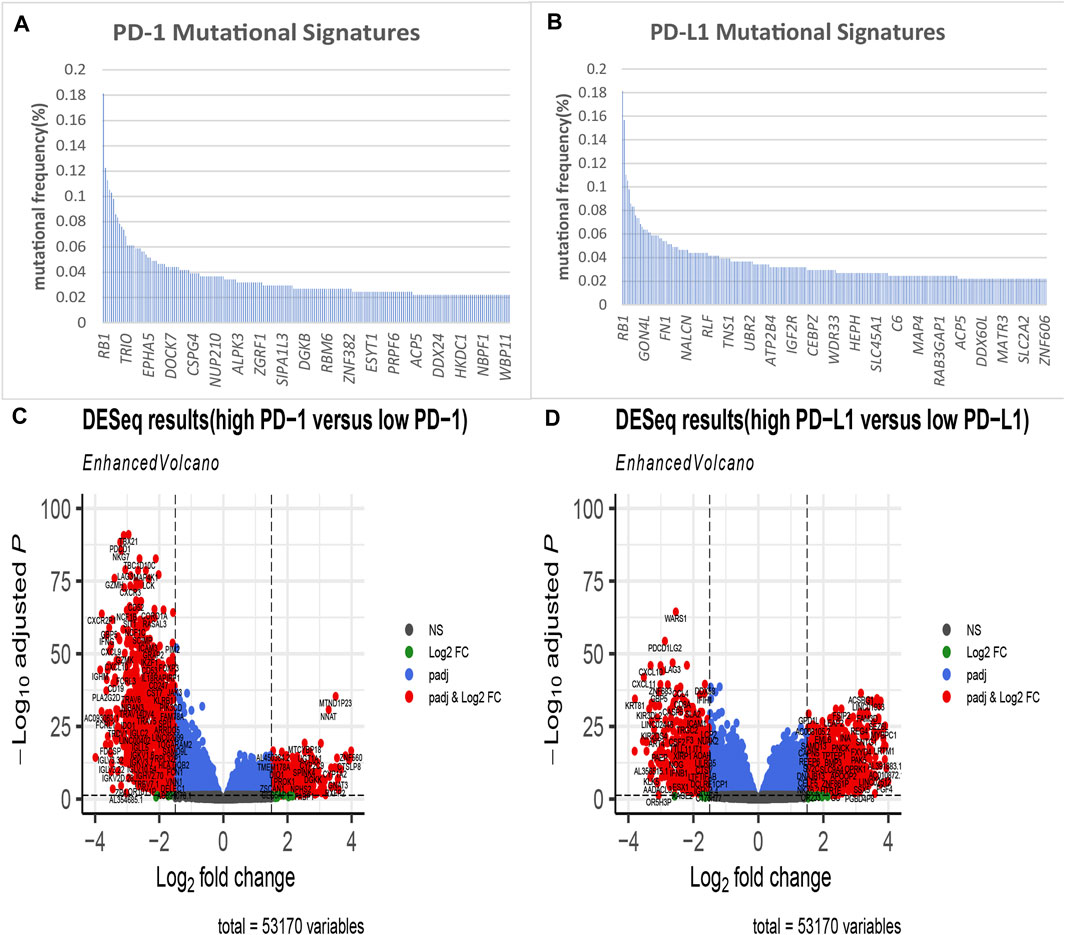
FIGURE 2. Identification of PD-1/PD-L1 mutational signatures and associated differentially expressed genes (DEGs) in bladder cancer. PD-1 (A)/PD-L1 (B) mutational signatures of TCGA bladder cancer ranked by mutation frequency (the abscissa labels manifest at random); (C) The volcano plot shows 1,867 differentially expressed genes DEGs as red dots between PD-1 high expression samples and PD-1 low expression tissues in bladder cancer (|log2-fold change|> 1.5, p < 0.05); (D) The volcano plot shows 2,135 differentially expressed genes as red dots between PD-L1 high expression samples and PD-L1 low expression tissues in bladder cancer (|log2-fold change|> 1.5, p < 0.05).
Next, using a minimum |log2FC| of 1.5 and adjusted p value of no more than 0.05, we performed a differential expression analysis between the PD-1/PD-L1 high-expression group (102 samples) and low-expression group (306 samples). As a result, we obtained a panel of DEGs out of 53,170 genes, which are described in Figures 2C,D.
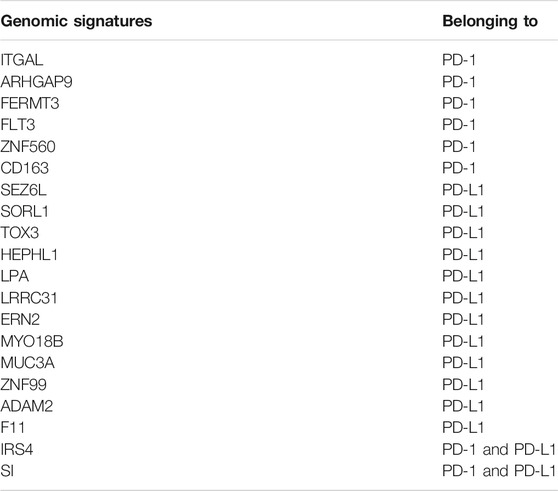
After Venn analysis, we obtained the PD-1 genomic signatures (n = 8) by calculating the intersection of the PD-1 mutational signature and the PD-1-related DEGs (Figure 3A). We carried out the same method for PD-L1 and obtained the PD-L1 genomic signatures (n = 14) (Figure 3B). Finally, we combined (n = 20) the PD-1- and PD-L1-related signatures (Figure 3C and Table 1).

FIGURE 3. Identification of PD-1/PD-L1 genomic signatures for bladder cancer. (A) We obtained a total of 8 PD-1 genomic signatures for bladder cancer by determining the intersection of 201 PD-1 mutational signatures and 1,867 PD-1-associated DEGs. (B) In the same way, we acquired 14 PD-L1 genomic signatures determining the intersection of 262 PD-L1 mutational signatures and 2,135 PD-L1-associated DEGs. (C) Finally, we obtained 20 overlapping PD-1/PD-L1 genomic signatures.
SORL1 Expression Predicts Favorable Prognosis in Bladder Cancer
Based on the clinical information obtained from the TCGA BLCA samples (401 cases), we performed the Kaplan-Meier survival analysis on 20 genes. Fortunately, we found that the genes TOX3 (p = 0.0043) and SORL1 (p = 0.042) were associated with BLCA (Figures 4A,B). To verify our finding, we applied Kaplan-Meier analysis to the GSE48075 cohort and found that the prognostic value of TOX3 lacked statistical significance (p = 0.17), but that BLCA patients in the SORL1 high-expression group showed significantly (p = 0.0065) more favourable OS outcomes than those in the SORL1 low-expression group in the GEO validation cohort (Figures 4C,D). In summary, SORL1 was identified as the only good prognostic indicator for BLCA overall survival outcomes.
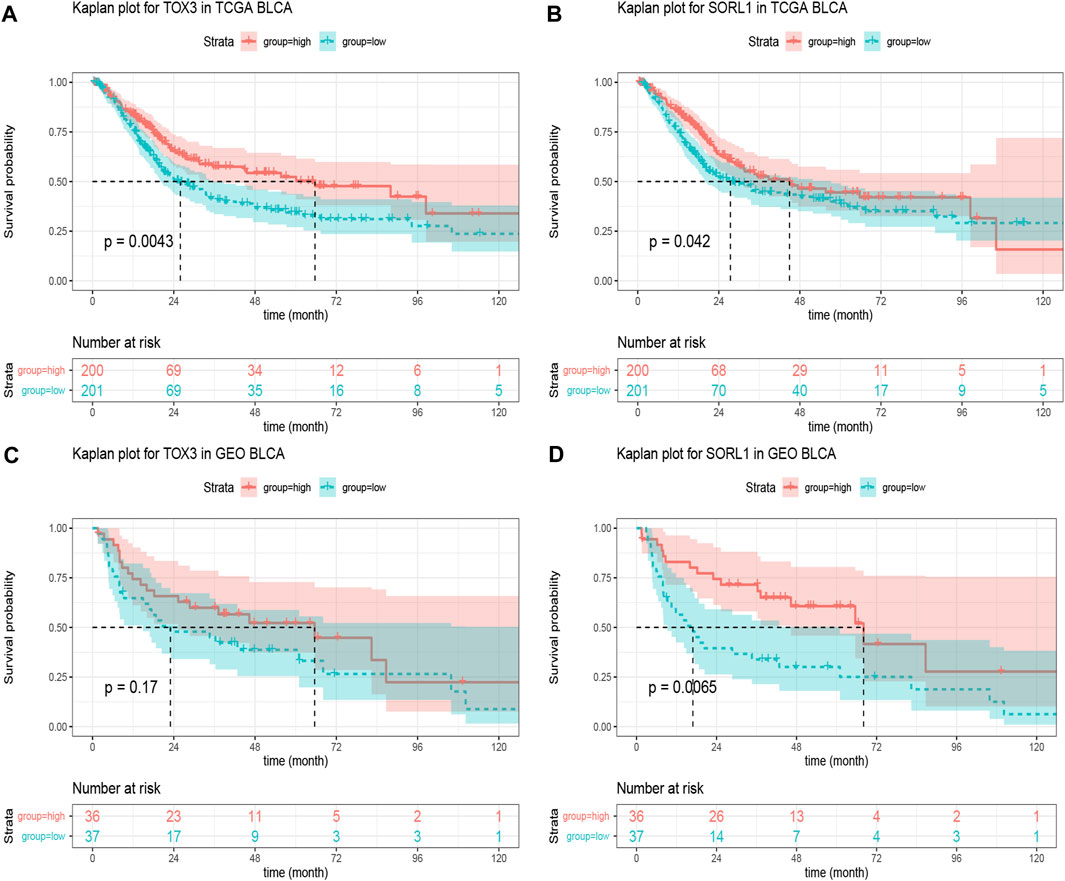
FIGURE 4. The prognostic assessment of the PD-1/PD-L1 genomic signatures in the TCGA and GSE48075 cohorts. (A) Kaplan-Meier curve of overall survival (OS) rates comparing prognosis between TOX3 high-expression and low-expression groups in the TCGA BLCA cohort; (B) OS Kaplan-Meier curve comparing prognosis for SORL1 high-expression and low-expression groups in the TCGA BLCA cohort. The difference in OS rates between groups with high and low expression levels of TOX3 (C) and SORL1 (D) in the GSE48075 cohort.
Association of mRNA and Protein Expression of SORL1 With Clinical Parameters
After identifying the survival-associated gene SORL1, we verified the relationship between the expression levels of SORL1 and the clinical parameters. From Figure 5A, we found there was a rough trend that the lower the level of SORL1 expression, the later the T staging (p < 0.01). However, there was no statistical significance in N staging (p > 0.05) (Figure 5B). Figure 5C shows the association between the SORL1 expression level and the pathological M stage of bladder carcinoma. A comparatively lower SORL1 expression level indicated a greater risk for metastasis (p < 0.05). Figure 5D depicts a higher expression level of SORL1 in the earlier stages of bladder cancer (p < 0.05). Figure 5E demonstrates the influence of the SORL1 expression level on primary therapeutic outcomes. However, the influence did not achieve statistical significance (p > 0.05). Figure 5F shows that the mRNA expression level of SORL1 was significantly reduced in tumours (p < 0.01). Furthermore, the protein levels of the SORL1 gene were lower in tumour tissues (Figure 5G) than in normal tissues (Figure 5H). Overall, these findings confirmed that the downregulation of SORL1 expression is associated with worse survival outcomes in BLCA patients.
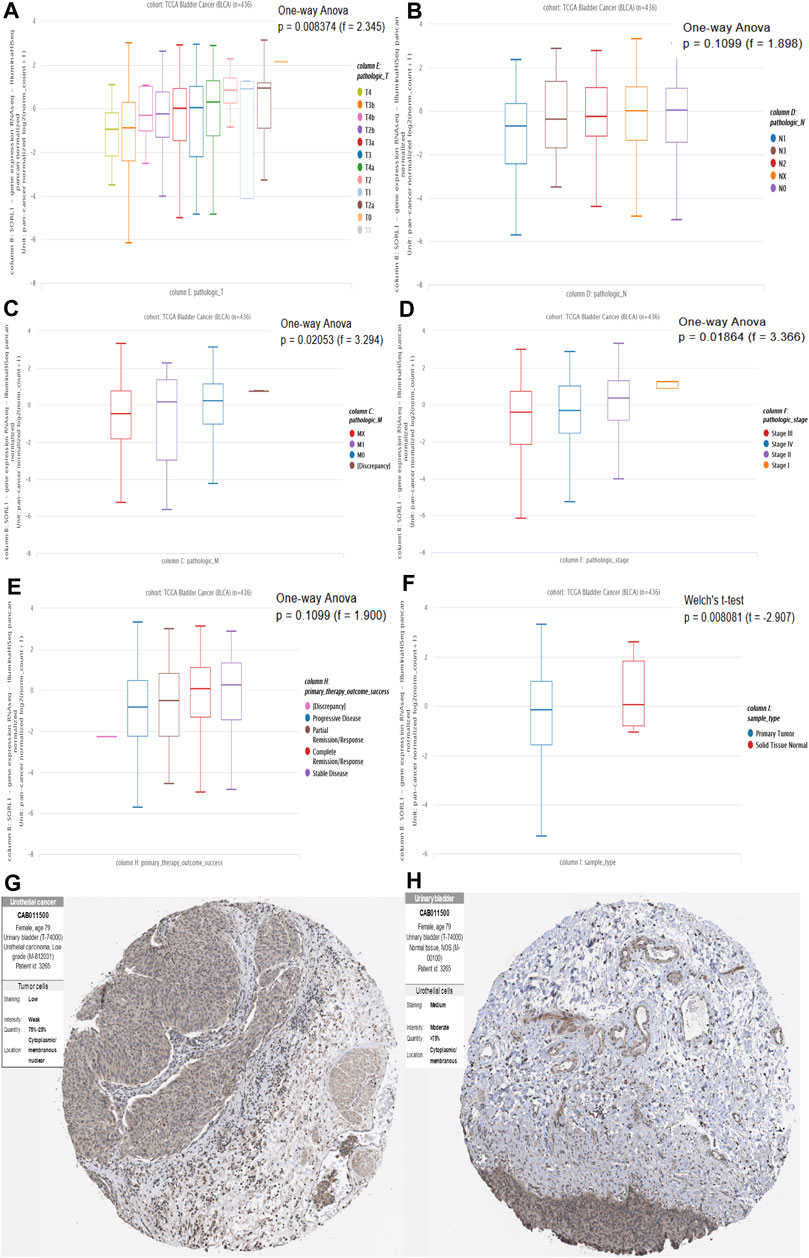
FIGURE 5. Association of mRNA and protein expression of SORL1 with clinical parameters. Association of SORL1 expression levels and pathologic T stage (A), pathologic N stage (B) and pathologic M stage (C) of BLCA patients; (D) The expression of SORL1 at different pathological stages; (E) The influence of the SORL1 expression level on primary therapy outcome success; (F) The expression level of SORL1 in tumor versus normal tissues in BLCA. (G) The protein expression level of SORL1 in BLCA tissues (patient ID: 3265; antibody CAB011500; staining: low; intensity: weak; quantity: 25–75%); (H) The protein expression level of SORL1 in normal tissues of BLCA patients (patient ID: 3265; antibody CAB011500; staining: medium; intensity: moderate; quantity: >75%).
Correlation Analysis Between SORL1 and PD-1/PD-L1 Expression
To investigate the association between the expression of SORL1 and the immune state, we performed a correlation analysis between SORL1 and PD-1/PD-L1 expression. We observed a negative correlation between the PD-1 (also named PDCD1) expression value and the PD-L1 (also named CD274) expression value with SORL1 expression. Additionally, the coefficients of the Spearman correlation were −0.32 (p = 2.83e−11) and −0.49 (p = 5.00e−26) (Figures 6A,B).
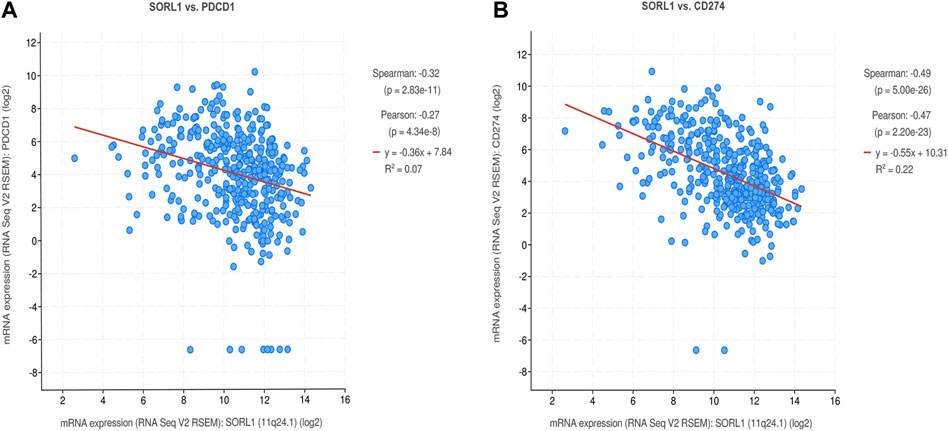
FIGURE 6. Correlation analysis between SORL1 and PD-1/PD-L1 expression. Correlation scatter plots show the correlation between the expression of PD-1 (A)/PD-L1 (B) and SORL1.
Determination of Tumor-Infiltrating Immune Cells Associated With SORL1 Expression
To clarify the survival mechanism related to the relationship between BLCA and the expression of SORL1, we explored the differences in tumour-infiltrating immune cells between patients with high and low SORL1 expression (the group allocation of patients was the same as the previous step). The results are depicted in Figures 7A,B. Figure 7C shows the landscapes of the proportions of 22 immune cells in the selected BLCA patients. We found that the differences in naïve B cells (p < 0.001), plasma cells (p < 0.001), activated memory CD4 T cells (p < 0.001), regulatory T cells (p < 0.001), resting NK cells (p < 0.001) and M2 macrophages (p = 0.002) were statistically significant. Additionally, the differences in naïve B cells, activated memory CD4 T cells, and M2 macrophages were much greater than the others (Figure 7B).
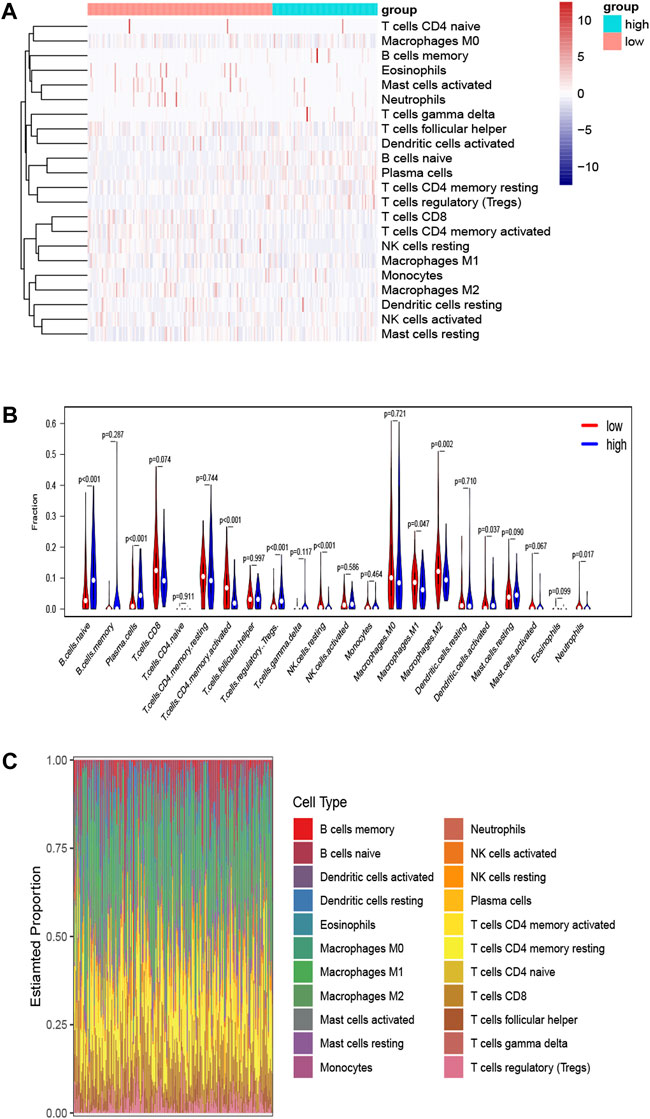
FIGURE 7. Determination of tumor-infiltrating immune cells associated with SORL1 expression. Heatmap (A) and violin plot (B) indicate the differences in the 22 immune cell distributions between SORL1 high expression and low expression in the BLCA cohort; (C) The histogram shows the landscapes of the proportions of 22 immune cells in the BLCA cohort.
Identification of Prognosis-Related Significantly Differentially Enriched Signatures
To investigate the SORL1-related pathway signatures, we performed GSVA on the TCGA bladder cancer samples.
Using GSVA, we obtained the enrichment scores of 50 hallmark signatures approximately in a normal distribution. We presented the association between the hallmark signatures and SORL1 expression level in the form of a heat map (Figure 8A). We identified three significantly differentially enriched signatures between the SORL1 high-expression and low-expression groups using the criteria of adjusted p-values < 0.001 and |log FC|>0.3. These were HALLMARK_ALLOGRAFT_REJECTION, HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION and HALLMARK_INFLAMMATORY_RESPONSE. Subsequently, the Kaplan-Meier analysis showed that the pathway signature EMT was associated with significantly worse survival outcomes (p = 0.0077) (Figure 8B).
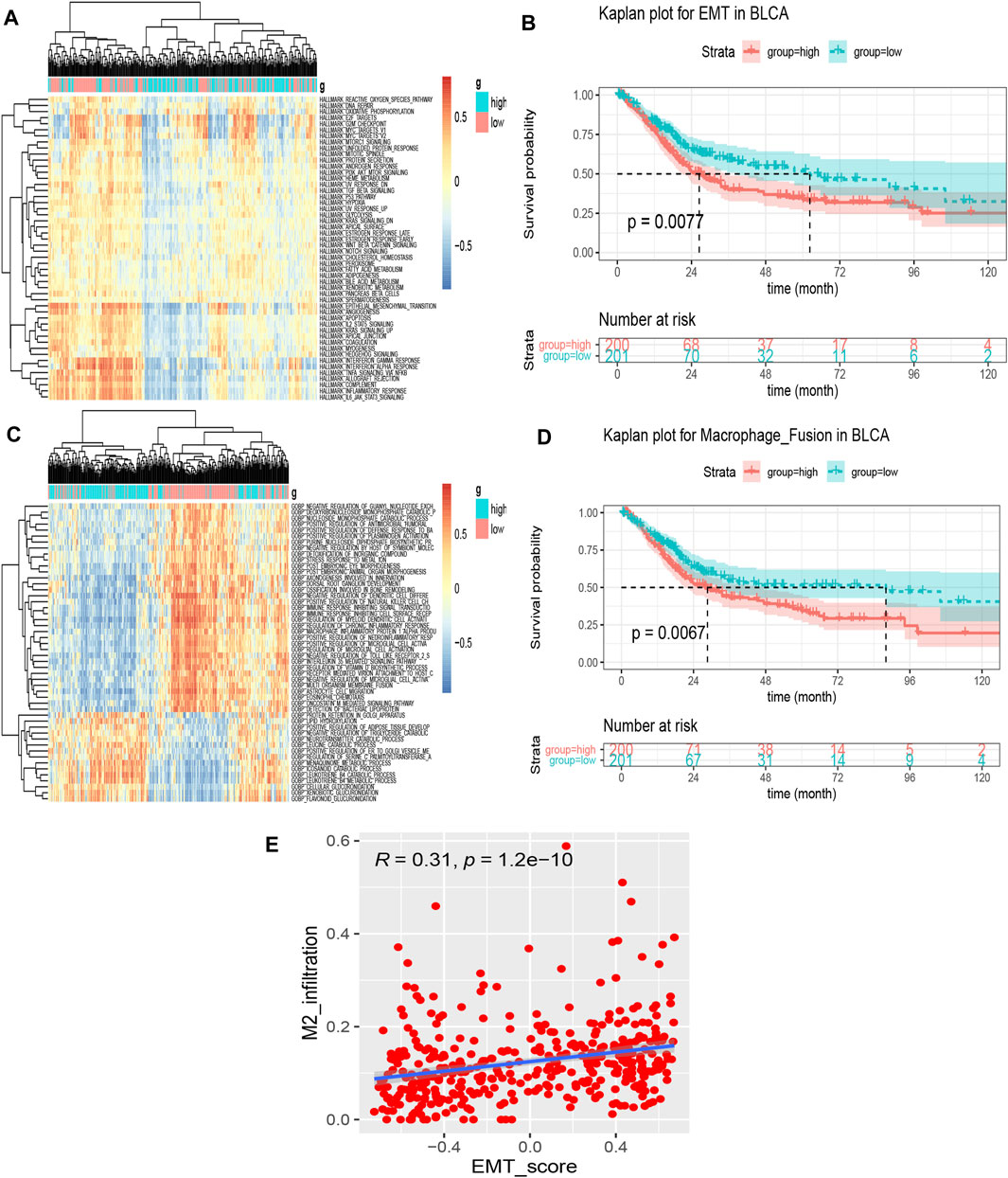
FIGURE 8. Identification of prognoses related to significant differentially enriched signatures. (A) Heatmap of 50 hallmark signature enrichment scores in 401 different bladder cancer samples; (B) Kaplan–Meier survival curve for the epithelial-mesenchymal transition (EMT) signature enrichment score based on the TCGA bladder cancer cohort (group cut-off = median); (C) Heatmap of the top 50 significantly differentially enriched signatures in the biological process signatures between SORL1 high-expression and low-expression groups by GSVA; (D) Kaplan–Meier survival curve for the signature—regulation of macrophage fusion—enrichment score based on the TCGA bladder cancer cohort (group cut-off = median); (E) Spearman’s correlation between the EMT score and M2 macrophage level.
For a set of biological process signatures, we also obtained 251 significantly differentially enriched signatures using the same criterion as the one described above. Figure 8C shows the top 50 differentially enriched pathways. We also applied Kaplan-Meier analysis to these 251 signatures and found that 14 signatures were associated with BLCA patient prognosis (Table 2). When we focused on the results, the signature regulation of macrophage fusion caught our attention. This signature was associated with poor prognosis among bladder cancer patients (p = 0.0067), as shown in Figure 8D.
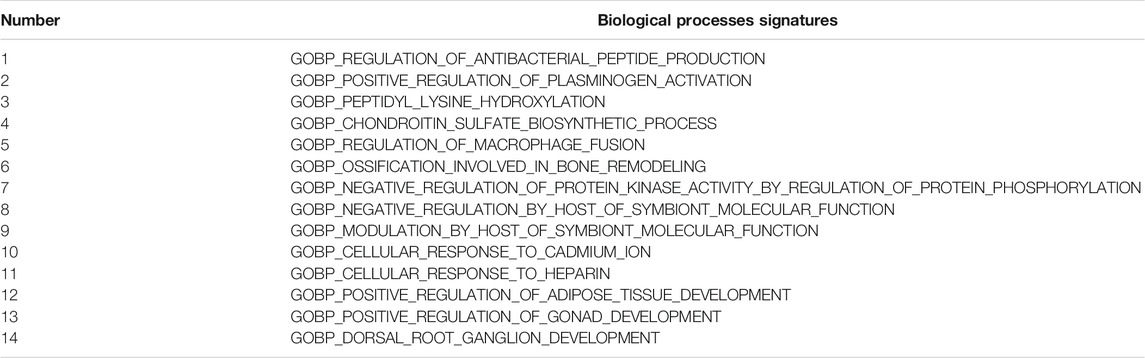
TABLE 2. 14 prognostic pathway signatures of biological processes associated with SORL1 expression in the TCGA bladder cancer cohort.
EMT Scores are Significantly Associated With the Abundance of M2 Macrophages
To further explore the correlation between EMT and M2 macrophages in bladder carcinoma, we performed a correlation analysis on the EMT score and M2 infiltration abundance. Figure 8E depicts a significant positive correlation between M2 macrophage levels and the EMT signature (cor = 0.31, p = 1.2e−10).
Discussion
Scientists widely acknowledge that immunotherapy is remarkably efficient for advanced bladder cancer, the most common malignancy of the urinary system (Kamat et al., 2016; Lopez-Beltran et al., 2021). Therefore, it is necessary to identify an immune-related biomarker to predict the prognosis of BLCA patients. In this study, we explored the genomic alterations present in high-expression PD-L1 and PD-1 samples, as well as the differentially expressed genes between PD-L1/PD-1 high- and low-expression samples. Herein, we aimed to seek out immune-related genomic signature predicting survival outcome of bladder cancer and attempted to explore its value as biomarker in clinical practice.
Firstly, we selected the PD-1/PD-L1 mutational signatures from 16,542 genes when their mutational frequency and the fold change value of mutant to wild met our expectation. Later we performed differential expression analysis between the top 25% of PD-1/PD-L1 expression and the remaining low expression samples, further screening condition of |log2FC| >1.5 with statistical significance determined PD-1/PD-L1 -associated DEGs. By analyzing the overlapping genes, we fortunately identified two immune microenvironment-related gene—sortilin related receptor 1 (SORL1) and TOX high mobility group box family member 3 (TOX3).
It is worth noting that a minor allele of TOX3 has been widely reported to implicate in an elevated risk of breast cancer (Stevens et al., 2011; Man et al., 2020) and be associated with the prognosis of patients (Fasching et al., 2012). Besides, research found TOX3 may play a vital part in the prognosis and treatment of gastric cancer (Zhang et al., 2013) as well as be a therapeutic target for clear renal cell carcinoma (Jiang et al., 2019) and colorectal cancer (Saleh et al., 2020). In TCGA database, it also predicts favorable survival outcomes for BLCA samples with p < 0.05, nevertheless, this underlying biomarker was denied by GSE48075 validation cohort (p > 0.05). Therefore, SORL1 is the only remaining genomic signature with prognostic value.
SORL1, also known as SORLA or LR11, encodes a protein belonging to the vacuolar protein sorting 10 (VPS10) domain-containing receptor family and the low-density lipoprotein receptor (LDLR) family. We found that mutation of this gene is associated with Alzheimer’s disease (Campion et al., 2019) and hematologic neoplasms (Sakai et al., 2012; Kawaguchi et al., 2013; Ohwada et al., 2015). According to Pietilä et al. (2019), the depletion of SORL1 triggers HER2 accumulation in dysfunctional lysosomes, and SORL1-silenced cells showed antiproliferative effects on not only breast cancer but also bladder cancer by regulating oncogenic receptor tyrosine kinase (RTK) signaling. This group further investigated the role of SORL1 in mediating targeted therapy resistance in breast cancer and discovered that SORL1 is necessary for HER2-HER3-driven oncogenic cell growth (Al-Akhrass et al., 2021). However, Michael et al. (2019) discovered that the downregulation of SORL1 expression facilitates tumor growth in a transplant tumor model and relatively low expression predicts a worse prognosis in human breast, lung, and gastric cancer patients. However, we have yet to determine the precise mechanism of this finding.
The results of our study indicate that SORL1 is a protective factor in bladder cancer. A comparatively lower SORL1 expression level increased metastasis risk and was associated with relatively advanced disease stages, while a higher expression level of SORL1 was associated with a favorable prognosis in BLCA patients. The difference in protein expression levels between tumor and normal tissues further demonstrates the reliability of our analysis.
We selected SORL1 from the PD-1/PD-L1-associated genomic signature. Consistently, a negative correlation was observed between the expression level of SORL1 and that of PD-1/PD-L1. PD-1 and PD-L1 are immunosuppressive molecules. Therefore, the upregulation of SORL1 expression inhibited the expression of PD-1/PD-L1 and then reduced the state of immune suppression, reactivating immune cells and allowing them to attack tumor cells. This may explain why SORL1 is a protective factor against bladder cancer.
Another reason for the protective role of SORL1 is that BLCA patients with high SORL1 expression generally showed lower M2 macrophage infiltration levels. GSVA also indicated that regulation of macrophage fusion was a significantly differentially enriched signature associated with SORL1 expression levels. Previous research indicated that M2 macrophages promote angiogenesis, tumor progression and metastasis (Pollard, 2004). Although few studies have covered M2 macrophage’s potential protective effects on bladder cancer (Aljabery et al., 2018), M2 macrophages have been associated with poor survival outcomes for bladder cancer patients in most studies (Ayari et al., 2009; Maniecki et al., 2012; Suriano et al., 2013; Martínez et al., 2017), along with in ours, as shown in Figure 8D. Additionally, GSVA revealed that comparatively lower SORL1 expression is associated with a higher EMT enrichment score, a cellular process during which epithelial cells acquire mesenchymal characteristics as a result of suppression of epithelial phenotypes (Yang et al., 2020). Studies have revealed that macrophages participate in EMT induction (Hu et al., 2021; Yang et al., 2021) and that EMT plays an important role in tumor metastasis and progression, including in bladder cancer (McConkey et al., 2009). Our study also indicated a positive correlation between M2 macrophages and the EMT score. Although no studies have revealed a direct relationship between EMT and M2 macrophages and the prognosis of bladder patients to date, we presume that the upregulation of SORL1 expression may predict a better prognosis among bladder patients as a result of SORL1-mediated inhibition of the M2 macrophage-induced EMT phenotype of tumor cells.
Although we identified a PD-1/PD-L1 genomic signature, SORL1, with favorable prognostic value for bladder cancer patients and explored the possible mechanism, there are several limitations in this study. First, almost all BLCA cohorts were mined from the TCGA database. Thus, we should expand our sample size to include different databases to verify our findings. Second, the comprehensive mechanism by which SORL1 affects BLCA prognosis remains unclear, besides, the detail of gene regulatory network for SORL1 which some literatures have introduced remains unexplored (Liu et al., 2021). Thus, further experimental exploration may contribute to providing direct evidence. Finally, the effects of the SORL1 gene on immunotherapy among bladder cancer patients in the real world are unclear.
Conclusion
In summary, we herein described a novel PD1/PD-L1-associated signature, SORL1, that was associated with favourable outcomes among bladder tumour patients. SORL1, as a protective factor, may alleviate the state of immune suppression and inhibit the M2 macrophage-induced EMT phenotype of tumour cells.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
YX, YC, and HS conceived, designed the study; YC, DC, and HS preformed software operation; YX, YC, XH, and HS performed validation and formal analysis; all authors participated in the data curation; YX, YC, DC, and HS drafted the paper; YX, YC, and HS participated in the review and editing of the paper; YC and LS visualized the data; HS performed supervision and project administration; HS and LS acquired funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Zhejiang Province Public Welfare Technology Application Research Project (number 2021KY782) and the Wenzhou Municipal Science and Technology Bureau (number Y2020151 and Y20190423).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BCG, bacillus Calmette-Guérin; BLCA, bladder urothelial carcinoma; CIBERSORT, cell-type identification by estimating relative subsets of RNA transcripts; CTLA4, cytotoxic T-lymphocyte antigen 4; DEGs, differentially expressed genes; EMT, epithelial-mesenchymal transition; FDA, Food and Drug Administration; FPKM, fragments per kilobase million; GEO, Gene Expression Omnibus; GSVA, gene set variation analysis; HPA, Human Protein Atlas; ICI, immune checkpoint inhibitor; IHC, immunohistochemistry; LDLR, low-density lipoprotein receptor; MSigDB, Molecular Signatures Database; NSCLC, non-small-cell lung cancer; OS, overall survival; PD-1/PD-L1, programmed cell death protein 1/its ligand; RTK: receptor tyrosine kinases; SORL1, sortilin related receptor 1; TCGA, The Cancer Genome Atlas; TPM, transcripts per kilobase million; VPS10, vacuolar protein sorting 10.
References
Al-Akhrass, H., Conway, J. R. W., Poulsen, A. S. A., Paatero, I., Kaivola, J., Padzik, A., et al. (2021). A Feed-Forward Loop between SorLA and HER3 Determines Heregulin Response and Neratinib Resistance. Oncogene 40, 1300–1317. doi:10.1038/s41388-020-01604-5
Aljabery, F., Olsson, H., Gimm, O., Jahnson, S., and Shabo, I. (2018). M2-macrophage Infiltration and Macrophage Traits of Tumor Cells in Urinary Bladder Cancer. Urol. Oncol. Semin. Original Invest. 36, e19–159. e26. doi:10.1016/j.urolonc.2017.11.020-
Ayari, C., LaRue, H., Hovington, H., Decobert, M., Harel, F., Bergeron, A., et al. (2009). Bladder Tumor Infiltrating Mature Dendritic Cells and Macrophages as Predictors of Response to Bacillus Calmette-Guérin Immunotherapy. Eur. Urol. 55, 1386–1396. doi:10.1016/j.eururo.2009.01.040
Bellmunt, J., Mullane, S. A., Werner, L., Fay, A. P., Callea, M., Leow, J. J., et al. (2015). Association of PD-L1 Expression on Tumor-Infiltrating Mononuclear Cells and Overall Survival in Patients with Urothelial Carcinoma. Ann. Oncol. 26, 812–817. doi:10.1093/annonc/mdv009
Campion, D., Charbonnier, C., and Nicolas, G. (2019). SORL1 Genetic Variants and Alzheimer Disease Risk: a Literature Review and Meta-Analysis of Sequencing Data. Acta Neuropathol. 138, 173–186. doi:10.1007/s00401-019-01991-4
Fasching, P. A., Pharoah, P. D., Cox, A., Nevanlinna, H., Bojesen, S. E., Karn, T., et al. (2012). The Role of Genetic Breast Cancer Susceptibility Variants as Prognostic Factors. Hum. Mol. Genet. 21, 3926–3939. doi:10.1093/hmg/dds159
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., et al. (2016). Atezolizumab versus Docetaxel for Patients with Previously Treated Non-small-cell Lung Cancer (POPLAR): a Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. The Lancet 387, 1837–1846. doi:10.1016/S0140-6736(16)00587-0
Hänzelmann, S., Castelo, R., and Guinney, J. (2013). GSVA: Gene Set Variation Analysis for Microarray and RNA-Seq Data. BMC bioinformatics 14, 7. doi:10.1186/1471-2105-14-7
Hu, Z., Cunnea, P., Zhong, Z., Lu, H., Osagie, O. I., Campo, L., et al. (2021). The Oxford Classic Links Epithelial-To-Mesenchymal Transition to Immunosuppression in Poor Prognosis Ovarian Cancers. Clin. Cancer Res. 27, 1570–1579. doi:10.1158/1078-0432.CCR-20-2782
Inman, B. A., Sebo, T. J., Frigola, X., Dong, H., Bergstralh, E. J., Frank, I., et al. (2007). PD-L1 (B7-H1) Expression by Urothelial Carcinoma of the Bladder and BCG-Induced Granulomata. Cancer 109, 1499–1505. doi:10.1002/cncr.22588
Jackson, C. M., Choi, J., and Lim, M. (2019). Mechanisms of Immunotherapy Resistance: Lessons from Glioblastoma. Nat. Immunol. 20, 1100–1109. doi:10.1038/s41590-019-0433-y
Jiang, B., Chen, W., Qin, H., Diao, W., Li, B., Cao, W., et al. (2019). TOX3 Inhibits Cancer Cell Migration and Invasion via Transcriptional Regulation of SNAI1 and SNAI2 in clear Cell Renal Cell Carcinoma. Cancer Lett. 449, 76–86. doi:10.1016/j.canlet.2019.02.020
Kamat, A. M., Hahn, N. M., Efstathiou, J. A., Lerner, S. P., Malmström, P.-U., Choi, W., et al. (2016). Bladder Cancer. The Lancet 388, 2796–2810. doi:10.1016/S0140-6736(16)30512-8
Kawaguchi, T., Ohwada, C., Takeuchi, M., Shimizu, N., Sakaida, E., Takeda, Y., et al. (2013). LR11: a Novel Biomarker Identified in Follicular Lymphoma. Br. J. Haematol. 163, a–n. doi:10.1111/bjh.12467
Krabbe, L.-M., Heitplatz, B., Preuss, S., Hutchinson, R. C., Woldu, S. L., Singla, N., et al. (2017). Prognostic Value of PD-1 and PD-L1 Expression in Patients with High Grade Upper Tract Urothelial Carcinoma. J. Urol. 198, 1253–1262. doi:10.1016/j.juro.2017.06.086
Lamm, D. L. (1992). Optimal BCG Treatment of Superficial Bladder Cancer as Defined by American Trials. Eur. Urol. 21 (Suppl. 2), 12–16. doi:10.1159/000474915
Leinwand, J., and Miller, G. (2020). Regulation and Modulation of Antitumor Immunity in Pancreatic Cancer. Nat. Immunol. 21, 1152–1159. doi:10.1038/s41590-020-0761-y
Liberzon, A., Birger, C., Thorvaldsdóttir, H., Ghandi, M., Mesirov, J. P., and Tamayo, P. (2015). The Molecular Signatures Database Hallmark Gene Set Collection. Cel Syst. 1, 417–425. doi:10.1016/j.cels.2015.12.004
Liu, W., Jiang, Y., Peng, L., Sun, X., Gan, W., Zhao, Q., et al. (2021). Inferring Gene Regulatory Networks Using the Improved Markov Blanket Discovery Algorithm. Interdiscip. Sci. Comput. Life Sci. 1, 1. doi:10.1007/s12539-021-00478-9
Lopez-Beltran, A., Cimadamore, A., Blanca, A., Massari, F., Vau, N., Scarpelli, M., et al. (2021). Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers 13, 131. doi:10.3390/cancers13010131
Man, Y., Zhao, R., Gao, X., Liu, Y., Zhao, S., Lu, G., et al. (2020). TOX3 Promotes Ovarian Estrogen Synthesis: An RNA-Sequencing and Network Study. Front. Endocrinol. 11, 615846. doi:10.3389/fendo.2020.615846
Maniecki, M. B., Etzerodt, A., Ulhøi, B. P., Steiniche, T., Borre, M., Dyrskjøt, L., et al. (2012). Tumor-promoting Macrophages Induce the Expression of the Macrophage-specific Receptor CD163 in Malignant Cells. Int. J. Cancer 131, 2320–2331. doi:10.1002/ijc.27506
Martínez, V. G., Rubio, C., Martínez-Fernández, M., Segovia, C., López-Calderón, F., Garín, M. I., et al. (2017). BMP4 Induces M2 Macrophage Polarization and Favors Tumor Progression in Bladder Cancer. Clin. Cancer Res. 23, 7388–7399. doi:10.1158/1078-0432.CCR-17-1004
Marzagalli, M., Ebelt, N. D., and Manuel, E. R. (2019). Unraveling the Crosstalk between Melanoma and Immune Cells in the Tumor Microenvironment. Semin. Cancer Biol. 59, 236–250. doi:10.1016/j.semcancer.2019.08.002
McConkey, D. J., Choi, W., Marquis, L., Martin, F., Williams, M. B., Shah, J., et al. (2009). Role of Epithelial-To-Mesenchymal Transition (EMT) in Drug Sensitivity and Metastasis in Bladder Cancer. Cancer Metastasis Rev. 28, 335–344. doi:10.1007/s10555-009-9194-7
Michael, I. P., Saghafinia, S., and Hanahan, D. (2019). A Set of microRNAs Coordinately Controls Tumorigenesis, Invasion, and Metastasis. Proc. Natl. Acad. Sci. USA 116, 24184–24195. doi:10.1073/pnas.1913307116
Newman, A. M., Liu, C. L., Green, M. R., Gentles, A. J., Feng, W., Xu, Y., et al. (2015). Robust Enumeration of Cell Subsets from Tissue Expression Profiles. Nat. Methods 12, 453–457. doi:10.1038/nmeth.3337
Ohwada, C., Yamazaki, A., Kawaguchi, T., Sugita, Y., Takeuchi, M., Shimizu, N., et al. (2015). Serum Soluble LR11, a Novel Tumor Derived Biomarker Associated with the Outcome of Patients with Diffuse Large B-Cell Lymphoma. Leuk. Lymphoma 56, 2982–2985. doi:10.3109/10428194.2015.1016930
Pardoll, D. M. (2012). The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 12, 252–264. doi:10.1038/nrc3239
Pietilä, M., Sahgal, P., Peuhu, E., Jäntti, N. Z., Paatero, I., Närvä, E., et al. (2019). SORLA Regulates Endosomal Trafficking and Oncogenic Fitness of HER2. Nat. Commun. 10, 2340. doi:10.1038/s41467-019-10275-0
Pollard, J. W. (2004). Tumour-educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer 4, 71–78. doi:10.1038/nrc1256
Robert, C., Schachter, J., Long, G. V., Arance, A., Grob, J. J., Mortier, L., et al. (2015). Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 372, 2521–2532. doi:10.1056/NEJMoa1503093
Sakai, S., Nakaseko, C., Takeuchi, M., Ohwada, C., Shimizu, N., Tsukamoto, S., et al. (2012). Circulating Soluble LR11/SorLA Levels Are Highly Increased and Ameliorated by Chemotherapy in Acute Leukemias. Clinica Chim. Acta 413, 1542–1548. doi:10.1016/j.cca.2012.06.025
Saleh, R., Taha, R. Z., Toor, S. M., Sasidharan Nair, V., Murshed, K., Khawar, M., et al. (2020). Expression of Immune Checkpoints and T Cell Exhaustion Markers in Early and Advanced Stages of Colorectal Cancer. Cancer Immunol. Immunothercii 69, 1989–1999. doi:10.1007/s00262-020-02593-w
Salmon, H., Remark, R., Gnjatic, S., and Merad, M. (2019). Host Tissue Determinants of Tumour Immunity. Nat. Rev. Cancer 19, 215–227. doi:10.1038/s41568-019-0125-9
Samstein, R. M., Lee, C.-H., Shoushtari, A. N., Hellmann, M. D., Shen, R., Janjigian, Y. Y., et al. (2019). Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 51, 202–206. doi:10.1038/s41588-018-0312-8
Soloway, M. S., Sofer, M., and Vaidya, A. (2002). Contemporary Management of Stage T1 Transitional Cell Carcinoma of the Bladder. J. Urol. 167, 1573–1583. doi:10.1016/s0022-5347(05)65157-9
Stevens, K. N., Vachon, C. M., Lee, A. M., Slager, S., Lesnick, T., Olswold, C., et al. (2011). Common Breast Cancer Susceptibility Loci Are Associated with Triple-Negative Breast Cancer. Cancer Res. 71, 6240–6249. doi:10.1158/0008-5472.CAN-11-1266
Sun, J., Zhang, Z., Bao, S., Yan, C., Hou, P., Wu, N., et al. (2020). Identification of Tumor Immune Infiltration-Associated lncRNAs for Improving Prognosis and Immunotherapy Response of Patients with Non-small Cell Lung Cancer. J. Immunother. Cancer 8, e000110. doi:10.1136/jitc-2019-000110
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Suriano, F., Santini, D., Perrone, G., Amato, M., Vincenzi, B., Tonini, G., et al. (2013). Tumor Associated Macrophages Polarization Dictates the Efficacy of BCG Instillation in Non-muscle Invasive Urothelial Bladder Cancer. J. Exp. Clin. Cancer Res. 32, 87. doi:10.1186/1756-9966-32-87
Uhlen, M., Oksvold, P., Fagerberg, L., Lundberg, E., Jonasson, K., Forsberg, M., et al. (2010). Towards a Knowledge-Based Human Protein Atlas. Nat. Biotechnol. 28, 1248–1250. doi:10.1038/nbt1210-1248
Xylinas, E., Robinson, B. D., Kluth, L. A., Volkmer, B. G., Hautmann, R., Küfer, R., et al. (2014). Association of T-Cell Co-regulatory Protein Expression with Clinical Outcomes Following Radical Cystectomy for Urothelial Carcinoma of the Bladder. Eur. J. Surg. Oncol. (Ejso) 40, 121–127. doi:10.1016/j.ejso.2013.08.023
Yang, C., Dou, R., Wei, C., Liu, K., Shi, D., Zhang, C., et al. (2021). Tumor-derived Exosomal microRNA-106b-5p Activates EMT-Cancer Cell and M2-Subtype TAM Interaction to Facilitate CRC Metastasis. Mol. Ther. 29, 2088–2107. doi:10.1016/j.ymthe.2021.02.006
Yang, J., Antin, P., Berx, G., Blanpain, C., Brabletz, T., Bronner, M., et al. (2020). Guidelines and Definitions for Research on Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cel Biol 21, 341–352. doi:10.1038/s41580-020-0237-9
Zhang, L., Yang, P., Feng, H., Zhao, Q., and Liu, H. (2021a). Using Network Distance Analysis to Predict lncRNA-miRNA Interactions. Interdiscip. Sci. Comput. Life Sci. 13, 535–545. doi:10.1007/s12539-021-00458-z
Zhang, X., Zhu, H., Wu, X., Wang, M., Gu, D., Gong, W., et al. (2013). A Genetic Polymorphism in TOX3 Is Associated with Survival of Gastric Cancer in a Chinese Population. PloS one 8, e72186. doi:10.1371/journal.pone.0072186
Zhang, Z., Bao, S., Yan, C., Hou, P., Zhou, M., and Sun, J. (2021b). Computational Principles and Practice for Decoding Immune Contexture in the Tumor Microenvironment. Brief. Bioinformatics 22, bbaa075. doi:10.1093/bib/bbaa075
Zhao, H., Gu, S., Bao, S., Yan, C., Zhang, Z., Hou, P., et al. (2021). Mechanistically Derived Patient-Level Framework for Precision Medicine Identifies a Personalized Immune Prognostic Signature in High-Grade Serous Ovarian Cancer. Brief. Bioinformatics 22, bbaa069. doi:10.1093/bib/bbaa069
Keywords: SORL1, bladder cancer, PD-L1, M2 macrophage, EMT, TCGA
Citation: Xu Y, Chen D, Shen L, Huang X, Chen Y and Su H (2021) Identification and Mechanism of the PD-1/PD-L1 Genomic Signature SORL1 as Protective Factor in Bladder Cancer. Front. Genet. 12:736158. doi: 10.3389/fgene.2021.736158
Received: 06 July 2021; Accepted: 17 November 2021;
Published: 16 December 2021.
Edited by:
Rahul Kumar, Indian Institute of Technology Hyderabad, IndiaReviewed by:
Qi Zhao, University of Science and Technology Liaoning, ChinaAbhinita S. Mohanty, Memorial Sloan Kettering Cancer Center, United States
Isha Monga, Columbia University Irving Medical Center, United States
Copyright © 2021 Xu, Chen, Shen, Huang, Chen and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Chen, eWkuY2hlbkBraS5zZQ==; Huafang Su, c3VodWFmYW5nQHd6aG9zcGl0YWwuY24=
†These authors have contributed equally to this work
 Yajing Xu1†
Yajing Xu1† Yi Chen
Yi Chen Huafang Su
Huafang Su