- Department of Obstetrics and Gynecology, Zhongnan Hospital of Wuhan University, Wuhan, China
Background: SPP1, secreted phosphoprotein 1, is a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family. Previous studies have proven SPP1 overexpressed in a variety of cancers and can be identified as a prognostic factor, while no study has explored the function and carcinogenic mechanism of SPP1 in cervical cancer.
Methods: We aimed to demonstrate the relationship between SPP1 expression and pan-cancer using The Cancer Genome Atlas (TCGA) database. Next, we validated SPP1 expression of cervical cancer in the Gene Expression Omnibus (GEO) database, including GSE7803, GSE63514, and GSE9750. The receiver operating characteristic (ROC) curve was used to evaluate the feasibility of SPP1 as a differentiating factor by the area under curve (AUC) score. Cox regression and logistic regression were performed to evaluate factors associated with prognosis. The SPP1-binding protein network was built by the STRING tool. Enrichment analysis by the R package clusterProfiler was used to explore potential function of SPP1. The single-sample GSEA (ssGSEA) method from the R package GSVA and TIMER database were used to investigate the association between the immune infiltration level and SPP1 expression in cervical cancer.
Results: Pan-cancer data analysis showed that SPP1 expression was higher in most cancer types, including cervical cancer, and we got the same result in the GEO database. The ROC curve suggested that SPP1 could be a potential diagnostic biomarker (AUC = 0.877). High SPP1 expression was associated with poorer overall survival (OS) (P = 0.032). Further enrichment and immune infiltration analysis revealed that high SPP1 expression was correlated with regulating the infiltration level of neutrophil cells and some immune cell types, including macrophage and DC.
Conclusion: SPP1 expression was higher in cervical cancer tissues than in normal cervical epithelial tissues. It was significantly associated with poor prognosis and immune cell infiltration. Thus, SPP1 may become a promising prognostic biomarker for cervical cancer patients.
1 Introduction
Cervical cancer remains the fourth most common cancer among women and accounts for 527,624 new diagnosed cases and 265,672 deaths in 2018 (Bray et al. (2018)). Cervical cancer continues to be the first or second leading cause of cancer-related death among women for many low- and middle-income countries (LMICs) (Wang et al. (2018)). Persistent HPV infection, especially types 16 and 18, is a high-risk factor but not the only one for cervical cancer (Revathidevi et al. (2020)). Host genetic factors may also be involved in tumor development. The major treatments for cervical cancer patients include surgery, chemotherapy, and radiotherapy. For patients with early-stage cervical cancer, 5-year survival is up to 91.5%, while the treatment of advanced cervical cancer is not ideal (Luan and Wang (2018)). The median survival time of metastatic cervical cancer patients is about 8–13 months, and the 5-year overall survival rate is only around 16.5% (Ferlay et al. (2013); van Meir et al. (2014)). Therefore, it is urgent to find more accurate biomarkers for early detection of cervical cancer and monitoring the disease progression.
Secreted phosphoprotein 1 (SPP1) is a secreted multifunctional phosphoprotein located in 4q13 with seven exons and six introns. SPP1, also known as osteopontin-like protein or early T-lymphocyte activation 1 protein, is a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family which can specifically bind and activate matrix metalloproteinases (MMPs) in cancer (Su et al. (2020)). Its main biological functions are involved in immune response, biomineralization, and tissue remodeling. SPP1 is also related to the growth, proliferation, migration, apoptosis, and chemotaxis of cells. Previous studies have proven that SPP1 is overexpressed in a variety of cancers and can be used to predict the adverse consequences, including ovarian cancer (Zeng et al. (2018)), glioblastoma (Kijewska et al. (2017)), hepatocellular carcinoma (Wang et al. (2019)), and gastric cancer (Song et al. (2019)). Recently, the relationship between the expression of SPP1 and chemotherapy resistance, such as prostate cancer and hepatocellular carcinoma, has also attracted the attention of researchers (Liu et al. (2016); Pang et al. (2019)), while no study has explored the correlation between SPP1 and cervical cancer. Therefore, our study aimed to explore the expression of SPP1 in cervical cancer tissues and its potential clinical values.
In our research, we utilized the cervical cancer RNA-seq data from The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), and Genotype-Tissue Expression databases to compare the differential expression of SPP1 between normal cervical tissues and cervical cancer samples. Next, we investigated the relationship between SPP1 expression levels and clinical pathological features of cervical cancer. Furthermore, we explored the prognostic value of SPP1 in cervical cancer. Besides, we performed gene enrichment analysis to reveal its potential functions. Finally, we analyzed the relationship between SPP1 expression and immune infiltration and comprehensively explored its mechanism in inducing and promoting cervical cancer.
2 Materials and Methods
2.1 RNA Sequencing Data Collection and Analysis
To evaluate the SPP1 expression level in pan-cancer, we downloaded data from the UCSC Xena (https://xenabrowser.net/datapages/). We selected samples from the TCGA database for the analysis of SPP1 expression in tumor tissues, while the combined analysis of TCGA and Genotype-Tissue Expression (GTEx) databases was used for the normal tissue samples. GSE7803 (Platform: GPL96), GSE63514 (Platform: GPL570), and GSE9750 (Platform: GPL96) downloaded from GEO were used to obtain cervical cancer microarray data.
2.2 Correlation and Gene Set Enrichment Analysis
We used data collected from TCGA to perform correlation analysis between SPP1 and other mRNAs in cervical cancer. To demonstrate the biological function of SPP1, we selected the top 100 genes most positively correlated with SPP1 for enrichment analysis. EnrichGO function in the R package “clusterProfiler” was used to perform gene ontology (GO) enrichment, including BP, CC, and MF. Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed using the EnrichKEGG function of the R package “clusterProfiler.”
2.3 Survival Prognosis Analysis
We used the R package “survival” (version 3.6) to obtain the overall survival (OS) survival plots of SPP1. Selecting the cutoff value of 50% as the dividing threshold, the cohorts were divided into high-expression and low-expression groups. To evaluate the value of SPP1 in predicting the prognosis of cervical cancer patients, we used the R package (version 3.6.3) “ROC” for analysis and “ggplot2” for visual.
2.4 Immune Cell Infiltration Analysis
We used the single-sample GSEA (ssGSEA) method from the R package GSVA (version 3.6) and Tumor Immune Estimation Resource (TIMER) database (http://timer.cistrome.org/) to comprehensively investigate molecular characterization of tumor–immune interactions in cervical cancer. In the literature, we examined the impact of SPP1 expression on immune cell infiltration using gene expression profiling data. To investigate the correlation between SPP1 expression and the abundances of tumor-infiltrating immune cells, p-values were calculated using the Wilcoxon rank-sum and Spearman’s rank correlation tests.
3 Results
3.1 The mRNA Expression Analysis of SPP1 in Pan-Cancer
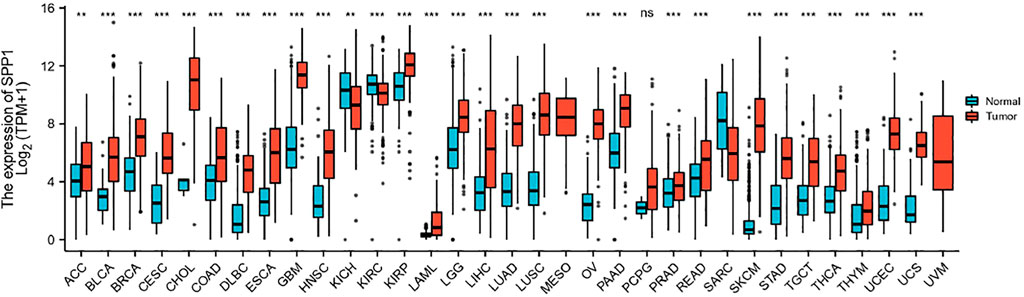
Data downloaded from TCGA and GTEx were used to analyze SPP1 expression in 33 types of cancer. The result revealed that SPP1 was overexpressed in most cancers, including ACC, BLCA, BRCA, CESC, CHOL, COAD, DLBC, ESCA, GBM, HNSC, KIRP, LAML, LGG, LIHC, LUAD, LUSC, OV, PAAD, PRAD, READ, SKCM, STAD, TGCT, THCA, THYM, UCEC, and UCS. However, the expression of SPP1 was low in KICH and KIRC (Figure 1). Furthermore, we assessed SPP1 expression in cervical cancer in the GEO database, including GSE7803 (Platform: GPL96), GSE63514 (Platform: GPL570), and GSE9750, and the results confirmed that SPP1 was overexpressed in cervical cancer tissues (Figures 2A–C). Additionally, we performed the receiver operating characteristic (ROC) curve to evaluate the feasibility of the SPP1 expression level to distinguish cervical cancer tissues from normal cervical tissues. The area under the ROC curve (AUC) was 0.877, representing the quality of the test.
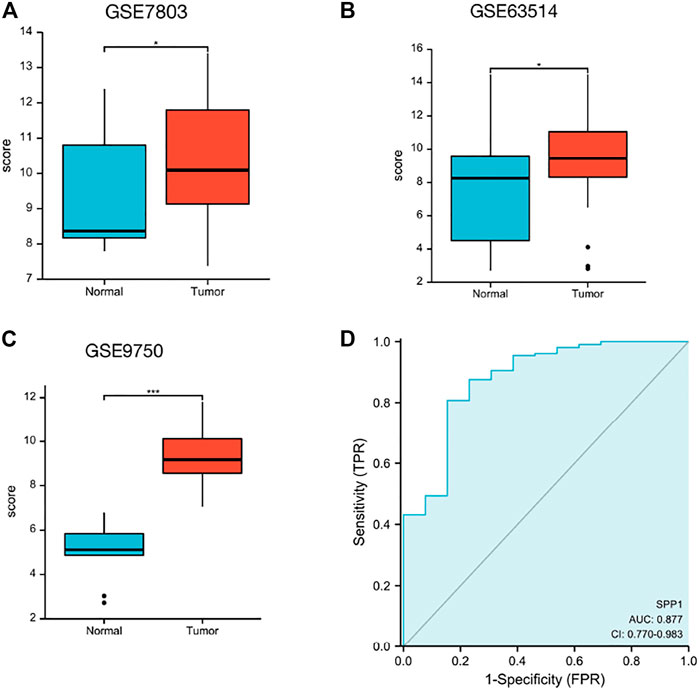
FIGURE 2. SPP1 expression in the GEO database. (A) SPP1 expression in normal and tumor tissues in cervical cancer from GSE7803. (B) SPP1 expression in normal cervical epithelial and cervical cancer tissues from GSE63514. (C) SPP1 expression in normal cervical tissues and cervical cancer epithelial component from GSE9750. (D) ROC curve of SPP1 in cervical cancer. X-axis represents false-positive rates, and Y-axis represents true-positive rates.
3.2 Clinical Relevance of the SPP1 Expression in Cervical Cancer Patients
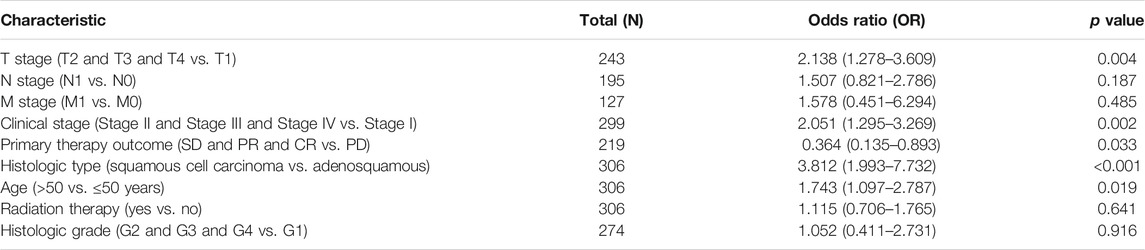
The characteristics of 306 primary cervical cancer patients with both clinical and gene expression data were downloaded from TCGA database. With the cutoff value of 50% as the dividing threshold, the patients were divided into a high–SPP1 expression group (n = 153) and a low–SPP1 expression group (n = 153). The correlation of the SPP1 expression level and patients’ clinicopathologic characteristics was explored. We found that SPP1 expression was significantly associated with T stage (P = 0.02), clinical stage (P = 0.02), and histologic type (P
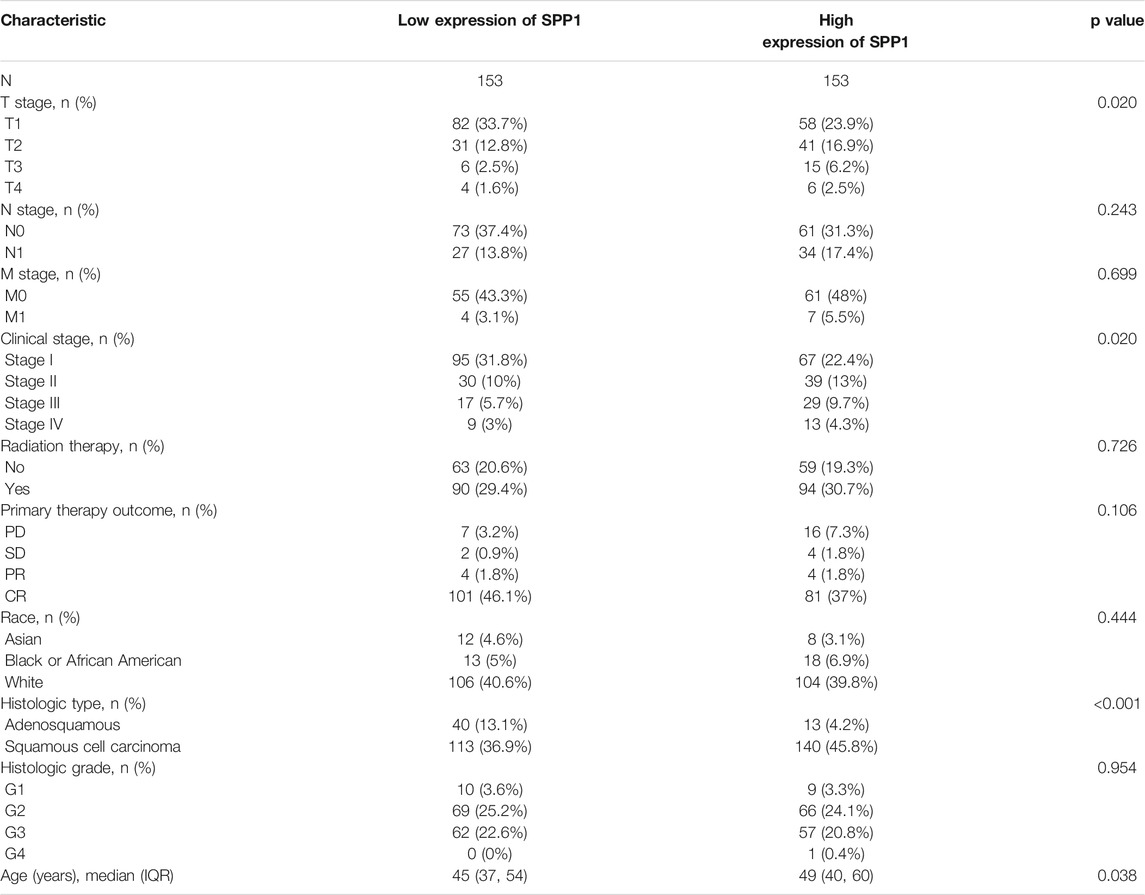
TABLE 1. Correlation analyzed between SPP1 expression and clinicopathologic characteristics in cervical cancer based on TCGA database.
We conducted the logistic regression method to further analyze the relationship between the SPP1 expression level and the clinicopathologic characteristics of cervical cancer. The results showed that the expression level of SPP1 was significantly associated with T stage (P = 0.004), clinical stage (P = 0.002), primary therapy outcome (P = 0.033), histologic type (P
Association Between SPP1 Expression and Cancer Patient Survival Prognosis
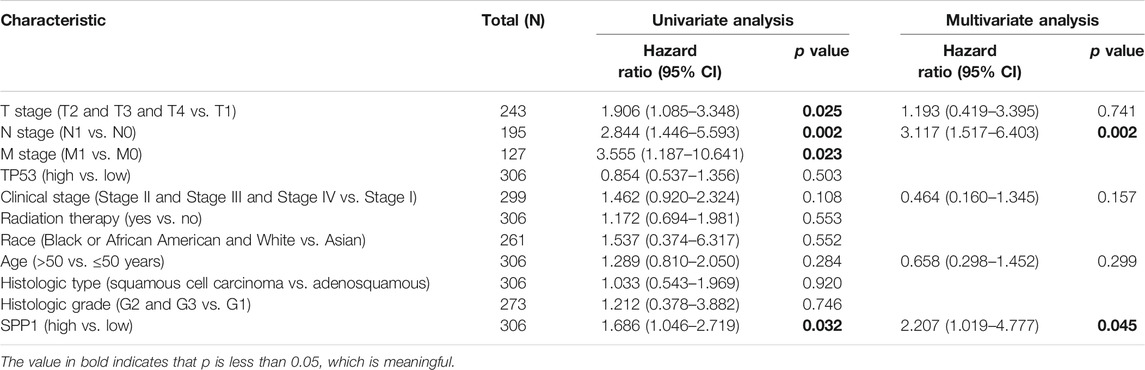
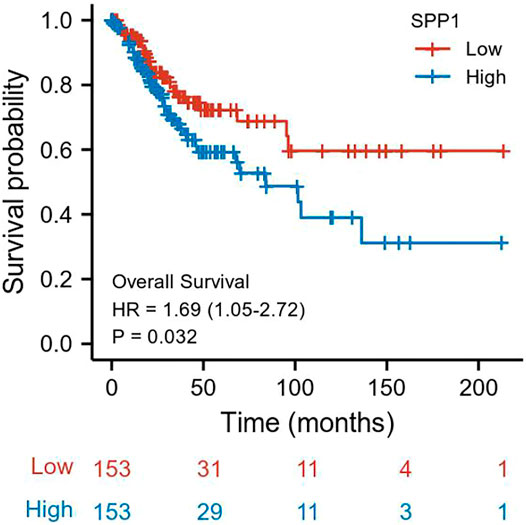
We performed univariate and multivariate Cox analyses of overall survival (OS) in cervical cancer patients, and results are shown in Table 3. In univariate Cox analysis of SPP1, T stage (P = 0.025), N stage (P = 0.002), M stage (P = 0.023), and SPP1 expression (P = 0.032) were associated with overall survival (OS) in cervical cancer patients. In the multivariate Cox model, we found that N stage (P = 0.002) and SPP1 expression (P = 0.045) were still relevant to worse prognosis. Furthermore, we investigated the relationship between SPP1 expression and overall survival (OS) of cervical cancer patients. According to the KM plot, patients with higher SPP1 mRNA expression showed poorer prognosis than the lower group (HR = 1.69, 95% CI: 1.05–2.72, P = 0.032) (Figure 3). Thus, SPP1 may become a promising prognostic biomarker for cervical cancer patients.
3.4 Correlation and SPP1-Related Gene Enrichment Analysis
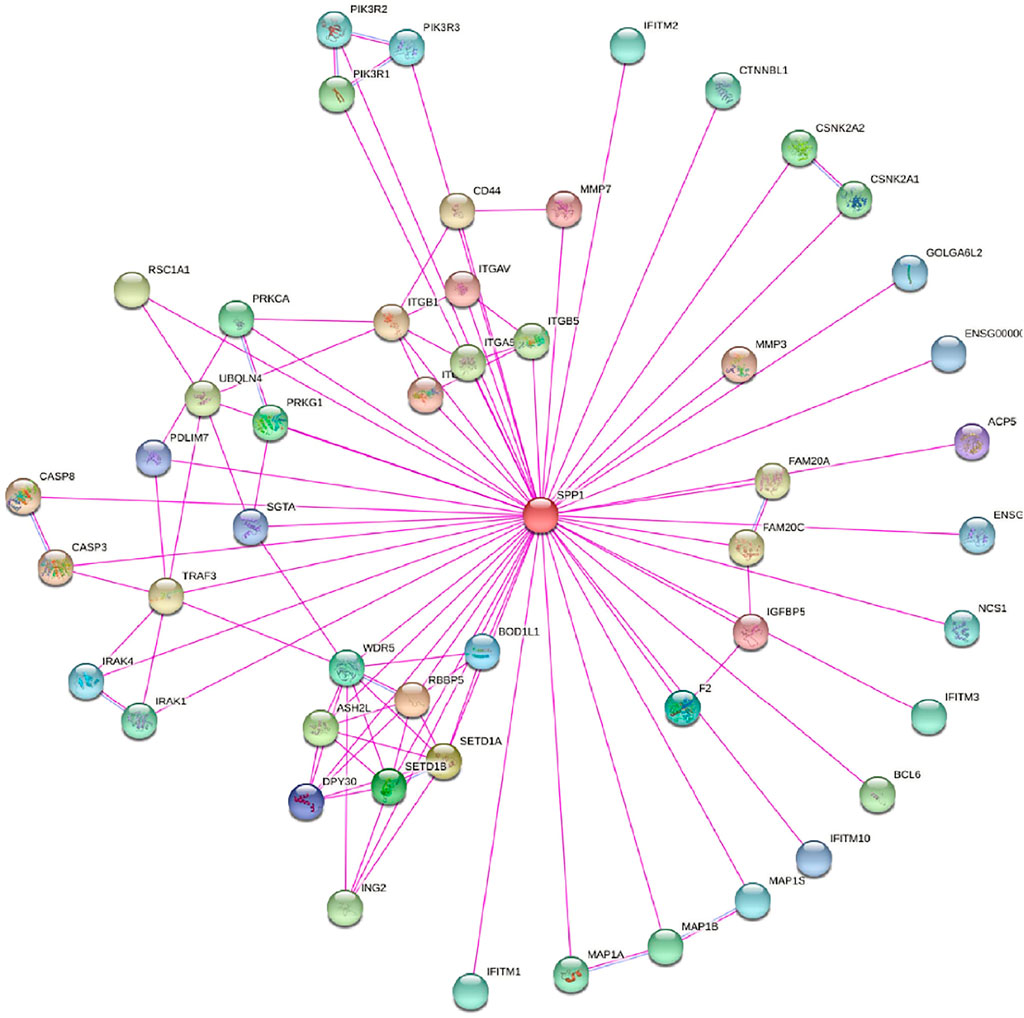
In this study, we only considered physically binding protein interactions and obtained 50 experimental supported SPP1-binding proteins from the STRING network (Figure 4). We downloaded data from TCGA database to further investigate the function of SPP1 and search SPP1 expression–correlated genes for related pathway analysis. We obtained the top 100 most positively correlated genes with SPP1 for GO and KEGG enrichment analysis by the “clusterProfile” R package. The GO analysis data showed that most of the genes were associated with neutrophil degranulation, neutrophil activation involved in immune response, neutrophil activation, and neutrophil-mediated immunity (Figure 5A). The KEGG data suggested that the “phagosome” may be related to the carcinogenic mechanism of SPP1 (Figure 5B).
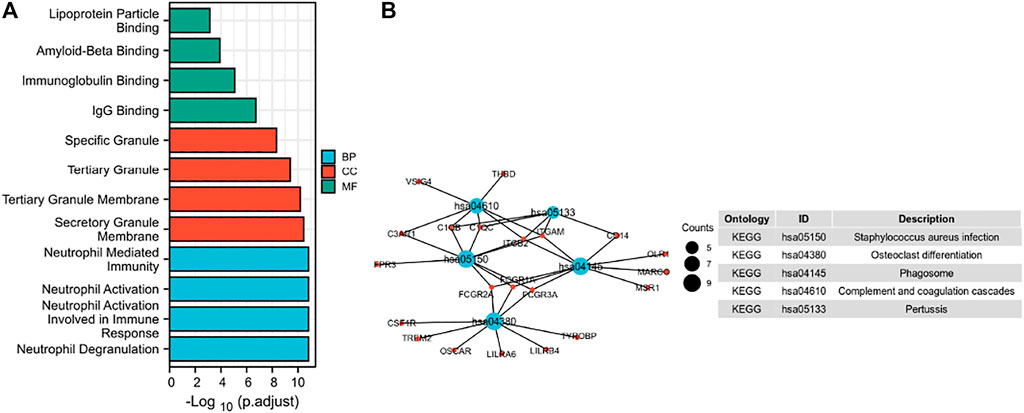
FIGURE 5. Function and pathway enrichment analysis of SPP1 in cervical cancer. (A) Significant Gene Ontology terms (including BP, MF, and CC) of the top 100 genes most positively associated with SPP1. (B) Significant KEGG pathway of the top 100 genes most positively associated with SPP1.
3.5 Relationship Between SPP1 Expression and Immune Cell Infiltration
Through the previous enrichment analysis, we found that SPP1 was mainly related to neutrophils and phagosomes. We hypothesized that there might be some relationship between SPP1 and immune cells. Thus, we further assessed whether the SPP1 expression level was associated with immune cell infiltration. We used ssGSEA from the R package with Spearman’s r to investigate the potential association between the SPP1 expression level and 24 types of immune cells. The result revealed that SPP1 expression had significant correlation with iDC, macrophages, neutrophils, NK CD56 bright cells, Th1 cells, DC, pDC, mast cells, and Treg cells (Figure 6). Further research showed that SPP1 expression was positively correlated with infiltration levels of iDC (Figure 7A) (r = 0.250, P
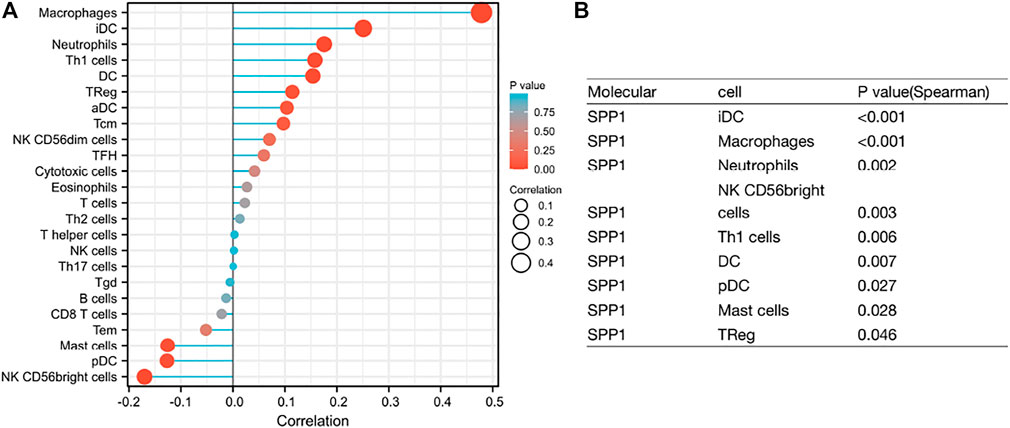
FIGURE 6. (A) Lollipop chart of SPP1 expression level in 24 immune cells. (B) The immune cell infiltration associated with SPP1 expression, P < 0.05, represents a significant result.
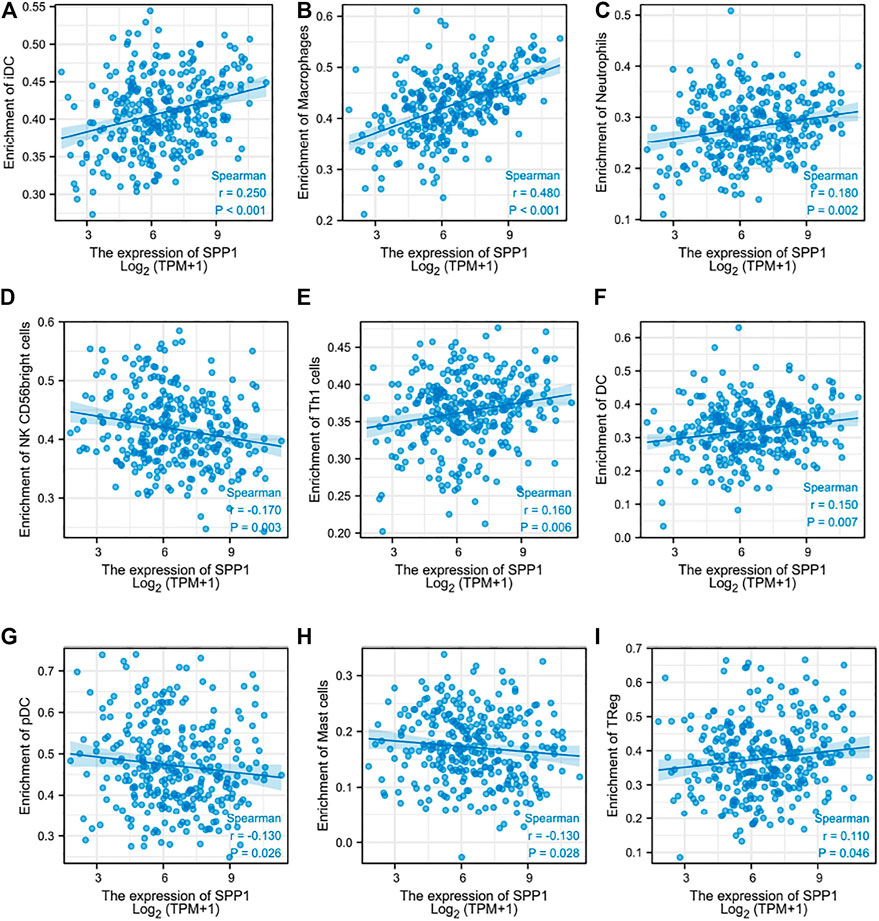
FIGURE 7. Correlation between SPP1 expression and immune cell infiltration. (A–I) Correlation between SPP1 expression and iDC, macrophages, neutrophils, NK CD56 bright cells, Th1 cells, DC, pDC, mast cells, and Treg cells.
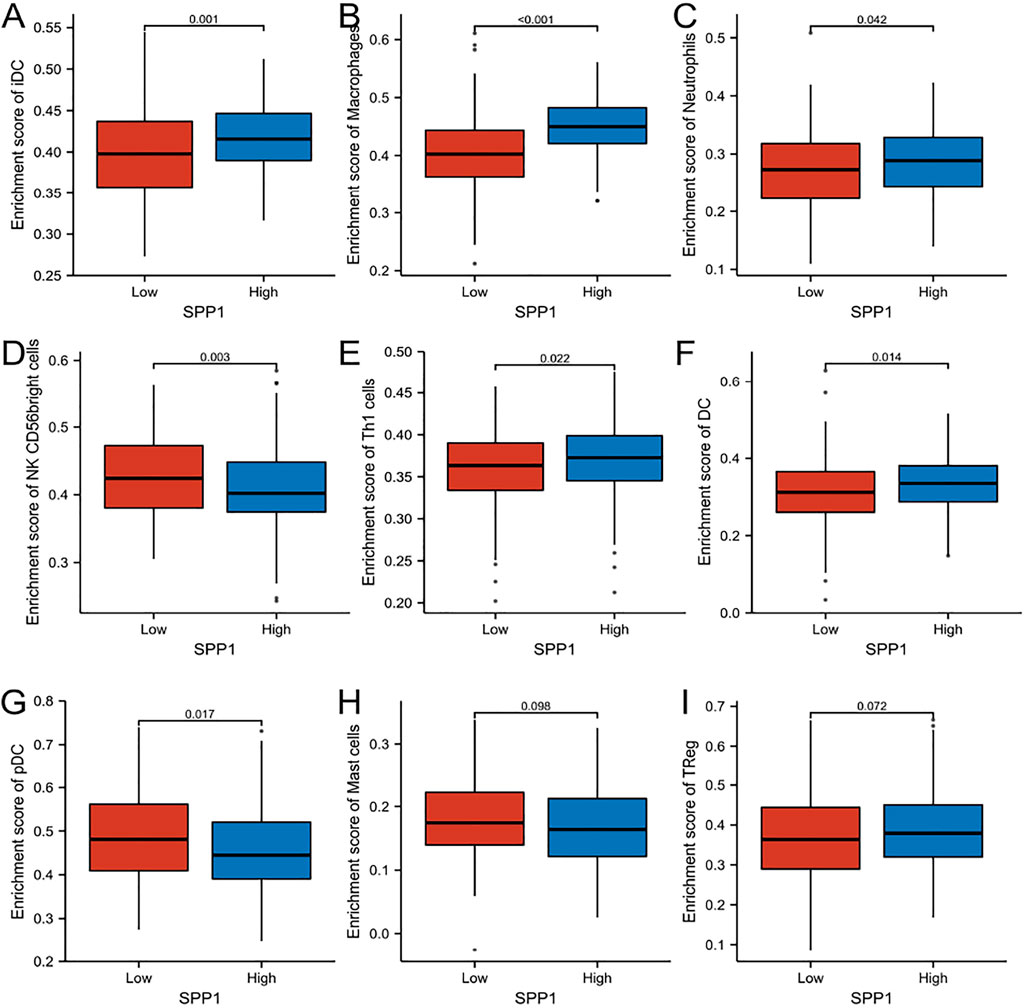
FIGURE 8. Comparison of immune cells between high– and low–SPP1 expression groups. (A–I) Histogram showing the difference of iDC, macrophages, neutrophils, NK CD56 bright cells, Th1 cells, DC, pDC, mast cells, and Treg cell infiltration level between high–and low–SPP1 expression groups.
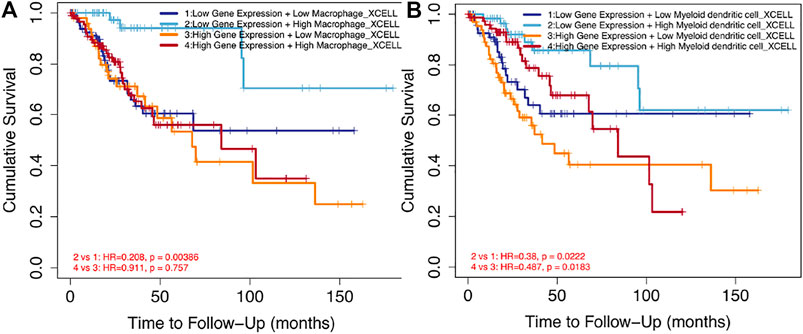
FIGURE 9. Impact of immune cell infiltration on prognosis in cervical cancer patients. (A) Clinical survival outcome of cervical cancer patients in the high-macrophage group. (B) Clinical survival outcome of cervical cancer patients in the high–DC cell group.
4 Discussion
Invasive cervical cancer remains the leading cause of cancer death among women worldwide (Shen et al. (2020)). Thus, it is necessary to find more accurate biomarkers to detect at an early stage and monitor disease progression. According to the previous studies, SPP1 is overexpressed in various cancer types (Xu et al. (2017); Choe et al. (2018); Zhang et al. (2020)) and identified as a prognostic factor (Li et al. (2018); Chen J et al. (2019); Guo et al. (2020)), while to our knowledge, no study has explored the relationship of SPP1 expression and cervical cancer. In our study, we attempted to explore the potential mechanism of SPP1 in promoting cervical cancer and its feasibility as a molecular biomarker.
In pan-cancer analysis, we found that SPP1 was upregulated in most cancer types. Further exploration revealed that higher SPP1 expression was associated with reduced overall survival (OS) in cervical cancer patients. We performed logistic regression to evaluate the relationship between the SPP1 expression level and the clinicopathologic characteristics of cervical cancer. The result showed that SPP1 was significantly correlated with clinical stages. In addition, univariate and multivariate Cox analyses indicated that SPP1 was an independent factor to predict prognosis of patients. All these aforementioned results and ROC analysis suggest that SPP1 may be a promising prognostic biomarker for cervical cancer patients.
The tumor microenvironment (TME), composed of various types of immune cells, played an important role in tumor progression, metastasis, and treatment resistance (Usui et al. (2016)). The composition of tumor-infiltrating immune cells strongly influenced the tumor microenvironment and the behavior of the tumor. Our gene enrichment analysis revealed that the main biological function of SPP1 was mainly involved in immune response. We next confirmed that SPP1 expression correlated with immune cell infiltration. Hence, we hypothesized that SPP1 may affect the tumor microenvironment by changing proportions of specific immune cell types, thereby promoting tumor progression and metastasis. It was, indeed, the case that SPP1 had recently been shown to be an important component in maintaining the tumor microenvironment in AML (Ruvolo et al. (2019)). Our research demonstrated the significant positive correlation between macrophages and the expression of SPP1. Macrophages are important components of the tumor microenvironment, and tumor-associated macrophages play complex roles in cancer pathophysiology (Gibson et al. (2019)). A previous study found that SPP1 was involved in the function, migration, and differentiation of macrophages (Zhang et al. (2017); Wei et al. (2019); Jaitin et al. (2019); Srirussamee et al. (2019)). A recent study also showed that SPP1 was essential for M2-like macrophage, the tumor-associated macrophage, and promoted tumor growth (Chen P et al. (2019)). Furthermore, we found that the increased level of macrophages and DC infiltration were correlated with poor prognosis. Our results were supported by the findings of similar studies about this topic (Long et al. (2016); Ndiaye et al. (2019)). Certainly, the tumor microenvironment had a high level of complexity in its regulation; other immune cell types in the tumor microenvironment may also influence tumor cell survival, including iDC, neutrophils, NK CD56 bright cells, Th1 cells, DC, and pDC. Future studies were needed to further explore the relationship between SPP1 expression and these cells.
In conclusion, we demonstrated that SPP1 expression was upregulated in cervical cancer and significantly related to poor survival outcome. In addition to this, SPP1 might participate in the occurrence and development of cervical cancer by influencing the infiltration level of immune cells. Therefore, our study revealed the role of SPP1 in cervical cancer and identified a promising prognostic biomarker.
Although our study is the first work to explore the relationship between SPP1 expression and cervical cancer, it also has some limitations. First, all of the data analyzed by bioinformatics methods in this study were downloaded directly from public databases, so it requires further validation by experimental investigations; second, the number of normal samples used as controls was considerably different from that of patients with tumor in the TCGA database; therefore, further studies based on an equal balance of sample size are necessary. Third, further validation studies with a long-term follow-up and larger cohorts of patients are needed to definitely validate SPP1 as an OS predictor. Last but not least, our study laid the foundation for detailed studies of the correlation between SPP1 and the tumor-associated immune microenvironment. However, more studies are required to explore the hypothesis in depth.
Statement
The cervical cancer cell lines (Siha and Hela) present in this study were obtained from the Scientific Research Center of Zhongnan Hospital of Wuhan University. And normal cervical epithelial cell (END1) was donated by Wuhan University Basic Medical College.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found freely from TCGA data portal (https://portal.gdc.cancer.gov/) and GEO database (https://www.ncbi.nlm.nih.gov/geo/).
Author Contributions
KZ and WZ contributed to the study conception and design. Material preparation, data collection, and analysis were performed by KZ and ZM. KZ contributed to the literature search. The first draft of the manuscript was written by KZ, and all authors commented on previous versions of the manuscript. WZ reviewed the article and gave suggestions on the revision of the article. All authors read and approved the final manuscript.
Funding
Our research was supported by the project of improving the ability of diagnosis and treatment of difficult diseases in Zhongnan Hospital of Wuhan University. The project number is ZLYNXM202019.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21492
Chen J, J., Hou, C., Zheng, Z., Lin, H., Lv, G., and Zhou, D. (2019). Identification of Secreted Phosphoprotein 1 (Spp1) as a Prognostic Factor in Lower-Grade Gliomas. World Neurosurg. 130, e775. doi:10.1016/j.wneu.2019.06.219
Chen P, P., Zhao, D., Li, J., Liang, X., Li, J., Chang, A., et al. (2019). Symbiotic Macrophage-Glioma Cell Interactions Reveal Synthetic Lethality in Pten-Null Glioma. Cancer cell 35, 868–884. doi:10.1016/j.ccell.2019.05.003
Choe, E. K., Yi, J. W., Chai, Y. J., and Park, K. J. (2018). Upregulation of the Adipokine Genes Adipor1 and Spp1 Is Related to Poor Survival Outcomes in Colorectal Cancer. J. Surg. Oncol. 117, 1833–1840. doi:10.1002/jso.25078
Ferlay, J., Steliarova-Foucher, E., Lortet-Tieulent, J., Rosso, S., Coebergh, J. W. W., Comber, H., et al. (2013). Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries in 2012. Eur. J. Cancer 49 (6), 1374–1403. doi:10.1016/j.ejca.2012.12.027
Gibson, E. M., Nagaraja, S., Ocampo, A., Tam, L. T., Wood, L. S., Pallegar, P. N., et al. (2019). Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 176, 43–55. doi:10.1016/j.cell.2018.10.049
Guo, Z., Huang, J., Wang, Y., Liu, X. P., Li, W., Yao, J., et al. (2020). Analysis of Expression and its Clinical Significance of the Secreted Phosphoprotein 1 in Lung Adenocarcinoma. Front. Genet. 11, 547. doi:10.3389/fgene.2020.00547
Jaitin, D. A., Adlung, L., Thaiss, C. A., Weiner, A., Li, B., Descamps, H., et al. (2019). Lipid-associated Macrophages Control Metabolic Homeostasis in a Trem2-dependent Manner. Cell 178, 686–698. doi:10.1016/j.cell.2019.05.054
Kijewska, M., Kocyk, M., Kloss, M., Stepniak, K., Korwek, Z., Polakowska, R., et al. (2017). The Embryonic Type of Spp1 Transcriptional Regulation Is Re-activated in Glioblastoma. Oncotarget 8, 16340–16355. doi:10.18632/oncotarget.14092
Li, S., Yang, R., Sun, X., Miao, S., Lu, T., Wang, Y., et al. (2018). Identification of Spp1 as a Promising Biomarker to Predict Clinical Outcome of Lung Adenocarcinoma Individuals. Gene 679, 398–404. doi:10.1016/j.gene.2018.09.030
Liu, G., Fan, X., Tang, M., Chen, R., Wang, H., Jia, R., et al. (2016). Osteopontin Induces Autophagy to Promote Chemo-Resistance in Human Hepatocellular Carcinoma Cells. Cancer Lett. 383 (2), 171–182. doi:10.1016/j.canlet.2016.09.033
Long, K. B., Gladney, W. L., Tooker, G. M., Graham, K., Fraietta, J. A., and Beatty, G. L. (2016). IFNγ and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 6 (4), 400–413. doi:10.1158/2159-8290.cd-15-1032
Luan, X., and Wang, Y. (2018). Lncrna Xloc_006390 Facilitates Cervical Cancer Tumorigenesis and Metastasis as a Cerna against Mir-331-3p and Mir-338-3p. J. Gynecol. Oncol. 29, e95. doi:10.3802/jgo.2018.29.e95
Ndiaye, P. D., Dufies, M., Giuliano, S., Douguet, L., Grépin, R., Durivault, J., et al. (2019). Vegfc Acts as a Double-Edged Sword in Renal Cell Carcinoma Aggressiveness. Theranostics 9, 661–675. doi:10.7150/thno.27794
Pang, X., Xie, R., Zhang, Z., Liu, Q., Wu, S., and Cui, Y. (2019). Identification of Spp1 as an Extracellular Matrix Signature for Metastatic Castration-Resistant Prostate Cancer. Front. Oncol. 9, 924. doi:10.3389/fonc.2019.00924
Revathidevi, S., Murugan, A. K., Nakaoka, H., Inoue, I., and Munirajan, A. K. (2020). Apobec: A Molecular Driver in Cervical Cancer Pathogenesis. Cancer Lett. 496, 104–116. doi:10.1016/j.canlet.2020.10.004
Ruvolo, P. P., Hu, C. W., Qiu, Y., Ruvolo, V. R., Go, R. L., Hubner, S. E., et al. (2019). Lgals3 Is Connected to Cd74 in a Previously Unknown Protein Network that Is Associated with Poor Survival in Patients with Aml. EBioMedicine 44, 126–137. doi:10.1016/j.ebiom.2019.05.025
Shen, S., Zhang, S., Liu, P., Wang, J., and Du, H. (2020). Potential Role of Micrornas in the Treatment and Diagnosis of Cervical Cancer. Cancer Genet. 248-249, 25–30. doi:10.1016/j.cancergen.2020.09.003
Song, S. Z., Lin, S., Liu, J. N., Zhang, M. B., Du, Y. T., Zhang, D. D., et al. (2019). Retracted : Targeting of SPP1 by microRNA‐340 Inhibits Gastric Cancer Cell Epithelial-Mesenchymal Transition through Inhibition of the PI3K/AKT Signaling Pathway. J. Cel Physiol. 234, 18587–18601. doi:10.1002/jcp.28497
Srirussamee, K., Mobini, S., Cassidy, N. J., and Cartmell, S. H. (2019). Direct Electrical Stimulation Enhances Osteogenesis by Inducing Bmp2 and Spp1 Expressions from Macrophages and Preosteoblasts. Biotechnol. Bioeng. 116, 3421–3432. doi:10.1002/bit.27142
Su, X., Xu, B., Zhou, D.-L., Ye, Z., He, H., Yang, X. H., et al. (2020). Polymorphisms in Matricellular Spp1 and Sparc Contribute to Susceptibility to Papillary Thyroid Cancer. Genomics 112, 4959. doi:10.1016/j.ygeno.2020.09.018
Usui, T., Sakurai, M., Enjoji, S., Kawasaki, H., Umata, K., Ohama, T., et al. (2016). Establishment of a Novel Model for Anticancer Drug Resistance in Three-Dimensional Primary Culture of Tumor Microenvironment. Stem Cell Int. 2016, 7053872. doi:10.1155/2016/7053872
van Meir, H., Kenter, G., Burggraaf, J., Kroep, J., Welters, M., Melief, C., et al. (2014). The Need for Improvement of the Treatment of Advanced and Metastatic Cervical Cancer, the Rationale for Combined Chemo-Immunotherapy. Anticancer Agents Med. Chem. 14 (2), 190–203. doi:10.2174/18715206113136660372
Wang, L., Zhao, Y., Wang, Y., and Wu, X. (2018). The Role of Galectins in Cervical Cancer Biology and Progression. Biomed. Res. Int. 2018, 2175927. doi:10.1155/2018/2175927
Wang, J., Hao, F., Fei, X., and Chen, Y. (2019). SPP1 Functions as an Enhancer of Cell Growth in Hepatocellular Carcinoma Targeted by Mir-181c. Am. J. Transl. Res. 11 (11), 6924–6937.
Wei, J., Marisetty, A., Schrand, B., Gabrusiewicz, K., Hashimoto, Y., Ott, M., et al. (2019). Osteopontin Mediates Glioblastoma-Associated Macrophage Infiltration and Is a Potential Therapeutic Target. J. Clin. Invest. 129, 137–149. doi:10.1172/JCI121266
Xu, C., Sun, L., Jiang, C., Zhou, H., Gu, L., Liu, Y., et al. (2017). Spp1, Analyzed by Bioinformatics Methods, Promotes the Metastasis in Colorectal Cancer by Activating Emt Pathway. Biomed. Pharmacother. 91, 1167–1177. doi:10.1016/j.biopha.2017.05.056
Zeng, B., Zhou, M., Wu, H., and Xiong, Z. (2018). Spp1 Promotes Ovarian Cancer Progression via Integrin β1/fak/akt Signaling Pathway. Onco. Targets Ther. 11, 1333–1343. doi:10.2147/ott.s154215
Zhang, Y., Du, W., Chen, Z., and Xiang, C. (2017). Upregulation of Pd-L1 by Spp1 Mediates Macrophage Polarization and Facilitates Immune Escape in Lung Adenocarcinoma. Exp. Cel Res. 359, 449–457. doi:10.1016/j.yexcr.2017.08.028
Zhang, Q., Li, L., Lai, Y., and Zhao, T. (2020). Silencing of Spp1 Suppresses Progression of Tongue Cancer by Mediating the Pi3k/akt Signaling Pathway. Technol. Cancer Res. Treat. 19, 1533033820971306. doi:10.1177/1533033820971306
Glossary
aDC activated DC
ACC adrenocortical carcinoma
BLCA bladder urothelial carcinoma
BRCA breast invasive carcinoma
CESC cervical squamous cell carcinoma and endocervical adenocarcinoma
CHOL cholangiocarcinoma
COAD colon adenocarcinoma
DLBC lymphoid neoplasm diffuse large B-cell lymphoma
ESCA esophageal carcinoma
GBM glioblastoma multiforme
GEO Gene Expression Omnibus
GO Gene Ontology
HNSC head and neck squamous cell carcinoma
iDC immature DC
KICH kidney chromophobe
KIRC kidney renal clear cell carcinoma
KIRP kidney renal papillary cell carcinoma
KEGG Kyoto Encyclopedia of Genes and Genomes
LAML acute myeloid leukemia
LGG lower grade glioma
LIHC liver hepatocellular carcinoma
LUAD lung adenocarcinoma
LUSC lung squamous cell carcinoma
OS overall survival
OV ovarian serous cystadenocarcinoma
pDC plasmacytoid DC
PAAD pancreatic adenocarcinoma
PRAD prostate adenocarcinoma
READ rectum adenocarcinoma
SKCM skin cutaneous melanoma
STAD stomach adenocarcinoma
SPP1 secreted phosphoprotein 1
Tcm T central memory
Tem T effector memory
Tfh T follicular helper
Tgd T gamma delta.
TCGA The Cancer Genome Atlas
TGCT testicular germ cell tumor
THCA thyroid carcinoma
THYM thymoma
UCEC uterine corpus endometrial carcinoma
UCS uterine carcinosarcoma
Keywords: SPP1, biomarker, cervical cancer, prognosis, immune infiltration
Citation: Zhao K, Ma Z and Zhang W (2022) Comprehensive Analysis to Identify SPP1 as a Prognostic Biomarker in Cervical Cancer. Front. Genet. 12:732822. doi: 10.3389/fgene.2021.732822
Received: 29 June 2021; Accepted: 03 December 2021;
Published: 04 January 2022.
Edited by:
Jian-Bing Fan, Illumina, United StatesReviewed by:
Lucia Tata-Chayeb, National Institute of Cancerology (INCAN), MexicoShanqiang Qu, Southern Medical University, China
Copyright © 2022 Zhao, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, em4wMDI2NDZAd2h1LmVkdS5jbg==
 Kaidi Zhao
Kaidi Zhao Zhou Ma
Zhou Ma