- 1Department of Molecular, Cell, and Developmental Biology, University of California, Los Angeles, Los Angeles, CA, United States
- 2Department of Integrative Biology and Physiology, University of California, Los Angeles, Los Angeles, CA, United States
- 3Molecular Biology Institute, University of California, Los Angeles, Los Angeles, CA, United States
A decline in mitochondrial function has long been associated with age-related health decline. Several lines of evidence suggest that interventions that stimulate mitochondrial autophagy (mitophagy) can slow aging and prolong healthy lifespan. Prohibitins (PHB1 and PHB2) assemble at the mitochondrial inner membrane and are critical for mitochondrial homeostasis. In addition, prohibitins (PHBs) have diverse roles in cell and organismal biology. Here, we will discuss the role of PHBs in mitophagy, oxidative phosphorylation, cellular senescence, and apoptosis. We will also discuss the role of PHBs in modulating lifespan. In addition, we will review the links between PHBs and diseases of aging. Finally, we will discuss the emerging concept that PHBs may represent an attractive therapeutic target to counteract aging and age-onset disease.
Introduction
There are two prohibitin subunits, prohibitin 1 (PHB1) and prohibitin 2 (PHB2), which together form a ring structured heterodimeric complex about 20–25 nm in diameter (Artal-Sanz and Tavernarakis, 2009a). PHBs are evolutionarily conserved and ubiquitously expressed in many types of tissues. PHB1 weighs 32 kDa and PHB2 weighs 34 kDa (Artal-Sanz and Tavernarakis, 2009a). Although PHBs are largely found in the inner mitochondrial membrane, they have also been found in the nucleus, cytosol, plasma membrane, endoplasmic reticulum, and macrophage phagosomes (Garin et al., 2001; Signorile et al., 2019).
PHBs were named “prohibitins” because they were initially found to prohibit initiation of DNA synthesis (McClung et al., 1989). PHBs have a diverse range of functions associated with aging, such as apoptosis, cellular senescence, cancer, and mitochondrial metabolism (Artal-Sanz and Tavernarakis, 2009a). PHBs also assist with protein folding of complexes in the electron transport chain (Nijtmans et al., 2002). PHB1 and PHB2 form a complex which acts as a chaperone by binding to products of mitochondrial translation and preventing their degradation by metalloproteases (Nijtmans et al., 2002). PHB2 acts as a protein-lipid scaffold (Artal-Sanz and Tavernarakis, 2009a). Both subunits of the PHB complex have N-terminal domains to anchor them in the inner mitochondrial membrane (Artal-Sanz and Tavernarakis, 2009a).
Prohibitins and Mitophagy
Mitophagy is the breakdown of damaged mitochondria via autophagy (Pickles et al., 2018). Mitophagy and mitochondrial dynamics (fission/fusion) are linked in maintaining mitochondrial quality control (Youle and van der Bliek, 2012). Excessive mitochondrial fusion/impaired mitochondrial fission is an underlying factor in the age-related decline in mitophagy, which is associated with senescence and aging (Rana et al., 2017; Aparicio et al., 2019; D'Amico et al., 2019). PHB2 binds to microtubule-associated protein 1A/1B-light chain 3 (LC3) to promote degradation of the mitochondria by an autophagosome (Tanida et al., 2008; Wei et al., 2017). Through oligomycin + antimycin (OA)-induced mitophagy, it was found that the mitochondrial membrane must be ruptured before PHB2 can bind to LC3 (Wei et al., 2017).
When there is a loss of mitochondrial membrane potential, PTEN-induced kinase 1 (PINK1) is unable to be degraded by presenilins-associated rhomboid-like protein (PARL), accumulates on the outer mitochondrial membrane, and recruits Parkin to mediate mitophagy (Jin et al., 2010). Parkin then ubiquitinates the mitochondrial fusion-promoting factor Mitofusin (Mfn) (Poole et al., 2010; Tanaka et al., 2010; Ziviani et al., 2010) and other mitochondrial proteins (Chan et al., 2011; Wang et al., 2011), promoting the segregation and autophagic turnover of the dysfunctional mitochondria (Youle and Narendra, 2011; Ashrafi and Schwarz, 2013).
A second study found further support that PHBs can promote mitophagy. This study found a new axis for mitophagy by PHB2: PHB2-PARL-PGAM5-PINK1 (Yan et al., 2020). Loss of PHB2 prevented mitophagy by destabilizing PINK1, which inhibited recruitment of Parkin, optineurin, and ubiquitin. Conversely, increasing PHB2 levels was found to increase mitophagy by promoting Parkin recruitment. Additionally, this study discovered that a synthesized ligand for PHBs, FL3, suppressed cancer by inhibition of mitophagy by PHB2. FL3 is a flavagline that has been shown to have a cardioprotective effect (Qureshi et al., 2015).
Prohibitins and Cellular Senescence
It is known that after a certain number of divisions, cells become senescent and stop dividing (Campisi and d'Adda di Fagagna, 2007). The number of senescent cells increases exponentially with age (He and Sharpless, 2017). As a result, a better understanding of the aging process may result from studying alterations in senescent cells. Levels of PHB2 decrease in senescing cells. The amount of mRNA coding for both PHB1 and PHB2 also decreases for yeast cells undergoing senescence (Piper et al., 2002). In addition, PHB1 and PHB2 levels decline for CEF chick embryo fibroblasts and HF19 human fibroblasts undergoing senescence (Coates et al., 2001). However, neither of these studies conclude that the decline in PHBs during senescence directly affects aging.
An early study on PHBs compared PHB1 levels in human cells between cells with low population doubling level (PDL) and cells with high PDL (Liu et al., 1994). PHB1 mRNA and protein levels were similar between low PDL cells and high PDL cells. However, a Western blot detected two PHB1 isoforms for low PDL cells, but only one PHB1 isoform for high PDL cells. This finding suggests that PHB1 in low PDL cells is post-translationally modified, but PHB1 in high PDL cells is not (Liu et al., 1994). The conclusion of Liu et al. (1994) that PHB1 mRNA and protein levels remain the same is also inconsistent with the results of Coates et al. (2001) and Piper et al. (2002).
PHB1 induces senescence in cells by synergizing with heterochromatin protein 1γ (HP1γ) to reduce transcription facilitated by E2F1 (Rastogi et al., 2006a). E2F1 is a transcription factor that acts as a regulator of promotors involved in cell division (DeGregori et al., 1995; Rastogi et al., 2006a). PHB1 recruits HP1γ to E2F1-controlled promoters to repress them. This recruitment was observed in senescent cells but not quiescent cells (Rastogi et al., 2006a). In addition, the study found that PHB1 depletion resulted in a reduced senescent phenotype.
Petunia flowers with silenced PhPHB1 senesce faster than unsilenced flowers (Chen et al., 2005). This finding suggests that PhPHB1 modulates the beginning of cellular senescence in flora. Silenced flowers also underwent fewer cellular divisions, with their petals containing only about 15% of the number of cells in the petals of the control group (Chen et al., 2005). Flowers with silenced PhPHB1 also had higher respiration rates and higher levels of catalase transcripts, which help protect cells from reactive oxygen species (ROS) (Chen et al., 2005). This finding suggests that there were higher ROS levels for the flowers with silenced PhPHB1. In endothelial cells, ROS have been shown through knockdown of PHB1 to lead to senescence (Schleicher et al., 2008).
Role of Prohibitins in Modulating Lifespan
PHB1 and PHB2 are the genes that encode the prohibitin proteins in yeast, Phb1p and Phb2p (Coates et al., 2001). Levels of Phb1p appear to be contingent on the levels of Phb2p in Saccharomyces cerevisiae. Deletion of PHB1 leads to a lack of Phb2p and deletion of PHB2 results in an absence of Phb1p (Berger and Yaffe, 1998). As a result, it was thought that depletion of either Phb1p or Phb2p would have the same effect on lifespan as the depletion of both Phb1p and Phb2p. However, it was discovered that the loss of both Phb1p and Phb2p had a greater effect on replicative lifespan than the loss of either alone (Piper et al., 2002). This finding was consistent with the results of Coates et al. (1997) but was inconsistent with Berger and Yaffe’s (1998) study, which found that the phb1-null phb2-null mutation had a less severe effect on replicative lifespan than the phb1-null mutation in MYY290 or MYY291 yeast cells with wild-type mitochondrial genomes.
There are different models of aging in yeast, which include replicative lifespan and chronological lifespan (Longo et al., 2012). Replicative lifespan measures the number of times a cell can divide, while chronological lifespan measures how long a non-dividing yeast cell stays alive (Longo et al., 2012). Deletion of PHB1 does not affect the chronological lifespan of S. cerevisiae cells stuck in G0 (Artal-Sanz and Tavernarakis, 2010). Piper et al. (2002) discovered that the chronological lifespan of yeast was relatively unchanged after the loss of Phb1p and Phb2p.
There are conflicting findings regarding the effect of PHB1 gene deletion on replicative lifespan in S. cerevisiae. One study observed that deletion of the PHB1 encoding gene in yeast haploid cells increases replicative lifespan by approximately 30%, while overexpression of the gene results in about a 20% decrease in lifespan (Franklin and Jazwinski, 1993). The deletion of the gene was thought to increase lifespan by allowing cells to divide for longer than they normally would have. In contrast, another study discovered that deletion of either PHB1 or PHB2 reduces the replicative lifespan in S. cerevisiae and leads to changes typical in aging, such as a longer duration of cell division and larger cell size (Coates et al., 1997; Smith et al., 2015). Since both Franklin and Jazwinski (1993) and Coates et al. (1997) were examining replicative lifespan, it may be beneficial to examine the factors that contribute to different results when measuring replicative lifespan in yeast. It has also been noted that different effects on lifespan can be observed when measuring the replicative lifespan of yeast, depending on the type of strain and the growth medium used (Longo et al., 2012).
In C. elegans, the relationship between knockdown of PHBs and longevity is dependent upon genotype (Artal-Sanz and Tavernarakis, 2010). Either PHB1 or PHB2 knockdown with RNAi decreases lifespan of wild type worms (Artal-Sanz and Tavernarakis, 2009b). However, knockdown of PHB1 through RNAi has been shown to increase the lifespan of certain C. elegans mutants. Notably, daf-2 mutants live 150% longer with either PHB1 or PHB2 knockdown (Artal-Sanz and Tavernarakis, 2009b). DAF-2 is a transmembrane insulin receptor kinase that modulates longevity in C. elegans (Kimura et al., 1997). Other C. elegans mutants found to live longer with knockdown of PHBs include electron transport chain mutants, mutants with changes in the metabolism of fat, and diet restriction mutants (Artal-Sanz and Tavernarakis, 2009b). In all these cases, knockdown of PHBs decreased intestinal fat and mitochondria levels. These findings suggest that although knockdown of PHBs decreases lifespan in wild type worms, knockdown of PHBs can increase lifespan in mutants with changed growth factor signaling, altered fat processing, or impaired mitochondrial performance (Artal-Sanz and Tavernarakis, 2010). As a result, the question of whether knockdown of PHBs increases or decreases lifespan in worms is dependent on the state of metabolism.
Although there are several studies examining the effects of depletion of PHBs on lifespan, experiments examining the effects of upregulation of PHBs on lifespan are notably lacking.
Prohibitins and Oxidative Phosphorylation
PHBs affect enzymes involved in metabolic processes, including oxidative phosphorylation. PHB1 acts as an inhibitor for pyruvate carboxylase and allows for insulin-stimulated regulation of glucose and fatty acid oxidation (Vessal et al., 2006). This regulation by PHB1 downregulates oxidative phosphorylation and instead supports metabolism through anaerobic glycolysis, which may have a role in aging. PHB1 can be modified through phosphorylation, but fibroblasts become less able to phosphorylate PHB1 as the cells undergo senescence (Liu et al., 1994). Since PHB1 regulates oxidative phosphorylation, this change in modifications over time may be related to the 40% decrease in oxidative activity of mitochondria as people age (Petersen et al., 2003).
PHB1’s role in metabolism may have importance in cancer because its role as an inhibitor of pyruvate carboxylate facilitates the shifting from oxidative phosphorylation to anaerobic glycolysis (Vessal et al., 2006). PHB1 overexpression has been reported in cancer cells (Yang et al., 2018). This overexpression may help rapidly growing cancer favor anaerobic glycolysis over oxidative phosphorylation, to reduce the high oxidative stress that would occur if oxidative phosphorylation was used (Vessal et al., 2006).
There are changes in PHB1 localization in reaction to oxidative stress. A study found that under oxidative stress, PHB1 relocated from the mitochondria to the cell nucleus, where it can regulate transcription (Sripathi et al., 2011). However, another study found that PHB1 translocated from the nucleus to the mitochondria in the presence of the ROS H2O2 (Lee H. et al., 2010). Another study found that overexpression of PHB1 made neonatal rat cardiomyocytes less susceptible to apoptosis in response to oxidative stress by H2O2 (Liu, X. et al., 2009).
PHBs also support complexes involved in the electron transport chain. PHB2 can help facilitate normal mitochondrial respiration through its interaction with sphingosine-1-phosphate (S1P), a lipid mediator, by supporting the proper assembly of complex IV of the electron transport chain (Strub et al., 2011). PHBs may also help support complex I of the electron transport chain. A study found that PHB1 can protect complex I from the rotenone inhibitor and upregulation of PHB1 is connected to decreased manufacture of ROS (Zhou et al., 2012).
Prohibitins in Age-Related Diseases
Degenerative diseases such as Alzheimer’s disease, Parkinson’s disease, diabetes, and cancer are often age-related. PHBs have been implicated in each of these diseases.
Prohibitins and Alzheimer’s Disease
In a 2007 paper, researchers observed the frontal cortex of sporadic Alzheimer’s disease (AD) cases and did not observe changes in PHB1 levels (Ferrer et al., 2007). This finding was consistent with another paper published in 2008 that also examined the frontal cortex and found that levels of PHB1 for AD cases were similar to the levels in control cases (Pérez-Gracia et al., 2008). However, a later study in 2017 found a change in PHB1 levels when studying the olfactory bulb (OB) (Lachén-Montes et al., 2017). An early sign of AD is olfactory dysfunction (Zou et al., 2016). In intermediary and progressive AD phases, Lachén-Montes et al. (2017) observed PHB2 depletion. There were also lower levels of PHB1 isoforms that were phosphorylated. This result is consistent with observations that fibroblasts become less able to phosphorylate PHB1 with age (Liu et al., 1994).
Lachén-Montes et al. (2017) found that AD is associated with PHB2 depletion, which is consistent with another study that observed neurodegeneration and cognitive and behavioral disablements in neuron-specific PHB2-deficient (Phb2NKO) mice (Merkwirth et al., 2012). Cognitive and behavioral disabilities in the mice were assessed using the Morris water maze paradigm, the elevated zero maze test, and open field tests. These impairments were associated with abnormal mitochondrial structure and hyperphosphorylated tau, a protein found mainly in neurons.
There is evidence that suggests the drug PDD005, which targets PHB1 and PHB2, can protect against neurodegenerative diseases such as AD and Parkinson’s disease (PD). PHB1 and PHB2 levels increase in the brains of aged mice when they are treated with PDD005 (Guyot et al., 2020). In contrast, the levels of the cytokine IL-1β decreased, suggesting that PDD005 and its interactions with PHB1 and PHB2 reduce inflammation in the brain. The authors discovered that PDD005 increases the expression of the signaling molecule GSK-3β, which can promote the destabilization of β-catenin through phosphorylation (Wu and Pan, 2010). This β-catenin interacts with nuclear factor-κ-light-chain-enhancer of activated B cell (NF-kβ) components to prevent transcription of proinflammatory molecules (Ma and Hottiger, 2016).
Prohibitins and Parkinson’s Disease
There is not much research studying the relationship between PHBs and Parkinson’s disease (PD). It is known that dysfunctional mitochondria play a significant role in the development of PD and in preventing the death of neuron cells (Pickrell and Youle, 2015). PHBs are involved in mitochondrial control by promoting mitophagy to prevent the buildup of dysfunctional mitochondria observed in aging, suggesting that PHBs may protect against PD (Wei et al., 2017). This idea has been supported by a study on PD demonstrating that decreased levels of PHB1 increased the susceptibility of dopamine sensitive neurons to 1-methyl-4-phenyl-pyridnium (MPP+) instigated death while overexpression of PHB1 made them less susceptible (Dutta et al., 2018). MPP+ acts as a neurotoxin and is the oxidized product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Sian et al., 1999). MPTP administration has been shown to result in dopaminergic neuron degeneration (Muñoz-Manchado et al., 2016). However, MPTP has the limitation of not inducing some of the characteristics of PD (Sian et al., 1999).
There are also lower PHB1 levels in the substantia nigra, a brain structure known to be vulnerable in PD, with an estimated 60% loss of neurons in this region by the time of presentation of motor symptoms (Ferrer et al., 2007).
Prohibitins and Diabetes
Another age-related disease that PHBs have been found to be implicated in is diabetes. Knockout of the gene for PHB2 in mouse pancreatic β-cells first leads to defective mitochondrial performance and defective secretion of insulin, then a decline in β-cells and continuing changes in glucose homeostasis, culminating in extreme diabetes (Supale et al., 2013).
Another study investigated the effects of PHB1 on diabetic cardiomyopathy. This study created a model of type 2 diabetes by feeding rats a diet high in fat while treating them with a low dose of streptozotocin, which is a toxin to pancreatic β-cells (Dong et al., 2016). PHB1 overexpression was achieved through lentiviral transduction. This transduction improved the deleterious effects in rats with diabetic cardiomyopathy, such as resistance to insulin, dysfunction of the left ventricle, fibrosis, and programmed cell death (Dong et al., 2016). However, the precise process in which PHB1 improves diabetic cardiomyopathy is not known.
Although increasing PHB1 levels helps reduce the effects of type 2 diabetes, it does not seem to be because PHB1 levels are lower in people with type 2 diabetes than in people who do not have type 2 diabetes. Levels of PHB1 in the serum of type 2 diabetic subjects are similar to the levels observed in control subjects (Kakarla et al., 2019).
Prohibitins, Cancer, and Apoptosis
There have been many studies describing the relationship between PHBs and cancer (Koushyar et al., 2015). However, there is disagreement between studies about whether PHBs repress or support cancer and whether PHBs protect cancer cells from apoptosis or make them more susceptible.
High levels of PHBs are commonly observed in tumors. Coates et al. (2001) discovered that there are Myc oncoprotein binding sites in the promoters for PHB1 and PHB2 and that increasing Myc levels induces expression of PHB1 and PHB2. These findings suggest that levels of PHBs are often increased in tumors due to upregulation by oncoproteins.
Overexpression of PHB1 and PHB2 has been observed in blood-related cancers. PHB1 and PHB2 levels in lymphoma and leukemia cells were compared to healthy peripheral blood mononuclear cells (Ross et al., 2017). PHB1 and PHB2 levels were higher in these cancer patients than in controls. The overexpression of PHBs also protected these cancer cells from apoptosis induced by ROS. This finding suggests that cancer cells, with their higher use of glycolysis and therefore higher generation of ROS, can survive through the overexpression of PHBs. This finding that PHBs protect cancer cells from apoptosis was supported by another study in which silencing the expression of PHB1 made ovarian cancer cells more susceptible to apoptosis (Gregory-Bass et al., 2008).
Depletion of PHBs has been observed to make cells more receptive to apoptosis, while overexpression of PHBs makes cells less susceptible (Peng et al., 2015). For example, depletion of PHB1 in mice results in lethality during development and high levels of apoptosis (Park et al., 2005; He et al., 2008; Merkwirth et al., 2012; Peng et al., 2015). For the cell line Kit225, PHB1 and PHB2 knockdown with siRNA results in increased cell death through the ROS H2O2 (Ross et al., 2017).
Although there are studies showing that PHBs protect cells from apoptosis, there are a few studies that have found the opposite. The finding of Gregory-Bass et al. (2008) that silencing of PHB1 increased tumor cell susceptibility to apoptosis was contradicted by another study demonstrating that PHB1 knockdown results in inhibition of apoptosis in NB4-R1 leukemia cells (Liu et al., 2012). Rather than protecting cells from apoptosis, PHB1 overexpression was found to encourage apoptosis of cells in leukemia with arsenic sulfide treatment (He et al., 2015).
There are also conflicting findings regarding PHB1 levels in gastric cancer. In one study, researchers found that in gastric cancer, the microRNA miR-27a is upregulated, targets PHB1, and acts as an oncogene (Liu T. et al., 2009). Downregulating miR-27a in these gastric cancer cells increased levels of PHB1 protein and transcripts. Although the study by Liu T. et al. (2009) revealed that the levels of PHB1 in gastric cancer cells were lower than in healthy cells, another study found that they were higher (He et al., 2004).
There are also conflicting conclusions regarding the potential of PHBs as a treatment for cancer. Synthetic PHB1 mRNA can prevent DNA synthesis in HeLa cells and healthy fibroblasts (Nuell et al., 1991), providing evidence that PHB1 transcripts could be a potential treatment for cancer by preventing the replication of DNA in cancer cells. This finding that PHB1 transcripts can prevent DNA synthesis was supported by another study demonstrating that the PHB1 3′ untranslated region (3′UTR) encodes RNA that suppresses breast cancer by preventing entry into S phase of the cell cycle (Jupe et al., 2001). Further studies examining breast cancer revealed that PHB1 increases p53-regulated transcription while decreasing transcription regulated by E2F1 (Fusaro et al., 2003). As a result, these studies suggest that PHB1 could be used as a treatment for cancer by suppressing cancer cell proliferation and increasing transcription regulated by tumor suppressor proteins such as p53. Although Nuell et al. (1991) found that synthetic PHB1 mRNA microinjection suppresses proliferation of cancer cells, another study found that silencing PHB1 helps make drug-resistant cancer cells drug-sensitive (Patel et al., 2010). This study found that drug-resistant cancer cells tend to have higher levels of PHB1 on their surface compared to drug-sensitive cells.
These conflicting findings between studies may be due to the relationship between PHBs, cellular senescence, and apoptosis. PHBs can inhibit the proliferation of cancer cells by preventing their entry into S phase and inducing senescence (Nuell et al., 1991). This senescence could protect cancer cells from apoptosis. The location of PHBs may also affect whether they act as tumor supporters or suppressors. PHB1 on the plasma membrane is associated with drug-resistant cancer cells, while PHB1 in the nucleus is associated with tumor suppression (Patel et al., 2010; Theiss and Sitaraman, 2011). The location of PHBs may affect whether the cell will undergo apoptosis. Cells were protected from programmed cell death when PHB1 transport to the cytoplasm was inhibited (Rastogi et al., 2006b).
Prohibitin as a Therapeutic Target
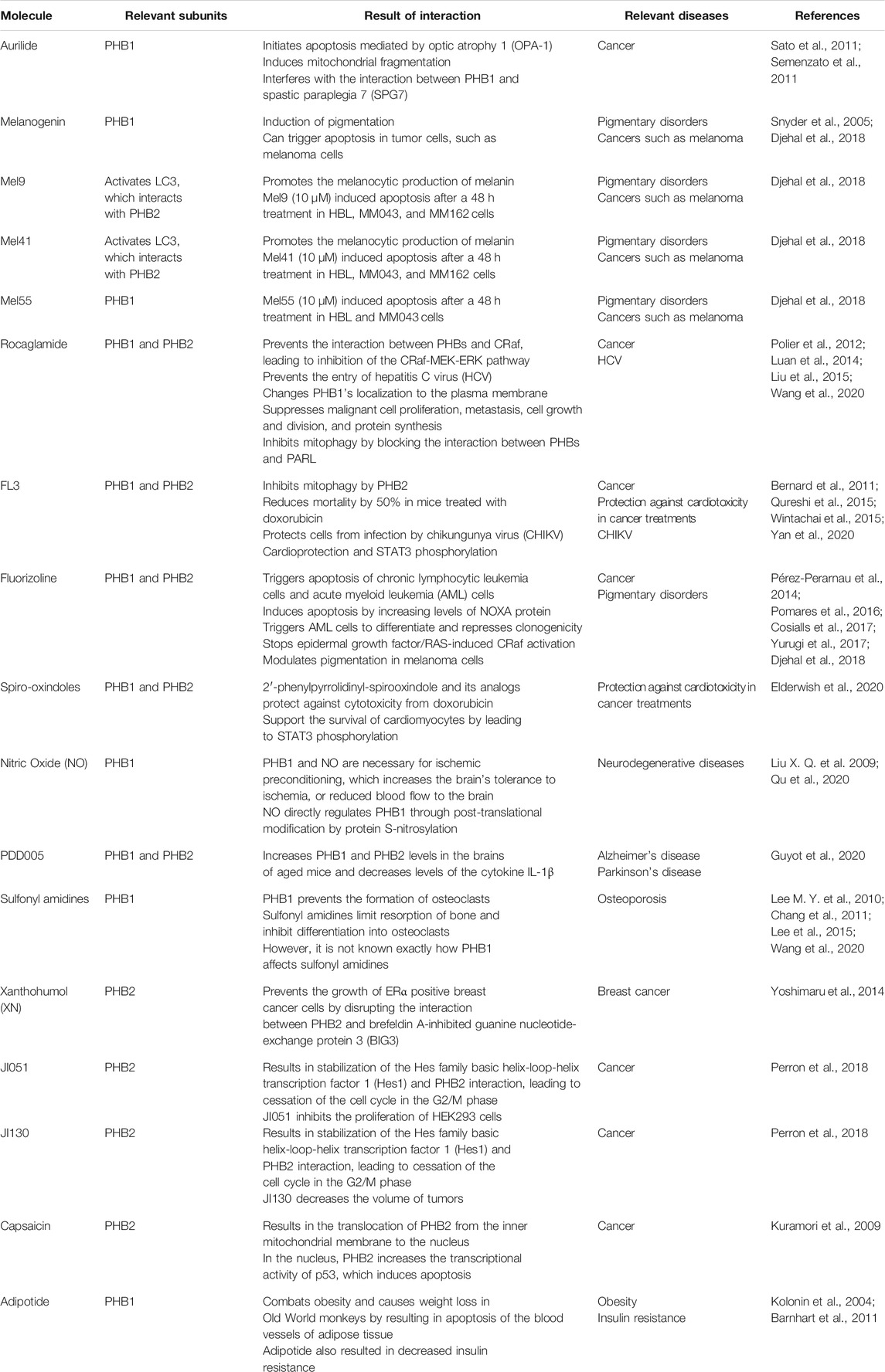
PHBs have been found to interact with a wide variety of molecules. Targeting the interactions between PHBs and these molecules may have therapeutic potential against age-related diseases. An outline of small molecules that interact with PHBs and their relevance to age-related diseases is provided in Table 1.
Prohibitins and Aurilide
The interactions of PHBs with small molecules can give insight into how PHBs modulate cellular processes such as apoptosis. Aurilide is a natural marine product from Dolabella auricularia and is cytotoxic (Suenaga et al., 2004). When PHB1 interacts with aurilide, it initiates apoptosis mediated by optic atrophy 1 (OPA-1) (Sato et al., 2011). The study by Sato et al. (2011) suggests aurilide can influence apoptosis through its interaction with PHB1. In contrast, PHB2 does not have affinity for aurilide (Sato et al., 2011; Semenzato et al., 2011).
Upregulating PHB1 makes cells less susceptible to aurilide (Sato et al., 2011). In contrast, partial downregulation of PHB1 through siRNA makes cells more susceptible. This finding suggests that aurilide interferes with the PHB complex. Sato et al. (2011) also discovered that aurilide induces mitochondrial fragmentation, which is similar to the mitochondrial morphology observed under PHB1 knockdown with siRNA.
Other studies revealed how aurilide’s interactions with PHB1 trigger apoptosis by affecting the morphology of mitochondria. PHB1 and spastic paraplegia 7 (SPG7) normally interact, but treatment with aurilide interferes with the interaction (Sato et al., 2011; Semenzato et al., 2011). SPG7 is part of the m-AAA protease which is involved in the processing of OPA-1 (Duvezin-Caubet et al., 2007). OPA-1 controls cristae dynamics, which involves a change in mitochondrial morphology (Griparic et al., 2004; Song et al., 2009).
OPA-1 is important for fusion of mitochondria (Cipolat et al., 2004; Song et al., 2009). Aurilide increased the formation of short (S) isoforms of OPA-1, which stop fusion and result in mitochondrial fragmentation (Duvezin-Caubet et al., 2006; Ishihara et al., 2006; Sato et al., 2011). This change in mitochondrial morphology enables cytochrome c to be released, which promotes apoptosis to activate proteases called caspases (Jiang and Wang, 2004; Wasilewski and Scorrano, 2009; Semenzato et al., 2011; Julien and Wells, 2017). These findings explain how aurilide triggers apoptosis through its interaction with PHB1 and suggest that aurilide results in fragmented mitochondria by inhibiting PHB1, eventually triggering apoptosis which kills cells. This apoptosis may have potential as a treatment against cancer.
Prohibitins and Melanogenin
Melanogenin is a ligand for PHBs (Djehal et al., 2018). Knockdown of PHB1 and PHB2 with siRNA demonstrated that PHBs are necessary for induction of pigmentation by melanogenin (Snyder et al., 2005). Two melanogenin analogs, Mel41 and Mel9, activate LC3, which interacts with PHB2 (Djehal et al., 2018). These melanogenin analogs have increased microphthalmia-associated transcription factor expression. Mel41 and Mel9 promote the melanocytic production of melanin. Another melanogenin analog, Mel55, interacts with PHB1 (Djehal et al., 2018).
The involvement of PHBs in melanin production suggests that targeting PHBs may be a promising therapeutic approach for pigmentary disorders, which can be common in the elderly population (Armenta et al., 2019). Melanogenin and melanogenin analogs also have potential as treatments against cancer since they can trigger apoptosis in tumor cells, such as melanoma cells (Djehal et al., 2018). Djehal et al. (2018) also found that in addition to interacting with melanogenin, PHBs appear to modulate pigmentation in melanoma cells by interacting with fluorizoline.
Prohibitins and Rocaglates
Rocaglates (also known as flavaglines) are from the genus Aglaia, which contains species that are used in traditional medicine (Ebada et al., 2011). Rocaglates interact with PHBs and have potential as a cancer treatment (Basmadjian et al., 2013). Rocaglamide is a rocaglate (Ebada et al., 2011). Rocaglamides interact directly with PHB1 and PHB2 and prevent their interaction with CRaf, leading to inhibition of the CRaf-MEK-ERK pathway (Polier et al., 2012). This inhibition suppresses malignant cell proliferation, cell growth and division, and protein synthesis. Polier et al. (2012) observed that the effect of rocaglamides on the CRaf-MEK-ERK cascade was similar to the effect of PHB1 knockdown.
Rocaglates also prevent energy production and mitophagy in cancer cells. The rocaglates FL3 and rocaglamide inhibit mitophagy by blocking the interaction between PHBs and PARL (Wang et al., 2020). These findings suggest rocaglamide could be an effective treatment against cancer through its interactions with PHBs.
Prohibitins and Fluorizoline
Using molecules to target PHBs and induce apoptosis could be a potential treatment for cancer. Fluorizoline is a synthetic ligand for both PHB1 and PHB2 and triggers apoptosis of chronic lymphocytic leukemia cells (Pérez-Perarnau et al., 2014; Cosialls et al., 2017). Through its interaction with PHBs, fluorizoline induces apoptosis by increasing levels of NOXA protein, which is a pro-apoptotic B-cell lymphoma 2 family member (Oda et al., 2000; Cosialls et al., 2017). PHBs are also necessary for fluorizoline to trigger this apoptosis (Moncunill-Massaguer et al., 2015). In addition, cells with PHB1 depletion are resistant against cell death from fluorizoline. Fluorizoline interacts with PHBs to trigger apoptosis in acute myeloid leukemia (AML) cells (Pomares et al., 2016). Fluorizoline also triggered these AML cells to differentiate and repressed the clonogenicity of these cells.
Rocaglamide and Fluorizoline in Cancer
Ligands for PHBs may also be potential treatments for lung cancer. PHB1 is upregulated in human non-small cell lung cancers (Jiang et al., 2013). Yurugi et al. (2017) observed that the higher the level of PHB1, the less likely the patient is to survive. This result is in line with a previous study which found that overexpressing PHB1 increased metastasis, increased mortality, and led to large cervical tumors in mice (Chiu et al., 2013).
Fluorizoline and rocaglamide stop epidermal growth factor/RAS-induced CRaf activation (Yurugi et al., 2017). This effect is a potential treatment against cancer since CRaf is necessary for tumorigenesis mediated by KRAS (Blasco et al., 2011). Rocaglamide prevents the migration and growth of lung tumor cells with mutated KRAS (Yurugi et al., 2017). Mutated KRAS often results in increased activity of ERK1/2 (Luan et al., 2014). PHB1 interacts directly with CRaf to activate ERK1/2 (Rajalingam et al., 2005). In melanoma cancer, this activation of ERK1/2 mediates resistance to the BRAF inhibitor drug vemurafenib and in melanoma cells, rocaglamide A was found to undo this resistance (Doudican and Orlow, 2017).
Rocaglamide and fluorizoline inhibit CRaf/ERK pathways by interfering with the interaction between CRaf and PHB1 (Luan et al., 2014; Yang et al., 2018). Rocaglamide was found to interfere with the interaction between PHB1 and CRaf by changing PHB1’s localization to the plasma membrane, leading to decreased RAS-ERK signaling. This decreased signaling suppresses metastasis and tumor growth (Polier et al., 2012; Luan et al., 2014). Luan et al. (2014) also found that in mice with tumors, treatment with rocaglamide lengthens lifespan.
Prohibitins and Spiro-Oxindoles
Spiro-oxindoles and their interaction with PHBs have relevance in cardiology and cancer treatments. Doxorubicin is an anthracycline that is used as an anticancer drug (Carvalho et al., 2009). However, doxorubicin can result in cardiotoxicity (Olson and Mushlin, 1990; Singal and Iliskovic., 1998). It was found that 2′-phenylpyrrolidinyl-spirooxindole and its analogs protect against cytotoxicity from doxorubicin (Elderwish et al., 2020).
Spiro-oxindoles bind both PHB1 and PHB2 (Elderwish et al., 2020). This binding supports the survival of cardiomyocytes by leading to STAT3 phosphorylation (Elderwish et al., 2020). Rocaglates (flavaglines) have a similar cardioprotective effect. In mice treated with doxorubicin, the flavagline FL3 was found to reduce mortality by 50% (Bernard et al., 2011). Knockdown of PHB1 and PHB2 with siRNA prevented FL3’s cardioprotection and STAT3 phosphorylation (Qureshi et al., 2015). These findings suggest that targeting interactions with PHBs can help protect against cardiotoxicity in cancer treatments.
Prohibitins and Nitric Oxide
The interactions between PHBs and small molecules could reveal the role of PHBs in neurodegenerative diseases. Upregulation of PHB1 increases neuroprotection in mice against brain damage from ischemia (Kahl et al., 2018). This finding is consistent with another study demonstrating that PHB1 is upregulated in ischemic preconditioning and decreases the number of neurons that die from injury (Zhou et al., 2012).
Brain ischemic preconditioning is an example of neuroprotection and increases the brain’s tolerance to ischemia, or reduced blood flow to the brain (Liu, X. Q. et al., 2009). Nitric oxide (NO), a neurotransmitter, regulates PHB1 and contributes to its neuroprotective effects (Qu et al., 2020). PHB1 and NO are necessary for ischemic preconditioning (Qu et al., 2020). NO directly regulates PHB1 through post-translational modification by protein S-nitrosylation (Qu et al., 2020). However, it is not known how protein S-nitrosylation of PHB1 by NO leads to neuroprotection.
Prohibitins, Sulfonyl Amidines, and Phosphoryl Amidines
Targeting PHBs also has potential as a treatment for osteoporosis. Osteoclastogenesis can contribute to osteoporosis through differentiation of hematopoietic stem cells into osteoclasts (Boyle et al., 2003). These osteoclasts can lead to decreased bone density by resorbing bone, which can contribute to osteoporosis if there is less formation of bone than resorption of bone (Boyle et al., 2003; Harada and Rodan, 2003).
Sulfonyl amidines and phosphoryl amidines have relevance in osteoporosis because they limit resorption of bone and inhibit differentiation into osteoclasts (Lee M. Y. et al., 2010). PHB1 binds to sulfonyl amidine compounds (Chang et al., 2011). PHB1 also prevents the formation of osteoclasts (Lee et al., 2015). However, it is not known exactly how PHB1 affects sulfonyl amidines (Wang et al., 2020).
Prohibitins and ERAP
Targeting PHB2 interactions with other molecules may be a potential treatment for breast cancer. ERα activity-regulator synthetic peptide (ERAP) disrupts the interaction between PHB2 and brefeldin A-inhibited guanine nucleotide-exchange protein 3 (BIG3) (Kim et al., 2009; Yoshimaru et al., 2013). Another molecule that directly interacts with PHB2 is xanthohumol (XN). Similar to ERAP, XN prevents the growth of breast cancer cells that are positive for ERα by disrupting the interaction between PHB2 and BIG3 (Yoshimaru et al., 2014). In most cases of breast cancer, there is BIG3 overexpression (Kim et al., 2009).
BIG3 affects the localization of PHB2. PHB2 interacts with ERα and suppresses transcription of ERα (Kim et al., 2009). This interaction occurs in the nucleus. In contrast, in breast cancer cells, PHB2 interacts with BIG3 in the cytoplasm. PHB2 is trapped in the cytoplasm due to its interaction with BIG3 (Kim et al., 2009; Yoshimaru et al., 2013). This interaction prevents PHB2 from suppressing transcription of ERα in the nucleus.
ERα has relevance in the severity of breast cancer. ERα interacts with E2 to increase metastasis and proliferation of breast cancer cells (Yager and Davidson, 2006). Using ERAP to disrupt the interaction between PHB2 and BIG3 can stop breast cancer cells that are positive for ERα from growing and make these cells more responsive to tamoxifen (Yoshimaru et al., 2013).
In addition to wild type (WT) ERα, PHB2 can bind to ERα mutants, such as D538G and Y537S, which are often present in breast cancers that are resistant to hormonal therapy (Chigira et al., 2015). As a result, treatment with ERAP could be used in breast cancer cells that are positive for ERα to undo resistance to hormonal therapies such as tamoxifen (Yoshimaru et al., 2013). This finding has importance since the majority of breast cancers that are positive for ERα become resistant to tamoxifen (Riggins et al., 2007).
A later study found that when ERAP disrupts the interaction between PHB2 and BIG3, PHB2 is able to interfere with the interaction of ER2α with a wide variety of other molecules, such as EGFR, human epidermal growth factor 2 (HER2), insulin-like growth factor 1 receptor beta (IGF-1Rβ), and PI3K (Yoshimaru et al., 2015). PHB2 repressed the proliferation of breast cancer cells that were positive for ERα by decreasing phosphorylated Akt, MAPK, and ERα, and by HER2, EGFR, and IGF-1Rβ inhibition.
Prohibitins, CRaf, Ras, and Akt
PHB1 interacts with CRaf (Raf-1), which can activate pathways involving Ras that support cancer and its invasiveness, such as the CRaf/ERK, PI3K/Akt, and RalGEF/Ral pathways (Karnoub and Weinberg, 2008). PHB1 is necessary for Ras to activate CRaf, which is important for controlling migration and cell adhesion for epithelial cells (Rajalingam et al., 2005). Without PHB1, Raf-1 kinase and activated Ras are unable to interact. Another study expanded on this finding by revealing that phosphorylated PHB1 at T258 is important for activating CRaf, for the direct interaction between CRaf and PHB1, and for cancer cell invasiveness (Chiu et al., 2013).
PHB1 is phosphorylated at T258 by Akt and phosphorylated PHB1 interacts with Akt, Ras, and Raf-1 (Han et al., 2008; Chiu et al., 2013). In contrast, dephosphorylating PHB1 at T258 reduced tumor cell invasiveness and metastasis and decreased epithelial-mesenchymal transition (Chiu et al., 2013). Together, these findings suggest that PHB1 phosphorylated at T258 by Akt can increase the invasiveness of cancer by direct interaction with Raf-1. This interaction allows Ras to interact with and activate Raf-1 to control signaling cascades (Rajalingam et al., 2005; Han et al., 2008; Karnoub and Weinberg, 2008; Chiu et al., 2013).
Prohibitins, Rocaglates, and Hepatitis C Virus
Older patients have a greater likelihood of developing chronic hepatitis C virus (HCV) infection (Reid et al., 2017). Interactions between small molecules and PHBs may be useful to protect against viruses. Both PHB1 and PHB2 are involved in HCV entry into human hepatocytes (Liu et al., 2015). PHB1 and PHB2 facilitate entry of HCV by associating with E2, a viral glycoprotein (Vieyres et al., 2010; Liu et al., 2015). E2, along with another glycoprotein called E1, are known to induce cells to take up the viral particle through endocytosis (Meertens et al., 2006). The use of rocaglates could be a useful protection against HCV entry (Liu et al., 2015).
Use of rocaglamide prevents the entry of HCV by preventing the interaction between PHB1, PHB2, and CRaf (Liu et al., 2015). Knockdown of PHBs does not alter HCV’s ability to bind to cells (Liu et al., 2015). However, PHB1 and PHB2 were found on the plasma membrane and were important for HCV entry after HCV binding to the cell (Liu et al., 2015). This study also found another rocaglate, aglaroxin C, which had a greater effect on preventing HCV entry than rocaglamide.
The study by Liu et al. (2015) suggests rocaglates targeting PHBs could be used to provide protection against viruses such as HCV. Similar to HCV, DENV 3, a serotype of the dengue virus (DENV), formed interactions with PHB1 and PHB2 to enter into cells (Clyde et al., 2006; Sharma et al., 2020). Other viruses, such as chikungunya virus and enterovirus 71, utilize PHB1 for entry into cells (Wintachai et al., 2012; Too et al., 2018).
Prohibitins, Rocaglates, and Chikungunya Virus
Rocaglates (flavaglines) could also be used to protect against the chikungunya virus (CHIKV). Like HCV, CHIKV utilizes PHBs to enter cells. The E2 protein of CHIKV interacts with PHB1 to allow the virus to infect microglial cells (Wintachai et al., 2012). The flavaglines FL3 and FL23 protect cells from infection by CHIKV (Wintachai et al., 2015). Increased viral replication of CHIKV has been observed in aged Rhesus macaques, which have a weaker immune response than adult Rhesus macaques (Messaoudi et al., 2013). Rocaglates may be able to be used to counteract this age-related effect. The rocaglate derivative silvestrol (Pan et al., 2014) inhibits replication of CHIKV and inhibits eIF4A, which is an RNA helicase (Henss et al., 2018).
Prohibitins and Coronaviruses
Older patients have a greater chance of developing life-threatening diseases from COVID-19 (Liu et al., 2020). PHBs interact with proteins of SARS-CoV-2, which is the virus responsible for COVID-19 (Andersen et al., 2020). SARS-CoV-2 has proteins called nonstructural proteins (nsps) numbered 1–16 (Chen et al., 2020). It was found that nsp2 interacts with both PHB1 and PHB2 (Cornillez-Ty et al., 2009). However, it is still not known exactly how nsp2’s interaction with PHB1 and PHB2 affects cells (Nebigil et al., 2020).
LC3 also interacts with PHBs. Coronaviruses lead to formation of double membrane vesicles with LC3-I, which enables the virus to take over intracellular membranes and to replicate (Reggiori et al., 2010). LC3 knockdown prevents cells from being infected by coronaviruses (Reggiori et al., 2010). LC3-I, PHBs, and nsp2 may interact as joint complex (Nebigil et al., 2020). This suggests that targeting PHBs could affect this joint complex and disrupt the entry of coronaviruses into cells.
Prohibitins, JI051, and JI130
Other compounds that interact with PHBs are JI051 and JI130. JI051 and JI130 are synthesized compounds that inhibit the proliferation and growth of cancer cells (Perron et al., 2018). During adulthood, there is an association between cancer development and abnormal signaling of Hes family basic helix-loop-helix transcription factor 1 (Hes1). JI051 interacts with PHB2 (Perron et al., 2018). This interaction results in stabilization of the Hes1 and PHB2 interaction, leading to cessation of the cell cycle in the G2/M phase. In addition, JI051 inhibits the proliferation of HEK293 cells and JI130 decreases the volume of tumors. These findings suggest JI051 and JI130 could be potential treatments for cancer.
Prohibitins and Capsaicin
Another compound that interacts with PHBs is capsaicin, which is found in hot chili peppers (Reyes-Escogido et al., 2011; Wang et al., 2020). Capsaicin can bind directly with PHB2 and result in the translocation of PHB2 from the inner mitochondrial membrane to the nucleus (Kuramori et al., 2009). In the nucleus, PHB2 increases the transcriptional activity of p53, which induces apoptosis. Capsaicin suppresses growth and triggers apoptosis in leukemic cells (Ito et al., 2004). This effect was greater for cells that were positive for p53 than for cells that were null for p53. Capsaicin also leads to mitochondrial membrane potential disruption and triggered cytochrome c release from the mitochondria, which is another sign of apoptosis (Kuramori et al., 2009).
Prohibitins and Adipotide
The risk of abdominal obesity increases with age and obesity can result in insulin resistance (Jura and Kozak, 2016). Targeting PHBs has been shown to undo obesity in animal models. The peptidomimetic adipotide interacts with PHB1 in the adipose vasculature in mice (Kolonin et al., 2004). This interaction was found to combat obesity and cause weight loss in Old World monkeys, by resulting in apoptosis of the blood vessels of adipose tissue (Barnhart et al., 2011). Adipotide also resulted in decreased insulin resistance (Barnhart et al., 2011). There has not been any additional research on adipotide since 2012 (Wang et al., 2020).
Future Directions
Much of the full function of PHBs in aging remains unknown. One of the most important questions that needs to be addressed is the number of conflicting studies about whether PHBs suppress or promote cancer and whether PHBs increase or decrease apoptosis. It would be beneficial to learn more about what factors make PHBs tumor supporters and what factors make them tumor suppressors.
More studies might be able to determine if PHBs could be used as a diagnostic tool for age-related diseases. There is potential for PHBs to be used as biomarkers for age-related problems such as oxidative stress and for diseases that increase with age such as diabetes (Lee H. et al., 2010; Supale et al., 2013; Dong et al., 2016). To further understand the role of PHBs in aging, it may be beneficial to perform observational studies on people with long health spans and lifespans to see how their expression of PHBs may differ from the general population. It would also be valuable to find out more information about the role of PHBs in Parkinson’s disease since there are only a few studies on this topic (Ferrer et al., 2007; Pickrell and Youle, 2015; Dutta et al., 2018).
An additional area to investigate is the effects of upregulation of PHBs on lifespan since many studies regarding the effect of PHBs on lifespan are only examining knockdown of PHBs (Artal-Sanz and Tavernarakis, 2010). It would also be beneficial to further examine the differences in strain genetic backgrounds that may contribute to the opposing effects on replicative lifespan in yeast (Kirchman et al., 1999). Another question deserving more attention is the relationship between PHB1 and PHB2. It should be determined why deletion of both subunits results in a greater decrease in lifespan than the deletion of only one or the other if a lack of PHB1 corresponds to a lack of PHB2 and vice versa (Berger and Yaffe, 1998). Little is known about the structure of the PHB complex, which is another area that could be studied in the future (Artal-Sanz and Tavernarakis, 2009a).
Another area of study is the relationship between apoptosis and senescence for PHBs and how it relates to cancer. It seems that induction of senescence by PHBs would be beneficial to treat cancer by preventing entry into S phase (Jupe et al., 2001). However, cells that are senescent are more resistant to apoptosis, suggesting that PHBs may also protect cancer cells from apoptosis (Marcotte et al., 2004).
There is an extensive amount of studies examining the targeting of PHBs as a potential treatment for cancer (Koushyar et al., 2015). However, there are not as many studies examining the therapeutic potential of PHBs in other diseases related to aging. For example, there are not many studies regarding the targeting of PHBs to treat neurodegenerative diseases. Nitric oxide has been found to be involved in neuroprotection, but its role in neurodegenerative diseases, as well as how its regulation of PHB1 results in neuroprotection, is not clear (Qu et al., 2020). There are also few studies that examine the therapeutic potential of PHBs in osteoporosis and how PHB1 affects sulfonyl amidines which limit bone resorption (Lee M. Y. et al., 2010; Chang et al., 2011). The therapeutic potential of PHBs in other age-related diseases such as Alzheimer’s disease, Parkinson’s disease, osteoarthritis, hypertension, diabetes, and cardiovascular diseases are all areas of research that could be expanded in the future.
Conclusion
PHBs are involved in aging through cellular senescence, apoptosis, and oxidative stress, and have functions in age-related diseases such as Alzheimer’s disease, Parkinson’s disease, diabetes, and cancer (Ferrer et al., 2007; Artal-Sanz and Tavernarakis, 2009a; Supale et al., 2013; Lachén-Montes et al., 2017).
One particularly compelling hypothesis is that PHBs can modulate aging through mitophagy to maintain mitochondrial quality control (Youle and van der Bliek, 2012). There is also evidence that PHBs can impact cellular senescence and shift oxidative phosphorylation to anaerobic respiration (Rastogi et al., 2006a; Vessal et al., 2006).
There are conflicting studies about whether PHBs induce apoptosis or suppress it (Liu et al., 2012; Peng et al., 2015). PHBs may have a potential role as a treatment for cancer. However, more research needs to be conducted to understand what factors may make PHBs tumor promoters and what factors may make PHBs tumor suppressors.
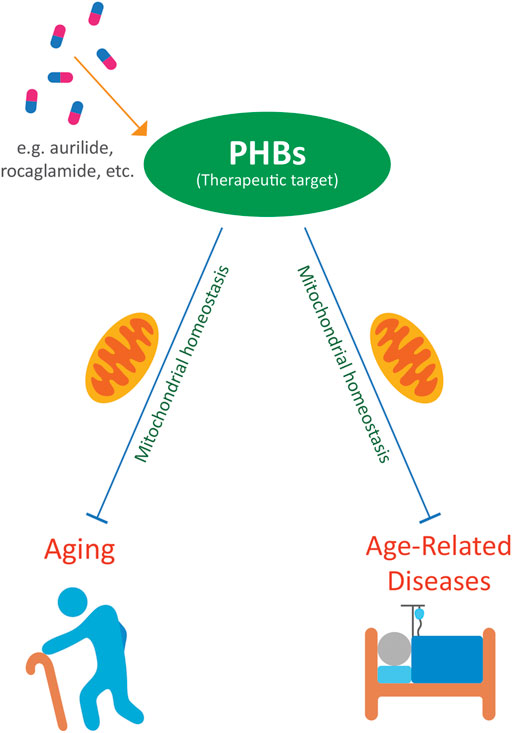
PHBs have therapeutic potential in a variety of age-related diseases. Targeting PHBs with compounds such as rocaglates, aurilide, fluorizoline, melanogenin, ERAP, capsaicin, JI051, and JI130 may be potential treatments against cancer (Ito et al., 2004; Sato et al., 2011; Yoshimaru et al., 2013; Cosialls et al., 2017; Yurugi et al., 2017; Djehal et al., 2018; Perron et al., 2018). Rocaglates may also protect against viral diseases that can be more severe in the elderly (Wintachai et al., 2015; Nebigil et al., 2020). Spiro-oxindoles can protect against cardiotoxicity in cancer patients (Elderwish et al., 2020). Nitric oxide is involved in neuroprotection, which may be relevant to neurodegenerative diseases (Qu et al., 2020). Sulfonyl amidines have relevance to osteoporosis and limit bone resorption (Lee M. Y. et al., 2010; Lee et al., 2015). Targeting PHBs with these compounds may be potential treatments against a wide variety of diseases related to aging (Figure 1).
Author Contributions
MB and DWW contributed to the structure of the manuscript. MB wrote the first draft and constructed the table. MB and DWW were involved in writing and editing the manuscript. MB and DWW designed the figure. MB did the artwork for the figure. We apologize to our colleagues whose work we were unable to cite, due to space and other limitations.
Funding
DWW is supported by NIH grants (R01AG037514, R01AG049157). MB is supported by a UCLA Undergraduate Research Fellowship Program (URFP) scholarship funded by the Wasserman Endowment.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks is given to Dr. Jung-Hoon Pyo for his encouragement.
References
Andersen, K. G., Rambaut, A., Lipkin, W. I., Holmes, E. C., and Garry, R. F. (2020). The Proximal Origin of SARS-CoV-2. Nat. Med. 26, 450–452. doi:10.1038/s41591-020-0820-9
Aparicio, R., Rana, A., and Walker, D. W. (2019). Upregulation of the Autophagy Adaptor p62/SQSTM1 Prolongs Health and Lifespan in Middle-Aged drosophila. Cell Rep. 28, 1029–1040. doi:10.1016/j.celrep.2019.06.070
Armenta, A. M., Henkel, E. D., and Ahmed, A. M. (2019). Pigmentation Disorders in the Elderly. Drugs Aging 36, 235–245. doi:10.1007/s40266-018-00633-w
Artal-Sanz, M., and Tavernarakis, N. (2010). Opposing Function of Mitochondrial Prohibitin in Aging. Aging 2, 1004–1011. doi:10.18632/aging.100246
Artal-Sanz, M., and Tavernarakis, N. (2009a). Prohibitin and Mitochondrial Biology. Trends Endocrinol. Metab. 20, 394–401. doi:10.1016/j.tem.2009.04.004
Artal-Sanz, M., and Tavernarakis, N. (2009b). Prohibitin Couples Diapause Signalling to Mitochondrial Metabolism During Ageing in C. elegans. Nature 461, 793–797. doi:10.1038/nature08466
Ashrafi, G., and Schwarz, T. L. (2013). The Pathways of Mitophagy for Quality Control and Clearance of Mitochondria. Cell Death Differ 20, 31–42. doi:10.1038/cdd.2012.81
Barnhart, K. F., Christianson, D. R., Hanley, P. W., Driessen, W. H., Bernacky, B. J., Baze, W. B., et al. (2011). A Peptidomimetic Targeting White Fat Causes Weight Loss and Improved Insulin Resistance in Obese Monkeys. Sci. Transl. Med. 3, 108ra112. doi:10.1126/scitranslmed.3002621
Basmadjian, C., Thuaud, F., Ribeiro, N., and Désaubry, L. (2013). Flavaglines: Potent Anticancer Drugs that Target Prohibitins and the Helicase eIF4A. Future Med. Chem. 5, 2185–2197. doi:10.4155/fmc.13.177
Berger, K. H., and Yaffe, M. P. (1998). Prohibitin Family Members Interact Genetically with Mitochondrial Inheritance Components in Saccharomyces cerevisiae. Mol. Cell. Biol. 18, 4043–4052. doi:10.1128/mcb.18.7.4043
Bernard, Y., Ribeiro, N., Thuaud, F., Türkeri, G., Dirr, R., Boulberdaa, M., et al. (2011). Flavaglines Alleviate Doxorubicin Cardiotoxicity: Implication of Hsp27. PLoS One 6, e25302. doi:10.1371/journal.pone.0025302
Blasco, R. B., Francoz, S., Santamaría, D., Cañamero, M., Dubus, P., Charron, J., et al. (2011). c‐Raf, but not B‐Raf, is Essential for Development of K‐Ras Oncogene‐Driven Non‐Small Cell Lung Carcinoma. Cancer Cell 19, 652–663. doi:10.1016/j.ccr.2011.04.002
Boyle, W. J., Simonet, W. S., and Lacey, D. L. (2003). Osteoclast Differentiation and Activation. Nature 423, 337–342. doi:10.1038/nature01658
Campisi, J., and D'adda Di Fagagna, F. (2007). Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 8, 729–740. doi:10.1038/nrm2233
Carvalho, C., Santos, R., Cardoso, S., Correia, S., Oliveira, P., Santos, M., et al. (2009). Doxorubicin: The Good, the Bad and the Ugly Effect. Cmc 16, 3267–3285. doi:10.2174/092986709788803312
Chan, N. C., Salazar, A. M., Pham, A. H., Sweredoski, M. J., Kolawa, N. J., Graham, R. L. J., et al. (2011). Broad Activation of the Ubiquitin-Proteasome System by Parkin Is Critical for Mitophagy. Hum. Mol. Genet. 20, 1726–1737. doi:10.1093/hmg/ddr048
Chang, S. Y., Bae, S. J., Lee, M. Y., Baek, S. H., Chang, S., and Kim, S. H. (2011). Chemical Affinity Matrix-Based Identification of Prohibitin as a Binding Protein to Anti-Resorptive Sulfonyl Amidine Compounds. Bioorg. Med. Chem. Lett. 21, 727–729. doi:10.1016/j.bmcl.2010.11.123
Chen, J. C., Jiang, C. Z., and Reid, M. S. (2005). Silencing a Prohibitin Alters Plant Development and Senescence. Plant J. 44, 16–24. doi:10.1111/j.1365-313x.2005.02505.x
Chen, Y., Liu, Q., and Guo, D. (2020). Emerging Coronaviruses: Genome Structure, Replication, and Pathogenesis. J. Med. Virol. 92, 418–423. doi:10.1002/jmv.25681
Chigira, T., Nagatoishi, S., and Tsumoto, K. (2015). Differential Binding of Prohibitin-2 to Estrogen Receptor α and to Drug-Resistant ERα Mutants. Biochem. Biophysical Res. Commun. 463, 726–731. doi:10.1016/j.bbrc.2015.06.002
Chiu, C. F., Ho, M. Y., Peng, J. M., Hung, S. W., Lee, W. H., Liang, C. M., et al. (2013). Raf Activation by Ras and Promotion of Cellular Metastasis Require Phosphorylation of Prohibitin in the Raft Domain of the Plasma Membrane. Oncogene 32, 777–787. doi:10.1038/onc.2012.86
Cipolat, S., de Brito, O. M., Dal Zilio, B., and Scorrano, L. (2004). OPA1 Requires Mitofusin 1 to Promote Mitochondrial Fusion. Proc. Natl. Acad. Sci. 101, 15927–15932. doi:10.1073/pnas.0407043101
Clyde, K., Kyle, J. L., and Harris, E. (2006). Recent Advances in Deciphering Viral and Host Determinants of Dengue Virus Replication and Pathogenesis. J. Virol. 80, 11418–11431. doi:10.1128/jvi.01257-06
Coates, P. J., Nenutil, R., Mcgregor, A., Picksley, S. M., Crouch, D. H., Hall, P. A., et al. (2001). Mammalian Prohibitin Proteins Respond to Mitochondrial Stress and Decrease During Cellular Senescence. Exp. Cell Res. 265, 262–273. doi:10.1006/excr.2001.5166
Coates, P. J., Jamieson, D. J., Smart, K., Prescott, A. R., and Hall, P. A. (1997). The Prohibitin Family of Mitochondrial Proteins Regulate Replicative Lifespan. Curr. Biol. 7, 607–610. doi:10.1016/s0960-9822(06)00261-2
Cornillez-Ty, C. T., Liao, L., Yates, J. R., Kuhn, P., and Buchmeier, M. J. (2009). Severe Acute Respiratory Syndrome Coronavirus Nonstructural Protein 2 Interacts with a Host Protein Complex Involved in Mitochondrial Biogenesis and Intracellular Signaling. J. Virol. 83, 10314–10318. doi:10.1128/jvi.00842-09
Cosialls, A. M., Pomares, H., Iglesias-Serret, D., Saura-Esteller, J., Núñez-Vázquez, S., González-Gironès, D. M., et al. (2017). The Prohibitin-Binding Compound Fluorizoline Induces Apoptosis in Chronic Lymphocytic Leukemia Cells through the Upregulation of NOXA and Synergizes with Ibrutinib, 5-Aminoimidazole-4-Carboxamide Riboside or Venetoclax. Haematologica 102, 1587–1593. doi:10.3324/haematol.2016.162958
D'amico, D., Mottis, A., Potenza, F., Sorrentino, V., Li, H., Romani, M., et al. (2019). The RNA-Binding Protein PUM2 Impairs Mitochondrial Dynamics and Mitophagy During Aging. Mol. Cell 73, 775–e10. doi:10.1016/j.molcel.2018.11.034
Degregori, J., Kowalik, T., and Nevins, J. R. (1995). Cellular Targets for Activation by the E2F1 Transcription Factor Include DNA Synthesis- and G1/S-Regulatory Genes. Mol. Cell Biol. 15, 4215–4224. doi:10.1128/mcb.15.8.4215
Djehal, A., Krayem, M., Najem, A., Hammoud, H., Cresteil, T., Nebigil, C. G., et al. (2018). Targeting Prohibitin with Small Molecules to Promote Melanogenesis and Apoptosis in Melanoma Cells. Eur. J. Med. Chem. 155, 880–888. doi:10.1016/j.ejmech.2018.06.052
Dong, W., Chao, M., Lu, Q., Chai, W., Zhang, W., Chen, X., et al. (2016). Prohibitin Overexpression Improves Myocardial Function in Diabetic Cardiomyopathy. Oncotarget 7, 66–80. doi:10.18632/oncotarget.6384
Doudican, N. A., and Orlow, S. J. (2017). Inhibition of the CRAF/Prohibitin Interaction Reverses CRAF-Dependent Resistance to Vemurafenib. Oncogene 36, 423–428. doi:10.1038/onc.2016.214
Dutta, D., Ali, N., Banerjee, E., Singh, R., Naskar, A., Paidi, R. K., et al. (2018). Low Levels of Prohibitin in Substantia Nigra Makes Dopaminergic Neurons Vulnerable in Parkinson's Disease. Mol. Neurobiol. 55, 804–821. doi:10.1007/s12035-016-0328-y
Duvezin-Caubet, S., Jagasia, R., Wagener, J., Hofmann, S., Trifunovic, A., Hansson, A., et al. (2006). Proteolytic Processing of OPA1 Links Mitochondrial Dysfunction to Alterations in Mitochondrial Morphology. J. Biol. Chem. 281, 37972–37979. doi:10.1074/jbc.m606059200
Duvezin-Caubet, S., Koppen, M., Wagener, J., Zick, M., Israel, L., Bernacchia, A., et al. (2007). OPA1 Processing Reconstituted in Yeast Depends on the Subunit Composition of the m-AAA Protease in Mitochondria. Mol. Biol. Cell 18, 3582–3590. doi:10.1091/mbc.e07-02-0164
Ebada, S. S., Lajkiewicz, N., Porco, J. A., Li-Weber, M., and Proksch, P. (2011). Chemistry and Biology of Rocaglamides (= Flavaglines) and Related Derivatives from Aglaia Species (Meliaceae). Prog. Chem. Org. Nat. Prod. 94, 1–58. doi:10.1007/978-3-7091-0748-5_1
Elderwish, S., Audebrand, A., Nebigil, C. G., and Désaubry, L. (2020). Discovery of 3,3′-Pyrrolidinyl-Spirooxindoles as Cardioprotectant Prohibitin Ligands. Eur. J. Med. Chem. 186, 111859. doi:10.1016/j.ejmech.2019.111859
Ferrer, I., Perez, E., Dalfó, E., and Barrachina, M. (2007). Abnormal Levels of Prohibitin and ATP Synthase in the Substantia Nigra and Frontal Cortex in Parkinson's Disease. Neurosci. Lett. 415, 205–209. doi:10.1016/j.neulet.2007.01.026
Franklin, D. S., and Jazwinski, S. M. (1993). A Yeast Homolog of the Rat Prohibitin Antiproliferative Gene Is Differentially Expressed and Determines Longevity in Saccharomyces cerevisiae. J. Cell Biochem. 17D, 159.
Fusaro, G., Dasgupta, P., Rastogi, S., Joshi, B., and Chellappan, S. (2003). Prohibitin Induces the Transcriptional Activity of P53 and Is Exported from the Nucleus upon Apoptotic Signaling. J. Biol. Chem. 278, 47853–47861. doi:10.1074/jbc.m305171200
Garin, J., Diez, R., Kieffer, S., Dermine, J. F., Duclos, S., Gagnon, E., et al. (2001). The Phagosome Proteome. J. Cell Biol. 152, 165–180. doi:10.1083/jcb.152.1.165
Gregory-Bass, R. C., Olatinwo, M., Xu, W., Matthews, R., Stiles, J. K., Thomas, K., et al. (2008). Prohibitin Silencing Reverses Stabilization of Mitochondrial Integrity and Chemoresistance in Ovarian Cancer Cells by Increasing Their Sensitivity to Apoptosis. Int. J. Cancer 122, 1923–1930. doi:10.1002/ijc.23351
Griparic, L., Van Der Wel, N. N., Orozco, I. J., Peters, P. J., and Van Der Bliek, A. M. (2004). Loss of the Intermembrane Space Protein Mgm1/OPA1 Induces Swelling and Localized Constrictions along the Lengths of Mitochondria. J. Biol. Chem. 279, 18792–18798. doi:10.1074/jbc.m400920200
Guyot, A. C., Leuxe, C., Disdier, C., Oumata, N., Costa, N., Roux, G. L., et al. (2020). A Small Compound Targeting Prohibitin with Potential Interest for Cognitive Deficit Rescue in Aging Mice and Tau Pathology Treatment. Sci. Rep. 10, 1143. doi:10.1038/s41598-020-57560-3
Han, E. K., Mcgonigal, T., Butler, C., Giranda, V. L., and Luo, Y. (2008). Characterization of Akt Overexpression in MiaPaCa-2 Cells: Prohibitin Is an Akt Substrate Both In Vitro and in Cells. Anticancer Res. 28, 957–963.
Harada, S., and Rodan, G. A. (2003). Control of Osteoblast Function and Regulation of Bone Mass. Nature 423, 349–355. doi:10.1038/nature01660
He, B., Feng, Q., Mukherjee, A., Lonard, D. M., Demayo, F. J., Katzenellenbogen, B. S., et al. (2008). A Repressive Role for Prohibitin in Estrogen Signaling. Mol. Endocrinol. 22, 344–360. doi:10.1210/me.2007-0400
He, P., Liu, Y., Qi, J., Zhu, H., Wang, Y., Zhao, J., et al. (2015). Prohibitin Promotes Apoptosis of Promyelocytic Leukemia Induced by Arsenic Sulfide. Int. J. Oncol. 47, 2286–2295. doi:10.3892/ijo.2015.3217
He, Q. Y., Cheung, Y. H., Leung, S. Y., Yuen, S. T., Chu, K. M., and Chiu, J. F. (2004). Diverse Proteomic Alterations in Gastric Adenocarcinoma. Proteomics 4, 3276–3287. doi:10.1002/pmic.200300916
He, S., and Sharpless, N. E. (2017). Senescence in Health and Disease. Cell 169, 1000–1011. doi:10.1016/j.cell.2017.05.015
Henss, L., Scholz, T., Grünweller, A., and Schnierle, B. (2018). Silvestrol Inhibits Chikungunya Virus Replication. Viruses 10, 592. doi:10.3390/v10110592
Ishihara, N., Fujita, Y., Oka, T., and Mihara, K. (2006). Regulation of Mitochondrial Morphology through Proteolytic Cleavage of OPA1. EMBO J. 25, 2966–2977. doi:10.1038/sj.emboj.7601184
Ito, K., Nakazato, T., Yamato, K., Miyakawa, Y., Yamada, T., Hozumi, N., et al. (2004). Induction of Apoptosis in Leukemic Cells by Homovanillic Acid Derivative, Capsaicin, through Oxidative Stress. Cancer Res. 64, 1071–1078. doi:10.1158/0008-5472.can-03-1670
Jiang, P., Xiang, Y., Wang, Y. J., Li, S. M., Wang, Y., Hua, H. R., et al. (2013). Differential Expression and Subcellular Localization of Prohibitin 1 Are Related to Tumorigenesis and Progression of Non-Small Cell Lung Cancer. Int. J. Clin. Exp. Pathol. 6, 2092–2101.
Jiang, X., and Wang, X. (2004). Cytochrome c-Mediated Apoptosis. Annu. Rev. Biochem. 73, 87–106. doi:10.1146/annurev.biochem.73.011303.073706
Jin, S. M., Lazarou, M., Wang, C., Kane, L. A., Narendra, D. P., and Youle, R. J. (2010). Mitochondrial Membrane Potential Regulates PINK1 Import and Proteolytic Destabilization by PARL. J. Cell Biol. 191, 933–942. doi:10.1083/jcb.201008084
Julien, O., and Wells, J. A. (2017). Caspases and Their Substrates. Cell Death Differ. 24, 1380–1389. doi:10.1038/cdd.2017.44
Jupe, E. R., Badgett, A. A., Neas, B. R., Craft, M. A., Mitchell, D. S., Resta, R., et al. (2001). Single Nucleotide Polymorphism in Prohibitin 3′untranslated Region and Breast-Cancer Susceptibility. The Lancet 357, 1588–1589. doi:10.1016/s0140-6736(00)04747-4
Jura, M., and Kozak, L. P. (2016). Obesity and Related Consequences to Ageing. Age 38, 23. doi:10.1007/s11357-016-9884-3,
Kahl, A., Anderson, C. J., Qian, L., Voss, H., Manfredi, G., Iadecola, C., et al. (2018). Neuronal Expression of the Mitochondrial Protein Prohibitin Confers Profound Neuroprotection in a Mouse Model of Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 38, 1010–1020. doi:10.1177/0271678x17720371
Kakarla, M., Puppala, V. K., Tyagi, S., Anger, A., Repp, K., Wang, J., et al. (2019). Circulating Levels of Mitochondrial Uncoupling Protein 2, but Not Prohibitin, Are Lower in Humans with Type 2 Diabetes and Correlate with Brachial Artery Flow-Mediated Dilation. Cardiovasc. Diabetol. 18, 148. doi:10.1186/s12933-019-0956-4
Karnoub, A. E., and Weinberg, R. A. (2008). Ras Oncogenes: Split Personalities. Nat. Rev. Mol. Cell Biol. 9, 517–531. doi:10.1038/nrm2438
Kim, J. W., Akiyama, M., Park, J. H., Lin, M. L., Shimo, A., Ueki, T., et al. (2009). Activation of an Estrogen/Estrogen Receptor Signaling by BIG3 through its Inhibitory Effect on Nuclear Transport of PHB2/REA in Breast Cancer. Cancer Sci. 100, 1468–1478. doi:10.1111/j.1349-7006.2009.01209.x
Kimura, K. D., Tissenbaum, H. A., Liu, Y., and Ruvkun, G. (1997). daf-2, an Insulin Receptor-Like Gene that Regulates Longevity and Diapause in Caenorhabditis elegans. Science 277, 942–946. doi:10.1126/science.277.5328.942
Kirchman, P. A., Kim, S., Lai, C. Y., and Jazwinski, S. M. (1999). Interorganelle Signaling Is a Determinant of Longevity in Saccharomyces cerevisiae. Genetics 152, 179–190. doi:10.1093/genetics/152.1.179
Kolonin, M. G., Saha, P. K., Chan, L., Pasqualini, R., and Arap, W. (2004). Reversal of Obesity by Targeted Ablation of Adipose Tissue. Nat. Med. 10, 625–632. doi:10.1038/nm1048
Koushyar, S., Jiang, W. G., and Dart, D. A. (2015). Unveiling the Potential of Prohibitin in Cancer. Cancer Lett. 369, 316–322. doi:10.1016/j.canlet.2015.09.012
Kuramori, C., Azuma, M., Kume, K., Kaneko, Y., Inoue, A., Yamaguchi, Y., et al. (2009). Capsaicin Binds to Prohibitin 2 and Displaces it from the Mitochondria to the Nucleus. Biochem. Biophysical Res. Commun. 379, 519–525. doi:10.1016/j.bbrc.2008.12.103
Lachén-Montes, M., González-Morales, A., Zelaya, M. V., Pérez-Valderrama, E., Ausín, K., Ferrer, I., et al. (2017). Olfactory Bulb Neuroproteomics Reveals a Chronological Perturbation of Survival Routes and a Disruption of Prohibitin Complex During Alzheimer's Disease Progression. Sci. Rep. 7, 9115. doi:10.1038/s41598-017-09481-x
Lee, C. H., Choi, S. W., Kim, J. Y., Kim, S. H., Yoon, K. H., Oh, J., et al. (2015). Overexpression of Prohibitin-1 Inhibits RANKL-Induced Activation of p38-Elk-1-SRE Signaling axis Blocking MKK6 Activity. Biochem. Biophysical Res. Commun. 463, 1028–1033. doi:10.1016/j.bbrc.2015.06.053
Lee, H., Arnouk, H., Sripathi, S., Chen, P., Zhang, R., Bartoli, M., et al. (2010). Prohibitin as an Oxidative Stress Biomarker in the Eye. Int. J. Biol. Macromolecules 47, 685–690. doi:10.1016/j.ijbiomac.2010.08.018
Lee, M. Y., Kim, M. H., Kim, J., Kim, S. H., Kim, B. T., Jeong, I. H., et al. (2010). Synthesis and SAR of Sulfonyl- and Phosphoryl Amidine Compounds as Anti-resorptive Agents. Bioorg. Med. Chem. Lett. 20, 541–545. doi:10.1016/j.bmcl.2009.11.104
Liu, K., Chen, Y., Lin, R., and Han, K. (2020). Clinical Features of COVID-19 in Elderly Patients: A Comparison with Young and Middle-Aged Patients. J. Infect. 80, e14–e18. doi:10.1016/j.jinf.2020.03.005
Liu, S., Wang, W., Brown, L. E., Qiu, C., Lajkiewicz, N., Zhao, T., et al. (2015). A Novel Class of Small Molecule Compounds that Inhibit Hepatitis C Virus Infection by Targeting the Prohibitin-CRaf Pathway. EBioMedicine 2, 1600–1606. doi:10.1016/j.ebiom.2015.09.018
Liu, T., Tang, H., Lang, Y., Liu, M., and Li, X. (2009). MicroRNA-27a Functions as an Oncogene in Gastric Adenocarcinoma by Targeting Prohibitin. Cancer Lett. 273, 233–242. doi:10.1016/j.canlet.2008.08.003
Liu, X., Ren, Z., Zhan, R., Wang, X., Wang, X., Zhang, Z., et al. (2009). Prohibitin Protects against Oxidative Stress-Induced Cell Injury in Cultured Neonatal Cardiomyocyte. Cell Stress and Chaperones 14, 311–319. doi:10.1007/s12192-008-0086-5
Liu, X. Q., Sheng, R., and Qin, Z. H. (2009). The Neuroprotective Mechanism of Brain Ischemic Preconditioning. Acta Pharmacol. Sin 30, 1071–1080. doi:10.1038/aps.2009.105
Liu, X. T., Stewart, C. A., King, R. L., Danner, D. A., Dellorco, R. T., and McClung, J. K. (1994). Prohibitin Expression During Cellular Senescence of Human Diploid Fibroblasts. Biochem. Biophysical Res. Commun. 201, 409–414. doi:10.1006/bbrc.1994.1716
Liu, Y., He, P., Zhang, M., and Wu, D. (2012). Lentiviral Vector-Mediated RNA Interference Targeted Against Prohibitin Inhibits Apoptosis of the Retinoic Acid-Resistant Acute Promyelocytic Leukemia Cell Line NB4-R1. Mol. Med. Rep. 6, 1288–1292. doi:10.3892/mmr.2012.1105
Longo, V. D., Shadel, G. S., Kaeberlein, M., and Kennedy, B. (2012). Replicative and Chronological Aging in Saccharomyces cerevisiae. Cell Metab. 16, 18–31. doi:10.1016/j.cmet.2012.06.002
Luan, Z., He, Y., Alattar, M., Chen, Z., and He, F. (2014). Targeting the Prohibitin Scaffold-CRAF Kinase Interaction in RAS-ERK-Driven Pancreatic Ductal Adenocarcinoma. Mol. Cancer 13, 38. doi:10.1186/1476-4598-13-38
Ma, B., and Hottiger, M. O. (2016). Crosstalk Between Wnt/β-Catenin and NF-Κb Signaling Pathway During Inflammation. Front. Immunol. 7, 378. doi:10.3389/fimmu.2016.00378
Marcotte, R., Lacelle, C., and Wang, E. (2004). Senescent Fibroblasts Resist Apoptosis by Downregulating Caspase-3. Mech. Ageing Dev. 125, 777–783. doi:10.1016/j.mad.2004.07.007
McClung, J. K., Danner, D. B., Stewart, D. A., Smith, J. R., Schneider, E. L., Lumpkin, C. K., et al. (1989). Isolation of a cDNA that Hybrid Selects Antiproliferative mRNA from Rat Liver. Biochem. Biophysical Res. Commun. 164, 1316–1322. doi:10.1016/0006-291x(89)91813-5
Meertens, L., Bertaux, C., and Dragic, T. (2006). Hepatitis C Virus Entry Requires a Critical Postinternalization Step and Delivery to Early Endosomes via Clathrin-Coated Vesicles. J. Virol. 80, 11571–11578. doi:10.1128/jvi.01717-06
Merkwirth, C., Martinelli, P., Korwitz, A., Morbin, M., Brönneke, H. S., Jordan, S. D., et al. (2012). Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration. Plos Genet. 8, e1003021. doi:10.1371/journal.pgen.1003021
Messaoudi, I., Vomaske, J., Totonchy, T., Kreklywich, C. N., Haberthur, K., Springgay, L., et al. (2013). Chikungunya Virus Infection Results in Higher and Persistent Viral Replication in Aged Rhesus Macaques Due to Defects in Anti-Viral Immunity. Plos Negl. Trop. Dis. 7, e2343. doi:10.1371/journal.pntd.0002343
Moncunill-Massaguer, C., Saura-Esteller, J., Pérez-Perarnau, A., Palmeri, C. M., Núñez-Vázquez, S., Cosialls, A. M., et al. (2015). A Novel Prohibitin-Binding Compound Induces the Mitochondrial Apoptotic Pathway through NOXA and BIM Upregulation. Oncotarget 6, 41750–41765. doi:10.18632/oncotarget.6154
Muñoz-Manchado, A. B., Villadiego, J., Romo-Madero, S., Suárez-Luna, N., Bermejo-Navas, A., Rodríguez-Gómez, J. A., et al. (2016). Chronic and Progressive Parkinson's Disease MPTP Model in Adult and Aged Mice. J. Neurochem. 136, 373–387. doi:10.1111/jnc.13409
Nebigil, C. G., Moog, C., Vagner, S., Benkirane-Jessel, N., Smith, D. R., and Désaubry, L. (2020). Flavaglines as Natural Products Targeting eIF4A and Prohibitins: From Traditional Chinese Medicine to Antiviral Activity against Coronaviruses. Eur. J. Med. Chem. 203, 112653. doi:10.1016/j.ejmech.2020.112653
Nijtmans, L. G. J., Artal Sanz, M., Grivell, L. A., and Coates, P. J. (2002). The Mitochondrial PHB Complex: Roles in Mitochondrial Respiratory Complex Assembly, Ageing and Degenerative Disease. Cell Mol. Life Sci. (Cmls) 59, 143–155. doi:10.1007/s00018-002-8411-0
Nuell, M. J., Stewart, D. A., Walker, L., Friedman, V., Wood, C. M., Owens, G. A., et al. (1991). Prohibitin, an Evolutionarily Conserved Intracellular Protein that Blocks DNA Synthesis in normal Fibroblasts and HeLa Cells. Mol. Cell Biol. 11, 1372–1381. doi:10.1128/mcb.11.3.1372
Oda, E., Ohki, R., Murasawa, H., Nemoto, J., Shibue, T., Yamashita, T., et al. (2000). Noxa, a BH3-Only Member of the Bcl-2 Family and Candidate Mediator of P53-Induced Apoptosis. Science 288, 1053–1058. doi:10.1126/science.288.5468.1053
Olson, R. D., and Mushlin, P. S. (1990). Doxorubicin Cardiotoxicity: Analysis of Prevailing Hypotheses. FASEB j. 4, 3076–3086. doi:10.1096/fasebj.4.13.2210154
Pan, L., Woodard, J. L., Lucas, D. M., Fuchs, J. R., and Douglas Kinghorn, A. (2014). Rocaglamide, Silvestrol and Structurally Related Bioactive Compounds from Aglaia Species. Nat. Prod. Rep. 31, 924–939. doi:10.1039/c4np00006d
Park, S. E., Xu, J., Frolova, A., Liao, L., O'malley, B. W., and Katzenellenbogen, B. S. (2005). Genetic Deletion of the Repressor of Estrogen Receptor Activity (REA) Enhances the Response to Estrogen in Target Tissues In Vivo. Mol. Cell Biol. 25, 1989–1999. doi:10.1128/mcb.25.5.1989-1999.2005
Patel, N., Chatterjee, S. K., Vrbanac, V., Chung, I., Mu, C. J., Olsen, R. R., et al. (2010). Rescue of Paclitaxel Sensitivity by Repression of Prohibitin1 in Drug-Resistant Cancer Cells. Proc. Natl. Acad. Sci. 107, 2503–2508. doi:10.1073/pnas.0910649107
Peng, Y. T., Chen, P., Ouyang, R. Y., and Song, L. (2015). Multifaceted Role of Prohibitin in Cell Survival and Apoptosis. Apoptosis 20, 1135–1149. doi:10.1007/s10495-015-1143-z
Pérez-Gracia, E., Torrejón-Escribano, B., and Ferrer, I. (2008). Dystrophic Neurites of Senile Plaques in Alzheimer's Disease Are Deficient in Cytochrome c Oxidase. Acta Neuropathol. 116, 261–268. doi:10.1007/s00401-008-0370-6
Pérez-Perarnau, A., Preciado, S., Palmeri, C. M., Moncunill-Massaguer, C., Iglesias-Serret, D., González-Gironès, D. M., et al. (2014). A Trifluorinated Thiazoline Scaffold Leading to Pro-Apoptotic Agents Targeting Prohibitins. Angew. Chem. Int. Ed. 53, 10150–10154. doi:10.1002/anie.201405758
Perron, A., Nishikawa, Y., Iwata, J., Shimojo, H., Takaya, J., Kobayashi, K., et al. (2018). Small-Molecule Screening Yields a Compound that Inhibits the Cancer-Associated Transcription Factor Hes1 via the PHB2 Chaperone. J. Biol. Chem. 293, 8285–8294. doi:10.1074/jbc.ra118.002316
Petersen, K. F., Befroy, D., Dufour, S., Dziura, J., Ariyan, C., Rothman, D. L., et al. (2003). Mitochondrial Dysfunction in the Elderly: Possible Role in Insulin Resistance. Science 300, 1140–1142. doi:10.1126/science.1082889
Pickles, S., Vigié, P., and Youle, R. J. (2018). Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 28, R170–R185. doi:10.1016/j.cub.2018.01.004
Pickrell, A. M., and Youle, R. J. (2015). The Roles of PINK1, Parkin, and Mitochondrial Fidelity in Parkinson's Disease. Neuron 85, 257–273. doi:10.1016/j.neuron.2014.12.007
Piper, P. W., Jones, G. W., Bringloe, D., Harris, N., Maclean, M., and Mollapour, M. (2002). The Shortened Replicative Life Span of Prohibitin Mutants of Yeast Appears to Be Due to Defective Mitochondrial Segregation in Old Mother Cells. Aging Cell 1, 149–157. doi:10.1046/j.1474-9728.2002.00018.x
Polier, G., Neumann, J., Thuaud, F., Ribeiro, N., Gelhaus, C., Schmidt, H., et al. (2012). The Natural Anticancer Compounds Rocaglamides Inhibit the Raf-MEK-ERK Pathway by Targeting Prohibitin 1 and 2. Chem. Biol. 19, 1093–1104. doi:10.1016/j.chembiol.2012.07.012
Pomares, H., Palmeri, C. M., Iglesias-Serret, D., Moncunill-Massaguer, C., Saura-Esteller, J., Núñez-Vázquez, S., et al. (2016). Targeting Prohibitins Induces Apoptosis in Acute Myeloid Leukemia Cells. Oncotarget 7, 64987–65000. doi:10.18632/oncotarget.11333
Poole, A. C., Thomas, R. E., Yu, S., Vincow, E. S., and Pallanck, L. (2010). The Mitochondrial Fusion-Promoting Factor Mitofusin Is a Substrate of the PINK1/Parkin Pathway. PLoS One 5, e10054. doi:10.1371/journal.pone.0010054
Qu, Y., Konrad, C., Anderson, C., Qian, L., Yin, T., Manfredi, G., et al. (2020). Prohibitin S-Nitrosylation Is Required for the Neuroprotective Effect of Nitric Oxide in Neuronal Cultures. J. Neurosci. 40, 3142–3151. doi:10.1523/jneurosci.1804-19.2020
Qureshi, R., Yildirim, O., Gasser, A., Basmadjian, C., Zhao, Q., Wilmet, J. P., et al. (2015). FL3, a Synthetic Flavagline and Ligand of Prohibitins, Protects Cardiomyocytes via STAT3 from Doxorubicin Toxicity. PLoS One 10, e0141826. doi:10.1371/journal.pone.0141826
Rajalingam, K., Wunder, C., Brinkmann, V., Churin, Y., Hekman, M., Sievers, C., et al. (2005). Prohibitin Is Required for Ras-Induced Raf-MEK-ERK Activation and Epithelial Cell Migration. Nat. Cell Biol. 7, 837–843. doi:10.1038/ncb1283
Rana, A., Oliveira, M. P., Khamoui, A. V., Aparicio, R., Rera, M., Rossiter, H. B., et al. (2017). Promoting Drp1-Mediated Mitochondrial Fission in Midlife Prolongs Healthy Lifespan of Drosophila melanogaster. Nat. Commun. 8, 448. doi:10.1038/s41467-017-00525-4
Rastogi, S., Joshi, B., Dasgupta, P., Morris, M., Wright, K., and Chellappan, S. (2006a). Prohibitin Facilitates Cellular Senescence by Recruiting Specific Corepressors to Inhibit E2F Target Genes. Mol. Cell Biol. 26, 4161–4171. doi:10.1128/mcb.02142-05
Rastogi, S., Joshi, B., Fusaro, G., and Chellappan, S. (2006b). Camptothecin Induces Nuclear Export of Prohibitin Preferentially in Transformed Cells through a CRM-1-Dependent Mechanism. J. Biol. Chem. 281, 2951–2959. doi:10.1074/jbc.m508669200
Reggiori, F., Monastyrska, I., Verheije, M. H., Calì, T., Ulasli, M., Bianchi, S., et al. (2010). Coronaviruses Hijack the LC3-I-Positive EDEMosomes, ER-Derived Vesicles Exporting Short-Lived ERAD Regulators, for Replication. Cell Host & Microbe 7, 500–508. doi:10.1016/j.chom.2010.05.013
Reid, M., Price, J. C., and Tien, P. C. (2017). Hepatitis C Virus Infection in the Older Patient. Infect. Dis. Clin. North America 31, 827–838. doi:10.1016/j.idc.2017.07.014
Reyes-Escogido, M., Gonzalez-Mondragon, E. G., and Vazquez-Tzompantzi, E. (2011). Chemical and Pharmacological Aspects of Capsaicin. Molecules 16, 1253–1270. doi:10.3390/molecules16021253
Riggins, R. B., Schrecengost, R. S., Guerrero, M. S., and Bouton, A. H. (2007). Pathways to Tamoxifen Resistance. Cancer Lett. 256, 1–24. doi:10.1016/j.canlet.2007.03.016
Ross, J. A., Robles-Escajeda, E., Oaxaca, D. M., Padilla, D. L., and Kirken, R. A. (2017). The Prohibitin Protein Complex Promotes Mitochondrial Stabilization and Cell Survival in Hematologic Malignancies. Oncotarget 8, 65445–65456. doi:10.18632/oncotarget.18920
Sato, S., Murata, A., Orihara, T., Shirakawa, T., Suenaga, K., Kigoshi, H., et al. (2011). Marine Natural Product Aurilide Activates the OPA1-Mediated Apoptosis by Binding to Prohibitin. Chem. Biol. 18, 131–139. doi:10.1016/j.chembiol.2010.10.017
Schleicher, M., Shepherd, B. R., Suarez, Y., Fernandez-Hernando, C., Yu, J., Pan, Y., et al. (2008). Prohibitin-1 Maintains the Angiogenic Capacity of Endothelial Cells by Regulating Mitochondrial Function and Senescence. J. Cell Biol. 180, 101–112. doi:10.1083/jcb.200706072
Semenzato, M., Cogliati, S., and Scorrano, L. (2011). Prohibitin(g) Cancer: Aurilide and Killing by Opa1-Dependent Cristae Remodeling. Chem. Biol. 18, 8–9. doi:10.1016/j.chembiol.2011.01.001
Sharma, A., Vasanthapuram, R., Venkataswamy, M. M., and Desai, A. (2020). Prohibitin 1/2 Mediates Dengue-3 Entry into Human Neuroblastoma (SH-Sy5y) and Microglia (CHME-3) Cells. J. Biomed. Sci. 27, 55. doi:10.1186/s12929-020-00639-w
Sian, J., Youdim, M. B. H., Riederer, P., and Gerlach, M. (1999). Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia, PA: Lippincott-Raven.
Signorile, A., Sgaramella, G., Bellomo, F., and De Rasmo, D. (2019). Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells 8, 71. doi:10.3390/cells8010071
Singal, P. K., and Iliskovic, N. (1998). Doxorubicin-Induced Cardiomyopathy. N. Eng. J. Med. 339, 900–905. doi:10.1056/nejm199809243391307
Smith, J., Wright, J., and Schneider, B. L. (2015). A Budding Yeast's Perspective on Aging: The Shape I'm In. Exp. Biol. Med. 240, 701–710. doi:10.1177/1535370215577584
Snyder, J. R., Hall, A., Ni-Komatsu, L., Khersonsky, S. M., Chang, Y. T., and Orlow, S. J. (2005). Dissection of Melanogenesis with Small Molecules Identifies Prohibitin as a Regulator. Chem. Biol. 12, 477–484. doi:10.1016/j.chembiol.2005.02.014
Song, Z., Ghochani, M., McCaffery, J. M., Frey, T. G., and Chan, D. C. (2009). Mitofusins and OPA1 Mediate Sequential Steps in Mitochondrial Membrane Fusion. Mol. Biol. Cell 20, 3525–3532. doi:10.1091/mbc.e09-03-0252
Sripathi, S. R., He, W., Atkinson, C. L., Smith, J. J., Liu, Z., Elledge, B. M., et al. (2011). Mitochondrial-Nuclear Communication by Prohibitin Shuttling under Oxidative Stress. Biochemistry 50, 8342–8351. doi:10.1021/bi2008933
Strub, G. M., Paillard, M., Liang, J., Gomez, L., Allegood, J. C., Hait, N. C., et al. (2011). Sphingosine-1-Phosphate Produced by Sphingosine Kinase 2 in Mitochondria Interacts with Prohibitin 2 to Regulate Complex IV Assembly and Respiration. FASEB J. 25, 600–612. doi:10.1096/fj.10-167502
Suenaga, K., Mutou, T., Shibata, T., Itoh, T., Fujita, T., Takada, N., et al. (2004). Aurilide, a Cytotoxic Depsipeptide from the Sea Hare Dolabella Auricularia: Isolation, Structure Determination, Synthesis, and Biological Activity. Tetrahedron 60, 8509–8527. doi:10.1016/j.tet.2004.06.125
Supale, S., Thorel, F., Merkwirth, C., Gjinovci, A., Herrera, P. L., Scorrano, L., et al. (2013). Loss of Prohibitin Induces Mitochondrial Damages Altering β-Cell Function and Survival and Is Responsible for Gradual Diabetes Development. Diabetes 62, 3488–3499. doi:10.2337/db13-0152
Tanaka, A., Cleland, M. M., Xu, S., Narendra, D. P., Suen, D. F., Karbowski, M., et al. (2010). Proteasome and P97 Mediate Mitophagy and Degradation of Mitofusins Induced by Parkin. J. Cell Biol. 191, 1367–1380. doi:10.1083/jcb.201007013
Tanida, I., Ueno, T., and Kominami, E. (2008). LC3 and Autophagy. Methods Mol. Biol. 445, 77–88. doi:10.1007/978-1-59745-157-4_4
Theiss, A. L., and Sitaraman, S. V. (2011). The Role and Therapeutic Potential of Prohibitin in Disease. Biochim. Biophys. Acta 1813, 1137–1143. doi:10.1016/j.bbamcr.2011.01.033
Too, I., Bonne, I., Tan, E. L., Chu, J., and Alonso, S. (2018). Prohibitin Plays a Critical Role in Enterovirus 71 Neuropathogenesis. Plos Pathog. 14, e1006778. doi:10.1371/journal.ppat.1006778
Vessal, M., Mishra, S., Moulik, S., and Murphy, L. J. (2006). Prohibitin Attenuates Insulin-Stimulated Glucose and Fatty Acid Oxidation in Adipose Tissue by Inhibition of Pyruvate Carboxylase. FEBS J. 273, 568–576. doi:10.1111/j.1742-4658.2005.05090.x
Vieyres, G., Thomas, X., Descamps, V., Duverlie, G., Patel, A. H., and Dubuisson, J. (2010). Characterization of the Envelope Glycoproteins Associated with Infectious Hepatitis C Virus. J. Virol. 84, 10159–10168. doi:10.1128/jvi.01180-10
Wang, D., Tabti, R., Elderwish, S., Abou-Hamdan, H., Djehal, A., Yu, P., et al. (2020). Prohibitin Ligands: A Growing Armamentarium to Tackle Cancers, Osteoporosis, Inflammatory, Cardiac and Neurological Diseases. Cell. Mol. Life Sci. 77, 3525–3546. doi:10.1007/s00018-020-03475-1
Wang, X., Winter, D., Ashrafi, G., Schlehe, J., Wong, Y. L., Selkoe, D., et al. (2011). PINK1 and Parkin Target Miro for Phosphorylation and Degradation to Arrest Mitochondrial Motility. Cell 147, 893–906. doi:10.1016/j.cell.2011.10.018
Wasilewski, M., and Scorrano, L. (2009). The Changing Shape of Mitochondrial Apoptosis. Trends Endorinol. Metab. 20, 287–294. doi:10.1016/j.tem.2009.03.007
Wei, Y., Chiang, W., Sumpter, R., Mishra, P., and Levine, B. (2017). Prohibitin 2 Is an Inner Mitochondrial Membrane Mitophagy Receptor. Cell 168, 224–238. doi:10.1016/j.cell.2016.11.042
Wintachai, P., Thuaud, F., Basmadjian, C., Roytrakul, S., Ubol, S., Désaubry, L., et al. (2015). Assessment of Flavaglines as Potential Chikungunya Virus Entry Inhibitors. Microbiol. Immunol. 59, 129–141. doi:10.1111/1348-0421.12230
Wintachai, P., Wikan, N., Kuadkitkan, A., Jaimipuk, T., Ubol, S., Pulmanausahakul, R., et al. (2012). Identification of Prohibitin as a Chikungunya Virus Receptor Protein. J. Med. Virol. 84, 1757–1770. doi:10.1002/jmv.23403
Wu, D., and Pan, W. (2010). GSK3: A Multifaceted Kinase in Wnt Signaling. Trends Biochem. Sci. 35, 161–168. doi:10.1016/j.tibs.2009.10.002
Yager, J. D., and Davidson, N. E. (2006). Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 354, 270–282. doi:10.1056/nejmra050776
Yan, C., Gong, L., Chen, L., Xu, M., Abou-Hamdan, H., and Tang, M. (2020). PHB2 (Prohibitin 2) Promotes PINK1-PRKN/Parkin Dependent Mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy 16, 419–434. doi:10.1080/15548627.2019.1628520
Yang, J., Li, B., and He, Q. Y. (2018). Significance of Prohibitin Domain Family in Tumorigenesis and its Implication in Cancer Diagnosis and Treatment. Cell Death Dis 9, 580. doi:10.1038/s41419-018-0661-3
Yoshimaru, T., Komatsu, M., Matsuo, T., Chen, Y. A., Murakami, Y., Mizuguchi, K., et al. (2013). Targeting BIG3-PHB2 Interaction to Overcome Tamoxifen Resistance in Breast Cancer Cells. Nat. Commun. 4, 2443. doi:10.1038/ncomms3443
Yoshimaru, T., Komatsu, M., Miyoshi, Y., Honda, J., Sasa, M., and Katagiri, T. (2015). Therapeutic Advances in BIG3-PHB2 Inhibition Targeting the Crosstalk Between Estrogen and Growth Factors in Breast Cancer. Cancer Sci. 106, 550–558. doi:10.1111/cas.12654
Yoshimaru, T., Komatsu, M., Tashiro, E., Imoto, M., Osada, H., Miyoshi, Y., et al. (2014). Xanthohumol Suppresses Oestrogen-Signalling in Breast Cancer through the Inhibition of BIG3-PHB2 Interactions. Sci. Rep. 4, 7355. doi:10.1038/srep07355
Youle, R. J., and Narendra, D. P. (2011). Mechanisms of Mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14. doi:10.1038/nrm3028
Youle, R. J., and Van Der Bliek, A. M. (2012). Mitochondria Fission, Fusion, and Stress. Science 337, 1062–1065. doi:10.1126/science.1219855
Yurugi, H., Marini, F., Weber, C., David, K., Zhao, Q., Binder, H., et al. (2017). Targeting Prohibitins with Chemical Ligands Inhibits KRAS-Mediated Lung Tumours. Oncogene 36, 4778–4789. doi:10.1038/onc.2017.93
Zhou, P., Qian, L., D'aurelio, M., Cho, S., Wang, G., Manfredi, G., et al. (2012). Prohibitin Reduces Mitochondrial Free Radical Production and Protects Brain Cells from Different Injury Modalities. J. Neurosci. 32, 583–592. doi:10.1523/jneurosci.2849-11.2012
Ziviani, E., Tao, R. N., and Whitworth, A. J. (2010). Drosophila Parkin Requires PINK1 for Mitochondrial Translocation and Ubiquitinates Mitofusin. Proc. Natl. Acad. Sci. USA 107, 5018–5023. doi:10.1073/pnas.0913485107
Keywords: prohibitin, aging, age-related diseases, PHB1, PHB2
Citation: Belser M and Walker DW (2021) Role of Prohibitins in Aging and Therapeutic Potential Against Age-Related Diseases. Front. Genet. 12:714228. doi: 10.3389/fgene.2021.714228
Received: 24 May 2021; Accepted: 21 September 2021;
Published: 29 October 2021.
Edited by:
Michael Rera, Centre de Recherches Interdisciplinaires (CRI), FranceReviewed by:
Maria Dudau, Victor Babes National Institute of Pathology (INCDVB), RomaniaPeter William Piper, The University of Sheffield, United Kingdom
Copyright © 2021 Belser and Walker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Misa Belser, belmisa@ucla.edu; David W. Walker, davidwalker@ucla.edu
 Misa Belser
Misa Belser