
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Genet. , 08 July 2021
Sec. Pharmacogenetics and Pharmacogenomics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.711943
This article is part of the Research Topic Pharmacogenomics: From Bench to Bedside and Back Again View all 8 articles
 Tessa A. M. Mulder1
Tessa A. M. Mulder1 Ruben A. G. van Eerden2
Ruben A. G. van Eerden2 Mirjam de With1,2
Mirjam de With1,2 Laure Elens1,3,4
Laure Elens1,3,4 Dennis A. Hesselink5,6
Dennis A. Hesselink5,6 Maja Matic1
Maja Matic1 Sander Bins2
Sander Bins2 Ron H. J. Mathijssen2
Ron H. J. Mathijssen2 Ron H. N. van Schaik1*
Ron H. N. van Schaik1*Cytochrome P450 3A4 (CYP3A4) is the most important drug metabolizing enzyme in the liver, responsible for the oxidative metabolism of ∼50% of clinically prescribed drugs. Therefore, genetic variation in CYP3A4 could potentially affect the pharmacokinetics, toxicity and clinical outcome of drug treatment. Thus far, pharmacogenetics for CYP3A4 has not received much attention. However, the recent discovery of the intron 6 single-nucleotide polymorphism (SNP) rs35599367C > T, encoding the CYP3A4∗22 allele, led to several studies into the pharmacogenetic effect of CYP3A4∗22 on different drugs. This allele has a relatively minor allele frequency of 3-5% and an effect on CYP3A4 enzymatic activity. Thus far, no review summarizing the data published on several drugs is available yet. This article therefore addresses the current knowledge on CYP3A4∗22. This information may help in deciding if, and for which drugs, CYP3A4∗22 genotype-based dosing could be helpful in improving drug therapy. CYP3A4∗22 was shown to significantly influence the pharmacokinetics of several drugs, with currently being most thoroughly investigated tacrolimus, cyclosporine, and statins. Additional studies, focusing on toxicity and clinical outcome, are warranted to demonstrate clinical utility of CYP3A4∗22 genotype-based dosing.
Cytochrome P450 enzymes (CYP450s) are responsible for the oxidative metabolism of many drugs, the most abundant enzyme being CYP3A4, and are involved in the metabolism of 50% of prescribed drugs (Wrighton et al., 2000; Danielson, 2002). Although analyzing genetic variation in CYP-genes is well known and nowadays used in clinical practice (Lauschke et al., 2017; Roden et al., 2019; van Schaik et al., 2020), single-nucleotide polymorphisms (SNPs) were thought to have a limited contribution to the observed variability in CYP3A4 activity, because of the unimodal distribution of enzyme activity (Lin et al., 2002) and the wide range of hepatic protein expression (Lamba et al., 2002a). Genetic variants changing amino acids are rare for CYP3A4 (Lamba et al., 2002b). The relatively abundant CYP3A4∗1B variant (3–5%) (van Schaik et al., 2000) has been associated with altered drug metabolism (Rebbeck et al., 1998; Westlind et al., 1999), but results are inconsistent and its function remains controversial (Rebbeck et al., 1998; Amirimani et al., 1999; Ball et al., 1999; García-Martín et al., 2002; Lamba et al., 2002b; Spurdle et al., 2002; Wojnowski and Kamdem, 2006). This could be due to linkage disequilibrium with CYP3A5∗1 (Zeigler-Johnson et al., 2004; Miao et al., 2009), suggesting that expression of CYP3A5 due to presence of CYP3A5∗1 accounts for the association between CYP3A metabolic activity and CYP3A4∗1B (Kuehl et al., 2001). However, in 2011 the CYP3A4 intron 6 SNP (rs35599367C > T, CYP3A4∗22) was described by Wang et al. (2011), using an allelic expression imbalance approach, explaining 12% of CYP3A4 enzyme activity variability. CYP3A4∗22 predominantly occurs in Europeans and admixed Americans, compared to Africans and Asians (MAF: 5%, 2.6%, < 0.1%, < 0.6%, respectively) (Elens et al., 2011b; Zhou et al., 2017), and proved to encode decreased mRNA, protein and enzymatic activity in vivo (Wang et al., 2011; Klein et al., 2012; Elens et al., 2013b; Okubo et al., 2013). In CYP3A4∗22, formation of a non-functional alternative splice variant (aSV) was >100% increased, with partial intron 6 retention (Wang and Sadee, 2016). Interestingly, CYP3A4 aSV was found in liver, but not in small intestine (Wang and Sadee, 2016). Since its discovery, CYP3A4∗22 effects have been described in several studies, but this information has not yet been summarized. This review addresses current knowledge on CYP3A4∗22, focusing on clinical studies. Unless otherwise indicated, statements on CYP3A4∗22 reflect comparisons to CYP3A4∗1/∗1 wild-type patients.
Elens et al. (2013b) used the CYP3A golden standard phenotyping probes midazolam and erythromycin as indicators for the in vivo effect of CYP3A4∗22 on drug metabolism, showing that cancer patients carrying CYP3A4∗22 had a 40% reduction in erythromycin clearance and a 21% lower midazolam metabolic ratio (MR) (Elens et al., 2013b) (Supplementary Table 1). This indicates that CYP3A4∗22 has potential clinically relevant effects.
Cyclosporine and tacrolimus are immunosuppressants used in solid organ transplantation, metabolized by CYP3A4 into less active compounds, and are characterized by highly variable pharmacokinetics and a narrow therapeutic index (Moes et al., 2014). To prevent overexposure (risking drug-related toxicity) or underexposure (risking transplant rejection), therapeutic drug monitoring is applied (Kahan et al., 2002; Lloberas et al., 2017). Pharmacogenetic testing may optimize the starting dose when pharmacokinetic steady states are not yet achieved.
Supra-therapeutic tacrolimus exposure is associated with toxicity (Miano et al., 2020), the main pharmacogenetic contributor being CYP3A5, first described by Hesselink et al. (2003). In 2011, Elens et al. (2011b) reported the influence of CYP3A4∗22, showing that CYP3A4∗22 carriers have 16–76% increased tacrolimus pre-dose concentrations (C0) (Elens et al., 2011b; Elens et al., 2013a; Guy-Viterbo et al., 2014; Pallet et al., 2015) and 29–100% increased dose-adjusted tacrolimus C0 (C0/D) (Elens et al., 2011b,c, 2013a; Kurzawski et al., 2014; Pallet et al., 2015; Lloberas et al., 2017; Gómez-Bravo et al., 2018) (Supplementary Table 2). Combining CYP3A4 and CYP3A5 genotype into poor, intermediate and extensive CYP3A metabolizers explains >60% of tacrolimus C0/D variability (Elens et al., 2011c). Four kidney transplant recipients with [CYP3A4∗22/∗22 + CYP3A5∗3/∗3] genotype showed a 91% increase in median tacrolimus C0/D compared to [CYP3A4∗1/∗1 + CYP3A5∗3/∗3] patients (3.05 vs. 1.60 ng/ml/mg, respectively) and a 342% increase compared to [CYP3A4∗1/∗1 + CYP3A5∗1/∗1] individuals (3.05 vs. 0.69 ng/ml/mg, respectively) (Scheibner et al., 2018). After 12 months, CYP3A4∗22 carriers had 36.8% lower mean steady-state clearance and 50% lower dose requirements (de Jonge et al., 2015), comparable to other studies with 30–33% lower mean tacrolimus dose requirement for CYP3A4∗22 carriers (Elens et al., 2011b; Gijsen et al., 2013; Pallet et al., 2015). Using a dosing algorithm, including CYP3A4∗22 and CYP3A5∗3 amongst other covariables, 58% of patients were on tacrolimus target at day 3 after transplantation (Francke et al., 2021) compared to 18.5–37.4% after initial bodyweight-based dosing (Thervet et al., 2010; Budde et al., 2014; Shuker et al., 2016). However, not all studies found a significant effect, but trends were observed (Santoro et al., 2013; Tavira et al., 2013; Lunde et al., 2014; Moes et al., 2014; Pulk et al., 2015; Debette-Gratien et al., 2016; Moes et al., 2016; Calvo et al., 2017; Madsen et al., 2017), which is possibly explained by low numbers of CYP3A4∗22 carriers or population stratification differences.
Cyclosporine treatment is mainly hampered by nephrotoxicity, limiting its clinical use (Kuroyanagi et al., 2018; Wu et al., 2018). In CYP3A4∗22 carriers, cyclosporine C0/D were 60% higher (Elens et al., 2011c) (Supplementary Table 2). Combining CYP3A4 and CYP3A5 genotypes, poor CYP3A-metabolizers presented cyclosporine C0/D 54% and 114% higher compared to intermediate and extensive CYP3A-metabolizers, respectively (Elens et al., 2011c). Two other studies could not confirm this correlation, possibly due to a smaller patient population [n = 47, (Cvetković et al., 2017)], whereas Debette-Gratien et al. studied 170 liver transplant recipients (Debette-Gratien et al., 2016). For CYP3A4∗22 carriers, a 53% higher dose-adjusted cyclosporine peak concentration (C2/D) (Lunde et al., 2014) and 15% lower cyclosporine clearance (Moes et al., 2014) were found. In total, 12% of interindividual variability in C2/D was explained by CYP3A4∗22 carriership and Lunde et al. (2014) estimated that recipients carrying one CYP3A4∗22 allele would need 50% less cyclosporine to reach therapeutic targets. Additionally, a significant association between CYP3A4∗22 and mean difference in second and first dose was found (El-Shair et al., 2019). Mixed-model analysis showed a 20% lower overall creatinine clearance, indicating cyclosporine-induced nephrotoxicity in CYP3A4∗22 patients receiving cyclosporine/mycophenolate mofetil (Elens et al., 2012). Clinically, CYP3A4∗22 carriers showed a significantly higher risk of delayed graft function, with an odds ratio (OR) of 6.34 (Elens et al., 2012).
Everolimus and sirolimus, immunosuppressive drugs inhibiting activation and proliferation of T-lymphocytes through mammalian Target Of Rapamycin (mTOR), are used to prevent transplant rejection as tacrolimus and cyclosporine-sparing agents (Cravedi et al., 2010; Moes et al., 2012; Woillard et al., 2013). Both drugs are metabolized by CYP3A4 and CYP3A5 into less active compounds (Woillard et al., 2013; Pascual et al., 2017). A 20% lower sirolimus metabolism was found in human liver microsomes of CYP3A4∗22 carriers (Woillard et al., 2013), whereas for everolimus, a trend of 7% lower clearance in CYP3A4∗22 carriers was observed (Moes et al., 2014) (Supplementary Table 2). Metastatic breast cancer patients carrying CYP3A4∗22 had 170% higher everolimus plasma concentrations (Pascual et al., 2017). It is important to realize that patients with metastatic breast cancer receive considerably higher doses than solid organ transplant recipients (Shipkova et al., 2016).
Ticagrelor, clopidogrel, and prasugrel are active platelet aggregation inhibitors metabolized by CYP3A4, used in cardiology and neurology. For ticagrelor, CYP3A4∗22 carriers showed an 89% higher area-under-the-plasma-concentration-time curve (AUC) and more pronounced platelet inhibition (43% vs. 21%) (Holmberg et al., 2019) (Supplementary Table 3). This increases risk of bleedings (Becker et al., 2011). No significant correlation of CYP3A4∗22 with active metabolites of clopidogrel or prasugrel was found, although the authors stated that differences in clopidogrel and prasugrel pharmacokinetics <50% cannot be ruled out (Holmberg et al., 2019). For sildenafil, metabolized by CYP3A4 in less active compounds, is used in patients with heart failure, CYP3A4∗22 carriers showed increased dose-adjusted concentrations (de Denus et al., 2018). For these drugs, only one study is currently available. Further studies are needed to confirm these findings.
For statins, CYP3A4∗22 carriers required significantly lower doses for optimal lipid control (Wang et al., 2011). Simvastatin and atorvastatin are primarily metabolized by CYP3A4 into less active metabolites (Vickers et al., 1990; Prueksaritanont et al., 2003). CYP3A4∗22 carriers demonstrated 49% higher simvastatin bioavailability (Tsamandouras et al., 2014), resulting in 20% higher simvastatin plasma concentrations (Kitzmiller et al., 2014) and 58% increased plasma 12-h concentrations (Luzum et al., 2015) (Supplementary Table 3). For atorvastatin, healthy CYP3A4∗22 carriers showed 35% decreased MR, confirming lower CYP3A4 activity (Klein et al., 2012). The 2-hydroxyatorvastatin/atorvastatin AUCinf ratio was 35% lower per copy of CYP3A4∗22 (Klein et al., 2012). CYP3A4∗22 carriers showed a greater reduction in total- and LDL-cholesterol levels (-0.31 and -0.34 mmol/l), consistent with expected higher simvastatin plasma concentrations, in Dutch Caucasians (Elens et al., 2011a). This finding could not be confirmed by Ragia et al. (2015) in Greek patients with hypercholesterolemia (n = 416), although the authors stated that potential confounders required further studies.
CYP2D6 pharmacogenetic based guiding of antidepressant drug therapy is nowadays quite accepted. Dosing advices, based on CYP2D6 and CYP2C19 genotype, are available at PharmGKB, CPIC and the Dutch Pharmacogenetic Working Group (DPWG) (Hicks et al., 2015). Thus far, CYP3A4 genotype-based dosing for psychotropic drugs has not yet been described. However, CYP3A4 does have a contribution in citalopram [CYP2C19, CYP2D6, CYP3A4 (Rochat et al., 1997)], escitalopram [CYP2C19, CYP3A4, CYP2D6 (von Moltke et al., 2001)] and mirtazapine [CYP2D6, CYP3A4, CYP1A2 (Störmer et al., 2000)] metabolism. The prominent role of CYP2C19 and CYP2D6 in the metabolism may complicate CYP3A4 studies in these drugs. Yet, compound heterozygotes for CYP2D6, CYP2C19, and CYP3A4 were found by us in patients with therapy related side effects, insufficiently explained by CYP2D6 and/or CYP2C19 genotype alone. Recently, combining CYP2C19, CYP2D6, and CYP3A4 genotypes proved to be a better predictor of citalopram/escitalopram blood levels as compared to individual genes (Shelton et al., 2020). We feel it would be beneficial to study the effect of CYP3A4∗22, in addition to CYP2D6 and CYP2C19, in antidepressants, but thus far, no clear indications for a prominent role for CYP3A4∗22 are available.
Alprazolam is one of the most commonly prescribed psychoactive agent for mood and anxiety disorders (Stahl, 2002). In patients with alcoholism and anxiety disorders, CYP3A4∗22 carriers had significantly increased active alprazolam concentration/dose ratios and a decreased treatment response, as reflected in HAMA scale scores (4.0 vs. 3.0) (Zastrozhin et al., 2020) (Supplementary Table 4).
Risperidone, a drug with CYP2D6 genotype-based dosing recommendations, showed CYP3A4∗22 carriers having 30% lower 9-hydroxyrisperidone (active metabolite, generated from risperidone by CYP2D6) clearance (Vandenberghe et al., 2015). Two other studies could not confirm this: the reason for this discrepancy could be sample size [n = 26, (Rafaniello et al., 2018)], although van der Weide et al. included 130 patients (van der Weide and van der Weide, 2015) (Supplementary Table 5). CYP3A4∗22 genotype was associated with serum levels active pimozide and C/D ratio, however, this association only explained 5% of total variation in a multiple regression analysis (van der Weide and van der Weide, 2015). For aripiprazole and haloperidol, no significant effect of CYP3A4∗22 was observed (van der Weide and van der Weide, 2015; Rafaniello et al., 2018), possibly because CYP2D6 plays a more prominent role (Fang et al., 1997; Fang et al., 1999; van der Weide and van der Weide, 2015). Multiple regression analysis demonstrated that 4–17% of the variation in concentration of these anti-psychotics was explained by CYP2D6 genotype (van der Weide and van der Weide, 2015). However, quetiapine, a drug metabolized by CYP3A4 (DeVane and Nemeroff, 2001), showed 150% higher serum concentrations and 67% higher dose-corrected quetiapine serum concentrations in CYP3A4∗22 carriers (van der Weide and van der Weide, 2014). Significantly more CYP3A4∗22 patients achieved serum levels above the therapeutic range of 500 μg/L (van der Weide and van der Weide, 2014).
For anticancer drugs, pharmacogenetic testing for CYP450 enzymes is currently limited to CYP2D6 testing for tamoxifen, which needs activation by CYP2D6 [for review, see Mulder et al. (2021)]. A good example of implementation of pharmacogenetic testing in oncology outside the CYP450 field, is DPYD analysis prior to capecitabine treatment, as well as TPMT testing for 6-mercaptopurine for treatment of Acute Lymphatic Leukemia (Henricks et al., 2018; Roden et al., 2019). Yet, CYP3A4 is involved in the metabolism of many anticancer drugs, and since efficacy and toxicity are important aspects in oncology, factors that may contribute to predicting toxicity should be examined for their potential clinical value. As indicated by Elens et al., decreased CYP3A4 metabolism due to CYP3A4∗22 could be demonstrated in cancer patients, as determined by midazolam and erythromycin metabolism (Elens et al., 2013b).
Tamoxifen, standard-of-care in (adjuvant) treatment of ER-positive breast cancer patients (FDA, 1977; Jordan, 2014), is metabolized by CYP3A4 into inactive N-desmethyltamoxifen. This is converted by CYP2D6 to the active endoxifen. While CYP2D6 contributes most to variation in systemic exposure to endoxifen (Goetz et al., 2018; Puszkiel et al., 2020), CYP3A4∗22 also affects this variation: CYP3A4∗22 carriers showed higher tamoxifen and 4-hydroxytamoxifen (Teft et al., 2013; Antunes et al., 2015), but also higher N-desmethyltamoxifen and endoxifen concentrations (Teft et al., 2013; Baxter et al., 2014) (Supplementary Table 6). Despite increased tamoxifen and endoxifen exposure, CYP3A4∗22 carriers were significantly less likely to experience hot flashes (OR: 8.87) (Baxter et al., 2014). Since these studies show conflicting results, no clear conclusions can be drawn and further research is needed. Exemestane, another drug used in breast cancer treatment, is also metabolized by CYP3A4. For this drug, 54% higher steady-state active exemestane concentrations were found in CYP3A4∗22 carriers (Hertz et al., 2017).
Paclitaxel and docetaxel are used for solid tumors. For paclitaxel, CYP2C8 has been the focus for CYP450 studies, but also CYP3A4 is involved in its metabolism (Harris et al., 1994). Neurotoxicity is often observed as side effect (Lee and Swain, 2006). In 261 cancer patients, CYP3A4∗22 carrier status was an independent predictive factor for development of paclitaxel-induced neurotoxicity, although no significant association with paclitaxel pharmacokinetics was found for reasons unknown to the authors (Supplementary Table 6) (de Graan et al., 2013). Interestingly, another CYP3A4 variant allele, CYP3A4∗20, was also associated with paclitaxel neuropathy (Apellániz-Ruiz et al., 2015). This supports the potential predictive value of CYP3A4 genotyping for paclitaxel neurotoxicity. For docetaxel, a study in 150 breast cancer patients showed that CYP3A4∗22 carriers were at increased risk of grade 3-4 toxicity (Sim et al., 2018). Docetaxel is mainly metabolized by CYP3A4 (Shou et al., 1998; Hirth et al., 2000), and large interindividual variability in docetaxel pharmacokinetics has been reported (Goh et al., 2002; Michael et al., 2012). Since the pharmacokinetic association of CYP3A4∗22 is lacking, more research on CYP3A4∗22 and docetaxel treatment is warranted.
Sunitinib and pazopanib are frequently prescribed tyrosine kinase inhibitors (TKIs) with established exposure-response relationships for renal cell carcinoma (Houk et al., 2010; Suttle et al., 2014). Both drugs are predominantly metabolized by CYP3A4 into less active metabolites (Sugiyama et al., 2011; Thorn et al., 2017). CYP3A4∗22 status was associated with a significantly decreased clearance of pazopanib (35%) (Bins et al., 2019) and a decreased clearance of sunitinib (22.5%) (Diekstra et al., 2014) (Supplementary Table 6). Bins et al. (2019) proposed that pazopanib dose adjustments based on CYP3A4∗22 status should be considered since their pharmacokinetic-model showed that 600 mg pazopanib in CYP3A4∗22 carriers would lead to similar pazopanib exposure as wild-type patients using 800 mg. Feasibility of CYP3A4∗22 genotype-guided dosing of TKIs in cancer patients is currently under investigation in a large prospective clinical trial; results are expected in 2022 (Dutch Trial Registry1).
Synthetic opioids, like alfentanil, sufentanil, remifentanil, and fentanyl, are all metabolized by CYP3A4 into inactive metabolites (Yun et al., 1992; Tateishi et al., 1996). Unfortunately, no studies regarding the association between CYP3A4∗22 and pharmacokinetics of alfentanil, sufentanil, and remifentanil are published. Regarding fentanyl, a positive association between CYP3A4∗22 carriers and increased exposure to fentanyl (higher AUC, lower clearance) was found in two studies with healthy individuals (Saiz-Rodríguez et al., 2019; Saiz-Rodríguez et al., 2020) (Supplementary Table 7). An association with fentanyl MR confirmed this influence, although the authors did not find a significant association with serum fentanyl concentration (Barratt et al., 2014).
Lopinavir and tenofovir alafenamide are metabolized by CYP3A4 into less active metabolites. Lopinavir is co-administered with ritonavir as a booster drug to enhance its oral bioavailability and plasma half-life by, in fact, inhibiting CYP3A4 activity (Ernest et al., 2005; Olagunju et al., 2014). In the presence of ritonavir-induced CYP3A4 inhibition, in 375 HIV-positive patients using 400/100 mg lopinavir/ritonavir, CYP3A4∗22/∗22 patients had lower lopinavir clearances compared to CYP3A4∗1/∗1 patient and compared to CYP3A4∗22 carriers (Olagunju et al., 2014). This resulted in 130% higher lopinavir C0 (Olagunju et al., 2014) (Supplementary Table 8). A trend of lower lopinavir clearance in CYP3A4∗22 carriers was also observed (Olagunju et al., 2014). CYP3A4∗22 carriers also had a 39% higher plasma tenofovir alafenamide AUC0–24 at day 56 (Cerrone et al., 2019) in patients receiving tenofovir alafenamide/emtricitabine in co-administration of rifampicin, a known CYP3A4-inducer (Niemi et al., 2003).
Although high interindividual variability is observed in CYP3A4 expression and activity, this could not be accounted for by genetic variability in CYP3A4, as polymorphisms that result in a significant altered CYP3A4 activity are rare. Therefore, the discovery that the more common observed CYP3A4∗22 has significant effects on the pharmacokinetics of several drugs was surprising. This argues in our opinion for further investigation of the potential clinical use of CYP3A4∗22 genotyping (Table 1), especially for tacrolimus, cyclosporine, midazolam and erythromycin for which the effects are strong. Ticagrelor, alprazolam, quetiapine, fentanyl, lopinavir, and various anticancer drugs should be considered, since several publications demonstrate effects of CYP3A4∗22 carrier status. For several drugs, like tacrolimus, tamoxifen, antidepressants, and antipsychotics, CYP3A4∗22 should be considered only in combination with other CYP genotyping analyses to generate a complete picture on CYP450 status. Since CYP3A4 is highly subject to food and drug induction/inhibition, this should also be taken into account. Effects of CYP3A4∗22 on simvastatin treatment were confirmed by several studies, yet the clinical sue seems limited due to easy effect measurement (cholesterol levels). For the purpose of this review, we have focused on clinical studies related to pharmacokinetics, and the discussion of in vitro investigation is not exhaustive. Correlations with clinical effects of CYP3A4∗22 regarding treatment toxicity or efficacy would need further investigation. Seeing the relations for CYP3A4∗22 on pharmacokinetics of several drugs, this seems a logical next step.
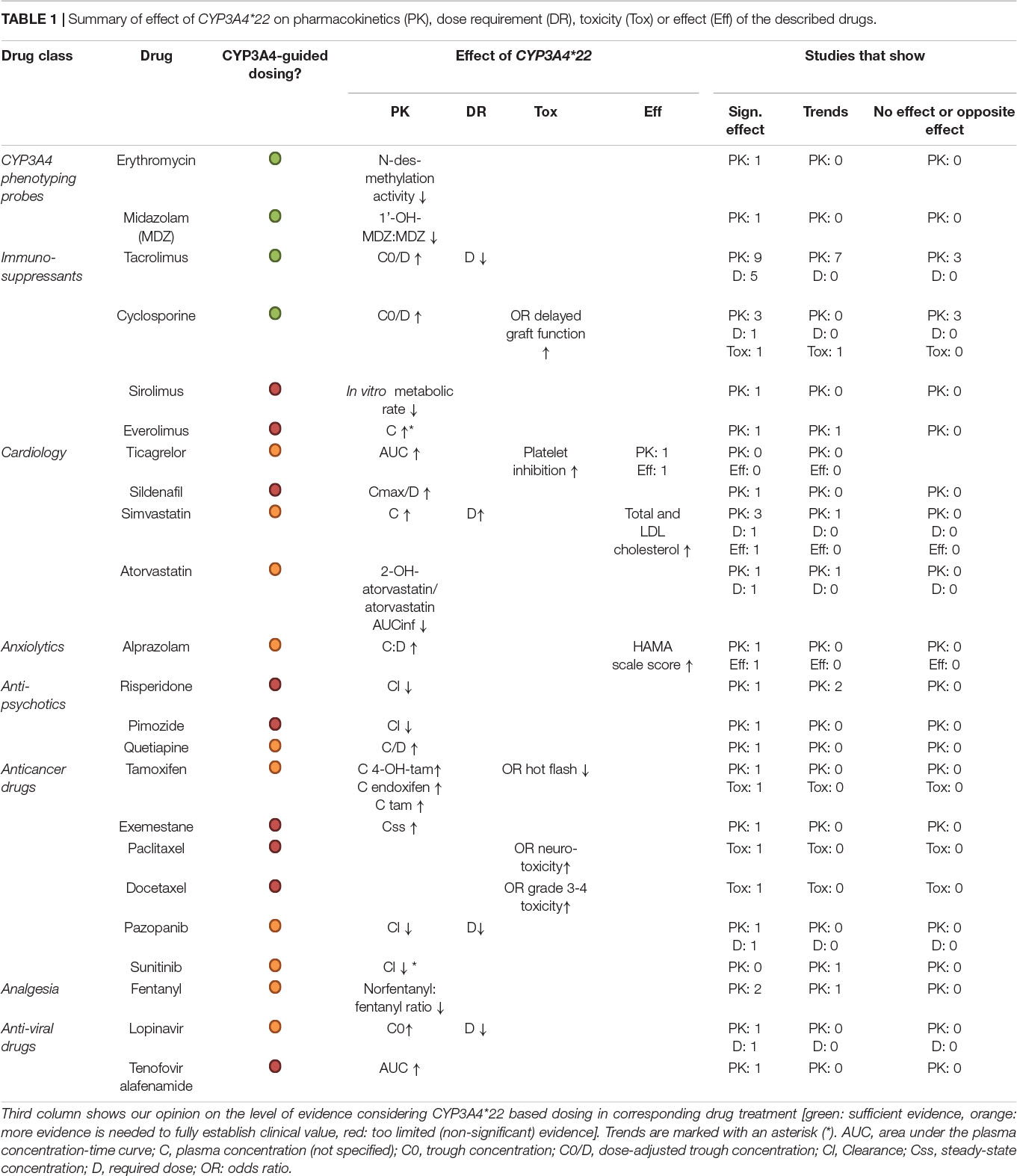
Table 1. Summary of effect of CYP3A4*22 on pharmacokinetics (PK), dose requirement (DR), toxicity (Tox) or effect (Eff) of the described drugs.
TM wrote the first draft of the manuscript and made all Supplementary Tables. TM and RE made Table 1. Supervision by RS. RE, MW, LE, DH, MM, SB, RM, and RS wrote sections of the manuscript and collected relevant references. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.711943/full#supplementary-material
Amirimani, B., Walker, A. H., Weber, B. L., and Rebbeck, T. R. (1999). RESPONSE: re: modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J. Natl. Cancer Inst. 91, 1588–1590. doi: 10.1093/jnci/91.18.1588
Antunes, M. V., De Oliveira, V., Raymundo, S., Staudt, D. E., Gössling, G., Biazús, J. V., et al. (2015). CYP3A4∗22 is related to increased plasma levels of 4-hydroxytamoxifen and partially compensates for reduced CYP2D6 activation of tamoxifen. Pharmacogenomics 16, 601–617. doi: 10.2217/pgs.15.13
Apellániz-Ruiz, M., Lee, M. Y., Sánchez-Barroso, L., Gutiérrez-Gutiérrez, G., Calvo, I., García-Estévez, L., et al. (2015). Whole-exome sequencing reveals defective CYP3A4 variants predictive of paclitaxel dose-limiting neuropathy. Clin. Cancer Res. 21, 322–328. doi: 10.1158/1078-0432.ccr-14-1758
Ball, S. E., Scatina, J., Kao, J., Ferron, G. M., Fruncillo, R., Mayer, P., et al. (1999). Population distribution and effects on drug metabolism of a genetic variant in the 5’ promoter region of CYP3A4. Clin. Pharmacol. Ther. 66, 288–294. doi: 10.1016/s0009-9236(99)70037-8
Barratt, D. T., Bandak, B., Klepstad, P., Dale, O., Kaasa, S., Christrup, L. L., et al. (2014). Genetic, pathological and physiological determinants of transdermal fentanyl pharmacokinetics in 620 cancer patients of the EPOS study. Pharmacogenet. Genom. 24, 185–194. doi: 10.1097/fpc.0000000000000032
Baxter, S. D., Teft, W. A., Choi, Y. H., Winquist, E., and Kim, R. B. (2014). Tamoxifen-associated hot flash severity is inversely correlated with endoxifen concentration and CYP3A4∗22. Breast Cancer Res. Treat. 145, 419–428. doi: 10.1007/s10549-014-2963-1
Becker, R. C., Bassand, J. P., Budaj, A., Wojdyla, D. M., James, S. K., Cornel, J. H., et al. (2011). Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur. Heart J. 32, 2933–2944. doi: 10.1093/eurheartj/ehr422
Bins, S., Huitema, A. D. R., Laven, P., Bouazzaoui, S. E., Yu, H., Van Erp, N., et al. (2019). Impact of CYP3A4∗22 on Pazopanib pharmacokinetics in cancer patients. Clin. Pharmacokinet. 58, 651–658. doi: 10.1007/s40262-018-0719-5
Budde, K., Bunnapradist, S., Grinyo, J. M., Ciechanowski, K., Denny, J. E., Silva, H. T., et al. (2014). Novel once-daily extended-release tacrolimus (LCPT) versus twice-daily tacrolimus in de novo kidney transplants: one-year results of Phase III, double-blind, randomized trial. Am. J. Transpl. 14, 2796–2806. doi: 10.1111/ajt.12955
Calvo, P. L., Serpe, L., Brunati, A., Nonnato, A., Bongioanni, D., Olio, D. D., et al. (2017). Donor CYP3A5 genotype influences tacrolimus disposition on the first day after paediatric liver transplantation. Br. J. Clin. Pharmacol. 83, 1252–1262. doi: 10.1111/bcp.13219
Cerrone, M., Alfarisi, O., Neary, M., Marzinke, M. A., Parsons, T. L., Owen, A., et al. (2019). Rifampicin effect on intracellular and plasma pharmacokinetics of tenofovir alafenamide. J. Antimicrob. Chemother. 74, 1670–1678. doi: 10.1093/jac/dkz068
Cravedi, P., Ruggenenti, P., and Remuzzi, G. (2010). Sirolimus for calcineurin inhibitors in organ transplantation: contra. Kidney Int. 78, 1068–1074. doi: 10.1038/ki.2010.268
Cvetković, M., Zivković, M., Bundalo, M., Gojković, I., Spasojević-Dimitrijeva, B., Stanković, A., et al. (2017). Effect of age and allele variants of CYP3A5, CYP3A4, and POR genes on the pharmacokinetics of Cyclosporin A in pediatric renal transplant recipients from serbia. Ther. Drug Monit. 39, 589–595. doi: 10.1097/ftd.0000000000000442
Danielson, P. B. (2002). The cytochrome P450 superfamily: biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 3, 561–597. doi: 10.2174/1389200023337054
de Denus, S., Rouleau, J. L., Mann, D. L., Huggins, G. S., Pereira, N. L., Shah, S. H., et al. (2018). CYP3A4 genotype is associated with sildenafil concentrations in patients with heart failure with preserved ejection fraction. Pharmacogenom. J. 18, 232–237. doi: 10.1038/tpj.2017.8
de Graan, A. J., Elens, L., Sprowl, J. A., Sparreboom, A., Friberg, L. E., Van Der Holt, B., et al. (2013). CYP3A4∗22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin. Cancer Res. 19, 3316–3324. doi: 10.1158/1078-0432.ccr-12-3786
de Jonge, H., Elens, L., De Loor, H., Van Schaik, R. H., and Kuypers, D. R. (2015). The CYP3A4∗22 C>T single nucleotide polymorphism is associated with reduced midazolam and tacrolimus clearance in stable renal allograft recipients. Pharmacogenom. J. 15, 144–152. doi: 10.1038/tpj.2014.49
Debette-Gratien, M., Woillard, J. B., Picard, N., Sebagh, M., Loustaud-Ratti, V., Sautereau, D., et al. (2016). Influence of donor and recipient CYP3A4, CYP3A5, and ABCB1 genotypes on clinical outcomes and nephrotoxicity in liver transplant recipients. Transplantation 100, 2129–2137. doi: 10.1097/tp.0000000000001394
DeVane, C. L., and Nemeroff, C. B. (2001). Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin. Pharmacokinet. 40, 509–522. doi: 10.2165/00003088-200140070-00003
Diekstra, M. H., Klümpen, H. J., Lolkema, M. P., Yu, H., Kloth, J. S., Gelderblom, H., et al. (2014). Association analysis of genetic polymorphisms in genes related to sunitinib pharmacokinetics, specifically clearance of sunitinib and SU12662. Clin. Pharmacol. Ther. 96, 81–89. doi: 10.1038/clpt.2014.47
Elens, L., Becker, M. L., Haufroid, V., Hofman, A., Visser, L. E., Uitterlinden, A. G., et al. (2011a). Novel CYP3A4 intron 6 single nucleotide polymorphism is associated with simvastatin-mediated cholesterol reduction in the Rotterdam Study. Pharmacogenet. Genom. 21, 861–866. doi: 10.1097/fpc.0b013e32834c6edb
Elens, L., Bouamar, R., Hesselink, D. A., Haufroid, V., Van Der Heiden, I. P., Van Gelder, T., et al. (2011b). A new functional CYP3A4 intron 6 polymorphism significantly affects tacrolimus pharmacokinetics in kidney transplant recipients. Clin. Chem. 57, 1574–1583. doi: 10.1373/clinchem.2011.165613
Elens, L., Van Schaik, R. H., Panin, N., De Meyer, M., Wallemacq, P., Lison, D., et al. (2011c). Effect of a new functional CYP3A4 polymorphism on calcineurin inhibitors’ dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenomics 12, 1383–1396. doi: 10.2217/pgs.11.90
Elens, L., Bouamar, R., Hesselink, D. A., Haufroid, V., Van Gelder, T., and Van Schaik, R. H. (2012). The new CYP3A4 intron 6 C>T polymorphism (CYP3A4∗22) is associated with an increased risk of delayed graft function and worse renal function in cyclosporine-treated kidney transplant patients. Pharmacogenet. Genom. 22, 373–380. doi: 10.1097/fpc.0b013e328351f3c1
Elens, L., Capron, A., Van Schaik, R. H., De Meyer, M., De Pauw, L., Eddour, D. C., et al. (2013a). Impact of CYP3A4∗22 allele on tacrolimus pharmacokinetics in early period after renal transplantation: toward updated genotype-based dosage guidelines. Ther. Drug Monit. 35, 608–616. doi: 10.1097/ftd.0b013e318296045b
Elens, L., Nieuweboer, A., Clarke, S. J., Charles, K. A., De Graan, A. J., Haufroid, V., et al. (2013b). CYP3A4 intron 6 C>T SNP (CYP3A4∗22) encodes lower CYP3A4 activity in cancer patients, as measured with probes midazolam and erythromycin. Pharmacogenomics 14, 137–149. doi: 10.2217/pgs.12.202
El-Shair, S., Al Shhab, M., Zayed, K., Alsmady, M., and Zihlif, M. (2019). Association between CYP3A4 and CYP3A5 genotypes and Cyclosporine’s blood levels and doses among jordanian kidney transplanted patients. Curr. Drug Metab. 20, 682–694. doi: 10.2174/1389200220666190806141825
Ernest, C. S. II, Hall, S. D., and Jones, D. R. (2005). Mechanism-based inactivation of CYP3A by HIV protease inhibitors. J. Pharmacol. Exp. Ther. 312, 583–591. doi: 10.1124/jpet.104.075416
Fang, J., Baker, G. B., Silverstone, P. H., and Coutts, R. T. (1997). Involvement of CYP3A4 and CYP2D6 in the metabolism of haloperidol. Cell Mol. Neurobiol. 17, 227–233.
Fang, J., Bourin, M., and Baker, G. B. (1999). Metabolism of risperidone to 9-hydroxyrisperidone by human cytochromes P450 2D6 and 3A4. Naunyn Schmiedeb. Arch. Pharmacol. 359, 147–151. doi: 10.1007/pl00005334
FDA (1977). SOLTAMOX® (tamoxifen citrate) Oral Solution. Silver Spring, MD: U.S. Food and Drug Administration.
Francke, M. I., Andrews, L. M., Le, H. L., Van De Wetering, J., Clahsen-Van Groningen, M. C., Van Gelder, T., et al. (2021). Avoiding Tacrolimus underexposure and overexposure with a dosing algorithm for renal transplant recipients: a single arm prospective intervention trial. Clin. Pharmacol. Ther. 110, 169–178.
García-Martín, E., Martínez, C., Pizarro, R. M., García-Gamito, F. J., Gullsten, H., Raunio, H., et al. (2002). CYP3A4 variant alleles in white individuals with low CYP3A4 enzyme activity. Clin. Pharmacol. Ther. 71, 196–204. doi: 10.1067/mcp.2002.121371
Gijsen, V. M., Van Schaik, R. H., Elens, L., Soldin, O. P., Soldin, S. J., Koren, G., et al. (2013). CYP3A4∗22 and CYP3A combined genotypes both correlate with tacrolimus disposition in pediatric heart transplant recipients. Pharmacogenomics 14, 1027–1036. doi: 10.2217/pgs.13.80
Goetz, M. P., Sangkuhl, K., Guchelaar, H. J., Schwab, M., Province, M., Whirl-Carrillo, M., et al. (2018). Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin. Pharmacol. Ther. 103, 770–777. doi: 10.1002/cpt.1007
Goh, B. C., Lee, S. C., Wang, L. Z., Fan, L., Guo, J. Y., Lamba, J., et al. (2002). Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J. Clin. Oncol. 20, 3683–3690. doi: 10.1200/jco.2002.01.025
Gómez-Bravo, M. A., Apellaniz-Ruiz, M., Salcedo, M., Fondevila, C., Suarez, F., Castellote, J., et al. (2018). Influence of donor liver CYP3A4∗20 loss-of-function genotype on tacrolimus pharmacokinetics in transplanted patients. Pharmacogenet. Genom. 28, 41–48. doi: 10.1097/fpc.0000000000000321
Guy-Viterbo, V., Baudet, H., Elens, L., Haufroid, V., Lacaille, F., Girard, M., et al. (2014). Influence of donor-recipient CYP3A4/5 genotypes, age and fluconazole on tacrolimus pharmacokinetics in pediatric liver transplantation: a population approach. Pharmacogenomics 15, 1207–1221. doi: 10.2217/pgs.14.75
Harris, J. W., Rahman, A., Kim, B. R., Guengerich, F. P., and Collins, J. M. (1994). Metabolism of taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res. 54, 4026–4035.
Henricks, L. M., Lunenburg, C., De Man, F. M., Meulendijks, D., Frederix, G. W. J., Kienhuis, E., et al. (2018). DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol. 19, 1459–1467. doi: 10.1016/s1470-2045(18)30686-7
Hertz, D. L., Kidwell, K. M., Seewald, N. J., Gersch, C. L., Desta, Z., Flockhart, D. A., et al. (2017). Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharmacogenom. J. 17, 521–527. doi: 10.1038/tpj.2016.60
Hesselink, D. A., Van Schaik, R. H., Van Der Heiden, I. P., Van Der Werf, M., Gregoor, P. J., Lindemans, J., et al. (2003). Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin. Pharmacol. Ther. 74, 245–254. doi: 10.1016/s0009-9236(03)00168-1
Hicks, J. K., Bishop, J. R., Sangkuhl, K., Müller, D. J., Ji, Y., Leckband, S. G., et al. (2015). Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 98, 127–134. doi: 10.1002/cpt.147
Hirth, J., Watkins, P. B., Strawderman, M., Schott, A., Bruno, R., and Baker, L. H. (2000). The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin. Cancer Res. 6, 1255–1258.
Holmberg, M. T., Tornio, A., Paile-Hyvärinen, M., Tarkiainen, E. K., Neuvonen, M., Neuvonen, P. J., et al. (2019). CYP3A4∗22 impairs the elimination of Ticagrelor, But has no significant effect on the Bioactivation of Clopidogrel or Prasugrel. Clin. Pharmacol. Ther. 105, 448–457. doi: 10.1002/cpt.1177
Houk, B. E., Bello, C. L., Poland, B., Rosen, L. S., Demetri, G. D., and Motzer, R. J. (2010). Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother. Pharmacol. 66, 357–371. doi: 10.1007/s00280-009-1170-y
Jordan, V. C. (2014). Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr. Relat. Cancer 21, R235–R246.
Kahan, B. D., Keown, P., Levy, G. A., and Johnston, A. (2002). Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin. Ther. 24, 330–350. doi: 10.1016/s0149-2918(02)85038-x
Kitzmiller, J. P., Luzum, J. A., Baldassarre, D., Krauss, R. M., and Medina, M. W. (2014). CYP3A4∗22 and CYP3A5∗3 are associated with increased levels of plasma simvastatin concentrations in the cholesterol and pharmacogenetics study cohort. Pharmacogenet. Genom. 24, 486–491. doi: 10.1097/fpc.0000000000000079
Klein, K., Thomas, M., Winter, S., Nussler, A. K., Niemi, M., Schwab, M., et al. (2012). PPARA: a novel genetic determinant of CYP3A4 in vitro and in vivo. Clin. Pharmacol. Ther. 91, 1044–1052. doi: 10.1038/clpt.2011.336
Kuehl, P., Zhang, J., Lin, Y., Lamba, J., Assem, M., Schuetz, J., et al. (2001). Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. 27, 383–391. doi: 10.1038/86882
Kuroyanagi, Y., Gotoh, Y., Kasahara, K., Nagano, C., Fujita, N., Yamakawa, S., et al. (2018). Effectiveness and nephrotoxicity of a 2-year medium dose of cyclosporine in pediatric patients with steroid-dependent nephrotic syndrome: determination of the need for follow-up kidney biopsy. Clin. Exp. Nephrol. 22, 413–419. doi: 10.1007/s10157-017-1444-3
Kurzawski, M., Dąbrowska, J., Dziewanowski, K., Domański, L., Perużyńska, M., and Droździk, M. (2014). CYP3A5 and CYP3A4, but not ABCB1 polymorphisms affect tacrolimus dose-adjusted trough concentrations in kidney transplant recipients. Pharmacogenomics 15, 179–188. doi: 10.2217/pgs.13.199
Lamba, J. K., Lin, Y. S., Schuetz, E. G., and Thummel, K. E. (2002a). Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug Deliv. Rev. 54, 1271–1294. doi: 10.1016/s0169-409x(02)00066-2
Lamba, J. K., Lin, Y. S., Thummel, K., Daly, A., Watkins, P. B., Strom, S., et al. (2002b). Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics 12, 121–132. doi: 10.1097/00008571-200203000-00006
Lauschke, V. M., Milani, L., and Ingelman-Sundberg, M. (2017). Pharmacogenomic biomarkers for improved drug therapy-recent progress and future developments. AAPS J. 20:4.
Lee, J. J., and Swain, S. M. (2006). Peripheral neuropathy induced by microtubule-stabilizing agents. J. Clin. Oncol. 24, 1633–1642. doi: 10.1200/jco.2005.04.0543
Lin, Y. S., Dowling, A. L., Quigley, S. D., Farin, F. M., Zhang, J., Lamba, J., et al. (2002). Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol. Pharmacol. 62, 162–172. doi: 10.1124/mol.62.1.162
Lloberas, N., Elens, L., Llaudó, I., Padullés, A., Van Gelder, T., Hesselink, D. A., et al. (2017). The combination of CYP3A4∗22 and CYP3A5∗3 single-nucleotide polymorphisms determines tacrolimus dose requirement after kidney transplantation. Pharmacogenet. Genom. 27, 313–322. doi: 10.1097/fpc.0000000000000296
Lunde, I., Bremer, S., Midtvedt, K., Mohebi, B., Dahl, M., Bergan, S., et al. (2014). The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur. J. Clin. Pharmacol. 70, 685–693. doi: 10.1007/s00228-014-1656-3
Luzum, J. A., Theusch, E., Taylor, K. D., Wang, A., Sadee, W., Binkley, P. F., et al. (2015). Individual and combined associations of genetic variants in CYP3A4, CYP3A5, and SLCO1B1 with Simvastatin and Simvastatin Acid Plasma concentrations. J. Cardiovasc. Pharmacol. 66, 80–85. doi: 10.1097/fjc.0000000000000246
Madsen, M. J., Bergmann, T. K., Brøsen, K., and Thiesson, H. C. (2017). The Pharmacogenetics of Tacrolimus in corticosteroid-sparse pediatric and adult kidney transplant recipients. Drugs R. D. 17, 279–286. doi: 10.1007/s40268-017-0177-9
Miano, T. A., Flesch, J. D., Feng, R., Forker, C. M., Brown, M., Oyster, M., et al. (2020). Early Tacrolimus concentrations after lung transplant are predicted by combined clinical and genetic factors and associated with acute kidney injury. Clin. Pharmacol. Ther. 107, 462–470. doi: 10.1002/cpt.1629
Miao, J., Jin, Y., Marunde, R. L., Gorski, C. J., Kim, S., Quinney, S., et al. (2009). Association of genotypes of the CYP3A cluster with midazolam disposition in vivo. Pharmacogenom. J. 9, 319–326. doi: 10.1038/tpj.2009.21
Michael, M., Cullinane, C., Hatzimihalis, A., O’kane, C., Milner, A., Booth, R., et al. (2012). Docetaxel pharmacokinetics and its correlation with two in vivo probes for cytochrome P450 enzymes: the C(14)-erythromycin breath test and the antipyrine clearance test. Cancer Chemother. Pharmacol. 69, 125–135. doi: 10.1007/s00280-011-1676-y
Moes, D. J., Press, R. R., Den Hartigh, J., Van Der Straaten, T., De Fijter, J. W., and Guchelaar, H. J. (2012). Population pharmacokinetics and pharmacogenetics of everolimus in renal transplant patients. Clin. Pharmacokinet. 51, 467–480. doi: 10.2165/11599710-000000000-00000
Moes, D. J., Swen, J. J., Den Hartigh, J., Van Der Straaten, T., Van Der Heide, J. J., Sanders, J. S., et al. (2014). Effect of CYP3A4∗22, CYP3A5∗3, and CYP3A combined genotypes on cyclosporine, Everolimus, and Tacrolimus pharmacokinetics in renal transplantation. CPT Pharmacometr. Syst. Pharmacol. 3:e100.
Moes, D. J., Van Der Bent, S. A., Swen, J. J., Van Der Straaten, T., Inderson, A., Olofsen, E., et al. (2016). Population pharmacokinetics and pharmacogenetics of once daily tacrolimus formulation in stable liver transplant recipients. Eur. J. Clin. Pharmacol. 72, 163–174.
Mulder, T. A. M., De With, M., Del Re, M., Danesi, R., Mathijssen, R. H. J., and Van Schaik, R. H. N. (2021). Clinical CYP2D6 genotyping to personalize adjuvant Tamoxifen treatment in er-positive breast cancer patients: current status of a controversy. Cancers 13:771. doi: 10.3390/cancers13040771
Niemi, M., Backman, J. T., Fromm, M. F., Neuvonen, P. J., and Kivistö, K. T. (2003). Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 42, 819–850. doi: 10.2165/00003088-200342090-00003
Okubo, M., Murayama, N., Shimizu, M., Shimada, T., Guengerich, F. P., and Yamazaki, H. (2013). CYP3A4 intron 6 C>T polymorphism (CYP3A4∗22) is associated with reduced CYP3A4 protein level and function in human liver microsomes. J. Toxicol. Sci. 38, 349–354. doi: 10.2131/jts.38.349
Olagunju, A., Schipani, A., Siccardi, M., Egan, D., Khoo, S., Back, D., et al. (2014). CYP3A4∗22 (c.522-191 C>T; rs35599367) is associated with lopinavir pharmacokinetics in HIV-positive adults. Pharmacogenet. Genom. 24, 459–463. doi: 10.1097/fpc.0000000000000073
Pallet, N., Jannot, A. S., El Bahri, M., Etienne, I., Buchler, M., De Ligny, B. H., et al. (2015). Kidney transplant recipients carrying the CYP3A4∗22 allelic variant have reduced tacrolimus clearance and often reach supratherapeutic tacrolimus concentrations. Am. J. Transpl. 15, 800–805. doi: 10.1111/ajt.13059
Pascual, T., Apellániz-Ruiz, M., Pernaut, C., Cueto-Felgueroso, C., Villalba, P., Álvarez, C., et al. (2017). Polymorphisms associated with everolimus pharmacokinetics, toxicity and survival in metastatic breast cancer. PLoS One 12:e0180192. doi: 10.1371/journal.pone.0180192
Prueksaritanont, T., Ma, B., and Yu, N. (2003). The human hepatic metabolism of simvastatin hydroxy acid is mediated primarily by CYP3A, and not CYP2D6. Br. J. Clin. Pharmacol. 56, 120–124. doi: 10.1046/j.1365-2125.2003.01833.x
Pulk, R. A., Schladt, D. S., Oetting, W. S., Guan, W., Israni, A. K., Matas, A. J., et al. (2015). Multigene predictors of tacrolimus exposure in kidney transplant recipients. Pharmacogenomics 16, 841–854. doi: 10.2217/pgs.15.42
Puszkiel, A., Arellano, C., Vachoux, C., Evrard, A., Le Morvan, V., Boyer, J. C., et al. (2020). Model-based quantification of impact of genetic polymorphisms and co-medications on pharmacokinetics of tamoxifen and six metabolites in breast cancer. Clin. Pharmacol. Ther. 109, 1244–1255. doi: 10.1002/cpt.2077
Rafaniello, C., Sessa, M., Bernardi, F. F., Pozzi, M., Cheli, S., Cattaneo, D., et al. (2018). The predictive value of ABCB1, ABCG2, CYP3A4/5 and CYP2D6 polymorphisms for risperidone and aripiprazole plasma concentrations and the occurrence of adverse drug reactions. Pharmacogenom. J. 18, 422–430. doi: 10.1038/tpj.2017.38
Ragia, G., Kolovou, V., Tavridou, A., Elens, L., Tselepis, A. D., Elisaf, M., et al. (2015). No effect of CYP3A4 intron 6 C>T polymorphism (CYP3A4∗22) on lipid-lowering response to statins in Greek patients with primary hypercholesterolemia. Drug Metab. Pers. Ther. 30, 43–48.
Rebbeck, T. R., Jaffe, J. M., Walker, A. H., Wein, A. J., and Malkowicz, S. B. (1998). Modification of clinical presentation of prostate tumors by a novel genetic variant in CYP3A4. J. Natl. Cancer Inst. 90, 1225–1229. doi: 10.1093/jnci/90.16.1225
Rochat, B., Amey, M., Gillet, M., Meyer, U. A., and Baumann, P. (1997). Identification of three cytochrome P450 isozymes involved in N-demethylation of citalopram enantiomers in human liver microsomes. Pharmacogenetics 7, 1–10. doi: 10.1097/00008571-199702000-00001
Roden, D. M., Mcleod, H. L., Relling, M. V., Williams, M. S., Mensah, G. A., Peterson, J. F., et al. (2019). Pharmacogenomics. Lancet 394, 521–532.
Saiz-Rodríguez, M., Almenara, S., Navares-Gómez, M., Ochoa, D., Román, M., Zubiaur, P., et al. (2020). Effect of the most relevant CYP3A4 and CYP3A5 polymorphisms on the pharmacokinetic parameters of 10 CYP3A substrates. Biomedicines 8:94. doi: 10.3390/biomedicines8040094
Saiz-Rodríguez, M., Ochoa, D., Herrador, C., Belmonte, C., Román, M., Alday, E., et al. (2019). Polymorphisms associated with fentanyl pharmacokinetics, pharmacodynamics and adverse effects. Basic Clin. Pharmacol. Toxicol. 124, 321–329. doi: 10.1111/bcpt.13141
Santoro, A. B., Struchiner, C. J., Felipe, C. R., Tedesco-Silva, H., Medina-Pestana, J. O., and Suarez-Kurtz, G. (2013). CYP3A5 genotype, but not CYP3A4∗1b, CYP3A4∗22, or hematocrit, predicts tacrolimus dose requirements in Brazilian renal transplant patients. Clin. Pharmacol. Ther. 94, 201–202. doi: 10.1038/clpt.2013.68
Scheibner, A., Remmel, R., Schladt, D., Oetting, W. S., Guan, W., Wu, B., et al. (2018). Tacrolimus elimination in four patients with a CYP3A5∗3/∗3 CYP3A4∗22/∗22 genotype combination. Pharmacotherapy 38, e46–e52.
Shelton, R. C., Parikh, S. V., Law, R. A., Rothschild, A. J., Thase, M. E., Dunlop, B. W., et al. (2020). Combinatorial pharmacogenomic algorithm is predictive of citalopram and escitalopram metabolism in patients with major depressive disorder. Psychiatry Res. 290:113017. doi: 10.1016/j.psychres.2020.113017
Shipkova, M., Hesselink, D. A., Holt, D. W., Billaud, E. M., Van Gelder, T., Kunicki, P. K., et al. (2016). Therapeutic drug monitoring of everolimus: a consensus report. Ther. Drug Monit. 38, 143–169. doi: 10.1097/ftd.0000000000000260
Shou, M., Martinet, M., Korzekwa, K. R., Krausz, K. W., Gonzalez, F. J., and Gelboin, H. V. (1998). Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics 8, 391–401. doi: 10.1097/00008571-199810000-00004
Shuker, N., Bouamar, R., Van Schaik, R. H., Clahsen-Van Groningen, M. C., Damman, J., Baan, C. C., et al. (2016). A randomized controlled trial comparing the efficacy of Cyp3a5 genotype-based with body-weight-based tacrolimus dosing after living donor kidney transplantation. Am. J. Transpl. 16, 2085–2096. doi: 10.1111/ajt.13691
Sim, S., Bergh, J., Hellström, M., Hatschek, T., and Xie, H. (2018). Pharmacogenetic impact of docetaxel on neoadjuvant treatment of breast cancer patients. Pharmacogenomics 19, 1259–1268. doi: 10.2217/pgs-2018-0080
Spurdle, A. B., Goodwin, B., Hodgson, E., Hopper, J. L., Chen, X., Purdie, D. M., et al. (2002). The CYP3A4∗1B polymorphism has no functional significance and is not associated with risk of breast or ovarian cancer. Pharmacogenetics 12, 355–366. doi: 10.1097/00008571-200207000-00003
Stahl, S. M. (2002). Don’t ask, don’t tell, but benzodiazepines are still the leading treatments for anxiety disorder. J. Clin. Psychiatry 63, 756–757. doi: 10.4088/jcp.v63n0901
Störmer, E., Von Moltke, L. L., Shader, R. I., and Greenblatt, D. J. (2000). Metabolism of the antidepressant mirtazapine in vitro: contribution of cytochromes P-450 1A2, 2D6, and 3A4. Drug Metab. Dispos. 28, 1168–1175.
Sugiyama, M., Fujita, K., Murayama, N., Akiyama, Y., Yamazaki, H., and Sasaki, Y. (2011). Sorafenib and sunitinib, two anticancer drugs, inhibit CYP3A4-mediated and activate CY3A5-mediated midazolam 1’-hydroxylation. Drug Metab. Dispos. 39, 757–762. doi: 10.1124/dmd.110.037853
Suttle, A. B., Ball, H. A., Molimard, M., Hutson, T. E., Carpenter, C., Rajagopalan, D., et al. (2014). Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br. J. Cancer 111, 1909–1916. doi: 10.1038/bjc.2014.503
Tateishi, T., Krivoruk, Y., Ueng, Y. F., Wood, A. J., Guengerich, F. P., and Wood, M. (1996). Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth. Analg. 82, 167–172. doi: 10.1213/00000539-199601000-00031
Tavira, B., Coto, E., Diaz-Corte, C., Alvarez, V., López-Larrea, C., and Ortega, F. (2013). A search for new CYP3A4 variants as determinants of tacrolimus dose requirements in renal-transplanted patients. Pharmacogenet. Genom. 23, 445–448. doi: 10.1097/fpc.0b013e3283636856
Teft, W. A., Gong, I. Y., Dingle, B., Potvin, K., Younus, J., Vandenberg, T. A., et al. (2013). CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res. Treat. 139, 95–105. doi: 10.1007/s10549-013-2511-4
Thervet, E., Loriot, M. A., Barbier, S., Buchler, M., Ficheux, M., Choukroun, G., et al. (2010). Optimization of initial tacrolimus dose using pharmacogenetic testing. Clin. Pharmacol. Ther. 87, 721–726.
Thorn, C. F., Sharma, M. R., Altman, R. B., and Klein, T. E. (2017). PharmGKB summary: pazopanib pathway, pharmacokinetics. Pharmacogenet. Genom. 27, 307–312. doi: 10.1097/fpc.0000000000000292
Tsamandouras, N., Dickinson, G., Guo, Y., Hall, S., Rostami-Hodjegan, A., Galetin, A., et al. (2014). Identification of the effect of multiple polymorphisms on the pharmacokinetics of simvastatin and simvastatin acid using a population-modeling approach. Clin. Pharmacol. Ther. 96, 90–100. doi: 10.1038/clpt.2014.55
van der Weide, K., and van der Weide, J. (2014). The influence of the CYP3A4∗22 polymorphism on serum concentration of quetiapine in psychiatric patients. J. Clin. Psychopharmacol. 34, 256–260. doi: 10.1097/jcp.0000000000000070
van der Weide, K., and van der Weide, J. (2015). The Influence of the CYP3A4∗22 Polymorphism and CYP2D6 polymorphisms on serum concentrations of Aripiprazole, Haloperidol, Pimozide, and Risperidone in psychiatric patients. J. Clin. Psychopharmacol. 35, 228–236. doi: 10.1097/jcp.0000000000000319
van Schaik, R. H., De Wildt, S. N., Van Iperen, N. M., Uitterlinden, A. G., Van Den Anker, J. N., and Lindemans, J. (2000). CYP3A4-V polymorphism detection by PCR-restriction fragment length polymorphism analysis and its allelic frequency among 199 Dutch Caucasians. Clin. Chem. 46, 1834–1836. doi: 10.1093/clinchem/46.11.1834
van Schaik, R. H. N., Müller, D. J., Serretti, A., and Ingelman-Sundberg, M. (2020). Pharmacogenetics in psychiatry: an update on clinical usability. Front. Pharmacol. 11:575540. doi: 10.3389/fphar.2020.575540
Vandenberghe, F., Guidi, M., Choong, E., Von Gunten, A., Conus, P., Csajka, C., et al. (2015). Genetics-based population pharmacokinetics and pharmacodynamics of risperidone in a psychiatric cohort. Clin. Pharmacokinet. 54, 1259–1272. doi: 10.1007/s40262-015-0289-8
Vickers, S., Duncan, C. A., Vyas, K. P., Kari, P. H., Arison, B., Prakash, S. R., et al. (1990). In vitro and in vivo biotransformation of simvastatin, an inhibitor of HMG CoA reductase. Drug Metab. Dispos. 18, 476–483.
von Moltke, L. L., Greenblatt, D. J., Giancarlo, G. M., Granda, B. W., Harmatz, J. S., and Shader, R. I. (2001). Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab. Dispos. 29, 1102–1109.
Wang, D., Guo, Y., Wrighton, S. A., Cooke, G. E., and Sadee, W. (2011). Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenom. J. 11, 274–286. doi: 10.1038/tpj.2010.28
Wang, D., and Sadee, W. (2016). CYP3A4 intronic SNP rs35599367 (CYP3A4∗22) alters RNA splicing. Pharmacogenet. Genom. 26, 40–43. doi: 10.1097/fpc.0000000000000183
Westlind, A., Löfberg, L., Tindberg, N., Andersson, T. B., and Ingelman-Sundberg, M. (1999). Interindividual differences in hepatic expression of CYP3A4: relationship to genetic polymorphism in the 5’-upstream regulatory region. Biochem. Biophys. Res. Commun. 259, 201–205. doi: 10.1006/bbrc.1999.0752
Woillard, J. B., Kamar, N., Coste, S., Rostaing, L., Marquet, P., and Picard, N. (2013). Effect of CYP3A4∗22, POR∗28, and PPARA rs4253728 on sirolimus in vitro metabolism and trough concentrations in kidney transplant recipients. Clin. Chem. 59, 1761–1769. doi: 10.1373/clinchem.2013.204990
Wojnowski, L., and Kamdem, L. K. (2006). Clinical implications of CYP3A polymorphisms. Expert Opin. Drug Metab. Toxicol. 2, 171–182.
Wrighton, S. A., Schuetz, E. G., Thummel, K. E., Shen, D. D., Korzekwa, K. R., and Watkins, P. B. (2000). The human CYP3A subfamily: practical considerations. Drug Metab. Rev. 32, 339–361. doi: 10.1081/dmr-100102338
Wu, Q., Wang, X., Nepovimova, E., Wang, Y., Yang, H., and Kuca, K. (2018). Mechanism of cyclosporine A nephrotoxicity: oxidative stress, autophagy, and signalings. Food Chem. Toxicol. 118, 889–907. doi: 10.1016/j.fct.2018.06.054
Yun, C. H., Wood, M., Wood, A. J., and Guengerich, F. P. (1992). Identification of the pharmacogenetic determinants of alfentanil metabolism: cytochrome P-450 3A4. An explanation of the variable elimination clearance. Anesthesiology 77, 467–474. doi: 10.1097/00000542-199209000-00011
Zastrozhin, M. S., Skryabin, V. Y., Smirnov, V. V., Petukhov, A. E., Pankratenko, E. P., Zastrozhina, A. K., et al. (2020). Effects of plasma concentration of micro-RNA Mir-27b and CYP3A4∗22 on equilibrium concentration of alprazolam in patients with anxiety disorders comorbid with alcohol use disorder. Gene 739:144513. doi: 10.1016/j.gene.2020.144513
Zeigler-Johnson, C., Friebel, T., Walker, A. H., Wang, Y., Spangler, E., Panossian, S., et al. (2004). CYP3A4, CYP3A5, and CYP3A43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer Res. 64, 8461–8467. doi: 10.1158/0008-5472.can-04-1651
Keywords: cytochrome P450, CYP3A4, CYP3A4∗22, genotyping, genotype-guided dosing, rs35599367, pharmacogenetics, personalized medicine
Citation: Mulder TAM, van Eerden RAG, de With M, Elens L, Hesselink DA, Matic M, Bins S, Mathijssen RHJ and van Schaik RHN (2021) CYP3A4∗22 Genotyping in Clinical Practice: Ready for Implementation? Front. Genet. 12:711943. doi: 10.3389/fgene.2021.711943
Received: 19 May 2021; Accepted: 17 June 2021;
Published: 08 July 2021.
Edited by:
Chad A. Bousman, University of Calgary, CanadaReviewed by:
Volker Martin Lauschke, Karolinska Institutet (KI), SwedenCopyright © 2021 Mulder, van Eerden, de With, Elens, Hesselink, Matic, Bins, Mathijssen and van Schaik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ron H. N. van Schaik, ci52YW5zY2hhaWtAZXJhc211c21jLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.