
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 29 July 2021
Sec. Genomic Assay Technology
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.698875
This article is part of the Research TopicWomen in Science: GeneticsView all 18 articles
Bacteria use K+-uptake transporters differentially for adaptation in varying growth conditions. In Mycobacterium tuberculosis, two K+-uptake systems, the Trk comprising the CeoB and CeoC proteins and the Kdp consisting of the two-component system (TCS), KdpDE and KdpFABC, have been characterized, but their selective utilization during bacterial growth has not been completely explored. In the current study, the roles of the M. tuberculosis KdpDE regulatory system alone and in association with the Trk transporters in bacterial growth were investigated by evaluating the growth of M. tuberculosis KdpDE-deletion and KdpDE/Trk (KT)-double knockout mutant strains in planktonic culture under standard growth conditions. The KT-double knockout mutant strain was first constructed using homologous recombination procedures and was evaluated together with the KdpDE-deletion mutant and the wild-type (WT) strains with respect to their rates of growth, K+-uptake efficiencies, and K+-transporter gene expression during planktonic growth. During growth at optimal K+ concentrations and pH levels, selective deletion of the TCS KdpDE (KdpDE-deletion mutant) led to attenuation of bacterial growth and an increase in bacterial K+-uptake efficiency, as well as dysregulated expression of the kdpFABC and trk genes. Deletion of both the KdpDE and the Trk systems (KT-double knockout) also led to severely attenuated bacterial growth, as well as an increase in bacterial K+-uptake efficiency. These results demonstrate that the KdpDE regulatory system plays a key role during bacterial growth by regulating K+ uptake via modulation of the expression and activities of both the KdpFABC and Trk systems and is important for bacterial growth possibly by preventing cytoplasmic K+ overload.
Growth of Mycobacterium tuberculosis is optimal in artificial culture media supplemented with potassium (K+) concentrations of around 14–15 millimolar (mM) at a pH of about 6.8 (Piddington et al., 2000; Cholo et al., 2015; Baker et al., 2019; Salina et al., 2019). Mycobacterium tuberculosis uses K+ to support cellular metabolic activities, such as cell wall biosynthesis, protein synthesis, lipid metabolism, and aerobic respiration, which are associated with logarithmic growth (Salina et al., 2014). However, in K+-limiting (Salina et al., 2014) and low pH environments (pH 5.5; Cholo et al., 2015), this bacterial pathogen alters its metabolic rates, transitioning to slow-growing, persistent-to-non-growing, and dormant phenotypes and allowing for bacterial survival in these unfavorable growth conditions.
Other studies have shown that bacteria adapt to these adverse growth conditions through differential utilization of K+-uptake transport systems (Epstein, 2003). For example, Escherichia coli utilizes the Trk system at elevated K+ concentrations in near-neutral pH, while the K+-uptake permease (Kup: TrkD) is used at low pH. However, at K+-limiting conditions at neutral pH, E. coli utilizes the Kdp system (Roe et al., 2000; Epstein, 2003). In this context, M. tuberculosis possesses two active K+-uptake transporters, namely the Trk and Kdp systems (Cole et al., 1998). The Trk consists of two TrkA proteins, CeoB and CeoC, encoded by highly homologous trk genes (ceoB and ceoC) of the ceoBC operon. We have previously shown that the Trk system is constitutively expressed in M. tuberculosis (Cholo et al., 2015), and has a lower affinity for K+ than the Kdp (Cholo et al., 2006). It plays a role in slowing bacterial growth in optimal conditions in standard 7H9 broth medium (15 mM K+, pH 6.8; Cholo et al., 2006). The Trk system has also been implicated in bacterial dormancy, with the CeoB protein being expressed at low K+ concentrations in biofilm cultures (Kerns et al., 2014; Hegde, 2020), while both trk genes are upregulated at low extracellular pH levels in planktonic culture (Cholo et al., 2015).
The M. tuberculosis Kdp system, on the other hand, is an inducible two-component system (TCS) comprised of the KdpDE sensor-regulator and the high-affinity K+-uptake transporter complex, KdpFABC (Cholo et al., 2006). The two Kdp components consist of six proteins encoded by a cluster of six genes, arranged in two operons, kdpDE and kdpFABC. These operons are divergently transcribed on the bacterial genome; being separated by an intergenic region (~234 bp) located between the kdpD and kdpF genes (Cole et al., 1998; Cholo et al., 2008). Activation of the Kdp system is mediated by the KdpDE, consisting of the sensor kinase KdpD and response regulator KdpE proteins (Steyn et al., 2003; Freeman et al., 2013). The KdpD and KdpE proteins interact with one another under basal conditions, with KdpD sensing the environmental stimulus and undergoing autophosphorylation at the histidine-642 residue, transferring the phosphoryl moiety to the KdpE subunit at the aspartate-52 residue, resulting in its phosphorylation, and leading to induction of the kdpFABC operon (Steyn et al., 2003; Agrawal and Saini, 2014). Low extracellular pH levels (Cholo et al., 2015), as well as K+-limiting conditions (Salina et al., 2014), are environmental stressors that lead to the induction of both the kdpDE and kdpFABC operons. These adverse growth conditions also result in acquisition of a dormant phenotype, seemingly implicating the involvement of the Kdp system in bacterial survival.
As with growth in adverse conditions of low extracellular K+ and pH, information on the role of the Kdp system during mycobacterial growth in optimal K+ concentrations and pH levels in vitro is also limited. In this context, we have previously reported that in the setting of optimal growth conditions, the Kdp system is repressed when the Trk system is functional, being induced and activated as a back-up when the Trk system is inactive (Cholo et al., 2006). Despite the limited information on growth, a few studies have identified that only the kdpE among the kdp genes, is necessary for optimal growth of M. tuberculosis (Sassetti et al., 2003; Griffin et al., 2011), as well as that of Mycobacterium smegmatis (Ali et al., 2017).
The issue of the differential utilization of these high- and low-affinity K+-uptake systems of M. tuberculosis during planktonic growth of the pathogen, including the seemingly modulatory role of KdpDE, have been explored in the current study. The research strategy used was based on a comparison of the growth of a selective KdpDE-deletion mutant strain and a recently constructed KdpDE/Trk (CeoBC; KT)-double knockout mutant strain with that of the wild-type (WT) strain of M. tuberculosis in standard growth conditions.
Unless indicated, all chemicals were purchased from the Sigma Chemical Co (St. Louis, MO, United States). Kanamycin and hygromycin antibiotics were used at 10 and 50 mg/L, respectively, for the selection of antibiotic-resistant colonies; 5-bromo-4-chloro-3-indolyl-B-D-galactoside (X-Gal) at 0.24 mg/L for blue colonies and sucrose at 2% (20 g/L) as a counter-selectable marker for sacB-expressing clones.
Rubidium-86 chloride (86Rb+) was purchased from PerkinElmer Life and Analytical Sciences, Du Pont-NEN Research Products, Boston, MA, United States.
All plasmids and bacterial strains used in this study are as shown in Table 1. The E. coli DH5α competent cells were used for cloning procedures for plasmid transformation. The pSOUP42 suicide-delivery vector (SDV) carrying the mutated M. tuberculosis kdpDE fragment and the KdpDE-deletion mutant strain of M. tuberculosis, were kindly provided by Professor N. Stoker, Royal Veterinary College, United Kingdom (Parish et al., 2003). The M. tuberculosis Trk-deletion mutant strain, which we had constructed previously (Cholo et al., 2006) was used for the construction of the KT-double knockout. The M. tuberculosis KdpDE-deletion (Parish et al., 2003) and the KT-double knockout (current study) mutant strains were compared with the WT strain for phenotypic and genotypic characteristics.
The Psi (Ψ)- broth medium (5 g Bacto yeast extract, 20 g Bacto tryptone, 5 g MgSO4/L, and pH 7.5) was used for preparation of competent E. coli cells and Luria-Bertani (LB) broth for growing plasmid-carrying E. coli bacteria, while the E. coli colonies were developed on Luria agar (LA) medium. For M. tuberculosis cultures, 7H9 broth and 7H10 agar (Difco) media supplemented with 10% oleic acid, dextrose, catalase (OADC), and 2/5% glycerol with/without 0.05% Tween 80 respectively, were used for liquid-based growth assays and colony development, respectively.
The KT-deletion mutant, which is characterized by inactivation of both the kdpDE and trk (ceoBC) operons, was constructed using homologous recombination following a two-step strategy (Parish and Stoker, 2000). Prior to electroporation, the hyg, PAg85-lacZ, Phsp60-sacB PacI cassette in the pSOUP42 SDV was replaced with the PAg85-lacZ, Phsp60-sacB PacI cassette from pGOAL17 to form pSOUP43 (Table 1). Briefly, approximately 5 μg of UV-pretreated pSOUP43 was electroporated into the M. tuberculosis Trk-deletion mutant, carrying the mutation of the ceoBC operon (Cholo et al., 2006) and plated onto 7H10 agar medium supplemented with hygromycin, kanamycin, and X-Gal for the isolation of blue single crossover (SCO) clones, followed by isolation of white double-crossovers (DCOs) on non-selection media. The DCO clones were characterized for absence of the plasmid phenotypically using sucrose and kanamycin sensitivity testing procedures. DNA samples from white sucrose-resistant, kanamycin-sensitive M. tuberculosis clones were extracted and mutations at the kdpDE and ceoBC operons were confirmed by a non-radioactive Southern blotting procedure using a digoxigenin (DIG)-labeled PCR-synthesized probe as described in the PCR DIG Probe Synthesis kit (Roche Molecular Biochemicals, Mannheim, Germany).
A bacterial inoculum of each strain was prepared as described, with minor modifications (Cholo et al., 2015; Mothiba et al., 2015). Briefly, a seed culture of M. tuberculosis cells was inoculated into 50 ml of 7H9 broth and grown to the mid-log phase at 37°C under stirring conditions. The bacterial cells were harvested by centrifugation at 2851 × g at room temperature (RT) for 15 min and the supernatant discarded. The pellet was washed twice and re-suspended in 7H9 broth, followed by adjustment of the optical density (OD) to 1.2 at 540 nm, yielding ca. 108–109 colony-forming units (cfu)/ml. An inoculum of ca. 105 cfu/ml was used in all of the assays.
For each strain, the bacterial cultures were prepared by inoculating approximately 105 cfu/ml cells into 7H9 broth followed by incubation of the culture at 37°C under stirring conditions until the early-, mid-, and late-log phases were reached, corresponding to ODs of 0.1–0.3, 0.4–0.6, and 2.0–2.3 at 540 nm, respectively (Cholo et al., 2015).
Cultures for determination of the rates of growth were prepared by inoculating ca. 105 cfu/ml of cells of the WT and mutant strains of M. tuberculosis into 7H9 broth. The cultures were thoroughly mixed followed by incubation at 37°C for 15 days in the dark with continuous stirring. The cultures were sampled every 3 days beginning at day 0 (D0) to day 15 (D15) and growth was determined spectrophotometrically at a wavelength of 540 nm. The rates of growth of each strain were determined as the time taken by the bacteria to reach the different logarithmic growth phases.
Uptake of K+ by the WT and mutant strains was determined at the mid- and late-log phases using 86Rb+ as a surrogate tracer for K+. Briefly, the bacteria were harvested from cultures grown to the two logarithmic growth phases and resuspended to ca. 106 cfu/ml in K+-free buffer (KONO) containing 2 mCi/L 86Rb+ and uptake of the radioisotope determined as absolute counts per minute (cpm) as previously described (Steel et al., 1999; Cholo et al., 2015).
The K+ concentrations and pH levels were determined at the early-, mid-, and late-log phases for each strain. Following culture preparation, the supernatants were harvested by centrifugation (2,851 × g, 15 min) followed by decontamination by heat treatment at 95°C for 60 min. The K+ concentrations and pH levels were measured in the undiluted samples by indirect potentiometry utilizing a K+-selective electrode in conjunction with a Na+-reference electrode using the Beckman Coulter Synchron LX 20 System (Beckman Coulter, Ireland Inc., Gateway, Ireland) and the Crison micropH2001 pH meter (Crison Instruments, Barcelona, Spain), respectively. These measurements were determined at the initial (D0), intermediate and final (early-, mid-, and late-log) growth phases, as well as in the processed and unprocessed bacteria-free 7H9 broth medium.
Gene expression was performed at the early-, mid-, and late-log growth phases in standard 7H9 liquid culture medium as described previously (Cholo et al., 2015). Briefly, RNA was extracted following the Trizol method, and complementary deoxyribonucleic acid (cDNA) was synthesized using the Sigma Enhanced Avian HS reverse transcriptase-PCR (RT-PCR) kit and amplified by quantitative (q)PCR using the LightCycler FastStart DNA Master SYBR Green I kit with the LightCycler 2.0 instrument (Roche Molecular Biochemicals, Mannheim, Germany). The quantities of the individual genes were determined using absolute (AQ) and relative (RQ) quantifications with sigA as the reference gene. The relative quantifications were determined based on quantification cycles (Cq) using the 2−ΔΔCq method.
All statistical analyses were performed using the INSTAT program and the unpaired and paired Student t-test/Mann–Whitney U-test for analysis of growth rates and gene expression data, respectively. The results are expressed as the means ± SDs. Significance levels were taken at a p ≤ 0.05.
In order to investigate the roles of the TCS KdpDE system on bacterial growth, alone and in association with the Trk system, acquisition of the K+-uptake mutant strains lacking the single KdpDE regulatory system (KdpDE-deletion mutant strain), as well as a combination of both the KdpDE and Trk systems (KT-double knockout mutant strain), was necessary.
The TCS KdpDE, together with the KdpFABC transporter, are the main components of the Kdp system, encoded by separate kdpDE and kdpFABC operons, transcribed in opposite directions, with the start codon of kdpD separated by 234 bp from the start codon of kdpF. This genomic arrangement of the kdp operons is similar to that of Mycobacterium bovis, but is different from those of other bacterial and mycobacterial species, which show both operons having similar transcriptional orientations with the kdpD being adjacent to the kdpC (Cole et al., 1998; Cholo et al., 2008; Agrawal and Saini, 2014).
The construction of the single KdpDE-deletion mutant of M. tuberculosis was as previously reported (Parish et al., 2003). Both the kdpD (Rv1028c: 2583 bp) and kdpE (Rv1027c: 681 bp) genes are transcribed in the negative direction with kdpE located at position 1148.427–1149.107 (681 bp) with its start codon overlapping the stop codon of kdpD found at position 1149.104–1051.686 on the chromosome. Mutation of the kdpDE operon was achieved by deletion of a 1,691-bp SphI fragment that spans both the kdpD and kdpE genes, resulting in inactivation of the sensor kinase/response regulator of the KdpFABC system. However, due to separation of the kdpDE and promoter-carrying kdpFABC operons on the M. tuberculosis genome, the promoter region of the kdpFABC operon remained genotypically intact.
In the case of the KT-double knockout strain of M. tuberculosis, we used the Trk-deletion mutant strain, which we had constructed previously (Cholo et al., 2006). The trk genes comprising the ceoB (Rv2691: 684 bp) and ceoC (Rv2692: 663 bp) genes are found on the ceoBC operon, with the ceoB located at position 3009.344–3010.027, with its stop codon overlapping the start codon of ceoC found at position 3010.024–3010.686 on the chromosome. Both trk genes are transcribed in the positive direction. The Trk-deletion mutant strain was characterized by inactivation of both the trk genes, resulting in a 348-bp deletion at ceoB gene and insertion of the 1746-bp BamHI-BglII hyg resistance cassette, derived from the plJ963 vector, at the NheI site of the ceoC gene. The KT-double knockout mutant was constructed by introducing a kdpDE-deletion fragment-carrying plasmid, pSOUP43, constructed as described (Parish et al., 2003; Table 1) into the Trk-deletion mutant (Cholo et al., 2006; Figure 1). Successful mutagenesis of the kdpDE operon in the KT-double knockout mutant strain was evident by detection of the 1996-bp XhoI-kdpDE fragment with the 1,010-bp PCR-synthesized kdpDE probe (Figure 1A; Table 2; Parish et al., 2003). For the ceoBC operon, mutation was revealed by detection of the 2,231-bp BclI-ceoBC fragment using the 714-bp PCR-synthesized ceoB probe (Figure 1B; Table 2; Cholo et al., 2006).
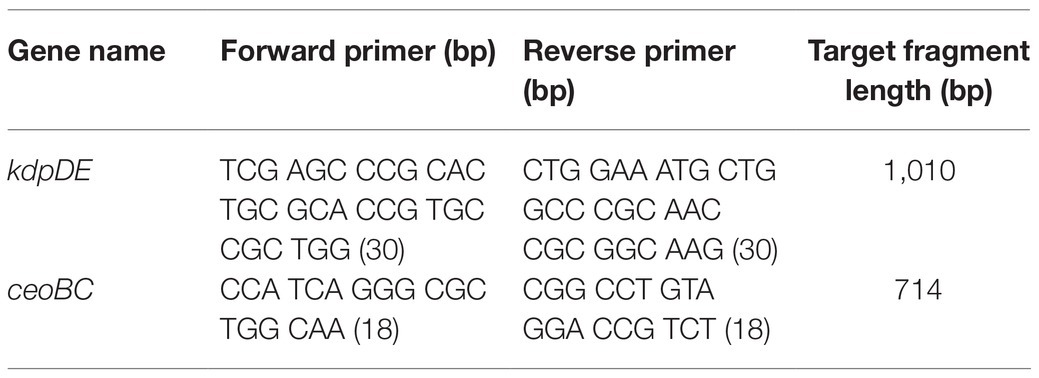
Table 2. Primers used for probe preparation for Southern blotting for KT-double knockout mutant strain construction.
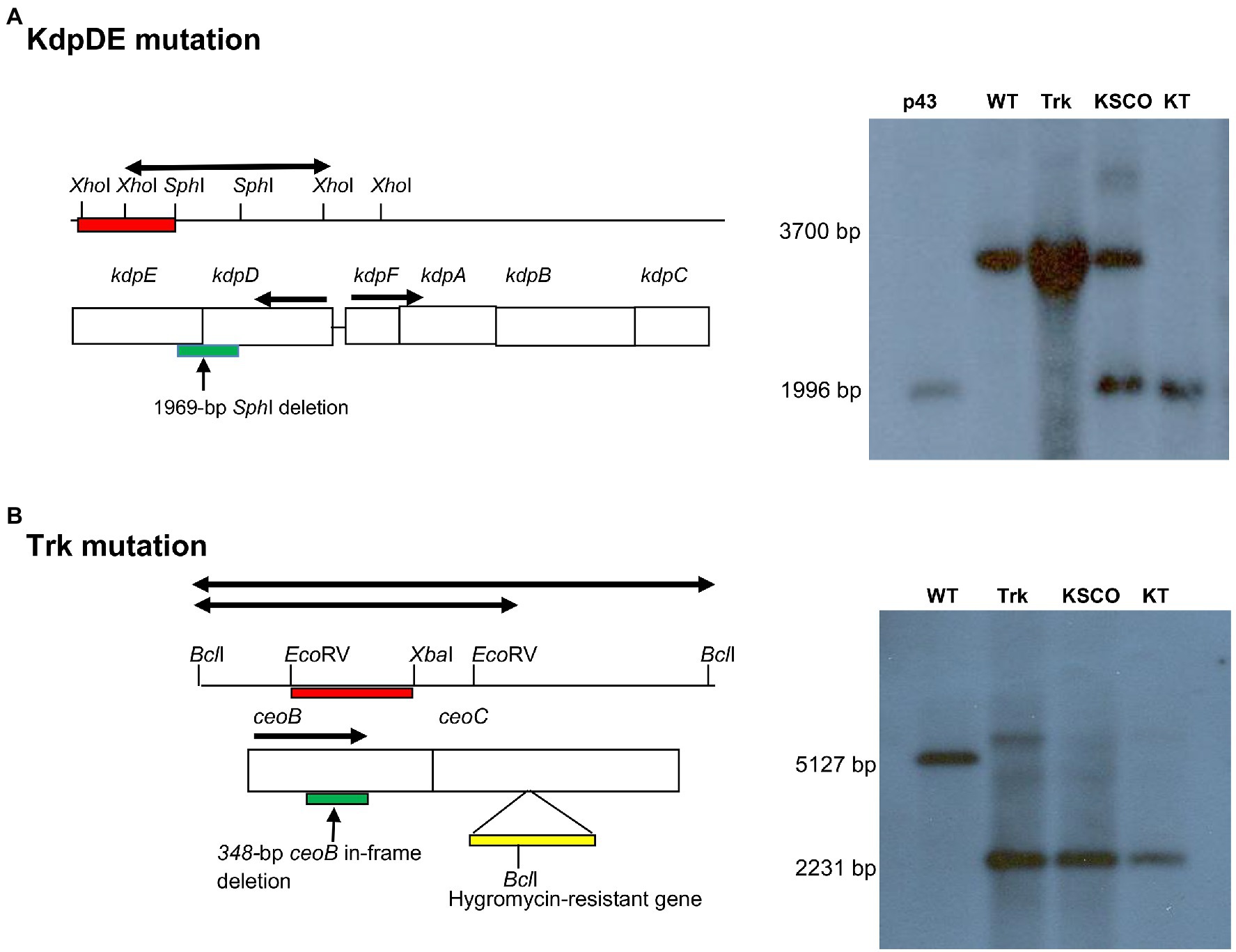
Figure 1. Schematic illustrations of allelic exchange mutagenesis of the kdpDE and ceoBC mutations in the KT-double knockout strain of M. tuberculosis as shown previously for inactivation of the single Trk- and KdpDE-deletion mutant strains (Parish et al., 2003; Cholo et al., 2006). The maps were not drawn to scale. DNA samples were digested with (A) XhoI and probed with 1,010-bp kdpDE PCR-synthesized fragments (red bar) for detection of the 1996-bp XhoI-kdpDE mutated fragment (thick black arrow) and (B) BclI and probed with 714-bp ceoB PCR-synthesized fragment (red bar) for detection of the 2,231-bp BclI-ceoBC mutated fragment (thick black arrow). Deletions in kdpDE and ceoB genes are shown by green bars, while insertion of the hyg gene in ceoC gene is shown by yellow bar. p43, pSOUP43; WT, wild type; Trk, Trk-deletion; KSCO, kdpDE single crossover; KT, KT-double knockout.
We used the WT and mutant strains to determine the role of the TCS KdpDE alone and in association with the Trk system on bacterial growth. This was achieved by assessing the rates of growth of the WT and the K+-uptake mutant strains in 7H9 broth medium (15 mM K+, pH 6.7) under aerobic conditions, sampled every 3 days beginning at D0 to D15 for OD determination. We have previously shown that the OD measurements of 0.1–0.3, 0.4–0.6, and 2.0–2.3 at 540 nm corresponded to the early-, mid-, and late-log phases, respectively (Cholo et al., 2015). The rate of growth of each strain was determined as the time, measured in days, taken by the bacteria to reach these growth phases, and the results are shown in Figure 2.
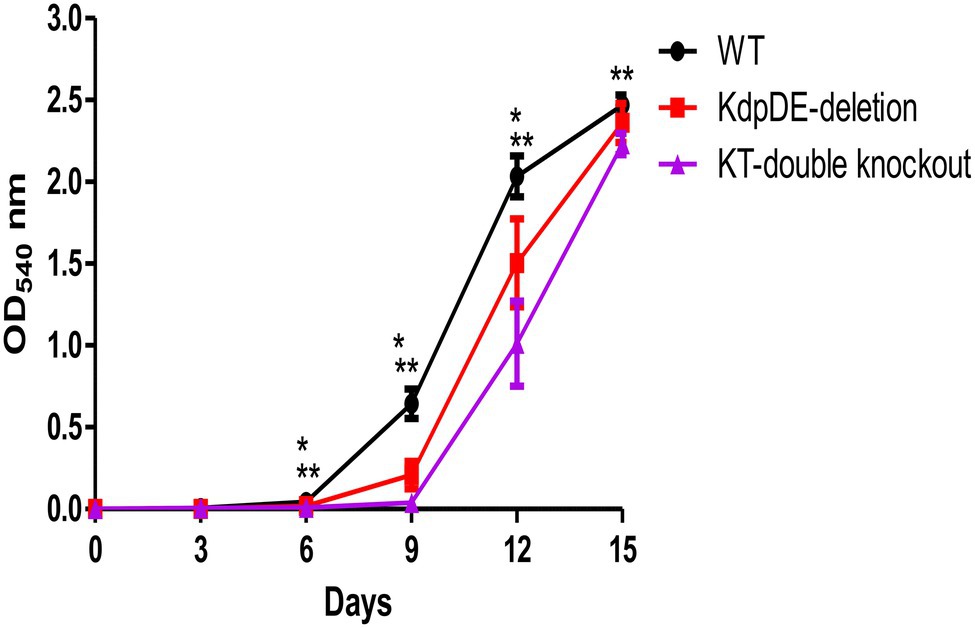
Figure 2. The rates of planktonic growth of the WT and mutant strains of M. tuberculosis measured over 15 days. The results are of three different experiments and are expressed as the mean optical density (OD) ± SD at 540 nm. The numbers of bacteria were 8.8 × 104 ± 7.5 × 104, 2.1 × 105 ± 1.8 × 105, and 9.7 × 104 ± 1.3 × 105 cfu/ml for the WT, KdpDE-deletion and KT-double knockout mutant strains at D0, respectively. The OD values for the WT, KdpDE-deletion and KT-double knockout mutants at D6 were: 0.044 ± 0.024, 0.018 ± 0.006, and 0.009 ± 0.006; at D9: 0.644 ± 0.09, 0.208 ± 0.084, and 0.04 ± 0.006; at D12: 2.034 ± 0.126, 1.505 ± 0.269, and 1.011 ± 0.259; and at D15: 2.468 ± 0.0064, 2.360 ± 0.118, and 2.232 ± 0.065, respectively. * and ** represent p ≤ 0.05 for the KdpDE-deletion and KT-double knockout mutants in relation to the WT, respectively. *Values of p between the WT and KdpDE-deletion mutant at D6, D9, and D12 were 0.026, 0.0022, and 0.0022 respectively; **Values of p between the WT and KT-double knockout at D6, D9, D12, and D15 were 0.0022 at each time point; Values of p between the KdpDE-deletion and KT-double knockout at D6, D9 and D12 were 0.0246, 0.0022, and 0.0260, respectively.
The numbers of bacteria were determined at D0 and were shown to be 8.8 × 104 ± 7.5 × 104, 2.1 × 105 ± 1.8 × 105, and 9.7 × 104 ± 1.3 × 105 cfu/ml for the WT, KdpDE-deletion, and KT-double knockout mutant strains, respectively. The results showed that the rates of growth of the WT and the mutant strains were different, entering the three log phases of growth at varying time points. The growth rates of the mutant strains were attenuated, showing prolonged early-log phases and reaching the early-, mid-, and late-log phases at D9, D12, and D15, respectively, while the WT reached these phases of growth at D6, D9, and D12. The rates of growth were significantly different between the WT and KdpDE-deletion mutant strain at D6, D9, and D12 (p < 0.05), while the rates of growth were comparable between the two strains at D15 (ODs: 2.468 ± 0.0064 and 2.360 ± 0.118 for the WT and KdpDE-deletion mutant, respectively, p = 0.132). However, the KT-double knockout strain was highly attenuated for growth, with the rates of growth of this mutant being significantly slower than those of the WT at D6, D9, D12, and D15 (p < 0.05).
Despite the KdpDE-deletion and KT-double knockout mutant strains reaching the early-, mid-, and late-log phases at the same time points (i.e., at D9, D12, and D15, respectively), the growth levels of the two mutant strains determined by comparing their OD measurements at the different time points, were nevertheless different, as shown in Figure 2, with the rate of growth of the KT-double knockout mutant strain at D6, D9, and D12 being significantly slower than that of the KdpDE-deletion mutant strain (p < 0.05).
As we have previously shown that M. tuberculosis utilizes the Trk K+-uptake transporter exclusively when cultured during standard growth conditions in 7H9 medium, suppressing the activity of the KdpFABC transporter (Cholo et al., 2006, 2015), these observations appear to implicate the KdpDE system in harmonizing the activities of both the Trk and KdpFABC systems to achieve optimum growth.
Following determination of growth rates, we then assessed the magnitudes of K+-uptake by measuring the uptake of 86Rb+ by the WT and mutant strains at the mid- and late-log phases of growth as described previously (Steel et al., 1999; Cholo et al., 2015) and the results are shown in Figure 3.
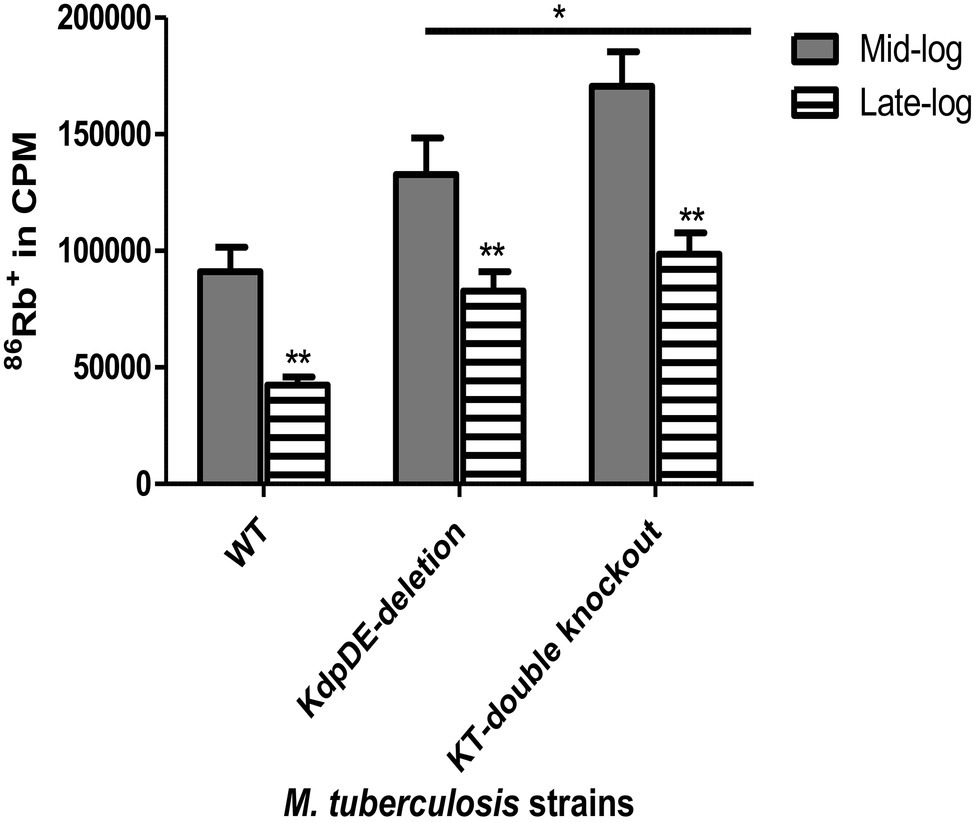
Figure 3. Rubidium (86Rb+) uptake by the WT and the K+-uptake-deletion mutant strains at the mid- and late-log phases. The results are of five experiments with three replicates for each time point represented as absolute counts per minute (cpm). The gray and striped bars represent uptake of 86Rb+ at the mid- and late-log phases, respectively. The cpm values were 91,041 ± 10464.87, 132748.3 ± 15,650, and 170577.3 ± 4877.88 at the mid-log phase and 42431.48 ± 3447.031, 82737.71 ± 8308.834, and 98569.04 ± 9211.451 at the late-log phase for H37Rv (WT), KdpDE-deletion, and KT-double knockout strains, respectively. Statistical differences at p < 0.05 are represented by * and **. *Values of p represent comparison of the responses of the WT strain relative to those of the mutant strains at the mid- and late-log phases and were 0.0397 and 0.0006 for the KdpDE-deletion and KT-double knockout mutant strains, respectively at the mid-log phase, while they were 0.0001 for both mutant strains at the late-log phase. The **values of p represent comparison of responses in each strain between mid- and late-log phases.
Using the 86Rb+-uptake model we have, previously shown that the Trk system has a low-affinity for K+ being responsible for K+ influx during bacterial growth at the mid- and late-log phases. On the other hand, the Kdp system has been characterized as the high-affinity K+ transporter, which is suppressed during the logarithmic phases of growth, being induced as a backup when the Trk system is not active (Cholo et al., 2006, 2015).
Somewhat surprisingly, the results revealed that the 86Rb+-uptake efficiencies of the mutant strains, were higher than those of the WT strain at both the mid- and late-log phases being highest at the mid-log phase for the KT-double knockout mutant (Figure 3). At the late-log phase, the 86Rb+-uptake efficiencies of the mutant strains were again higher than that of the WT strain, but not significantly different from one another (82,738 ± 8,309 and 98,569 ± 9,212 cpm for the KdpDE-deletion and KT-double knockout mutant strains, respectively, p = 0.126). These findings indicate that deletion of the KdpDE system results in K+ overload, presumably due to dysregulation of the Trk and KdpFABC K+ transporters, an event that may disrupt mycobacterial cytoplasmic pH, cellular metabolism, and growth.
For all the strains, the 86Rb+-uptake efficiencies were significantly reduced at the late-log phase, being 46, 62, and 57% of the corresponding efficiencies noted at the mid-log phase for the WT, KdpDE-deletion and KT-double knockout mutant strains, respectively, most probably due to decreased extracellular pH (Cholo et al., 2015).
Alterations in the extracellular K+ concentrations and pH levels that occur during the early-, mid-, and late-log phases of growth of the WT and mutant strains of M. tuberculosis under optimal culture conditions in 7H9 broth (15 mM K+ and pH 6.8) were determined. In the case of the extracellular K+ concentrations of the culture supernatants, these remained unchanged (14–15.5 mM) with respect to all three strains of M. tuberculosis and were comparable during the three log phases (Supplementary Table S1). The lack of significant alterations in extracellular K+ may reflect recycling of K+ during bacterial growth.
As shown in Figure 4, the extracellular pH values of the growth media increased at the early- to mid-log phases and decreased dramatically during the late-log phase, achieving statistical significance in comparison with the corresponding D0 pH value (pH 6.711) for all three strains. The extracellular pH levels of the two mutant strains were comparable at the three log phases of growth, but were higher than those of the WT strain.
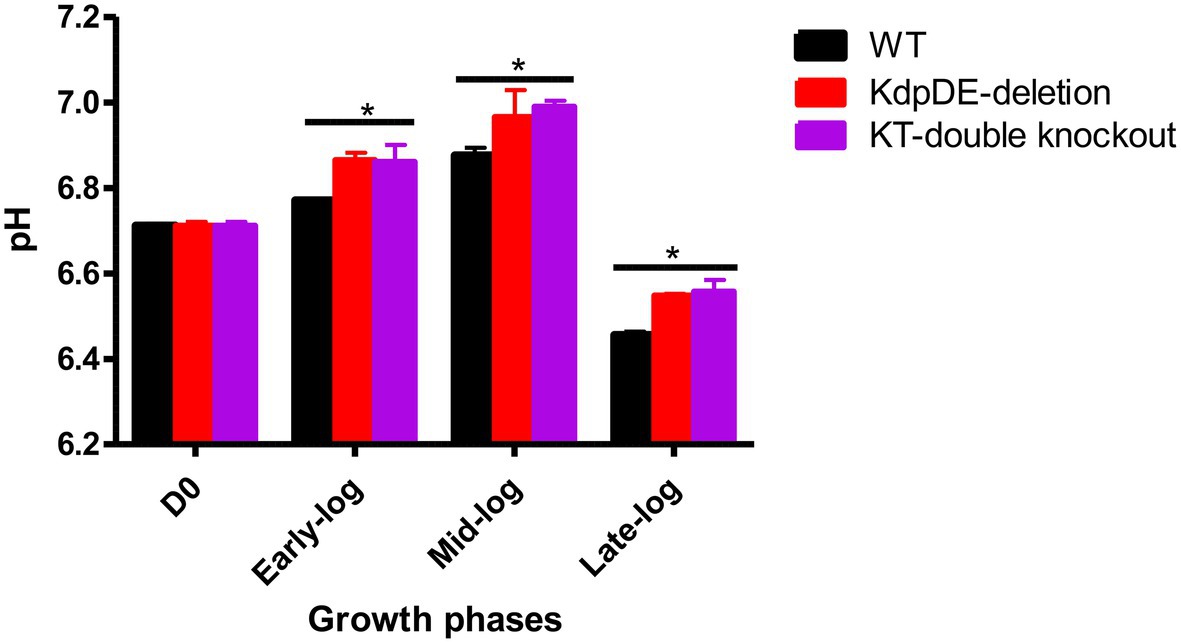
Figure 4. The extracellular pH levels at the three growth phases. The results are of three experiments performed in triplicate. The pH levels were similar at D0 for all the strains 6.711 ± 0.01. The pH levels for the WT, KdpDE-deletion and KT-double knockout mutant strains at early-log were: 6.77 ± 0.011, 6.865 ± 0.017, and 6.86 ± 0.04; at mid-log: 6.876 ± 0.045, 6.965 ± 0.064, and 6.99 ± 0.04; and at late-log: 6.455 ± 0.022, 6.548 ± 0.004, and 6.557 ± 0.028, respectively. Statistically significant differences between D0 vs. the log phases are shown with an (*) representing p < 0.05.
The effects of the KdpDE regulatory system alone and in the presence of the Trk system on the expression of the K+-uptake genes during bacterial growth were explored by determining the expression levels of all six kdp and two trk genes in the WT and both mutant strains at the early-, mid-, and late-log growth phases in standard 7H9 liquid culture medium using RT-PCR. We confirmed mutations of the kdpDE (kdpD and kdpE) and ceoBC (ceoB and ceoC) genes by formation of non-specific fragments, with different melting temperatures from those of targeted gene-specific fragments using melting curve analysis data (Supplementary Table S2).
The results were analyzed as absolute amounts (μg/ml; AQ) and relative quantifications (RQ) using sigA as the reference gene (Figure 5; Supplementary Table S3). As shown in our previous study under similar conditions (Cholo et al., 2015), the expression levels of the sigA gene were comparable between the WT and the KdpDE-deletion and the KT-knockout mutants between the early- and mid-log phases. However, at the late-log phase, expression levels of sigA were significantly increased in all the strains, possibly indicative of low pH-induced stress. Similarly, all the K+-uptake genes in all the strains were elevated during the late-log phase of growth, highlighting responses to alterations in the environmental conditions in the growth medium. Despite this, the levels of increased expression of the sigA gene in the WT were much lower than those of the K+-uptake genes illustrating the constant expression of the sigA gene at the logarithmic phase. However, the levels of sigA were excessively high in the mutants, increasing by 2–2.5-fold relative to those of the WT at the late-log phase due to low pH stress (Cholo et al., 2015), which are clearly shown by AQ data (Figure 5A; Supplementary Tables S3.1–S3.3).
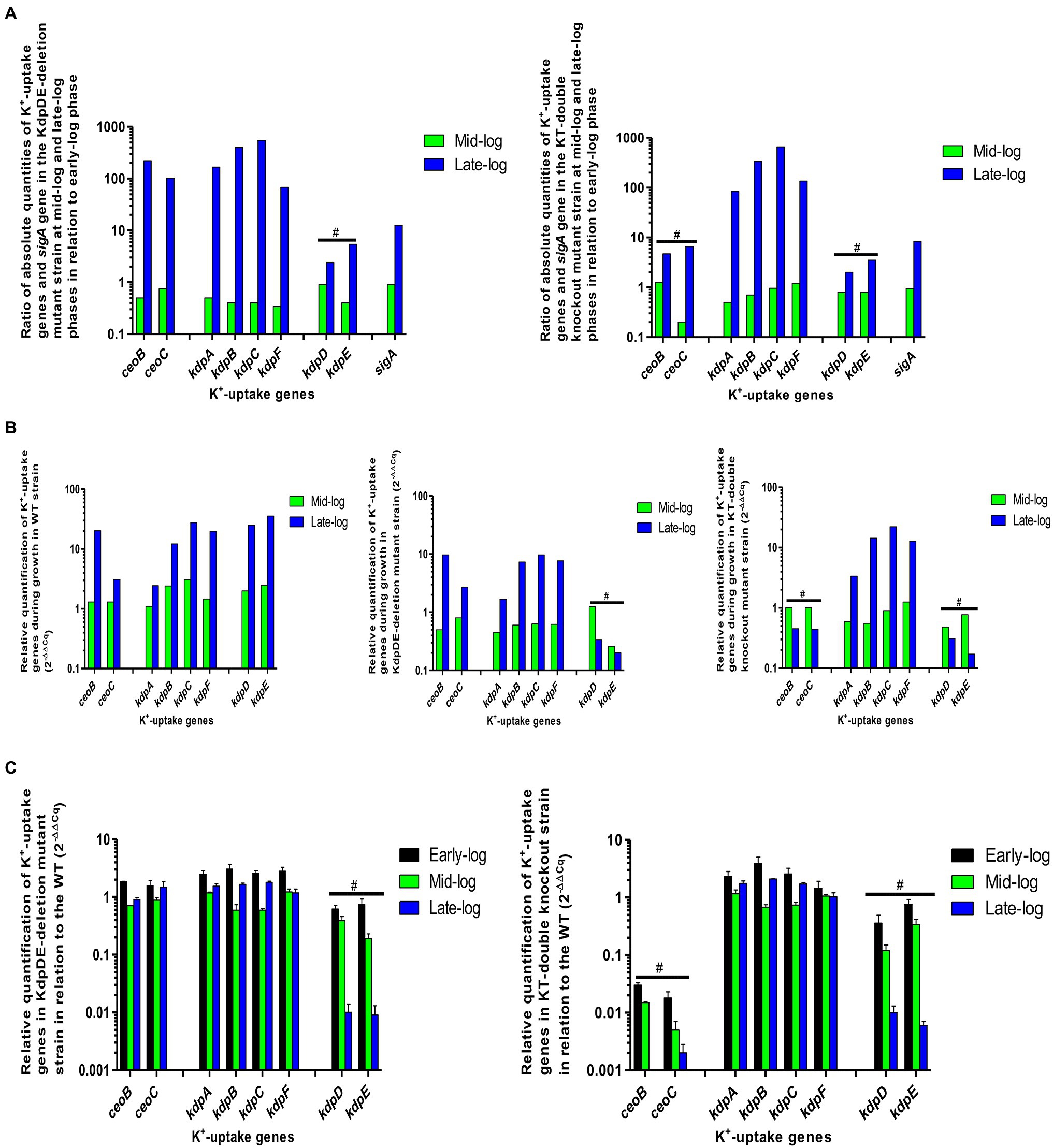
Figure 5. Gene expression measured during the various phases of bacterial growth for the WT and mutant strains. (A) Ratio of gene expression of absolute quantities of the K+-uptake genes and the sigA gene in the mutant strains during growth at mid- and late-log phases in relation to early-log phase, (B) relative quantification of K+-uptake genes in the mutant strains during growth relative to early-log phase (2−ΔΔCq), and (C) relative quantification of K+-uptake genes in mutant strains relative to the WT (2−ΔΔCq). #represents mutations of the individual genes.
For each strain, the expression of all the measured K+-uptake genes during growth was determined by comparing the expression levels of each gene at the mid- and late-log phases relative to those at the early-log phase.
The AQ data for the WT strain were the same as we have previously reported (Cholo et al., 2015). In summary, during growth, at the early- and mid-log phases, the kdp and the trk genes were expressed at minimum levels and were upregulated at the late-log phase, with the ceoB gene being the most prominently induced gene among all the K+-uptake genes, followed by kdpD and kdpF (Figure 5A; Supplementary Table S3.3). Despite upregulation of the K+-uptake genes, bacterial growth was slow at the late-log phase, due presumably to low extracellular pH levels (Cholo et al., 2015). Similar findings have been reported in E. coli, revealing that expression levels of the trk and the kdp genes are dependent on the extracellular pH (Epstein, 2003).
The RQ values of the genes in the WT strain were determined during growth at the mid- and late-log phases in relation to the early-log phase (Figure 5B; Supplementary Table S3.4). Expression of the kdpDE and kdpBC genes was significantly increased at the mid-log phase, while all the kdp genes were upregulated at the late-log phase. In the case of the trk genes, expression levels of both genes were unchanged at the mid-log phase, while both genes were upregulated at the late-log phase, particularly the ceoB gene.
During growth, expression levels of all the kdpFABC and trk genes were minimal at the early- and mid-log phases. However, the kdpFABC genes were upregulated at early-log phase probably as a response to the decrease in extracellular pH (6.7), while they were downregulated at mid-log phase at elevated pH level of 6.9. These genes were significantly increased by ≥1,000-fold at the late-log phase at the lower pH of 6.5 (Figure 3; Supplementary Tables S3.1 and S3.3). In relation to the WT (Cholo et al., 2015), the kdp genes were increased, while the trk genes were decreased at early-log phase. Both kdpFABC and trk genes were downregulated at mid-log phase. However, with the exception of the ceoB gene, and similar to those of the WT, the expression levels of the ceoC and kdpFABC genes were significantly increased by at least 1.5 and up to 7-fold relative to those of the WT at the late-log phase.
During growth, all the kdpFABC and trk genes were downregulated at the mid-log phase and upregulated at the late-log phase (Figure 5B; Supplementary Table S3.4). In relation to the WT strain (Figure 5C; Supplementary Table S3.5), all the kdpFABC and trk genes were increased at the early-log phase, decreased at mid-log phase, and upregulated at the late-log phase, showing dependency on extracellular pH levels for their expression.
The AQ and RQ results appear to show that inactivation of the KdpDE system leads to dysregulation of both the kdpFABC and trk genes, resulting in constitutive expression of both operons.
Only the kdpFABC genes were evaluated as the kdpDE and trk genes were deleted in this mutant strain as shown by Tm of non-specific fragments due to mutations of the targeted genes (Supplementary Table S1).
The expression levels and patterns of the kdpFABC genes were similar to those of the KdpDE-deletion mutant at the three growth phases (Figure 5A; Supplementary Tables S3.2 and S3.3).
During growth, the kdpA and kdpB genes were downregulated, while expression of the kdpC and kdpF genes remained unchanged at the mid-log phase (Figure 5B; Supplementary Table S3.4). All the kdpFABC genes were upregulated at the late-log phase. In relation to the WT strain (Figure 5C; Supplementary Table S3.6) and similar to the KdpDE-deletion mutant, all the kdpFABC genes were increased at the early-log phase, decreased at the mid-log phase, and upregulated at the late-log phase.
Mycobacterium tuberculosis is able to grow and adapt to varying environmental conditions. We have previously demonstrated that this bacterial pathogen grows exponentially at the mid-log phase at optimum K+ concentrations and pH levels (14–15 mM K+, pH 6.8–7.0), but is attenuated for growth, acquiring slow growth-to-dormant status at low pH levels (pH 5.5–6.0), despite maintaining elevated K+ concentrations at the late-log phase (Cholo et al., 2015).
Most bacteria adapt to varying extracellular K+ concentrations and pH levels by utilizing different K+-uptake transporters. Bacterial species, such as E. coli and Salmonella species, in which these K+ transporters have been extensively studied, utilize the Trk at elevated K+ concentrations and neutral pH levels and the Kup at low pH levels (pH 5.5), while they use the Kdp in K+-limiting conditions at neutral pH (Epstein, 2003; Liu et al., 2013). We have previously shown that M. tuberculosis, which encodes the Trk and Kdp as the predominant K+-uptake transporters (Cole et al., 1998), utilize the Trk system at mid-log (15 mM K+, pH 6.8; Cholo et al., 2006) and late-log phases (15 mM K+, pH 6.5; Cholo et al., 2015). However, in the case of the Kdp system, we have observed that the KdpFABC K+-uptake transporter is suppressed at mid-log while, similar to the Trk transporter, it is upregulated at the late-log phase (Cholo et al., 2015).
However, information on the differential utilization of these K+-uptake systems during bacterial growth is limited. This was investigated in the current study, in which we determined the role of the TCS KdpDE system in regulating the activities of the two K+-uptake transporters, the KdpFABC and Trk, during bacterial growth. We achieved this by first constructing the KT-double knockout mutant by transforming the kdpDE-deletion fragment constructed previously (Parish et al., 2003; Table 1) into the Trk-deletion mutant, also constructed previously (Cholo et al., 2006). Acquisition of the KdpDE-deletion and KT-double knockout mutant strains of M. tuberculosis were essential prerequisites to enable us to probe the involvement of the KdpDE system in harmonizing the activities of the KdpFABC and Trk transporters.
The findings of the current study revealed that the TCS KdpDE of M. tuberculosis is mechanistically involved in accelerating the rate of bacterial growth by shortening the duration of the early-log phase. This contention is supported by the observation that selective inactivation of the KdpDE system of M. tuberculosis caused attenuation of growth that was associated with prolongation of the early-log phase and delayed progression to the mid- and late-log phases, even in the presence of favorable extracellular K+ concentrations and near-neutral pH, both of which are conducive to exponential growth. Involvement of the KdpDE regulatory system during the exponential phase of bacterial growth was also evident, as demonstrated by the upregulation of both the kdpD and kdpE genes in the WT strain at the mid-log phase (Figure 5B; Supplementary Table S3.4). Other studies in M. tuberculosis and M. smegmatis have emphasized the essentiality of the kdpE gene during bacterial growth in standard growth conditions in vitro (Sassetti et al., 2003; Griffin et al., 2011; Ali et al., 2017).
As shown previously, M. tuberculosis cultured in these conditions utilizes the Trk system as the main K+-uptake transporter for growth (Cholo et al., 2006). Interestingly, however, in the current study, the growth of M. tuberculosis expressing an intact Trk system in the absence of KdpDE system (selective kdpDE-gene knockout mutant strain) was significantly attenuated, seemingly, implicating the KdpDE regulatory system in modulating the activity of the Trk system during growth. Not surprisingly, dual inactivation of the KdpDE and the Trk systems resulted in the most severe attenuation of growth relative to both the WT and KdpDE-deletion single knockout mutant. These observations not only underscore the interaction between these systems in promoting optimum bacterial growth, but also the seemingly key involvement of KdpDE in regulating the Trk, as well as the KdpFABC systems.
Using the 86Rb+ uptake procedure to determine bacterial K+-uptake efficiency, we demonstrated that during growth in optimal conditions, M. tuberculosis uses the KdpDE system to regulate K+ uptake by the KdpFABC and Trk transporters. In our previous studies, using the Trk-deletion mutant strain (Cholo et al., 2006, 2015), we demonstrated that at elevated K+ concentrations, bacteria utilize the low-affinity Trk system for K+ uptake, while the high-affinity Kdp system is suppressed, being induced as a back-up in the absence of Trk, conferring high K+-uptake efficiency on the Trk-deletion mutant (Cholo et al., 2006, 2015). The increase in K+-uptake efficiency of the KdpDE-deletion mutant strain in the presence of both KdpFABC and Trk K+-uptake transporters observed in the current study appears to demonstrate failure of the bacteria to differentially regulate the utilization of the two transporters, resulting in both systems being simultaneously operative. Excessive uptake of K+ is likely to result in dysregulation of bacterial cytoplasmic pH creating an intracellular environment unfavorable for cellular metabolism for growth.
However, at late-log phase (15 mM K+, pH 6.5), despite bacterial requirements of both the Kdp and Trk systems, the Trk has been shown to be the main K+-uptake transporter responsible for uptake of the cation. This contention is supported by the 86Rb+ uptake data, showing low K+-uptake efficiency of the WT in relation to the Trk-deletion mutant, together with gene expression data (AQ) in the WT strain showing that the ceoB gene is the most highly induced gene among all the K+-uptake genes at late-log phase (Cholo et al., 2015). In the current study, we have shown that in these conditions, as with optimal growth, the bacteria use the KdpDE regulatory system to regulate the activities and expression of both K+-uptake transporters. This has been demonstrated by the findings of increased uptake of 86Rb+ consistent with dysregulated, simultaneous, excessive functioning of both the Kdp and Trk K+-uptake transporters, in the absence of the KdpDE regulatory system.
These findings illustrate the constitutive activation of the KdpFABC transporter in the absence of its inducer KdpDE (Epstein, 2003; Steyn et al., 2003; Freeman et al., 2013; Agrawal and Saini, 2014). Similar findings have been demonstrated in previous studies in KdpDE mutant strains of E. coli (Asha and Gowrishankar, 1993; Sardesai and Gowrishankar, 2001; Epstein, 2003). These suggest a spontaneous induction of this operon in the absence of its inducer, or alternatively, the presence of an additional mechanism(s) of induction. While these have not been identified in M. tuberculosis, such mechanisms that bypass KdpDE have been described in E. coli and involve utilization of the histone-like nucleoid-structuring (H-NS) protein, thioredoxin 1, and thioredoxin reductase (Cole et al., 1998; Sardesai and Gowrishankar, 2001; Epstein, 2016). Although present in M. tuberculosis, the involvement of these mechanisms in activation of KdpFABC has not been described (Cole et al., 1998).
We do concede that our findings on the expression of these two mycobacterial K+-uptake transporters at various stages of growth in the absence of the TCS KdpDE system, are based on quantitation of their mRNA expression, which represents a potential limitation of our study. Nevertheless, we do believe that our findings demonstrate a novel and potentially important dual regulatory role of the KdpDE system in harmonizing the activities of the Trk and Kdp K+ transporters to ensure stringent control of cytoplasmic pH and growth. We also believe that these findings represent a platform that enables progression to additional confirmatory studies that would include target gene promoter expression using the β-galactosidase assay in this difficult and slow-growing pathogen.
These findings of the current study highlight the critical roles played by the K+-uptake transporters of M. tuberculosis during infection of the host. For example, in macrophages, the primary targets of the pathogen, in high intracellular K+ concentrations (phagosomal vacuolar K+ concentration: 19–50 mM; Wagner et al., 2005) and pH levels (pH 6.8; Piddington et al., 2000; Vandal et al., 2009), are probably conducive to bacterial growth. Presumably in this setting, the KdpDE and Trk systems are utilized by M. tuberculosis to establish infection (Haydel and Clark-Curtiss, 2004; Rengarajan et al., 2005; MacGilvary et al., 2019), possibly playing a role in bacterial virulence. However, at low pH levels, M. tuberculosis bacteria may utilize both the Kdp and Trk systems for survival (Figure 6). In this context, absence of the KdpDE system alone, and particularly in combination with the Trk system, is clearly detrimental to bacterial growth, underscoring the potential of these K+ transporters to serve as potential targets for development of anti-TB drugs.
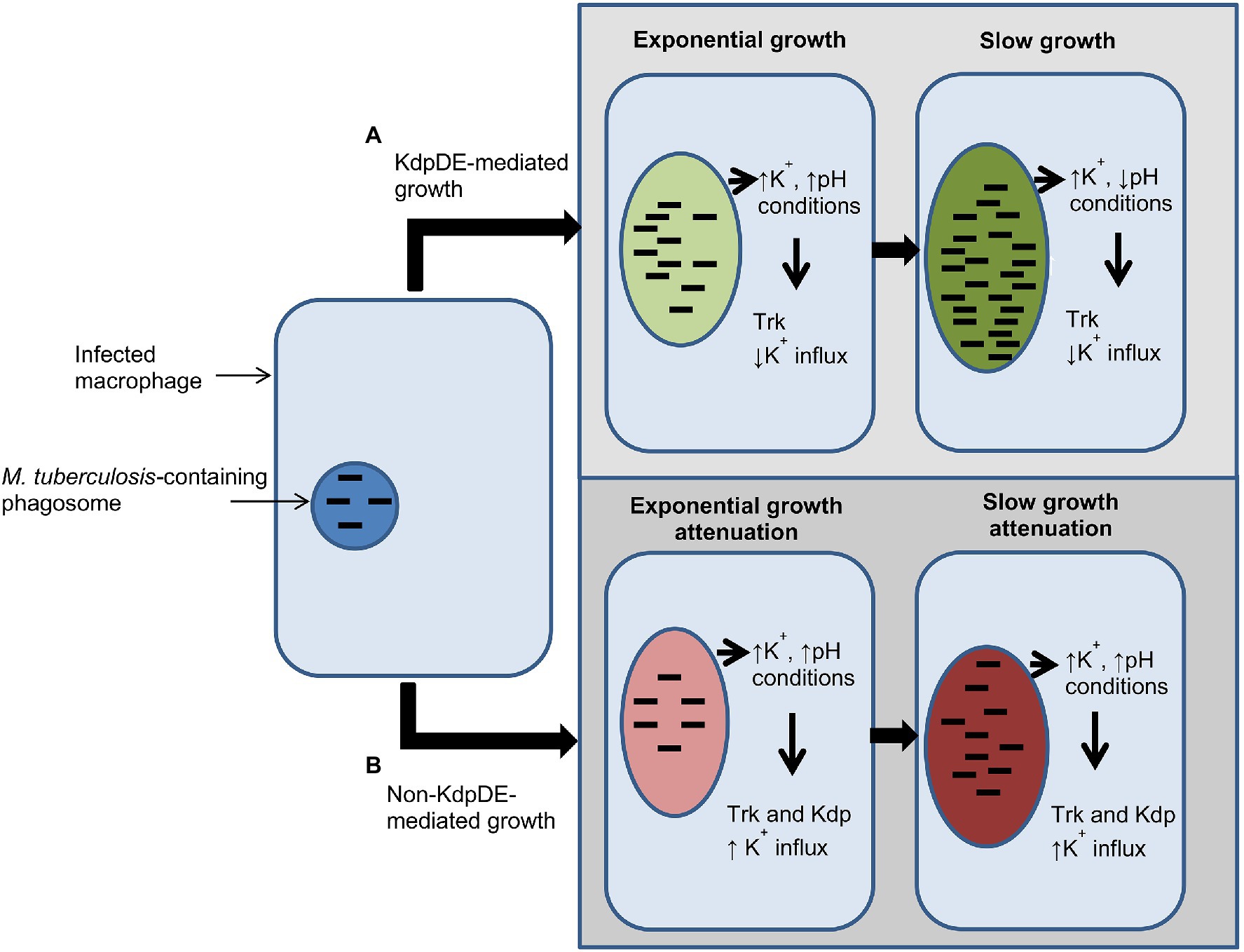
Figure 6. Schematic illustration summarizing events involved in bacterial growth in macrophages in the presence and absence of the two-component system (TCS) KdpDE. (A) In the presence of the KdpDE system, bacteria utilize the Trk transporter for K+ uptake suppressing the KdpFABC transporter, while (B) in the absence of the KdpDE both the KdpFABC and the Trk systems are constitutively activated, resulting in excessive influx of K+, which is detrimental to the bacteria, leading to attenuation of bacterial growth. ↑K+, phagosomal potassium concentration (19–50 mM); ↑pH, pH 6.7–6.8; ↓pH, pH 6.5.
In conclusion, in M. tuberculosis, the KdpDE system plays a key modulatory role in controlling the activities of the KdpFABC and Trk K+ uptake transporters to regulate growth.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MC and RA contributed to the conception and design of the study and wrote, edited and reviewed the manuscript. MC constructed the mutant strain. MC, MM, and AO performed the phenotypic experiments. MC, MM, AO, and RA contributed to interpretation of the data. All authors contributed to the article and approved the submitted version.
This study was supported by the South African National Research Foundation grant 87649 to MC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the TB Platform of the South African Medical Research Council (SAMRC) for the provision of the TB laboratory facility and the SAMRC/NHLS/WITS Molecular Mycobacteriology Research Unit, DST-NRF Centre of Excellence for Biomedical Research, School of Pathology, University of the Witwatersrand and National Health Laboratory Service, Johannesburg, South Africa for assistance in generation of the KT-double knockout mutant strain. We would also like to thank N. Stoker for critically reading the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.698875/full#supplementary-material
Agrawal, R., and Saini, D. K. (2014). Rv1027c-Rv1028c encode functional KdpDE two-component system in Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 446, 1172–1178. doi: 10.1016/j.bbrc.2014.03.066
Ali, M. K., Li, X., Tang, Q., Liu, X., Chen, F., Xiao, J., et al. (2017). Regulation of inducible potassium transporter KdpFABC by the KdpD/KdpE two-component system in Mycobacterium smegmatis. Front. Microbiol. 8:570. doi: 10.3389/fmicb.2017.00570
Asha, H., and Gowrishankar, J. (1993). Regulation of kdp operon expression in Escherichia coli: evidence against turgor as signal for transcriptional control. J. Bacteriol. 175, 4528–4537. doi: 10.1128/jb.175.14.4528-4537.1993
Baker, J. J., Dechow, S. J., and Abramovitch, R. B. (2019). Acid fasting: modulation of Mycobacterium tuberculosis metabolism at acidic pH. Trends Microbiol. 27, 942–953. doi: 10.1016/j.tim.2019.06.005
Cholo, M. C., Boshoff, H. I., Steel, H. C., Cockeran, R., Matlola, N. M., Downing, K. J., et al. (2006). Effects of clofazimine on potassium uptake by a Trk-deletion mutant of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 57, 79–84. doi: 10.1093/jac/dki409
Cholo, M. C., van Rensburg, E. J., and Anderson, R. (2008). Potassium uptake systems of Mycobacterium tuberculosis: genomic and protein organisation and potential roles in microbial pathogenesis and chemotherapy. South Afr. J. Epidemiol. Infect. 23, 13–16. doi: 10.1080/10158782.2008.11441327
Cholo, M. C., van Rensburg, E. J., Osman, A. G., and Anderson, R. (2015). Expression of the genes encoding the Trk and Kdp potassium transport systems of Mycobacterium tuberculosis during growth in vitro. Biomed. Res. Int. 2015:608682. doi: 10.1155/2015/608682
Cole, S. T., Brosch, R., Parkhill, J., Garnier, T., Churcher, C., Harris, D., et al. (1998). Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544.
Epstein, W. (2003). The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75, 293–320. doi: 10.1016/s0079-6603(03)75008-9
Epstein, W. (2016). The KdpD sensor kinase of Escherichia coli responds to several distinct signals to turn on expression of the Kdp transport system. J. Bacteriol. 198, 212–220. doi: 10.1128/JB.00602-15
Freeman, Z. N., Drus, S., and Waterfield, N. R. (2013). The KdpD/KdpE two-component system: integrating K+ homeostasis and virulence. PLoS Pathog. 9:e1003201. doi: 10.1371/journal.ppat.1003201
Griffin, J. E., Gawronski, J. D., DeJesus, M. A., Ioerger, T. R., Akerley, B. J., and Sassetti, C. M. (2011). High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 7:e1002251. doi: 10.1371/journal.ppat.1002251
Haydel, S. E., and Clark-Curtiss, J. E. (2004). Global expression analysis of two-component system regulator genes during Mycobacterium tuberculosis growth in human macrophages. FEMS Microbiol. Lett. 236, 341–347. doi: 10.1111/j.1574-6968.2004.tb09667.x
Hegde, S. R. (2020). Computational identification of the proteins associated with quorum sensing and biofilm formation in Mycobacterium tuberculosis. Front. Microbiol. 10:3011. doi: 10.3389/fmicb.2019.03011
Kerns, P. W., Ackart, D. F., Basaraba, R. J., Leid, J., and Shirtliff, M. E. (2014). Mycobacterium tuberculosis pellicles express unique proteins recognized by the host humoral response. Pathog Dis. 70, 347–358. doi: 10.1111/2049-632X.12142
Liu, Y., Ho, K. K., Su, J., Gong, H., Chang, A. C., and Lu, S. (2013). Potassium transport of Salmonella is important for type III secretion and pathogenesis. Microbiology 159, 1705–1719. doi: 10.1099/mic.0.068700-0
MacGilvary, N. J., Kevorkian, Y. L., and Tan, S. (2019). Potassium response and homeostasis in Mycobacterium tuberculosis modulates environmental adaptation and is important for host colonization. PLoS Pathog. 15:e1007591. doi: 10.1371/journal.ppat.1007591
Mothiba, M. T., Anderson, R., Fourie, B., Germishuizen, W. A., and Cholo, M. C. (2015). Effects of clofazimine on planktonic and biofilm growth of Mycobacterium tuberculosis and Mycobacterium smegmatis. J. Glob. Antimicrob. Resist. 3, 13–18. doi: 10.1016/j.jgar.2014.12.001
Parish, T., Smith, D. A., Kendall, S., Casali, N., Bancroft, G. J., and Stoker, N. G. (2003). Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect. Immun. 71, 1134–1140. doi: 10.1128/IAI.71.3.1134-1140.2003
Parish, T., and Stoker, N. G. (2000). Use of a flexible cassette method to generate a double unmarked Mycobacterium tuberculosis tlyA plcABC mutant by gene replacement. Microbiology 146, 1969–1975. doi: 10.1099/00221287-146-8-1969
Piddington, D. L., Kashkouli, A., and Buchmeier, N. A. (2000). Growth of Mycobacterium tuberculosis in a defined medium is very restricted by acid pH and Mg2+ levels. Infect. Immun. 68, 4518–4522. doi: 10.1128/IAI.68.8.4518-4522.2000
Rengarajan, J., Bloom, B. R., and Rubin, E. J. (2005). Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 102, 8327–8332. doi: 10.1073/pnas.0503272102
Roe, A. J., McLaggan, D., O’Byrne, C. P., and Booth, I. R. (2000). Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol. Microbiol. 35, 1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x
Salina, E. G., Grigorov, A. S., Bychenko, O. S., Skvortsova, Y. V., Mamedov, I. Z., Azhikina, T. L., et al. (2019). Resuscitation of dormant “non-culturable” Mycobacterium tuberculosis is characterized by immediate transcriptional burst. Front. Cell. Infect. Microbiol. 9:272. doi: 10.3389/fcimb.2019.00272
Salina, E. G., Waddell, S. J., Hoffmann, N., Rosenkrands, I., Butcher, P. D., and Kaprelyants, A. S. (2014). Potassium availability triggers Mycobacterium tuberculosis transition to, and resuscitation from, non-culturable (dormant) states. Open Biol. 4:140106. doi: 10.1098/rsob.140106
Sardesai, A. A., and Gowrishankar, J. (2001). Trans-acting mutations in loci other than KdpDE that affect kdp operon regulation in Escherichia coli: effects of cytoplasmic thiol oxidation status and nucleoid protein H-NS on kdp expression. J. Bacteriol. 183, 86–93. doi: 10.1128/JB.183.1.86-93.2001
Sassetti, C. M., Boyd, D. H., and Rubin, E. J. (2003). Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84. doi: 10.1046/j.1365-2958.2003.03425.x
Steel, H. C., Matlola, N. M., and Anderson, R. (1999). Inhibition of potassium transport and growth of mycobacteria exposed to clofazimine and B669 is associated with a calcium-independent increase in microbial phospholipase A2 activity. J. Antimicrob. Chemother. 44, 209–216. doi: 10.1093/jac/44.2.209
Steyn, A. J., Joseph, J., and Bloom, B. R. (2003). Interaction of the sensor module of Mycobacterium tuberculosis H37Rv KdpD with members of the Lpr family. Mol. Microbiol. 47, 1075–1089. doi: 10.1046/j.1365-2958.2003.03356.x
Vandal, O. H., Nathan, C. F., and Ehrt, S. (2009). Acid resistance in Mycobacterium tuberculosis. J. Bacteriol. 191, 4714–4721. doi: 10.1128/JB.00305-09
Keywords: Mycobacterium tuberculosis, two-component system KdpDE, KdpFABC system, Trk system, K+-uptake systems, K+ concentration, pH level, gene expression
Citation: Cholo MC, Matjokotja MT, Osman AG and Anderson R (2021) Role of the kdpDE Regulatory Operon of Mycobacterium tuberculosis in Modulating Bacterial Growth in vitro. Front. Genet. 12:698875. doi: 10.3389/fgene.2021.698875
Received: 22 April 2021; Accepted: 12 July 2021;
Published: 29 July 2021.
Edited by:
Silvia Calo, Pontificia Universidad Católica Madre y Maestra, Dominican RepublicReviewed by:
Monde Ntwasa, University of South Africa, South AfricaCopyright © 2021 Cholo, Matjokotja, Osman and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moloko C. Cholo, bW9sb2tvLmNob2xvQHVwLmFjLnph
†ORCID: Moloko C. Cholo, orcid.org/0000-0002-0958-2401
Ronald Anderson, orcid.org/0000-0002-5925-6452
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.