- Department of Obstetrics and Gynecology, Third Xiangya Hospital of Central South University, Changsha, China
Ovarian cancer is one of the most common gynecological malignancies with highest mortality rate among all gynecological malignant tumors. Advanced ovarian cancer patients can obtain a survival benefit from chemotherapy, including platinum drugs and paclitaxel. In more recent years, the administration of poly-ADP ribose polymerase inhibitor to patients with BRCA mutations has significantly improved the progression-free survival of ovarian cancer patients. Nevertheless, primary drug resistance or the acquisition of drug resistance eventually leads to treatment failure and poor outcomes for ovarian cancer patients. The mechanism underlying drug resistance in ovarian cancer is complex and has not been fully elucidated. Interestingly, different non-coding RNAs (ncRNAs), such as circular RNAs, long non-coding RNAs and microRNAs, play a critical role in the development of ovarian cancer. Accumulating evidence has indicated that ncRNAs have important regulatory roles in ovarian cancer resistance to chemotherapy reagents and targeted therapy drugs. In this review, we systematically highlight the emerging roles and the regulatory mechanisms by which ncRNAs affect ovarian cancer chemoresistance. Additionally, we suggest that ncRNAs can be considered as potential diagnostic and prognostic biomarkers as well as novel therapeutic targets for ovarian cancer.
Background
Ovarian cancer is one of the most deadly gynecologic malignancy, there are approximately 313,959 new cases and more than 207,252 deaths annually worldwide (Sung et al., 2021). Unfortunately, due to lack of effective early screening methods, 5-year survival rate was only 20–40% (Lheureux et al., 2019). Currently, the main methods used for clinical treatment of ovarian cancer are still based on cytoreductive surgery and multidrug combination chemotherapy based on platinum drugs (Armstrong et al., 2021). Chemotherapy is the main treatment option available for advanced or recurrent ovarian cancers, and the commonly used chemotherapeutic agents include platinum drugs and paclitaxel (PTX). In addition, the administration of poly-ADP ribose polymerase inhibitor (PARPi) to BRCA mutation patients has significantly improved the progression-free survival (PFS) of ovarian cancer (Tew et al., 2020). Although chemotherapy in combination with targeted therapy prolongs the overall survival of ovarian cancer patients, acquired multidrug resistance (MDR) hinders its clinical benefits. Therefore, patients with ovarian cancer frequently have a poor prognosis. The complicated mechanisms involved in MDR ovarian cancer include decreased drug uptake into the cell, increased drug efflux, intracellular drug inactivation, DNA damage repair, resistance to drug-induced apoptosis, activation of cancer stem cells, and epithelial-mesenchymal transition (EMT) (Christie et al., 2019; Liang et al., 2019; Belur Nagaraj et al., 2021; Chiappa et al., 2021). While progress has been made in understanding the pathogenesis of ovarian cancer, the detailed mechanisms of MDR remain elusive.
Non-coding RNAs (ncRNAs) are a kind of DNA transcription product that cannot be encoded into proteins. NcRNAs can be classified according to their length and shape into tiny/short ncRNAs, long ncRNAs (lncRNAs) which is larger than 200 nucleotides (nt), and circular RNA (circRNAs). Various small ncRNAs have been identified, such as microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), and small nuclear RNAs (snRNAs) (Kristensen et al., 2019; Shuai et al., 2019; Jin et al., 2020; Cui et al., 2021; Han et al., 2021; Luo et al., 2021; Tsitsipatis et al., 2021). NcRNAs have been proven to have important regulatory potential, both in transcription and post transcription, instead of just being “transcription noise” or “transcription garbage.” There is ample evidence that ncRNAs are of crucial importance in the regulation of gene expression. Meanwhile, ncRNAs participate in many biological functions, such as cell proliferation, cell cycle progression, and apoptosis (Cocquerelle et al., 1993; Memczak et al., 2013; Cech and Steitz, 2014; Li et al., 2021; Ramat and Simonelig, 2021; Statello et al., 2021). In addition, a large number of studies have shown that abnormally expressed ncRNAs participate in tumor cell invasion, metastasis, drug resistance and radiotherapy resistance (Bi et al., 2020; Chen et al., 2020; Wang P. et al., 2020). Similarly, previous research suggested that ncRNAs are dysregulated when drug resistance develops, which indicates that in ovarian cancer, multiple ncRNAs might play a vital role in drug resistance.
In this review, we summarized the detailed mechanisms by which miRNAs, lncRNAs, and circRNAs affect ovarian cancer drug resistance. The potential mechanisms of ncRNAs related to drug-resistance in ovarian cancer are summarized in Figure 1. NcRNAs have potential as diagnostic and prognostic biomarkers as well as novel therapeutic targets for ovarian cancer in the future.
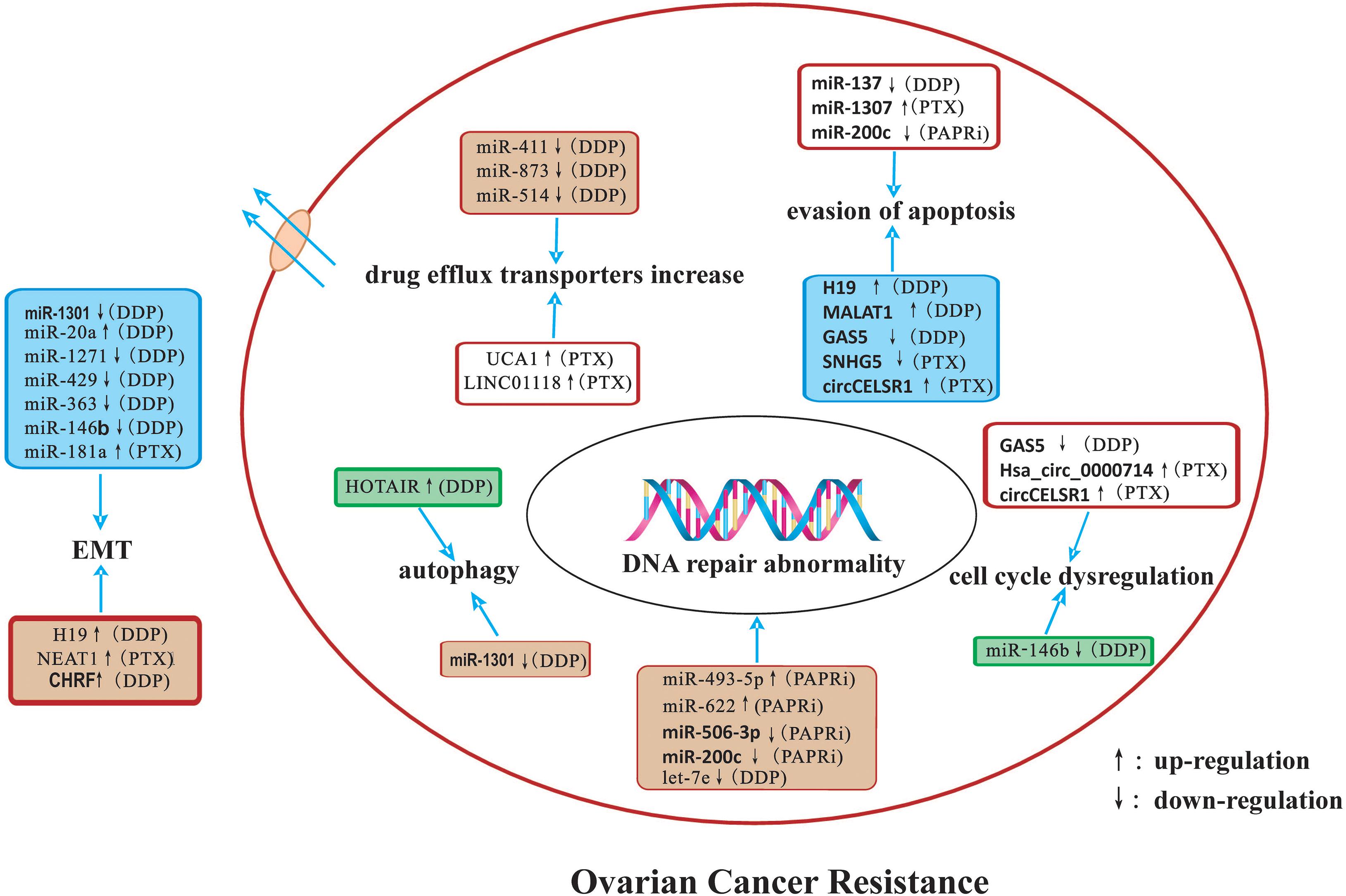
Figure 1. A summary diagram of miRNAs, lncRNAs, and circRNAs involved in the drug resistance of ovarian cancer. Several ncRNAs could participate in drug resistance of ovarian cancer by influencing cell apoptosis, proliferation, cell cycle, autophagy, DNA repair, and epithelial-mesenchymal transition through modulating the expression of downstream target genes and related signaling pathway.
MiRNAs and Drug Resistance
MicroRNAs are a class of small ncRNAs containing 20–24 nt that can post transcriptionally suppress gene expression by binding to the 3′-untranslated region (3′-UTR) of multiple target messenger RNAs (mRNAs) and/or other RNAs (Wang X. et al., 2021). MiRNAs are key molecules that are involved in many different kinds of fundamental cellular processes, including cell differentiation and proliferation, cell cycle regulation, angiogenesis, metabolic stress, and other functions (He et al., 2019; Komoll et al., 2021; Xing et al., 2021). It has been found that multiple miRNAs are dysregulated in ovarian cancer and are closely related to its occurrence, development, metastasis and drug resistance (Mak et al., 2017; Tung et al., 2020; Zhang Z. et al., 2020). Significant changes in miRNA expression profiles have been observed in drug-resistant cancer cells in comparison with parental drug-sensitive cancer cells. The involvement of miRNAs in ovarian cancer resistance to platinum drugs, PTX, ADR, and PARPi is summarized below.
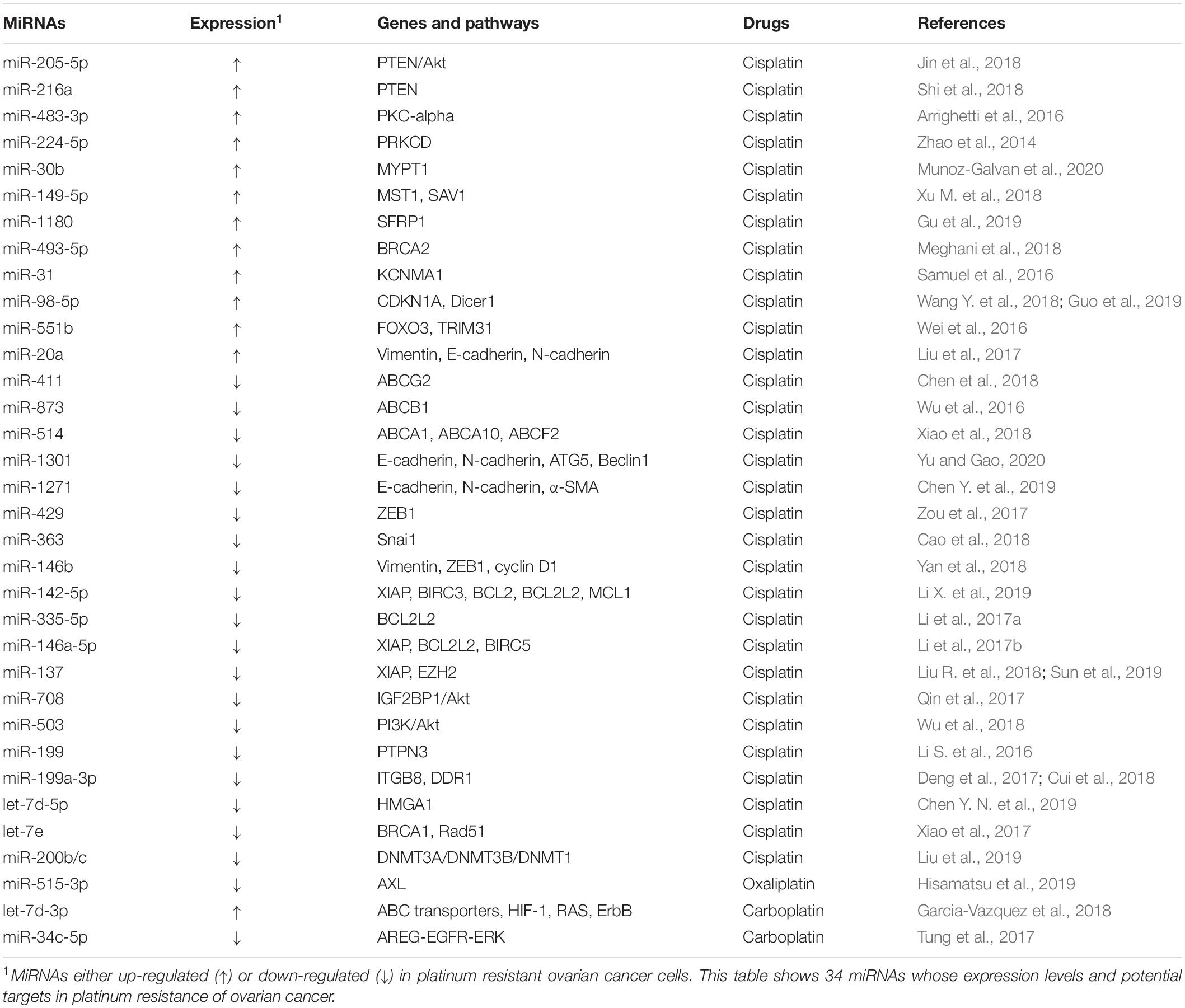
MiRNAs and Resistance to Platinum
Platinum drugs are cell cycle non-specific drugs that are widely used in the clinic. They induce DNA damage or ribosome biosynthesis stress and activate tumor cell death by apoptosis or necrosis. However, a series of complex mechanisms lead to platinum resistance (Bruno et al., 2017; Huang et al., 2019). The commonly used platinum drugs include the first generation of drug cisplatin (DDP), the second generation of drug carboplatin, as well as the third-generation drugs oxaliplatin and Lopatin. Many miRNAs are related to the resistance to platinum drugs in ovarian cancer (Table 1).
Several oncogenic miRNAs can promote resistance to platinum drugs in ovarian cancer cells. For example, miR-205-5p and miR-216a confer DDP resistance by suppressing the PTEN (phosphatase and tensin homolog)/Akt signaling pathway in ovarian cancer cells (Jin et al., 2018; Shi et al., 2018). Similarly, miR-483-3p and miR-224-5p have also been found to promote DDP resistance by silencing protein kinase C (PRKC) family members (Zhao et al., 2014; Arrighetti et al., 2016). Studies have shown that miR-30b and miR-149-5p are involved in the Hippo signaling pathway and promote DDP resistance by downregulating the target genes protein phosphatase 1 regulatory subunit 12A (PPP1R12A), STE20-like kinase 1 (MST1), and protein salvador homolog 1 (SAV1), respectively (Xu M. et al., 2018; Munoz-Galvan et al., 2020). In addition, oncogenic miR-1180, miR-493-5p, and miR-31 confer DDP resistance to ovarian cancer cells through silencing secreted frizzled-related protein 1(SFRP1), BRCA2, and potassium calcium-activated channel subfamily M alpha 1 (KCNMA1), respectively (Samuel et al., 2016; Meghani et al., 2018; Gu et al., 2019). In animal models, miR-98-5p can potentiate the resistance of ovarian cancer to DDP, suggesting that miR-98-5p is a possible therapeutic target of ovarian cancer (Wang Y. et al., 2018; Guo et al., 2019). MiR-551b functions through the suppression of forkhead box O3 (FOXO3) and tripartite motif containing 31 (TRIM31), two important tumor suppressors. It was also found that elevated expression of miR-551b is significantly associated with worse survival of xenograft ovarian cancer models (Wei et al., 2016). Additionally, miR-20a could enhance DDP resistance of OVCAR3 ovarian cancer cells by altering the expression of EMT markers (E-cadherin, N-cadherin, and vimentin) (Liu et al., 2017).
In contrast, multiple tumor suppressor miRNAs have been found to be able to reverse DDP resistance in ovarian cancer. For instance, tumor suppressors miR-411, miR-873, and miR-514 have been confirmed to be involved in DDP resistance of ovarian cancer by modulating the expression/function of the ABC transporters family members (Wu et al., 2016; Chen et al., 2018; Xiao et al., 2018). In the meantime, miR-1301, miR-1271, miR-429, miR-363, and miR-146b can sensitize ovarian cancer cells to DDP by inhibiting the expression of multiple EMT-related genes (Zou et al., 2017; Cao et al., 2018; Yan et al., 2018; Chen Y. et al., 2019; Yu and Gao, 2020). By inhibiting the Bcl-2 signaling pathway, several tumor suppressor miRNAs, including miR-142-5p, miR-335-5p, miR-146a-5p, and miR-137 have been confirmed to sensitize ovarian cancer cells to DDP (Li et al., 2017a,b; Liu R. et al., 2018; Li X. et al., 2019). In addition, exogenous expression of miR-137 can also strongly promote DDP chemosensitivity through downregulating the expression of X-linked inhibitor of apoptosis (XIAP) and the zeste homolog 2 (EZH2) (Sun et al., 2019). Similarly, miR-708 and miR-503 can modulate ovarian cancer resistance to cisplatin through regulating the Akt pathway (Qin et al., 2017; Wu et al., 2018).Recently, emerging evidence has shown that miRNAs are aberrantly expressed in ovarian cancer, and some of them regulate different mRNAs and inhibit cisplatin resistance. Abnormal expression of the miR-199 cluster, for example, has been confirmed to increase the sensitivity of ovarian cancer cells to DDP through silencing the expression of protein tyrosine phosphatase non-receptor type 3 (PTPN3), integrin subunit beta 8 (ITGB8) and discoidin domain receptor 1 (DDR1) (Li S. et al., 2016; Deng et al., 2017; Cui et al., 2018). Additionally, ectopic miR-let-7 cluster expression can weaken DDP resistance in ovarian cancer cells by inhibiting high mobility group AT-hook 1 (HMGA1), RAD51 recombinase (RAD51), and BRCA1, indicating that the miR-let-7 cluster might be a candidate biomarker to predict ovarian cancer responders to DDP treatment (Xiao et al., 2017; Chen Y. N. et al., 2019). Moreover, the miR-200b/c cluster can improve the sensitivity of ovarian cancer cells to cisplatin by inhibiting the expression of DNA methyltransferase (DNMT) (Liu et al., 2019).
Studies on carboplatin and oxaliplatin are far less extensive than cisplatin. Tumor suppressor miR-515-3p can regulate oxaliplatin sensitivity by targeting AXL Receptor Tyrosine Kinase (AXL) (Hisamatsu et al., 2019). Similarly, let-7d-3p could enhance carboplatin-resistance (Garcia-Vazquez et al., 2018). Tumor suppressors miR-634 and miR-34c-5p have been proven to be involved in the regulation of carboplatin sensitivity through the MAPK pathway (Tung et al., 2017).
MiRNAs and PTX Resistance
Paclitaxel is one of the first-line chemotherapy drugs used to treat ovarian cancer. It is highly cytotoxic against tubulin. It induces and promotes the polymerization of tubulin and microtubule assembly, and it prevents depolymerization, stabilizing microtubules, and inhibiting the mitosis of cancer cells, leading to cell cycle arrest in G2/M. This effectively prevents the proliferation of cancer cells. It has been reported that various miRNAs are involved in PTX-resistance of ovarian cancer (Table 2).
Several oncogenic miRNAs can facilitate PTX resistance, such as miR-21 and miR-630. Exogenous expression of miR-21 and miR-630 enhanced PTX resistance of ovarian cancer cells by silencing apoptotic peptidase activating factor 1 (APAF1) (Au Yeung et al., 2016; Eoh et al., 2018). Similarly, miR-1307, a highly expressed miRNA in ovarian cancer tissues and cell lines, has been demonstrated to be positively correlated with PTX resistance. By targeting the capicua transcriptional repressor (CIC) and the inhibitor of growth family member 5 (ING5), miR-1307 could dramatically inhibit apoptosis induced by PTX (Chen W. T. et al., 2017; Zhou et al., 2019). Moreover, the miR-181a level in chemoresistant cancer tissues is significantly higher than in chemosensitive cancer tissues and in normal tissue, and its upregulation is associated with an increased level of EMT and decreased cell apoptosis induced by PTX treatment (Li L. et al., 2016).
In contrast, several tumor suppressor miRNAs may reverse PTX resistance in ovarian cancer. The Bcl-2 family participates in the chemoresistance of malignancies, including ovarian cancer. Tumor suppressors miR-215 can promote PTX-induced apoptosis of ovarian cancer cells by silencing the expression of XIAP (Ge et al., 2016). Activation of the EMT pathway has also been observed to regulate PTX resistance of ovarian cancer. A variety of miRNAs, such as miR-200b and miR-200c, have been observed to be involved in the EMT pathway mediated PTX resistance of ovarian cancer (Duran et al., 2017). By inhibiting the signal transducer and activator of transcription 3 (STAT3) signaling pathway, several tumor suppressor miRNAs, including miR-92 and miR-503-5p, have been found to sensitize ovarian cancer cells to PTX. In animal models, targeting STAT3 in combination with paclitaxel can synergistically reduce intraperitoneal dissemination and prolong the survival of mice with ovarian cancer (Chen M. W. et al., 2017; Park and Kim, 2019). Similarly, tumor suppressors miR-136, miR-383-5p, and miR-874 have been reported to conquer PTX resistance of ovarian cancer cells by silencing NOTCH3, tripartite motif containing 27 (TRIM27), and salt inducible kinase 2 (SIK2), respectively (Jeong et al., 2017; Xia et al., 2018; Jiang et al., 2019).
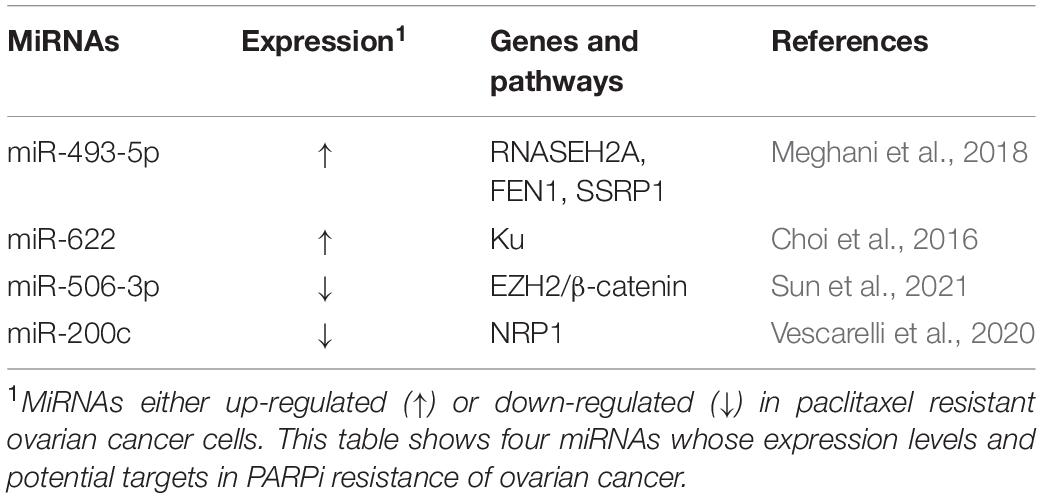
MiRNAs and PARPi Resistance
Poly-ADP ribose polymerase inhibitor have emerged as exciting new chemotherapy options for women with ovarian cancer, especially for patients with BRCA1 or BRCA2 mutations or non-functional homologous recombination repair pathways. The most advantageous feature of PARPi is its mechanism of action. PARPi is able to eliminate the function of PARP, leading to the accumulation of single-stranded breaks (SSB), which in turn can be converted into double-strand breaks (DSB) that the cell cannot repair, leading to cancer cell death (Wiltshire et al., 2010). Moreover, PARPi can enhance the efficacy of radiotherapy and chemotherapy with docetaxel and platinum drugs. Three PARPis have been approved for the treatment of recurrent epithelial ovarian cancer in the United States: olaparib, rucaparib, and niraparib. However, long-term use of PARPis may cause PARPi resistance. In ovarian cancer cells, multiple miRNAs were found to be involved in PARPi resistance (Table 3).
Multiple oncogene miRNAs can promote PARPi resistance. According to a recent report, miR-493-5p is significantly upregulated in BRCA2-mutated ovarian cancer cells and it participates in the PARPi resistance process by regulating ribonuclease H2 subunit A (RNASEH2A), flap structure-specific endonuclease 1 (FEN1), and structure specific recognition protein 1 (SSRP1). miR-493-5p can reduce single-strand annealing (SSA), stabilize the replication fork, and thus induce PARPi tolerance (Meghani et al., 2018). In addition, miR-622 is highly expressed in BRCA1-deficient high-grade serous ovarian carcinomas (HGSOCs), which can rescue the homologous recombination repair (HRR) defect of BRCA1 mutant ovarian cancer and promote PARPi resistance by regulating the expression of Ku complex and inhibiting HR and non-homologous end joining (NHEJ) (Choi et al., 2016).
In contrast, multiple tumor suppressor miRNAs can reverse the PARPi resistance of ovarian cancer. For instance, miR-506-3p acts as a vital regulator in the sensitivity to PARPis and cisplatin by targeting EZH2/β-catenin pathway in ovarian cancers (Sun et al., 2021). Additionally, ectopic miR-200c expression can increase apoptosis and weaken the resistance to olaparib in the ovarian cancer cells SKOV3/PARPi by silencing Neuropilin 1 (NRP1) (Vescarelli et al., 2020).
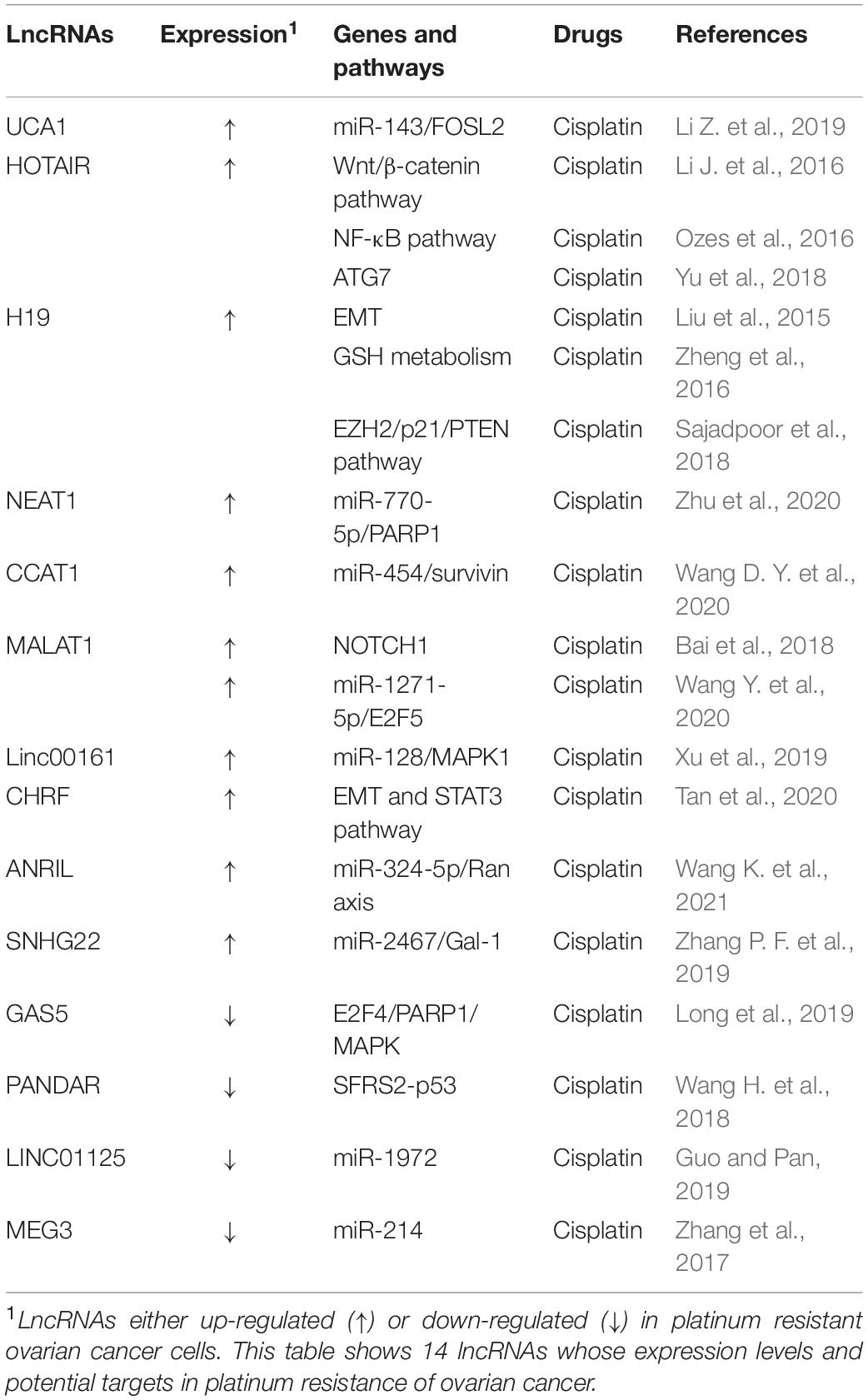
LncRNAs and Therapy Resistance
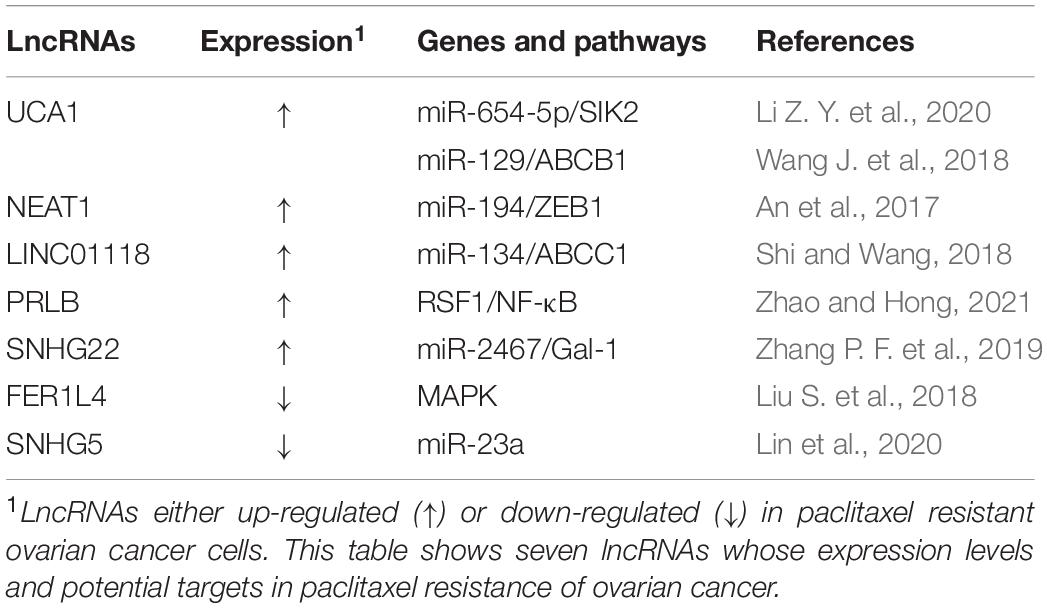
Long non-coding RNAs are a category of RNA transcripts longer than 200 nt without coding capacity, which are transcribed by RNA Polymerase II (RNAP II) and expressed in a tissue-specific manner (Quinn and Chang, 2016). At present, it is known that lncRNAs can regulate the malignant biological behavior of cells by acting as a competitive endogenous RNA (ceRNA), recruiting downstream molecules, serving as protein scaffolds, transmitting regulatory signals (Wong et al., 2018), and regulating endolysosome pH (Miller et al., 2018). A number of lncRNAs have a close relationship to the development of ovarian cancer metastasis, recurrence, and chemotherapy resistance (Winham et al., 2019; Zhang M. et al., 2019; Sun et al., 2020). Aberrantly expressed lncRNAs may participate in ovarian cancer progression through various mechanisms, including inducing autophagy, increasing DNA damage repair, changing cell cycle progression and checkpoints, inducing anti-apoptosis, regulating cell signaling pathways, and promoting EMT (Liu et al., 2015; Yan et al., 2017; Xu Q. F. et al., 2018; Wu et al., 2019). Several lncRNAs have been found to be involved in drug resistance in ovarian cancer (Tables 4, 5).
It has been reported that lncRNA UCA1 (urothelial cancer associated 1) is significantly upregulated in PTX-resistant ovarian cancer tissues and cell lines and confers ovarian cancer resistance to PTX. UCA1 promote tumor progression both in vitro and in vivo. SIK2 protein is involved in the separation of centrosomes during mitosis, which can lead to ovarian cancer drug resistance (Ahmed et al., 2010; Zhou et al., 2017). In ovarian cancer cells, UCA1 can induce SIK2 expression via endogenous sponging of miR-654-5p and thus antagonize chemosensitivity to PTX (Li Z. Y. et al., 2020). Additionally, ABCB1 (ATP binding cassette subfamily B member 1) is one of the members of the superfamily of ABC transporters that are involved in MDR. In ovarian cancer cells, UCA1 can also induce ABCB1 expression though endogenous sponging of miR-129 to enhance PTX tolerance (Wang J. et al., 2018). In recent years, lncRNA UCA1 has also been found to be involved in cisplatin resistance in ovarian cancer and blood UCA1 levels are upregulated in patients after cisplatin treatment. Via binding to the 3′-UTRs of FOS-like 2 (FOSL2), miR-143 can negatively regulate FOSL2 expression, suggesting that the UCA1/miR-143 axis may have potential therapeutic value for the treatment of cisplatin resistance in ovarian cancer patients (Li Z. et al., 2019).
Long non-coding RNAs HOTAIR (HOX antisense intergenic RNA) is one of the most well-studied lncRNAs, which is transcribed from the antisense strand of the HOXC gene cluster present on chromosome 12 with a length of 2.2 kb. HOTAIR, a highly expressed lncRNA in ovarian cancer tissues and cell lines, has been found to be positively correlated with advanced tumor stages, high histological grade, lymph node metastasis, drug resistance, and poor prognosis of ovarian cancer patients (Qiu et al., 2014; Wang et al., 2015). Moreover, it has been reported that exogenous HOTAIR overexpression in ovarian cancer cells significantly promoted cisplatin resistance by regulating the Wnt/β-catenin signaling pathway as well as the NF-κB-HOTAIR axis, indicating that HOTAIR may act as a regulator of cisplatin resistance (Li J. et al., 2016; Ozes et al., 2016). Similarly, knockdown of HOTAIR can inhibit autophagy via decreasing autophagy related 7 (ATG7) expression, and the inhibition of cisplatin-induced autophagy by silencing HOTAIR has been shown to enhance the chemotherapeutic efficacy of cisplatin in ovarian cancer (Yu et al., 2018).
Increasing findings indicate that lncRNA H19 plays an important role in chemotherapy drug resistance of ovarian cancer. In the OVCAR3/DDP resistant ovarian cancer cell, silencing lncRNA H19 can significantly increase E-cadherin expression and reduce twist, slug, and snail expression, indicating that lncRNA H19 induces cisplatin resistance via EMT (Wu et al., 2019). In addition, lncRNA H19 can also confer resistance to cisplatin to ovarian cancer cells by promoting glutathione (GSH) metabolism (Zheng et al., 2016). It has been reported that valproic acid (VPA) acts on A2780/CP resistant cells, which negatively regulates the expression of lncRNA H19, and then induces cell apoptosis and inhibits cell proliferation, thereby making A2780 resistant cells sensitive to cisplatin (Sajadpoor et al., 2018). These findings suggest that lncRNA H19 has potential as a new target for overcoming drug resistance in ovarian cancer.
Long non-coding RNAs NEAT1 (nuclear paraspeckle assembly transcript 1) was reported to be correlated with clinically poor paclitaxel response ovarian cancer. It has been found that lncRNA NEAT1 promotes paclitaxel resistance via competitively binding miR-194 to facilitate ZEB1 expression in ovarian cancer cells (An et al., 2017). Recently, LncRNA NEAT1 is also found to play a part in cisplatin resistance of ovarian cancer. NEAT1 is significantly upregulated in ovarian cancer, associates with cisplatin resistance and FIGO stage. Knockdown of NEAT1 suppresses cisplatin resistance of ovarian cancer cells in vitro and in vivo. LncRNA NEAT1 contributes to DDP resistance of ovarian cancer cells by regulating PARP1 expression via miR-770-5p (Zhu et al., 2020).
In addition, some other lncRNAs were found to be involved in platinum-based chemotherapy resistance in ovarian cancer. On the one hand, lncRNAs can promote platinum resistance. For instance, lncRNA CCAT1 (colon cancer associated transcript 1) is upregulated in A2780/DDP and SKOV3/DDP resistant ovarian cancer cells, and it can confer resistance to DDP by modulating the miR-454/survivin axis (Wang D. Y. et al., 2020). LncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been reported to be upregulated and to contribute to ovarian cancer tumorigenesis. Knockdown of MALAT1 could enhance cisplatin-induced apoptosis and improve the chemosensitivity of ovarian cancer cells to cisplatin through inhibiting the notch1 signaling pathway (Bai et al., 2018). Besides, MALAT1 could regulate ovarian cancer progression and DDP- resistance by miR-1271-5p/E2F5 Axis (Wang Y. et al., 2020). Moreover, it has been found that lncRNA linc0161 functions as a ceRNA of microRNA-128 and promotes drug resistance through blocking MAPK1 (Xu et al., 2019). In addition, CHRF contributes to cisplatin resistance of ovarian cancer cells by regulating EMT and STAT3 signaling via miR-10b (Tan et al., 2020). ANRIL could modulate the progression, drug resistance and tumor stem cell-like characteristics of ovarian cancer cells via miR-324-5p/Ran Axis (Wang K. et al., 2021).
Some tumor suppressor lncRNAs can reverse platinum drug resistance of ovarian cancer. LncRNA GAS5 expression in SKOV3/DDP cells has been found to be significantly reduced compared to that in drug-sensitive cells, and it has been reported that GAS5 can sensitize ovarian cancer cells to DDP by leading to G0/G1 cell cycle arrest and increasing apoptosis. Further research showed that GAS5 could inhibit DDP-resistance and tumor progression of ovarian cancer via the GAS5-E2F4-PARP1-MAPK axis (Long et al., 2019). It has been reported that lncRNA PANDAR dictates the chemoresistance of ovarian cancer by regulating SFRS2-mediated p53 phosphorylation (Wang H. et al., 2018). Interestingly, lncRNA linc01125 can inhibit ovarian cancer cell proliferation and it enhances the cytotoxicity of DDP in ovarian cancer cells. Tumor suppressor linc01125 has been shown to enhance the cisplatin sensitivity of ovarian cells by sponging miR-1972 (Guo and Pan, 2019). In addition, the literature shows that curcumin inhibits cisplatin resistance development partly by regulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer (Zhang et al., 2017).
There are several novel lncRNAs that have been found to play crucial functions in ovarian cancer PTX resistance. For instance, it has been reported that lncRNA linc0118 is significantly upregulated in PTX-resistant ovarian cancer tissues and cell lines and confers ovarian cancer resistance to PTX. Linc0118 can promote tumor progression in vitro and in vivo. In ovarian cancer cells, linc0118 can induce ABCC1 expression via endogenous sponging of miR-134 and, thus, antagonize chemosensitivity to PTX (Shi and Wang, 2018). In ovarian cancer cells, lncRNA-PRLB have been found to promote TAX resistance by suppressing miR-150-5p and activating NF-κB signaling. Moreover, PRLB has been found to inhibit TAX in ovarian cancer cells through enhancing RSF1 expression, whereas elevated PRLB expression has been found to be associated with a poor response to TAX treatment (Zhao and Hong, 2021). LncRNA SNHG22 is another chemoresistance-related gene and it has been found to promote DDP resistance and PTX resistance through regulating the miR-2467/galectin 1 (Gal-1) axis and it is correlated with poor patient outcomes (Zhang P. F. et al., 2019).
In contrast, a number of tumor suppressor lncRNAs can reverse PTX drug resistance in ovarian cancer. In comparison with normal ovarian epithelial cells, lncRNA FER1L4 is downregulated in SKOV3/PTX resistant cells. Overexpression of the lncRNA FER1L4 can inhibit paclitaxel tolerance of ovarian cancer cells through regulating MAPK signaling pathway (Liu S. et al., 2018). Recently, significantly diminished expression of lncRNA SNHG5 was observed in SKOV3/PTX and HeyA-8/PTX PTX-resistant ovarian cancer cells. Exogenous expression of lncRNA SNHG5 has been found to promote apoptosis, inhibit cell proliferation and enhance PTX sensitivity of ovarian cancer cells by sponging miR-23a (Lin et al., 2020).
CircRNAs and Chemoresistance in Ovarian Cancer
Circular RNAs are crucial members of the ncRNA family, and those related to animal physiologies have been widely studied in recent years. CircRNAs have a closed-loop structure because of a covalent junction between their 3′ and 5′ ends. CircRNAs show stability, conservation, abundance, and tissue and cell specificity (Salzman et al., 2013; Ashwal-Fluss et al., 2014; Maass et al., 2017; Xia et al., 2017). CircRNAs play important roles in biological functions by acting as a “microRNA sponge,” regulating gene transcription and interacting with RNA binding proteins in most cases (Fan et al., 2021; Shen et al., 2021; Zeng et al., 2021). Accumulating evidences have shown that circRNAs are abnormally expressed in various malignant tumors, and circRNAs can act as both proto-oncogenes and tumor suppressors. It has been reported that circRNAs in tumors not only contribute to multiple processes of malignancy, including cell differentiation, proliferation, invasion, and metastasis but are also involved in the mechanism of chemotherapy resistance (Ding et al., 2020; Hong et al., 2020; Ou et al., 2020; Table 6).
Several circRNAs are known to be involved in PTX-resistant ovarian cancer. The cancer-related circTNPO3 has, for example, been found to function as an oncogene in ovarian cancer and confer PTX resistance. CircTNPO3 associates with advanced FIGO stage and histological type. CircTNPO3 promotes PTX resistance of ovarian cancer cells in vitro and in vivo. CircTNPO3 promotes PTX resistance via competitively binding miR-1299 to upregulate NEK2 (Xia et al., 2020). Moreover, circNRIP1 was up-regulated in PTX-resistant ovarian cancer tissues and cells. Silencing of circNRIP1 suppressed the PTX resistance of ovarian cancer cells in vitro and in vivo. Oncogenic CircNRIP1 could contribute to PTX resistance of ovarian cancer by modulating expression of the miR-211-5p/HOXC8 axis (Li M. et al., 2020). Additionally, Hsa_circ_0000714 is an up-regulated circRNA in PTX resistant cells SKOV3/PTX and A2780/PTX, which is contributed to PTX resistance by influencing cell cycle G1/S transition and colony formation. Hsa_circ_0000714 mediates PTX resistance in ovarian cancer cells by sponging miR-370-3p and regulating the expression of RAB17 (Guo et al., 2020). Meanwhile, the cancer-related circCELSR1 (hsa_circ_0063809) has also been identified to be upregulated in SKOV3/PTX and HeyA-8/PTX PTX-resistant ovarian cancer cell lines. Inhibiting circCELSR1 can cause ovarian cancer cell cycle G0/G1 arrest and an increase in apoptosis. CircCELSR1 has been shown to contribute to PTX resistance by modulating forkhead box R2 (FOXR2) expression through miR-1252 (Zhang S. et al., 2020). On the contrary, tumor suppressor circRNAs can reverse PTX resistance in ovarian cancer. circEXOC6B shows notably decreased expression in ovarian cancer tissues and is associated with long survival time of ovarian cancer patients. In ovarian cancer cells, circEXOC6B could suppress FOXO3 expression via endogenous sponging miR-376c-3p and, thus, elevate chemosensitivity to PTX (Zheng et al., 2020).
Also, several circRNAs have been found to be involved in ovarian cancer DDP chemoresistance. Significantly decreased expression levels of circRNA Cdr1as have been observed in both tissues and serum exosomes of Cisplatin-Resistant ovarian cancer patients. It has been confirmed that downregulating suppressor of cancer cell invasion (SCAI) by sponging miR-1270, Cdr1as can conquer DDP resistance of ovarian cancer cells (Zhao et al., 2019). Recently, circulating exosomal circFoxp1, whose expression is positively associated with International Federation of Gynecology and Obstetrics stage, primary tumor size, lymphatic metastasis, distant metastasis, residual tumor diameter, and clinical response, has been reported to promote resistance to DDP of ovarian cancer cells through up-regulating expression of CCAAT enhancer binding protein gamma (CEBPG) and formin like 3 (FMNL3) through miR-22 and miR-150-3p (Luo and Gui, 2020).
Conclusion and Future Perspectives
Ovarian cancer is a comprehensive disease, but the pathogenesis has not been completely elucidated. Although substantial progress has been made in the diagnosis and treatment of ovarian cancer, unfortunately, the prognosis remains unsatisfactory. A growing number of ncRNAs have been identified to be involved in chemoresistance of ovarian cancer. Targeting ncRNAs, in combination with traditional chemotherapy or targeted therapy, may be a promising choice to combat drug resistance in advanced ovarian cancers. NcRNAs affect cell drug resistance through multiple mechanisms. In ovarian cancer, we reviewed EMT, drug efflux transporters, autophagy, cell cycle dysregulation, and DNA repair abnormality. At present, it has also received widespread attention that ncRNAs mediate exosomes to cause cell drug resistance.
A variety of methods are used to identify ncRNA that affect drug resistance, and the more commonly used methods include high-throughput analysis, silicon analysis, integrated analysis, bioinformatics, and expression arrays (Hartmaier et al., 2017; Cen et al., 2021). These technologies enable researchers to target the direction of tumor research, explore the mechanism of tumor occurrence and development, and explore the mechanism of clinical drug resistance. However, it is still a great challenge to select the critical target ncRNAs from the large number of candidates and there is still a long way to go for ncRNA to be used as clinical drug targets. Further translational studies or clinical trials are indispensable to develop ncRNAs-based therapeutics, which may ultimately provide potential approaches for overcoming ovarian cancer drug resistance.
Author Contributions
XZ and SX designed the study. HL analyzed and interpreted the data, and wrote the original draft. JYu wrote this manuscript. DZ, CL, XG, and JYo edited and revised the manuscript. All authors have seen and approved the final version of the manuscript.
Funding
This work was supported by the Independent Exploration and Innovation Project for Graduate Students of Central South University (2021zzts1080), Philosophy and Social Science Foundation Project of Hunan Province (19YBA349), Clinical Medical Technology Innovation Guidance Plan of Hunan Province (2020SK53607), and Natural Science Foundation of Changsha (kq2014261).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PTX, paclitaxel; ADR, adriamycin; PARPi, poly-ADP ribose polymerase inhibitor; PFS, progression-free survival; ncRNAs, non-coding RNAs; miRNAs, microRNAs; lncRNAs, long non-coding RNAs; circRNAs, circular RNAs; MDR, multidrug resistance; EMT, epithelial-mesenchymal transition; piRNAs, PIWI-interacting RNAs; snoRNAs, small nucleolar RNAs; snRNAs, small nuclear RNAs; 3′ -UTR, 3′ -untranslated region; mRNAs, messenger RNAs; DDP, cisplatin; PRKC, protein kinase C; PTEN, phosphatase and tensin homolog; PPP1R12A, protein phosphatase 1 regulatory subunit 12A; MST, STE20-like kinase; SAV1, protein salvador homolog 1; SFRP1, secreted frizzled-related protein 1; KCNMA1, potassium calcium-activated channel subfamily M alpha 1; FOXO3, forkhead box O3; TRIM31, tripartite motif containing 31; EZH2, zeste homolog 2; PTPN3, protein tyrosine phosphatase non-receptor type 3; ITGB8, integrin subunit beta 8; DDR1, discoidin domain receptor 1; NOTCH1, notch receptor 1; HMGA1, high mobility group AT-hook 1; RAD51, RAD51 recombinase; DNMT, DNA methyltransferase; AXL, AXL Receptor Tyrosine Kinase; APAF1, apoptotic peptidase activating factor 1; CIC, capicua transcriptional repressor; ING5, inhibitor of growth family member 5; XIAP, X-linked inhibitor of apoptosis; STAT3, signal transducer and activator of transcription; TRIM27, tripartite motif containing 27; SIK2, salt inducible kinase 2; DSB, double-strand breaks; RNASEH2A, ribonuclease H2 subunit A; FEN1, flap structure-specific endonuclease 1; SSRP1, structure specific recognition protein 1; SSA, single-strand annealing; HGSOCs, high-grade serous ovarian carcinomas; NHEJ, non-homologous end joining; NRP1, Neuropilin 1; RNAP II, RNA Polymerase II; ceRNA, competitive endogenous RNA; UCA1, urothelial cancer associated 1; ABC, ATP binding cassette; HOTAIR, HOX antisense intergenic RNA; ATG7, autophagy related 7; VPA, valproic acid; CCAT1, colon cancer associated transcript 1; MALAT1, metastasis-associated lung adenocarcinoma transcript 1; ZEB1, zinc finger E-box binding homeobox 1; Gal-1, galectin 1; FOXR2, forkhead box R2; SCAI, suppressor of cancer cell invasion.
References
Ahmed, A. A., Lu, Z., Jennings, N. B., Etemadmoghadam, D., Capalbo, L., Jacamo, R. O., et al. (2010). SIK2 is a centrosome kinase required for bipolar mitotic spindle formation that provides a potential target for therapy in ovarian cancer. Cancer Cell 18, 109–121. doi: 10.1016/j.ccr.2010.06.018
An, J., Lv, W., and Zhang, Y. (2017). LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther. 10, 5377–5390. doi: 10.2147/OTT.S147586
Armstrong, D. K., Alvarez, R. D., Bakkum-Gamez, J. N., Barroilhet, L., Behbakht, K., Berchuck, A., et al. (2021). Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 19, 191–226. doi: 10.6004/jnccn.2021.0007
Arrighetti, N., Cossa, G., De Cecco, L., Stucchi, S., Carenini, N., Corna, E., et al. (2016). PKC-alpha modulation by miR-483-3p in platinum-resistant ovarian carcinoma cells. Toxicol. Appl. Pharmacol. 310, 9–19. doi: 10.1016/j.taap.2016.08.005
Ashwal-Fluss, R., Meyer, M., Pamudurti, N. R., Ivanov, A., Bartok, O., Hanan, M., et al. (2014). circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 56, 55–66. doi: 10.1016/j.molcel.2014.08.019
Au Yeung, C. L., Co, N. N., Tsuruga, T., Yeung, T. L., Kwan, S. Y., Leung, C. S., et al. (2016). Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 7:11150. doi: 10.1038/ncomms11150
Bai, L., Wang, A., Zhang, Y., Xu, X., and Zhang, X. (2018). Knockdown of MALAT1 enhances chemosensitivity of ovarian cancer cells to cisplatin through inhibiting the Notch1 signaling pathway. Exp. Cell Res. 366, 161–171. doi: 10.1016/j.yexcr.2018.03.014
Belur Nagaraj, A., Knarr, M., Sekhar, S., Connor, R. S., Joseph, P., Kovalenko, O., et al. (2021). The miR-181a-SFRP4 axis regulates Wnt activation to drive stemness and platinum resistance in ovarian cancer. Cancer Res. 81, 2044–2055. doi: 10.1158/0008-5472.CAN-20-2041
Bi, Z., Li, Q., Dinglin, X., Xu, Y., You, K., Hong, H., et al. (2020). Nanoparticles (NPs)-meditated LncRNA AFAP1-AS1 silencing to block Wnt/beta-catenin signaling pathway for synergistic reversal of radioresistance and effective cancer radiotherapy. Adv. Sci. 7:2000915. doi: 10.1002/advs.202000915
Bruno, P. M., Liu, Y., Park, G. Y., Murai, J., Koch, C. E., Eisen, T. J., et al. (2017). A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat. Med. 23, 461–471. doi: 10.1038/nm.4291
Cao, L., Wan, Q., Li, F., and Tang, C. E. (2018). MiR-363 inhibits cisplatin chemoresistance of epithelial ovarian cancer by regulating snail-induced epithelial-mesenchymal transition. BMB Rep. 51, 456–461.
Cech, T. R., and Steitz, J. A. (2014). The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157, 77–94. doi: 10.1016/j.cell.2014.03.008
Cen, J., Liang, Y., Huang, Y., Pan, Y., Shu, G., Zheng, Z., et al. (2021). Circular RNA circSDHC serves as a sponge for miR-127-3p to promote the proliferation and metastasis of renal cell carcinoma via the CDKN3/E2F1 axis. Mol. Cancer 20:19. doi: 10.1186/s12943-021-01314-w
Chen, F. D., Chen, H. H., Ke, S. C., Zheng, L. R., and Zheng, X. Y. (2018). SLC27A2 regulates miR-411 to affect chemo-resistance in ovarian cancer. Neoplasma 65, 915–924. doi: 10.4149/neo_2018_180122N48
Chen, L. Y., Wang, L., Ren, Y. X., Pang, Z., Liu, Y., Sun, X. D., et al. (2020). The circular RNA circ-ERBIN promotes growth and metastasis of colorectal cancer by miR-125a-5p and miR-138-5p/4EBP-1 mediated cap-independent HIF-1alpha translation. Mol. Cancer 19:164. doi: 10.1186/s12943-020-01272-9
Chen, M. W., Yang, S. T., Chien, M. H., Hua, K. T., Wu, C. J., Hsiao, S. M., et al. (2017). The STAT3-miRNA-92-Wnt signaling pathway regulates spheroid formation and malignant progression in ovarian cancer. Cancer Res. 77, 1955–1967. doi: 10.1158/0008-5472.CAN-16-1115
Chen, W. T., Yang, Y. J., Zhang, Z. D., An, Q., Li, N., Liu, W., et al. (2017). MiR-1307 promotes ovarian cancer cell chemoresistance by targeting the ING5 expression. J. Ovarian Res. 10:1. doi: 10.1186/s13048-016-0301-4
Chen, Y., Wang, L., and Zhou, J. (2019). Effects of microRNA-1271 on ovarian cancer via inhibition of epithelial-mesenchymal transition and cisplatin resistance. J. Obstet. Gynaecol. Res. 45, 2243–2254. doi: 10.1111/jog.14079
Chen, Y. N., Ren, C. C., Yang, L., Nai, M. M., Xu, Y. M., Zhang, F., et al. (2019). MicroRNA let7d5p rescues ovarian cancer cell apoptosis and restores chemosensitivity by regulating the p53 signaling pathway via HMGA1. Int. J. Oncol. 54, 1771–1784. doi: 10.3892/ijo.2019.4731
Chiappa, M., Guffanti, F., Bertoni, F., Colombo, I., and Damia, G. (2021). Overcoming PARPi resistance: preclinical and clinical evidence in ovarian cancer. Drug Resist. Updat. 55:100744. doi: 10.1016/j.drup.2021.100744
Choi, Y. E., Meghani, K., Brault, M. E., Leclerc, L., He, Y. J., Day, T. A., et al. (2016). Platinum and PARP inhibitor resistance due to overexpression of MicroRNA-622 in BRCA1-mutant ovarian cancer. Cell Rep. 14, 429–439. doi: 10.1016/j.celrep.2015.12.046
Christie, E. L., Pattnaik, S., Beach, J., Copeland, A., Rashoo, N., Fereday, S., et al. (2019). Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nat. Commun. 10:1295. doi: 10.1038/s41467-019-09312-9
Cocquerelle, C., Mascrez, B., Hetuin, D., and Bailleul, B. (1993). Mis-splicing yields circular RNA molecules. FASEB J. 7, 155–160. doi: 10.1096/fasebj.7.1.7678559
Cui, C., Liu, Y., Gerloff, D., Rohde, C., Pauli, C., Kohn, M., et al. (2021). NOP10 predicts lung cancer prognosis and its associated small nucleolar RNAs drive proliferation and migration. Oncogene 40, 909–921. doi: 10.1038/s41388-020-01570-y
Cui, Y., Wu, F., Tian, D., Wang, T., Lu, T., Huang, X., et al. (2018). miR-199a-3p enhances cisplatin sensitivity of ovarian cancer cells by targeting ITGB8. Oncol. Rep. 39, 1649–1657. doi: 10.3892/or.2018.6259
Deng, Y., Zhao, F., Hui, L., Li, X., Zhang, D., Lin, W., et al. (2017). Suppressing miR-199a-3p by promoter methylation contributes to tumor aggressiveness and cisplatin resistance of ovarian cancer through promoting DDR1 expression. J. Ovarian Res. 10:50. doi: 10.1186/s13048-017-0333-4
Ding, C., Yi, X., Wu, X., Bu, X., Wang, D., Wu, Z., et al. (2020). Exosome-mediated transfer of circRNA CircNFIX enhances temozolomide resistance in glioma. Cancer Lett. 479, 1–12. doi: 10.1016/j.canlet.2020.03.002
Duran, G. E., Wang, Y. C., Moisan, F., Francisco, E. B., and Sikic, B. I. (2017). Decreased levels of baseline and drug-induced tubulin polymerisation are hallmarks of resistance to taxanes in ovarian cancer cells and are associated with epithelial-to-mesenchymal transition. Br. J. Cancer 116, 1318–1328. doi: 10.1038/bjc.2017.102
Eoh, K. J., Lee, S. H., Kim, H. J., Lee, J. Y., Kim, S., Kim, S. W., et al. (2018). MicroRNA-630 inhibitor sensitizes chemoresistant ovarian cancer to chemotherapy by enhancing apoptosis. Biochem. Biophys. Res. Commun. 497, 513–520. doi: 10.1016/j.bbrc.2018.02.062
Fan, Y., Wang, J., Jin, W., Sun, Y., Xu, Y., Wang, Y., et al. (2021). CircNR3C2 promotes HRD1-mediated tumor-suppressive effect via sponging miR-513a-3p in triple-negative breast cancer. Mol. Cancer 20:25. doi: 10.1186/s12943-021-01321-x
Garcia-Vazquez, R., Gallardo Rincon, D., Ruiz-Garcia, E., Meneses Garcia, A., Hernandez De La Cruz, O. N., Astudillo-De La Vega, H., et al. (2018). let-7d-3p is associated with apoptosis and response to neoadjuvant chemotherapy in ovarian cancer. Oncol. Rep. 39, 3086–3094. doi: 10.3892/or.2018.6366
Ge, G., Zhang, W., Niu, L., Yan, Y., Ren, Y., and Zou, Y. (2016). miR-215 functions as a tumor suppressor in epithelial ovarian cancer through regulation of the X-chromosome-linked inhibitor of apoptosis. Oncol. Rep. 35, 1816–1822. doi: 10.3892/or.2015.4482
Gu, Z. W., He, Y. F., Wang, W. J., Tian, Q., and Di, W. (2019). MiR-1180 from bone marrow-derived mesenchymal stem cells induces glycolysis and chemoresistance in ovarian cancer cells by upregulating the Wnt signaling pathway. J. Zhejiang Univ. Sci. B 20, 219–237. doi: 10.1631/jzus.B1800190
Guo, H., Ha, C., Dong, H., Yang, Z., Ma, Y., and Ding, Y. (2019). Cancer-associated fibroblast-derived exosomal microRNA-98-5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell. Int. 19:347. doi: 10.1186/s12935-019-1051-3
Guo, J., and Pan, H. (2019). Long noncoding RNA LINC01125 enhances cisplatin sensitivity of ovarian cancer via miR-1972. Med. Sci. Monit. 25, 9844–9854. doi: 10.12659/MSM.916820
Guo, M., Li, S., Zhao, X., Yuan, Y., Zhang, B., and Guan, Y. (2020). Knockdown of circular RNA Hsa_circ_0000714 can regulate RAB17 by sponging miR-370-3p to reduce paclitaxel resistance of ovarian cancer through CDK6/RB pathway. Onco Targets Ther. 13, 13211–13224. doi: 10.2147/OTT.S285153
Han, H., Fan, G., Song, S., Jiang, Y., Qian, C., Zhang, W., et al. (2021). piRNA-30473 contributes to tumorigenesis and poor prognosis by regulating m6A RNA methylation in DLBCL. Blood 137, 1603–1614. doi: 10.1182/blood.2019003764
Hartmaier, R. J., Albacker, L. A., Chmielecki, J., Bailey, M., He, J., Goldberg, M. E., et al. (2017). High-throughput genomic profiling of adult solid tumors reveals novel insights into cancer pathogenesis. Cancer Res. 77, 2464–2475. doi: 10.1158/0008-5472.CAN-16-2479
He, L., Zhu, W., Chen, Q., Yuan, Y., Wang, Y., Wang, J., et al. (2019). Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 9, 8206–8220. doi: 10.7150/thno.37455
Hisamatsu, T., McGuire, M., Wu, S. Y., Rupaimoole, R., Pradeep, S., Bayraktar, E., et al. (2019). PRKRA/PACT expression promotes chemoresistance of mucinous ovarian cancer. Mol. Cancer Ther. 18, 162–172. doi: 10.1158/1535-7163.MCT-17-1050
Hong, X., Liu, N., Liang, Y., He, Q., Yang, X., Lei, Y., et al. (2020). Circular RNA CRIM1 functions as a ceRNA to promote nasopharyngeal carcinoma metastasis and docetaxel chemoresistance through upregulating FOXQ1. Mol. Cancer 19:33. doi: 10.1186/s12943-020-01149-x
Huang, X., Li, Z., Zhang, Q., Wang, W., Li, B., Wang, L., et al. (2019). Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol. Cancer 18:71. doi: 10.1186/s12943-019-0969-3
Jeong, J. Y., Kang, H., Kim, T. H., Kim, G., Heo, J. H., Kwon, A. Y., et al. (2017). MicroRNA-136 inhibits cancer stem cell activity and enhances the anti-tumor effect of paclitaxel against chemoresistant ovarian cancer cells by targeting Notch3. Cancer Lett. 386, 168–178. doi: 10.1016/j.canlet.2016.11.017
Jiang, J., Xie, C., Liu, Y., Shi, Q., and Chen, Y. (2019). Up-regulation of miR-383-5p suppresses proliferation and enhances chemosensitivity in ovarian cancer cells by targeting TRIM27. Biomed. Pharmacother. 109, 595–601. doi: 10.1016/j.biopha.2018.10.148
Jin, H., Ma, J., Xu, J., Li, H., Chang, Y., Zang, N., et al. (2020). Oncogenic role of MIR516A in human bladder cancer was mediated by its attenuating PHLPP2 expression and BECN1-dependent autophagy. Autophagy 17, 840–854. doi: 10.1080/15548627.2020.1733262
Jin, P., Liu, Y., and Wang, R. (2018). STAT3 regulated miR-216a promotes ovarian cancer proliferation and cisplatin resistance. Biosci. Rep. 38:BSR20180547. doi: 10.1042/BSR20180547
Komoll, R. M., Hu, Q., Olarewaju, O., von Dohlen, L., Yuan, Q., Xie, Y., et al. (2021). MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J. Hepatol. 74, 122–134. doi: 10.1016/j.jhep.2020.07.039
Kristensen, L. S., Andersen, M. S., Stagsted, L. V. W., Ebbesen, K. K., Hansen, T. B., and Kjems, J. (2019). The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 20, 675–691. doi: 10.1038/s41576-019-0158-7
Lheureux, S., Braunstein, M., and Oza, A. M. (2019). Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J. Clin. 69, 280–304. doi: 10.3322/caac.21559
Li, B., Zhu, L., Lu, C., Wang, C., Wang, H., Jin, H., et al. (2021). circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat. Commun. 12:295. doi: 10.1038/s41467-020-20527-z
Li, J., Yang, S., Su, N., Wang, Y., Yu, J., Qiu, H., et al. (2016). Overexpression of long non-coding RNA HOTAIR leads to chemoresistance by activating the Wnt/beta-catenin pathway in human ovarian cancer. Tumour Biol. 37, 2057–2065. doi: 10.1007/s13277-015-3998-6
Li, L., Xu, Q. H., Dong, Y. H., Li, G. X., Yang, L., Wang, L. W., et al. (2016). MiR-181a upregulation is associated with epithelial-to-mesenchymal transition (EMT) and multidrug resistance (MDR) of ovarian cancer cells. Eur. Rev. Med. Pharmacol. Sci. 20, 2004–2010.
Li, M., Cai, J., Han, X., and Ren, Y. (2020). Downregulation of circNRIP1 suppresses the paclitaxel resistance of ovarian cancer via regulating the miR-211-5p/HOXC8 axis. Cancer Manag. Res. 12, 9159–9171. doi: 10.2147/CMAR.S268872
Li, S., Cao, J., Zhang, W., Zhang, F., Ni, G., Luo, Q., et al. (2016). Protein tyrosine phosphatase PTPN3 promotes drug resistance and stem cell-like characteristics in ovarian cancer. Sci. Rep. 6:36873. doi: 10.1038/srep36873
Li, X., Chen, W., Jin, Y., Xue, R., Su, J., Mu, Z., et al. (2019). miR-142-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes. Biochem. Pharmacol. 161, 98–112. doi: 10.1016/j.bcp.2019.01.009
Li, X., Chen, W., Zeng, W., Wan, C., Duan, S., and Jiang, S. (2017a). microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP. Br. J. Cancer 116, 66–76. doi: 10.1038/bjc.2016.379
Li, X., Jin, Y., Mu, Z., Chen, W., and Jiang, S. (2017b). MicroRNA146a5p enhances cisplatininduced apoptosis in ovarian cancer cells by targeting multiple antiapoptotic genes. Int. J. Oncol. 51, 327–335. doi: 10.3892/ijo.2017.4023
Li, Z., Niu, H., Qin, Q., Yang, S., Wang, Q., Yu, C., et al. (2019). lncRNA UCA1 mediates resistance to cisplatin by regulating the miR-143/FOSL2-signaling pathway in ovarian cancer. Mol. Ther. Nucleic Acids 17, 92–101. doi: 10.1016/j.omtn.2019.05.007
Li, Z. Y., Wang, X. L., Dang, Y., Zhu, X. Z., Zhang, Y. H., Cai, B. X., et al. (2020). Long non-coding RNA UCA1 promotes the progression of paclitaxel resistance in ovarian cancer by regulating the miR-654-5p/SIK2 axis. Eur. Rev. Med. Pharmacol. Sci. 24, 591–603. doi: 10.26355/eurrev_202001_20035
Liang, F., Ren, C., Wang, J., Wang, S., Yang, L., Han, X., et al. (2019). The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis 8:59. doi: 10.1038/s41389-019-0165-8
Lin, H., Shen, L., Lin, Q., Dong, C., Maswela, B., Illahi, G. S., et al. (2020). SNHG5 enhances Paclitaxel sensitivity of ovarian cancer cells through sponging miR-23a. Biomed. Pharmacother. 123:109711. doi: 10.1016/j.biopha.2019.109711
Liu, E., Liu, Z., Zhou, Y., Mi, R., and Wang, D. (2015). Overexpression of long non-coding RNA PVT1 in ovarian cancer cells promotes cisplatin resistance by regulating apoptotic pathways. Int. J. Clin. Exp. Med. 8, 20565–20572.
Liu, J., Zhang, X., Huang, Y., Zhang, Q., Zhou, J., Zhang, X., et al. (2019). miR-200b and miR-200c co-contribute to the cisplatin sensitivity of ovarian cancer cells by targeting DNA methyltransferases. Oncol. Lett. 17, 1453–1460. doi: 10.3892/ol.2018.9745
Liu, R., Guo, H., and Lu, S. (2018). MiR-335-5p restores cisplatin sensitivity in ovarian cancer cells through targeting BCL2L2. Cancer Med. 7, 4598–4609. doi: 10.1002/cam4.1682
Liu, S., Zou, B., Tian, T., Luo, X., Mao, B., Zhang, X., et al. (2018). Overexpression of the lncRNA FER1L4 inhibits paclitaxel tolerance of ovarian cancer cells via the regulation of the MAPK signaling pathway. J. Cell Biochem. [Epub ahead of print]. doi: 10.1002/jcb.28032
Liu, Y., Han, S., Li, Y., Liu, Y., Zhang, D., Li, Y., et al. (2017). MicroRNA-20a contributes to cisplatin-resistance and migration of OVCAR3 ovarian cancer cell line. Oncol. Lett. 14, 1780–1786. doi: 10.3892/ol.2017.6348
Long, X., Song, K., Hu, H., Tian, Q., Wang, W., Dong, Q., et al. (2019). Long non-coding RNA GAS5 inhibits DDP-resistance and tumor progression of epithelial ovarian cancer via GAS5-E2F4-PARP1-MAPK axis. J. Exp. Clin. Cancer Res. 38:345. doi: 10.1186/s13046-019-1329-2
Luo, Y., and Gui, R. (2020). Circulating exosomal circFoxp1 confers cisplatin resistance in epithelial ovarian cancer cells. J. Gynecol. Oncol. 31:e75. doi: 10.3802/jgo.2020.31.e75
Luo, Y., Zheng, S., Wu, Q., Wu, J., Zhou, R., Wang, C., et al. (2021). Long noncoding RNA (lncRNA) EIF3J-DT induces chemoresistance of gastric cancer via autophagy activation. Autophagy [Epub ahead of print]. doi: 10.1080/15548627.2021.1901204
Maass, P. G., Glazar, P., Memczak, S., Dittmar, G., Hollfinger, I., Schreyer, L., et al. (2017). A map of human circular RNAs in clinically relevant tissues. J. Mol. Med. 95, 1179–1189. doi: 10.1007/s00109-017-1582-9
Mak, C. S., Yung, M. M., Hui, L. M., Leung, L. L., Liang, R., Chen, K., et al. (2017). MicroRNA-141 enhances anoikis resistance in metastatic progression of ovarian cancer through targeting KLF12/Sp1/survivin axis. Mol. Cancer 16:11. doi: 10.1186/s12943-017-0582-2
Meghani, K., Fuchs, W., Detappe, A., Drane, P., Gogola, E., Rottenberg, S., et al. (2018). Multifaceted impact of MicroRNA 493-5p on genome-stabilizing pathways induces platinum and parp inhibitor resistance in BRCA2-mutated carcinomas. Cell Rep. 23, 100–111. doi: 10.1016/j.celrep.2018.03.038
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. doi: 10.1038/nature11928
Miller, C. M., Wan, W. B., Seth, P. P., and Harris, E. N. (2018). Endosomal escape of antisense oligonucleotides internalized by stabilin receptors is regulated by Rab5C and EEA1 during endosomal maturation. Nucleic Acid Ther. 28, 86–96. doi: 10.1089/nat.2017.0694
Munoz-Galvan, S., Felipe-Abrio, B., Verdugo-Sivianes, E. M., Perez, M., Jimenez-Garcia, M. P., Suarez-Martinez, E., et al. (2020). Downregulation of MYPT1 increases tumor resistance in ovarian cancer by targeting the Hippo pathway and increasing the stemness. Mol. Cancer 19:7. doi: 10.1186/s12943-020-1130-z
Ou, R., Mo, L., Tang, H., Leng, S., Zhu, H., Zhao, L., et al. (2020). circRNA-AKT1 Sequesters miR-942-5p to upregulate AKT1 and promote cervical cancer progression. Mol. Ther. Nucleic Acids 20, 308–322. doi: 10.1016/j.omtn.2020.01.003
Ozes, A. R., Miller, D. F., Ozes, O. N., Fang, F., Liu, Y., Matei, D., et al. (2016). NF-kappaB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene 35, 5350–5361. doi: 10.1038/onc.2016.75
Park, G. B., and Kim, D. (2019). MicroRNA-503-5p inhibits the CD97-mediated JAK2/STAT3 pathway in metastatic or paclitaxel-resistant ovarian cancer cells. Neoplasia 21, 206–215. doi: 10.1016/j.neo.2018.12.005
Qin, X., Sun, L., and Wang, J. (2017). Restoration of microRNA-708 sensitizes ovarian cancer cells to cisplatin via IGF2BP1/Akt pathway. Cell Biol. Int. 41, 1110–1118. doi: 10.1002/cbin.10819
Qiu, J. J., Lin, Y. Y., Ye, L. C., Ding, J. X., Feng, W. W., Jin, H. Y., et al. (2014). Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol. Oncol. 134, 121–128. doi: 10.1016/j.ygyno.2014.03.556
Quinn, J. J., and Chang, H. Y. (2016). Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 17, 47–62. doi: 10.1038/nrg.2015.10
Ramat, A., and Simonelig, M. (2021). Functions of PIWI proteins in gene regulation: new arrows added to the piRNA quiver. Trends Genet. 37, 188–200. doi: 10.1016/j.tig.2020.08.011
Sajadpoor, Z., Amini-Farsani, Z., Teimori, H., Shamsara, M., Sangtarash, M. H., Ghasemi-Dehkordi, P., et al. (2018). Valproic acid promotes apoptosis and cisplatin sensitivity through downregulation of H19 noncoding RNA in ovarian A2780 cells. Appl. Biochem. Biotechnol. 185, 1132–1144. doi: 10.1007/s12010-017-2684-0
Salzman, J., Chen, R. E., Olsen, M. N., Wang, P. L., and Brown, P. O. (2013). Cell-type specific features of circular RNA expression. PLoS Genet. 9:e1003777. doi: 10.1371/journal.pgen.1003777
Samuel, P., Pink, R. C., Caley, D. P., Currie, J. M., Brooks, S. A., and Carter, D. R. (2016). Over-expression of miR-31 or loss of KCNMA1 leads to increased cisplatin resistance in ovarian cancer cells. Tumour Biol. 37, 2565–2573. doi: 10.1007/s13277-015-4081-z
Shen, P., Yang, T., Chen, Q., Yuan, H., Wu, P., Cai, B., et al. (2021). CircNEIL3 regulatory loop promotes pancreatic ductal adenocarcinoma progression via miRNA sponging and A-to-I RNA-editing. Mol. Cancer 20:51. doi: 10.1186/s12943-021-01333-7
Shi, C., and Wang, M. (2018). LINC01118 modulates paclitaxel resistance of epithelial ovarian cancer by regulating miR-134/ABCC1. Med. Sci. Monit. 24, 8831–8839. doi: 10.12659/MSM.910932
Shi, X., Xiao, L., Mao, X., He, J., Ding, Y., Huang, J., et al. (2018). miR-205-5p mediated downregulation of PTEN contributes to cisplatin resistance in C13K human ovarian cancer cells. Front. Genet. 9:555. doi: 10.3389/fgene.2018.00555
Shuai, S., Suzuki, H., Diaz-Navarro, A., Nadeu, F., Kumar, S. A., Gutierrez-Fernandez, A., et al. (2019). The U1 spliceosomal RNA is recurrently mutated in multiple cancers. Nature 574, 712–716. doi: 10.1038/s41586-019-1651-z
Statello, L., Guo, C. J., Chen, L. L., and Huarte, M. (2021). Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 22, 96–118. doi: 10.1038/s41580-020-00315-9
Sun, J., Cai, X., Yung, M. M., Zhou, W., Li, J., Zhang, Y., et al. (2019). miR-137 mediates the functional link between c-Myc and EZH2 that regulates cisplatin resistance in ovarian cancer. Oncogene 38, 564–580. doi: 10.1038/s41388-018-0459-x
Sun, Y., Wu, J., Dong, X., Zhang, J., Meng, C., and Liu, G. (2021). MicroRNA-506-3p increases the response to PARP inhibitors and cisplatin by targeting EZH2/beta-catenin in serous ovarian cancers. Transl. Oncol. 14:100987. doi: 10.1016/j.tranon.2020.100987
Sun, Z., Gao, S., Xuan, L., and Liu, X. (2020). Long non-coding RNA FEZF1-AS1 induced progression of ovarian cancer via regulating miR-130a-5p/SOX4 axis. J. Cell. Mol. Med. 24, 4275–4285. doi: 10.1111/jcmm.15088
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Tan, W. X., Sun, G., Shangguan, M. Y., Gui, Z., Bao, Y., Li, Y. F., et al. (2020). Novel role of lncRNA CHRF in cisplatin resistance of ovarian cancer is mediated by miR-10b induced EMT and STAT3 signaling. Sci. Rep. 10:14768. doi: 10.1038/s41598-020-71153-0
Tew, W. P., Lacchetti, C., Ellis, A., Maxian, K., Banerjee, S., Bookman, M., et al. (2020). PARP inhibitors in the management of ovarian cancer: ASCO guideline. J. Clin. Oncol. 38, 3468–3493. doi: 10.1200/JCO.20.01924
Tsitsipatis, D., Grammatikakis, I., Driscoll, R. K., Yang, X., Abdelmohsen, K., Harris, S. C., et al. (2021). AUF1 ligand circPCNX reduces cell proliferation by competing with p21 mRNA to increase p21 production. Nucleic Acids Res. 49, 1631–1646. doi: 10.1093/nar/gkaa1246
Tung, C. H., Kuo, L. W., Huang, M. F., Wu, Y. Y., Tsai, Y. T., Wu, J. E., et al. (2020). MicroRNA-150-5p promotes cell motility by inhibiting c-Myb-mediated Slug suppression and is a prognostic biomarker for recurrent ovarian cancer. Oncogene 39, 862–876. doi: 10.1038/s41388-019-1025-x
Tung, S. L., Huang, W. C., Hsu, F. C., Yang, Z. P., Jang, T. H., Chang, J. W., et al. (2017). miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis 6:e326. doi: 10.1038/oncsis.2017.25
Vescarelli, E., Gerini, G., Megiorni, F., Anastasiadou, E., Pontecorvi, P., Solito, L., et al. (2020). MiR-200c sensitizes Olaparib-resistant ovarian cancer cells by targeting Neuropilin 1. J. Exp. Clin. Cancer Res. 39:3. doi: 10.1186/s13046-019-1490-7
Wang, D. Y., Li, N., and Cui, Y. L. (2020). Long non-coding RNA CCAT1 sponges miR-454 to promote chemoresistance of ovarian cancer cells to cisplatin by regulation of surviving. Cancer Res. Treat. 52, 798–814. doi: 10.4143/crt.2019.498
Wang, H., Fang, L., Jiang, J., Kuang, Y., Wang, B., Shang, X., et al. (2018). The cisplatin-induced lncRNA PANDAR dictates the chemoresistance of ovarian cancer via regulating SFRS2-mediated p53 phosphorylation. Cell Death Dis. 9:1103. doi: 10.1038/s41419-018-1148-y
Wang, J., Ye, C., Liu, J., and Hu, Y. (2018). UCA1 confers paclitaxel resistance to ovarian cancer through miR-129/ABCB1 axis. Biochem. Biophys. Res. Commun. 501, 1034–1040. doi: 10.1016/j.bbrc.2018.05.104
Wang, K., Hu, Y. B., Zhao, Y., and Ye, C. (2021). LncRNA ANRIL regulates ovarian cancer progression and tumor stem cell-like characteristics via miR-324-5p/Ran axis. Onco Targets Ther. 14, 565–576. doi: 10.2147/OTT.S273614
Wang, P., Yang, Z., Ye, T., Shao, F., Li, J., Sun, N., et al. (2020). lncTUG1/miR-144-3p affect the radiosensitivity of esophageal squamous cell carcinoma by competitively regulating c-MET. J. Exp. Clin. Cancer Res. 39:7. doi: 10.1186/s13046-019-1519-y
Wang, X., He, Y., Mackowiak, B., and Gao, B. (2021). MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 70, 784–795. doi: 10.1136/gutjnl-2020-322526
Wang, Y., Bao, W., Liu, Y., Wang, S., Xu, S., Li, X., et al. (2018). miR-98-5p contributes to cisplatin resistance in epithelial ovarian cancer by suppressing miR-152 biogenesis via targeting Dicer1. Cell Death Dis. 9:447. doi: 10.1038/s41419-018-0390-7
Wang, Y., Wang, H., Song, T., Zou, Y., Jiang, J., Fang, L., et al. (2015). HOTAIR is a potential target for the treatment of cisplatinresistant ovarian cancer. Mol. Med. Rep. 12, 2211–2216. doi: 10.3892/mmr.2015.3562
Wang, Y., Wang, X., Han, L., and Hu, D. (2020). LncRNA MALAT1 regulates the progression and cisplatin resistance of ovarian cancer cells via modulating miR-1271-5p/E2F5 axis. Cancer Manag. Res. 12, 9999–10010. doi: 10.2147/CMAR.S261979
Wei, Z., Liu, Y., Wang, Y., Zhang, Y., Luo, Q., Man, X., et al. (2016). Downregulation of Foxo3 and TRIM31 by miR-551b in side population promotes cell proliferation, invasion, and drug resistance of ovarian cancer. Med. Oncol. 33:126. doi: 10.1007/s12032-016-0842-9
Wiltshire, T. D., Lovejoy, C. A., Wang, T., Xia, F., O’Connor, M. J., and Cortez, D. (2010). Sensitivity to poly(ADP-ribose) polymerase (PARP) inhibition identifies ubiquitin-specific peptidase 11 (USP11) as a regulator of DNA double-strand break repair. J. Biol. Chem. 285, 14565–14571. doi: 10.1074/jbc.M110.104745
Winham, S. J., Larson, N. B., Armasu, S. M., Fogarty, Z. C., Larson, M. C., McCauley, B. M., et al. (2019). Molecular signatures of X chromosome inactivation and associations with clinical outcomes in epithelial ovarian cancer. Hum. Mol. Genet. 28, 1331–1342. doi: 10.1093/hmg/ddy444
Wong, C. M., Tsang, F. H., and Ng, I. O. (2018). Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat. Rev. Gastroenterol. Hepatol. 15, 137–151. doi: 10.1038/nrgastro.2017.169
Wu, D., Lu, P., Mi, X., and Miao, J. (2018). Downregulation of miR-503 contributes to the development of drug resistance in ovarian cancer by targeting PI3K p85. Arch. Gynecol. Obstet. 297, 699–707. doi: 10.1007/s00404-018-4649-0
Wu, D. D., Li, X. S., Meng, X. N., Yan, J., and Zong, Z. H. (2016). MicroRNA-873 mediates multidrug resistance in ovarian cancer cells by targeting ABCB1. Tumour Biol. 37, 10499–10506. doi: 10.1007/s13277-016-4944-y
Wu, Y., Zhou, Y., He, J., Sun, H., and Jin, Z. (2019). Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT. Int. J. Clin. Exp. Pathol. 12, 2506–2515.
Xia, B., Lin, M., Dong, W., Chen, H., Li, B., Zhang, X., et al. (2018). Upregulation of miR-874-3p and miR-874-5p inhibits epithelial ovarian cancer malignancy via SIK2. J. Biochem. Mol. Toxicol. 32:e22168. doi: 10.1002/jbt.22168
Xia, B., Zhao, Z., Wu, Y., Wang, Y., Zhao, Y., and Wang, J. (2020). Circular RNA circTNPO3 regulates paclitaxel resistance of ovarian cancer cells by miR-1299/NEK2 signaling pathway. Mol. Ther. Nucleic Acids 21, 780–791. doi: 10.1016/j.omtn.2020.06.002
Xia, S., Feng, J., Lei, L., Hu, J., Xia, L., Wang, J., et al. (2017). Comprehensive characterization of tissue-specific circular RNAs in the human and mouse genomes. Brief Bioinform. 18, 984–992. doi: 10.1093/bib/bbw081
Xiao, M., Cai, J., Cai, L., Jia, J., Xie, L., Zhu, Y., et al. (2017). Let-7e sensitizes epithelial ovarian cancer to cisplatin through repressing DNA double strand break repair. J. Ovarian Res. 10, 24. doi: 10.1186/s13048-017-0321-8
Xiao, S., Zhang, M., Liu, C., and Wang, D. (2018). MiR-514 attenuates proliferation and increases chemoresistance by targeting ATP binding cassette subfamily in ovarian cancer. Mol. Genet. Genomics 293, 1159–1167. doi: 10.1007/s00438-018-1447-0
Xing, S., Tian, Z., Zheng, W., Yang, W., Du, N., Gu, Y., et al. (2021). Hypoxia downregulated miR-4521 suppresses gastric carcinoma progression through regulation of IGF2 and FOXM1. Mol. Cancer 20:9. doi: 10.1186/s12943-020-01295-2
Xu, M., Xiao, J., Chen, M., Yuan, L., Li, J., Shen, H., et al. (2018). miR1495p promotes chemotherapeutic resistance in ovarian cancer via the inactivation of the Hippo signaling pathway. Int. J. Oncol. 52, 815–827. doi: 10.3892/ijo.2018.4252
Xu, M., Zhou, K., Wu, Y., Wang, L., and Lu, S. (2019). Linc00161 regulated the drug resistance of ovarian cancer by sponging microRNA-128 and modulating MAPK1. Mol. Carcinog. 58, 577–587. doi: 10.1002/mc.22952
Xu, Q. F., Tang, Y. X., and Wang, X. (2018). LncRNA EBIC promoted proliferation, metastasis and cisplatin resistance of ovarian cancer cells and predicted poor survival in ovarian cancer patients. Eur. Rev. Med. Pharmacol. Sci. 22, 4440–4447. doi: 10.26355/eurrev_201807_15495
Yan, H., Xia, J. Y., and Feng, F. Z. (2017). Long non-coding RNA ENST00000457645 reverses cisplatin resistance in CP70 ovarian cancer cells. Genet. Mol. Res. 16:gmr16019411. doi: 10.4238/gmr16019411
Yan, M., Yang, X., Shen, R., Wu, C., Wang, H., Ye, Q., et al. (2018). miR-146b promotes cell proliferation and increases chemosensitivity, but attenuates cell migration and invasion via FBXL10 in ovarian cancer. Cell Death Dis. 9:1123. doi: 10.1038/s41419-018-1093-9
Yu, J. L., and Gao, X. (2020). MicroRNA 1301 inhibits cisplatin resistance in human ovarian cancer cells by regulating EMT and autophagy. Eur. Rev. Med. Pharmacol. Sci. 24, 1688–1696. doi: 10.26355/eurrev_202002_20343
Yu, Y., Zhang, X., Tian, H., Zhang, Z., and Tian, Y. (2018). Knockdown of long non-coding RNA HOTAIR increases cisplatin sensitivity in ovarian cancer by inhibiting cisplatin-induced autophagy. J. BUON 23, 1396–1401.
Zeng, Z., Xia, L., Fan, S., Zheng, J., Qin, J., Fan, X., et al. (2021). Circular RNA CircMAP3K5 acts as a MicroRNA-22-3p sponge to promote resolution of intimal hyperplasia via TET2-mediated smooth muscle cell differentiation. Circulation 143, 354–371. doi: 10.1161/CIRCULATIONAHA.120.049715
Zhang, J., Liu, J., Xu, X., and Li, L. (2017). Curcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer. Cancer Chemother. Pharmacol. 79, 479–487. doi: 10.1007/s00280-017-3238-4
Zhang, M., Liu, S., Fu, C., Wang, X., Zhang, M., Liu, G., et al. (2019). LncRNA KB-1471A8.2 overexpression suppresses cell proliferation and migration and antagonizes the paclitaxel resistance of ovarian cancer cells. Cancer Biother. Radiopharm. 34, 316–324. doi: 10.1089/cbr.2018.2698
Zhang, P. F., Wu, J., Luo, J. H., Li, K. S., Wang, F., Huang, W., et al. (2019). SNHG22 overexpression indicates poor prognosis and induces chemotherapy resistance via the miR-2467/Gal-1 signaling pathway in epithelial ovarian carcinoma. Aging 11, 8204–8216. doi: 10.18632/aging.102313
Zhang, S., Cheng, J., Quan, C., Wen, H., Feng, Z., Hu, Q., et al. (2020). circCELSR1 (hsa_circ_0063809) contributes to paclitaxel resistance of ovarian cancer cells by regulating FOXR2 expression via miR-1252. Mol. Ther. Nucleic Acids 19, 718–730. doi: 10.1016/j.omtn.2019.12.005
Zhang, Z., Zhang, L., Wang, B., Wei, R., Wang, Y., Wan, J., et al. (2020). MiR-337-3p suppresses proliferation of epithelial ovarian cancer by targeting PIK3CA and PIK3CB. Cancer Lett. 469, 54–67. doi: 10.1016/j.canlet.2019.10.021
Zhao, H., Bi, T., Qu, Z., Jiang, J., Cui, S., and Wang, Y. (2014). Expression of miR-224-5p is associated with the original cisplatin resistance of ovarian papillary serous carcinoma. Oncol. Rep. 32, 1003–1012. doi: 10.3892/or.2014.3311
Zhao, Y., and Hong, L. (2021). lncRNA-PRLB confers paclitaxel resistance of ovarian cancer cells by regulating RSF1/NF-kappaB signaling pathway. Cancer Biother. Radiopharm. 36, 202–210. doi: 10.1089/cbr.2019.3363
Zhao, Z., Ji, M., Wang, Q., He, N., and Li, Y. (2019). Circular RNA Cdr1as upregulates SCAI to suppress cisplatin resistance in ovarian cancer via miR-1270 suppression. Mol. Ther. Nucleic Acids 18, 24–33. doi: 10.1016/j.omtn.2019.07.012
Zheng, Y., Li, Z., Yang, S., Wang, Y., and Luan, Z. (2020). CircEXOC6B suppresses the proliferation and motility and sensitizes ovarian cancer cells to paclitaxel through miR-376c-3p/FOXO3 axis. Cancer Biother. Radiopharm. [Epub ahead of print]. doi: 10.1089/cbr.2020.3739
Zheng, Z. G., Xu, H., Suo, S. S., Xu, X. L., Ni, M. W., Gu, L. H., et al. (2016). The essential role of H19 contributing to cisplatin resistance by regulating glutathione metabolism in high-grade serous ovarian cancer. Sci. Rep. 6:26093. doi: 10.1038/srep26093
Zhou, J., Alfraidi, A., Zhang, S., Santiago-O’Farrill, J. M., Yerramreddy Reddy, V. K., Alsaadi, A., et al. (2017). A novel compound ARN-3236 inhibits salt-inducible kinase 2 and sensitizes ovarian cancer cell lines and xenografts to paclitaxel. Clin. Cancer Res. 23, 1945–1954. doi: 10.1158/1078-0432.CCR-16-1562
Zhou, Y., Wang, M., Shuang, T., Liu, Y., Zhang, Y., and Shi, C. (2019). MiR-1307 influences the chemotherapeutic sensitivity in ovarian cancer cells through the regulation of the CIC transcriptional repressor. Pathol. Res. Pract. 215:152606. doi: 10.1016/j.prp.2019.152606
Zhu, M., Yang, L., and Wang, X. (2020). NEAT1 knockdown suppresses the cisplatin resistance in ovarian cancer by regulating miR-770-5p/PARP1 axis. Cancer Manag. Res. 12, 7277–7289. doi: 10.2147/CMAR.S257311
Keywords: ovarian cancer, drug resistance, microRNA, long non-coding RNA, circular RNA
Citation: Lan H, Yuan J, Zeng D, Liu C, Guo X, Yong J, Zeng X and Xiao S (2021) The Emerging Role of Non-coding RNAs in Drug Resistance of Ovarian Cancer. Front. Genet. 12:693259. doi: 10.3389/fgene.2021.693259
Received: 10 April 2021; Accepted: 28 June 2021;
Published: 20 August 2021.
Edited by:
Rais Ahmad Ansari, Nova Southeastern University, United StatesReviewed by:
Nabab Khan, University of North Dakota, United StatesYilin Zhang, University of Chicago, United States
Copyright © 2021 Lan, Yuan, Zeng, Liu, Guo, Yong, Zeng and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Songshu Xiao, eGlhb3NvbmdzaHVAY3N1LmVkdS5jbg==; Xiangyang Zeng, NDQxNzgzOTA3QHFxLmNvbQ==
 Hua Lan
Hua Lan Jing Yuan
Jing Yuan Chu Liu
Chu Liu Songshu Xiao
Songshu Xiao