- 1Department of Clinical Biological Resource Bank, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 2Department of Blood Transfusion and Clinical Laboratory, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 3School of Medicine, South China University of Technology, Guangzhou, China
Background: The main symptoms of Kawasaki disease (KD) are inflammatory vasculitis characterized by fever lasting 1–2 weeks, failure to respond to antibiotic treatment, conjunctivitis, redness of the lips and mouth, strawberry tongue, and painless enlargement of the neck lymph nodes. Studies have been shown that tumor necrosis factor (TNF) and TNF receptor family members are abnormally expressed in the acute phase of Kawasaki disease, also revealing that these two play a significant role in the pathogenesis of KD. The purpose of our study is to determine the relationship between TNFRSF11A rs7239667 and the pathogenesis of KD and Coronary artery lesions in KD.
Methods and Results: In this study, TNFRSF11A (rs7239667) genotyping was performed in 1396 patients with KD and 1673 healthy controls. Our results showed that G > C polymorphism of TNFRSF11A (rs7239667) was not associated with KD susceptibility. In addition, the patients with KD were divided into CAA and NCAA groups according to whether they had coronary artery aneurysm (CAA) or not, and the TNFRSF11A rs7239667 genotyping was performed in the two groups. After gender and age calibration, We found that genotype CC of TNFRSF11A may be a protective factor in KD coronary artery damage (adjusted OR = 0.69 95% CI = 0.49–0.99 P = 0.0429) and is more significant in children with KD ≤ 60 months (adjusted OR = 0.49 95% CI = 0.49–0.93 P = 0.0173).
Conclusion: Our study suggests that TNFRSF11A rs7239667 G > C polymorphism maybe play a protective gene role for the severity of KD coronary artery injury and is related to age, which has not been previously revealed.
Introduction
Kawasaki disease is an immune angioinflammatory disease characterized by a fever that persists for 1–2 weeks, conjunctivitis, redness of the lips and mouth, enlarged non-suppurative lymph nodes in the neck, and peeling of the hands and feet (Kato et al., 1975; Newburger et al., 2004). Coronary artery disease is the most common and intractable complication of KD. In the most serious cases, it can become coronary artery aneurysm (CAA) and endanger the life of patients (Tacke et al., 2014). Coronary artery lesion (CAL) caused by KD have became the most common cause of acquired heart disease in children in some countries (Newburger et al., 2004; Tacke et al., 2014; Singh et al., 2015; Kumrah et al., 2020). Therefore, intravenous gamma globulin is often used for the anti-inflammatory treatment of acute phase of KD in clinic (Gupta et al., 2001; Kumrah et al., 2020). At present, it has been more than 50 years since the first diagnosed KD case, and researches on its etiology and pathogenesis continue continuously, but its etiology and pathogenesis are still an unsolved mystery (Tacke et al., 2012). A growing number of studies have claimed that genetic variations associated with immune response function are associated with increased susceptibility to KD and development of CAL (Kuo et al., 2015; Kumrah et al., 2020).
Tumor necrosis factor α(TNF-α), a member of Tumor necrosis factor superfamily (Aggarwal, 2003), is one of the cytokines produced by immune cells during inflammation (Gupta et al., 2001; Wang et al., 2011; Kumrah et al., 2020). It is believed that it plays an indispensable role in the body’s resistance to infection and immune response (Yamaji et al., 2019; Halim et al., 2021). Numerous reports have confirmed that high level of TNF can induce inflammatory response in children with KD, and is closely related to vascular endothelial damage and the incidence of CAA in KD closely (Fiers, 1991; Aeschlimann and Yeung, 2016). In addition, Furukawa et al. (1994) showed that TNF-TNF receptor signals were abnormally activated in the acute phase of KD. Yasumura et al. (2020) found that ratio of sTNFR-I/II was lower in both the acute and non-acute phases of TNF-recurrent syndrome than that in autoinflammatory diseases including KD Mizuta et al. (2021) showed that serum levels of STNFR-1, STNFR-2, and STNFR-I/II were significantly higher in patients with KD complicated with macrophage activation syndrome than that in patients with acute KD (Jinkawa et al., 2019). Stringer and Yeung (2008) suggested that TNFRs contained several functional motifs which were interacted with intracellular proteins, directed intracellular signal transduction, and further activated transcription factors, which ultimately led to increasing expression of pro-inflammatory cytokines and leukocyte recruitment. Weiss first reported that the TNFR blocker infliximab can be used in children with KD who were resistant to Immunoglobulin C (Weiss et al., 2004). Moreover, some studies have further verified in animal experiments that the mouse model without tumor necrosis factor or using tumor necrosis factor blocker were not easy to develop CAL (Oharaseki et al., 2013). While Chien et al. (2003) found no significant correlation between TNF-α promoter region gene polymorphism and susceptibility to KD or CAL in Taiwan population. In addition, TNF and TNFRs promoter region gene polymorphisms may be associated with the occurrence of a variety of tumors (Gupta et al., 2008). More and more studies, have led us to speculate about the association between polymorphisms in other TNFR loci and KD. As a member of the tumor necrosis factor receptor superfamily, TNFRSF11A is also known as nuclear factor-κB receptor activator (RANK) (Yang et al., 2004). The RANK/RANK ligand (RANKL)/osteoprotection axis (RRO axis) was first identified in the immune system and skeletal system (Anderson et al., 1997; Lacey et al., 1998). As we all know, KD is also an immune vasculitis disease (Che et al., 2018b). However, there haven’t any reports on the relationship between TNFRSF11A gene polymorphism and susceptibility to KD and CAL. In the present study, we aimed to explore the association between TNFRSF11A (rs7239667) gene polymorphism and genetic susceptibility to KD and CAL in southern Chinese population.
Materials and Methods
Study Subjects
To investigate the effect of TNFRSF11A (rs7239667) gene polymorphism on the severity of coronary complications associated with KD, we enrolled 1396 patients with KD diagnosed at Guangzhou Women and Children’s Medical Center. All of KD patients who diagnosed according to the criteria of American Heart Association were enrolled from Guangzhou Women and Children’s Medical Center between January 2014 and December 2019 (Newburger et al., 2004; McCrindle et al., 2017). In addition, 1673 healthy age- and sex- matched children who underwent physical examinations at the hospital were selected as controls with the informed consent of each control person’s guardian. Each control donated 2 ml of blood for genomic DNA extraction. This study was approved by Children’s Medical Center of Guangzhou Women and Women’s Affairs Commission (2014073009).
DNA Extraction and SNPs Genotyping
We melted all the collected whole blood, and then ensured that each tube was 200 μl of whole blood, according to the DNA extraction kit manufacturer instructions (Tiangen, Beijing, China) for DNA extraction using DNA quality, finally will be extracted to save until later use DNA −80°C. As above, after A extraction kit (Tiangen, Beijing, China), TaqMan method was used to genotype TNFRSF11A rs7239667 polymorphisms. The PCR mixture (total volume was 10 μg, including 2× multiplex PCR mixture + template DNA to be amplified and PCR primers) was added to the 384-well plate, and the related detection was performed by ABI-Q6 PCR instrument. The primers were purchased from Thermo Fisher Scientific reagent company. The specific steps can be referred to our previous literature (Che et al., 2018b).
Statistical Analysis
In this study, the genotype frequency and demographic variable χ2 test of each SNP were compared between KD cases and healthy controls. The Hardy – Weinberg equilibrium (HWE) of the samples was calculated using the chi-square goodness of fit test. The association between TNFRSF11A (rs7239667) polymorphism and KD susceptibility and coronary artery disease severity was assessed by calculation of odds ratio (OR) and 95% confidence interval (CI). Univariate unconditional logistic regression analysis was taken. The adjusted ORS were calculated by multivariate analysis adjusted for gender. SAS software was used for all statistical analyses (version 9.1; SAS Institute), P < 0.05 was deemed statistically significant.
Results
Clinical Characteristics of the Study Population
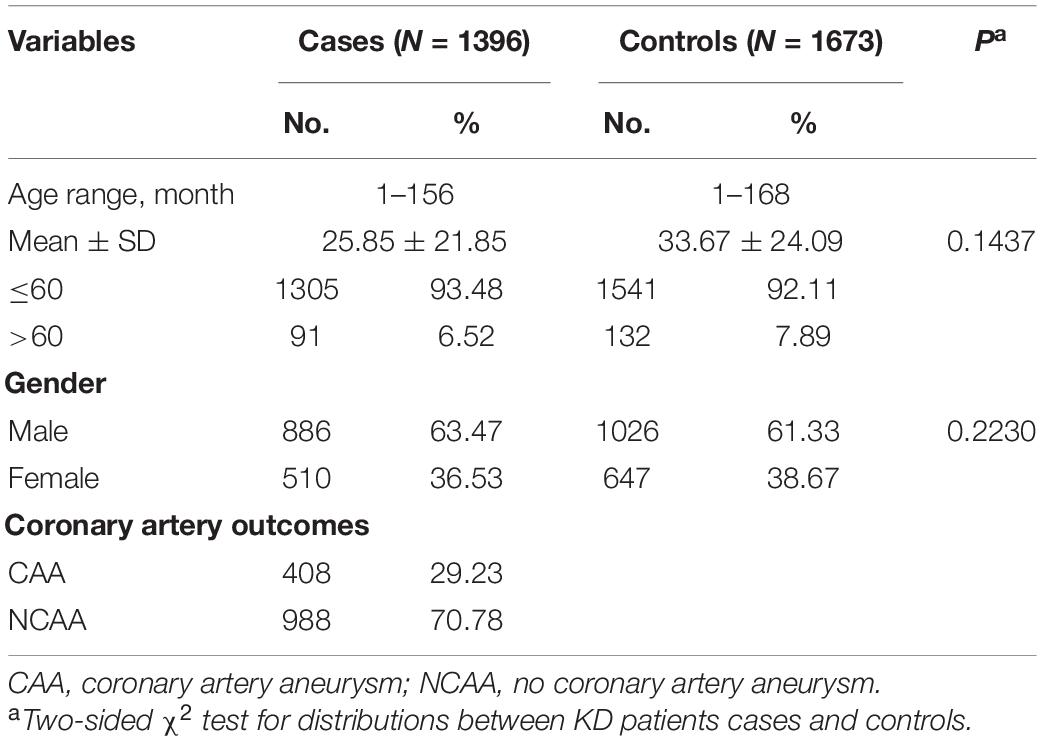
The distribution of age and sex between KD patients and healthy control group are shown in Table 1. The mean age and age distribution range of KD patients and healthy control group was 25.85 months (±21.85, range 1–156 months) and 33.67 months (±24.09, range 1–168 months), respectively. There was no significant difference in age (P = 0.1437) and gender (P = 0.2230) between KD patients and healthy control group.
Correlations Between TNFRSF11A Polymorphisms With the Risk of KD and the Severity of Coronary Complications in KD Patients
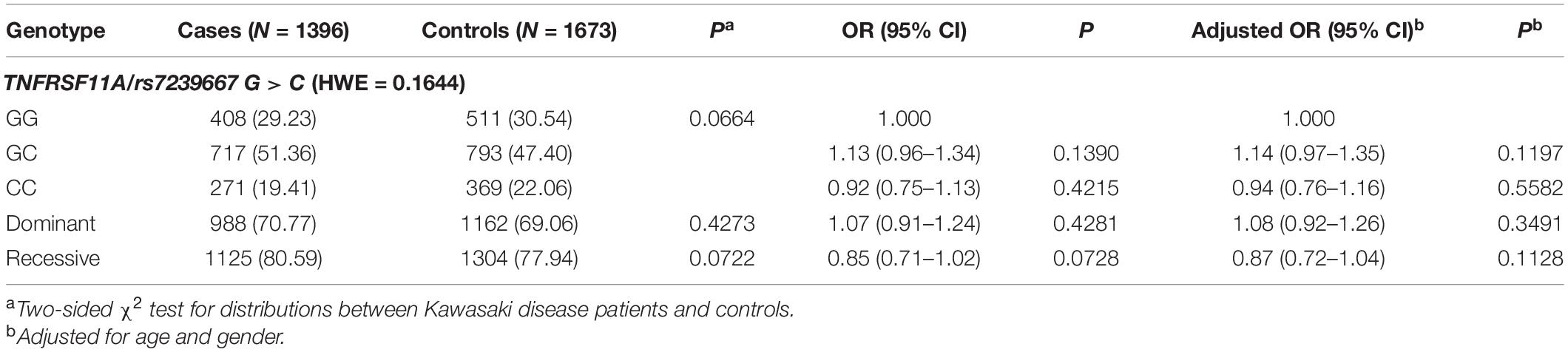
The SNP genotype distribution of selected TNFRSF11A rs7239667 G/C and its correlation with KD risk are shown in Table 2. The genotype frequency of the samples conforms to Hardy–Weinberg law. Unfortunately, we did not observe any significant association between the risk of SNP and KD. Patients with KD were then divided into CAA group and NCAA group according to whether they had coronary aneurysm (CAA) or not, and TNFRSF11A rs7239667 genotyping was performed in the two groups (Table 3). After further adjustment for gender and age, we found that genotype CC of TNFRSF11A may be a protective factor for KD coronary artery injury (adjusted OR = 0.69 95% CI = 0.49–0.99 P = 0.0429) (Table 4). It was more significant in KD patients ≤60 months (adjusted OR = 0.49 95% CI = 0.49–0.93 P = 0.0173).
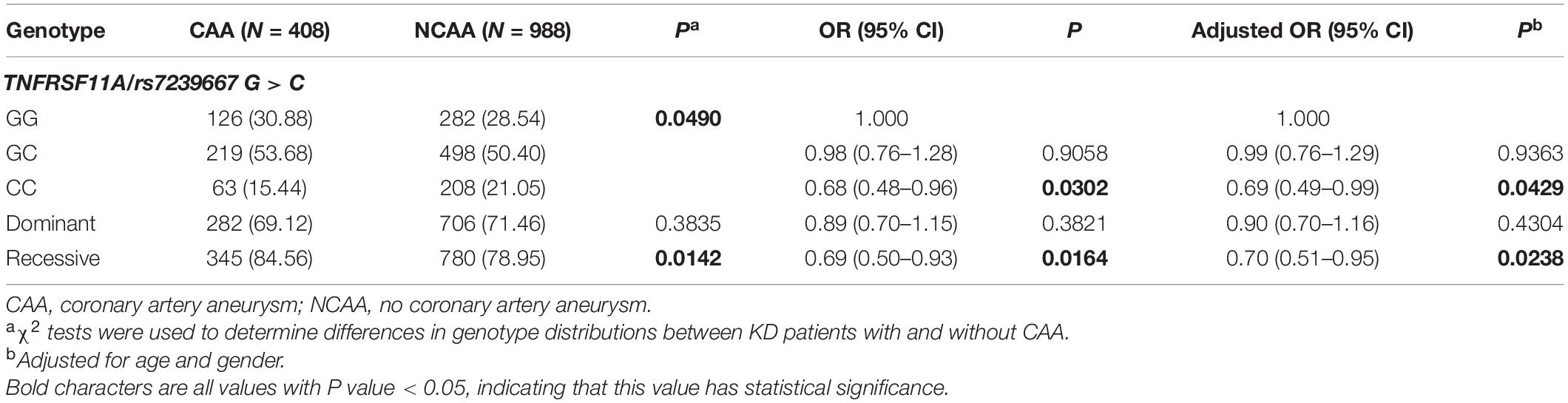
Table 4. TNFRSF11A rs7239667 G > C polymorphism and genotype distribution in KD patients with different coronary outcomes.
Stratified Analysis
Then we further explored the relationship between TNFRSF11A gene and the prevalence of KD and the degree of coronary artery damage in patients with KD considering age and gender (Tables 5, 6). In terms of age, since KD occurs more frequently in children aged less than 60 months (Chu et al., 2017), we conducted stratified analysis of age by taking ≤60 months as the limit (Aeschlimann and Yeung, 2016). We found that male patients with KD (OR = 0.79, 95% CI = 0.64–0.99, P = 0.0425, adjusted OR = 0.80, 95% CI = 0.63–1.00 P = 0.0533) and patients with KD with CAA (OR = 0.65, 95% CI = 0.48–0.86, P = 0.0033, adjusted OR = 0.65, 95% CI = 0.48–0.88, P = 0.045), the risk of developing KD in children with rs7239667 CC genotype was significantly lower than that in children with GG/GC genotype. In addition, children with rs7239667 CC genotype with KD are less likely to develop coronary aneurysms. However, no such correlation is found in Table 5.
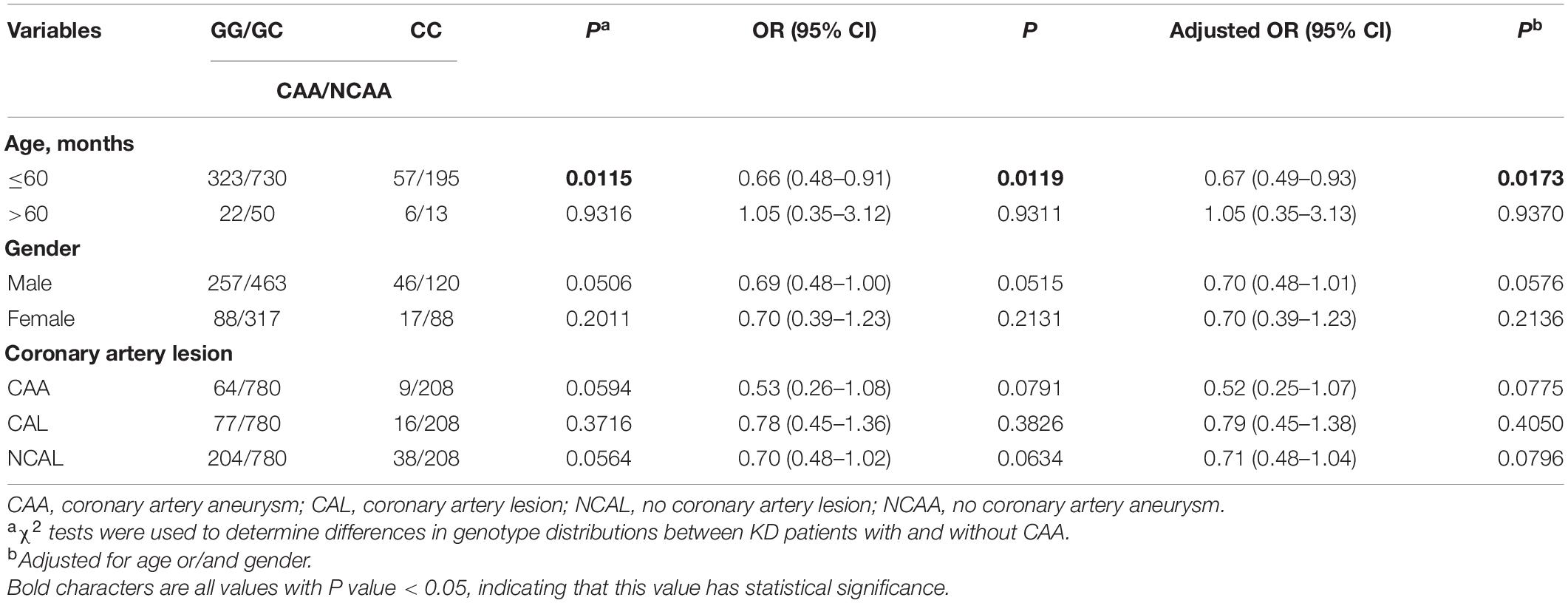
Table 5. Stratified analysis of the association between TNFRSF11A polymorphism and the risk of KD patients with CAA in a population of southern China.
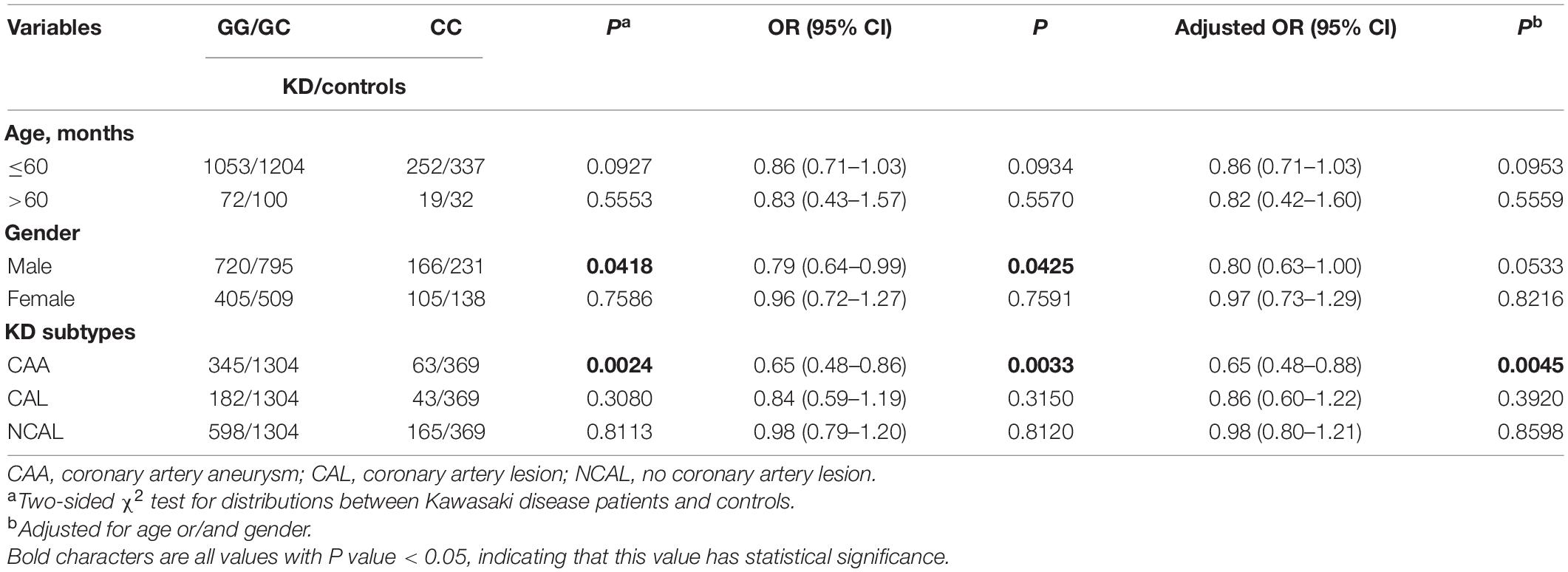
Table 6. Stratified analysis of the association between TNFRSF11A polymorphism and KD risk in southern China.
Discussion
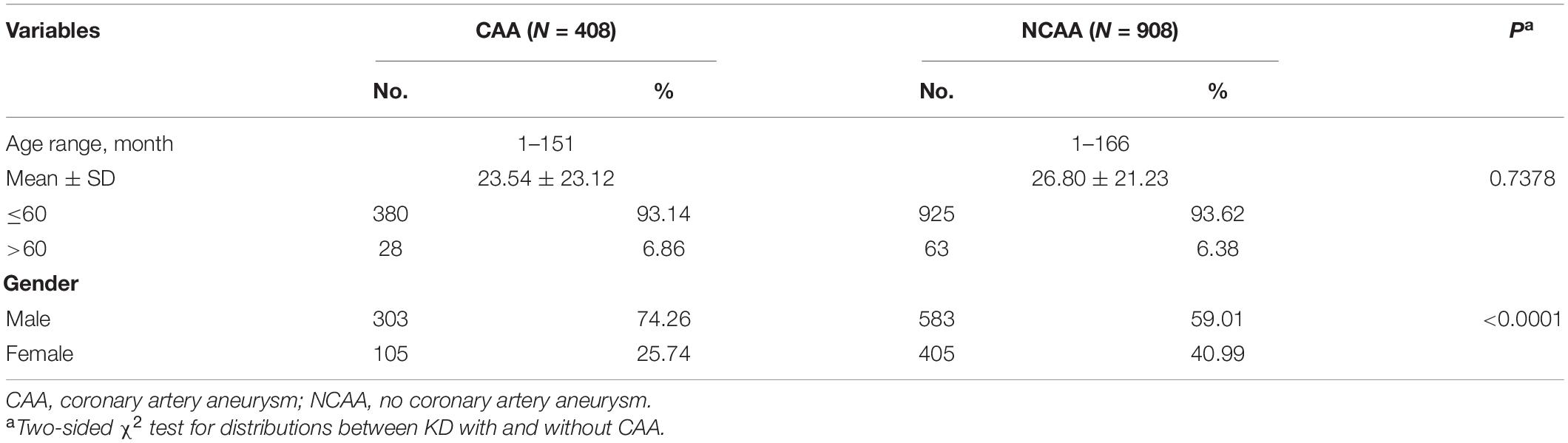
In the present study, we evaluated the association between TNFRSF11A gene polymorphism (rs7239667 C) and susceptibility to KD and the severity of coronary artery damage in children with KD in 1396 patients (408 with CAA and 988 without CAA) and 1673 healthy controls. Our results showed that TNFRSF11A (rs7239667), the selected SNP, was not associated with KD susceptibility in children from southern China. Interestingly, when we compared KD patients with or without CAA, we found that TNFRSF11A (rs7239667 C) variant genotype significantly reduced the degree of coronary artery damage in KD patients.
Although the etiology and pathogenesis of KD remain unclear, systemic vascular disease is the most prominent manifestation of KD (Wang et al., 2011). A large number of studies have shown that in the occurrence of KD, TNF-α, IL-6, TNFR, and other cytokines exist, and these cytokines lead to vascular endothelial cell damage in KD patients, and eventually causing vasculitis (Furukawa et al., 1994; Wang et al., 2011; Kumrah et al., 2020). Tumor necrosis factor (TNF) can bind to TNFR and initiate inflammatory response and physiological functions (Halim et al., 2021). TNFR family is composed of TNFRSF8, OPG, DCR3, etc., and is a growing superfamily with extracellular homologous sequences (Darnay and Aggarwal, 1999; Inoue et al., 2000; Aggarwal, 2003). As an activator of NF-κB receptor, TNFRSF11A is also a member of the tumor necrosis factor receptor superfamily (Yang et al., 2019; Glasnovic et al., 2020). Yang et al. (2019) indicated that RANKL (a specific ligand of TNFRSF11A) rs2277438 polymorphism increased the risk of rheumatoid disease. Petean et al. (2019) showed that TNFRSF11A (rs3826620) and its ligand RANKL (rs9594738) gene polymorphisms were associated with Persistent Apical Periodontitis. Wu et al. (2019) deemed that TNFRSF11B (rs2073617) gene polymorphism may increase chronic infection with HCV. In a study on osteoporosis, Richards et al. (2009) showed that polymorphism at the TNFRSF11A SNP site was significantly associated with fracture risk. Omar et al. (2015) suggested that the rs2073618 gene locus polymorphism of OPG, a key protein downstream of RANK-RANKL signaling pathway, was associated with the development of breast cancer to some degree. These studies suggested that TNFRSF11A gene polymorphism may play a role in the occurrence and development of different diseases.
With the development of molecular genetic methods, it is possible to identify susceptibility genes of complex diseases using GWAS and candidate gene methods (Kumrah et al., 2020). Numerous studies have shown that many candidate genes, including FCGR2A (Kuo et al., 2015), ITPKC (Onouchi et al., 2008), VEGF (Ohno et al., 2000), IL-1B (Fu et al., 2019), and ABCC4 (Che et al., 2018a), have been identified as susceptibility genes that increase the risk of KD or coronary complications from KD. However, there is a lack of research on TNFRSF11A gene polymorphism and its relationship with KD. In our case-control study, we found that TNFRSF11A rs7239667C allele is a protective factor for CAL of KD. To our knowledge, this is the first study to validate the association between rs7239667 C allele and KD coronary complications in a population of southern China. We considered that rs7239667 C allele may play a significant role in the pathogenesis of CAL of KD.
Although this is the first study to evaluate the association between TNFRSF11A gene polymorphism and the risk of KD in children in southern China, there are some limitations that should be noted. First, we focus only on the allele associated with rs7239667 G > C of TNFRSF11A. Polymorphisms at other loci of TNFRSF11A gene were ignored. Secondly, due to the nature of the retrospective study design, we only collected cases and controls consistent with race, geography, age and gender, ignoring other factors such as the environment of parents and the presence or absence of infection in eating habits. Third, the sample size of this study is limited and this study lacks functional studies on TNFRSF11A (rs7239667). Future studies require larger sample sizes to confirm the role of TNFRSF11A in susceptibility to KD and relevant functional studies also will be included in our research plan in the follow-up work.
In conclusion, although the current study suggested an association between TNFRSF11A gene rs7239667 and the severity of coronary artery damage in KD. We still need to conduct big data, multi-center studies on the polypeptide properties of TNFRSF11A gene to further confirm our current results.
Data Availability Statement
The original data supporting the relevant research in this article is in the supplementary material. For further inquiries, please contact the corresponding authors of this article directly.
Ethics Statement
This study was approved by the Medical Ethics Committee of Guangzhou Women and Children’s Medical Center (2014073009). Informed written consent was obtained from the guardians of patients.
Author Contributions
LZ, KL, YW, and XG designed and organized the study and supervised the whole project. LP, HM, JL, ZJ, and BW contributed to field survey, data collection, laboratory detection, and quality control. DC, LF, YX, and HY performed the data cleansing and statistical analysis. XG, HY, DC, and LZ wrote and critically revised the manuscript. All authors contributed constructively to the editing and drafting of the manuscript and read and approved the final manuscript.
Funding
This study was supported by the Guangdong Natural Science Fund, China (grant numbers 2019A1515012061 and 2021A1515011207), the Guangzhou Science and Technology Program Project, China (grant numbers 201904010486 and 202102010197), Ost-doctoral Fund from Guangzhou Service Center for Scholarly Exchange, China (grant number 011302026), Postdoctoral Research Initiation Fund from Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center (grant number 3001127), and the Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center Fund, China (grant numbers GCP-2019-003, GCP-2019-006, and YIP-2019-050).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks all the enrolled patients and the Clinical Biological Resource Bank of Guangzhou Women and Children’s Medical Center for providing the clinical samples used in this study.
References
Aeschlimann, F. A., and Yeung, R. S. M. (2016). TNF and IL-1 targeted treatment in kawasaki disease. Curr. Treatment Opt. Rheumatol. 2, 283–295. doi: 10.1007/s40674-016-0053-58
Aggarwal, B. B. (2003). Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756. doi: 10.1038/nri1184
Anderson, D. M., Maraskovsky, E., Billingsley, W. L., Dougall, W. C., Tometsko, M. E., Roux, E. R., et al. (1997). A homologue of the TNFreceptor and its ligandenhance T-cell growthand dendritic-cell function. Nature 390, 175–179.
Che, D., Pi, L., Fang, Z., Xu, Y., Cai, M., Fu, L., et al. (2018a). ABCC4 variants modify susceptibility to kawasaki disease in a southern chinese population. Dis. Markers 2018:8638096. doi: 10.1155/2018/8638096
Che, D., Pi, L., Xu, Y., Fu, L., Zhou, H., Wang, Z., et al. (2018b). TBXA2R rs4523 G allele is associated with decreased susceptibility to Kawasaki disease. Cytokine 111, 216–221. doi: 10.1016/j.cyto.2018.08.029
Chien, Y. H., Chang, K. W., Yang, Y. H., Lu, M. Y., Lin, Y. T., and Chiang, B. L. (2003). Association between levels of tnf-α and tnf-α promoter –308 a/a polymorphism in children with kawasaki disease. J. Formos. Med. Assoc. 102, 147–150.
Chu, M., Wu, R., Qin, S., Hua, W., Shan, Z., Rong, X., et al. (2017). Bone marrow-derived MicroRNA-223 works as an endocrine genetic signal in vascular endothelial cells and participates in vascular injury from Kawasaki disease. J. Am. Heart Assoc. 6:e004878. doi: 10.1161/JAHA.116.004878
Darnay, B., and Aggarwal, B. (1999). Signal transduction by tumour necrosis factor and tumour necrosis factor related ligands and their receptors. Ann. Rheum. Dis. 58, (Suppl. 1), I2–I13. doi: 10.1136/ard.58.2008.i2
Fiers, W. (1991). Tumor necrosis factor characterization at the molecular, cellular and in vivo level. FEBS Lett. 289, 199–212.
Fu, L. Y., Qiu, X., Deng, Q. L., Huang, P., Pi, L., Xu, Y., et al. (2019). The IL-1B gene polymorphisms rs16944 and rs1143627 contribute to an increased risk of coronary artery lesions in southern chinese children with Kawasaki disease. J. Immunol. Res. 2019:4730507. doi: 10.1155/2019/4730507
Furukawa, S., Matsubara, T., Umezawa, Y., Okumura, K., and Yabuta, K. (1994). Serum levels of p60 soluble tumor necrosis factor receptor during acute Kawasaki disease. J. Pediatr. 124, 721–725.
Glasnovic, A., O’Mara, N., Kovacic, N., Grcevic, D., and Gajovic, S. (2020). RANK/RANKL/OPG signaling in the brain: a systematic review of the literature. Front. Neurol. 11:590480. doi: 10.3389/fneur.2020.590480
Gupta, M., Noel, G. J., Schaefer, M., Friedman, D., Bussel, J., and Johann-Liang, R. (2001). Cytokine modulation with immune g-globulin in peripheral blood of normal children and its implications in Kawasaki disease treatment. J. Clin. Immunol. 21, 193–199.
Gupta, R., Sharma, S. C., and Das, S. N. (2008). Association of TNF-alpha and TNFR1 promoters and 3’ UTR region of TNFR2 gene polymorphisms with genetic susceptibility to tobacco-related oral carcinoma in Asian Indians. Oral Oncol. 44, 455–463. doi: 10.1016/j.oraloncology.2007.06.003
Halim, S. A., Sikandari, A. G., Khan, A., Wadood, A., Fatmi, M. Q., Csuk, R., et al. (2021). Structure-Based virtual screening of tumor necrosis factor-alpha inhibitors by cheminformatics approaches and bio-molecular simulation. Biomolecules 11:329. doi: 10.3390/biom11020329
Inoue, J., Ishida, T., Tsukamoto, N., Kobayashi, N., Naito, A., Azuma, S., et al. (2000). Tumor necrosis factor receptor-associated factor (TRAF) family: adapter proteins that mediate cytokine signaling. Exp. Cell Res. 254, 14–24. doi: 10.1006/excr.1999.4733
Jinkawa, A., Shimizu, M., Nishida, K., Kaneko, S., Usami, M., Sakumura, N., et al. (2019). Cytokine profile of macrophage activation syndrome associated with Kawasaki disease. Cytokine 119, 52–56. doi: 10.1016/j.cyto.2019.03.001
Kato, H., Koike, S., Yamamoto, M., Ito, Y., and Yano, E. (1975). Coronary aneurysms in infants and young children with acute febrile mucocutaneous lymph node syndrome. J. Pediatr. 1975, 892–898.
Kumrah, R., Vignesh, P., Rawat, A., and Singh, S. (2020). Immunogenetics of Kawasaki disease. Clin. Rev. Allergy. Immunol. 59, 122–139. doi: 10.1007/s12016-020-08783-8789
Kuo, H. C., Chang, J. C., Kuo, H. C., Yu, H. R., Wang, C. L., Lee, C. P., et al. (2015). Identification of an association between genomic hypomethylation of FCGR2A and susceptibility to Kawasaki disease and intravenous immunoglobulin resistance by DNA methylation array. Arthritis Rheumatol. 67, 828–836. doi: 10.1002/art.38976
Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T., et al. (1998). Osteoprotegerin ligand is a cytokinethat regulates osteoclast differentiationand activation. Cell 93, 165–176.
McCrindle, B. W., Rowley, A. H., Newburger, J. W., Burns, J. C., Bolger, A. F., Gewitz, M., et al. (2017). Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the american heart association. Circulation 135, e927–e999. doi: 10.1161/CIR.0000000000000484
Mizuta, M., Shimizu, M., Irabu, H., Usami, M., Inoue, N., Nakagishi, Y., et al. (2021). Comparison of serum cytokine profiles in macrophage activation syndrome complicating different background rheumatic diseases in children. Rheumatology (Oxford) 60, 231–238. doi: 10.1093/rheumatology/keaa299
Newburger, J. W., Takahashi, M., Gerber, M. A., Gewitz, M. H., Tani, L. Y., Burns, J. C., et al. (2004). Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and kawasaki disease, council on cardiovascular disease in the young, american heart association. Circulation 110, 2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78
Oharaseki, T., Yokouchi, Y., Yamada, H., Mamada, H., Muto, S., Sadamoto, K., et al. (2013). The role of TNF-α in a murine model of Kawasaki disease arteritis induced with a Candida albicans cell wall polysaccharide. Modern Rheumatol. 24, 120–128. doi: 10.1007/s10165-013-0865-864
Ohno, T., Igarashi, H., Inoue, K., Akazawa, K., Joho, K., and Hara, T. (2000). Serum vascular endothelial growth factor: a new predictive indicator for the occurrence of coronary artery lesions in Kawasaki disease. Eur. J. Pediatr. 159, 424–429.
Omar, H. S., Shaker, O. G., Nassar, Y. H., Marzouk, S. A., and ElMarzouky, M. S. (2015). The association between RANKL and Osteoprotegerin gene polymorphisms with breast cancer. Mol. Cell Biochem. 403, 219–229. doi: 10.1007/s11010-015-2352-z
Onouchi, Y., Gunji, T., Burns, J. C., Shimizu, C., Newburger, J. W., Yashiro, M., et al. (2008). ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat. Genet. 40, 35–42. doi: 10.1038/ng.2007.59
Petean, I. B. F., Kuchler, E. C., Soares, I. M. V., Segato, R. A. B., Silva, L., Antunes, L. A. A., et al. (2019). Genetic polymorphisms in RANK and RANKL are associated with persistent apical periodontitis. J. Endod. 45, 526–531. doi: 10.1016/j.joen.2018.10.022
Richards, J. B., Kavvoura, F. K., Rivadeneira, F., Styrkársdóttir, U., Estrada, K., Halldórsson, B. V., et al. (2009). Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann. Int. Med. 151, 528–537.
Singh, S., Vignesh, P., and Burgner, D. (2015). The epidemiology of Kawasaki disease: a global update. Arch. Dis. Child. 100, 1084–1088. doi: 10.1136/archdischild-2014-307536
Stringer, E., and Yeung, R. S. M. (2008). Pathogenesis of Kawasaki disease: the central role of TNF-α. Fut. Rheumatol. 3, 69–71. doi: 10.2217/17460816.3.1.69
Tacke, C. E., Breunis, W. B., Pereira, R. R., Breur, J. M., Kuipers, I. M., and Kuijpers, T. W. (2014). Five years of Kawasaki disease in the Netherlands: a national surveillance study. Pediatr. Infect. Dis. J. 33, 793–797. doi: 10.1097/INF.0000000000000271
Tacke, C. E., Burgner, D., Kuipers, I. M., and Kuijpers, T. W. (2012). Management of acute and refractory Kawasaki disease. Expert Rev. Anti. Infect. Ther. 10, 1203–1215. doi: 10.1586/eri.12.101
Wang, G. B., Li, C. R., Yang, J., Wen, P. Q., and Jia, S. L. (2011). A regulatory polymorphism in promoter region of TNFR1 gene is associated with Kawasaki disease in Chinese individuals. Hum. Immunol. 72, 451–457. doi: 10.1016/j.humimm.2011.02.004
Weiss, J. E., Eberhard, B. A., Chowdhury, D., and Gottlieb, B. S. (2004). Infliximab as a novel therapy for refractory Kawasaki disease. J. Rheumatol. 31, 808–810.
Wu, J., Huang, P., Yue, M., Wang, C., Wu, C., Shao, J., et al. (2019). [Association between TNFRSF11A and TNFRSF11B gene polymorphisms and the outcome of hepatitis C virus infection]. Zhonghua Liu Xing Bing Xue Za Zhi 40, 1291–1295. doi: 10.3760/cma.j.issn.0254-6450.2019.10.022
Yamaji, N., da Silva, Lopes, K., Shoda, T., Ishitsuka, K., Kobayashi, T., et al. (2019). TNF-alpha blockers for the treatment of Kawasaki disease in children. Cochrane Database. Syst. Rev. 8:CD012448. doi: 10.1002/14651858.CD012448
Yang, C. R., Wang, J. H., Hsieh, S. L., Wang, S. M., Hsu, T. L., and Lin, W. W. (2004). Decoy receptor 3 (DcR3) induces osteoclast formation from monocyte/macrophage lineage precursor cells. Cell Death Differ. 11, (Suppl. 1), S97–S107. doi: 10.1038/sj.cdd.4401403
Yang, H., Liu, W., Zhou, X., Rui, H., Zhang, H., and Liu, R. (2019). The association between RANK, RANKL and OPG gene polymorphisms and the risk of rheumatoid arthritis: a case-controlled study and meta-analysis. Biosci. Rep. 39:BSR20182356. doi: 10.1042/bsr20182356
Yasumura, J., Shimizu, M., Toma, T., Yashiro, M., Yachie, A., and Okada, S. (2020). Clinical Significance of serum soluble TNF receptor I/II ratio for the differential diagnosis of tumor necrosis factor receptor-associated periodic syndrome from other autoinflammatory diseases. Front. Immunol. 11:576152. doi: 10.3389/fimmu.2020.576152
Keywords: Kawasaki disease (KD), coronary artery lesion (CAL), tumor necrosis factor receptor superfamily, single nucleotide polymorphisms (SNP), tumor necrosis factor superfamily
Citation: Zhang L, Lin K, Wang Y, Yu H, Li J, Fu L, Xu Y, Wei B, Mai H, Jiang Z, Che D, Pi L and Gu X (2021) Protective Effect of TNFRSF11A rs7239667 G > C Gene Polymorphism on Coronary Outcome of Kawasaki Disease in Southern Chinese Population. Front. Genet. 12:691282. doi: 10.3389/fgene.2021.691282
Received: 16 April 2021; Accepted: 23 June 2021;
Published: 17 August 2021.
Edited by:
Mario Capasso, University of Naples Federico II, ItalyReviewed by:
Gilberto Vargas Alarcón, Instituto Nacional de Cardiología Ignacio Chávez, MexicoYanina Timasheva, Institute of Biochemistry and Genetics of Ufa Scientific Centre (RAS), Russia
Copyright © 2021 Zhang, Lin, Wang, Yu, Li, Fu, Xu, Wei, Mai, Jiang, Che, Pi and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqiong Gu, Z3V4aWFvcWlvbmdAZ3djbWMub3Jn; Lei Pi, cGlsZWlAZ3djbWMub3Jn
†These authors have contributed equally to this work
 Linyuan Zhang
Linyuan Zhang Kun Lin
Kun Lin Yishuai Wang
Yishuai Wang Hongyan Yu
Hongyan Yu Jinqing Li
Jinqing Li Lanyan Fu2
Lanyan Fu2 Yufen Xu
Yufen Xu Bing Wei
Bing Wei Hanran Mai
Hanran Mai Zhiyong Jiang
Zhiyong Jiang Di Che
Di Che Lei Pi
Lei Pi Xiaoqiong Gu
Xiaoqiong Gu