
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 15 June 2021
Sec. Statistical Genetics and Methodology
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.689897
Background: Observational studies have implied an association between polycystic ovary syndrome (PCOS) and psychiatric disorders. Here we examined whether PCOS might contribute causally to such disorders, focusing on anxiety disorder (AD), bipolar disorder (BIP), major depression disorder (MDD), obsessive compulsive disorder (OCD), and schizophrenia (SCZ).
Methods: Causality was explored using two-sample Mendelian randomization (MR) with genetic variants as instrumental variables. The genetic variants were from summary data of genome-wide association studies in European populations. First, potential causal effects of PCOS on each psychiatric disorder were evaluated, and then potential reverse causality was also assessed once PCOS was found to be causally associated with any psychiatric disorder. Causal effects were explored using inverse variance weighting, MR-Egger analysis, simulation extrapolation, and weighted median analysis.
Results: Genetically predicted PCOS was positively associated with OCD based on inverse variance weighting (OR 1.339, 95% CI 1.083–1.657, p = 0.007), simulation extrapolation (OR 1.382, 95% CI 1.149–1.662, p = 0.009) and weighted median analysis (OR 1.493, 95% CI 1.145–1.946, p = 0.003). However, genetically predicted OCD was not associated with PCOS. Genetically predicted PCOS did not exert causal effects on AD, BIP, MDD, or SCZ.
Conclusions: In European populations, PCOS may be a causal factor in OCD, but not AD, BIP, MDD, or SCZ.
Polycystic ovary syndrome (PCOS) affects 5–15% of women of reproductive age, making it one of the most common endocrine disorders in that group. It is characterized mainly by hirsutism, elevated serum testosterone, oligo-/amenorrhea, and polycystic ovaries (Rosenfield and Ehrmann, 2016). Because of its high prevalence and association with infertility and long-term metabolic complications (diabetes, cardiovascular disease), PCOS is a major threat to women's health and a burden on the economy in the amount of 4.36 billion USD annually (Azziz et al., 2005).
Several studies have revealed a relationship between PCOS and mental illness. For instance, women with PCOS are at increased risk for psychiatric disorders, including anxiety disorder (AD), bipolar disorder (BIP), major depression disorder (MDD), obsessive compulsive disorder (OCD), and schizophrenia (SCZ) (Hung et al., 2014; Blay et al., 2016; Cesta et al., 2016; Brutocao et al., 2018). PCOS is a lifelong morbidity, and the onset of mental illness at any time in a women's life can further aggravate her disease burden. Therefore, early detection of psychiatric disorders in women with PCOS is important in order to enable timely intervention to prevent progression or recurrence of such disorders.
Whether the association between PCOS and psychiatric disorders reflects a causal relationship is unclear. This is due, in part, to the heterogeneity of PCOS and the presence of unavoidable confounders, including obesity, hyperandrogenism, insulin resistance, and inflammation. A method for exploring such potential causality in an unbiased way is two-sample Mendelian randomization (MR), which relies on genetic variants as instrumental variables (IVs) to evaluate causality between an exposure and an outcome (Davies et al., 2018). After random allocation during meiosis, single-nucleotide polymorphisms (SNPs) remain stable and unmodified throughout a lifetime of environmental exposure, making them independent of confounders or reverse causation (Sekula et al., 2016). MR has been already proven powerful for resolving controversies about PCOS: while epidemiologic studies suggested that women with PCOS were more likely to experience type 2 diabetes mellitus, coronary heart disease, or stroke, MR detected no genetic causality between these diseases and PCOS (Zhu et al., 2020). MR has also suggested that PCOS is positively associated with breast cancer (Wen et al., 2020) and negatively associated with ovarian cancer (Harris et al., 2019), despite earlier studies showing no such associations (Barry et al., 2014).
Therefore, we applied MR analysis to summary data from European genome-wide association studies (GWAS) in order to examine potential causal associations of PCOS with five common psychiatric disorders: AD, BIP, MDD, OCD, and SCZ.
GWAS data for PCOS were taken from Apollo (https://doi.org/10.17863/CAM.27720), while GWAS data for AD, BIP, MDD, OCD, and SCZ were taken from the Psychiatric Genomics Consortium (https://www.med.unc.edu/pgc/results-and-downloads) (Table 1). All subsequent analyses were restricted to “European” as the ethnic cohort. The ID of SNPs, the associated effect size (β), standard error (se) of the effect size, and effect allele were extracted from each GWAS summary dataset.
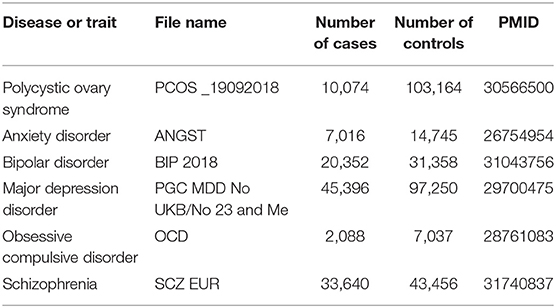
Table 1. Overview of genome-wide association datasets for polycystic ovary syndrome and five psychiatric disorders.
The original articles that published GWAS exposure and outcome data were obtained to retrieve detailed information about study design and sample collection. Participants recruited from the same consortium or hospital in the exposure and outcome studies were regarded as duplicates. The maximized overlap was calculated as n/N, where n referred to the number of potentially repetitive recruiters, and N to the total sample number in the larger dataset (Burgess et al., 2016).
Several quality control steps were conducted to select eligible SNPs as IVs from the exposure data. First, SNPs had to meet genome-wide significance, defined as p < 5 × 10−8. Second, the SNPs had to be independent of each other, so clumping (criteria: r2 = 0.001, kb = 10,000) was performed to exclude linkage disequilibrium (LD) between the SNPs. Among SNP-shaving LD, only those with the lowest p-values were retained. Third, SNPs related to a confounder-associated phenotype were removed. The potentially related phenotypes were detected using the online database “PhenoScanner” (www.phenoscanner.medschl.cam.ac.uk/phenoscanner), by filtration of r2 > 0.8 and p < 5 × 10−8.
Before statistical analysis of causal relationships, we ensured that GWAS exposure and outcome data matched well. First, information about exposure-associated SNPs was extracted from each outcome dataset. Any exposure-associated SNP absent from the outcome dataset was substituted with a proxy SNP; that is, if it existed, and it was in LD (r2 > 0.8, MAF for palindromes <0.3) with the requested one. Second, the exposure and outcome data were harmonized to ensure that the effect of the SNP was on the same allele. Otherwise, the SNP was deleted.
Third, the statistical significance of the matched exposure-outcome summary data was analyzed by several methods. Inverse variance weighted (IVW) analysis assumed no or balanced pleiotropy. We used the random-effects model to avoid heterogeneity bias, which was measured using Cochran's Q test. The MR-Egger method not only allowed for, but also detected, horizontal pleiotropy based on its intercept with a y-axis. When the intercept was not zero, there was horizontal pleiotropy. The MR-Egger method was based on the “instrument strength independent of the direct effects” (INSIDE) and “no measurement error in the SNP exposure effects” (NOME) assumptions, and it also used the random-effects model. In addition, when the regression dilution I2 statistic was <90%, indicating violation with the NOME assumption, the simulation extrapolation (SIMEX) correction was performed (Bowden et al., 2016). The WM method was used to generate unbiased results when ≥50% SNPs were valid variants.
Fourth, SNP pleiotropy and sensitivity were assessed using pleiotropy tests, forest plots, funnel plots, and leave-one-out plots. The forest plot estimated the causal effect of each SNP on the outcome by using Wald ratio analysis. The Funnel plot was used to assess heterogeneity by depicting the reciprocal of the se of the SNP against SNP effects on the outcome. The leave-one-out plot ascertained whether an association was disproportionately influenced by a single SNP. In such a plot, each black point represented the IVW analysis after exclusion of that particular SNP.
We investigated whether PCOS-SNPs could induce major mental illness (AD, BIP, MDD, OCD, and SCZ), so the direction of MR analysis was defined as forward when PCOS was the exposure and psychiatric disorders were the outcomes. When PCOS was found to be causally associated with any outcome, reverse MR analysis was performed, in which the precise psychiatric disorder was the exposure and PCOS was the outcome. This allowed us to eliminate bias due to reverse causation.
Analyses were conducted using the “sqldf,” “biomaRt,” “TwoSampleMR,” “MendelianRandomization,” or “simex” modules in the R package (version 4.0.3). In general, statistical significance was defined as p < 0.05. In the case of multiple testing, the threshold for statistical significance was adjusted by the conservative Bonferroni correction according to the formula p < 0.05/n, where n referred to the number of MR tests. Since the present study included five forward and one reverse two-sample MR analyses, the adjusted p-values for the forward and reverse MR tests were, respectively, 0.01 (0.05/5) and 0.05 (0.05/1). When a p-value for an association was below the adjusted cut-off value, the association was considered statistically significant as a causal association. When a p-value was below 0.05 but equal to or greater than the adjusted cut-off value, the association was considered one of potential causality needing further confirmation. When a p-value was equal to or >0.05, it was considered not to be causal.
Ethics approval was not required since the data were published GWAS summary statistics from public databases.
Two-sample MR analysis requires that the exposure and outcome studies be conducted in two independent, non-overlapping cohorts from the same population. High participant overlap can increase type I error, biasing the MR results (Burgess et al., 2016). The estimated participant overlap between PCOS and each psychiatric disorder was low: 5.8028% for AD, 0.2296% for BIP, 5.2101% for MDD, 0% for OCD, and 2.6174% for SCZ (Table 2). We considered such low overlap as unlikely to bias subsequent analyses.
Among the most significant 10,000 SNPs of the largest GWAS meta-analysis in European women with PCOS (Supplementary Material), 14 remained after filtering for genome-wide significance (p < 5 × 10−8) and eliminating SNPs in LD (Table 3). Then whether these 14 SNPs had other confounder-associated phenotypes was detected. Four SNPs were found to be related to other phenotypes: rs11031005, rs13164856, rs7864171, rs2271194. The first three SNPs were related to irrelevant secondary phenotypes. Although rs2271194 was related to body mass index and asthma, the effect sizes of rs2271194 on body mass index (β = 0.01348) and asthma (0.005336) were too small to bias the results. Hence none of them were excluded and the final set of PCOS-IVs contained 14 SNPs (Table 3).
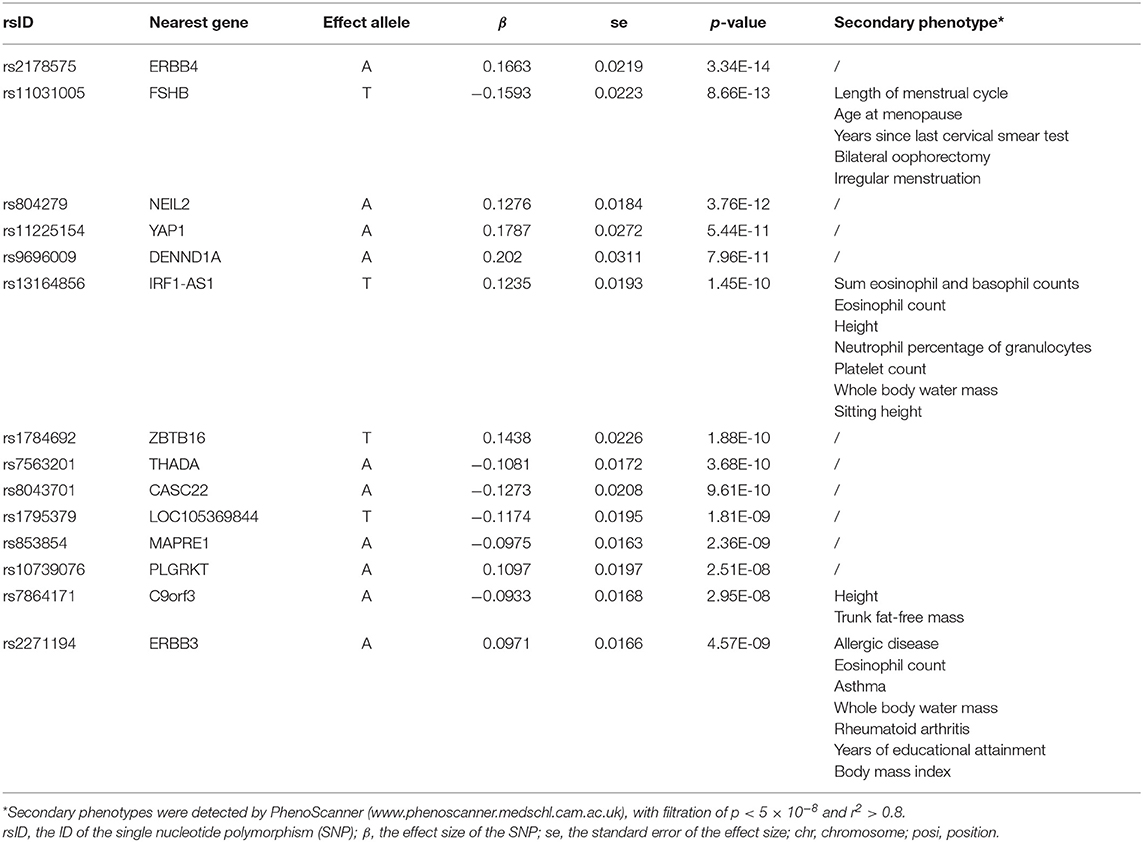
Table 3. The 14 single-nucleotide polymorphisms (SNPs) used as instrumental variables (IVs) of polycystic ovary syndrome.
During data harmonization before MR, rs804279, rs8043701, rs853854, and rs2271194 were discarded because they were found to be palindromic SNPs with intermediate allele frequencies. MR analyses involving different methods are detailed in Figure 1, and the sensitivity analyses are displayed in Figure 2. The I2 statistic determined by the MR-Egger method in each MR was <90%, suggesting NOME violation and regression dilution, which can inflate type I error (Bowden et al., 2016). Therefore, the results from the MR-Egger method were considered inaccurate and therefore corrected with the SIMEX method (Bowden et al., 2016).
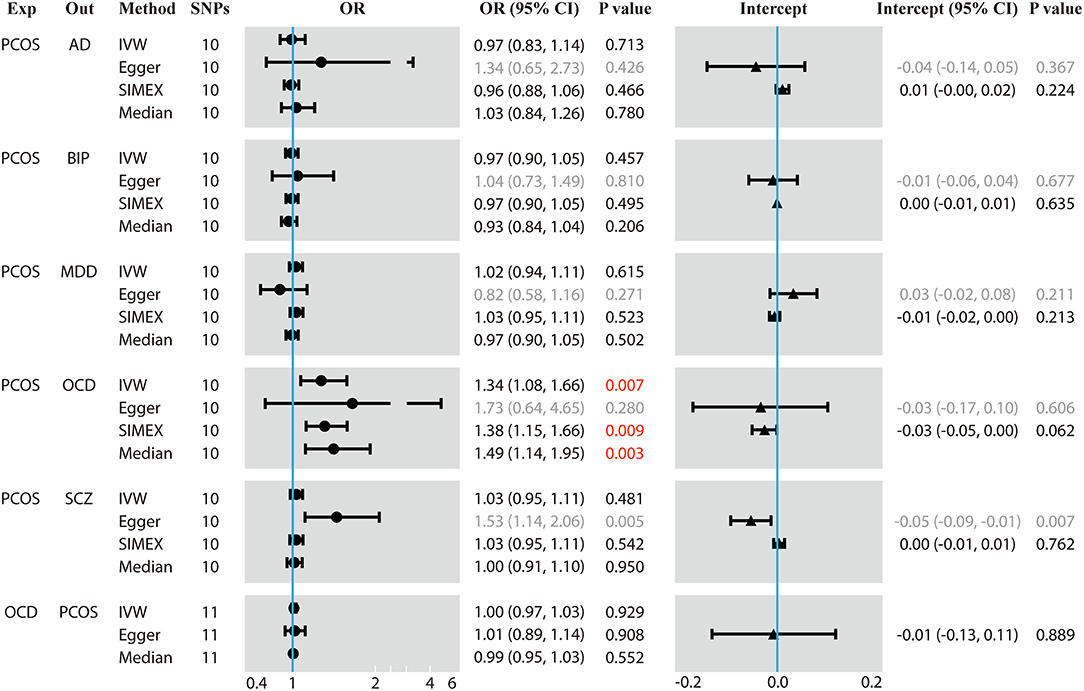
Figure 1. Results of forward and reverse Mendelian randomization (MR) analyses. Two-sample MR analysis showing the effect of the exposure on the outcome using single-nucleotide polymorphisms (SNPs) significant at p < 5 × 10−8. Exp, exposure; Out, outcome; SNPs, number of the SNPs used in MR analysis; OR, odds ratio; CI, confidence interval; PCOS, polycystic ovary syndrome; AD, anxiety disorder; BIP, bipolar disorder; MDD, major depression disorder; OCD, obsessive compulsive disorder; SCZ, schizophrenia. The regression dilution I2 statistics between PCOS exposure and each of the five psychiatric disorders were <90%, which made the results of the Egger method inaccurate. Therefore, simulation extrapolation (SIMEX) correction was applied. OR values are binary, while the intercept values are continuous. Values in red mean that they are statistically significant.
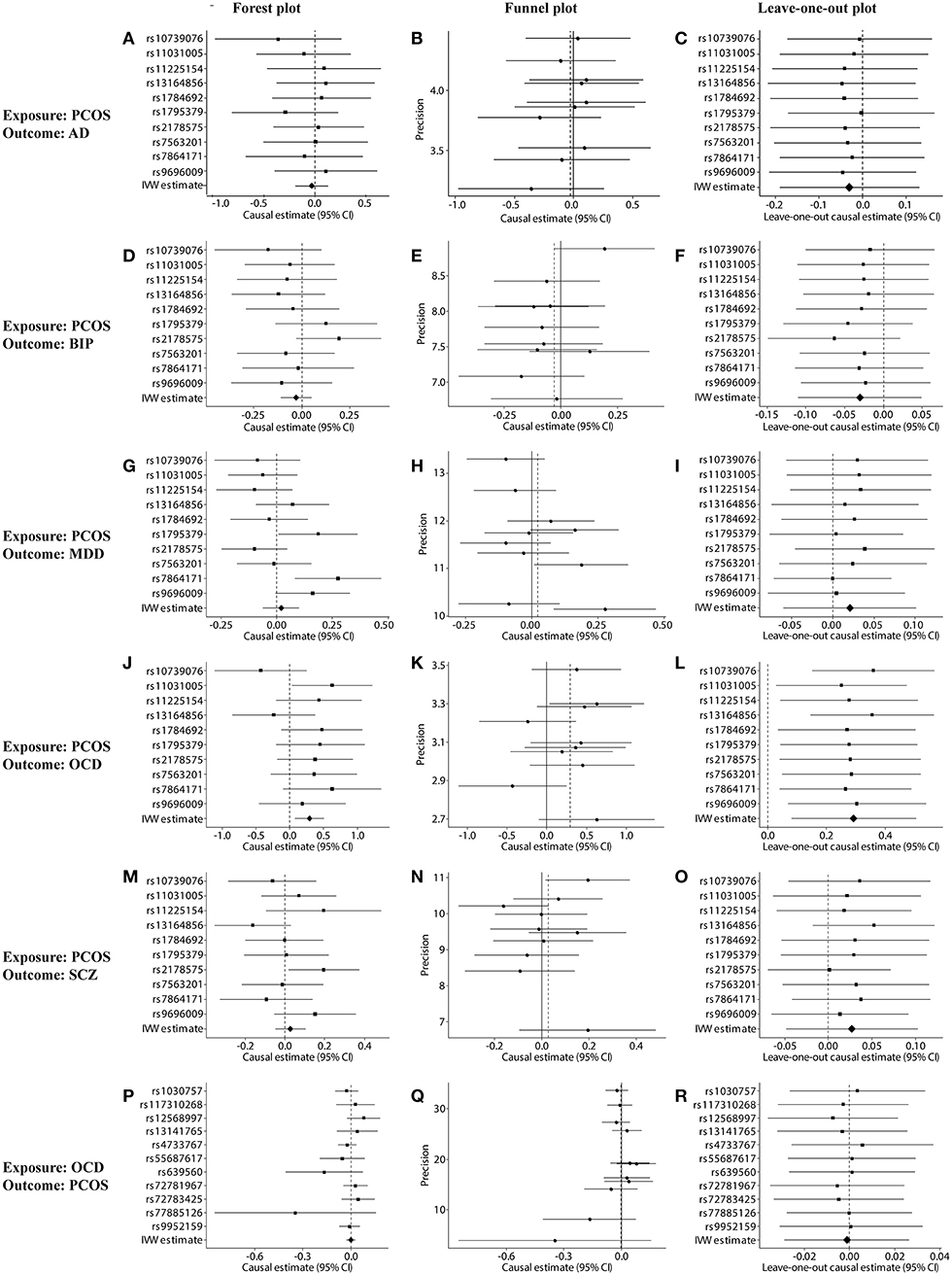
Figure 2. Sensitivity tests of forward and reverse Mendelian randomization (MR) analyses. (A–O) Forest, funnel, and leave-one-out plots for MR analysis between PCOS (exposure) and one of the following outcomes: (A–C) AD, (D–F) BIP, (G–I) MDD, (J–L) OCD, or (M–O) SCZ. (P–R) Forest, funnel, and leave-one-out plots for MR analysis between OCD (exposure) and PCOS (outcome). Plots were generated based on the effect size of the SNP (beta) and its standard error (se). PCOS, polycystic ovary syndrome; AD, anxiety disorder; BIP, bipolar disorder; MDD, major depression disorder; OCD, obsessive compulsive disorder; SCZ, schizophrenia.
Genetically predicted PCOS was positively associated with OCD according to models based on random-effect IVW (OR 1.339, 95% CI 1.083–1.657, p = 0.007), SIMEX (OR 1.382, 95% CI 1.149–1.662, p = 0.009) and WM (OR 1.493, 95% CI 1.145–1.946, p = 0.003). The causal estimates from the three methods were consistent in direction and magnitude, which is unlikely to be a coincidence. In addition, no significant heterogeneity was detected by Cochran's Q statistic in the IVW analysis (3.2554, p = 0.3308) or MR-Egger analysis (2.4418, p = 0.2708), and no significant horizontal pleiotropy was detected based on the SIMEX intercept (β = −0.027, p = 0.062). The funnel plot showed symmetric distribution, further validating the absence of heterogeneity. The leave-one-out plot showed that excluding any single SNP from the IVs did not substantially alter the results. This suggests that the association between PCOS and OCD was not driven by any single SNP and is therefore likely to be reliable.
In contrast, genetically predicted PCOS was not related to AD, BIP, MDD or SCZ. The results from all three types of MR analyses were consistent, no significant heterogeneity or horizontal pleiotropy was observed, and no single SNP strongly drove the overall effect. Hence, these negative associations are likely to be reliable.
To eliminate the bias of reverse causation, the causal effect from OCD to PCOS was assessed (Figures 1, 2). No SNPs were found to be associated with OCD with genome-wide significance (p < 5 × 10−8), so we relaxed the threshold to p < 5 × 10−6, leading to a set of 15 independent SNPs as IVs (Table 4). The SNPs rs1163477, rs1256899, rs5634380, and rs909701 were discarded during data harmonization because they were found to be palindromic SNPs with intermediate allele frequencies. The I2 of the MR-Egger method was 98.8% (>90%), so the results were not corrected using SIMEX.
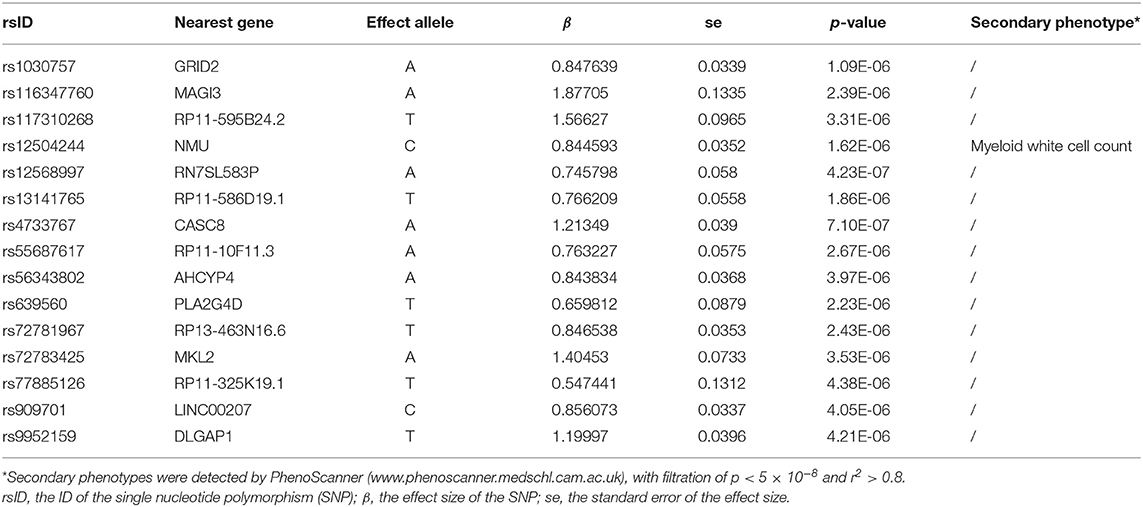
Table 4. The 15 single-nucleotide polymorphisms (SNPs) used as instrumental variables of obsessive compulsive disorder (OCD).
Genetically predicted OCD showed no association with PCOS based on models involving random-effect IVW (OR 0.999, 95% CI 0.971–1.026, p = 0.929), MR-Egger (OR 1.007, 95% CI 0.890–1.139, p = 0.908) or WM (OR 0.989, 95% CI 0.953–1.026, p = 0.552). We found no evidence of significant heterogeneity or horizontal pleiotropy that might bias the results, and no single SNP strongly drove the overall effect. We conclude that the relationship between PCOS and OCD is not likely to be biased by reverse causation.
In this study, summary GWAS data from European populations were used to investigate the potential causality between PCOS and five common psychiatric disorders. In our analysis, genetically predicted PCOS increased the risk for OCD, but it was not related to AD, BIP, MDD, and SCZ.
Several factors lead us to consider our results as likely to be reliable. First, participant overlap, which would increase false positives, was rather low, ranging from 0 to 5.8028%. The SNPs from PCOS-IVs were strongly associated with PCOS, reflected in F statistic values >30 (Zhu et al., 2020), much higher than the cut-off of >10 for a strong association (Pierce et al., 2011). This should help reduce bias. In fact, using the metric of Burgess et al. (2016), in which the overlap rate is divided by the F statistic, we estimate that the relative bias in the present study ranged from 0 to 0.1934%, since our maximal overlap rate was 5.8028% and the minimal F statistic was 30. This implies minimal bias in our MR results. Second, our IVs satisfied all three assumptions needed for MR analysis (Emdin et al., 2017): all IVs were closely associated with the exposure (“relevance assumption”), they were not associated with any other risk factors (“independence assumption”), and the IVs affected the outcome exclusively through exposure (“exclusion restriction assumption”). Third, the three types of MR analysis gave consistent causal estimates, further implying reliability. Nevertheless, due to the complexity of PCOS and psychiatric disorders, further investigations are still needed to confirm our results.
Our results showed that the genetically predicted PCOS positively associated with OCD, whereas the genetically predicted OCD was irrelevant to PCOS, confirming that the genetic causal direction was from PCOS to OCD. Consistent with our results, a previous study found that women with PCOS were more susceptible to OCD (OR 1.37, 95% CI 1.22–1.55) and their OCD symptoms were more severe (Brutocao et al., 2018). Similarly, a cross-sectional study of an Australian population showed increased prevalence of OCD among women with PCOS (adjusted OR 1.8, 95% CI 1.2–2.5) (Tay et al., 2020). A study of an Indian population found OCD prevalence of 6.36% among women with PCOS and of 2.5% among controls (Hussain et al., 2015).
Although PCOS regarded as an ovarian disease, the aberrant brain circuits concerning hyperactive gonadotropin-releasing hormone (GnRH), highlights the neuroendocrinal role in PCOS etiology (Moore and Campbell, 2017; Ruddenklau and Campbell, 2019). The GnRH neurons, located dispersedly in the rostral forebrain, receive inputs from upstream afferent neurons and regulate the downstream pulsatile release of luteinizing hormone (LH) in anterior pituitary. The gamma-Aminobutyric acid (GABA) and kisspeptin/neurokinin B/dynorphin (KNDy) neurons in the arcuate nucleus are two important afferent neurons (Ozgen and Yildiz, 2021). In prenatally androgenized (PNA) PCOS-like mice, the GABA neuron axon inputs and GABAergic post-synaptic currents to GnRH neurons were increased, causing hyperactive GnRH impulses (Moore et al., 2015; Coutinho and Kauffman, 2019). The kisspeptin and neurokinin B (NKB) signaling have been found to increase GnRH release (Terasawa et al., 2018; Coutinho and Kauffman, 2019). In PNA PCOS-like rats, the number of kisspeptin- and NKB-positive cells, as well as the expression of Kiss1 (gene for kisspeptin) and Tac2 (gene for NKB), were increased in arcuate nucleus (Yan et al., 2014; Osuka et al., 2017), which possibly increased GnRH-neuron activity.
How PCOS may contribute to OCD is unknown. In fact, little is known about how OCD occurs. It is a chronic, debilitating psychiatric illness, characterized by obsessions (intrusive and disturbing thoughts), compulsions (repetitive behaviors), or both. OCD appears to involve the cortico-striato-thalamo-cortical (CSTC) circuit, as well as the fronto-limbic, fronto-parietal, and cerebellar networks (Stein et al., 2019; Bellia et al., 2021). Within the CSTC circuit, defects in neurotransmission involving serotonin, catecholamine/dopamine and glutamate appear to contribute to OCD (Stein et al., 2019). Indeed, several genetic polymorphisms have been linked to OCD involving serotonin signaling (in the genes SLC6A4, HTR2A, HTR2C, HTR1B, TPH1, TPH2), catecholamine/dopamine system (COMT, SLC6A3, MAOA, DRD2, DRD3, DRD4), and glutamate system (SLC1A1, GRIN2B, GRIK2, GRIK3) (Bellia et al., 2021).
Some SNPs among the PCOS-IVs in the present study play roles in neurotransmitter systems. For instance, rs2178575 lies in an intron of the gene encoding Erb-B2 receptor tyrosine kinase 4 (ERBB4), which is expressed in GABAergic interneurons but also in midbrain dopaminergic neurons, where it regulates dopamine levels by binding to neuregulin 1 (NRG1) (Skirzewski et al., 2020). Mice lacking ErbB4 show regional imbalances in basal dopamine levels and fail to increase dopamine release in response to local NRG1 infusion in the dorsal hippocampus, medial pre-frontal cortex and dorsal striatum (Skirzewski et al., 2018). The SNP rs11225154 also lies in an intron of the gene encoding Yes1-associated transcriptional regulator (YAP1), which promotes survival of dopaminergic neurons by reducing the synthesis of the apoptotic protein PTEN (Zhang et al., 2017). The SNP rs10739076 lies in an intron upstream of plasminogen receptor with a C-terminal lysine (PLGRKT). This protein is expressed on the surface of catecholaminergic cells, where it stimulates plasminogen activation and regulates catecholamine release (Bai et al., 2011). Future studies should explore whether and how these PCOS-SNPs may contribute to the pathophysiology of OCD.
In addition, future work should explore how our results may help explain the potential association of OCD with certain epigenetic changes, including in DNA methylation and miRNA profiles (Kandemir et al., 2015; Privitera et al., 2015; Nissen et al., 2016; Yue et al., 2016). The PCOS SNP rs1784692 in the present study lies in an intron of the protein “zinc finger and BTB domain containing 16” (ZBTB16), which can alter histone modifications and DNA methylation (Puszyk et al., 2013). Studies should examine whether and how ZBTB16 participates in OCD.
Our results may identify PCOS-related genes unique to OCD, given that we found a significant association of PCOS with OCD, but not with four other psychiatric disorders – even though all five disorders show considerable genetic overlap (6,786,993 SNPs) (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019). For example, OCD shows genetic correlation (rg) of 0.22 with BIP, 0.21 with MDD, and 0.35 with SCZ (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2019). One study estimated that about 37% of genes are unique to OCD, and that such genes are enriched in neuroactive ligand-receptor interactions and signaling pathways involving G protein-coupled receptors (O'Connell et al., 2018). Among our PCOS-IVs, rs11031005 lies in an intergenic region of the gene encoding subunit beta of follicle-stimulating hormone, whose receptor is a classical G protein-coupled receptor expressed in neurons. Knocking down this receptor induces anxiety- or depression-like behaviors in mice (Bi et al., 2020). Future studies should explore the roles of follicle-stimulating hormone and its receptor in OCD.
Our results suggest that the positive associations of PCOS with AD (OR 2.75, 95% CI 2.10–3.60), BIP (OR 1.78, 95% CI 1.43–2.23), MDD (OR 2.79, 95% CI 2.23–3.50), and SCZ (OR 1.36, 95% CI 1.12–1.70) reported in observational studies (Cesta et al., 2016; Brutocao et al., 2018) may not reflect causality. One possible explanation is that residual confounders, rather than PCOS itself, increase the risk of psychiatric disorders. These confounders include high testosterone level, obesity (high body mass index), insulin resistance, and inflammation. Prenatal exposure to dihydrotestosterone can induce anxiety- and autism-like behaviors in rats (Domonkos et al., 2018; Xiang et al., 2020). Testosterone is elevated in the cerebrospinal fluid of schizophrenia patients (Misiak et al., 2018), and the androgen receptor is up-regulated in patients with bipolar disorder (Owens et al., 2019). Obesity increases risk of anxiety and depression in children and adolescents (Lindberg et al., 2020), and higher body mass index increases risk of attention-deficit/hyperactivity disorder (Martins-Silva et al., 2019). Insulin resistance has been positively associated with BIP, MDD, SCZ, and cognitive defects (Kullmann et al., 2016; Agarwal et al., 2020; Cuperfain et al., 2020; Zou et al., 2020), reflecting roles of insulin in energy metabolism and dopamine release in the central nervous system, contributing to memory and learning. Several inflammation-related factors have been recognized as biomarkers of psychiatric disorders (Yuan et al., 2019). Co-occurrence of OCD with other lifetime psychiatric disorders, which occurs in as many as 90% of patients with OCD (Stein et al., 2019), may also contribute to confounding. Stress can be another confounder: for example, stress due to such PCOS symptoms as hirsutism, acne, and infertility may make patients manifest anxiety- or depression-like symptoms.
Our findings should be interpreted with caution in light of several limitations. First, our IVs were derived from the largest European PCOS GWAS, which applied diverse diagnostic criteria: 51.4% of patients were diagnosed based on ad hoc, study-specific criteria, while 34.0% were diagnosed based on the Rotterdam criteria and 14.6% based on US National Institutes of Health criteria (Day et al., 2018; Zhu et al., 2020). This would increase the clinical heterogeneity of the patients. Second, there were relatively small numbers of OCD patients (2,088) and controls (7,037) in our sample. Third, all exposure and outcome data came from European populations, which may make our findings less generalizable to other ethnic groups or geographic areas.
Despite these limitations, our analysis suggests that, based solely on genetic factors, PCOS is a potentially causal factor for OCD, but not AD, BIP, MDD, or SCZ, in European populations. Appropriate screening for OCD, based on questionnaires or interviews, may be useful for European women with PCOS, which might enable early interventions involving a multidisciplinary approach (ZareMobini et al., 2018).
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LJ designed the study. LJ, JY, YC, and HP acquired and analyzed the data. LJ and JY drafted the manuscript. JS and HH obtained funding and revised the manuscript. All authors reviewed, contributed to, and approved the final manuscript.
This study was financed by the National Key Research and Development Program of China (2018YFC1004402, 2017YFC1001001), the National Natural Science Foundation of China (82003496), and the Fundamental Research Funds for the Central Universities (2021FZZX002-10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the Psychiatric Genomics Consortium for opening up access to their datasets. We thank Wei Zhao and Jiabao Sun from Zhejiang University for their help in R code to get rsIDs of SNPs by their chromosome and position information.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.689897/full#supplementary-material
Agarwal, S. M., Caravaggio, F., Costa-Dookhan, K. A., Castellani, L., Kowalchuk, C., Asgariroozbehani, R., et al. (2020). Brain insulin action in schizophrenia: something borrowed and something new. Neuropharmacology 163:107633. doi: 10.1016/j.neuropharm.2019.05.010
Azziz, R., Marin, C., Hoq, L., Badamgarav, E., and Song, P. (2005). Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J. Clin. Endocrinol. Metab. 90, 4650–4658. doi: 10.1210/jc.2005-0628
Bai, H., Baik, N., Kiosses, W. B., Krajewski, S., Miles, L. A., and Parmer, R. J. (2011). The novel plasminogen receptor, plasminogen receptor(KT) (Plg-R(KT)), regulates catecholamine release. J. Biol. Chem. 286, 33125–33133. doi: 10.1074/jbc.M111.218693
Barry, J. A., Azizia, M. M., and Hardiman, P. J. (2014). Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 20, 748–758. doi: 10.1093/humupd/dmu012
Bellia, F., Vismara, M., Annunzi, E., Cifani, C., Benatti, B., Dell'Osso, B., et al. (2021). Genetic and epigenetic architecture of obsessive-compulsive disorder: in search of possible diagnostic and prognostic biomarkers. J. Psychiatr. Res. 137, 554–571. doi: 10.1016/j.jpsychires.2020.10.040
Bi, W. K., Luan, S. S., Wang, J., Wu, S. S., Jin, X. C., Fu, Y. L., et al. (2020). FSH signaling is involved in affective disorders. Biochem. Biophys. Res. Commun. 525, 915–920. doi: 10.1016/j.bbrc.2020.03.039
Blay, S. L., Aguiar, J. V., and Passos, I. C. (2016). Polycystic ovary syndrome and mental disorders: a systematic review and exploratory meta-analysis. Neuropsychiatr. Dis. Treat. 12, 2895–2903. doi: 10.2147/NDT.S91700
Bowden, J., Del, G. M. F., Minelli, C., Davey, S. G., Sheehan, N. A., and Thompson, J. R. (2016). Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45, 1961–1974. doi: 10.1093/ije/dyw220
Brutocao, C., Zaiem, F., Alsawas, M., Morrow, A. S., Murad, M. H., and Javed, A. (2018). Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine 62, 318–325. doi: 10.1007/s12020-018-1692-3
Burgess, S., Davies, N. M., and Thompson, S. G. (2016). Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 40, 597–608. doi: 10.1002/gepi.21998
Cesta, C. E., Mansson, M., Palm, C., Lichtenstein, P., Iliadou, A. N., and Landen, M. (2016). Polycystic ovary syndrome and psychiatric disorders: co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrino. 73, 196–203. doi: 10.1016/j.psyneuen.2016.08.005
Coutinho, E. A., and Kauffman, A. S. (2019). The role of the brain in the pathogenesis and physiology of polycystic ovary syndrome (PCOS). Med Sci (Basel). 7:84. doi: 10.3390/medsci7080084
Cross-Disorder Group of the Psychiatric Genomics Consortium (2019). Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482. doi: 10.1016/j.cell.2019.11.020
Cuperfain, A. B., Kennedy, J. L., and Goncalves, V. F. (2020). Overlapping mechanisms linking insulin resistance with cognition and neuroprogression in bipolar disorder. Neurosci. Biobehav. Rev. 111, 125–134. doi: 10.1016/j.neubiorev.2020.01.022
Davies, N. M., Holmes, M. V., and Davey, S. G. (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601. doi: 10.1136/bmj.k601
Day, F., Karaderi, T., Jones, M. R., Meun, C., He, C., Drong, A., et al. (2018). Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 14:e1007813. doi: 10.1371/journal.pgen.1007813
Domonkos, E., Hodosy, J., Ostatnikova, D., and Celec, P. (2018). On the role of testosterone in Anxiety-Like behavior across life in experimental rodents. Front. Endocrinol. 9:441. doi: 10.3389/fendo.2018.00441
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian randomization. JAMA 318, 1925–1926. doi: 10.1001/jama.2017.17219
Harris, H. R., Cushing-Haugen, K. L., Webb, P. M., Nagle, C. M., Jordan, S. J., Risch, H. A., et al. (2019). Association between genetically predicted polycystic ovary syndrome and ovarian cancer: a Mendelian randomization study. Int. J. Epidemiol. 48, 822–830. doi: 10.1093/ije/dyz113
Hung, J. H., Hu, L. Y., Tsai, S. J., Yang, A. C., Huang, M. W., Chen, P. M., et al. (2014). Risk of psychiatric disorders following polycystic ovary syndrome: a nationwide population-based cohort study. PLoS ONE 9:e97041. doi: 10.1371/journal.pone.0097041
Hussain, A., Chandel, R. K., Ganie, M. A., Dar, M. A., Rather, Y. H., Wani, Z. A., et al. (2015). Prevalence of psychiatric disorders in patients with a diagnosis of polycystic ovary syndrome in kashmir. Indian J. Psychol. Med. 37, 66–70. doi: 10.4103/0253-7176.150822
Kandemir, H., Erdal, M. E., Selek, S., Izci, A. O., Karababa, I. F., Ay, M. E., et al. (2015). Microribonucleic acid dysregulations in children and adolescents with obsessive-compulsive disorder. Neuropsychiatr. Dis. Treat. 11, 1695–1701. doi: 10.2147/NDT.S81884
Kullmann, S., Heni, M., Hallschmid, M., Fritsche, A., Preissl, H., and Haring, H. U. (2016). Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 96, 1169–1209. doi: 10.1152/physrev.00032.2015
Lindberg, L., Hagman, E., Danielsson, P., Marcus, C., and Persson, M. (2020). Anxiety and depression in children and adolescents with obesity: a nationwide study in Sweden. BMC Med. 18:30. doi: 10.1186/s12916-020-1498-z
Martins-Silva, T., Vaz, J., Hutz, M. H., Salatino-Oliveira, A., Genro, J. P., Hartwig, F. P., et al. (2019). Assessing causality in the association between attention-deficit/hyperactivity disorder and obesity: a Mendelian randomization study. Int. J. Obes. 43, 2500–2508. doi: 10.1038/s41366-019-0346-8
Misiak, B., Frydecka, D., Loska, O., Moustafa, A. A., Samochowiec, J., Kasznia, J., et al. (2018). Testosterone, DHEA and DHEA-S in patients with schizophrenia: a systematic review and meta-analysis. Psychoneuroendocrino. 89, 92–102. doi: 10.1016/j.psyneuen.2018.01.007
Moore, A. M., and Campbell, R. E. (2017). Polycystic ovary syndrome: understanding the role of the brain. Front. Neuroendocrinol. 46:2. doi: 10.1016/j.yfrne.2017.05.002
Moore, A. M., Prescott, M., Marshall, C. J., Yip, S. H., and Campbell, R. E. (2015). Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome. Proc. Natl. Acad. Sci. U. S. A. 112, 596–601. doi: 10.1073/pnas.1415038112
Nissen, J. B., Hansen, C. S., Starnawska, A., Mattheisen, M., Borglum, A. D., Buttenschon, H. N., et al. (2016). DNA methylation at the neonatal state and at the time of diagnosis: preliminary support for an association with the estrogen receptor 1, Gamma-Aminobutyric acid b receptor 1, and myelin oligodendrocyte glycoprotein in female adolescent patients with OCD. Front. Psychiatry 7:35. doi: 10.3389/fpsyt.2016.00035
O'Connell, K. S., McGregor, N. W., Lochner, C., Emsley, R., and Warnich, L. (2018). The genetic architecture of schizophrenia, bipolar disorder, obsessive-compulsive disorder and autism spectrum disorder. Mol. Cell. Neurosci. 88, 300–307. doi: 10.1016/j.mcn.2018.02.010
Osuka, S., Iwase, A., Nakahara, T., Kondo, M., Saito, A., Bayasula, et al. (2017). Kisspeptin in the hypothalamus of 2 rat models of polycystic ovary syndrome. Endocrinology 158, 367–377. doi: 10.1210/en.2016-1333
Owens, S. J., Purves-Tyson, T. D., Webster, M. J., and Shannon, W. C. (2019). Evidence for enhanced androgen action in the prefrontal cortex of people with bipolar disorder but not schizophrenia or major depressive disorder. Psychiatry Res. 280:112503. doi: 10.1016/j.psychres.2019.112503
Ozgen, S. B., and Yildiz, B. O. (2021). Polycystic ovary syndrome and brain: an update on structural and functional studies. J. Clin. Endocrinol. Metab. 106, e430–e441. doi: 10.1210/clinem/dgaa843
Pierce, B. L., Ahsan, H., and Vanderweele, T. J. (2011). Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 40, 740–752. doi: 10.1093/ije/dyq151
Privitera, A. P., Distefano, R., Wefer, H. A., Ferro, A., Pulvirenti, A., and Giugno, R. (2015). OCDB: a database collecting genes, miRNAs and drugs for obsessive-compulsive disorder. Database 2015:v69. doi: 10.1093/database/bav069
Puszyk, W., Down, T., Grimwade, D., Chomienne, C., Oakey, R. J., Solomon, E., et al. (2013). The epigenetic regulator PLZF represses L1 retrotransposition in germ and progenitor cells. EMBO J. 32, 1941–1952. doi: 10.1038/emboj.2013.118
Rosenfield, R. L., and Ehrmann, D. A. (2016). The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520. doi: 10.1210/er.2015-1104
Ruddenklau, A., and Campbell, R. E. (2019). Neuroendocrine impairments of polycystic ovary syndrome. Endocrinology 160, 2230–2242. doi: 10.1210/en.2019-00428
Sekula, P., Del, G. M. F., Pattaro, C., and Kottgen, A. (2016). Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27, 3253–3265. doi: 10.1681/ASN.2016010098
Skirzewski, M., Cronin, M. E., Murphy, R., Fobbs, W., Kravitz, A. V., and Buonanno, A. (2020). ErbB4 null mice display altered mesocorticolimbic and nigrostriatal dopamine levels as well as deficits in cognitive and motivational behaviors. eNeuro 7:ENEURO.0395-19.2020. doi: 10.1523/ENEURO.0395-19.2020
Skirzewski, M., Karavanova, I., Shamir, A., Erben, L., Garcia-Olivares, J., Shin, J. H., et al. (2018). ErbB4 signaling in dopaminergic axonal projections increases extracellular dopamine levels and regulates spatial/working memory behaviors. Mol. Psychiatry 23, 2227–2237. doi: 10.1038/mp.2017.132
Stein, D. J., Costa, D., Lochner, C., Miguel, E. C., Reddy, Y., Shavitt, R. G., et al. (2019). Obsessive-compulsive disorder. Nat. Rev. Dis. Primers 5:52. doi: 10.1038/s41572-019-0102-3
Tay, C. T., Teede, H. J., Loxton, D., Kulkarni, J., and Joham, A. E. (2020). Psychiatric comorbidities and adverse childhood experiences in women with self-reported polycystic ovary syndrome: an Australian population-based study. Psychoneuroendocrino. 116:104678. doi: 10.1016/j.psyneuen.2020.104678
Terasawa, E., Garcia, J. P., Seminara, S. B., and Keen, K. L. (2018). Role of kisspeptin and neurokinin b in puberty in female Non-Human primates. Front. Endocrinol. 9:148. doi: 10.3389/fendo.2018.00148
Wen, Y., Wu, X., Peng, H., Li, C., Jiang, Y., Su, Z., et al. (2020). Breast cancer risk in patients with polycystic ovary syndrome: a Mendelian randomization analysis. Breast Cancer Res. Treat. 185, 799–806. doi: 10.1007/s10549-020-05973-z
Xiang, D., Lu, J., Wei, C., Cai, X., Wang, Y., Liang, Y., et al. (2020). Berberine ameliorates prenatal dihydrotestosterone Exposure-Induced Autism-Like behavior by suppression of androgen receptor. Front. Cell. Neurosci. 14:87. doi: 10.3389/fncel.2020.00087
Yan, X., Yuan, C., Zhao, N., Cui, Y., and Liu, J. (2014). Prenatal androgen excess enhances stimulation of the GNRH pulse in pubertal female rats. J. Endocrinol. 222, 73–85. doi: 10.1530/JOE-14-0021
Yuan, N., Chen, Y., Xia, Y., Dai, J., and Liu, C. (2019). Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 9:233. doi: 10.1038/s41398-019-0570-y
Yue, W., Cheng, W., Liu, Z., Tang, Y., Lu, T., Zhang, D., et al. (2016). Genome-wide DNA methylation analysis in obsessive-compulsive disorder patients. Sci. Rep. 6:31333. doi: 10.1038/srep31333
ZareMobini, F., Kazemi, A., and Farajzadegan, Z. (2018). A comprehensive mental health care program for women with polycystic ovary syndrome: protocol for a mixed methods study. Reprod. Health. 15:46. doi: 10.1186/s12978-018-0488-5
Zhang, D., Yang, S., Toledo, E. M., Gyllborg, D., Salto, C., Carlos, V. J., et al. (2017). Niche-derived laminin-511 promotes midbrain dopaminergic neuron survival and differentiation through YAP. Sci. Signal. 10:eaal4165. doi: 10.1126/scisignal.aal4165
Zhu, T., Cui, J., and Goodarzi, M. O. (2020). Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, and stroke. Diabetes 70, 627–637. doi: 10.2337/db20-0800
Keywords: Mendelian randomization, polycystic ovary syndrome, psychiatric disorders, causality, single nucleotide polymorphism
Citation: Jin L, Yu J, Chen Y, Pang H, Sheng J and Huang H (2021) Polycystic Ovary Syndrome and Risk of Five Common Psychiatric Disorders Among European Women: A Two-Sample Mendelian Randomization Study. Front. Genet. 12:689897. doi: 10.3389/fgene.2021.689897
Received: 01 April 2021; Accepted: 25 May 2021;
Published: 15 June 2021.
Edited by:
Jijun Tang, University of South Carolina, United StatesReviewed by:
Zi-Jiang Chen, Shandong University, ChinaCopyright © 2021 Jin, Yu, Chen, Pang, Sheng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hefeng Huang, aGhmNTdAemp1LmVkdS5jbg==; Jianzhong Sheng, c2hlbmdqekB6anUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.