
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Genet., 14 June 2021
Sec. Genetics of Common and Rare Diseases
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.671682
This article is part of the Research TopicGenetic Studies on Spondyloarthritis: from Disease Predictors to Therapeutic TargetsView all 10 articles
Ankylosing spondylitis (AS) is a common, highly heritable inflammatory arthritis affecting the mainly axial joints in both East Asia and Europe. To date, the pathogenesis of AS is still unknown, although we know that genetics play a vital role in it. The HLA-B27 allele is found in over 85% of AS patients. However, strong evidence suggests that other major histocompatibility complex (MHC) and non-MHC genes are also involved in the pathogenesis. In addition, current data showed that there were significant differences in both genomics and metagenomics among the different ethnic populations. The investigation of the key role of the microbiome in AS pathogenesis also highlighted the host–microbiome genetic interactions. Here, we systematically review current AS genetic research data and further compare genetic differences, especially between East Asian and European groups, which may highlight the challenge in future genetic studies.
Ankylosing spondylitis (AS) is one of the commonest rheumatic diseases in both Asia and Europe. It is a highly heritable chronic inflammatory disease that mainly affects the axial joints, but also has peripheral joints and various organs involvement (Brown et al., 1997, 2000). Pathogenesis of AS is still unknown. The worldwide distribution of AS is closely related to the carrier rate of human leukocyte antigen (HLA)-B27 in population. The prevalence of the disease is about 0.55% of Caucasian population (Braun et al., 1998) and 0.26% in Chinese (Wigley et al., 1994) but less common in Japanese and Africans, mostly attributing to the parallel carrier status of HLA-B27 alleles in these ancestry groups. While the HLA-B27 allele is found in over 85% of patients (Caffrey and James, 1973), there is strong evidence indicating that other major histocompatibility complex (MHC) and non-MHC genes also jointly play roles in the pathogenesis of the disease. Interestingly, current data showed an obvious disease-associated genetic discrepancy across different populations. The observed genetic heterogeneity across divergent populations at several risk loci is by differences in allele frequencies, linkage disequilibrium patterns, effect sizes of associated polymorphisms, or a combination of these factors (International Genetics of Ankylosing Spondylitis Consortium et al., 2013). The objective of this review is mainly to summarize the currently available genetic data and to further make comparisons of this disease genetically between East Asian and European populations.
Associations between HLA-B27 and AS were first reported in 1972 (which were some of the earliest described genetic associations), and it remains the most substantial risk factor for AS (Stokes et al., 1972). There are significant differences in the worldwide distribution of HLA-B27 and its subtypes (Khan et al., 2007; Gragert et al., 2013). The prevalence of HLA-B27 positivity in the Chinese and Korean populations has been reported to be from 4 to 8% and 2.3 to 7%, respectively (Kim and Kim, 2010; Yang et al., 2013), which is lower than that in Caucasians but much higher than that in the Japanese population (1%) (Feltkamp et al., 2001).
HLA-B27 plays a pivotal role in the pathogenesis of AS. To date, in both Europeans and Asians, the most accurate tag SNP of HLA-B27 is rs116488202 (International Genetics of Ankylosing Spondylitis Consortium et al., 2013), which is superior to previously reported tag SNP rs4349859 and rs13202464 in Asian populations (Lin et al., 2011). To date, there are 213 known alleles of HLA-B27 at the nucleotide sequence level, while at the translated protein level, there are 160 known subtypes based on one or more amino acid sequence differences (Khan, 2017). The frequencies of HLA-B27 alleles vary in different race groups (Supplementary Table 1 and Figure 1; Khan et al., 2007; Gragert et al., 2013). Like other ethnic groups, more than 80% of Chinese AS patients are HLA-B27 positive, but the primary subtype is HLA-B∗27:04, followed by HLA-B∗27:05, which is a predominate subtype for Caucasian cases (Liu et al., 2010). The distribution of HLA-B27 subtypes also reveals substantial demographic and geographic diversity in China. Although HLA-B∗27:04 is a major subtype in Chinese individuals, it has been reported that the proportion of HLA-B∗27:04 carriers in AS patients were higher in southern China than in northern China, whereas HLA-B∗27:05 positivity was the reverse (Rong and Jieruo, 2013). Being consistent with Mainland Chinese Han Cases, Taiwan Han population is also dominated by HLA-B∗27:04 (Yang et al., 2004; Liu et al., 2010). As for Korea being up north of China geographically, it makes sense that B∗27:05 is the predominant subtype in Koreans, which is similar to Caucasians but different from other Asians down south (Park et al., 2009).
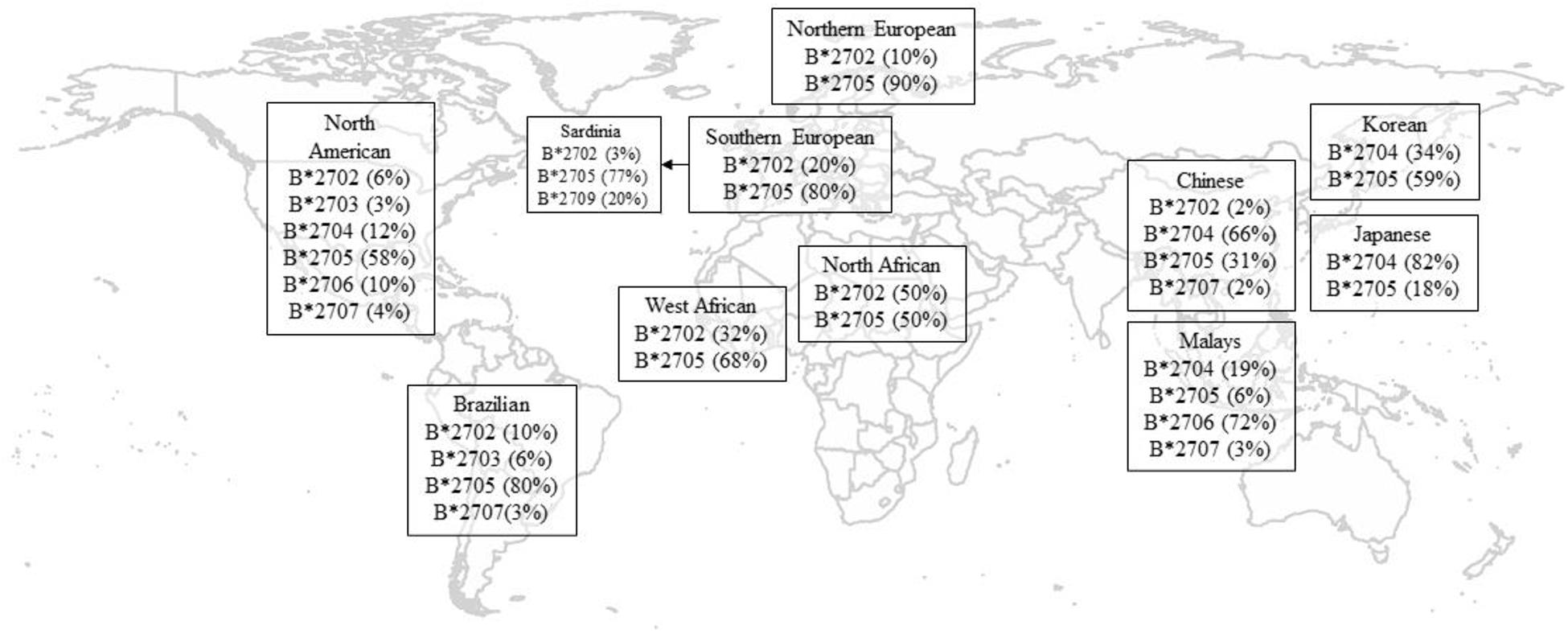
Figure 1. Geographical distribution of HLA-B27 subtypes. Only the most frequent alleles were shown, and statistics are rounded off for simplicity and indicate percentage in healthy controls. Data of North American were adapted from Gragert et al. (2013; Supplementary Table 1). Data for Korean were adapted from Park et al. (2009). Data of other populations were adapted from Khan et al. (2007).
HLA-B∗27:06 has a relatively weak and negative association with AS compared to the HLA-B∗27:04 subtype in Southeast Asia (Nasution et al., 1997; Garcia-Fernandez et al., 2001; Chen et al., 2002), which is more prevalent in Malay descendants (Lopez-Larrea et al., 1995; Gonzalez-Roces et al., 1997; Ren et al., 1997; Hou et al., 2007). In addition, B∗27:09 was found not associated with AS in Sardinia (D’Amato et al., 1995). Mathieu et al. (2008a, b) have proposed a possible evolutionary effect of genetic selection by malaria infection, which could explain the absence of risk haplotypes for AS where malaria was endemic.
Recently, a large-scale study in European case–control cohorts has been initially genotyped by Illumina Immunochip (International Genetics of Ankylosing Spondylitis Consortium et al., 2013), taking the lead in fine-mapping MHC region of HLA classic alleles, SNP, and amino-acid residues in European AS cases and controls. This study suggested the associations with HLA-B40 and multiple other class I and II alleles (Cortes et al., 2015). It also demonstrated that in that Caucasian the amino-acid sequence of HLA-B at position 97 in the B-peptide-binding pocket is the crucial determinant of HLA associations with AS. After controlling for the associated haplotypes in HLA-B, independent associations with variants in the HLA-A, HLA-DPB1, and HLA-DRB1 loci were observed.
As for East Asian populations, several loci associated with AS in the Chinese population have been identified, including HLA-B60 and MHC I chain-related gene A (MICA) (Ho and Chen, 2013; Zhou et al., 2014). A recent case–control study in Korean additionally identified the association with AS at HLA-C∗15:02 (Kim et al., 2015). In our recent study, we analyzed the associations of AS across the MHC aiming to identify potentially causal SNPs, amino acids, or haplotypes using an extended cohort of East Asian ancestry (1,637 Chinese, Taiwanese, and Korean AS cases and 1,589 ethnically matched controls). We have assessed the MHC association of AS in an expanded East Asian cohort. Imputation of the MHC region was conducted in order to assess the variants, HLA classical alleles, and amino-acid residue HLA proteins. This study suggests that the HLA-B associations (B27 and B40) are mainly driven by the amino acids at positions 70 and 97, which locate in the B-pocket of HLA-B peptide-binding groove. Except for HLA-B associations, previously reported East Asian-specific association at HLA-C∗15 had been validated. In addition, a novel association at HLA-DQB1∗04 has been identified (Wang et al., 2020). However, our study needs further validation in larger cohort and conduct with better MHC imputation reference panel of the Pan-Asian population.
As discussed above, AS is strongly associated with variants in the MHC region and HLA alleles. However, HLA-B27 and other MHC genes contribute no more than one-third of the genetic risk. It has been intensely investigated that genetic factor non-MHC variants contribute to disease susceptibility. So far, at least 36 genetic variants in non-MHC regions have been identified as associated with AS in genome-wide association study (GWAS) (Brown et al., 2016).
A large-scale multi-ethnic case–control association study performed with Illumina Immunochip microarray provided a new perspective on the similarities and differences in AS susceptibility between East Asian and Caucasians. A total of 13 loci had at least nominal levels of association in East Asians (Chinese, Koreans, Taiwanese), whereas 23 achieved genome-wide significance in white Europeans (International Genetics of Ankylosing Spondylitis Consortium et al., 2013; Ellinghaus et al., 2016; Robinson et al., 2016). Additional studies have been performed in European cohorts. An exome-wide study further identified a novel genome-wide significant association at CDKAL1, and several suggestive and secondary loci (Robinson et al., 2016). Ellinghaus et al. (2016) conducted a case–control Immunochip study of five closely associated conditions, including AS, primary sclerosing cholangitis, psoriasis, Crohn’s disease, and ulcerative colitis, in a cohort of European ancestry, delineated the genetic overlap between the conditions, and identified 17 new genome-wide significant susceptibility loci of AS. It also showed that comorbidities between AS and the other chronic inflammatory diseases were mostly attributed to genetic pleiotropy. However, the promising findings of the susceptibility and pleiotropy in Caucasians need to be validated in Asian cohorts.
On account of the limited sample size of the East Asian cohort in the Immunochip study, the power of the East Asian cohort was much inferior to that of the European cohort. However, there is hitherto more than 40% overall of the associated loci that have been validated in East Asian (Figure 2), including ERAP1, GPR35, HHAT, HLA-B, ICOSLG, IL23R, IL27, NOS2, NPEPPS, RUNX3, TBX21, TYK2, UBE2E3, UBE2L3, and ZMIZ1, and two intergenic regions (2p15 and 21q22) (Brown et al., 2016). Besides, a GWAS study in Han Chinese identified two AS associated loci (HAPLN1-EDIL3 and ANO6) (Lin et al., 2011). However, few signals of the association have been observed on these two loci in an independent Immunochip study of both East Asians and Caucasians.
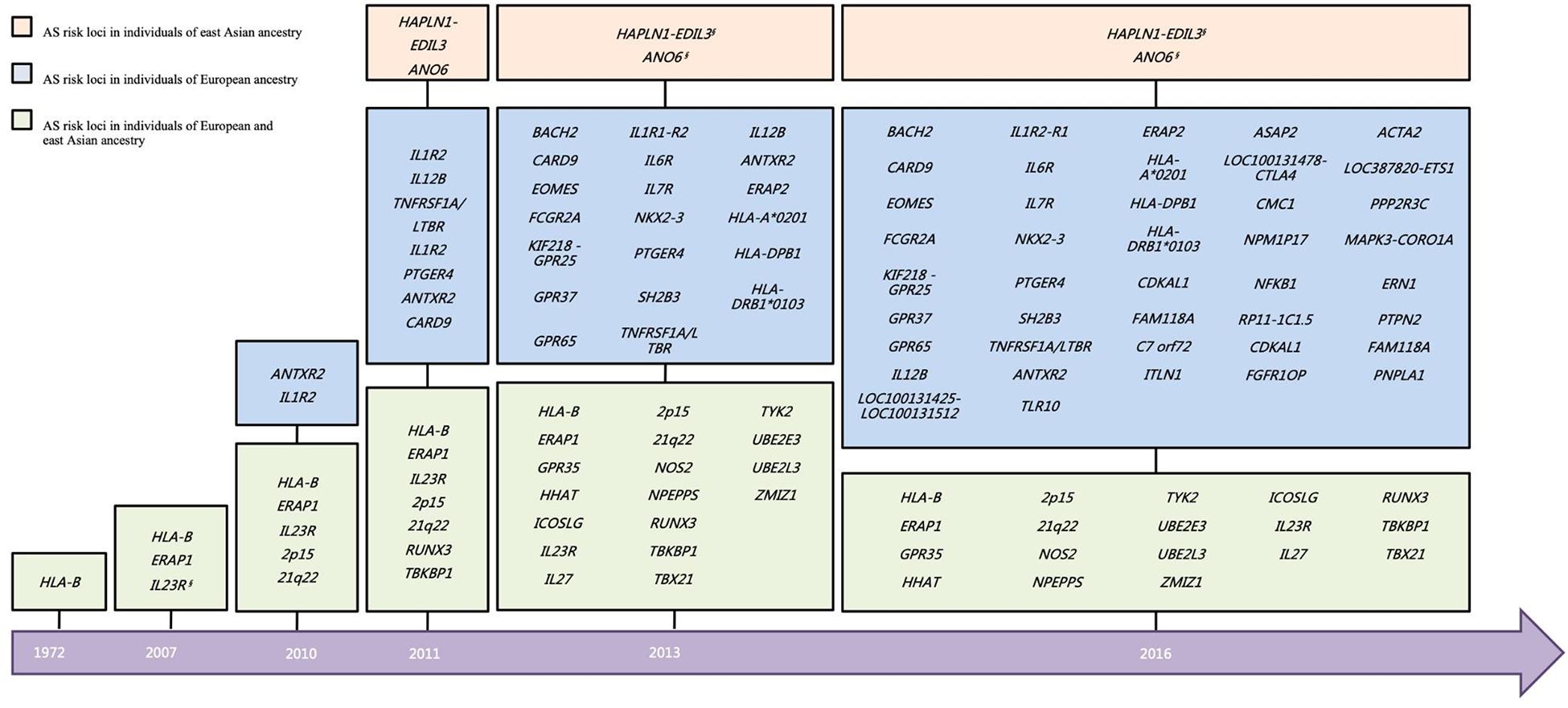
Figure 2. Historical overview of disease susceptibility polymorphisms identified in AS. Risk loci selected with a genome-wide significance threshold of P < 5 × 10–7 as well as secondary associations (P < 5 × 10–4 after conditioned for the primary associated SNP), in the previous studies are included. *But different SNP. §No independent support for the two loci in other East Asian cohort. AS, ankylosing spondylitis.
Even though most large-scale GWASs have been disproportionately investigated in cohorts of European descent and similar patterns of predisposition were observed between East Asians and Europeans, which is consistent with a shared ancestor origin of the disease-associated SNP, genetic differences exist between ethnic groups pointing to differences in ethnicity-specific etiopathogenesis. Taking the interleukin-23 receptor (IL23R) gene, for example, the primarily associated variants indicated diversity between Europe and Asia. rs11209026, a critical non-synonymous SNP in IL23R associated with AS in Caucasians, was not polymorphic in East Asians (Davidson et al., 2009). No other common SNPs on IL23R found to be significantly associated with AS in Caucasian European populations. It was suggested that IL23R might be a Caucasian-specific associated gene. However, a low-frequency visitant in IL23R, rs76418789, has been reported to potentially attenuate the protective effects of IL23R against AS in Han Chinese (Davidson et al., 2013). The same SNP was also nominally associated with AS in Europeans. On the contrary, the minor allele frequency (MAF) of rs76418789 was about 3.7% in East Asians, while only 0.34% in Europeans. In addition to the rare variants on IL23R, the SNPs on STAT3 were also found associated with AS in the Chinese population, which is a downstream molecule of IL-23R in the IL-23 signaling pathway involving the differentiation of Th17 cell populations (Davidson et al., 2011). It may indicate that the sharing effect on Th17 cells is attributed to different mechanisms of disease pathogenesis. Among the other AS-associated loci defined in Europeans but not in East Asians, only the primary associated variant rs17765610 on BACH2 presents a different frequency of over six times greater in Europeans than in East Asians (East Asians, 1.8%; Europeans, 11.8%) (International Genetics of Ankylosing Spondylitis Consortium et al., 2013). The findings might indicate that the different associations between East Asia and Europe were not attributed to the various frequencies of associated variants at most loci.
It has been drawing increasing attention that gut inflammation plays a pivotal role in the pathogenesis of AS. IBD, as a paradigm for microbiota effects on the pathogenesis of the immune-mediated disease, is strongly related and significantly overlaps with AS in genetic predisposition. HLA-B27 transgenic rats did not develop gut and joint inflammation when bred in a germ-free environment, while inflammations present when they were exposed to healthy gut bacteria (Rath et al., 1996).
Several studies have investigated how the microbiome as a key role in driving the pathogenesis of AS. Intestinal dysbacteriosis may affect the permeability of the intestinal wall, the expression of related inflammatory factors, the intestinal mucosal immune status in AS patients, and molecular mimicry of HLA-B27 (Ebringer and Ghuloom, 1986; Ciccia et al., 2009, 2014, 2015; Wright et al., 2016). Intestinal flora imbalance can also mediate host metabolism and immune function imbalance via a series of cytokines, thus participating in the pathogenesis and progress of AS.
Studies have revealed several notable differences in bacterial species and in abundance. Costello et al. (2015) have compared the terminal ileum microbial communities in AS patients with healthy controls in Australia, and Lachnospiraceae, Veillonellaceae, Ruminococcaceae, Rikenellaceae, Bacteroidaceae, Porphyromonadaceae, and Prevotellaceae were significantly enriched or decreased in abundance in AS patients. Wen et al. (2017) have reported a quantitative metagenomics study suggesting that discrete gut microbial signature is associated with the pathogenesis of AS in Chinese, suggesting consistent findings with the report of Costello, such as Prevotellaceae. It also showed some discordance with previous reports in Europeans, like Bacteroidetes, and identified other novel biomarkers that might be involved in the development of AS in the Chinese population (Wen et al., 2017). To further investigate the roles of microbiome in AS pathogenesis, Yin et al. (2019) conducted a case–control metagenomic analysis of 250 Han Chinese. In addition to confirmation of previously reported gut dysbiosis and species differences in AS, the results also indicate that treatment with TNF inhibitor (TNFi) normalizes the gut microbiome. The AS gut microbiome is enriched for bacterial peptides that have previously been shown to be presented by HLA-B27, and that this enrichment is also normalized by TNFi treatment. Bacterial peptides presented by HLA-B27 have been found enriched in gut microbiome in AS patients, which is also normalized by TNFi treatment. Relative to untreated patients, TNFi therapy of AS patients was also associated with a reduction of potentially arthritogenic bacterial peptides, which are enriched bacterial peptides homologous to HLA-B27-presented epitopes. Host-bacteria genetic interactions were also observed between an AS-associated SNP (RUNX3) and microbiome, highlighting a non-MHC host genotype influencing AS via the microbiome potentially (Yin et al., 2019).
Over the last few decades, Asia, especially China, has experienced rapid urbanization, resulting in massive changes of dietary habits, which directly links to the changes of the microbiome (Yang et al., 2016). Gut microbiota is both an important therapeutic target for AS and an essential biomarker for disease surveillance. The reconstruction of gut microbiota has a potential therapeutic effect on AS patients, and the relationship between intestinal flora and AS deserves further study.
To date, clinical practice data show that the average diagnostic delay of AS is 6–10 years and early treatment, such as anti-TNFα biological agents, have been proved to improve disease outcomes. So, the early prediction of AS is challenging, causing noteworthy. However, the genome-wide significant associated SNPs only represent a trivial fraction of total heritability. A polygenic risk score (PRS) is aiming to use genotype data from thousands of genetic variants to quantify an individual’s genetic risk for specific diseases and has potential as a diagnostic and screening test for common heritable diseases, including AS (Torkamani et al., 2018). In our recent research (Li et al., 2021), we used GWAS data from 15,083 AS cases and 20,902 controls and then developed and validated PRS in European-descent and East Asian ethnicity subjects. PRS showed greater discriminatory capacity and accuracy than HLA-B27 testing, MRI scanning, or CRP testing, and could be used to assist in diagnosing AS among chronic back pain patients, as well as screening populations to identify subjects at increased risk of the disease. When the PRS was derived and tested in individuals of primarily their ancestries, the area under the curves (AUC) of the European and East Asian GRSs were 0.92 and 0.95, respectively. The discriminant capacities were attenuated cross-ethnic validations (AUC = 0.79 for European model in the East Asian cohorts, AUC = 0.88 for East Asian model in the European cohorts). It suggests that the performance of the PRS does vary between ethnic groups. The PRS developed specifically for the East Asian population performed considerably better in that population than did the European PRS, elucidating the different genetic landscapes between East Asian and European.
The similarities and differences in the genetic features of AS between East Asia and other ethnic populations have demonstrated the utility of gene mapping in probing the genetic diversity among different ancestry groups. It is of great importance to confirm the associated loci in populations of different ancestries, which is a crucial indicator of the overall significance of defining the true disease-causing variant. The identification of genetic signatures in both the East Asian and European populations will provide additional details for unraveling the genetic basis of AS and other autoimmune diseases.
PRS will be of clinical use, particularly for a disease of low prevalence and high heritability, like AS. Given the low cost of microarray, even next-generation sequencing, PRS makes it possible for population screening. Modified PRS models of ethnic specificity and multi-omics, including epigenomics and metagenomics, need more comprehensive training cohorts and more accurate evidence for better disease prediction of AS. To date, most genetics studies have been undertaken in European ancestry. Therefore, further studies of multi-omics analyses, microbiota, and environmental factors will require undertaking or expanding trans-ethnic cohorts.
XW, GW, LZ, and HX made substantial contributions to draft and revise the manuscript.
This work was supported by grants from the National Natural Science Foundation of China (31770988 to XW and 31821003 to HX), the Innovative Clinical Research Project of Changzheng Hospital (2020YCXYJ-QN04 to XW), the China Ministry of Science and Technology (2018AAA0100302 to HX), the Shanghai Municipal Key Clinical Specialty Fund (shslczdzk02602 to HX), and Shanghai Science and Technology Development Funds (2020-SH-XY-2).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.671682/full#supplementary-material
Braun, J., Bollow, M., Remlinger, G., Eggens, U., Rudwaleit, M., Distler, A., et al. (1998). Prevalence of spondylarthropathies in HLA-B27 positive and negative blood donors. Arthritis Rheum. 41, 58–67.
Brown, M. A., Kenna, T., and Wordsworth, B. P. (2016). Genetics of ankylosing spondylitis–insights into pathogenesis. Nat. Rev. Rheumatol. 12, 81–91. doi: 10.1038/nrrheum.2015.133
Brown, M. A., Kennedy, L. G., MacGregor, A. J., Darke, C., Duncan, E., Shatford, J. L., et al. (1997). Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum. 40, 1823–1828.
Brown, M. A., Laval, S. H., Brophy, S., and Calin, A. (2000). Recurrence risk modelling of the genetic susceptibility to ankylosing spondylitis. Ann. Rheum. Dis. 59, 883–886.
Caffrey, M. F., and James, D. C. (1973). Human lymphocyte antigen association in ankylosing spondylitis. Nature 242:121.
Chen, I.-H., Yang, K. L., Lee, A., Huang, H. H., Lin, P. Y., and Lee, T. D. (2002). Low frequency of HLA-B*2706 in Taiwanese patients with ankylosing spondylitis. Eur. J. Immunogenet. 29, 435–438. doi: 10.1046/j.1365-2370.2002.00353.x
Ciccia, F., Accardo-Palumbo, A., Rizzo, A., Guggino, G., Raimondo, S., Giardina, A., et al. (2014). Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann. Rheum. Dis. 73, 1566–1574. doi: 10.1136/annrheumdis-2012-202925
Ciccia, F., Bombardieri, M., Principato, A., Giardina, A., Tripodo, C., Porcasi, R., et al. (2009). Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 60, 955–965. doi: 10.1002/art.24389
Ciccia, F., Guggino, G., Rizzo, A., Saieva, L., Peralta, S., Giardina, A., et al. (2015). Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 74, 1739–1747. doi: 10.1136/annrheumdis-2014-206323
Cortes, A., Pulit, S. L., Leo, P. J., Pointon, J. J., Robinson, P. C., Weisman, M. H., et al. (2015). Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat. Commun. 6:7146. doi: 10.1038/ncomms8146
Costello, M. E., Ciccia, F., Willner, D., Warrington, N., Robinson, P. C., Gardiner, B., et al. (2015). Brief report: intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 67, 686–691. doi: 10.1002/art.38967
D’Amato, M., Fiorillo, M. T., Carcassi, C., Mathieu, A., Zuccarelli, A., Bitti, P. P., et al. (1995). Relevance of residue 116 of HLA-B27 in determining susceptibility to ankylosing spondylitis. Eur. J. Immunol. 25, 3199–3201. doi: 10.1002/eji.1830251133
Davidson, S. I., Jiang, L., Cortes, A., Wu, X., Glazov, E. A., Donskoi, M., et al. (2013). Brief report: high-throughput sequencing of IL23R reveals a low-frequency, nonsynonymous single-nucleotide polymorphism that is associated with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 65, 1747–1752. doi: 10.1002/art.37976
Davidson, S. I., Liu, Y., Danoy, P. A., Wu, X., Thomas, G. P., Jiang, L., et al. (2011). Association of STAT3 and TNFRSF1A with ankylosing spondylitis in Han Chinese. Ann. Rheum. Dis. 70, 289–292. doi: 10.1136/ard.2010.133322
Davidson, S. I., Wu, X., Liu, Y., Wei, M., Danoy, P. A., Thomas, G., et al. (2009). Association of ERAP1, but not IL23R, with ankylosing spondylitis in a Han Chinese population. Arthritis Rheum. 60, 3263–3268. doi: 10.1002/art.24933
Ebringer, A., and Ghuloom, M. (1986). Ankylosing spondylitis, HLA-B27, and klebsiella: cross reactivity and antibody studies. Ann. Rheum. Dis. 45, 703–704.
Ellinghaus, D., Jostins, L., Spain, S. L., Cortes, A., Bethune, J., Han, B., et al. (2016). Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 48, 510–518. doi: 10.1038/ng.3528
Feltkamp, T. E., Mardjuadi, A., Huang, F., and Chou, C. T. (2001). Spondyloarthropathies in eastern Asia. Curr. Opin. Rheumatol. 13, 285–290.
Garcia-Fernandez, S., Gonzalez, S., Mina Blanco, A., Martinez-Borra, J., Blanco-Gelaz, M., Lopez-Vazquez, A., et al. (2001). New insights regarding HLA-B27 diversity in the Asian population. Tissue Antigens 58, 259–262. doi: 10.1034/j.1399-0039.2001.580407.x
Gonzalez-Roces, S., Alvarez, M. V., Gonzalez, S., Dieye, A., Makni, H., Woodfield, D. G., et al. (1997). HLA-B27 polymorphism and worldwide susceptibility to ankylosing spondylitis. Tissue Antigens 49, 116–123.
Gragert, L., Madbouly, A., Freeman, J., and Maiers, M. (2013). Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Hum. Immunol. 74, 1313–1320. doi: 10.1016/j.humimm.2013.06.025
Ho, H. H., and Chen, J. Y. (2013). Ankylosing spondylitis: chinese perspective, clinical phenotypes, and associated extra-articular systemic features. Curr. Rheumatol. Rep. 15:344. doi: 10.1007/s11926-013-0344-0
Hou, T. Y., Chen, H. C., Chen, C. H., Chang, D. M., Liu, F. C., and Lai, J. H. (2007). Usefulness of human leucocyte antigen-B27 subtypes in predicting ankylosing spondylitis: taiwan experience. Intern. Med. J. 37, 749–752. doi: 10.1111/j.1445-5994.2007.01450.x
International Genetics of Ankylosing Spondylitis Consortium, Cortes, A., Hadler, J., Pointon, J. P., Robinson, P. C., Karaderi, T., et al. (2013). Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730–738. doi: 10.1038/ng.2667
Khan, M. A. (2017). An update on the genetic polymorphism of HLA-B∗27 With 213 alleles encompassing 160 subtypes (and still counting). Curr. Rheumatol. Rep. 19:9. doi: 10.1007/s11926-017-0640-1
Khan, M. A., Mathieu, A., Sorrentino, R., and Akkoc, N. (2007). The pathogenetic role of HLA-B27 and its subtypes. Autoimmun. Rev. 6, 183–189. doi: 10.1016/j.autrev.2006.11.003
Kim, K., Bang, S. Y., Lee, S., Lee, H. S., Shim, S. C., Kang, Y. M., et al. (2015). An HLA-C amino-acid variant in addition to HLA-B∗27 confers risk for ankylosing spondylitis in the Korean population. Arthritis Res. Ther. 17:342. doi: 10.1186/s13075-015-0855-3
Kim, T. J., and Kim, T. H. (2010). Clinical spectrum of ankylosing spondylitis in Korea. Joint Bone Spine 77, 235–240. doi: 10.1016/j.jbspin.2009.11.015
Li, Z., Wu, X., Leo, P. J., de Guzman, E., Akkoc, N., Breban, M., et al. (2021). Polygenic risk scores have high diagnostic capacity in ankylosing spondylitis. Ann. Rheum. Dis. doi: 10.1136/annrheumdis-2020-219446 [Epub ahead of print].
Lin, Z., Bei, J. X., Shen, M., Li, Q., Liao, Z., Zhang, Y., et al. (2011). A genome-wide association study in Han Chinese identifies new susceptibility loci for ankylosing spondylitis. Nat. Genet. 44, 73–77. doi: 10.1038/ng.1005
Liu, Y., Jiang, L., Cai, Q., Danoy, P., Barnardo, M. C., Brown, M. A., et al. (2010). Predominant association of HLA-B∗2704 with ankylosing spondylitis in Chinese Han patients. Tissue Antigens 75, 61–64. doi: 10.1111/j.1399-0039.2009.01379.x
Lopez-Larrea, C., Sujirachato, K., Mehra, N. K., Chiewsilp, P., Isarangkura, D., Kanga, U., et al. (1995). HLA-B27 subtypes in Asian patients with ankylosing spondylitis. Evidence for new associations. Tissue Antigens 45, 169–176.
Mathieu, A., Cauli, A., Fiorillo, M. T., and Sorrentino, R. (2008a). HLA-B27 and ankylosing spondylitis geographic distribution as the result of a genetic selection induced by malaria endemic? A review supporting the hypothesis. Autoimmun. Rev. 7, 398–403. doi: 10.1016/j.autrev.2008.03.013
Mathieu, A., Cauli, A., Fiorillo, M. T., and Sorrentino, R. (2008b). HLA-B27 and ankylosing spondylitis geographic distribution versus malaria endemic: casual or causal liaison? Ann. Rheum. Dis. 67, 138–140. doi: 10.1136/ard.2007.072488
Nasution, A. R., Mardjuadi, A., Kunmartini, S., Suryadhana, N. G., Setyohadi, B., Sudarsono, D., et al. (1997). HLA-B27 subtypes positively and negatively associated with spondyloarthropathy. J. Rheumatol. 24, 1111–1114.
Park, S. H., Kim, J., Kim, S. G., Kim, S. K., Chung, W. T., and Choe, J. Y. (2009). Human leucocyte antigen-B27 subtypes in Korean patients with ankylosing spondylitis: higher B∗2705 in the patient group. Int. J. Rheum. Dis. 12, 34–38. doi: 10.1111/j.1756-185X.2009.01377.x
Rath, H. C., Herfarth, H. H., Ikeda, J. S., Grenther, W. B., Hamm, T. E. Jr., Balish, E., et al. (1996). Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Invest. 98, 945–953. doi: 10.1172/JCI118878
Ren, E. C., Koh, W. H., Sim, D., Boey, M. L., Wee, G. B., and Chan, S. H. (1997). Possible protective role of HLA-B∗2706 for ankylosing spondylitis. Tissue Antigens 49, 67–69.
Robinson, P. C., Leo, P. J., Pointon, J. J., Harris, J., Cremin, K., Bradbury, L. A., et al. (2016). Exome-wide study of ankylosing spondylitis demonstrates additional shared genetic background with inflammatory bowel disease. NPJ Genom. Med. 1:16008. doi: 10.1038/npjgenmed.2016.8
Rong, J., and Jieruo, G. (2013). Spondyloarthritis in China. Curr. Opin. Rheumatol. 25, 460–467. doi: 10.1097/BOR.0b013e3283621b8c
Stokes, P. L., Asquith, P., Holmes, G. K., Mackintosh, P., and Cooke, W. T. (1972). Histocompatibility antigens associated with adult coeliac disease. Lancet 2, 162–164. doi: 10.1016/s0140-6736(72)91330-x
Torkamani, A., Wineinger, N. E., and Topol, E. J. (2018). The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 19:581. doi: 10.1038/s41576-018-0018-x
Wang, G., Kim, T. H., Li, Z., Cortes, A., Kim, K., Bang, S.-Y., et al. (2020). MHC associations of ankylosing spondylitis in East Asians are complex and involve non-HLA-B27 HLA contributions. Arthritis Res. Ther. 22:74. doi: 10.1186/s13075-020-02148-5
Wen, C., Zheng, Z., Shao, T., Liu, L., Xie, Z., Le Chatelier, E., et al. (2017). Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 18:142. doi: 10.1186/s13059-017-1271-6
Wigley, R. D., Zhang, N. Z., Zeng, Q. Y., Shi, C. S., Hu, D. W., Couchman, K., et al. (1994). Rheumatic diseases in China: ILAR-China study comparing the prevalence of rheumatic symptoms in northern and southern rural populations. J. Rheumatol. 21, 1484–1490.
Wright, P. B., McEntegart, A., McCarey, D., McInnes, I. B., Siebert, S., and Milling, S. W. (2016). Ankylosing spondylitis patients display altered dendritic cell and T cell populations that implicate pathogenic roles for the IL-23 cytokine axis and intestinal inflammation. Rheumatology (Oxford) 55, 120–132. doi: 10.1093/rheumatology/kev245
Yang, K. L., Chen, I. H., Hsiao, C. K., Cherng, J. M., Yang, K. Z., Chang, C. C., et al. (2004). Polymorphism of HLA-B27 in Taiwanese Chinese. Tissue Antigens 63, 476–479. doi: 10.1111/j.0001-2815.2004.00197.x
Yang, M., Xu, M., Pan, X., Hu, Z., Li, Q., Wei, Y., et al. (2013). Epidemiological comparison of clinical manifestations according to HLA-B∗27 carrier status of Chinese ankylosing spondylitis patients. Tissue Antigens 82, 338–343. doi: 10.1111/tan.12186
Yang, Y., Owyang, C., and Wu, G. D. (2016). East meets west: the increasing incidence of inflammatory bowel disease in asia as a paradigm for environmental effects on the pathogenesis of immune-mediated disease. Gastroenterology 151, e1–e5. doi: 10.1053/j.gastro.2016.10.034
Yin, J., Sternes, P. R., Wang, M., Morrison, M., Song, J., Li, T., et al. (2019). Shotgun metagenomics reveals an enrichment of potentially cross-reactive bacterial epitopes in ankylosing spondylitis patients, as well as the effects of TNFi therapy and the host’s genotype upon microbiome composition. bioRxiv [Preprint] doi: 10.1101/571430 bioRxiv: 571430,
Keywords: ankylosing spondylitis, genetics, polygenetic risk score, East Asia, Europe
Citation: Wu X, Wang G, Zhang L and Xu H (2021) Genetics of Ankylosing Spondylitis—Focusing on the Ethnic Difference Between East Asia and Europe. Front. Genet. 12:671682. doi: 10.3389/fgene.2021.671682
Received: 24 February 2021; Accepted: 04 May 2021;
Published: 14 June 2021.
Edited by:
Fabiana Paladini, Sapienza University of Rome, ItalyReviewed by:
Alessandro Mathieu, University of Cagliari, ItalyCopyright © 2021 Wu, Wang, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huji Xu, eHVodWppQHNtbXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.