- 1Department of Biochemistry and Cancer Institute of the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Key Laboratory of Cancer Prevention and Intervention of China National Ministry of Education, Hangzhou, China
- 3Department of Orthopedic Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Orthopedic Research Institute of Zhejiang University, Hangzhou, China
Background: Observational studies indicate that phospholipid fatty acids (FAs) have an impact on the etiology in cancers, but the results are conflicting. We aimed to investigate the causal association of phospholipid FAs with breast cancer and prostate cancer.
Methods: Fourteen single nucleotide polymorphisms (SNPs) were selected as instrumental variables to predict the level of 10 phospholipid FAs from Genome-wide association studies (GWAS). We obtained the summary statistics for the latest and largest GWAS datasets for breast cancer (113,789 controls and 133,384 cases) and prostate cancer (61,106 controls and 79,148 cases) from the Breast Cancer Association Consortium (BCAC) and Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium. Two-sample Mendelian randomization analysis was applied.
Results: The results demonstrate that the 10 individual plasma phospholipid FAs are not significantly associated with breast cancer risk and prostate cancer risk.
Conclusion: The evidence is insufficient to support the causal association of the 10 individual plasma phospholipid FAs with breast cancer and prostate cancer.
Introduction
Fatty acids (FAs) are involved in various physiological processes, including maintaining cell membrane stability, forming raft lipid to regulate signal transduction, the process of inflammation, and even hormone synthesis (Lands, 2014; Fritsche, 2015; Calder, 2020). The two hormone-related cancers, breast cancer and prostate cancer, are the leading causes of death and the most common cancers among women and men (Bray et al., 2018; Siegel et al., 2019). As FAs are modifiable factors that strongly relate to dietary intake, whether FAs play a role in tumorigenesis has been a focus of investigation. However, overall findings from the epidemiological studies are conflicting.
Epidemiological studies suggested an inverse association of ω-3 polyunsaturated fatty acids (PUFA) (Zheng et al., 2013; Hirko et al., 2018; Yang et al., 2019), a positive association of ω-6 PUFA (Fabian et al., 2015; Kiyabu et al., 2015), and saturated fatty acids (SFA) with breast cancer risk (Brennan et al., 2015; Xia et al., 2015; Hirko et al., 2018). However, ω-3 PUFA is shown to be a risk factor for prostate cancer, whereas ω-6 PUFA and SFA are the protective factors by several observational studies (Dahm et al., 2012; Brasky et al., 2013; Crowe et al., 2014). Furthermore, several studies indicate that both the above FAs and monounsaturated fatty acids (MUFA) are not associated with breast cancer risk (Sanders, 2014; Cao et al., 2016; Zhou et al., 2016). These contradictory results are largely based on observational studies that are prone to reverse causality and residual confounding. A recent large randomized control trial (RCT, n = 25,871) with follow-up for an average of 5.3 years concluded that ω-3 PUFA supplementation did not reduce major cardiovascular events and overall cancer incidence (Manson et al., 2019), while the specific data of large RCT investigating the effects of PUFA, MUFA, and SFA on the two hormone-related cancer is limited.
To overcome the aforementioned problems, Mendelian randomization (MR) analysis is widely applied to investigate the causality between the exposure and outcome. MR analysis uses single nucleotide polymorphisms (SNPs) for predicting the level of exposure to determine its causal effects on the outcome (Emdin et al., 2017; Gala and Tomlinson, 2020). According to the Mendel’s genetic law, SNPs are randomly assigned during gamete formation. The MR approach, therefore, can be thought of as a “natural” RCT (Gala and Tomlinson, 2020). Herein, we used the MR approach to investigate the causal association of 10 individual circulating phospholipid FAs with breast cancer and prostate cancer, including α-linolenic acid (ALA), docosahexaenoic acid (DHA), oleic acid (OA), palmitic acid (PA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), linoleic, acid (LA), arachidonic acid (AA), palmitoleic acid (POA), and stearic acid (SA).
Materials and Methods
Selection of SNPs
The instrumental variables (IVs) were built on the largest genomewide association studies (GWAS) on circulating phospholipid FAs from the Cohorts for Heart and Aging Research in Genomic Epidemiology consortium, in which the participants are of European origin (Lemaitre et al., 2011; Wu et al., 2013; Guan et al., 2014). The IVs are associated with 10 individual plasma phospholipid FAs mentioned above. SNPs at the genomewide significance (P < 5 × 10–8) of the association with the phospholipid FAs were subsequently searched on the PhenoScanner V2 website to determine whether the SNPs were related to the potential confounders between plasma phospholipid FAs and the two types of cancer. Furthermore, a primary assumption in MR analysis is that the IVs influence the outcome only via the exposure, while other pathways involved between the IVs and outcome can cause a biased result. Given the above, the two SNPs with high pleiotropy are associated with body mass index and alcohol consumption were excluded (rs780093 and rs780094). To ensure the selected SNPs were independently (r2<0.005) associated with the corresponding FAs, we performed a linkage disequilibrium test on the LD-link website (population: CEU)1 (Supplementary Tables 1, 2). Finally, 14 distinct SNPs were selected as the previous studies (Yuan et al., 2019; Yuan and Larsson, 2020) to predict the serum phospholipid level of ω-3 PUFAs (n = 8,866) (Lemaitre et al., 2011), ω-6 PUFAs (n = 8,631) (Guan et al., 2014), and MUFA and SFAs (n = 8,961) (Wu et al., 2013). The characteristics of the IVs are shown in Table 1, and the brief overview of the MR study design is shown in Figure 1.
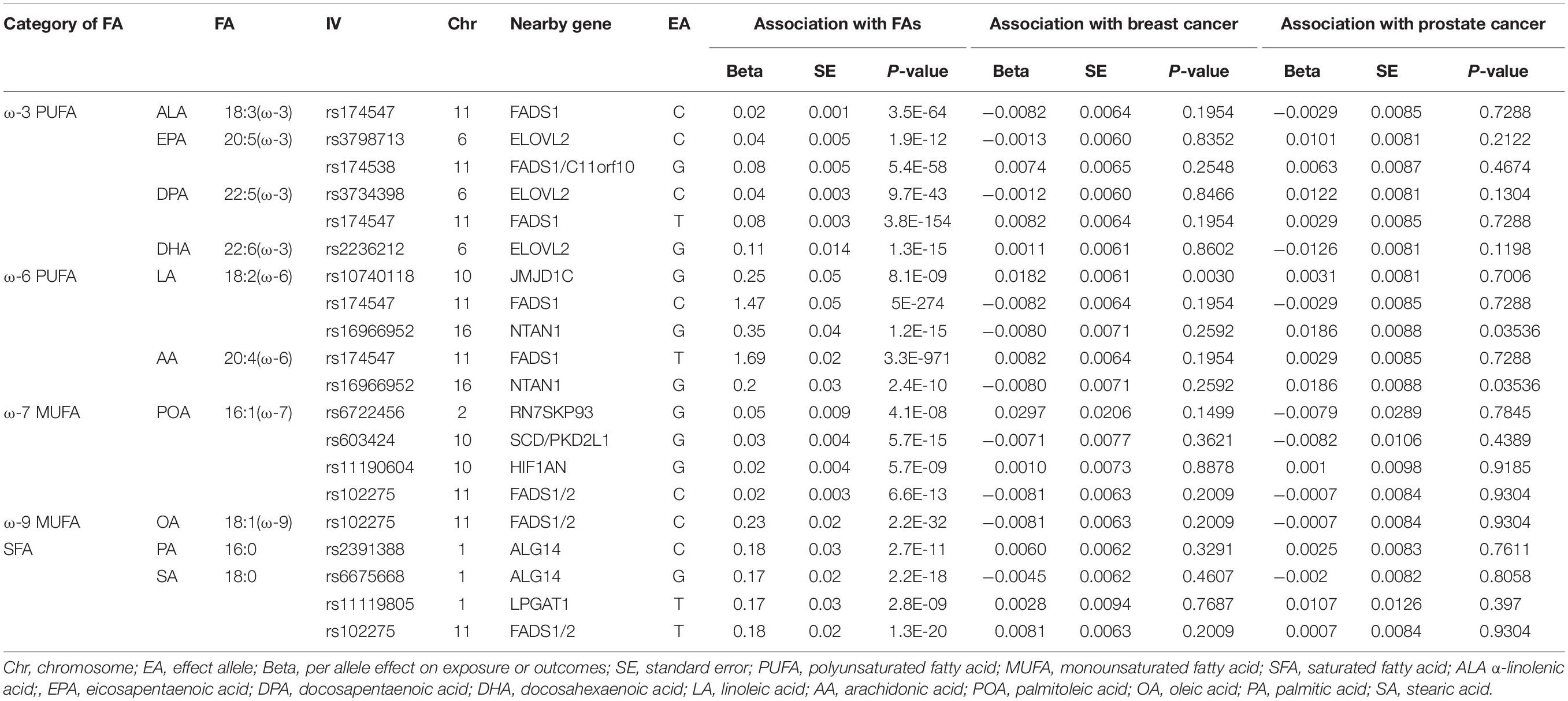
Table 1. Characteristics of the instrumental variables (IVs) associated with plasma phospholipid fatty acids (FAs) and the associations with breast cancer and prostate cancer.
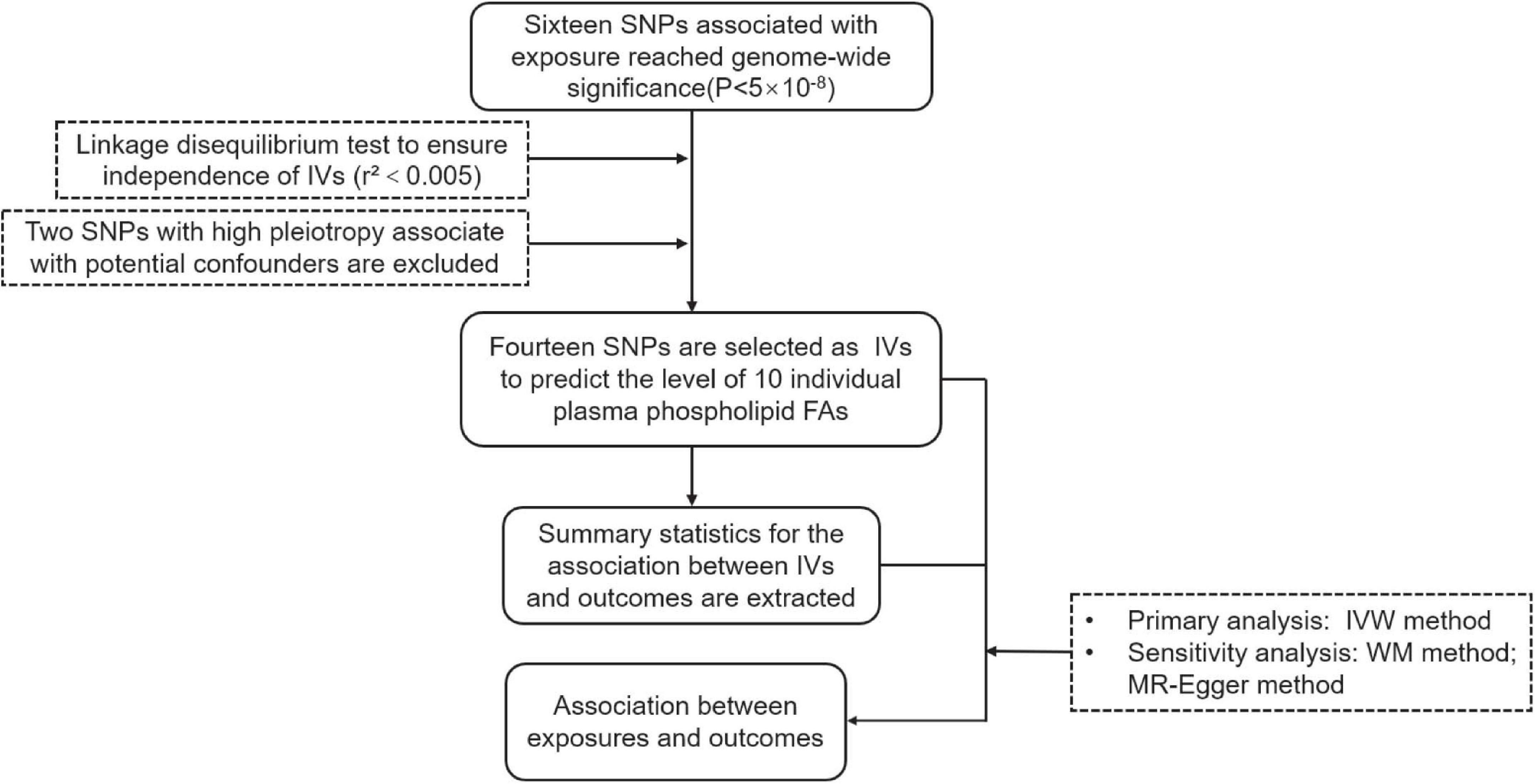
Figure 1. The flow chart of this study. IV, Instrumental variable; IVW, Inverse-variance-weighted; SNP, Single nucleotide polymorphism; WM, Weighted Median.
Genetic Association With Outcomes
Summary statistics for breast cancer were obtained from the largest and latest GWAS meta-analysis from The Breast Cancer Association Consortium comprising of 133,384 cases and 113,789 controls2 (Zhang et al., 2020). The participants were limited to women of European ancestry. The summary data for prostate cancer was derived from the hitherto largest meta-GWAS with 79,148 cases and 61,106 controls of European ancestry from Prostate Cancer Association Group to Investigate Cancer-Associated Alterations in the Genome consortium3 (Schumacher et al., 2018). The summary information of GWAS on outcomes are displayed in Supplementary Table 3. The genetic association between plasma phospholipid FA and the outcomes are shown in Table 1.
The participants have provided written consent, and all the studies contributing data to our MR analysis were approved by the relevant ethical review boards.
Statistical Analysis
The primary MR analysis of the causal association between plasma phospholipid FAs and cancers was performed by inverse-variance-weighted (IVW) approach to estimate each IV’s combined causal effects. The IVW approach evaluates the combined effect by calculating the Wald ratio of each SNPs and then using the corresponding inverse variance as weights for meta-analysis (Lee et al., 2016). There is only one SNP as IV for ALA, DHA, OA, and PA; therefore, the Wald ratio was directly used to calculate effect in the IVW analysis. By calculating the median value of the IVs’ estimates, a weighted median (WM) analysis was used in sensitivity analysis (Bowden et al., 2016). Furthermore, we test the pleiotropic effects through the MR-Egger approach, which examines whether the intercept of the association between plasma phospholipids and cancers differs from zero (Bowden et al., 2015).
All statistical analyses were conducted by R version 4.0.2 and R package “MendelianRandomization.”
Results
Plasma Phospholipid FAs and Breast Cancer
We used IVW method as primary analysis, the results of which show that the 10 individual FAs examined are not significantly associated with breast cancer: ALA (OR = 0.66, 95%CI: 0.35, 1.24), EPA (OR = 1.07, 95%CI: 0.93, 1.23), DPA (OR = 1.08, 95%CI: 0.94, 1.23), DHA (OR = 1.01, 95%CI: 0.91, 1.13), LA (OR = 1.00, 95%CI: 0.99, 1.00), AA (OR = 1.00, 95%CI:1.00, 1.01), POA (OR = 0.91, 95%CI: 0.63, 1.32), OA (OR = 0.97, 95%CI: 0.91, 1.02), PA (OR = 1.03, 95%CI: 0.97, 1.11), SA (OR = 1.01, 95%CI: 0.97, 1.06) (Figure 2).
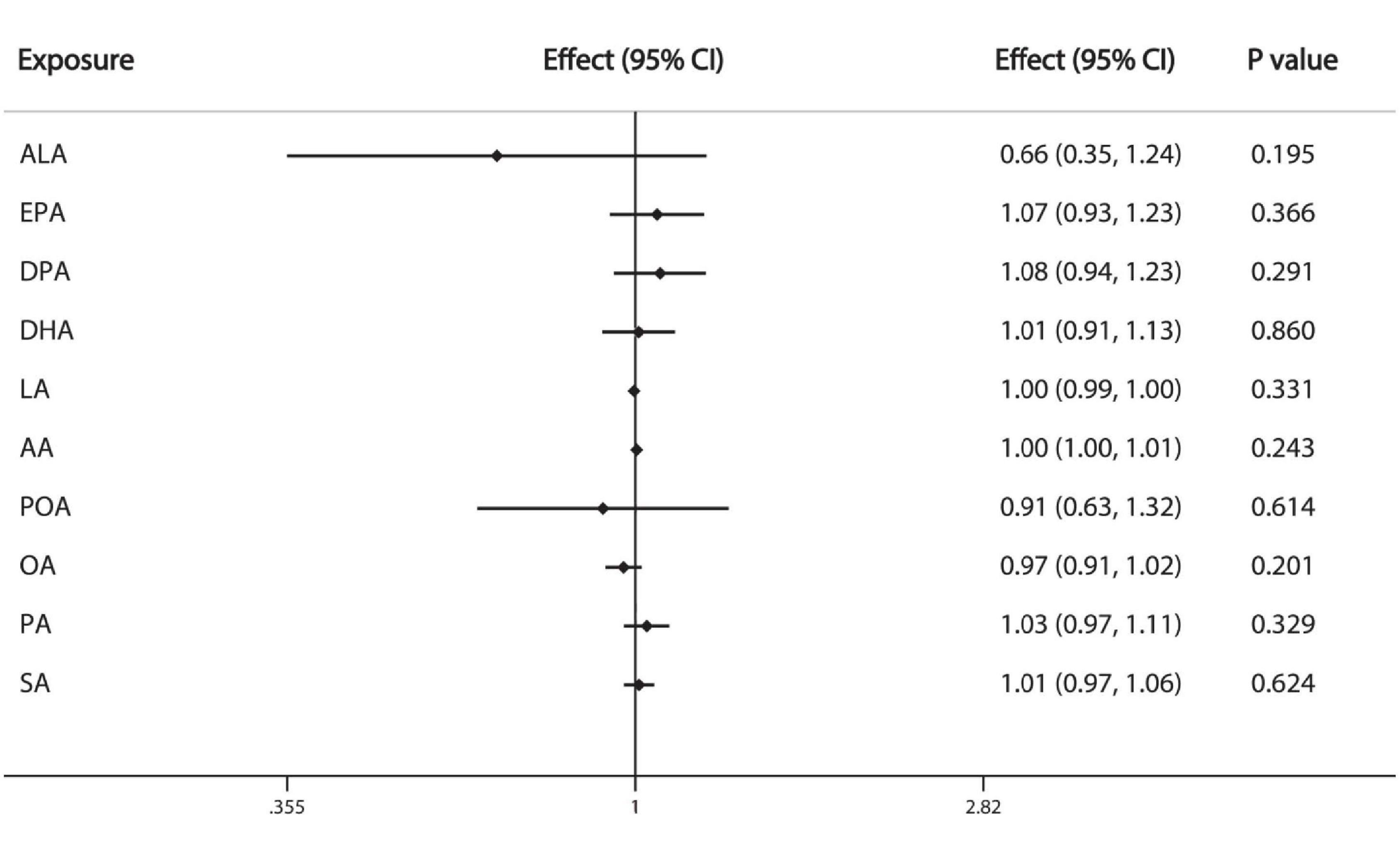
Figure 2. Causal associations between plasma phospholipid FAs and breast cancer risk. CI: confidence interval; Effect: Odds ratio.
Plasma Phospholipid FAs and Prostate Cancer
The results of IVW analysis demonstrate that the FAs are not causally associated with prostate cancer: ALA (OR = 0.87, 95%CI: 0.38, 1.99), EPA (OR = 1.12, 95%CI: 0.93, 1.36), DPA (OR = 1.10, 95%CI: 0.91, 1.32), DHA (OR = 0.89, 95%CI: 0.77, 1.03), LA (OR = 1.00, 95%CI: 0.98, 1.02), AA (OR = 1.00, 95%CI:0.98, 1.02), POA (OR = 0.88, 95%CI: 0.57, 1.35), OA (OR = 1.00, 95%CI: 0.93, 1.07), PA (OR = 1.01, 95%CI: 0.93, 1.11), SA (OR = 1.01, 95%CI: 0.95, 1.07) (Figure 3).
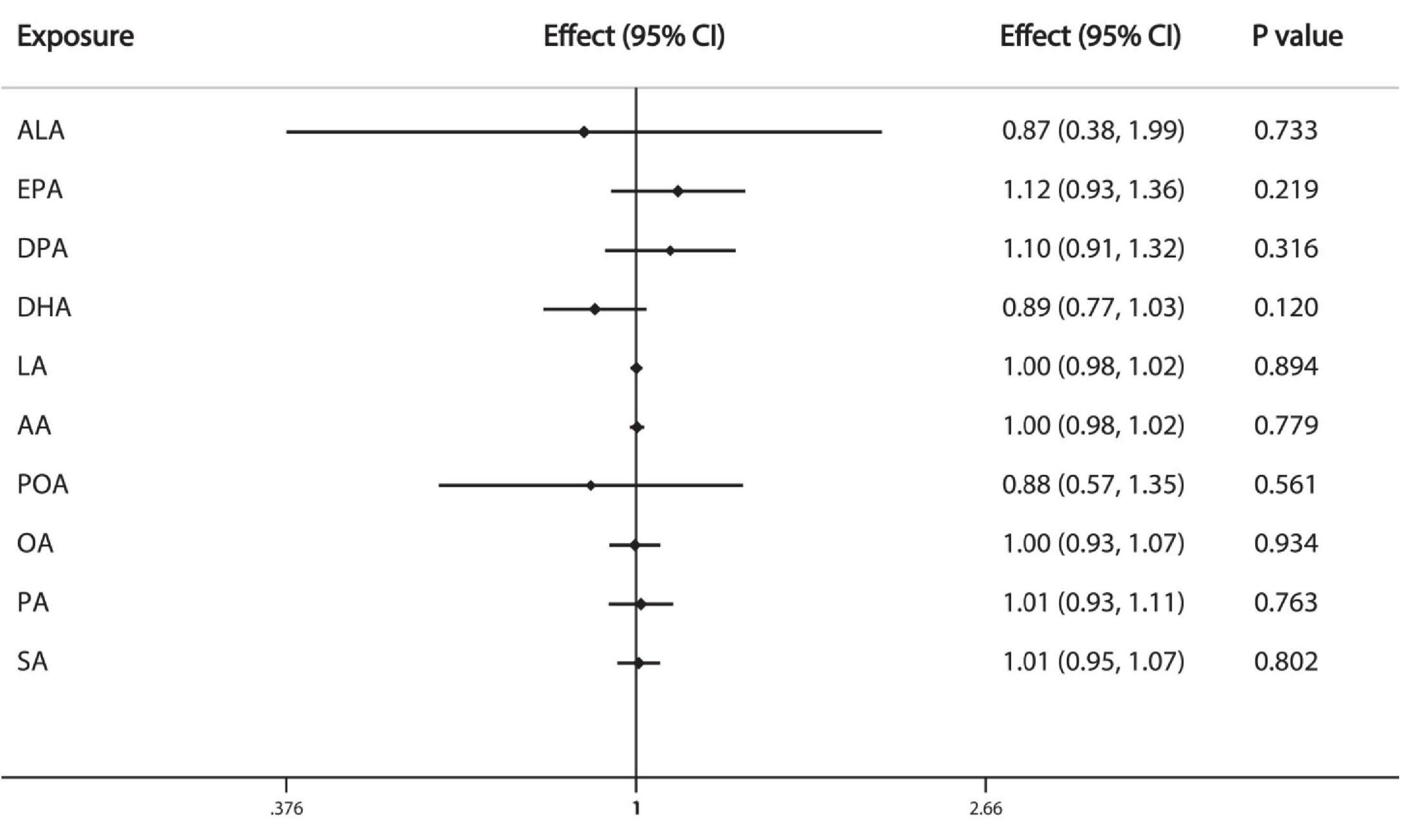
Figure 3. Causal associations between plasma phospholipid FAs and prostate cancer risk. CI: confidence interval; Effect: Odds ratio.
Sensitivity Analysis
WM and MR-Egger analysis could not be performed to test the pleiotropy of EPA, DPA, DHA, LA, AA, OA, and PA because the IVs were less than three SNPs. The sensitivity analysis was conducted only to LA, POA, and SA; the estimated effects of IVW analyses were similar to the results calculated by WM, MR-Egger, and MR-Egger intercept methods (Supplementary Tables 4, 5). The intercept values of the MR-Egger analysis did not significantly differ from zero. The sensitivity analysis suggested that the pleiotropy did not bias our results.
Discussion
We used the MR approach to investigate the causal association of the 10 individual plasma phospholipid FAs with breast cancer and prostate cancer, including MUFA, SFA, ω-3, and ω-6 PUFA. Our MR analysis indicates the 10 individual FAs we examined are not significantly associated with the breast cancer risk and prostate cancer risk.
Among the different types of FAs, ω-3 PUFA is the most controversial and has been considered as a protective factor for breast cancer (Zheng et al., 2013; Fabian et al., 2015; Hirko et al., 2018; Yang et al., 2019). Higher dietary intake of ω-3 PUFA has been shown to negatively associated with the breast cancer risk in meta-analyses (Zheng et al., 2013; Yang et al., 2019). Given the complexity of digestion, absorption and conversion of the dietary FAs, the level of circulating FAs and dietary intake of FAs are not completely equal. Circulating FAs constitute the endogenous exposure of bioavailable FA to individuals (Jenab et al., 2009). In a large case-control study (Ncase = Ncontrol = 2,982) nested within the European Prospective Investigation into Cancer and Nutrition study, the researchers found no significant association between plasma phospholipid ω-3 PUFA between breast cancer risk (Chajes et al., 2017). Another nested case-control (Ncase = Ncontrol = 794) study in Nurses’ Health Study II demonstrated that erythrocyte membrane FAs, which reflected dietary intake for several months were not associated with breast cancer overall, while the subgroup analysis suggested a protective effect of DPA and EPA but not DHA among overweight women (Hirko et al., 2018). A more recently published meta-analysis indicates circulating ω-3 PUFA is significant associated with a lower breast cancer risk (Yang et al., 2019). However, the large RCT in New England of Medicine in 2019, Manson et al. reported that ω-3 PUFA supplementation (460 mg of EPA and 380 mg of DHA per day) did not reduce the overall cancer risk (HR:1.03, 95%CI:0.93-1.13), as well as the breast cancer risk (HR:0.90, 95%CI:0.70-1.16) and prostate cancer risk (HR:1.15, 95%CI:0.94-1.39) (Manson et al., 2019). Our MR analysis also demonstrates that ω-3 PUFA is not causally associated with breast cancer and prostate cancer (Figures 2, 3).
Whether FAs play a role in prostate carcinogenesis is similarly controversial. Meta-analyses have attempted to clarify the association between FAs and prostate cancer risk; a meta-analysis demonstrates that dietary intake of ALA reduces the risk (Fu et al., 2015), while several meta-analyses indicate that dietary intake of PUFA or SFA is not associated with prostate cancer incidence (Szymanski et al., 2010; Alexander et al., 2015; Aucoin et al., 2017). Given that the interpretation on the results of meta-analyses was complicated by interstudy heterogeneity, Wu et al. recently reported the data from a 24-year prospective cohort study (n = 47,885), which suggests that higher intake of ALA is not associated with the lethal prostate cancer risk after the introduction of PSA screening (Wu et al., 2018). However, the findings of studies on circulating FAs are contrary to the expected anti-inflammatory effect of ω-3 PUFA. Observational studies suggest that total ω-3 PUFA or individual ω-3 PUFA (i.e., ALA, DPA, EPA, DHA) are risk factors for prostate cancer (Crowe et al., 2008, 2014; Simon et al., 2009; Brasky et al., 2011, 2013; Dahm et al., 2012; Fu et al., 2015; Huang et al., 2019), whereas stearic acid (Crowe et al., 2008, 2014) and ω-6 PUFA (Brasky et al., 2013) are marginally inversely associated with prostate cancer. Relatively recent meta-analysis results do not support an association between serum ω-3 PUFA with prostate cancer risk (Alexander et al., 2015). Our MR analysis results demonstrate that plasma phospholipid FAs, including ω-3 and ω-6 PUFA, SFA, and MUFA, may not have a role in prostate cancer etiology.
Researchers have applied the MR approach to investigate the causal association between plasma phospholipid FAs and several diseases, including atrial fibrillation (Yuan and Larsson, 2019), orthopedic diseases (Yuan et al., 2020), cardiovascular diseases (Yuan et al., 2019), and type 2 diabetes (Yuan and Larsson, 2020). To our knowledge, we first used MR analysis to address this highly controversial issue, which avoids the reverse causality and confounders that result in bias in observational studies. Previous cohort studies assessing the association of FAs with tumorigenesis primarily used dietary questionnaires to estimate specific FA intake or directly detected the level of FAs through a single collection of blood samples several years ago. Instead, we used GWAS datasets with a large sample size and measured the genetic predisposing level of FAs by SNPs that reflects a long-term bioavailable exposure, which enabled a relatively reliable result. However, a major limitation of our study is that some IVs (rs174547, rs16966952, rs102275) are related to more than one individual plasma phospholipid FAs, which leads to a lower probability to unravel the association between specific FAs and cancers. Another limitation is that seven exposures (DPA, DHA, EPA, ALA, AA, OA, and PA) were excluded in the sensitivity analysis because the IVs associated with them is less than three, which did not reach the requirement of MR-Egger and WM analysis. Given that we performed the MR analysis using GWAS datasets of European descendants, the conclusion we made may not be applicable to other populations.
In conclusion, our MR analysis indicates that little evidence supports the causal association of the 10 plasma phospholipid FAs with breast cancer and prostate cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZY, JL, YS, YLu, and XZ conceived the study design and drafted the manuscript. ZY, JL, YS, and ZQ participated in data extraction and data analysis. YLi, LZ, QH, XJ, and MA did the data checking and analysis. All authors critically reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by the China National 973 Project (2014CB542003), the China Natural Sciences Foundation Project (81372179), and the Zhejiang Provincial SciTech Commission Project (2014C03048-2) to YLu. The breast cancer genome-wide association analyses for BCAC and CIMBA were supported by Cancer Research UK (C1287/A10118, C1287/A16563, C1287/A10710, C12292/A20861, C12292/A11174, C1281/A12014, C5047/A8384, C5047/A15007, C5047/A10692, and C8197/A16565), The National Institutes of Health (CA128978, X01HG007492-the DRIVE consortium), the PERSPECTIVE project supported by the Government of Canada through Genome Canada and the Canadian Institutes of Health Research (Grant GPH-129344) and the Ministère de l’Économie, Science et Innovation du Québec through Genome Québec and the PSRSIIRI-701 grant, the Quebec Breast Cancer Foundation, the European Community’s Seventh Framework Programme under grant agreement no. 223175 (HEALTH-F2-2009-223175) (COGS), the European Union’s Horizon 2020 Research and Innovation Programme (634935 and 633784), the Post-Cancer GWAS initiative (U19 CA148537, CA148065, and CA148112—the GAME-ON initiative), the Department of Defence (W81XWH-10-1-0341), the Canadian Institutes of Health Research (CIHR) for the CIHR Team in Familial Risks of Breast Cancer (CRN-87521), the Komen Foundation for the Cure, the Breast Cancer Research Foundation, and the Ovarian Cancer Research Fund. All studies and funders are listed in Zhang et al. (2020).
Acknowledgments
We thank Chaojie Zhang (New York University), Sisi Xiao (The Chinese University of Hong Kong), and Minyue Zhu (Wuhan University) for technical help in R code. We also thank Wei He (Department of Public Health and Preventive Medicine, Zhejiang University) for his helpful suggestions.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.664498/full#supplementary-material
Abbreviations
AA, Arachidonic acid; ALA, α -linolenic acid; CI, Confidence interval; DHA, Docosahexaenoic acid; DPA, Docosapentaenoic acid; EPA, Eicosapentaenoic acid; FA, Fatty acid; GWAS, Genome-wide association studies; IV, Instrumental variable; IVW, Inverse-variance-weighted; LA, linoleic acid; MR, Mendelian randomization; MUFA, Monounsaturated fatty acid; OR, Odds ratio; OA, Oleic acid; PA, Palmitic acid; PSA, Prostate-specific antigen; POA, Palmitoleic acid; PUFA, Polyunsaturated fatty acid; RCT, Randomized controlled trial; SA, Stearic acid; SFA, Saturated fatty acid; SNP, Single nucleotide polymorphism; WM, Weighted median.
Footnotes
- ^ https://ldlink.nci.nih.gov/
- ^ http://bcac.ccge.medschl.cam.ac.uk/
- ^ http://practical.icr.ac.uk/blog/
References
Alexander, D. D., Bassett, J. K., Weed, D. L., Barrett, E. C., Watson, H., and Harris, W. (2015). Meta-analysis of long-chain omega-3 polyunsaturated fatty acids (LComega-3PUFA) and prostate cancer. Nutr. Cancer 67, 543–554. doi: 10.1080/01635581.2015.1015745
Aucoin, M., Cooley, K., Knee, C., Fritz, H., Balneaves, L. G., Breau, R., et al. (2017). Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr. Cancer Ther. 16, 32–62. doi: 10.1177/1534735416656052
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi: 10.1002/gepi.21965
Brasky, T. M., Darke, A. K., Song, X., Tangen, C. M., Goodman, P. J., Thompson, I. M., et al. (2013). Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 105, 1132–1141.
Brasky, T. M., Till, C., White, E., Neuhouser, M. L., Song, X., Goodman, P., et al. (2011). Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am. J. Epidemiol. 173, 1429–1439. doi: 10.1093/aje/kwr027
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Brennan, S. F., Woodside, J. V., Lunny, P. M., Cardwell, C. R., and Cantwell, M. M. (2015). Dietary fat and breast cancer mortality: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 57, 1999–2008. doi: 10.1080/10408398.2012.724481
Calder, P. C. (2020). n-3 PUFA and inflammation: from membrane to nucleus and from bench to bedside. Proc. Nutr. Soc. 1–13. doi: 10.1017/S0029665120007077 [Epub ahead of print]
Cao, Y., Hou, L., and Wang, W. (2016). Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: a meta-analysis of prospective cohort studies. Int. J. Cancer 138, 1894–1904. doi: 10.1002/ijc.29938
Chajes, V., Assi, N., Biessy, C., Ferrari, P., Rinaldi, S., Slimani, N., et al. (2017). A prospective evaluation of plasma phospholipid fatty acids and breast cancer risk in the EPIC study. Ann. Oncol. 28, 2836–2842. doi: 10.1093/annonc/mdx482
Crowe, F. L., Allen, N. E., Appleby, P. N., Overvad, K., Aardestrup, I. V., Johnsen, N. F., et al. (2008). Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 88, 1353–1363.
Crowe, F. L., Appleby, P. N., Travis, R. C., Barnett, M., Brasky, T. M., Bueno-de-Mesquita, H. B., et al. (2014). Circulating fatty acids and prostate cancer risk: individual participant meta-analysis of prospective studies. J. Natl. Cancer Inst. 106
Dahm, C. C., Gorst-Rasmussen, A., Crowe, F. L., Roswall, N., Tjonneland, A., Drogan, D., et al. (2012). Fatty acid patterns and risk of prostate cancer in a case-control study nested within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 96, 1354–1361.
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian RANDOMIZATION. JAMA 318, 1925–1926.
Fabian, C. J., Kimler, B. F., and Hursting, S. D. (2015). Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 17:62.
Fu, Y. Q., Zheng, J. S., Yang, B., and Li, D. (2015). Effect of individual omega-3 fatty acids on the risk of prostate cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. J. Epidemiol. 25, 261–274. doi: 10.2188/jea.je20140120
Gala, H., and Tomlinson, I. (2020). The use of Mendelian randomisation to identify causal cancer risk factors: promise and limitations. J. Pathol. 250, 541–554. doi: 10.1002/path.5421
Guan, W., Steffen, B. T., Lemaitre, R. N., Wu, J. H. Y., Tanaka, T., Manichaikul, A., et al. (2014). Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ. Cardiovasc. Genet. 7, 321–331. doi: 10.1161/circgenetics.113.000208
Hirko, K. A., Chai, B., Spiegelman, D., Campos, H., Farvid, M. S., Hankinson, S. E., et al. (2018). Erythrocyte membrane fatty acids and breast cancer risk: a prospective analysis in the nurses’ health study II. Int. J. Cancer. 142, 1116–1129. doi: 10.1002/ijc.31133
Huang, J., Mondul, A. M., Weinstein, S. J., Derkach, A., Moore, S. C., Sampson, J. N., et al. (2019). Prospective serum metabolomic profiling of lethal prostate cancer. Int. J. Cancer 145, 3231–3243. doi: 10.1002/ijc.32218
Jenab, M., Slimani, N., Bictash, M., Ferrari, P., and Bingham, S. A. (2009). Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum. Genet. 125, 507–525. doi: 10.1007/s00439-009-0662-5
Kiyabu, G. Y., Inoue, M., Saito, E., Abe, S. K., Sawada, N., Ishihara, J., et al. (2015). Fish, n – 3 polyunsaturated fatty acids and n – 6 polyunsaturated fatty acids intake and breast cancer risk: the Japan Public Health Center-based prospective study. Int. J. Cancer 137, 2915–2926. doi: 10.1002/ijc.29672
Lands, B. (2014). Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog. Lipid Res. 55, 17–29. doi: 10.1016/j.plipres.2014.04.002
Lee, C. H., Cook, S., Lee, J. S., and Han, B. (2016). Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of Z-scores. Genomics Inform. 14, 173–180. doi: 10.5808/gi.2016.14.4.173
Lemaitre, R. N., Tanaka, T., Tang, W., Manichaikul, A., Foy, M., Kabagambe, E. K., et al. (2011). Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE consortium. PLoS Genet. 7:e1002193. doi: 10.1371/journal.pgen.1002193
Manson, J. E., Cook, N. R., Lee, I. M., Christen, W., Bassuk, S. S., Mora, S., et al. (2019). Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 380, 23–32.
Sanders, T. A. (2014). Protective effects of dietary PUFA against chronic disease: evidence from epidemiological studies and intervention trials. Proc. Nutr. Soc. 73, 73–79. doi: 10.1017/s0029665113003789
Schumacher, F. R., Al Olama, A. A., Berndt, S. I., Benlloch, S., Ahmed, M., Saunders, E. J., et al. (2018). Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 50, 928–936.
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34.
Simon, J. A., Chen, Y.-H., and Bent, S. (2009). The relation of α-linolenic acid to the risk of prostate cancer: a systematic review and meta-analysis. Am. J. Clin. Nutr. 89, 1558S–1564S.
Szymanski, K. M., Wheeler, D. C., and Mucci, L. A. (2010). Fish consumption and prostate cancer risk: a review and meta-analysis. Am. J. Clin. Nutr. 92, 1223–1233. doi: 10.3945/ajcn.2010.29530
Wu, J., Wilson, K. M., Stampfer, M. J., Willett, W. C., and Giovannucci, E. L. (2018). A 24-year prospective study of dietary alpha-linolenic acid and lethal prostate cancer. Int. J. Cancer 142, 2207–2214. doi: 10.1002/ijc.31247
Wu, J. H. Y., Lemaitre, R. N., Manichaikul, A., Guan, W., Tanaka, T., Foy, M., et al. (2013). Genome-wide association study identifies novel loci associated with concentrations of four plasma phospholipid fatty acids in the de novo lipogenesis pathway. Circ. Cardiovasc. Genet. 6, 171–183.
Xia, H., Ma, S., Wang, S., and Sun, G. (2015). Meta-analysis of saturated fatty acid intake and breast cancer risk. Medicine (Baltimore) 94:e2391. doi: 10.1097/md.0000000000002391
Yang, B., Ren, X. L., Wang, Z. Y., Wang, L., Zhao, F., Guo, X. J., et al. (2019). Biomarker of long-chain n-3 fatty acid intake and breast cancer: accumulative evidence from an updated meta-analysis of epidemiological studies. Crit. Rev. Food Sci. Nutr. 59, 3152–3164. doi: 10.1080/10408398.2018.1485133
Yuan, S., Back, M., Bruzelius, M., Mason, A. M., Burgess, S., and Larsson, S. (2019). Plasma phospholipid fatty acids, FADS1 and risk of 15 cardiovascular diseases: a mendelian randomisation study. Nutrients 11:3001. doi: 10.3390/nu11123001
Yuan, S., and Larsson, S. C. (2019). Plasma phospholipid fatty acids and risk of atrial fibrillation: a mendelian randomization study. Nutrients 11:1651. doi: 10.3390/nu11071651
Yuan, S., and Larsson, S. C. (2020). Association of genetic variants related to plasma fatty acids with type 2 diabetes mellitus and glycaemic traits: a Mendelian randomisation study. Diabetologia 63, 116–123. doi: 10.1007/s00125-019-05019-0
Yuan, S., Lemming, E. W., Michaëlsson, K., and Larsson, S. C. (2020). Plasma phospholipid fatty acids, bone mineral density and fracture risk: evidence from a Mendelian randomization study. Clin. Nutr. 39, 2180–2186. doi: 10.1016/j.clnu.2019.09.005
Zhang, H., Ahearn, T. U., Lecarpentier, J., Barnes, D., Beesley, J., Qi, G., et al. (2020). Genome-wide association study identifies 32 novel breast cancer susceptibility loci from overall and subtype-specific analyses. Nat. Genet. 52, 572–581.
Zheng, J. S., Hu, X. J., Zhao, Y. M., Yang, J., and Li, D. (2013). Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ 346:f3706. doi: 10.1136/bmj.f3706
Keywords: risk factors, Mendelian randomization, prostate cancer, breast cancer, plasma phospholipid fatty acids
Citation: Yang Z, Li J, Sun Y, Qu Z, Lin Y, Zhang L, He Q, Jia X, Ahmad M, Zhang X and Luo Y (2021) Using Genetic Variants to Evaluate the Causal Effect of Plasma Phospholipid Fatty Acids on Breast Cancer and Prostate Cancer: A Mendelian Randomization Study. Front. Genet. 12:664498. doi: 10.3389/fgene.2021.664498
Received: 05 February 2021; Accepted: 12 May 2021;
Published: 30 June 2021.
Edited by:
Yufang Pei, Soochow University Medical College, ChinaReviewed by:
Tomas Drgon, United States Food and Drug Administration, United StatesGangqiang Ding, National Institute for Nutrition and Health, China
Copyright © 2021 Yang, Li, Sun, Qu, Lin, Zhang, He, Jia, Ahmad, Zhang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueyun Zhang, MDYyMDQ5N0B6anUuZWR1LmNu; Yan Luo, bHVveWFuMjAxMUB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Ze Yang
Ze Yang Jingjia Li1,2†
Jingjia Li1,2† Yandi Sun
Yandi Sun Zihao Qu
Zihao Qu Lihong Zhang
Lihong Zhang Qian He
Qian He Yan Luo
Yan Luo