- 1Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
- 2Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China
Objective: To examine the associations between sleep quality and health span using a prospective cohort design based on the UK Biobank (UKB).
Materials and Methods: This longitudinal cohort study enrolled 328,850 participants aged between 37 and 73 years from UKB to examine the associations between sleep quality and risk of terminated health span. End of health span was defined by eight events strongly associated with longevity (cancer, death, congestive heart failure, myocardial infarction, chronic obstructive pulmonary disease, stroke, dementia, and diabetes), and a sleep score was generated according to five sleep behavioral factors (sleep duration, chronotype, sleeplessness, daytime sleepiness, and snoring) to characterize sleep quality. The hazard ratio (HR) and 95% confidence intervals (CIs) were calculated by multivariate-adjusted Cox proportional hazards model. Moreover, we calculated population attributable risk percentage (PAR%) to reflect the public health significance of healthy sleep quality.
Results: Compared with poor sleep quality, participants with healthy sleep quality had a 15% (HR: 0.85, 95% CI: 0.81–0.88) reduced risk of terminated health span, and those of less-healthy sleep quality had a 12% (HR: 0.88, 95% CI: 0.85–0.92) reduced risk. Linear trend results indicated that the risk of terminated health span decreased by 4% for every additional sleep score. Nearly 15% health span termination events in this cohort would have been prevented if a healthy sleep behavior pattern was adhered to (PAR%: 15.30, 95% CI: 12.58–17.93).
Conclusion: Healthy sleep quality was associated with a reduced risk of premature end of health span, suggesting healthy sleep behavior may extend health span. However, further studies are suggested for confirmation of causality and potential mechanism.
Introduction
Health span is a significant phenotype that enables individuals to age in good health without chronic diseases or disability (Zenin et al., 2019). Although global life expectancy has increased (Gbd 2016 Mortality Collaborators, 2017), aging populations often suffer functional health loss, and absolute expansion of morbidity (Jagger et al., 2008; Gbd 2015 DALYs and Hale Collaborators, 2016). Due to the significance of sleep and the fact that humans spend one-third of their lives asleep, there is growing interest in sleep behavior as a determinant of health span (Kay and Dzierzewski, 2015). Moreover, an alarming number of individuals suffer from sleeping problems and sleep deprivation worldwide. It was estimated that over 36% of the global population are suffering from sleep loss (Kryger et al., 2017), and nearly 50–70 million Americans chronically suffer from sleeplessness and sleep-related disorders, which hinder daily functioning and adversely affect health and longevity (Institute of Medicine Committee on Sleep Medicine and Research, 2006).
Poor sleep moderates biological responses such as increased oxidative stress, altered inflammatory and coagulatory responses, neural autonomic control changes, and accelerated atherosclerosis (Kryger et al., 2017; Tobaldini et al., 2017), which also show the profound impact of sleep on maintaining individual health status. Recent studies revealed that sleep quality is associated with cardiometabolic health and mortality (Karthikeyan et al., 2019; Fan et al., 2020), as well as epigenetic and skin aging, frailty, and mental health (Lo et al., 2014; Oyetakin-White et al., 2015; Carskadon et al., 2019; Sun et al., 2020). Abnormal sleep duration (both short and long sleep duration) was associated with a higher risk of total cardiovascular diseases (CVDs), chronic heart disease (CHD), stroke, and myocardial infarction (MI) (Mesas et al., 2010; Cappuccio et al., 2011; Daghlas et al., 2019). Therefore, it is essential to pay particular attention to sleep problems.
Although the associations of sleep behavioral factors with morbidity and mortality risk are documented (Merikanto et al., 2013), the evidence related to health span is still insufficient and uncertain, especially from the perspective of integrating multiple sleep behaviors. Most of the studies were limited by their modest sample sizes, the inclusion of patients with certain diseases at baseline, short follow-up, or insufficient confounder control, leading to inconsistency in the findings (Cappuccio et al., 2011; Yin et al., 2017). To fill this void, we integrated eight predominant health span-terminating events (Zenin et al., 2019) and adopted a sleep score consisting of five sleep behavioral factors (chronotype, sleep duration, sleeplessness/insomnia, snoring, and daytime sleepiness) as a measurement for sleep quality. Therefore, our study aimed to assess the associations between sleep quality and health span based on a large-scale prospective cohort [UK Biobank (UKB)].
Materials and Methods
Study Population
The study population was composed of 328,850 participants of the UKB, a large-scale prospective cohort study with over 500,000 participants recruited between 2006 and 2010 across the United Kingdom. A detailed description of the UKB project is reported elsewhere (Sudlow et al., 2015). Briefly, the participants (aged from 37 to 73 years) attended one of 22 assessment centers in England, Wales, and Scotland, where they completed baseline questionnaires, underwent various physical assessments, and reported medical conditions. The North West Multicenter Research Ethical Committee approved the UKB project, and participants’ consent was obtained.
Before performing the analysis in this study, we pruned the data for suitability. First, we excluded 72,477 and 29,027 participants whose health span had terminated prior to the baseline according to in-patient hospital admissions data (UKB data category 2000) and self-reported diagnoses obtained via verbal interview (UKB data category 100074), respectively. Additionally, 72,153 participants with missing sleep-related data were excluded. Finally, 328,850 participants of the UKB were included in this study (Supplementary Figure 1).
Ascertainment of Sleep Behaviors
The self-reported sleep behaviors (chronotype, sleep duration, sleeplessness/insomnia, snoring, and daytime sleepiness) were measured in the UKB using a standardized questionnaire. Except for sleep duration and snoring, the responses were measured on Likert scales from “never/rarely” to “usually” experiences (Fan et al., 2020). Chronotype means the tendency for earlier or later timing of sleep. An individual who prefers going to bed and waking earlier is considered a “morning person,” while a person who prefers going to bed and waking late is considered an “evening person” (Jones et al., 2019). Chronotype preference was assessed with the question “Do you consider yourself to be (i) “definitely a morning person,” (ii) “more a morning than evening person,” (iii) “more an evening than morning person,” or (iv) “definitely an evening person.” For sleep duration, participants responded to the question “About how many hours sleep do you get in every 24 h? (including naps)” with responses in hourly increments. Experience of sleeplessness/insomnia symptom was assessed with the question “Do you have trouble falling asleep at night or do you wake up in the middle of the night?” and the responses were given in 3-point Likert scale (never/rarely; sometimes; usually). Habitual snoring was assessed with the question “Does your partner or a close relative or friend complain about your snoring?” with responses of (i) yes or (ii) no. The question for subjective daytime sleepiness was “How likely are you to doze off or fall asleep during the daytime when you don’t mean to (for example, when working, reading, or driving)?,” with responses of (i) never/rarely, (ii) sometimes, (iii) often, or (iv) all of the time.
Definition of Sleep Score and Sleep Quality
According to an epidemiologic study associated with sleep patterns and incident cardiovascular disease (Fan et al., 2020), for each sleep behavior, participants with the low-risk sleep behavior were assigned a score of 1, while those classified as high risk earn the score of zero (0). Then, all component scores were summed to acquire a total sleep score ranging from 0 to 5, with higher scores indicating healthier sleep patterns. Furthermore, we defined “sleep quality” as three levels: “healthy” (sleep scores 4–5), “less-healthy” (sleep scores 2–3), and “poor” (sleep scores 0–1) (Fan et al., 2020).
Here, the low-risk sleep behaviors include early chronotype (“morning” or “morning than evening”) (Merikanto et al., 2013), sleep duration 7–8 h per day (Gallicchio and Kalesan, 2009; Itani et al., 2017), never or rarely experience sleeplessness/insomnia symptoms (Hsu et al., 2015; Javaheri and Redline, 2017), no self-reported snoring (Li et al., 2014), and no frequent daytime sleepiness (“never/rarely” or “sometimes”) (Gangwisch et al., 2014).
Ascertainment of Outcome
Health span is defined generally as aging without functional health loss (GBD, 2015; Li et al., 2020). In this study, health span was defined based on eight predominant health-terminating events strongly associated with longevity, such as congestive heart failure (CHF), myocardial infarction (MI), chronic obstructive pulmonary disease (COPD), stroke, dementia, diabetes, cancer, and death (Zenin et al., 2019). Health span was considered “terminated” for only participants first time diagnosed with any of these conditions during the UKB follow-up.
For each selected condition, except for cancer and death, we compiled a list of hospital data codes [International Classification of Diseases, 10th Revision (ICD-10)] and self-reported data codes (UKB data coding 6) to define these conditions in our study (Supplementary Table 1). We used the “National cancer registries linkage to UKB” (UKB data category 100092) to define cancer, and the “National death registries linkage to UKB” (UKB data category 100093) to define death event. The National cancer registries linkage to UKB was updated until December 14, 2016, earlier than the other two databases (inpatient hospital admissions data: March 31, 2017; National death registries linkage to UKB: February 14, 2018). To ensure consistency for the three databases, we set December 14, 2016, as the end date of follow-up in this study. Therefore, we calculated the personal follow-up time from the date of attending assessment center until the date of health span termination or December 14, 2016, whichever occurred first.
Statistical Analysis
We applied descriptive statistics (mean, SD, and proportion) to explore the baseline characteristics of the participants and estimated multivariate-adjusted hazard ratio (HR) for terminated health span using Cox proportional hazards regression models (Chandola et al., 2010; Boden-Albala et al., 2012). The proportional hazards assumptions for the Cox model were tested using Schoenfeld residuals method (Weisberg, 2010). In the basic model, we adjusted for age, sex, and ethnicity and further adjusted, in the fully adjusted model, for Townsend Deprivation Index, education, body mass index (BMI), smoking status, alcohol consumption, physical activity, healthy diet, family history of diseases [cancer and cardiac–cerebrovascular disease (CCVD)], and medication (sleep-related drugs and aspirin/ibuprofen). More details of the covariates can be found in the section “Supplementary Method”). Furthermore, we calculated the population attributable risk percentage (PAR%) for high-risk sleep behaviors using the “epi2by2” function in “epiR” package of R (Stevenson et al., 2020). Stratified analyses were conducted according to age (<50, 50–60, and >60 years), gender (male and female), BMI (<30 and ≥30 kg/m2), smoking status (never and ever), alcohol intake frequency (≥once a week and <once a week), physical activity (low and moderate&high), healthy diet intake (yes and no), college degree (yes and no), and Townsend Deprivation Index (≥median and <median) to examine heterogeneity across these subgroups.
Additionally, in sensitivity analysis, we constructed a weighted sleep score of five sleep behaviors using the following equation: weighted sleep score = (β1 × factor 1 + β2 × factor 2 +. + β5 × factor 5) × (5/sum of the β coefficients) to evaluate the reliability of the results (Fan et al., 2020). To validate the robustness of our findings, we further performed sensitivity analyses: (1) excluding participants with terminated health span within the first 2 years of follow-up, (2) excluding those with poor self-reported health status at baseline, (3) further adjustment for principal components (PC1–3) and genotype chip. All analyses were performed using R (version 4.1.0), and statistical significance was defined as two-sided p-value ≤ 0.05.
Results
In total, 49,772 participants of the 328,850 participants had terminated health span during the follow-up period, and approximately half of the events were caused by cancer (46.38%), followed by MI (17.73%) and death (10.99%) (Supplementary Table 2). The median follow-up time was 7.66 years (interquartile range: 6.80–8.42 years).
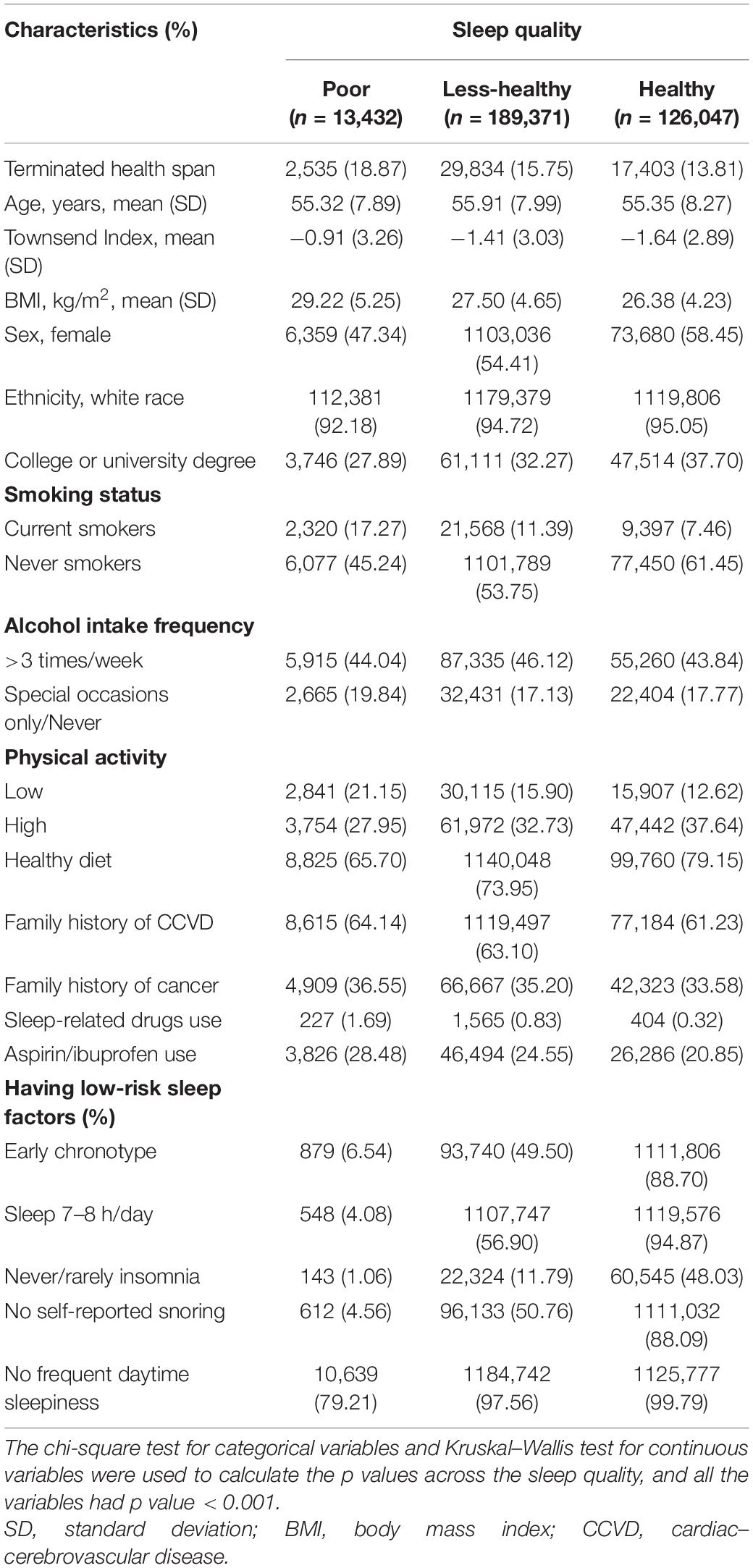
The baseline characteristics of 328,850 participants are summarized in Table 1. Overall, 4.08% of the participants had poor sleep quality (sleep scores 0–1), 57.59% had less-healthy sleep quality (sleep scores 2–3), and 38.33% had healthy sleep quality (sleep scores 4–5), with corresponding 18.87%, 15.75%, and 13.81% terminated health span, respectively. The female population was slightly higher among the healthy sleep quality group (58.45%). More participants with healthy (37.70%) sleep quality attained higher education than those with less-healthy (32.27%) or poor (27.89%) sleep quality. Besides, participants in the healthy sleep quality group had a relatively lower mean BMI (26.38 kg/m2), and approximately 37.64% of them engaged in high physical exercise. Participants who reported “currently smoking” seldom had healthy sleep quality (7.46%) compared to “never smoked” (61.45%). Participants with healthy sleep quality were more likely to have a healthy diet intake (79.15%) and less likely to have a family history of cardiovascular diseases (61.23%) and cancer (33.58%). Similarly, compared to poor sleep quality, participants with less-healthy and healthy sleep quality were less likely to take sleep-related drugs and aspirin/ibuprofen.
In Table 2, associations for sleep quality with risk of terminated health span were exhibited. Compared to poor sleep quality, participants with healthy sleep quality and less-healthy sleep quality had 15% (HR: 0.85, 95% CI: 0.81–0.88) and 12% (HR: 0.88, 95% CI: 0.85–0.92) reduced risk of terminated health span, respectively. The corresponding PAR% for less-healthy and healthy sleep quality was 1.29% (PAR%: 1.29, 95% CI: 1.01–1.58) and 3.41% (PAR%: 3.41; 95% CI: 2.95–3.88), respectively.
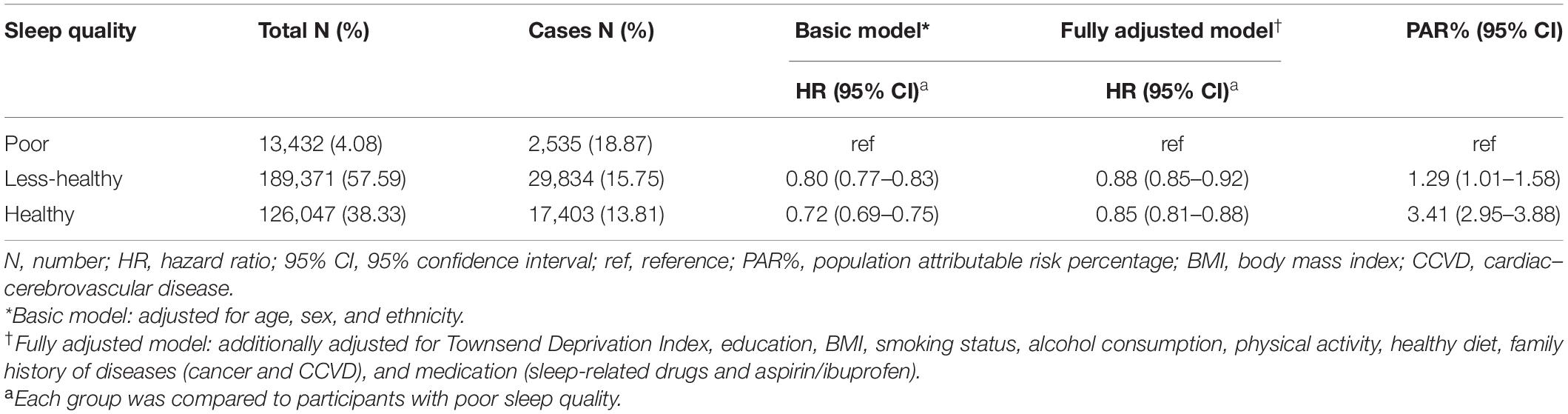
Table 2. Associations for sleep quality with risk of terminated health span among 328,850 participants.
From the perspective of sleep score, we found the participants with the score 5 had the lowest risk of premature end of health span (HR: 0.84, 95% CI: 0.80–0.88), and the trend analysis also revealed that the risk of terminated health span decreased by 4% (HR: 0.96, 95% CI: 0.96–0.97) for every additional sleep score (Figure 1A). Moreover, the corresponding PAR% for score 5 was nearly 15% (PAR%:14.31; 95% CI: 12.45–16.13) compared to those with the lowest sleep scores (Figure 1B).
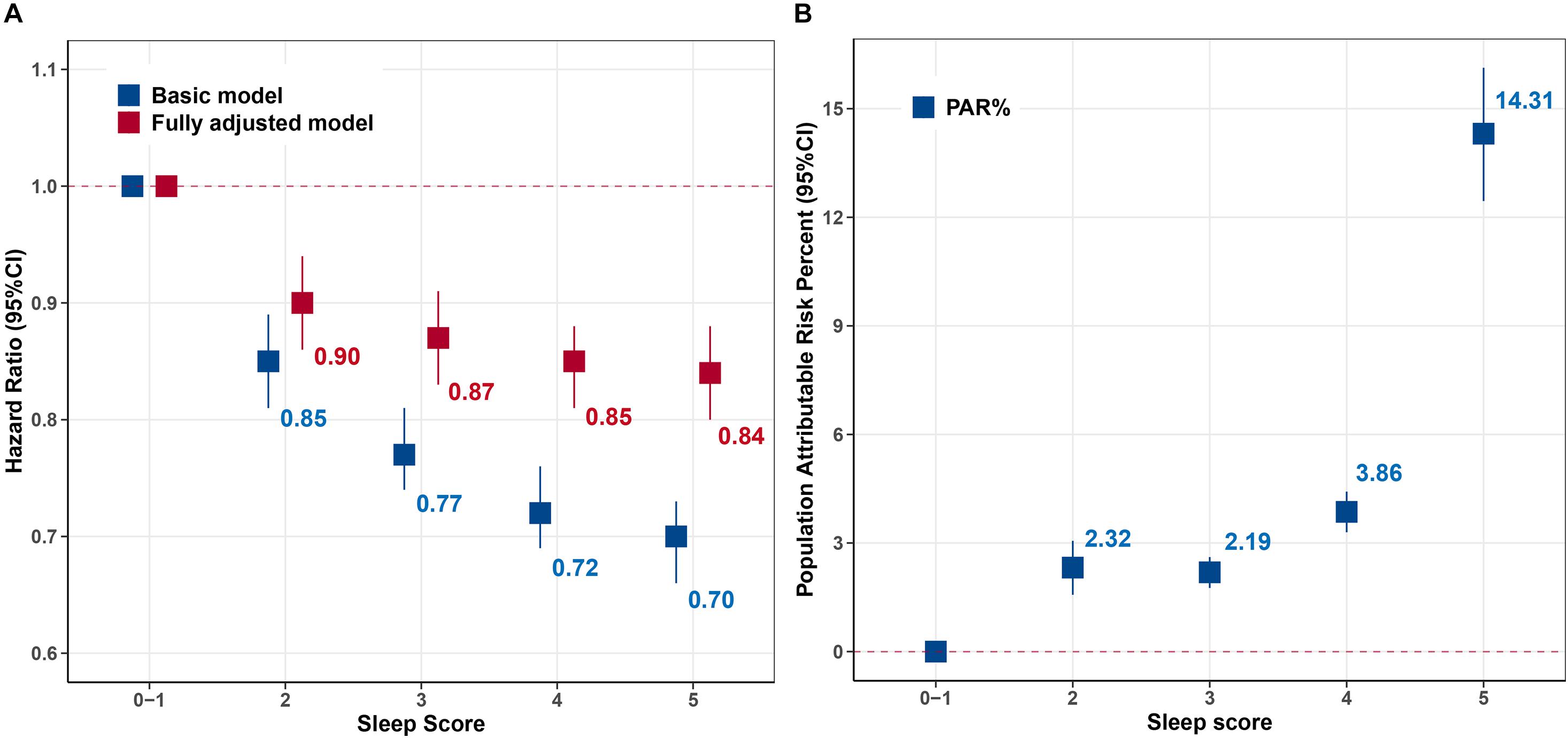
Figure 1. Hazard ratio (HR) and population attributable risk percentage (PAR%) for risk of terminated health span according to sleep score. (A) HR [95% confidence interval (95% CI)]. (B) Population attributable risk percentage (PAR%). Basic model: adjusted for age, sex, and ethnicity. Fully adjusted model further adjusted for Townsend Deprivation Index, education, body mass index (BMI), smoking status, alcohol consumption, physical activity, healthy diet, family history of diseases [cancer and cardiac–cerebrovascular disease (CCVD)], and medication (sleep-related drugs and aspirin/ibuprofen). Sleep scores 0–1 were the reference. The values were the point estimation of HRs and PAR%.
Additionally, we also demonstrated the cumulative hazard curves between sleep situation and terminated health span. Figure 2A showed that with increasing follow-up time, the cumulative hazard of terminated health span increased more in individuals with poor sleep quality than those with less-healthy or healthy sleep quality. Similar results were observed for sleep score, showing distinct risk between sleep scores 0–1 and high scores (Figure 2B).
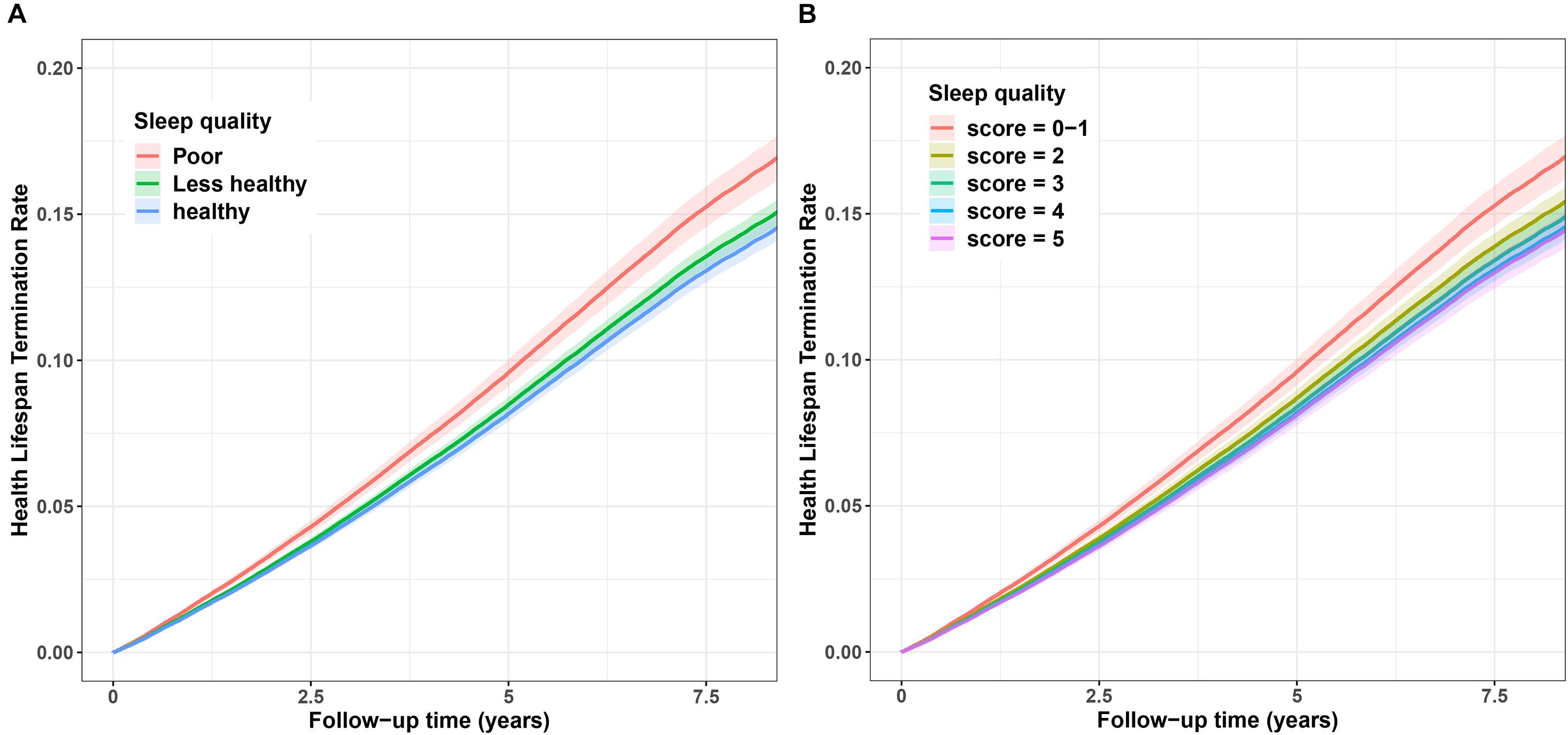
Figure 2. Cumulative hazard curve for the associations between sleep quality and risk of terminated health span. (A) Sleep quality (poor, less–healthy, and healthy). (B) Sleep score (scores 0–1, 2, 3, 4, and 5). The Y-axis represents cumulative hazard of terminated health span, the while X-axis represents the follow-up time (years). Shaded regions represent the 95% confidence intervals (95% CIs). Cumulative hazard curves were based on the fully adjusted model.
Then, we further explored the effects of related sleep traits on health span (Table 3). Participants with the low-risk sleep behaviors such as “sleep duration 7–8 h/day” (HR: 0.94, 95% CI: 0.92-0.95), “Never/rarely insomnia” (HR: 0.94, 95% CI: 0.92–0.96), and “rarely daytime sleepiness” (HR: 0.83, 95% CI: 0.79–0.87) had decreased risk of terminated health span. Furthermore, PAR% for terminated health span suggests that nearly 15% (PAR%: 15.30, 95% CI: 12.58–17.93) of terminated health span in this cohort would not have occurred if all participants had been in the low-risk group for all five sleep factors.
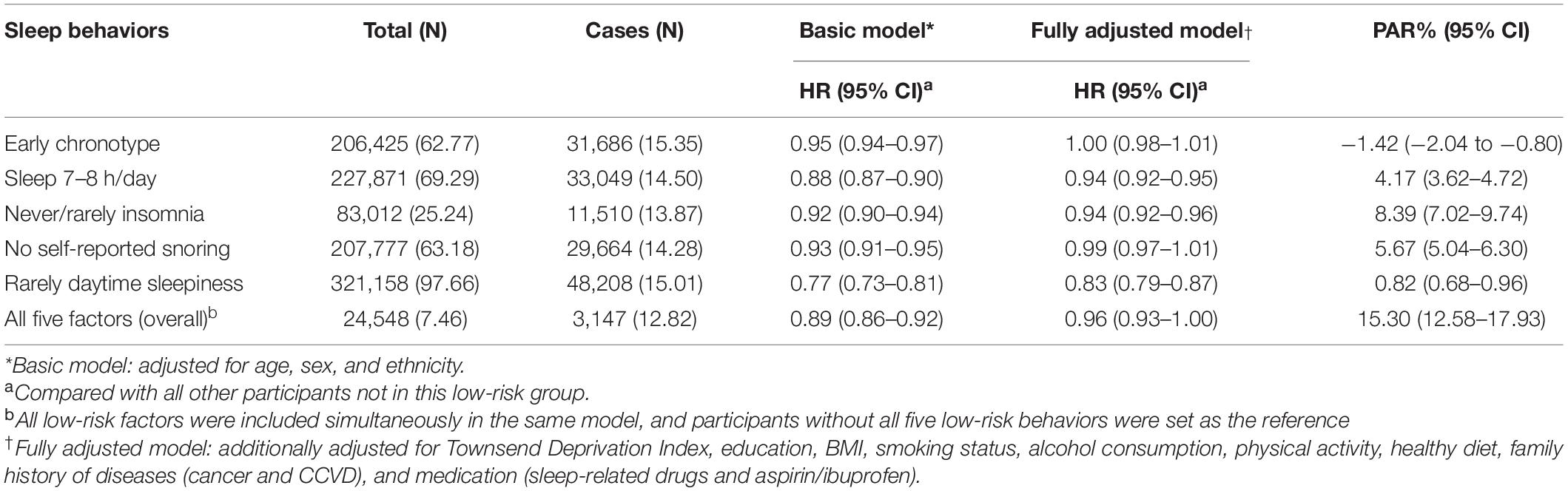
Table 3. Associations for low-risk sleep behaviors with risk of terminated health span among 328,850 participants.
In stratified analyses, we observed that the associations of sleep quality with the risk of terminated health span were largely consistent across subgroups, except the smoking status. The ever smokers had a stronger association than never smokers (Supplementary Figure 2). Additionally, we constructed a weighted sleep score to reevaluate its association with the risk of terminated health span. We found that high-grade weighted sleep score (weighted sleep score 4∼5 vs. 0∼<1, HR: 0.76, 95% CI: 0.71–0.82) also reduced the risk of terminated health span (Table 4).
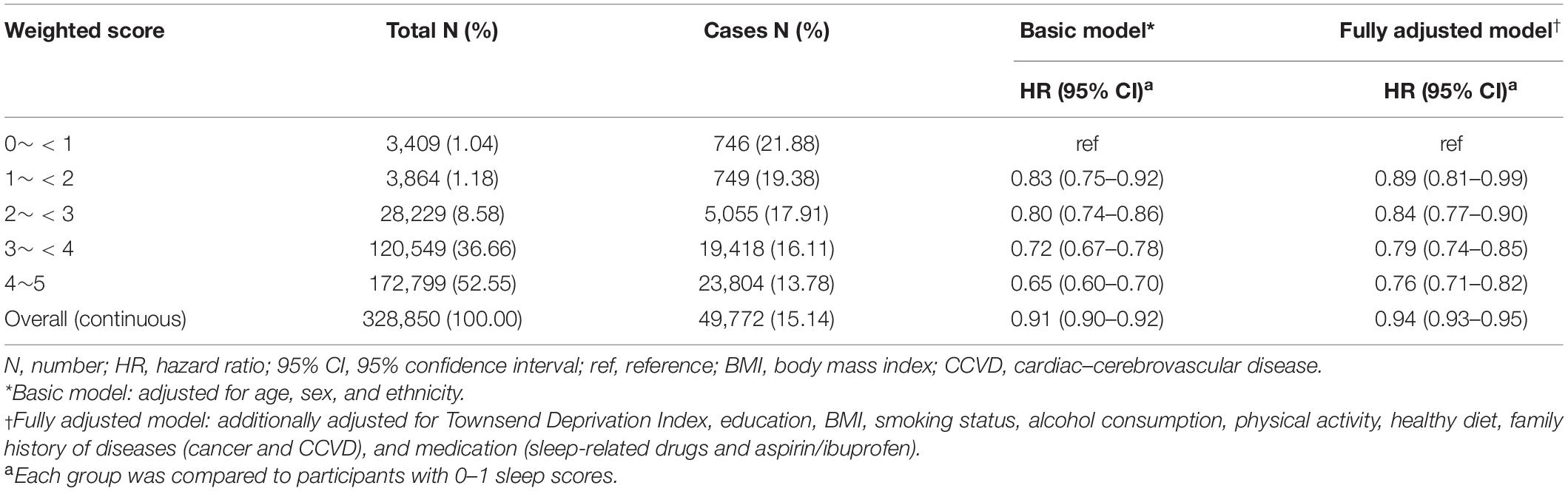
Table 4. Associations for weighted sleep score with risk of terminated health span among 328,850 participants.
Moreover, further sensitivity analyses were performed by respectively excluding participants with terminated health span within the first 2 years of follow-up and those with poor self-reported health status at baseline. The associations were largely similar to our previous findings (Supplementary Tables 4, 5). Given that complicated structure of population in UKB, we additionally adjusted the top 3 principal components (PC1–3) and genotype chip to offset the potential effect. Similarly, the results were consistent and supported the robustness of the observed findings in our study (Supplementary Table 6).
Discussion
In this large-scale prospective cohort study, we examined the associations of sleep quality/sleep score with risk of terminated health span based on 328,850 participants of the UKB. Participants with a healthy sleep quality had a 15% lower risk of terminated health span. The PAR% further suggested that nearly 15% of terminated health span in this cohort would not have occurred if all participants had low-risk sleep behavior for all five sleep behavioral factors. Besides, four sensitivity analyses implemented in this study indicated that the associations we found are robust and reliable to some degree.
Our results are in line with other comparable findings that sleep behavior affects health and wellbeing (Cappuccio et al., 2011; Gangwisch et al., 2014). Although sleep behavioral factors separately have a bearing on health, it was significant to evaluate a combination of sleep behavioral factors due to their synchrony that could jointly increase the risk of health span termination (Javaheri and Redline, 2017). For instance, a previous study showed that insomnia/sleeplessness was related to sleep duration and excessive daytime sleepiness (EDS), and late chronotype reduced sleep duration (Depner et al., 2014). Thus, we generated a sleep score integrating five sleep behaviors to comprehensively assess sleep quality and its association with health span, which was characterized by a host of eight health events that are commonly involved in health span termination (Zenin et al., 2019). Our study showed that healthy sleep quality reduced the risk of terminated health span, suggesting that healthy sleep behavior can improve health span. In agreement with our finding, previous studies showed that insomnia accompanied short sleep duration (Hsu et al., 2015; He et al., 2017; Javaheri and Redline, 2017), and habitual snoring with EDS increased the risk of hypertension, lung cancer (Liu et al., 2019; Campos et al., 2020), vascular death (Blachier et al., 2012; Boden-Albala et al., 2012), atherosclerosis (Sands et al., 2013; Javaheri and Redline, 2017), and diabetes (Li et al., 2015).
We also observed a decreased risk of terminated health span for single low-risk sleep behaviors, such as “sleep duration 7–8 h/day,” “no daytime sleepiness,” “never/rarely insomnia/sleeplessness,” and “early chronotype.” Similarly, high-risk sleep behavioral factors including “late chronotype” (Merikanto et al., 2013; Erren et al., 2016), “abnormal sleep duration” (Mesas et al., 2010; Cappuccio et al., 2011; Rudnicka et al., 2017; Daghlas et al., 2019), “frequently experience insomnia/sleeplessness” (Hsu et al., 2015; Javaheri and Redline, 2017), “habitual snoring” (Seidel et al., 2012; Sands et al., 2013; Li et al., 2015), and “excessive daytime sleepiness” (Blachier et al., 2012; Boden-Albala et al., 2012; Barfield et al., 2019) were associated with increased risk of chronic disease morbidity and mortality. If all these five high-risk sleep behaviors were rectified appropriately, nearly 15% of terminated health span would have been prevented, highlighting the importance of adhering to healthy sleep behaviors. However, it is worth noting that due to the multiple-center and large-scale design of UKB, these 328,850 participants aged from 37 to 73 years were nationwide. Besides, sleep traits were collected by trained volunteers according to a standard questionnaire. Therefore, the exposure distribution could represent the general population of United Kingdom, indicating the reliability of the PAR% we calculated at some degree, although a further validation in other cohorts was still necessary.
Biologically, sleep regulates many important pathways in the human physiology, such as autonomic, sympathetic, cardiometabolic, and immunologic responses (Cappuccio et al., 2011; Tobaldini et al., 2017), which support that adopting healthy sleep behavior to the circadian rhythm would enhance health and quality of life (Backhaus et al., 2015; Jones et al., 2019). On the other hand, poor sleep quality affects functional health in both young and adult, including disruption of cognitive performance and diurnal alertness (Skeldon et al., 2016). Moreover, it is essential to be cautious about shared and non-shared environmental determinants of ill-sleep habit (Gregory et al., 2016), including lifestyle, such as alcohol dependence, smoking, obesity, physical inactivity, and stress, that could upset healthy sleep behaviors (Chakravorty et al., 2016; Christie et al., 2016; Liao et al., 2019; Garcia-Marin et al., 2020).
Moreover, sleep disorders and poor sleep habits are alarming health threats warranting more public attention and are necessary to take appropriate action to promote sleep quality and health status, particularly for those with irregular sleep patterns, such as shift workers (Palermo et al., 2015; Redeker et al., 2019). A previous study among shift workers showed that resting and napping lowered the levels of sleepiness at the end of the shift (Barthe et al., 2015), which means more efficiency at work and less chances of accidents due to sleepiness (Ruggiero and Redeker, 2014; Geiger-Brown et al., 2016) and ultimately beneficial for the extension of health span. In addition to potential contributions to the individual’s life quality, a healthy sleep quality may also mitigate extravagant medical costs associated with chronic disease morbidity as well as lighten the heavy burden of social demands on health services. Therefore, we not only aim to investigate the potential effects of sleep quality on health span but also hope to call for more attention to individual sleep problems and correct improper sleep patterns as far as possible.
Here, the definition of health span we adopted is a promising longevity phenotype, reflecting individual aging and health status. Based on the richness and accuracy of clinical information in UKB, the construction of the health span phenotype is reliable and robust. Thus, we have a chance to assess the associations between sleep quality and risk of premature health span termination for the first time. The sleep score constructed by five sleep behaviors is an effective way to measure the sleep quality quantitatively. Meanwhile, the reliable data, large-scale sample, and long-term follow-up time of UKB provide sufficient power for our study. However, all the sleep behaviors are self-reported, which may lead to misclassification of exposures inevitably. To our knowledge, misclassification will underestimate the associations we observed. Although we have adjusted the sociodemographic characteristics, lifestyles, and other confounding factors in the full model, residual confounding from unknown or unmeasured factors still remains possible. Thus, the effects of associations and the PAR% we calculated are essential to be further validated in other perspective cohorts. Thirdly, a single measurement of sleep behaviors at baseline is not satisfactory to reflect the dynamic change of sleep factors during the following time, which means that the evaluation of effects of changing sleep patterns on health span requires repeated measurements of sleep traits. Moreover, in our observational study, the potential causality is hard to determine, and further work is necessary. Finally, most of the study participants are white, and generalizing the findings to other populations should warrant caution.
In summary, we tentatively explored the effect of sleep quality on health life span in this study. A healthy sleep quality plays an important role in individual health status, aging, and diseases. Sleep problem is not only related to individual physical and mental health but also a public health and social problem, which deserves more attention and early intervention.
Conclusion
In this large-scale prospective study that enrolled 328,850 participants, we found that healthy sleep quality was associated with a reduced risk of premature end of health life span, suggesting that healthy sleep behaviors may be beneficial to extend health life span. Therefore, sleep problems deserve more attention and early intervention. However, further studies are suggested for confirmation of causality and potential mechanism.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the North West Multi-Centre Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JD contributed to the conception and design of the study. MS, XZ, and TH conducted the statistical analysis and wrote the first draft. TH, JF, and TB helped apply for the permission to use data and offered technical support during the study. MZ, CW, DH, and YJ critically revised the manuscript for important intellectual content. All authors reviewed and approved the final manuscript.
Funding
This work was funded by the National Natural Science of China (81941020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was conducted using the UK Biobank resource (Application Number 64689). We thank the study participants and research staff for their contributions and commitment to this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.663449/full#supplementary-material
References
Backhaus, W., Kempe, S., and Hummel, F. C. (2015). The effect of sleep on motor learning in the aging and stroke population - a systematic review. Restor. Neurol. Neurosci. 34, 153–164. doi: 10.3233/rnn-150521
Barfield, R., Wang, H., Liu, Y., Brody, J. A., Swenson, B., Li, R., et al. (2019). Epigenome-wide association analysis of daytime sleepiness in the multi-ethnic study of atherosclerosis reveals African-American-specific associations. Sleep 42:zsz101.
Barthe, B., Tirilly, G., Gentil, C., and Toupin, C. (2015). Job demands and resting and napping opportunities for nurses during night shifts: impact on sleepiness and self-evaluated quality of healthcare. Ind. Health 54, 157–162. doi: 10.2486/indhealth.2015-0002
Blachier, M., Dauvilliers, Y., Jaussent, I., Helmer, C., Ritchie, K., Jouven, X., et al. (2012). Excessive daytime sleepiness and vascular events: the three city study. Ann. Neurol. 71, 661–667. doi: 10.1002/ana.22656
Boden-Albala, B., Roberts, E. T., Bazil, C., Moon, Y., Elkind, M. S., Rundek, T., et al. (2012). Daytime sleepiness and risk of stroke and vascular disease: findings from the Northern manhattan study (NOMAS). Circ. Cardiovasc. Qual. Outcomes 5, 500–507. doi: 10.1161/circoutcomes.111.963801
Campos, A. I., García-Marín, L. M., Byrne, E. M., Martin, N. G., Cuéllar-Partida, G., and Rentería, M. E. (2020). Insights into the aetiology of snoring from observational and genetic investigations in the UK Biobank. Nat. Commun. 11:817.
Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P., and Miller, M. A. (2011). Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492. doi: 10.1093/eurheartj/ehr007
Carskadon, M. A., Chappell, K. R., Barker, D. H., Hart, A. C., Dwyer, K., Gredvig-Ardito, C., et al. (2019). A pilot prospective study of sleep patterns and DNA methylation-characterized epigenetic aging in young adults. BMC Res. Notes 12:583.
Chakravorty, S., Chaudhary, N. S., and Brower, K. J. (2016). Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol. Clin. Exp. Res. 40, 2271–2282. doi: 10.1111/acer.13217
Chandola, T., Ferrie, J. E., Perski, A., Akbaraly, T., and Marmot, M. G. (2010). The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the whitehall II cohort. SLEEP 33, 739–744. doi: 10.1093/sleep/33.6.739
Christie, A. D., Seery, E., and Kent, J. A. (2016). Physical activity, sleep quality, and self-reported fatigue across the adult lifespan. Exp. Gerontol. 77, 7–11. doi: 10.1016/j.exger.2016.02.001
Daghlas, I., Dashti, H. S., Lane, J., Aragam, K. G., Rutter, M. K., Saxena, R., et al. (2019). Sleep duration and myocardial infarction. J. Am. Coll. Cardiol. 74, 1304–1314. doi: 10.1016/j.jacc.2019.07.022
Depner, C. M., Stothard, E. R., and Wright, K. P. (2014). Metabolic consequences of sleep and circadian disorders. Curr. Diab. Rep. 14:507.
Erren, T. C., Morfeld, P., Foster, R. G., Reiter, R. J., Groß, J. V., and Westermann, I. K. (2016). Sleep and cancer: synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol. Int. 33, 325–350. doi: 10.3109/07420528.2016.1149486
Fan, M., Sun, D., Zhou, T., Heianza, Y., Lv, J., Li, L., et al. (2020). Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur. Heart J. 41, 1182–1189. doi: 10.1093/eurheartj/ehz849
Gallicchio, L., and Kalesan, B. (2009). Sleep duration and mortality: a systematic review and meta-analysis. J. Sleep Res. 18, 148–158. doi: 10.1111/j.1365-2869.2008.00732.x
Gangwisch, J. E., Rexrode, K., Forman, J. P., Mukamal, K., Malaspina, D., and Feskanich, D. (2014). Daytime sleepiness and risk of coronary heart disease and stroke: results from the Nurses’ Health study II. Sleep Med. 15, 782–788. doi: 10.1016/j.sleep.2014.04.001
Garcia-Marin, L. M., Campos, A. I., Martin, N. G., Cuellar-Partida, G., and Renteria, M. E. (2020). Inference of causal relationships between sleep-related traits and 1,527 phenotypes using genetic data. Sleep 44:zsaa154.
Gbd 2015 DALYs and Hale Collaborators (2016). Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1603–1658.
Gbd 2016 Mortality Collaborators (2017). Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970-2016: a systematic analysis for the Global burden of disease study 2016. Lancet 390, 1084–1150.
GBD (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800.
Geiger-Brown, J. P., Sagherian, K., Zhu, S., Blair, L., Warren, J., et al. (2016). Napping on the night shift: a two-hospital implementation project. Am. J. Nursing 116, 26–33. doi: 10.1097/01.naj.0000482953.88608.80
Gregory, A. M., Rijsdijk, F. V., Eley, T. C., Buysse, D. J., Schneider, M. N., Parsons, M., et al. (2016). A longitudinal twin and sibling study of associations between insomnia and depression symptoms in young adults. Sleep 39, 1985–1992. doi: 10.5665/sleep.6228
He, Q., Sun, H., Wu, X., Zhang, P., Dai, H., Ai, C., et al. (2017). Sleep duration and risk of stroke: a dose-response meta-analysis of prospective cohort studies. Sleep Med. 32, 66–74. doi: 10.1016/j.sleep.2016.12.012
Hsu, C. Y., Chen, Y. T., Chen, M. H., Huang, C. C., Chiang, C. H., Huang, P. H., et al. (2015). The association between insomnia and increased future cardiovascular events: a nationwide population-based study. Psychosom. Med. 77, 743–751. doi: 10.1097/psy.0000000000000199
Institute of Medicine Committee on Sleep Medicine and Research (2006). “The national academies collection: reports funded by national institutes of health,” in Sleep Disorders and Sleep Deprivation: an Unmet Public Health Problem, eds H. R. Colten and B. M. Altevogt (Washington, DC: National Academies Press).
Itani, O., Jike, M., Watanabe, N., and Kaneita, Y. (2017). Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 32, 246–256. doi: 10.1016/j.sleep.2016.08.006
Jagger, C., Gillies, C., Moscone, F., Cambois, E., Van Oyen, H., Nusselder, W., et al. (2008). Inequalities in healthy life years in the 25 countries of the European Union in 2005: a cross-national meta-regression analysis. Lancet 372, 2124–2131. doi: 10.1016/s0140-6736(08)61594-9
Javaheri, S., and Redline, S. (2017). Insomnia and risk of cardiovascular disease. Chest 152, 435–444. doi: 10.1016/j.chest.2017.01.026
Jones, S. E., Lane, J. M., Wood, A. R., van Hees, V. T., Tyrrell, J., Beaumont, R. N., et al. (2019). Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat. Commun. 10:343.
Karthikeyan, R., Spence, D. W., and Pandi-Perumal, S. R. (2019). The contribution of modern 24-hour society to the development of type 2 diabetes mellitus: the role of insufficient sleep. Sleep Sci. 12, 227–231.
Kay, D. B., and Dzierzewski, J. M. (2015). Sleep in the context of healthy aging and psychiatric syndromes. Sleep Med. Clin. 10, 11–15. doi: 10.1016/j.jsmc.2014.11.012
Kryger, M. H., ROth, T., and Dement, W. C. (2017). Principles and Practice of Sleep Medicine. Amsterdam: Elsevier.
Li, D., Liu, D., Wang, X., and He, D. (2014). Self-reported habitual snoring and risk of cardiovascular disease and all-cause mortality. Atherosclerosis 235, 189–195. doi: 10.1016/j.atherosclerosis.2014.04.031
Li, M., Li, K., Zhang, X. W., Hou, W. S., and Tang, Z. Y. (2015). Habitual snoring and risk of stroke: a meta-analysis of prospective studies. Int. J. Cardiol. 185, 46–49. doi: 10.1016/j.ijcard.2015.03.112
Li, Y., Schoufour, J., Wang, D. D., Dhana, K., Pan, A., Liu, X., et al. (2020). Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. Bmj 368:l6669. doi: 10.1136/bmj.l6669
Liao, Y., Xie, L., Chen, X., Kelly, B. C., Qi, C., Pan, C., et al. (2019). Sleep quality in cigarette smokers and nonsmokers: findings from the general population in central China. BMC Public Health 19:808.
Liu, W., Luo, M., Fang, Y. Y., Wei, S., Zhou, L., and Liu, K. (2019). Relationship between occurrence and progression of lung cancer and nocturnal intermittent hypoxia. apnea and daytime sleepiness. Curr. Med. Sci. 39, 568–575. doi: 10.1007/s11596-019-2075-6
Lo, J. C., Sim, S. K., and Chee, M. W. (2014). Sleep reduces false memory in healthy older adults. Sleep 37, 665–671, 671A.
Merikanto, I., Lahti, T., Puolijoki, H., Vanhala, M., Peltonen, M., Laatikainen, T., et al. (2013). Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol. Int. 30, 470–477. doi: 10.3109/07420528.2012.741171
Mesas, A. E., López-García, E., León-Muñoz, L. M., Guallar-Castillón, P., and Rodríguez-Artalejo, F. (2010). Sleep duration and mortality according to health status in older adults. J. Am. Geriatr. Soc. 58, 1870–1877. doi: 10.1111/j.1532-5415.2010.03071.x
Oyetakin-White, P., Suggs, A., Koo, B., Matsui, M. S., Yarosh, D., Cooper, K. D., et al. (2015). Does poor sleep quality affect skin ageing? Clin. Exp. Dermatol. 40, 17–22. doi: 10.1111/ced.12455
Palermo, T. A., Rotenberg, L., Zeitoune, R. C., Silva-Costa, A., Souto, E. P., and Griep, R. H. (2015). Napping during the night shift and recovery after work among hospital nurses. Rev. Lat. Am. Enfermagem 23, 114–121. doi: 10.1590/0104-1169.0147.2532
Redeker, N. S., Caruso, C. C., Hashmi, S. D., Mullington, J. M., Grandner, M., and Morgenthaler, T. I. (2019). Workplace interventions to promote sleep health and an alert, healthy workforce. J. Clin. Sleep Med. 15, 649–657. doi: 10.5664/jcsm.7734
Rudnicka, A. R., Nightingale, C. M., Donin, A. S., Sattar, N., Cook, D. G., Whincup, P. H., et al. (2017). Sleep duration and Risk of Type 2 diabetes. Pediatrics 140, 529–537.
Ruggiero, J. S., and Redeker, N. S. (2014). Effects of napping on sleepiness and sleep-related performance deficits in night-shift workers: a systematic review. Biol. Res. Nursing 16, 134–142. doi: 10.1177/1099800413476571
Sands, M., Loucks, E. B., Lu, B., Carskadon, M. A., Sharkey, K., Stefanick, M., et al. (2013). Self-reported snoring and risk of cardiovascular disease among postmenopausal women (from the women’s health initiative). Am. J. Cardiol. 111, 540–546. doi: 10.1016/j.amjcard.2012.10.039
Seidel, S., Frantal, S., Oberhofer, P., Bauer, T., Scheibel, N., Albert, F., et al. (2012). Morning headaches in snorers and their bed partners: a prospective diary study. Cephalalgia 32, 888–895. doi: 10.1177/0333102412453950
Skeldon, A. C., Derks, G., and Dijk, D. J. (2016). Modelling changes in sleep timing and duration across the lifespan: changes in circadian rhythmicity or sleep homeostasis? Sleep Med. Rev. 28, 96–107. doi: 10.1016/j.smrv.2015.05.011
Stevenson, M., Nunes, T., Heuer, C., Marshall, J., Sanchez, J., and Thornton, R. (2020). epiR: Tools for the Analysis of Epidemiological Data. Avaialble online at: https://CRAN.R-project.org/package=epiR
Sudlow, C., Gallacher, J., Allen, N., Beral, V., Burton, P., Danesh, J., et al. (2015). UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12:e1001779. doi: 10.1371/journal.pmed.1001779
Sun, X. H., Ma, T., Yao, S., Chen, Z. K., Xu, W. D., Jiang, X. Y., et al. (2020). Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population Rugao longevity and ageing study. BMC Geriatr. 20:9.
Tobaldini, E., Costantino, G., Solbiati, M., Cogliati, C., Kara, T., Nobili, L., et al. (2017). Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 74, 321–329. doi: 10.1016/j.neubiorev.2016.07.004
Weisberg, S., and Fox, J. (2018). Cox Proportional-Hazards Regression for Survival Data in R. Appendix to an R Companion to Applied Regression, 3rd Edn. Available online at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/appendices/Appendix-Cox-Regression.pdf
Yin, J., Jin, X., Shan, Z., Li, S., Huang, H., Li, P., et al. (2017). Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart. Assoc. 6:e005947.
Keywords: sleep quality, sleep score, health span, aging, population attributable risk percent, UK Biobank
Citation: Sambou ML, Zhao X, Hong T, Fan J, Basnet TB, Zhu M, Wang C, Hang D, Jiang Y and Dai J (2021) Associations Between Sleep Quality and Health Span: A Prospective Cohort Study Based on 328,850 UK Biobank Participants. Front. Genet. 12:663449. doi: 10.3389/fgene.2021.663449
Received: 02 February 2021; Accepted: 10 May 2021;
Published: 15 June 2021.
Edited by:
Yuanwei Zhang, University of Science and Technology of China, ChinaReviewed by:
Shaohua Xie, Karolinska Institutet (KI), SwedenLiangdan Sun, Anhui Medical University, China
Copyright © 2021 Sambou, Zhao, Hong, Fan, Basnet, Zhu, Wang, Hang, Jiang and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juncheng Dai, ZGpjQG5qbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Muhammed Lamin Sambou
Muhammed Lamin Sambou Xiaoyu Zhao1†
Xiaoyu Zhao1† Dong Hang
Dong Hang Juncheng Dai
Juncheng Dai