
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 11 May 2021
Sec. Computational Genomics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.650213
This article is part of the Research Topic Network Based Multi-Omics Analyses to Reveal Mechanisms and Biomarkers of Complex Diseases View all 8 articles
Background: Valvular heart disease is obtaining growing attention in the cardiovascular field and it is believed that calcific aortic valve disease (CAVD) is the most common valvular heart disease (VHD) in the world. CAVD does not have a fully effective treatment to delay its progression and the specific molecular mechanism of aortic valve calcification remains unclear.
Materials and Methods: We obtained the gene expression datasets GSE12644 and GSE51472 from the public comprehensive free database GEO. Then, a series of bioinformatics methods, such as GO and KEGG analysis, STING online tool, Cytoscape software, were used to identify differentially expressed genes in CAVD and healthy controls, construct a PPI network, and then identify key genes. In addition, immune infiltration analysis was used via CIBERSORT to observe the expression of various immune cells in CAVD.
Results: A total of 144 differential expression genes were identified in the CAVD samples in comparison with the control samples, including 49 up-regulated genes and 95 down-regulated genes. GO analysis of DEGs were most observably enriched in the immune response, signal transduction, inflammatory response, proteolysis, innate immune response, and apoptotic process. The KEGG analysis revealed that the enrichment of DEGs in CAVD were remarkably observed in the chemokine signaling pathway, cytokine-cytokine receptor interaction, and PI3K-Akt signaling pathway. Chemokines CXCL13, CCL19, CCL8, CXCL8, CXCL16, MMP9, CCL18, CXCL5, VCAM1, and PPBP were identified as the hub genes of CAVD. It was macrophages that accounted for the maximal proportion among these immune cells. The expression of macrophages M0, B cells memory, and Plasma cells were higher in the CAVD valves than in healthy valves, however, the expression of B cells naïve, NK cells activated, and macrophages M2 were lower.
Conclusion: We detected that chemokines CXCL13, CXCL8, CXCL16, and CXCL5, and CCL19, CCL8, and CCL18 are the most important markers of aortic valve disease. The regulatory macrophages M0, plasma cells, B cells memory, B cells naïve, NK cells activated, and macrophages M2 are probably related to the occurrence and the advancement of aortic valve stenosis. These identified chemokines and these immune cells may interact with a subtle adjustment relationship in the development of calcification in CAVD.
Valvular heart disease is obtaining growing attention in the cardiovascular field and it is believed that calcific aortic valve disease (CAVD) is the most common valvular heart disease (VHD) worldwide (Iung and Vahanian, 2011; Liu et al., 2017). It is also the predominant causation of aortic valve stenosis (Iung and Vahanian, 2011). CAVD presents with an escalating fibrous calcification remodeling and pathological incrassation of aortic valves, which evolves for a long time, inducing a serious block of cardiac outflow and ultimately reducing the mobility of leaflets (Lindman et al., 2016). Aortic stenosis (AS), the most common clinical manifestation of CAVD, its prevalence increases with age, impacting about 9% of patients older than 80 years. In addition, aortic sclerosis is obviously more widespread, impacting 25–30% of patients older than 65 years, and progresses to AS at a ratio of approximately 2% every year (Coffey et al., 2014; Otto and Prendergast, 2014). It is assumed that CAVD is becoming a major societal and economic burden for the aging world that is likely to be confirmed in the near future (Osnabrugge et al., 2013). In spite of the progress on radical therapies, such as valve replacement and intervention therapy already used for aortic stenosis, still, the specific molecular mechanism of aortic valve calcification remains unclear. Therefore, it is of great necessity to understand the evolution of CAVD and explore the key molecules for better treatment of aortic stenosis.
As is well-known, CAVD develops slowly and the severity of each stage is different. In the beginning, there is only mild valve thickening while at terminal stage, grievous calcification is accompanied by impaired leaflet movement, aortic valve stenosis and clinical symptoms such as heart pits, dyspnea, and angina (Freeman and Otto, 2005; Rajamannan et al., 2007). In the past, CAVD was deemed to be a retrograde procedure owing to the time-conditioned abrasion of valves and calcification deposition passively. At present, lots of convincing evidences have implied that CAVD was an unasked, multifaceted process, which is concerned with the sedimentation of chylomicrons, VLDL, LDL, active valve calcification, ossification of valvular interstitial cells, and so on (Freeman and Otto, 2005). More and more researches suggested that signal transduction channels referred to the progress of aortic calcification consisted of various growth factors, cytokines, and tumor necrosis factors (O’Brien et al., 2002; Jian et al., 2003; Kaden et al., 2005). Since statins and angiotensin-converting enzyme inhibitors failed to retard CAVD progression, there were no effective medical therapies (Lindman et al., 2013; Nishimura et al., 2014). When CAVD progresses to an advanced stage with terrible clinical manifestations, the most effective measures are surgery and transcatheter aortic valve replacement (TAVR), but the actual situation is that these procedures are related to higher costs, unavoidable risk of death, perioperative and long-run complications such as complications of lifelong anti-coagulation therapy and reoperation of artificial valve insufficiency (Lindman et al., 2016).
Hence, it is necessary for us to make a deeper discernment of the latent mechanisms of CAVD and mine certain key genes which perhaps are curative targets. In the recent years, the high-throughput sequencing information acquired via chip techniques helped us confirm the differentially expressed genes (DEGs) and their functions, excavate their pathways related to the advancement of various complex disorders (Zhao et al., 2018). Through comprehensive bioinformatics analysis of public genetic data, we could be able to conduct a secondary data digging and identify biomarkers related to diseases (Lei et al., 2019). Furthermore, we investigated the correlation between key molecules and immune cells to deeply and comprehensively comprehend the immune mechanism among the developing process in CAVD.
The gene expression profiles with series numbers GSE12644 and GSE51472 were obtained from the Expression Omnibus Gene (GEO) database1, 2 NCBI (Lei et al., 2019). These two datasets were genetic expression profiles of aortic valves from patients with calcified aortic valve diseases and healthy controls, which were detected on the GPL570 platform and founded on the Affymetrix Human Genome U133 Plus 2.0 array (Li et al., 2015). GSE12644 contains gene expression profile of 10 normal and 10 calcified stenotic human aortic valves from Canada. GSE51472 contains 15 human aortic valves from during the different stages of development of calcified aortic valve disease (normal, sclerotic, calcified) from Finland. All the participants were white male. In order to analyze it more conveniently, we removed the five sclerotic valve samples after merging and batch calibration from all these data. Moreover, we also obtained two data sets related to CAVD (GSE83453 and GSE26953) from GEO to further verify and analyze the results of immune infiltration. GSE83453 included 10 calcified aortic valves, nine stenotic aortic valves, and eight normal aortic valves, and GSE26953 contained 24 mRNAs of human aortic valve endothelial cells under the following conditions: laminar flow and shear stress.
We obtained the original data of these two chips, GSE12644 and GSE51472, and then read them through the R package (version: 4.2.0). Subsequently, the two data sets were merged and batch corrected. In addition, we made use of the annotation configuration file offered by the platform to annotate probes and discard probes unmatched.(Zhang et al., 2019). Limma package in R was applied to screen the DEGs of CAVD with P value < 0.05, | log2 (Fold Change)| ≥ = 1. Finally, we distinguished 49 genes with an increased expression and 95 genes with a decreased expression in the combined matrix. By processing the heatmap package of R, we accomplished hierarchical clustering and visualization. As for GSE83453 and GSE26953, we just utilized the annotation configuration file to annotate probes.
The powerful online bioinformatics database, Database for Annotation, Visualization, and Integrated Discovery (DAVID3), furnishes investigators with an all-round range of functional annotation tools to solve the biological implications of large gene lists (Yin et al., 2020). In this research, we used DAVID to aggregate the functions of DEGs including the screening of significant biological processes, molecular functions, cellular components, and as well as, identifying pathways related to these DEGs (Zou Z. B. et al., 2019). In order to obtain the visual results of the function and pathway terms, we used the R package clusterProfiler to obtain the histogram of the top six most important GO functions separately and the bubble chart of the signal pathways.
On account of the Search tool for the retrieval of interacting genes/proteins (STRING4), our PPI network among the DEGs-encoded proteins were established successfully (Zhao et al., 2018). Using the Network Analyzer available in the Cytoscape software (version: 3.7.2), the PPIs network was visualized. Using the Network Analyzer provided in the Cytoscape software (version: 3.7.2), you can see the PPI network. CytoHubba in Cytoscape is mainly used to rank nodes in the network through its network function, and provides 11 topological analysis methods (Ali et al., 2016). Of these 11 means, MCC can predict the essential proteins in the PPI network more accurately and finally, we obtained the 10 core genes of the PPI network according to the degree score.
CIBERSORT5, a robust online tool, utilizes a deconvolution algorithm to analyze the relationship between gene expression and infiltrating immune cells (Ali et al., 2016). Combined and corrected micro array matrix was uploaded to the CIBERSORT algorithm running to deduce the percentages of 22 kinds of infiltrating immunocytes and then selected samples with P < 0.05 to the final study cohort (Deng et al., 2020). The analyzed immunocytes contained macrophages, dendritic cells (DC), B cells, CD4+ T cells, CD8+ T cells, neutrophils, and several naive cells. Subsequently, based on R, the results of the immune infiltration analysis are visualized in different ways like bar plot, heatmap, and co-heatmap. ESTIMATE algorithm calculates the immune score and stromal score from the matrix of GSE83453 and GSE26953 data sets; in this algorithm, the immune and stromal scores are calculated by analyzing the specific gene expression characteristics of immune and stromal cells to predict the infiltration of non-tumor cells.
In order to analyze the relationship between hub genes and lipid metabolism related biomarkers, firstly, we used the script in the Perl6 software to extract the identified hub genes and metabolism-related biomarkers. Subsequently, the limma package containing the Cor function of the R language was used to analyze the correlation coefficients and draw correlation pictures between the two variables (Tang et al., 2020). According to statistical standards, the correlation coefficient r greater than 0.4 is a positive correlation, less than −0.4 is a negative correlation, and it is considered that the p value that is less than 0.05 has a statistical difference (Wang et al., 2019).
Firstly, after merging the GSE12644 and GSE51472 datasets, we removed the batch differences between the two gene expression data sets (Figure 1). Subsequently, there are 144 DEGs altogether satisfying the criterion of | log2FC| ≥ = 1 and P value < 0.05, containing 49 genes with an increased expression and 95 genes with a decreased expression in CAVD compared to the healthy samples. Through both the heat map and volcano plot (Figures 2A,B), we can see the remarkable difference in the distribution between each data set.
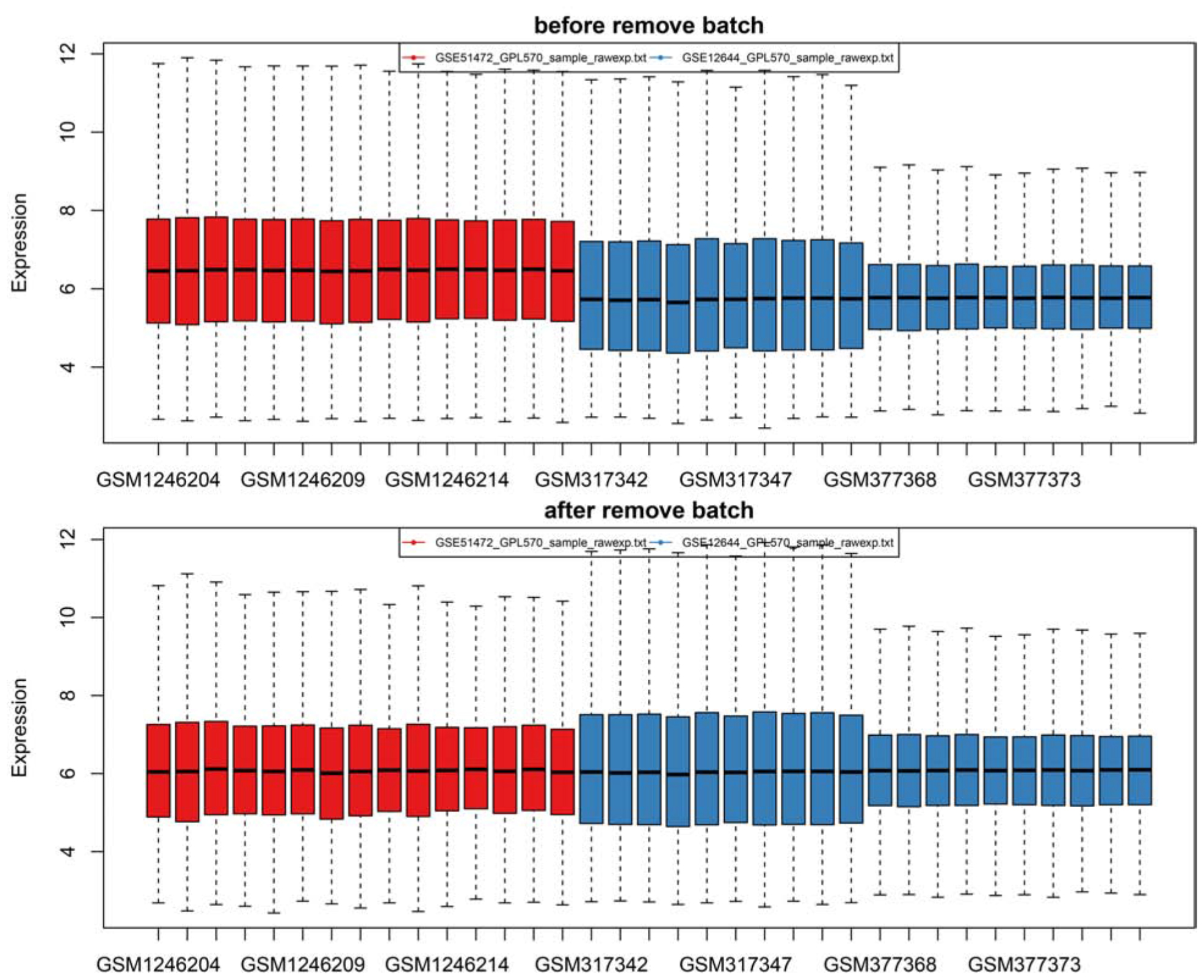
Figure 1. There are two batches of samples in the original data as revealed by the box plot and the expression profile was successfully standardized to a comparable level by pretreatment and batch-effects adjustment.
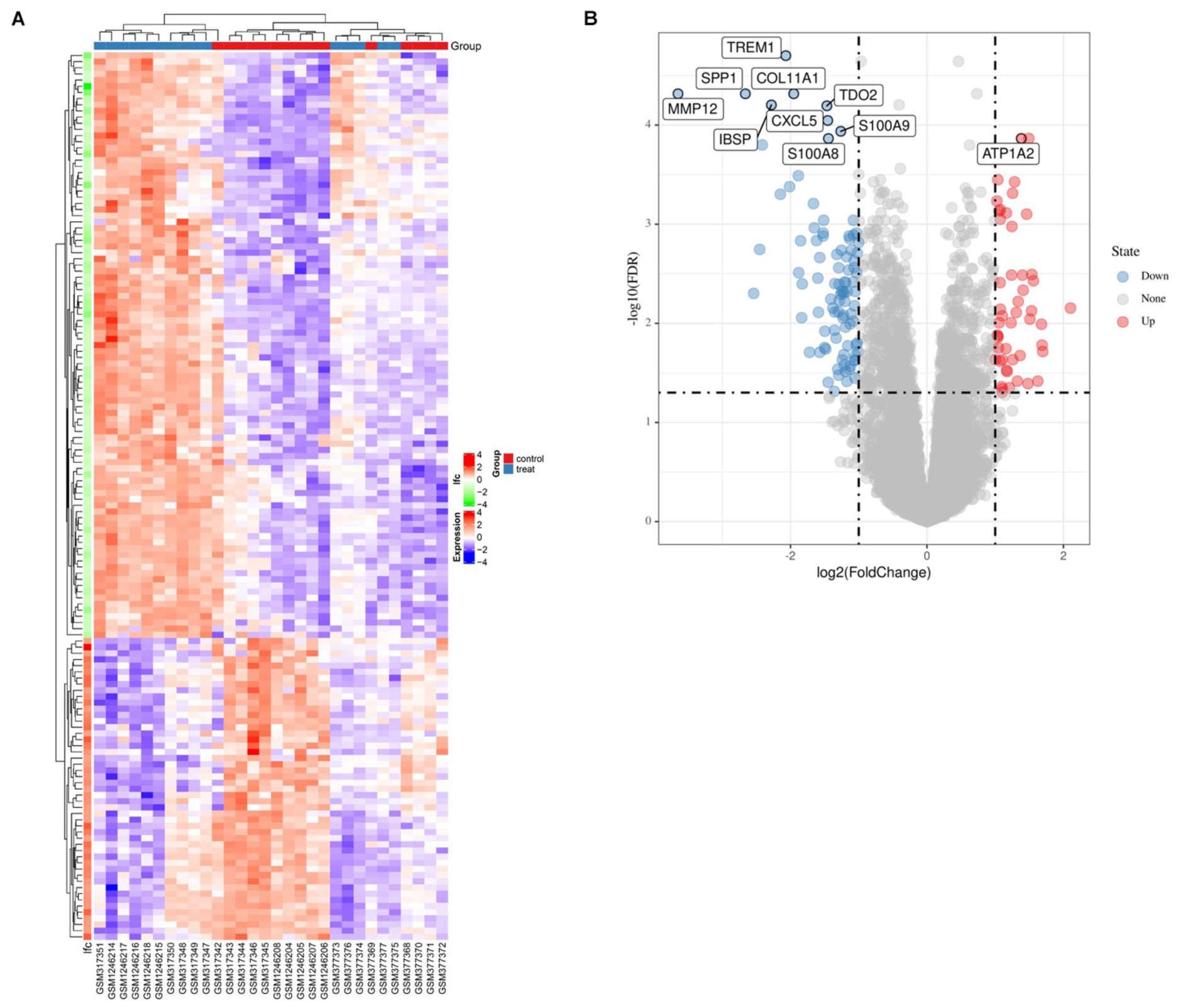
Figure 2. The identification of EDGs. (A) The heat-map of differential expression genes (P < 0.05, | logFC| > 1). Up-regulated genes were in red and down-regulated genes were in blue. (B) Volcano plot of genes detected in CAVD, red dots represent upregulated genes and blue dots represent downregulated genes.
Based on DAVID, we executed the GO and KEGG functional annotation analysis for investigating the specific biological function classification of DEGs globally (Zou R. et al., 2019). GO function annotation results displayed that changes at biological process (BP) were observably focused in immune response, signal transduction, inflammatory response, proteolysis, innate immune response, and apoptotic process (Figure 3A). The most enriched molecular function (MF) annotations were serine-type endopeptidase activity, protein homodimerization activity, calcium ion binding, chemokine activity, antigen binding, and metallo endopeptidase activity. Changes of DEGs significantly in cell component (CC) were mostly in the plasma membrane, extracellular region, extracellular space, extracellular exosome, integral component of plasma membrane, and proteinaceous extracellular matrix. In addition, the results of the KEGG pathway analysis in bubble chart revealed that DEGs were remarkably concentrated in the chemokine signaling pathway, cytokine-cytokine receptor interaction, PI3K-Akt signaling pathway, focal adhesion, ECM-receptor interaction, and protein digestion and absorption (Figure 3B). Some more details of the GO and KEGG analyses can be seen in Tables 1, 2.
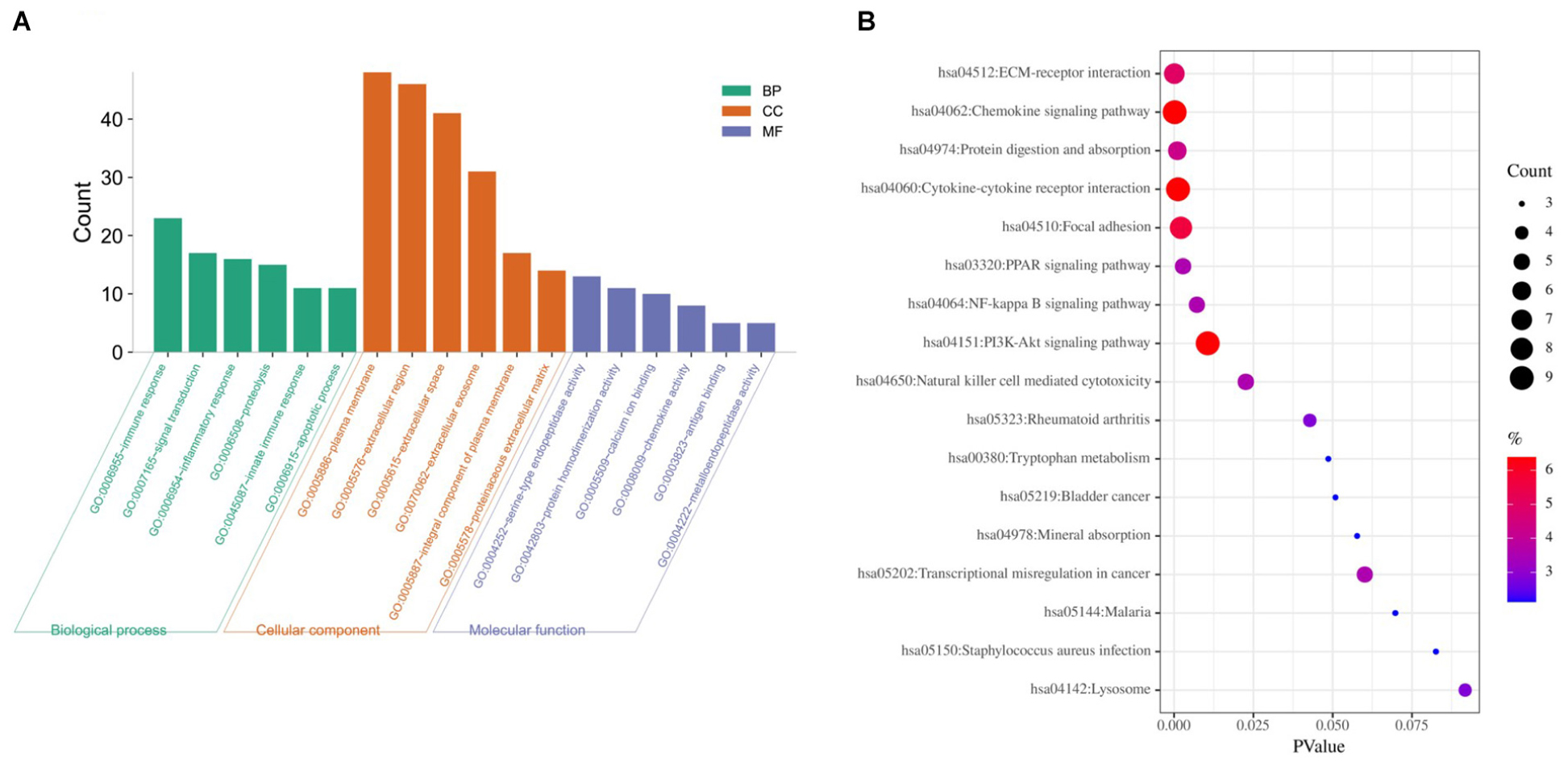
Figure 3. GO and KEGG pathway enrichment analysis of DEGs between CAVD and normal controls. (A) The top six GO analysis results of DEGs. (B) Pathway analyses results of DEGs (top 20 according to adjusted P value). GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
The precise PPI network was constructed based on the online tool STRING and then, was adjusted and visualized via the Cytoscape software (Cai et al., 2020). Ultimately, there were 344 edges and 102 nodes among this PPI network. The red dots represent the up-regulated genes and the green dots represent the down-regulated genes (Figure 4A). CytoHubba, a plug-in of the Cytoscape, was utilized to filter the most important genes and modules from the network. Top 10 genes (CXCL13, CCL19, CCL8, CXCL8, CXCL16, MMP9, CCL18, CXCL5, VCAM1, and PPBP) ranked by degree were selected (Figure 4B) as core molecules, the names, abbreviations, and functions for which are displayed in Table 3. In addition, BPs analysis showed that DEGs were related to the chemokine-mediated signaling pathway, inflammatory response, immune response, G-protein coupled receptor signaling pathway, cellular response to tumor necrosis factor, and chemotaxis, while CCs analysis showed that DEGs were chiefly concentrated on the extracellular space, extracellular region, and cell. As for MFs, the DEGs were mainly enriched in the chemokine activity, CCR chemokine receptor binding, CCR10 chemokine receptor binding, CXCR chemokine receptor binding, and heparin binding (Figure 4C).
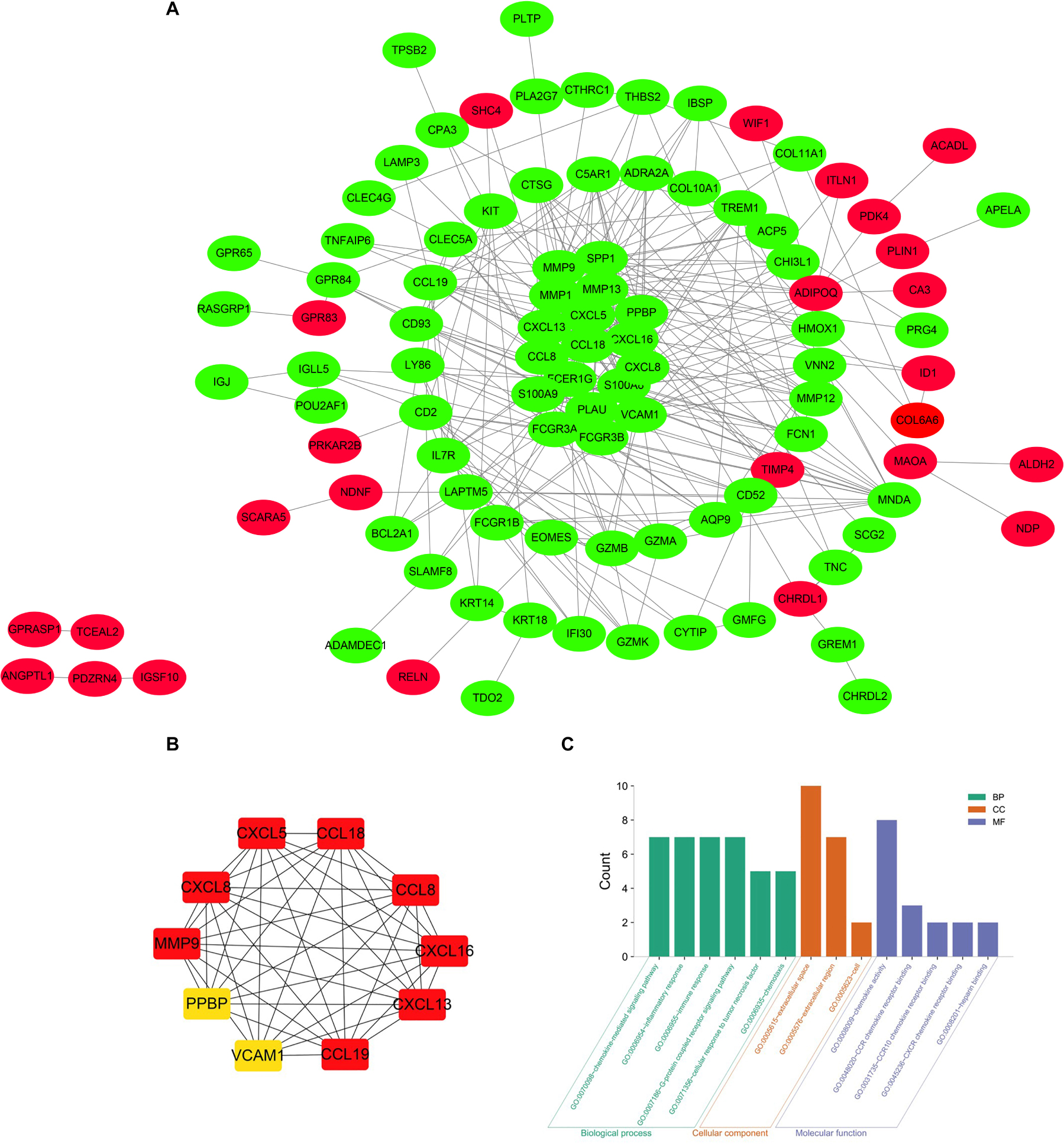
Figure 4. (A) The PPI network of DEGs identified from the two microarray datasets was constructed using Cytoscape, including 49 up-regulated genes and 95 down-regulated genes. The upregulated genes are marked in red and downregulated genes are marked in green. (B) The top 10 most significant genes were obtained from PPI network. DEG, differentially expressed gene; PPI protein, protein interaction. (C) The top 10 most significantly enriched GO terms of DEGs.
Based on CIBERSORT7, distinctions of 22 subpopulations of infiltration immune cells between the CAVD and normal valve tissues were identified (Zhao et al., 2020). Figures 5A,B could demonstrate the proportion of 22 kinds of immune cells vividly from 15 normal controls and 15 CAVD patients. In addition, we discovered that it was macrophages that accounted for the maximal proportion among these immune cells. Supplementary Table 1 showed the results of the immune cells infiltration specifically. Results of the correlation analysis between immune cells found that NK cells resting and mast cells activated had the most intense positive relationship with r = −0.65, yet mast cells activated and mast cells resting had the most obvious negative correlation with r = 0.73 (Figure 5C). The result of the analysis of the change in the trend of various immune cells showed that the infiltration of macrophages M0, B cells memory, and plasma cells in CAVD patients was higher compared with the healthy controls, but the infiltration of B cells naïve, NK cells activated, and macrophages M2 was lower (Figure 5D; p < 0.05). As shown in Figure 6A, the immune score of the normal aortic valve group was significantly lower than that of the calcification group and the stenosis group, but the immune scores of the laminar flow group and the shear stress group were not very different (Figure 6B). Similarly, the stromal scores of the normal group were significantly lower than that of the calcification and stenosis group (Figure 6C), but there was still no significant difference between the laminar flow group and the shear stress group (Figure 6D).
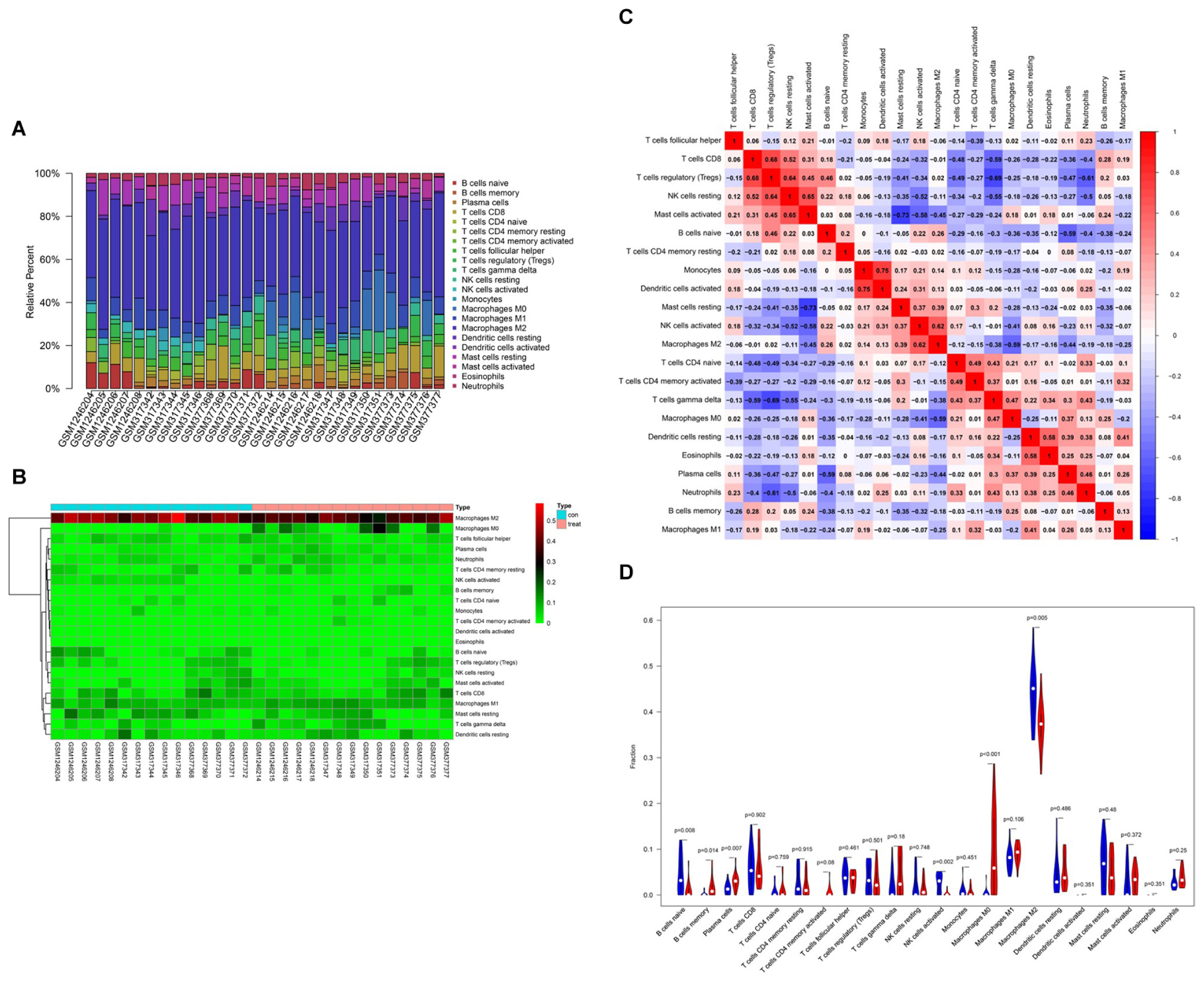
Figure 5. Summary of inferred immune cell subsets between CAVD and normal controls. (A) The relative percentage of 22 subpopulations of immune cells in 30 samples from GSE13985and GSE6054 datasets. (B) The heat-map of the 22 subpopulations of immune cells. (C) Correlation analysis based on 22 immune cell subpopulations. (D) The difference of immune infiltration between VACD and normal controls. (The normal controls group was marked as blue color and CAVD group was marked as red color. P values < 0.05 were considered as statistically significant).
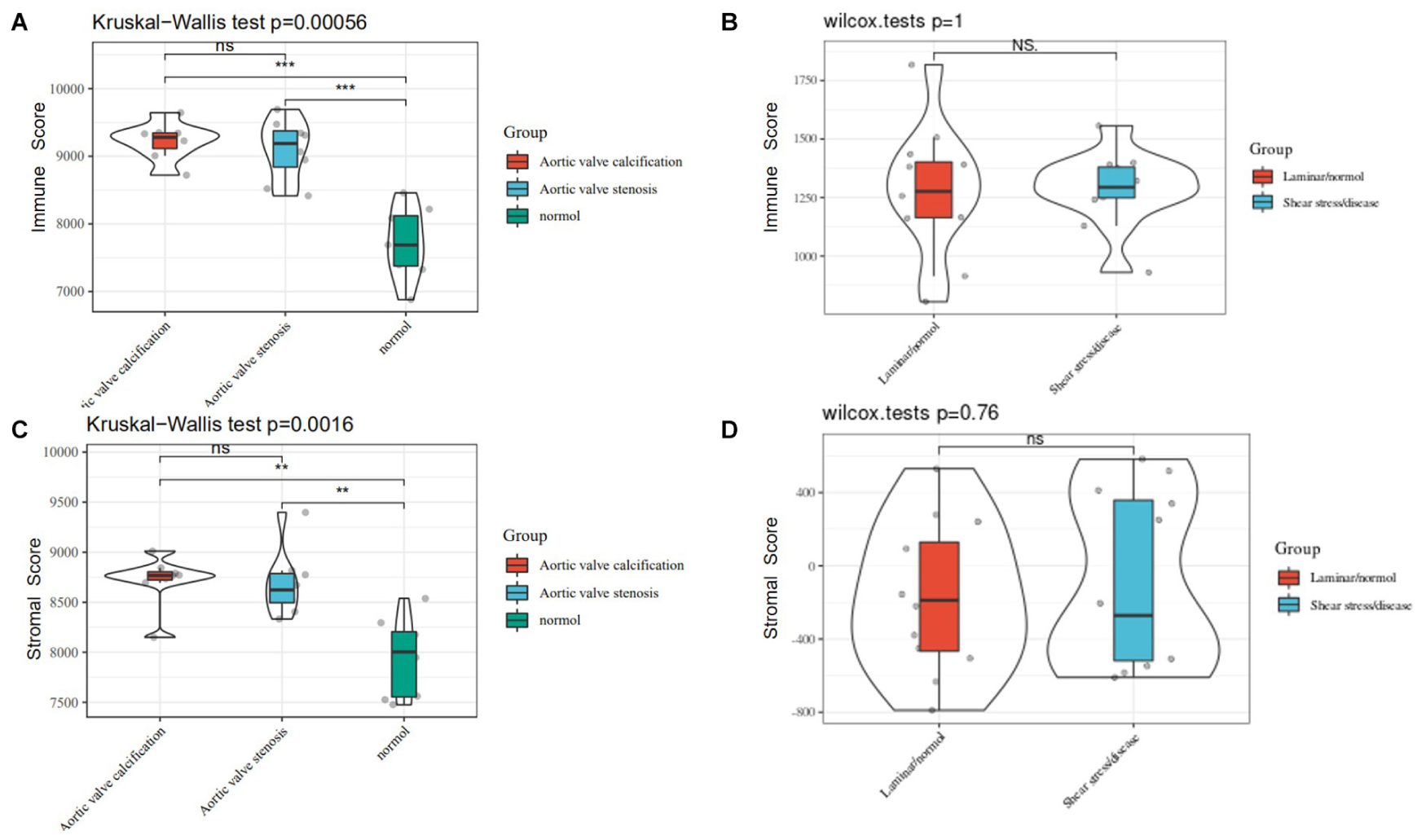
Figure 6. (A) The immune score of the normal, calcification, and stenosis groups of GSE83453. (B) The immune scores of the laminar flow group and the shear stress group in GSE26953. (C) The stromal scores of the normal, calcification, and stenosis groups. (D) The stromal scores of the laminar flow group and the shear stress group.
Pearson correlation analysis shows that CCL8, CCL18, CCL19, CXCL16, and VCAM1 are positively correlated with fatty acid synthase (FAS) gene and hydroxymethylglutaryl coenzyme A reductase (HMGCR) gene among the 10 hub genes (Figures 7A,B), yet CXCL13, CXCL5, and CXCL8 have a strong negative correlation with the lecithin cholesterol acyltransferase (CAT) gene (Figure 7C). As we all know, the calcification process of CAVD involves lipid infiltration, FAS is a key factor in fat synthesis and HMGCR is a key enzyme that catalyzes the de novo synthesis of cholesterol in the body while CAT participates in reverse cholesterol transport (Zechner et al., 2012). This result further illustrates the close relationship between chemokines, lipid metabolism, and CAVD. More information on the 10 hub genes and metabolism-associated genes are shown in Supplementary Material.
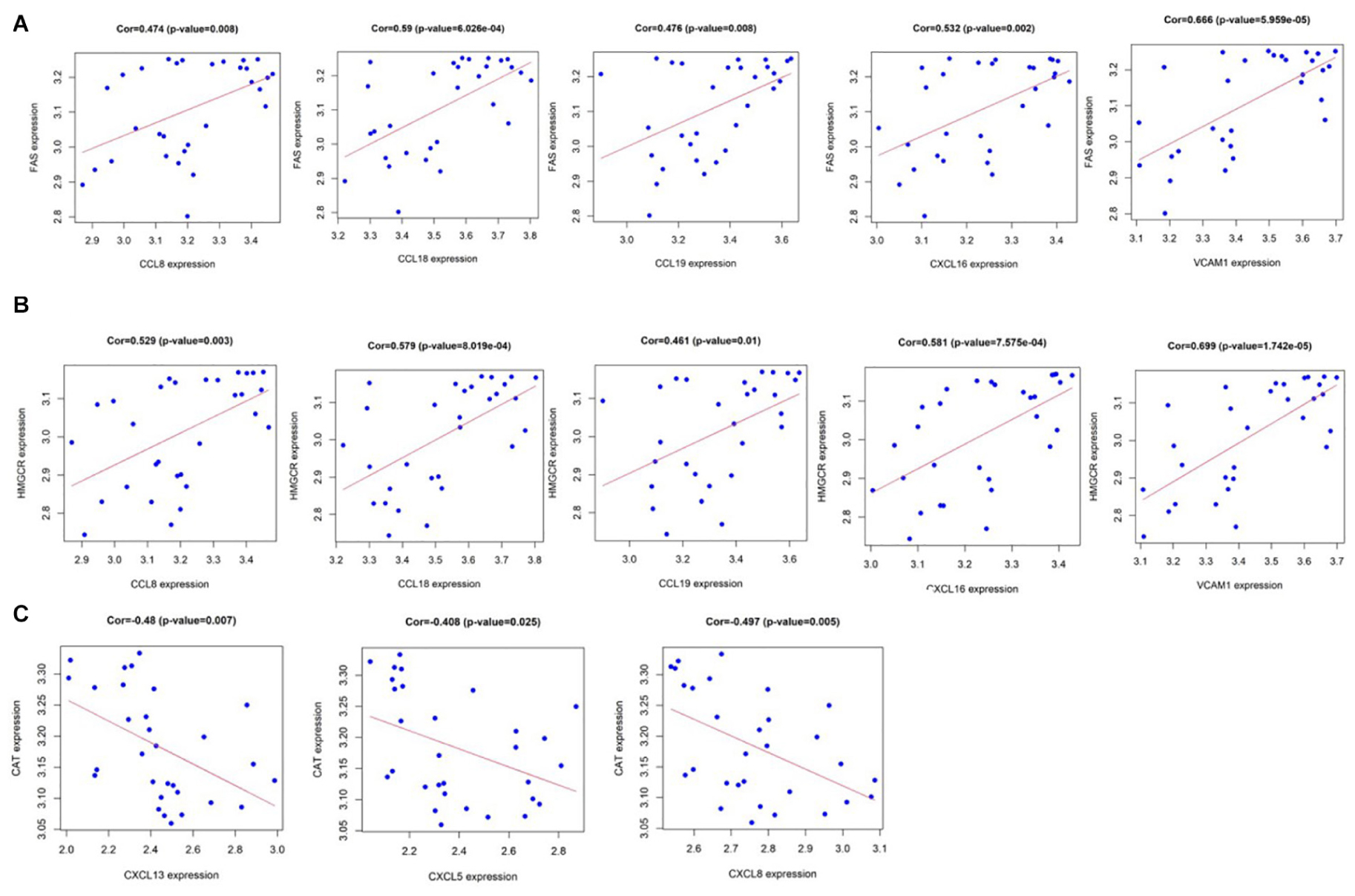
Figure 7. (A) CCL8, CCL18, CCL19, CXCL16, and VCAM1 are positively correlated with fatty acid synthase (FAS); and (B) CCL8, CCL18, CCL19, CXCL16, and VCAM1 are positively correlated with hydroxymethylglutaryl coenzyme A reductase (HMGCR). (C) CXCL13, CXCL5, and CXCL8 have a strong negative correlation with lecithin cholesterol acyltransferase (CAT). P value < 0.05.
Calcific aortic valve disease stands for a disease that covers the process from aortic valve sclerosis to aortic valve stenosis (Freeman and Otto, 2005). Incremental proofs verified that CAVD is an irreversible valvular lesion that remains irrecoverable though tremendous improvement in the surgical treatment strategy (Pawade et al., 2015). CAVD was primitively regarded as a retrograde procedure due to the gradual calcium sedimentation in aortic valve while getting older. It is generally accepted that CAVD is a complicated and active process and various physiological and pathological conditions are involved in the regulation like lipoprotein deposition, inflammatory response, activating RAAS, transformation of ECM, and cellular ossification (Mathieu and Boulanger, 2014; Kostyunin et al., 2019). Previous researches showed that valvular disease was widespread in most industrialized countries, where a decrement in the prevalence of rheumatic heart disease (RHD) has been associated with an enhancement in the popularity of degenerative etiologies (Coffey et al., 2014). Some studies have shown that valvular disease and coronary disease have some common risk factors, including hypertension, diabetes mellitus, dyslipidemia, cigarette, and so on (Nishimura et al., 2014). The pathophysiological process of CAVD principally devastates endothelial valve cells and valvular interstitial cells, exacerbating valvular calcification, making for stenosis of valve orifice, and blocking the left ventricular outflow tract (Dutta and Lincoln, 2018). The loading of valvular disease is anticipated to ascend, in consideration of the prolonged life expectancy and the current insufficiency of effective preventive measures, emphasizing the need to deepen our discernment of the pathophysiology of retrogressive heart valve diseases (Gould et al., 2013; Lindman et al., 2013). In recent decades, gene sequencing and bioinformatics technologies have developed dramatically, so it became possible to further analyze and utilize plentiful sequencing data (Zhao et al., 2020). In the present study, we screened significant DEGs between the aortic stenosis of CAVD and control samples from the GEO database. Furthermore, quite a lot of bioinformatics analysis was performed. The GO function annotation demonstrated that those DEGs were enriched in some BPs such as immune response, signal transduction, inflammatory response, proteolysis, innate immune response, and apoptotic process. KEGG pathway enrichment analysis uncovered that DEGs markedly related to chemokine signaling pathway, cytokine-cytokine receptor interaction, and PI3K-Akt signaling pathway. To explore molecular mechanism of CAVD, ten hub genes (CXCL13, CCL19, CCL8, CXCL8, CXCL16, MMP9, CCL18, CXCL5, VCAM1, and PPBP) connected with CAVD occurrence were explored out based on the STRING and Cytoscape software. We speculate that these important DEGs and their potential functions bring about the progression of CAVD in theory.
Among these hub genes, two kinds of chemokine ligands, CXCLs and CCLs, are very conspicuous. As we all know, the chemokine ligands family contains chemotactic cytokines or ligands and they are significant ingredients of the cross-communication among the assorted cell types in the inflammation or tumor microenvironment (Lacalle et al., 2017). Chemokine ligands mediate their activity by way of interacting with G protein coupled receptors that have unique 7 transmembrane architecture, hence becoming chemokine ligand/receptor pairs (Hussain et al., 2019). Chemokine ligand/receptor interactions include huge signal plasticity and complexity, which are crucial for fine-tuning chemotaxis of specific leukocyte subsets (Borroni et al., 2010). Firstly, the C-X-C motif chemokine ligands CXCL13, CXCL8, CXCL16, and CXCL5 are insiders of the CXC chemokine family. The protein encoded by CXCL13 is a chemokine ligand that plays a role in the homing of B lymphocytes to lymphoid follicles. Activated germinal center helper T cells are expressed at a high level. Cells with an up-regulated expression of CXCL13 receptor CXCL5 can induce chemotaxis. It induces B cells to enter follicles, and induces memory T cells in the paracortical area to return to follicles. Anne et al. reported a crucial function of CXCL13 and its receptor CXCR5 in durability and maintenance of cardiac structure in the case of pressure overload, possibly via modulating small leucine-rich proteoglycans (SLRPs) in ECM (Waehre et al., 2011). CXCL8, also known as interleukin-8, is a cytokine secreted by macrophages and epithelial cells and has a chemotactic effect on neutrophils to achieve its regulation of inflammation. It was reported that this proinflammatory protein may act on coronary artery disease even endothelial dysfunction and CXCL8 classically associates with an effective angiogenic role. The combination of CXCL8 with its receptors (IL-8RB/CXCR2) raises vascular permeability, and the increase of IL-8 level is positively correlated with the severity of prognosis of many diseases. Observations from Kokje et al. (2018) recognized that the high expression of CXCL8 is a clear distinguishing feature among abdominal aortic aneurysm disease (AAA) and atherosclerosis, and proved that there is a transcription mechanism demanding CXCL8 signal transduction in AAA. CXCL16 (C-X-C motif chemokine ligand 16) protein exists in two forms, transmembrane-bound type and soluble secretion type. The two forms have different biological effects and are related to each other. The transmembrane-bound type mainly functions as a scavenger receptor, and the soluble secreted type mainly functions as a chemokine. CXCL16 explicitly combines with Ox LDL, indicating that it probably relates to atherosclerosis (Shimaoka et al., 2000). There was indeed research deeming CXCL16 can have athero-protective effect but might make plaque unstable, yet CXCR6 can prevent myocardial ischemia (Izquierdo et al., 2014). Furthermore, the CXCL16 also induces a strong chemotactic response and calcium mobilization that may have a potential effect on the progression of CAVD. CXCL5 bound to G-protein coupled receptor and transmits cell signals recruiting neutrophils promoting angiogenesis and rebuilding connective tissue. In addition, CXCL5/ENA-78 is a pro-inflammatory chemokine pair that mediates CXCR2-dependent neutrophil transportation in humans (Layhadi et al., 2018). Researchers believed that platelets are the major source of CXCL5 in homeostasis, nevertheless, CXCL5 is mainly generated by lung epithelial cells in fierce infection (Koltsova and Ley, 2010). Performer studies showed that CXCL5 induces EMT to promote CRC cell migration via activating ERK/Elk-1/Snail, and enhances infiltration by activating AKT/GSK3β, thereby inhibiting β-catenin degradation (Zhao et al., 2017). Interestingly, Ravi et al. (2017) found that the plasma CXCL5 level is negatively correlated with CHD severity from clinical evidence.
Besides, the CC cytokines CCL19, CCL8, and CCL18 screened in the network are proteins containing two neighboring cysteine. CCL19 can function in lymphocyte recirculation and homing and is also a significant regulatory factor, which induces T cell activation, immune tolerance, and inflammatory reaction in the course of sequential immune monitoring and homeostasis. It reported that CCL19 and the paired CCR7 were are associated with autoimmune diseases like rheumatoid arthritis and enteropathy (Burman et al., 2005). Damås et al. (2007) found that CCL19 was expressed in the vascular plaques of ApoE -/- mice with atherosclerosis, homo’s carotid plaque and plasma of sufferers with CAD. Moreover, they also testified the plasma level of CCL19 clearly increased in cases with unstable angina pectoris than the sick with stable angina pectoris and healthy control patients (Damås et al., 2007). CCL8 is another member of the CC subfamily, which CCL8 has chemotactic activity on mononuclear cells, leukomonocytes, basophilic granulocytes, and eosinophilic granulocytes. Through enlisting white blood cells to inflammatory places, CCL8 contributes to tumor-interrelated neutrophils infiltration and antivirotic condition in opposition to HIV (Lacalle et al., 2017). The cytokine CCL18 exerts obvious chemoattractant activities on immature T cells, CD4+ and CD8+ T cells, and resting lymphocytes. It recruits immature T lymphocytes to DCs and actuated macrophages in lymph glands and functions in humoral and cellular immune processes (Murphy et al., 2000). Matrix metalloproteinase (MMP) are related to the decomposition of ECM in natural physiological events like fetal development, engendering, and tissue reconstitution, along with in clinical courses like arthrophlogosis and metastatic tumor (Kluger et al., 2013). The function of MMP9 is to debase collagen IV and V. Researches on macaques have shown that MMP9 is concerned with IL-8-elicited mobilization of bone marrow-derived blood ancestral cells and additionally, some studies indicated that MMP9 acted on tumor-related tissue reconstitution (Pruijt et al., 1999). Vascular cell adhesion molecule 1 (VCAM1), belongs to the Ig superfamily, encodes sialoglycoprotein on endothelial cell surface activated by cytokine. VCAM1 is a type I membrane protein as well mediating the adhesion of leukocytes to endothelial cell and signaling transduction and functioning in the development of atherosclerosis and rheumatoid arthritis (Dessein et al., 2005). It is reported that VCAM1/ITGA4/ITGB1 interactivity possibly worked on the pathophysiological process of immunological reactions and leukocyte traffic to inflammatory spots. Pro-platelet basic protein (PPBP), alternately named as CXCL7, is set free abundantly from activated platelets and participates in vascular injury responses (Stankiewicz et al., 2014). PPBP is also an effective chemotactic agent and neutrophil activator. What is more, researched suggested that it stimulated a variety of cellular physiological courses containing DNA synthesis, mitosis, anaerobic glycolysis, cytosolic cAMP amassing, PG-E2 release, and so on (Brown et al., 2017). In a nutshell, it seems that the inflammatory response is very active during the calcification of CAVD.
To search immunocytes infiltration in the microenvironment of CAVD, CIBERSORT was employed to manage a thorough estimation of the data obtained from GEO previously. A growing infiltration of macrophages M0, B cells memory, plasma cells and a dropping infiltration of B cells naïve, NK cells activated, macrophages M2 were identified that may be connected with the incidence and progression of CAVD. Former researchers have demonstrated the existence of inflammatory T cells which accounted for atherosclerotic lesions forming at an early stage of aortic valve disease and the presence of C-reactive protein in valve samples of candidates suffering from aortic stenosis after percutaneous aortic valve replacement illustrating an inflammatory state during calcification (Galante et al., 2001; Rajamannan et al., 2005). Notably, we found that the macrophage0 population were significantly high expression in the calcified valve. This is consistent with previous research results (Cote et al., 2013). In another study, aortic valve interstitial cells (AVICs), the main components of valves, were treated with conditioned media from M1 macrophages. The results displayed that expression of osteogenic calcification markers increased at transcription and translation levels, antibody barricade the inflammatory factors TNF and/or IL-6 decreased the calcification ex vivo indicating a pro-calcific effect of macrophages (Li et al., 2017). Research showed that chronic inflammation with infiltration of lymphocytes, phagocytes, tissue cells, and mastocytes existed in calcific aortic stenosis (Steiner et al., 2012). In addition, it was reported that NK cells gathered in the valve and circulation of patients with CAVD, which is related to the addition of valve pressure gradient (Mazur et al., 2018). From another perspective, correlation analysis in our study showed that the identified key genes CCL8, CCL18, CCL19, CXCL16, and VCAM1 were related to lipid metabolism biomarkers, which were closely related to the occurrence of CAVD from a biochemical point of view. A study on senile calcific aortic valve disease showed that plasma lipoprotein(a) was an independent risk factor for CAVD (Zhanjing et al., 2019). It can be speculated that cytokines and lipid metabolites may also have lipid-cytokine-chemical cascades that is similar to neutrophil-driven joint inflammation in immune complex-mediated arthritis and eosinophilic pneumonia driven by TH2 cells (Sadik and Luster, 2012). Additionally, experiments have shown that the expression of chemokines can directly affect the production of cytokines and cellular immune responses (Murphy et al., 2000). From the results of our study, we reasonably believe that chemokines and immune cells interact with each other during inflammation and even calcification of CAVD. For example, CXCL13 obviously leads to the recruitment of B cells and the production of LT-a β. In turn, LT-a β can stimulate the secretion of CXCL13 (Lalani et al., 1999). In addition, many chemokines secreted by lymphocytes have their own chemotactic effects. The concentration of lymphocytes in the microenvironment can be significantly expanded. Chemokines are also expressed on the surface of immune cells such as T or B cells. The expression of CCR7 causes dendritic cells to accumulate in the lymph. The fluid is infused into the human tube and T cells are distributed in the lymph nodes. Both CCL21 and CCL19 can bind CCR7, they can induce the targeted migration of dendritic cells, and move to a dense area of T cells. On the other hand, dendritic cells chemotaxis through secretion factors CCL18, CCL19, and CCI21 can also recruit natural T cells, Dendritic cells, and activated T cells (Stein et al., 2000). Chemokines provide an effective mechanism for the mutual positioning of immune cells to ensure the smooth progress of the immune response. The migration of immune cells is closely related to the functional role it plays, and chemokine receptors provide better characteristic markers for functional lymphocyte subsets during the progressive inflammatory calcification of AS.
Above all, we found that the chemokines CXCL13, CXCL8, CXCL16, CXCL5, CCL19, CCL8, and CCL18 are the most important markers of CAVD. We detected that regulatory macrophages M0, B cells memory, plasma cells, B cells naïve, NK cells activated, and macrophages M2 possibly referred to the occurrence and progress of CAVD. As the number and status of so many immune cells are significantly changed in CAVD, despite how the immune system affects AV calcification is not clear, we can infer that the immune system produces a major effect on the proceeding of calcification. In addition, these chemokines and immune cells may interact in the development of calcification in CAVD. The limitation of this study is that we did not conduct experimental studies and, thus, a deeper investigation of the immune cells can ascertain the ideal immunotherapy target and heighten autologous immunomodulatory for sufferers with CAVD.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
DW was agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and drafted the manuscript. BL and TX made substantial contributions to conception and design and revised the manuscript critically for important intellectual content. JW and KX made substantial contributions to acquisition of data. WY made substantial contributions to analysis and interpretation of data. QS gave final approval of the version to be published. All authors read and approved the final manuscript.
This research was supported by grants from the National Natural Science Foundation of China (81770251), Social Undertakings and People’s Livelihood Protection Technology Innovation of Chongqing Science Commission (cstc2017shmsA130086), and Chongqing city Yuzhong District Science and Technology Basic and Advanced Research Projects (20170107).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.650213/full#supplementary-material
Supplementary Table 1 | Immune Cell Infiltration of CAVD with 22 subpopulations of immune cells in 30 samples.
Supplementary Table 2 | Correlation coefficients of 10 hub genes and lipid metabolism markers.
AAA, abdominal aortic aneurysm disease; AS, aortic stenosis; AVIC, aortic valve interstitial cells; BP, biological process; CAD, coronary artery disease; CAS, calcific aortic stenosis; CAT, cholesterol acyltransferase; CAVD, calcific aortic valve disease; CC, cell component; CCL, C-C Motif chemokine ligand; CCR, C-C motif chemokine receptor; CXCL, C-X -C Motif chemokine ligand; CXCR, C-X -C motif chemokine receptor; DEG, differentially expressed gene; ECM, extracellular matrix; FAS, fatty acid synthase; GO, gene ontology; HMGCR, hydroxymethylglutaryl coenzyme A reductase; KEGG, Kyoto encyclopedia of genes and genomes; LDL, low-density lipoprotein; MF, molecular function; MMP, matrix metalloproteinase; PPBP, pro-platelet basic protein; PPI, protein-protein interaction; RHD, rheumatic heart disease; TAVR, transcatheter aortic valve replacement; VCAM1, vascular cell adhesion molecule 1.
Ali, H. R., Chlon, L., Pharoah, P. D. P., Markowetz, F., and Caldas, C. (2016). Patterns ofImmune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS Med. 13:e1002194. doi: 10.1371/journal.pmed.1002194
Borroni, E. M., Mantovani, A., Locati, M., and Bonecchi, R. (2010). Chemokine receptors intracellular trafficking. Pharmacol. Ther. 127, 637–658.
Brown, A. J., Joseph, P. R., Sawant, K. V., and Rajarathnam, K. (2017). Chemokine CXCL7 heterodimers: structural insights, cxcr2 receptor function, and glycosaminoglycan interactions. Int. J. Mol. Sci. 18:748. doi: 10.3390/ijms18040748
Burman, A., Haworth, O., Hardie, D. L., Amft, E. N., Siewert, C., and Jackson, D. G. (2005). A chemokine-dependent stromal induction mechanism for aberrant lymphocyte accumulation and compromised lymphatic return in rheumatoid arthritis. J. Immunol. 174, 1693–1700. doi: 10.4049/jimmunol.174.3.1693
Cai, W., Li, H., Zhang, Y., and Han, G. (2020). Identification of key biomarkers and immune infiltration in the synovial tissue of osteoarthritis by bioinformatics analysis. PeerJ 8:e8390. doi: 10.7717/peerj.8390
Coffey, S., Cox, B., and Williams, M. J. (2014). The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 63, 2852–2861. doi: 10.1016/j.jacc.2014.04.018
Cote, N., Mahmut, A., Bosse, Y., Couture, C., Pagé, S., Trahan, S., et al. (2013). Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation 36, 573–581.
Damås, J., Smith, C., Øie, E., Fevang, B., Halvorsen, B., Waehre, T., et al. (2007). Enhanced expression of the homeostatic chemokines CCL19 and CCL21 in clinical and experimental atherosclerosis: possible pathogenic role in plaque destabilization. Arterioscler. Thromb. Vasc. Biol. 27, 614–620. doi: 10.1161/01.atv.0000255581.38523.7c
Deng, Y. J., Ren, E. H., Yuan, W. H., Zhang, G. Z., Wu, Z. L., and Xie, Q. Q. (2020). GRB10 and E2F3 as diagnostic markers of osteoarthritis and their correlation with immune infiltration. Diagnostics 10:171. doi: 10.3390/diagnostics10030171
Dessein, P. H., Joffe, B. I., and Singh, S. (2005). Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res. Ther. 7, R634–R643.
Dutta, P., and Lincoln, J. (2018). Calcific aortic valve disease: a developmental biology perspective. Curr. Cardiol. Rep. 20:21.
Freeman, R. V., and Otto, C. M. (2005). Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111, 3316–3326. doi: 10.1161/circulationaha.104.486738
Galante, A., Pietroiusti, A., Vellini, M., Piccolo, P., Possati, G., De Bonis, M., et al. (2001). C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J. Am. Coll. Cardiol. 38, 1078–1082. doi: 10.1016/s0735-1097(01)01484-x
Gould, S. T., Srigunapalan, S., Simmons, C. A., and Anseth, K. S. (2013). Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ. Res. 113, 186–197. doi: 10.1161/circresaha.112.300154
Hussain, M., Adah, D., Tariq, M., Lu, Y., Zhang, J., and Liu, J. (2019). CXCL13/CXCR5 signaling axis in cancer. Life Sci. 227, 175–186. doi: 10.1016/j.lfs.2019.04.053
Iung, B., and Vahanian, A. (2011). Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 8, 162–172. doi: 10.1038/nrcardio.2010.202
Izquierdo, M. C., Martin-Cleary, C., Fernandez-Fernandez, B., Elewa, U., Sanchez-Niño, M. D., and Carrero, J. J. (2014). CXCL16 in kidney and cardiovascular injury. Cytokine Growth Factor. Rev. 25, 317–325. doi: 10.1016/j.cytogfr.2014.04.002
Jian, B., Narula, N., Li, Q., Mohler, E., and Levy, R. J. (2003). Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Throc. Surg. 75, 457–465. doi: 10.1016/s0003-4975(02)04312-6
Kaden, J., Kiliç, R., Sarikoç, A., Hagl, S., Lang, S., Hoffmann, U., et al. (2005). Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int. J. Mol. Med. 16, 869–872.
Kluger, M. A., Zahner, G., Paust, H. J., Schaper, M., Magnus, T., Panzer, U., et al. (2013). Leukocyte-derived MMP9 is crucial for the recruitment of proinflammatory macrophages in experimental glomerulonephritis. Kidney Int. 83, 865–877. doi: 10.1038/ki.2012.483
Kokje, V. B. C., Gabel, G., Dalman, R. L., Koole, D., Northoff, B. H., and Holdt, L. M. (2018). CXCL8 hyper-signaling in the aortic abdominal aneurysm. Cytokine 108, 96–104. doi: 10.1016/j.cyto.2018.03.031
Koltsova, E. K., and Ley, K. (2010). The mysterious ways of the chemokine CXCL5. Immunity 33, 7–9. doi: 10.1016/j.immuni.2010.07.012
Kostyunin, A. E., Yuzhalin, A. E., Ovcharenko, E. A., and Kutikhin, A. G. (2019). Development of calcific aortic valve disease: do we know enough for new clinical trials? J. Mol. Cell Cardiol. 132, 189–209. doi: 10.1016/j.yjmcc.2019.05.016
Lacalle, R. A., Blanco, R., Carmona-Rodriguez, L., Martin-Leal, A., Mira, E., and Manes, S. (2017). Chemokine receptor signaling and the hallmarks of cancer. Int. Rev. Cell Mol. Biol. 331, 181–244. doi: 10.1016/bs.ircmb.2016.09.011
Lalani, A. S., Masters, J., Zeng, W., Barrett, J., Pannu, R., and Everett, H. (1999). Arendt, grant mcfadden. use of chemokine receptors by poxviruses. Science 286, 1968–1971. doi: 10.1126/science.286.5446.1968
Layhadi, J. A., Turner, J., Crossman, D., and Fountain, S. J. (2018). ATP evokes Ca(2+) responses and CXCL5 secretion via P2X4 receptor activation in human monocyte-derived macrophages. J. Immunol. 200, 1159–1168. doi: 10.4049/jimmunol.1700965
Lei, F., Zhang, H., and Xie, X. (2019). Comprehensive analysis of an lncRNA-miRNA-mRNA competing endogenous RNA network in pulpitis. PeerJ 7:e7135. doi: 10.7717/peerj.7135
Li, G., Qiao, W., Zhang, W., Li, F., Shi, J., and Dong, N. (2017). The shift of macrophages toward M1 phenotype promotes aortic valvular calcification. J. Thorac. Cardiovasc. Surg. 153, 1318–1327 e113181327e1.
Li, G., Wu, X. J., Kong, X.-Q., and Wang, L. (2015). Cytochrome c oxidase subunit VIIb as a potential target in familial hypercholesterolemia by bioinformatical analysis. Eur. Rev. Med. Pharmacol. Sci. 2015, 4139–4145.
Lindman, B. R., Bonow, R. O., and Otto, C. M. (2013). Current management of calcific aortic stenosis. Circ. Res. 113, 223–237. doi: 10.1001/jama.1963.03700100151094
Lindman, B. R., Clavel, M. A., Mathieu, P., Iung, B., Lancellotti, P., Otto, C. M., et al. (2016). Calcific aortic stenosis. Nat. Rev. Dis. Primers 2:16006.
Liu, M., Luo, M., Sun, H., Ni, B., and Shao, Y. (2017). Integrated bioinformatics analysis predicts the key genes involved in aortic valve calcification: from hemodynamic changes to extracellular remodeling. Tohoku J. Exp. Med. 243, 263–273. doi: 10.1620/tjem.243.263
Mathieu, P., and Boulanger, M. C. (2014). Basic mechanisms of calcific aortic valve disease. Can. J. Cardiol. 30, 982–993. doi: 10.1016/j.cjca.2014.03.029
Mazur, P., Mielimonka, A., Natorska, J., Wypasek, E., Gawêda, B., Sobczyk, D., et al. (2018). Lymphocyte and monocyte subpopulations in severe aortic stenosis at the time of surgical intervention. Cardiovasc. Pathol. 35, 1–7. doi: 10.1016/j.carpath.2018.03.004
Murphy, P. M., Baggiolini, M., Charo, I. F., Hébert, C. A., Horuk, R., and Matsushima, K. (2000). International union of pharmacology. XXII. nomenclature for chemokine receptors. Pharmacol. Rev. 52, 145–176.
Nishimura, R. A., Otto, C. M., and Bonow, R. O. (2014). AHA/ACC guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 63, 1486–1588.
O’Brien, K., Shavelle, D., Caulfield, M., McDonald, T. O., Olin-Lewis, K., Otto, C. M., et al. (2002). Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 106, 2224–2230. doi: 10.1161/01.cir.0000035655.45453.d2
Osnabrugge, R. L., Mylotte, D., Head, S. J., Van Mieghem, N. M., Nkomo, V. T., LeReun, C. M., et al. (2013). Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J. Am. Coll. Cardiol. 62, 1002–1012.
Otto, C. M., and Prendergast, B. (2014). Aortic-valve stenosis–from patients at risk to severe valve obstruction. N. Engl. J. Med. 371, 744–756. doi: 10.1056/nejmra1313875
Pawade, T. A., Newby, D. E., and Dweck, M. R. (2015). Calcification in aortic stenosis: the skeleton key. J. Am. Coll. Cardiol. 66, 561–577.
Pruijt, J. F., Fibbe, W. E., Laterveer, L., Pieters, R. A., Lindley, I. J., and Paemen, L. (1999). Prevention of interleukin-8-induced mobilization of hematopoietic progenitor cells in rhesus monkeys by inhibitory antibodies against the Metalloproteinase gelatinase B (MMP-9). Proc. Natl. Acad. Sci. U.S.A. 96, 10863–10868. doi: 10.1073/pnas.96.19.10863
Rajamannan, N. M., Bonow, R. O., and Rahimtoola, S. H. (2007). Calcific aortic stenosis: an update. Nat. Clin. Pract. Cardiovasc. Med. 4, 254–262.
Rajamannan, N. M., Nealis, T. B., Subramaniam, M., Pandya, S., Stock, S. R., Ignatiev, C. I., et al. (2005). Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation 111, 3296–3301. doi: 10.1161/circulationaha.104.473165
Ravi, S., Schuck, R. N., Hilliard, E., Lee, C. R., Dai, X., Lenhart, K., et al. (2017). Clinical evidence supports a protective role for CXCL5 in coronary artery disease. Am. J. Pathol. 187, 2895–2911. doi: 10.1016/j.ajpath.2017.08.006
Sadik, C. D., and Luster, A. D. (2012). Lipid-cytokine-chemokine cascades orchestrate leukocyte recruitment in inflammation. J. Leukoc. Biol. 91, 207–215. doi: 10.1189/jlb.0811402
Shimaoka, T., Kume, N., Minami, M., Hayashida, K., Kataoka, H., and Kita, T. (2000). Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J. Biol. Chem. 275, 40663–40666. doi: 10.1074/jbc.c000761200
Stankiewicz, A. M., Goscik, J., Swiergiel, A. H., Majewska, A., Wieczorek, M., and Juszczak, G. R. (2014). Social stress increases expression of hemoglobin genes in mouse prefrontal cortex. BMC Neurosci. 15:130.
Stein, J. V., Rot, A., Luo, Y., Narasimhaswamy, M., Nakano, H., and Gunn, M. D. (2000). The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, Exodus-2) triggers lymphocyte function–associated antigen 1–mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76. doi: 10.1084/jem.191.1.61
Steiner, I., Krbal, L., Rozkos, T., Harrer, J., and Laco, J. (2012). Calcific aortic valve stenosis: immunohistochemical analysis of inflammatory infiltrate. Pathol. Res. Pract. 208, 231–234. doi: 10.1016/j.prp.2012.02.009
Tang, J., Yang, Q., Cui, Q., Zhang, D., Kong, D., Liao, X., et al. (2020). Weighted gene correlation network analysis identifies RSAD2, HERC5, and CCL8 as prognostic candidates for breast cancer. J. Cell. Physiol. 235, 394–407. doi: 10.1002/jcp.28980
Waehre, A., Halvorsen, B., Yndestad, A., Husberg, C., Sjaastad, I., Nygård, S., et al. (2011). Lack of chemokine signaling through CXCR5 causes increased mortality, ventricular dilatation and deranged matrix during cardiac pressure overload. PLoS One 6:e18668. doi: 10.1371/journal.pone.0018668
Wang, X., Liao, X., Yang, C., Huang, K., Yu, T., Yu, L., et al. (2019). Identification of prognostic biomarkers for patients with hepatocellular carcinoma after hepatectomy. Oncol. Rep. 41, 1586–1602.
Yin, Y., Zou, Y. F., Xiao, Y., Wang, T. X., Wang, Y. N., Dong, Z. C., et al. (2020). Identification of potential hub genes of atherosclerosis through bioinformatic analysis. J. Comput. Biol. 28, 60–78. doi: 10.1089/cmb.2019.0334
Zechner, R., Zimmermann, R., Eichmann, T. O., Kohlwein, S. D., Haemmerle, G., Lass, A., et al. (2012). FAT SIGNALS–lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 15, 279–291. doi: 10.1016/j.cmet.2011.12.018
Zhang, N., Chen, Y., Lou, S., Shen, Y., and Deng, J. (2019). A six-gene-based prognostic model predicts complete remission and overall survival in childhood acute myeloid leukemia. Onco Targets Ther. 12, 6591–6604. doi: 10.2147/ott.s218928
Zhanjing, S. Z., Hong, W. H., Can, C., Peiguang, L., Jing, L., and Weiwei, Z. (2019). Relationship between plasma lipoprotein a and senile calcified aortic valve disease. Chin. J. Evid. Based Cardiovasc. Med. 2, 167–170.
Zhao, J., Lv, T., Quan, J., Zhao, W., Song, J., Li, Z., et al. (2018). Identification of target genes in cardiomyopathy with fibrosis and cardiac remodeling. J. Biomed. Sci. 25:63.
Zhao, J., Ou, B., Han, D., Wang, P., Zong, Y., and Zhu, C. (2017). Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3beta/beta-catenin pathways. Mol. Cancer 16:70.
Zhao, Y., Xia, Z., Lin, T., and Yin, Y. (2020). Significance of hub genes and immune cell infiltration identified by bioinformatics analysis in pelvic organ prolapse. PeerJ 8:e9773. doi: 10.7717/peerj.9773
Zou, R., Zhang, D., Lv, L., Shi, W., Song, Z., and Yi, B. (2019). Bioinformatic gene analysis for potentialbiomarkers and therapeutic targets of atrialfibrillation-related stroke.pdf. J. Trans. Med. 17:45.
Keywords: CAVD, chemokines, immune cells, CXCL13, CCL19, CXCL8, macrophages, bioinformatics analysis
Citation: Wang D, Xiong T, Yu W, Liu B, Wang J, Xiao K and She Q (2021) Predicting the Key Genes Involved in Aortic Valve Calcification Through Integrated Bioinformatics Analysis. Front. Genet. 12:650213. doi: 10.3389/fgene.2021.650213
Received: 06 January 2021; Accepted: 05 March 2021;
Published: 11 May 2021.
Edited by:
Ben-gong Zhang, Wuhan Textile University, ChinaReviewed by:
Ming Yi, China University of Geosciences Wuhan, ChinaCopyright © 2021 Wang, Xiong, Yu, Liu, Wang, Xiao and She. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang She, cXNoZTk4QGNxbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.